Abstract
Experiments of nature are crucial for informing scientific discovery. Nearly 30 years ago we began to investigate an outbreak of rachitic bone disease in adolescent New World primates residing at the Los Angeles Zoo. Our investigation of this experiment of nature and that of an adolescent human female with a similar phenotype led us to the discovery of a novel means for relative resistance to vitamin D in primates, including man. We coined this resistance-causing protein the vitamin D response element-binding protein or VDRE-BP for its ability to compete in trans with the liganded vitamin D receptor (VDR) for its cognate response elements. VDRE-BP is now identified as a nucleic acid-binding protein(s) in the heterogeneous nuclear ribonucleoprotein C (hnRNPC) family. The purpose of this review is to examine the role of the VDRE-BP and other associated intracellular proteins that regulate the expression of vitamin D-controlled genes in nonhuman and human primates.
Man with all his noble qualities…with his god-like intellect which has penetrated into the movements and constitution of the solar system…still bears in his bodily frame, the indelible stamp of his lowly origin.
Charles Robert Darwin, in the Descent of Man
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Vitamin D
- Resistance
- 1,25-Dihydroxyvitamin D
- Monkeys
- Ribonucleoprotein
- Primate evolution
- Steroid hormone
- New World monkeys
- Vitamin D response element
-
Nearly 30 years ago we began to investigate an outbreak of rachitic bone disease in adolescent New World primates residing at the Los Angeles Zoo.
-
Our investigation of this “experiment of nature” and that of an adolescent human female with a similar phenotype led us to the discovery of a novel means for relative resistance to vitamin D in primates, including man.
-
We coined this resistance-causing protein the vitamin D response element-binding protein or VDRE-BP for its ability to compete in trans with the liganded vitamin D receptor (VDR) for its cognate response elements.
-
VDRE-BP is now identified as a nucleic acid-binding protein(s) in the heterogeneous nuclear ribonucleoprotein C (hnRNPC) family.
-
The purpose of this review is to examine the role of the VDRE-BP and other associated intracellular proteins that regulate the expression of vitamin D-controlled genes in nonhuman and human primates.
1 Early Primate Evolution
The three major primate infraorders, platyrrhines or New World primates, catarrhines or Old World primates, and lemurs, evolved independently of one another [1] (Fig. 28.1a) in the Eocene period, 50–100 million years ago owing to rupture of the great southern hemispheric landmass, Pangea. These tectonic events resulted in the American land mass and Madagascar moving away from Africa. Because this continental separation occurred early in the process of primate evolution, primordial primates in these three infraorders, were trapped in what we now know as South America, Africa, and Madagascar, respectively. Compared to Old World primates, including man, which have populated virtually every land mass on our planet over the course of last 50 million years, New World primates have remained confined to Central and South America, ±20° north and south of the equator. As such and in comparison to terrestrial Old World primate like gorillas, New World primates have evolved to be smaller in stature; a characteristic well suited to their lifestyle as plant-eating, arboreal sunbathers, residing in the canopy of the periequatorial rain forests of the Americas.
New World primate evolution and rachitic bone lesion. Panel (a) describes in geographic terms the independent evolution of the three primate suborders, Platyrrhini, Catarrhini, and Lemuridae, in South America (the New World), Africa (the Old World), and Madagascar, respectively. Panel (b) displays the characteristic “cupping” and “fraying” of the tibial metaphysis (arrows) in a rachitic New World primate resident of the Los Angeles Zoo
2 Skeletal Disease in Captive New World Primates
The appearance of generalized metabolic bone disease in captive primates has been recognized for the last 150 years [2]. The disease, which has yet to be well studied from a histopathological standpoint, carries the clinical and radiological stigmata of rickets in adolescent primates and osteomalacia in adults, especially females [3] (Fig. 28.1b). Compared to Old World primates reared in captivity, New World primates are particularly susceptible to the disease. The disorder affects primarily young, growing animals and results in muscle weakness, skeletal fragility, and in many instances death of the affected individual. Rachitic bone disease of this sort has long presented a problem to veterinarians caring for captive platyrrhines, particularly in North American and European zoos [4, 5], because death of preadolescent and adolescent primates prior to sexual maturity severely limits on-site breeding programs.
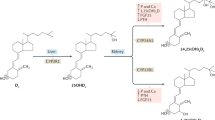
Because the disease was reported to be ameliorated by either the oral administration of vitamin D3 in large doses or by ultraviolet B (UVB) irradiation of affected primates, it was presumed to be caused by vitamin D deficiency [4]. The frequent occurrence of rickets and osteomalacia in New World primates was also ascribed to the relative inability of platyrrhines, compared to Old World primates including man, to effectively employ vitamin D2 in their diet [6]; a similar observation had been made for chickens [7]. Realizing that in order to be active as a hormone vitamin D must first be converted to the pro-hormone 25-hydroxyvitamin D [25(OH)D] and then to the hormone 1,25-dihydroxyvitamin D [1,25(OH)2D] (Fig. 28.2) and using an assay technology that does not discriminate between 25-hydroxylated vitamin D2 and vitamin D3 metabolites, Marx and colleagues [8] determined that 25(OH)D levels were two- to threefold higher when platyrrhines were dosed with supplemental vitamin D3 than with vitamin D2. These data suggested that 25-hydroxylation of vitamin D substrate in New World primates was much more effective when vitamin D3 was employed as substrate. However, in the same study, two species of Old World primates demonstrated similar discrimination against vitamin D2 in favor of vitamin D3 to promote significantly more 25(OH)D produced.
Scheme of vitamin D synthesis and metabolism in New World primates. The bold arrows describe the means by which high 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] levels are achieved and maintained in New World primates. Ultraviolet B photon (UVB) exposure is increased in the natural habitat of New World primates, the canopy of the equatorial rain forests of Central and South America. Increased cutaneous vitamin D3 synthesis results in increased production, via one of the many hepatic vitamin D-25-hydroxylases (25-OHase), of 25-hydroxyvitamin D3 [25(OH)D3]. Elevated 1,25(OH)2D3 levels are achieved by increased synthesis of the hormone via the CYP27B1-hydroxylase (1-OHase) as well as by diminished catabolism to scheme 24,25(OH)2D3 via the CYP24A1. In this way 1,25(OH)2D3 becomes available to the VDR in relatively large quantities to compensate for the hormone-resistant state characteristic of New World primates
The above results seemed to indicate that all subhuman primates, whether from Old or New World genera, were relatively resistant to vitamin D2 in terms of its ability to engender an increase in total serum levels of 25(OH)D. This led Hay and colleagues [9] to suggest that New World primates may transport 25(OH)D2 and 25(OH)D3 in the serum by means that are dissimilar from those encountered in Old World primate species. The Hay hypothesis was subsequently disproven by Bouillon et al. [10], who showed that the serum vitamin D-binding protein (DBP) was the major carrier of 25(OH)D2 and 25(OH)D3 in the serum of both New and Old World primates. It has been subsequently shown that 25(OH)D2 does have shorter half-life in serum than does 25(OH)D3, regardless of species examined, owing to a relative reduction in the affinity of DBP for vitamin D2 metabolites [10].
Other possible explanations for the greatly increased susceptibility of New World compared to Old World primates to develop vitamin D-deficient skeletal disease is that New World primates in captivity somehow failed to convert the pre-pro-hormone vitamin D to the pro-hormone 25(OH)D effectively (see Fig. 28.2) and/or free-living New World primates employ a dietary source of vitamin D that is distinct from what is normally provided to the same species in captivity. The former has been shown not to be the case; there are now several reports of free-living New World primates possessing serum concentrations of 25(OH)D that approximate those encountered in captive primates of the same genera given supplemental dietary vitamin D or exposed to supplemental UVB [11]. The latter possibility is likely. It is now recognized that many genera of New World primates consume fungi in the wild [12–15]. When exposed to UVB, fungi are the richest natural source of vitamin D2 on the planet [16].
3 Vitamin D and Steroid Hormone Resistance in New World Primates
The question of why platyrrhines were more susceptible to vitamin D deficiency than were catarrhines began to be answered with the detection of extraordinarily high circulating levels of the active vitamin D metabolite, 1,25-dihydroxyvitamin D [1,25(OH)2D] in New World primates [17, 18]. These data pointed to the fact that New World primates were resistant to the active vitamin D hormone. Beginning in 1985, our research team was asked to investigate an outbreak of rickets among New World primate species at the Los Angeles Zoo. The index case in our original studies was a preadolescent female New World primate of the Emperor tamarin species. When investigated radiographically (Fig. 28.1b), this tamarin and those like her displayed classical rickets complete with growth retardation, metaphyseal cupping, and fraying characteristic of rickets. In order to investigate this rachitic syndrome, blood and urine was collected from involved monkeys as well as from control, nonrachitic New and Old World primates. Compared to Old World primates and as shown in Fig. 28.3a, that comparison yielded a biochemical phenotype that was most remarkable for an elevated serum 1,25(OH)2D level in rachitic New World primates [18]. In fact, with the exception of nocturnal primates in the genus Aotus, New World primates in all other genera had vitamin D hormone levels ranging up to two orders of magnitude higher than that observed in Old World primates, including man [19–21].
Biochemical phenotype of rachitic New World primates. Panel (a) demonstrates biochemical indices of bone health in New World primate suffering from rickets compared to developmental age- and sex-matched nonrachitic Old World primates. The outstanding characteristic is a 1,25-dihydroxyvitamin D [1,25(OH)2D] level 2–3 orders of magnitude greater than that observed in Old World primates, including man. Panel B shows the mean 25-hydroxyvitamin D (left) and 1,25-dihydroxyvitamin D levels (right) in seven different rachitic New World primates before (pre) and after (post) exposure to 6 months of artificial sunlight in their enclosures. The upper limits of the normal human Old World primate range is described by the dotted line. Both substrate and product rose significantly with light therapy and resulted in cure of rickets. (Data from ref. [22])
In the initial analysis New World primates affected with rickets were those with the lowest 1,25(OH)2D levels, while their healthy counterparts were those with the highest serum 1,25(OH)2D levels. These data were interpreted to mean that most New World primate genera were naturally resistant to the vitamin D hormone, and that the resistant state could be compensated by maintenance of high 1,25(OH)2D levels. If this was true, then an increase in the serum 1,25(OH)2D concentration in affected primates should result in biochemical compensation for the resistant state and resolution of their rachitic bone disease. When rachitic New World primates were exposed to artificial sunlight of 6 months duration in their enclosures, both substrate serum 25(OH)D and product 1,25(OH)2D levels rose dramatically, resulting in cure of rickets [22] (Fig. 28.3b). New World primates are periequitorial sunbathers for a reason. As depicted by the oversized arrows in a simplified scheme of vitamin D synthesis and metabolism (Fig. 28.3), New World primates require a lot of cutaneous vitamin D synthesis in order to push their 25(OH)D and 1,25(OH)2D levels high enough to interact effectively with the vitamin D receptor (VDR). The question remained as to why these primates are resistant to all but the highest levels of the vitamin D hormone.
The concept of generalized steroid hormone resistance in New World primates was first revealed by Brown et al. in 1970 [23]. These investigators discovered greatly elevated serum cortisol levels in platyrrhini compared to catarrhini. Despite biochemical evidence of resistance to glucocorticoids, platyrrhines affected with high cortisol levels showed no sign of glucocorticoid deficiency or toxicity at the level of the target organ; glucose homeostasis, electrolyte balance, blood pressure, and life expectancy were all similar to that observed in Old World primates [24]. These data indicated that glucocorticoid resistance in New World primates was physiologically compensated by increased synthesis of the hormone. Increased production of the hormone was achieved by lack of feedback inhibition of pituitary adrenocorticotropic hormone (ACTH) production [25], adrenal (zona fasiculata) hypertrophy [26], and increased enzymatic synthesis and decreased catabolism of cortisol [27, 28]. A relative increase in the availability of glucocorticoid to target tissues was also proposed to occur in New World primates [29] and to participate in the counter-response to cortisol resistance. What is the proximate cause of glucocorticoid resistance in New World primates? Early studies from the group of Lipsett and Loriaux [24, 30, 31] suggested that resistance was caused by expression of a glucocorticoid receptor (GR) in New World primates with a lower affinity for cortisol. Most recently, relative overexpression of FKBP51, the FK506-binding immunophilin that normally interacts with the heat shock protein 90 (hsp90)-GR complex, was postulated to be the cause of lowered affinity of the New World primate GR for its cognate ligand [32, 33] and by sequestration of the GR in the cytoplasm of the cell [34, 35]. It remains to be determined whether constitutive overexpression of the New World primate FKBP51 in Old World primate cells will squelch GR-directed transactivation in host cells.
New World primates are also resistant to steroid hormones produced by the ovary [25]. Until recently, it was considered that estrogen and progesterone resistance resulted from a diminishment of the estrogen receptor (ER) and progesterone receptor (PR) population in target tissues. A similar mechanism was proposed for vitamin D resistance [36]. As will be discussed below, it now appears that the steroid hormone and vitamin D hormone receptor complement of New World primates is not functionally distinct from that of their Old World primate counterparts. What is different is the relative overexpression in New World primate cells of at least two distinct families of intracellular proteins, the heterogeneous nuclear ribonucleoproteins (hnRNPs) and the heat-shock proteins 70 (hsp70), which conspire to legislate, by receptor-independent means, the degree of steroid/sterol hormone resistance among the various New World primate species.
4 Investigating the Biochemical Nature of Vitamin D Resistance in New World Primates
In order to answer the above question, cultured fibroblasts and immortalized cell lines from both resistant and hormone-responsive New and Old World primates were used to track, step by step, the path taken by the vitamin D hormone from the serum vitamin D-binding protein (DBP) in the blood in route to the nucleus and transactivation of hormone-responsive genes [19–22, 37–42] (Fig. 28.4). It was determined that the movement of hormone from DBP across the cell membrane and through the cell cytoplasm and nuclear membrane in New World primate cells was indistinguishable from that observed in Old World primate cells.
Pathway of hormone 1,25-dihydroxyvitamin D (1,25-D) from the blood to nucleus of the target cell in the vitamin D-resistant New World primate. In Old World primate cells (Panel a) the vitamin D hormone (dark triangles) moves normally from the circulating vitamin D-binding protein (DBP), through the cell membrane and cytoplasm, and onto the vitamin D receptor (VDR) paired with the retinoid X receptor (RXR) forming a heterodimeric complex in the cell nucleus. This heterodimeric complex then interacts with its cognate cis element, the vitamin D response element (VDRE), and initiates transcription of vitamin D-regulated genes. Panel (b) demonstrates similar events as they occur in New World primate cells. The jagged line at the VDRE represents a relative inability of the heterodimeric receptor complex to engage the cis element. This and the accumulation of hormone in the cell cytoplasm represent salient disparities in hormone handling and action in the New World and Old World primate cells
It was also determined that the ability of the New World primate VDR to bind to 1,25(OH)2D3 or 1,25(OH)2D2 and induce receptor dimerization with the retinoid X receptor (RXR) was normal. In fact, when removed from the intranuclear environment and in distinction to previous reports [36], the VDR in New World primates was similar to the Old World primate VDR in all biochemical and functional respects [40]. What was not the same in New World primate cells (see Fig. 28.4b) was the reduced ability of VDR-RXR complex to bind to its cognate cis element and transactivate gene expression. In addition to this failure was the apparent buildup of hormone in the cytoplasm of the New World primate cell.
In order to elucidate nuclear receptor events in New World primate cells, the nuclei of New World primate cells were isolated and extracted. In addition to the VDR–RXR, it was determined that these extracts contained a second protein that was bound by a consensus vitamin D response element (VDRE). This protein was coined the vitamin D response element-binding protein (VDRE-BP) [43]. In electromobility shift assay (EMSA) using the VDRE as probe (Fig. 28.5a), Old World primate cell extract contained only the VDR–RXR bound to the VDRE probe, while the New World primate extract contained two probe-reactive bands, one compatible with the VDR–RXR and a second, more pronounced VDRE–BP–VDRE band. This VDRE–BP–VDRE binding reaction was specific, as the VDRE–BP was competed away from VDRE probe by the addition of excess unlabeled VDRE. These data suggested that VDRE–BP might function as a dominant-negative inhibitor of receptor-response element binding by competing in trans with receptor, “knocking it off” the VDRE (Fig. 28.5b). That was the case. When recombinant human VDR and RXR were permitted to interact in EMSA with increasing amounts of nuclear extract from either vitamin D-resistant cells containing a VDRE–BP or from normal vitamin D-responsive cells, the addition of more control extract acted only to amplify the VDR–RXR–retarded probe on the gel. By contrast, increasing amounts of the hormone-resistant extract competed away VDR–RXR–probe binding in favor VDRE–BP–probe binding.
Evidence for the dominant-negative action of the New World primate vitamin D response element-binding protein (VDRE-BP). Panel (a) shows an electromobility shift assay using consensus vitamin D response element as probe, showing the presence of a second trans-binding protein, in addition to the vitamin D receptor (VDR)–retinoid X receptor (RXR), in nuclear extracts of vitamin D-resistant New World primate cells. Addition of excess unlabeled VDRE is shown in lanes 3 and 7. Addition of anti-RXRalpha antibody (lane 4) supershifts, while addition of either anti-VDR antibody (lane 5) or New World primate nuclear extract containing the VDRE-BP (lane 6) competes away probe-VDR-RXR binding; these data are reprinted with permission of the authors from ref. [43]. Panel (b) is a cartoon representing the proposed competition for binding to the VDRE between the VDRE–BP and vitamin D receptor (VDR)
5 Response Element-Binding Proteins
The VDRE–BPs in New World primates have been identified [44, 45]. Previously considered to be only single-strand mRNA-binding proteins [46] the VDRE-BPs are members of the heterogeneous nuclear ribonucleoprotein C (hnRNP C) family, hnRNP C1 and hnRNPC2 [47], that acts by binding to nucleic acid substrates as a tetramer (C13C21) [48]. However, as pointed out (see Fig. 28.5a), VDRE–BPs can also bind specifically to double-strand DNA. In fact, it is by virtue of their ability to bind DNA that they can be distinguished from traditional corepressor proteins [49]. When overexpressed, they can effectively squelch VDR-directed transactivation. This ability to squelch transactivation is shown in Fig. 28.6. Depicted is VDRE-directed reporter activity in four different subclones of wild-type Old World primate cells stably overexpressing the New World primate VDRE–BP as well as New World primate cells that are naturally hormone-resistant. In all instances, stable overexpression of VDRE–BP squelched VDRE-directed luciferase activity substantially compared to the untransfected, wild-type host cell to levels observed in hormone-resistant New World primate cells that naturally overexpress the protein. These data provide strong confirmatory evidence that when naturally overexpressed in vivo, VDRE–BP is the cause of vitamin D resistance in these monkeys.
Vitamin D response element-binding protein (VDRE-BP) squelched transactivation. Shown is significant squelching (p < 0.001) of VDR–RXR-directed, VDRE–reporter-driven transactivation in four different clones of Old World primate (OWP) cells after stable transfection with the New World primate VDRE–BP; reporter activity in untransfected New World primate (NWP) cells is shown for comparison. (Data from ref. [43])
As previously noted New World primates appear to be resistant to a host of steroid/sterol hormones, including estrogen, androgen, progesterone, glucocorticoids, and mineralocorticoids, not just vitamin D. We have isolated and characterized the functional equivalent of the VDRE-BP for estrogens. This estrogen response element-binding protein (ERE-BP) is also an hnRNP, hnRNPD [48, 50–53].
6 Intracellular Vitamin D-Binding Proteins
On the way to the discovery of the VDRE–BPs in New World primate cells, it was also observed that these cells were extraordinarily efficient at accumulating 25-hydroxylated vitamin D metabolites in the cytoplasmic space (see Fig. 28.4b). Accumulation here was the result of expression of a second set of resistance-associated proteins. These intracellular vitamin D-binding proteins [54, 55], or IDBPs as they have come to be called, exhibit both high capacity and high affinity for 25-hydroxylated vitamin D metabolites. In fact, among all of the vitamin D metabolites that have been tested, IDBP purified from vitamin D-resistant New World primate cells binds 25(OH)D3 and 25(OH)D2 best [21, 55]; in a competitive displacement assay using radioinert 25(OH)D3 as competitor and [3H]25(OH)D3 as labeled ligand, the concentration of metabolite required to achieve half-maximal displacement of labeled hormone (EC50) < 1 nM. Although normally present in Old World primate, including human cells, these proteins can be overexpressed some 50-fold in New World primate cells. They are highly homologous to proteins in the human (Old World primate) heat-shock protein-70 family [56]. The first three members of this family cloned and characterized by our laboratory, IDBP-1, -2, and -3, bear a high degree of sequence identity with constitutively expressed human heat-shock protein-70, heat-shock-inducible heat-shock protein-70, and mitochondrial-targeted grp-75, respectively. The general domain structure of the IDBPs [56] is shown in Fig. 28.7a. They all contain an ATP-binding-ATPase domain ahead of a protein–protein interaction domain. Some, such as IDBP-3, also harbor an N-terminal organelle-targeting domain. The vitamin D ligand (substrate)-binding domain is in the middle of the molecule [57].
The hsp-70-related intracellular vitamin D-binding proteins and their proposed function in vitamin D-resistant New World primate cells. Panel (a) shows the general domain structure of these hsp-70-like proteins. They all contain an ATP-binding ATPase domain ahead of two protein–protein interaction domains. Some also harbor an N-terminal organelle-targeting domain. Two countervailing hypotheses for the function of these proteins were considered (Panel b). One hypothesis held that these IDBPs were “sink” molecules that worked in cooperation with the response element-binding proteins to exert vitamin D resistance by disallowing access of the hormone to the vitamin receptor (VDR) and the nucleus of the cell. The opposing hypothesis held that these were “swim” molecules, promoting the delivery of ligand to the vitamin D receptor, improving the ability of the VDR to dimerize and bind to the vitamin D response element (VDRE–BP), antagonizing the actions of the vitamin D response element-binding protein that is overexpressed in New World primate cells
What are these IDBPs doing inside the hormone-resistant New World primate cell? Two countervailing hypotheses were considered to explain the function of these proteins (Fig. 28.7b). One hypothesis held that these IDBPs were “sink” molecules that worked in cooperation with the VDRE–BP in the nucleus to exert vitamin D resistance by disallowing access of the hormone to the VDR and the nucleus of the cell. The opposing hypothesis held that these were “swim” molecules that actually promoted the delivery of ligand to the vitamin D receptor, improving the ability of the VDR to dimerize and bind to DNA, antagonizing the actions of the VDRE–BP that was overexpressed in New World primate cells. In order to determine which of these hypotheses was correct, we stably overexpressed the most abundant of these IDBPs, IDBP-1, or hsc70, in wild-type Old World primate cells and demonstrated that IDBP-1 imparted protransactivating potential [57]; the endogenous transcriptional activity of three different 1,25(OH)2D-responsive genes, the vitamin CYP24-hydroxylase, osteopontin, and osteocalcin genes, in Old World primate wild-type cells was markedly enhanced (Fig. 28.8a). It was concluded from these studies, at least for the function of transactivation, that IDBP-1 was a “swim” molecule for the vitamin D hormone, promoting delivery of ligand to the VDR.
Consequences of stable overexpression of members of the New World primate intracellular vitamin D-binding protein (IDBP) family in vitamin D-responsive Old World primate cells. Panel (a) depicts the 1,25-dihydroxyvitamin D concentration-dependent relative endogenous expression level, by Northern blot analysis, of three hormone-responsive genes in Old World primate cells before (open squares) and after (filled squares) stable overexpression of IDBP-1. Panel B shows the 1,25-dihydroxyvitamin D synthetic capacity of Old World primate (wild-type) before and after and stable overexpression of IDBP-1; IDBP-1.1 and -1.2 represent different clones of cells stably transfected with IDBP-1. (Data from refs. [57, 58])
Considering the facts that New World primates are required to maintain very high serum levels of 1,25(OH)2D in order to avert rickets (see Figs. 28.2 and 28.3), it was also hypothesized that the IDBPs, which are known to bind 25(OH)D even better than 1,25(OH)2D, will also promote the synthesis of the active vitamin D metabolite via promotion of the CYP27B-1-hydroxylase. Evidence that this is the case is provided in Fig. 28.8b. When human kidney cells expressing the CYP27B-1-hydroxylase gene were stably transfected with IDBP-1 and incubated with substrate 25(OH)D3, 1,25(OH)2D3 production went up 4-8-fold compared to untransfected wild-type cells [58]. This increase in specific 25(OH)D-1-hydroxylase activity occurred independent of a change in expression of the CYP27B-1-hydroxylase gene [58]. In fact, data [58] now strongly indicate that this increase in hormone production is the result of the ability of IDBPs to promote the delivery of substrate 25(OH)D to the inner mitochondrial membrane and the CYP27B-1-hydroxylase stabled there.
7 A New Model for Intracellular Vitamin D Trafficking
Dogma has held that sterol/steroid hormones such as vitamin D, by nature of their lipid solubility, move through the plasma membrane of the target cell and “ping-pong” around the cell interior until they encounter another specific binding protein such as the CYP27B-1-hydroxylase or the VDR with which to bind. Recent results, developed from a compendium of confocal imaging studies with fluorescently labeled IDBPs and vitamin D metabolites as well as with gst-pull-down, co-immunoprecipitation, and yeast 2-hybrid binding experiments [45], indicate that the hormone does not haphazardly “ping-pong” around the cell interior. Rather, the hormone enters the cell and is distributed to specific intracellular destinations by a series of protein–protein interactions that involve the hsp family of intracellular vitamin D-binding proteins.
For example, it is now know from the work of Willnow and coworkers [59, 60] that vitamin Ds can enter some target cells (e.g., the proximal tubular epithelial cell of the kidney, the principal site of endocrine-acting 1,25(OH)2D synthesis) via internalized vesicles (Fig. 28.9). The vitamin D stays bound to the serum vitamin D-binding protein (DBP), which is in turn bound by megalin and cubulin, members of the LDL superfamily of proteins. Once inside the cell there is interaction between the C-terminal domain of megalin, which protrudes into the cytoplasm, and the N-terminal domain of at least two different IDBPs, IDBP-1 and -3 [61, 62]. Upon acidification of the vesicle interior and denaturation of DBP, 25(OH)D is free to interact with the IDBP.
Proposed roles for the intracellular vitamin D-binding proteins (IDBPs) in the intracellular trafficking of vitamin D metabolites. Vitamin Ds enter target cells via internalized vesicles. The vitamin D remains bound to the serum vitamin D-binding protein (DBP), which is, in turn, bound by members of the LDL superfamily of proteins, megalin and cubulin. Once inside the cell there is the potential for interaction between the C-terminal domain of megalin, which protrudes into the cytoplasm, and the N-terminal domain of at least two different IDBPs, IDBP-1 and -3. It is proposed that if the interaction is with IDBP-1, then there is transfer of the vitamin D cargo to IDBP-1. IDBP-1 can undergo protein–protein interaction with the vitamin D receptor (VDR), resulting in delivery of its vitamin D cargo to the receptor and promotion of transactivation. On the other hand, if the megalin interaction is with IDBP-3, which possesses an amino-terminal mitochondrial targeting sequence (mito), then there exists the potential for the IDBP-3 and its cargo to enter mitochondrial. A protein–protein interaction between the CYP27B-1-hydroxylase and IDBP-3 can result in transfer of the vitamin D cargo to the enzyme for metabolism
If one overexpresses either IDBP-1, the hsc-70 homolog, or IDBP-3, a mitochondrially-targeted hsp, and incubates transfected IDBP-overexpressing cells with a fluorescently labeled 25-hydroxylated vitamin D metabolite, one will observe a significant increase in the uptake of the labeled hormone [63]. Moreover, if the protein–protein interaction is between megalin and IDBP-1 and the ligand is 1,25(OH)2D3, then the ultimate destination for that hormone and its chaperone is the unliganded VDR [45] residing in the perinuclear region of the cell. If, on the other hand, megalin interacts with IDBP-3, which contains an N-terminal targeting sequence for the inner mitochondrial membrane, then the ultimate destination for the hormone is the mitochondria. Confirmation of a protein–protein interaction between a substrate-carrying IDBP molecule and a target enzyme, the CYP27B-1-hydroxylase, has been accomplished with gst “pull-down” assays using the carboxy-terminal domain of the CYP27B-1-hydroxylase as bait [61]; this is the portion of the CYP27B-1-hydroxylase that is exposed to the intermembrane space of the mitochondria. Employing this substrate-accessible part of the enzyme to capture CYP27B-1-hydroxylase-interacting proteins, it has recently been shown that the grp-75-like IDBP-3, but not the hsc-like IDBP-1, interacts with the 25(OH)D-1-hydroxylase (Chun, unpublished).
8 Conclusion
In summary, it is proposed that these hsp-mediated-chaperoning events are normally active in man but overly active in hormone-resistant New World primates, where they function to compensate for the VDRE-BP (hnRNP C1/C2)-directed vitamin D-resistant state by augmenting receptor function and increasing vitamin D hormone synthesis. We anticipate that further analysis of these events will more clearly define the chaperone mediated vitamin D trafficking circuits that contribute to a determination of the action and metabolism of vitamin D metabolites in target cells harboring the VDR and/or vitamin D hydroxylases.
References
Pilbeam D. The descent of hominoids and hominoids and hominoids. Sci Am. 1984;250:84–96.
Bland Sutton JB. Observation on rickets etc. in wild animals. J Anat. 1884;18:363–97.
Krook L, Barrett RB. Simian bone disease—a secondary hyperparathyroidism. Cornell Vet. 1962;52:459–92.
Hershkovitz P. Living new world monkeys (Platyrrhini): with an introduction to primates. Chicago, IL: University of Chicago Press; 1977.
Jarcho MR, Power ML, Layne-Colon DG, Tardif SD. Digestive efficiency mediated by serum calcium predicts bone mineral density in the common marmoset (Callithrix jacchus). Am J Primatol. 2013;75(2):153–60.
Hunt RD, Garcia FG, Hegsted DM. A comparison of vitamin D2 and D3 in New World primates. I. Production and regression of osteodystrophia fibrosa. Lab Anim Care. 1967;17:222–34.
Steenbock H, Kletzein SWF, Halpin JG. Reaction of chicken irradiated ergosterol and irradiated yeast as contrasted with natural vitamin D of fish liver oils. J Biol Chem. 1932;97:249–66.
Marx SJ, Jones G, Weinstein RS, Chrousos GP, Renquist DM. Differences in mineral metabolism among nonhuman primates receiving diets with only vitamin D3 or only vitamin D2. J Clin Endocrinol Metab. 1989;69:1282–90.
Hay AW. The transport of 25-hydroxycholecalciferol in a New World monkey. Biochem J. 1975;151:193–6.
Bouillon R, Van Baelen H, De Moor P. The transport of vitamin D in the serum of primates. Biochem J. 1976;159:463–6.
Teixeira DS, Castro LC, Nóbrega YK, Almeida RC, Gandolfi L, Pratesi R. 25-Hydroxy-vitamin D levels among Callithrix penicillata primate species raised in captivity. J Med Primatol. 2010;39(2):77–82.
Prado F, Valladares-Padua C. Feeding ecology of a group of black-headed lion tamarin, Leontopithecus caissara (Primates: Callithrichidae), in the Superagui National Park, Southern Brazil. Primatol Brasil. 2004;8:145–54.
Correa HKM, Coutinho PEG, Ferrari SF. Between-year differences in the feeding ecology of highland marmosets (Callithrix aurita and Callithrix flaviceps) in southeastern Brazil. J Zool. 2000;252:421–7.
Hilario RR. Padra˜o de actividades, dieta e uso de ha´bitat por Callithrix flaviceps na Reserva Biolo´gica Augusto Ruschi, Santa Teresa, ES, Masters Thesis, Universidade Federal de Minas Gerais. 2008.
Porter LM, Garber PA. Mycophagy and its influence on habitat use and ranging patterns in Callimico goeldii. Am J Phys Anthropol. 2010;142(3):468–75.
Hewison M, Adams JS. Update in vitamin D. J Clin Endocrinol Metab. 2010;95:471–8.
Shinki T, Shiina N, Takahashi Y, Tamoika H, Koizumi H, Suda T. Extremely high circulating levels of 1,25-dihydroxy vitamin D3 in the marmoset, a New World Monkey. Biochem Biophys Res Commun. 1983;114:452–7.
Adams JS, Gacad MA, Baker AJ, Gonzales B, Rude RK. Serum concentrations of 1,25-dihydroxyvitamin D in Platyrrhini and Catarrhini: a phylogenetic appraisal. Am J Primatol. 1985;9:219–24.
Adams JS, Gacad MA, Rude RK, Endres DB, Mallette LE. Serum concentrations of immunoreactive parathyroid hormone in Platyrrhini and Catarrhini: a comparative analysis with three different antisera. Am J Primatol. 1987;13:425–33.
Adams JS, Gacad MA, Baker AJ, Keuhn G, Rude RK. Diminished internalization and action of 1,25-dihydroxyvitamin D in dermal fibroblasts cultured from New World primates. Endocrinology. 1985;116:2523–7.
Gacad MA, Adams JS. Evidence for endogenous blockage of cellular 1,25-dihydroxyvitamin D-receptor binding in New World primates. J Clin Invest. 1991;87:996–1001.
Gacad MA, Adams JS. Influence of ultraviolet B radiation on vitamin D3 metabolism in vitamin D3-resistant New World primates. Am J Primatol. 1992;28:263–70.
Brown GM, Grota LJ, Penney DP, Reichlin S. Pituitary-adrenal function in the squirrel monkey. Endocrinology. 1970;86:519–29.
Brandon DD, Markwick AJ, Chrousos GP, Loriaux DL. Glucocoorticoid resistance in humans and nonhuman primates. Cancer Res. 1989;49:2203–13.
Chrousos GP, Brandon D, Renquist DM, et al. Uterine estrogen and progesterone receptors in estrogen and progesterone-resistant primates. J Clin Endocrinol Metab. 1984;58:516–20.
Albertson BD, Maronian NC, Frederick KL, et al. The effect of ketoconazole on steroidogenesis. II. Adrenocortical enzyme activity in vitro. Res Commun Chem Pathol Pharmacol. 1988;61:27–34.
Albertson BD, Frederick KL, Maronian NC, et al. The effect of ketoconazole on steroidogenesis: I. Leydig cell enzyme activity in vitro. Res Commun Chem Pathol Pharmacol. 1988;61:17–26.
Moore CC, Mellon SH, Murai J, Siiteri PK, Miller WL. Structure and function of the hepatic form of 11 beta-hydroxysteroid dehydrogenase in the squirrel monkey, an animal model of glucocorticoid resistance. Endocrinology. 1993;133:368–75.
Klosterman LL, Murai JT, Siiteri PK. Cortisol levels, binding, and properties of corticosteroidbinding globulin in the serum of primates. Endocrinology. 1986;118:424–34.
Chrousos GP, Renquist DM, Brandon D, Fowler D, Loriaux DL, Lipsett MB. The squirrel monkey: receptor-mediated end-organ resistant to progesterone? J Clin Endocrinol Metab. 1982;55:364–8.
Brandon DD, Markwick AJ, Flores M, Dixon K, Albertson BD, Loriaux DL. Genetic variation of the glucocorticoid receptor from a steroid-resistant primate. Mol Endocrinol. 1991;7:89–96.
Scammell JG, Denny WB, Valentine DL, Smith DF. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. Gen Comp Endocrinol. 2001;124:152–65.
Stechschulte LA, Sanchez ER. FKBP51-a selective modulator of glucocorticoid and androgen sensitivity. Curr Opin Pharmacol. 2011;11(4):332–7.
Reynolds PD, Ruan Y, Smith DF, Scammell JG. Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. J Clin Endocrinol Metab. 1999;84(2):663–9.
Davies TH, Ning YM, Sánchez ER. A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J Biol Chem. 2002;277(7):4597–600.
Liberman UA, de Grange D, Marx SJ. Low affinity of the receptor for 1 alpha, 25-dihydroxyvitamin D3 in the marmoset, a New World monkey. FEBS Lett. 1985;182:385–8.
Adams JS, Gacad MA. Phenotypic diversity of the cellular 1,25-dihydroxyvitamin D-receptor interaction among different genera of New World primates. J Clin Endocrinol Metab. 1988;66:224–9.
Gacad MA, Adams JS. Specificity of steroid binding in New World primate cells with a vitamin D-resistant phenotype. Endocrinology. 1992;131:2581–7.
Gacad MA, Adams JS. Identification of a competitive binding component in vitamin D-resistant New World primate cells with a low affinity but high capacity for 1,25-dihydroxyvitmain D3. J Bone Miner Res. 1993;8:27–35.
Chun RF, Chen H, Boldrick L, Sweet C, Adams JS. Cloning, sequencing and functional characterization of the vitamin D receptor in vitamin D-resistant New World primates. Am J Primates. 2001;54:107–18.
Arbelle JE, Chen H, Gacad MA, Allegretto EA, Pike JW, Adams JS. Inhibition of vitamin D receptor-retinoid X receptor-vitamin D response element complex formation by nuclear extracts of vitamin D-resistant New World primate cells. Endocrinology. 1996;137:786–9.
Chen H, Arbelle JE, Gacad MA, Allegretto EA, Adams JS. Vitamin D and gonadal steroid-resistant New World primate cells express an intracellular protein which competes with the estrogen receptor for binding to the estrogen response element. J Clin Invest. 1997;99:769–75.
Chen H, Hu B, Allegretto EA, Adams JS. The vitamin D response element binding proteins: novel dominant-negative-acting regulators of vitamin D-directed transactivation. J Biol Chem. 2000;275:35557–64.
Chen H, Hewison M, Hu B, Adams JS. Heterogeneous nuclear ribonucleoprotein (hnRNP)-binding to hormone response elements: a cause of vitamin D resistance. Proc Natl Acad Sci U S A. 2003;100:6109–14.
Adams JS, Chen H, Chun R, Gacad MA, Encinas C, Ren S, Nguyen L, Wu S. Hewison, Barsony J. Response element binding proteins and intracellular vitamin D binding proteins: novel regulators of vitamin D trafficking, action and metabolism. J Ster Biochem Mol Biol. 2004;89–90:461–5.
Dreyfuss G, Matunis MJ, Pinol-Roma S, Burd CG. hnRNP proteins and the biogenesis of mRNA. Annu Rev Biochem. 1993;62:289–321.
Chen H, Hewison M, Adams JS. Functional characterization of heterogeneous nuclear ribonuclear protein C1/C2 in vitamin D resistance: a novel response element-binding protein. J Biol Chem. 2006;281:39114–20.
Lisse TS, Hewison M, Adams JS. Hormone response element binding proteins: novel regulators of vitamin D and estrogen signaling. Steroids. 2011;76(4):331–9.
Horwitz KB, Jackson TA, Bain DL, Richer JK, Takamoto GS, Tung L. Nuclear receptors coactivators and corepressors. Mol Endocrinol. 1996;10:1167–77.
Chen H, Stuart W, Hu B, Nguyen L, Huang G, Clemens TL, Adams JS. Creation of estrogen resistance in vivo by transgenic overexpression of the heterogeneous nuclear ribonucleoprotein-related estrogen response element binding protein. Endocrinology. 2005;146:4266–73.
Chen H, Hewison M, Adams JS. Control of estradiol-directed gene transactivation by an intracellular estrogen-binding protein and an estrogen response element-binding protein. Mol Endocrinol. 2008;22:559–69.
Chen H, Clemens TL, Hewison M, Adams JS. Estradiol and tamoxifen mediate rescue of the dominant-negative effects of estrogen response element-binding protein in vivo and in vitro. Endocrinology. 2009;150:2429–35.
Chen H, Gilbert LC, Lu X, Liu Z, You S, Weitzmann MN, Nanes MS, Adams JS. Estrogen response element binding protein stimulates osteoclastogenesis and bone resorption. J Bone Min Res. 2011;26:2537–47.
Gacad MA, LeBon TR, Chen H, Arbelle JE, Adams JS. Functional characterization and purification of an intracellular vitamin D binding protein in vitamin D resistant New World primate cells: amino acid sequence homology with proteins in the hsp-70 family. J Biol Chem. 1997;272:8433–40.
Gacad MA, Adams JS. Proteins in the heat shock-70 family specifically bind 25-hydroxylated vitamin D metabolites and 17β-estradiol. J Clin Endocrinol Metab. 1998;83:1264–7.
Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;3381:571–9.
Wu S, Ren S-Y, Gacad MA, Adams JS. Intracellular vitamin D binding proteins: novel facilitators of vitamin D-directed transactivation. Mol Endocrinol. 2000;14:1387–97.
Wu S, Chun R, Ren S, Chen H, Adams JS. Regulation of 1,25-dihydroxyvitamin D synthesis by intracellular vitamin D binding protein-1. Endocrinology. 2002;143:4135–8.
Christensen EI, Willnow TE. Essential role of megalin in renal proximal tubule for vitamin homeostasis. J Am Soc Nephrol. 1999;10:2224–36.
Nykjaer A, Dragun D, Walther D, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25(OH) vitamin D3. Cell. 1999;96:507–15.
Chun R, Gacad MA, Hewison M, Adams JS. Adenosine 5’-triphosphate-dependent vitamin D sterol binding to heat shock protein-70 chaperones. Endocrinology. 2005;146:5540–4.
Adams JS. “Bound” to work: the free hormone hypothesis revisited. Cell. 2005;122:647–9.
Adams JS, Chen H, Chun RF, Nguyen L, Wu S, Ren SY, Barsony J, Gacad MA. Novel regulators of vitamin D action and metabolism: lessons learned at the Los Angeles Zoo. J Cell Biol. 2003;88:308–14.
Acknowledgments
This work was supported by National Institutes of Health grants AR37399 and DK58891 to John S. Adams. The authors would like to acknowledge the useful discussions and critiques of this work provided over the years by Dr. Thomas Clemens and the late Dr. Bayard “Skip” Catherwood.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Adams, J.S., Chen, H., Chun, R.F., Lisse, T.S., Garcia, A., Hewison, M. (2015). Vitamin D Utilization in Subhuman Primates. In: Holick, M., Nieves, J. (eds) Nutrition and Bone Health. Nutrition and Health. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-2001-3_28
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2001-3_28
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-2000-6
Online ISBN: 978-1-4939-2001-3
eBook Packages: MedicineMedicine (R0)