Abstract
The branched chain amino acids (BCAAs) have recently been recognized as having functions other than simple nutrition. The signaling action of leucine in protein synthesis has been well studied, but the pharmacological effects of isoleucine and valine have not been clarified. It has recently been reported that, among the BCAAs, leucine and isoleucine act as signals in glucose metabolism. We revealed that isoleucine stimulates both glucose uptake in the muscle and whole body glucose oxidation, in addition to depressing gluconeogenesis in the liver, thereby leading to a hypoglycemic effect in rats. The major focus of this chapter is on the role of BCAAs in regulating glucose metabolism.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Key Points
-
BCAAs have recently been recognized as having functions other than simple nutrition.
-
The signaling action of leucine in protein synthesis has been well studied, but the pharmacological effects of isoleucine and valine have not been clarified.
-
It has recently been reported that, among the BCAAs, leucine and isoleucine act as signals in glucose metabolism.
-
We revealed that isoleucine stimulates both glucose uptake in the muscle and whole body glucose oxidation, in addition to depressing gluconeogenesis in the liver, thereby leading to a hypoglycemic effect in rats.
-
We speculate that isoleucine signaling accelerates catabolism of incorporated glucose for energy production and consumption.
Abbreviations
- AMPK:
-
5′-AMP-activated protein kinase
- ChREBP:
-
Carbohydrate responsive element binding protein
- DPP-4:
-
Dipeptidyl peptidase-4
- FAS:
-
Fatty acid synthase
- G6Pase:
-
Glucose-6-phosphatase
- GLUT:
-
Glucose transporter
- GSK-3:
-
Glycogen synthase kinase-3
- HbA1c:
-
Hemoglobin A1c
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- IRS:
-
Insulin receptor substrates
- L-GK:
-
Liver-type glucokinase
- LXR:
-
Liver X receptor
- mTOR:
-
Mammalian target of rapamycin
- PEPCK:
-
Phosphoenolpyruvate carboxykinase
- PI3K:
-
Phosphoinositide 3-kinase
- PKB/Akt:
-
Protein kinase B
- PKC:
-
Protein kinase C
- PPAR:
-
Peroxisome proliferator-activated receptor
- S6K1:
-
70-kDa ribosomal protein S6 kinase
- SREBP:
-
Sterol regulatory element binding protein
Introduction
The branched chain amino acids (BCAAs) leucine, isoleucine, and valine are the most abundant of the essential amino acids. In addition to their critical role as substrates for protein synthesis, these amino acids play a variety of roles in the body. It is believed that BCAAs contribute to energy metabolism during exercise as energy sources and substrates to expand the pool of citric acid-cycle intermediates (anaplerosis) and for gluconeogenesis. Moreover, BCAAs serve as regulatory (signaling) molecules that modulate numerous cellular functions (Table 6.1). BCAAs, acting as nutrient signals, regulate protein synthesis and degradation, and insulin secretion, and have been implicated in central nervous system control of food intake and energy balance. Of the BCAAs, leucine has been the most thoroughly investigated as a signaling molecule. In particular, the signaling action of leucine in protein synthesis has been well studied, and the mechanism is currently under investigation [1]. Leucine appears to be the specific effector of protein synthesis in several tissues including skeletal muscle [2], liver [3], and adipose tissue [4]. However, the pharmacological effects of the other BCAAs, isoleucine and valine, have not been well clarified. We have focused on the pharmacological effects of BCAAs for the last 10 years, and found that among the BCAAs, isoleucine acts as a nutrient regulator of glucose metabolism [5, 6]. Another group has also showed that leucine plays a key role in regulating glucose homeostasis [7]. Today, it is generally accepted that one of the features of BCAA administration is modification of glucose metabolism.
The major focus of this chapter is on the role of BCAAs in regulating glucose metabolism in rats.
Effects of Amino Acids on Glucose Metabolism
BCAAs, particularly leucine, play essential roles in hormonal secretion and action, as well as in intracellular signaling. In glucose metabolism, despite the fact that amino acids can stimulate the release of insulin, it has been shown that amino acid infusion actually inhibits glucose utilization. Previous studies have also shown that amino acids, particularly leucine, inhibit insulin-stimulated glucose uptake [8–11]. One mechanism through which this could occur is the preferential oxidation of amino acids leading to glucose sparing. As an alternative to glucose oxidation, amino acids may serve as fuel, and amino acids, including the glucogenic amino acids (alanine, valine, or glutamine), are considered to be able to increase glucose production and blood glucose levels. More recent work has begun to identify intracellular mechanisms through which amino acids appear to control glucose metabolism. This inhibitory action is mediated through the attenuated tyrosine phosphorylation of insulin receptor substrates (IRS)-1 and IRS-2 and subsequent interaction with the regulatory subunit of phosphoinositide 3-kinase (PI3K), leading to decreased activity of PI3K, protein kinase B (PKB/Akt), and mammalian target of rapamycin (mTOR) [11–13].
Of the amino acids, leucine is involved in glucose uptake in isolated muscle [7], in glycogen synthesis via the inactivation of glycogen synthase kinase-3 (GSK-3) [14], and in the insulin-secretion effect in the pancreas [15]. On the other hand, leucine, but not isoleucine or valine, also inhibits insulin-stimulated glucose uptake in L6 cells by degrading IRS-1 via activation of the mTOR/S6K1 signaling pathway, leading to desensitization of insulin signaling [11, 13, 16]. In addition, leucine reduces the duration of insulin-induced IRS-1-associated PI3K activity in rat skeletal muscle [17]. Given these results, it is to be expected that amino acids will decrease glucose oxidation and lead to amino acid-induced insulin resistance. However, it has been reported that amino acid infusion causes a decrease in blood glucose levels and an increase in glucose oxidation in humans [18, 19], although there have been few investigations of this hypoglycemic effect to date. These changes appear to occur via the action of insulin, as leucine, but not isoleucine or valine, stimulates insulin release from the pancreas, thereby decreasing blood glucose [20, 21]. Thus, this contradicts the amino acid-induced insulin resistance described above, and this issue therefore remains controversial.
Hypoglycemic Effect of Isoleucine
Some studies have demonstrated that a BCAAs mixture decreases plasma glucose levels in vivo. Oral administration of a BCAAs mixture has been shown to ameliorate hyperglycemia in a virus-induced noninsulin-dependent diabetes mellitus mouse model [22]. In streptozotocin-induced rats, oral administration of a BCAAs mixture (0.75–1.5 g/kg body weight) significantly decreased plasma glucose levels [23]. However, it was unknown whether this reflected glucose metabolism caused by leucine, isoleucine, or valine, and the mechanism of action of the individual BCAAs was not understood in vivo or in vitro.
Our collaborators reported that isoleucine prevents a rise in the plasma glucose concentration and that the effect of isoleucine is greater than that of leucine or valine in oral glucose tolerance tests in normal 7-week-old rats [24]. Oral administration of isoleucine (0.30 g/kg body weight) significantly alleviated the increase of plasma glucose at 30 and 60 min after glucose infusion in these young rats compared to saline-administered control rats. In contrast, the administration of valine (0.30 g/kg body weight) significantly increased the glucose level at 30 min after glucose administration, which suggests that valine, a glycogenic amino acid, is used as a substrate for gluconeogenesis in the liver. Leucine had a similar effect at 120 min compared to saline-administered control rats. Oral administration of leucine, isoleucine, and valine (0.30 g/kg body weight) in these rats did not alter plasma insulin at 30, 60, or 120 min after oral glucose administration compared to the control. Moreover, a dose-dependent effect that lowered plasma glucose levels by isoleucine was observed in 18-week-old rats. In these older rats, oral administration of isoleucine (0.30 g/kg body weight) significantly lowered plasma glucose levels at 30, 60, and 120 min after glucose infusion, whereas oral administration of lower amounts of isoleucine (0.05 or 0.10 g/kg body weight) caused no significant changes in plasma glucose levels. The plasma insulin levels in the isoleucine-administered 18-week-old rats were below those in the controls at 30 and 60 min after glucose infusion. The hypoglycemic effect of isoleucine was recently confirmed in a human study using oral administration of isoleucine [25]. In C2C12 myotubes, leucine and isoleucine stimulate glucose uptake in an insulin-independent manner, and the effect of isoleucine is greater than that of leucine [24]. In such cells, signaling pathway analysis using a PI3K inhibitor (LY294002), a protein kinase C (PKC) inhibitor (GF109203X), and an mTOR inhibitor (rapamycin) suggests that PI3K and PKC, but not mTOR, are involved in the enhancement of glucose uptake by isoleucine. These data suggest that isoleucine assumes the role of a signal for glucose metabolism, thereby stimulating insulin-independent and mTOR-independent glucose transport in cultured skeletal muscle cells.
We focused on the blood glucose-lowering effects of isoleucine and examined whether isoleucine decreased the plasma glucose concentration in food-deprived rats, and whether isoleucine increased glucose uptake in skeletal muscles in vivo. Valine was excluded from the scope of this research, as valine caused an increase in plasma glucose levels. Oral administration of isoleucine, but not leucine, significantly decreased plasma glucose concentrations in food-deprived rats [5]. Glucose uptake in the skeletal muscle did not differ after leucine administration, but glucose uptake in the muscles of rats given isoleucine was 73 % greater than that in food-deprived controls, suggesting that isoleucine increases skeletal muscle glucose uptake in vivo. These results indicate a relationship between the reduction in blood glucose and the increase in skeletal muscle glucose uptake that occur with isoleucine administration in rats (Table 6.2).
Furthermore, we investigated the possible involvement of the energy sensor 5′-AMP-activated protein kinase (AMPK) in the modulation of glucose uptake in skeletal muscle, which is independent of insulin, and also in isoleucine-stimulated glucose uptake [5]. AMPK is a serine/threonine kinase consisting of a catalytic subunit (α) and two regulatory subunits (β and γ). The catalytic α subunit occurs in two distinct isoforms in mammals. AMPK α1 is widely expressed, whereas the α2 isoform is expressed predominantly in the liver, heart, and skeletal muscle [26]. AMPK α1 activity in skeletal muscle was not affected by leucine or isoleucine administration (Table 6.3). However, isoleucine, but not leucine, significantly decreased AMPK α2 activity (Table 6.3). These results indicate that isoleucine-stimulated glucose uptake increases in the absence of increased AMPK α1 and α2 activity in skeletal muscle in food-deprived rats.
Effects of Isoleucine on Glycogen Synthesis and Glucose Oxidation
As mentioned above, the administration of isoleucine leads to an increase in glucose uptake in skeletal muscle in vivo [5]. However, it remains unknown how the glucose incorporated by isoleucine is metabolized. Leucine stimulates glycogen synthesis through the inactivation of GSK-3 in L6 muscle cells in a manner that is dependent on mTOR and independent of insulin [14]. Our collaborators reported that leucine causes a significant increase in d-[U-14C] glucose incorporation into intracellular glycogen in myotube cells in vitro, whereas isoleucine does not affect glycogen synthesis when compared to controls [24]. Therefore, we first examined the effects of isoleucine on glycogen synthesis in skeletal muscle.
Muscle glycogen synthesis, as determined by [U-14C] glucose incorporation into glycogen, was significantly increased by administration of leucine, but not isoleucine, in rat skeletal muscles in vivo when compared with food-deprived control rats (Table 6.2). Although leucine has less of an effect on glucose uptake in skeletal muscle, it stimulates glycogen synthesis in skeletal muscle (Table 6.2). In contrast, isoleucine stimulates glucose uptake, although it has less of an effect on glycogen synthesis (Table 6.2) [5]. We also measured the contents of high-energy phosphate metabolites (AMP, ADP, and ATP) in the skeletal muscles of rats administered with leucine and isoleucine to evaluate the cellular energy state [5]. Oral administration of leucine or isoleucine did not alter the ADP or ATP contents in the skeletal muscle when compared with control rats (Table 6.3). Although isoleucine caused a decrease in AMP content in skeletal muscle when compared with the control and leucine groups, the AMP content was not affected after administration of leucine when compared with the control group (Table 6.3). Furthermore, although leucine did not change the AMP:ATP ratio, isoleucine caused a significant decrease in this ratio in the skeletal muscle when compared with the control group (Table 6.3). We assume that a depletion of cellular AMP would result in a decrease in the AMP:ATP ratio and improve the availability of ATP in the skeletal muscle without any marked increase in ATP concentration, thereby resulting in an improvement in the cellular energy state.
In order to determine whether the hypoglycemic effect of isoleucine affects whole body glucose oxidation, we examined the effects of isoleucine on the expiratory excretion of 14CO2 from [U-14C] glucose in vivo at a dose where the hypoglycemic effect was greatest [6]. The expiratory excretion of 14CO2 of rats treated with isoleucine was significantly elevated between 60 and 90 min after administration when compared with controls. Based on the above results, muscle glucose uptake in isoleucine-administered rats was elevated at 60 min. As the time to achieve maximum plasma isoleucine levels was 60 min [5, 24], this indicates a strong correlation among the increase in muscle glucose uptake, the decrease in blood glucose, and the subsequent increase in glucose oxidation. In contrast, it has been reported that isoleucine suppresses 14CO2 production from [1-14C] pyruvate in isolated skeletal muscle [8]. As a potential explanation, the suppression might have been caused by an increase in total pyruvate content in the skeletal muscle [27] due to increased glucose uptake by isoleucine [5, 24], which results in a diluting effect for [1-14C] pyruvate and an increase in total CO2 production. Meanwhile, glycogen synthesis in isoleucine-administered rats was not altered in skeletal muscle [5], thus suggesting that when there is a lowering of blood glucose levels, isoleucine increases glucose uptake in skeletal muscle with the incorporated glucose mainly oxidized in the muscle immediately after uptake (Table 6.4).
Effects of Isoleucine on Glucose Uptake in Peripheral Tissues and Hepatic Glucose Production
Generally, the maintenance of blood glucose levels is due to an optimal balance between glucose uptake by peripheral tissues and glucose production occurring mainly in the liver. Therefore, we examined the effects of isoleucine administration on glucose uptake in peripheral tissues and hepatic glucose production.
Glucose uptake was significantly increased in the muscles of isoleucine-administered rats when compared with controls at the most effective dose of isoleucine. In contrast, there were no significant differences in the glucose uptake in liver or adipose tissue in isoleucine-administered rats when compared with controls. These results suggest that skeletal muscle is the major organ contributing to the hypoglycemic effects of isoleucine on glucose uptake (Fig. 6.1). Leucine has a stimulatory effect on insulin secretion [9]. As a temporal increase in plasma insulin levels after oral administration of leucine was observed in this study, the effect of leucine on glucose metabolism may primarily be insulin dependent. On the other hand, the hypoglycemic effect of isoleucine was more potent than that of leucine, although significant changes in plasma insulin and glucagon levels were not observed after isoleucine administration. Furthermore, isoleucine had an additive effect on insulin-stimulated glucose uptake via PI3K [24], in contrast to leucine, which inhibited insulin-stimulated glucose uptake in skeletal muscle cells [11]. Although the molecular basis of increased glucose uptake by isoleucine remains unclear, a previous study by another group revealed that glucose uptake by isoleucine is involved in increased glucose transporter (GLUT)1 and GLUT4 translocation in the skeletal muscles of rats with liver cirrhosis [28]. These data suggest that isoleucine improves insulin sensitivity in skeletal muscle via an intracellular signaling pathway.
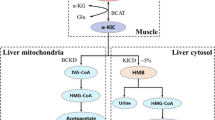
A possible mechanism for the glucose-lowering effects of isoleucine in rats. Glucose uptake was significantly increased in the muscles of isoleucine-administered rats when compared with controls. In contrast, there were no significant differences in the glucose uptake in liver or adipose tissue in isoleucine-administered rats. Glycogen synthesis in isoleucine-administered rats was not altered in skeletal muscle, thus suggesting that when there is a lowering of blood glucose levels, isoleucine increases glucose uptake in skeletal muscle with the incorporated glucose mainly oxidized immediately after uptake. Isoleucine also reduces hepatic glucose production in vitro and the expression and activity of hepatic gluconeogenic enzymes both in vitro and in vivo
The liver plays a role in the uptake of blood glucose as with skeletal muscles. The primary transporter for glucose uptake in the liver is GLUT2. Glucose is taken up into the hepatocytes through GLUT2, and liver-type glucokinase (L-GK) traps glucose in the cytoplasm by phosphorylation. Therefore, GLUT2 and L-GK play important roles in the liver as a glucose-sensing apparatus [29]. We have not evaluated the effect of leucine or isoleucine on the glucose-sensing apparatus in the liver, because there were no significant differences in the glucose uptake in liver in leucine-administered or isoleucine-administered rats when compared with controls. Recently, it was demonstrated that BCAAs strongly accelerated GLUT2 and L-GK mRNA expression in HepG2 cells in a glucose-dependent manner, and dose-dependently enhanced the mRNA levels of L-GK in rat liver [30]. The glucose-sensing apparatus enables glucose to regulate the expression of glucose-responsive genes such as L-type pyruvate kinase, S14, fatty acid synthase (FAS), and GLUT2 [31]. Thus, the glucose-sensing apparatus exerts a strong influence on glucose utilization and glycogen synthesis. The biological activity of the glucose-sensing apparatus is tightly regulated by transcriptional mechanisms. The transcriptional factors sterol regulatory element binding protein (SREBP)-1c and peroxisome proliferator-activated receptor (PPAR)-γ are thought to be involved in the transcriptional regulation of the glucose-sensing apparatus, because the functional binding sites for SREBP-1c and PPAR-γ have been identified in the GLUT2 and L-GK promoter regions, and the mRNA expression of these genes is upregulated by SREBP-1c and PPAR-γ [29, 32–34]. BCAAs markedly upregulated the expression of SREBP-1c, carbohydrate responsive element binding protein (ChREBP), and liver X receptor (LXR)α in HepG2 cells, and upregulated SREBP-1c and LXRα in rat liver [30]. Because LXRα is known to transactivate SREBP-1c and ChREBP in the presence of glucose or glucose-6-phosphate, as direct agonists, the increased glucose or glucose-6-phosphate, through the activation of the glucose-sensing apparatus by BCAAs, may have a role in the activation of LXRα. From these results, it has been speculated that the LXRα-induced SREBP-1c-dependent mechanism may be the main signaling pathway for BCAAs-induced transactivation of the glucose-sensing apparatus [30].
Hepatic gluconeogenesis, as well as glucose utilization by peripheral and hepatic tissues, may be a possible mechanism by which amino acids lower blood glucose levels. During the fasting state, glucose production is largely a result of gluconeogenesis as opposed to hepatic glycogenolysis [35]. Therefore, we examined the effects of isoleucine on the gluconeogenic rate-limiting enzymes in vivo. The mRNA levels of phosphoenolpyruvate carboxykinase (PEPCK), which is a well-researched key gluconeogenic enzyme, parallel both PEPCK activity and the rate of gluconeogenesis [36, 37]. The data showed that expression levels of hepatic PEPCK mRNA were lower in isoleucine-administered rats than in controls, suggesting that PEPCK activity was lower in isoleucine-administered rats and that the inhibitory effects of isoleucine on PEPCK are regulated at the transcriptional level [6]. Furthermore, we demonstrated that expression levels of hepatic glucose-6-phosphatase (G6Pase) mRNA, and G6Pase activity were also lower in isoleucine-administered rats [6]. Enzyme activity is regulated by controlling both protein expression and existing enzymes. Although whether isoleucine regulates existing enzymes is unknown, we believe that isoleucine inhibits G6Pase activity by decreasing G6Pase mRNA expression. These findings suggest that isoleucine also downregulates G6Pase activity and associated mRNA, in addition to inhibiting gluconeogenesis in the liver in vivo (Fig. 6.1).
Under in vitro conditions, there is an inhibitory effect of isoleucine on the expression of PEPCK and G6Pase in isolated hepatocytes [6]. It has also been demonstrated that the G6Pase activity is lower in isoleucine-added cells when compared with controls [6]. These findings suggest that isoleucine downregulates the transcription of gluconeogenic enzymes and inhibits glucose production in the liver under insulin-free conditions. This suggests that the inhibitory effects of gluconeogenesis by isoleucine involve an insulin-independent signaling pathway as well as the insulinotropic effects of isoleucine in vivo.
Although we can calculate the glucose uptake and endogenous glucose production values at the same time through the use of a tracer, the value of endogenous glucose production may be underestimated in experiments in which glucose uptake values are markedly elevated. Therefore, we measured glucose production by using isolated hepatocytes in order to determine the mechanism underlying the hypoglycemic effects of isoleucine [6]. As a glucogenic substrate, we examined alanine, because plasma alanine levels were found to be significantly higher in the 0.45 g/kg body weight isoleucine group, which was also the most effective dose for decreasing plasma glucose when compared with controls [6]. Isoleucine significantly inhibited glucose production when alanine was used as a glucogenic substrate in isolated hepatocytes [6]. In addition, phenylalanine, which is a neutral amino acid transported via the same neutral amino acid transport system as alanine, leucine, and isoleucine [38, 39], also significantly reduced glucose production [6]. These results indicate that the inhibitory effects of isoleucine on glucose production with alanine may be due to a competitive inhibitory effect with alanine for transport via the neutral amino acid transporter.
In conclusion, isoleucine administration stimulates both glucose uptake in the muscles and whole body glucose oxidation, in addition to depressing gluconeogenesis in the liver, thereby leading to a hypoglycemic effect in rats (Fig. 6.2).
Schematic diagram of the effects of isoleucine on glucose metabolism. Isoleucine stimulates glucose uptake in skeletal muscle and the incorporated glucose is oxidized without significant elevation of plasma insulin levels. In the liver, isoleucine decreases hepatic gluconeogenic enzyme activity and glucose production. These mechanisms are responsible for the hypoglycemic effect of isoleucine that improves the energy state of the muscle and liver, and that may improve insulin resistance in vivo. This figure is partially modified from an original reported by Doi et al. [6]
The Clinical Utility of BCAAs as Regulators of Glucose Metabolism
The clinical importance of BCAAs as regulators of glucose metabolism has been studied. Nuttall et al. have systematically evaluated the insulin and glucose responses to individual amino acids ingested with and without glucose in healthy nondiabetic volunteers. When ingested alone, isoleucine did not affect the insulin concentration but did decrease the glucose concentration [25]. It increased glucagon modestly. When isoleucine was ingested with glucose it resulted in a smaller increase in glucose concentration, but a similar increase in insulin. The data suggest that this was associated with an increased removal rate of peripheral circulating glucose. They also determined whether leucine stimulates insulin and/or glucagon secretion, and whether when ingested with glucose it modifies the glucose, insulin, or glucagon response [40]. Leucine at a dose equivalent to that present in a high-protein meal had little effect on serum glucose or insulin concentrations but did increase the glucagon concentration. When leucine was ingested with glucose, it attenuated the serum glucose response and strongly stimulated additional insulin secretion. Leucine also attenuated the decrease in glucagon expected when glucose alone is ingested. The data suggest that a rise in glucose concentration is necessary for leucine to stimulate significant insulin secretion. This in turn reduces the glucose response to ingested glucose.
The blood glucose-lowering effects of BCAAs in patients with chronic liver disease have been reported [41, 42]. BCAAs supplementation improved the homeostasis model assessment of insulin resistance (HOMA-IR) [41] and decreased plasma glucose levels in a glucose tolerance test [42]. Chronic liver disease, especially hepatitis C, is associated with insulin resistance and diabetes. The effects of BCAAs on glucose tolerance and insulin sensitivity in patients with chronic hepatitis C and insulin resistance have been reported [43]. BCAAs supplementation therapy did not have adverse effects on glucose tolerance or insulin sensitivity in patients with chronic hepatitis C and insulin resistance. BCAAs did not significantly improve overall glycemic control in this study. However, BCAAs therapy may exert a beneficial effect on hemoglobin A1c (HbA1c) values in patients with marked insulin resistance in skeletal muscles. Based on these preliminary observations, future clinical trials should also evaluate the effect of BCAAs in patients with type 2 diabetes.
Conclusions
Numerous hormones play important roles in the metabolic regulation of nutrients. Among these, insulin functions to regulate the metabolism of all macronutrients (e.g., proteins, carbohydrates, and fats), making it a crucial metabolic hormone. Insulin acts anabolically on protein metabolism by stimulating protein synthesis and inhibiting its breakdown. Similarly, leucine functions to stimulate protein synthesis and inhibit its breakdown. The role of isoleucine has been overshadowed by leucine, but it has also been demonstrated to have glucose metabolism-regulating functions similar to insulin, such as stimulation of glucose uptake into cells and inhibition of gluconeogenesis. These findings suggest that leucine and isoleucine share metabolic regulatory functions with insulin. Recent studies suggest a close relationship between BCAAs and insulin resistance and demonstrate that BCAAs may play a major role in the modulation of insulin action [44]. In addition, it has been reported that certain BCAA-containing bioactive peptides derived from whey protein can reduce postprandial glucose levels and stimulate insulin release in healthy subjects and in subjects with type 2 diabetes by reducing dipeptidyl peptidase-4 (DPP-4) activity in the proximal bowel, hence increasing intact incretin levels [45]. Although we have not been able to find reports on the effects of single BCAAs on DPP-4 activity, the possibility that a single BCAA can regulate glucose metabolism by affecting the activity of DPP4 cannot be excluded.
As BCAAs serve to regulate the metabolism of major nutrients, similarly to insulin, the value of their use as biological regulators cannot be overestimated. BCAAs are nutrients that should therefore be a focus of investigation as next-generation biological regulators.
References
Yoshizawa F. Regulation of protein synthesis by branched-chain amino acids in vivo. Biochem Biophys Res Commun. 2004;313:417–22.
Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–9.
Anthony TG, Anthony JC, Yoshizawa F, Kimball SR, Jefferson LS. Oral administration of leucine stimulates ribosomal protein mRNA translation but not global rates of protein synthesis in the liver of rats. J Nutr. 2001;131:1171–6.
Lynch CJ, Patson BJ, Anthony J, Vaval A, Jefferson LS, Vary TC. Leucine is a direct-acting nutrient signal that regulates protein synthesis in adipose tissue. Am J Physiol Endocrinol Metab. 2002;283:E503–13.
Doi M, Yamaoka I, Nakayama M, Mochizuki S, Sugahara K, Yoshizawa F. Isoleucine, a blood glucose-lowering amino acid, increases glucose uptake in rat skeletal muscle in the absence of increases in AMP-activated protein kinase activity. J Nutr. 2005;135:2103–8.
Doi M, Yamaoka I, Nakayama M, Sugahara K, Yoshizawa F. Hypoglycemic effect of isoleucine involves increased muscle glucose uptake and whole body glucose oxidation and decreased hepatic gluconeogenesis. Am J Physiol Endocrinol Metab. 2007;292:E1683–93.
Nishitani S, Matsumura T, Fujitani S, Sonaka I, Miura Y, Yagasaki K. Leucine promotes glucose uptake in skeletal muscles of rats. Biochem Biophys Res Commun. 2002;299:693–6.
Chang TW, Goldberg AL. Leucine inhibits oxidation of glucose and pyruvate in skeletal muscles during fasting. J Biol Chem. 1978;253:3696–701.
Flakoll PJ, Wentzel LS, Rice DE, Hill JO, Abumrad NN. Short-term regulation of insulin-mediated glucose utilization in four-day fasted human volunteers: role of amino acid availability. Diabetologia. 1992;35:357–66.
Tessari P, Inchiostro S, Biolo G, et al. Hyperaminoacidaemia reduces insulin-mediated glucose disposal in healthy man. Diabetologia. 1985;28:870–2.
Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J Biol Chem. 2001;276:38052–60.
Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR. Bidirectional modulation of insulin action by amino acids. J Clin Invest. 1998;101:1519–29.
Takano A, Usui I, Haruta T, et al. Mammalian target of rapamycin pathway regulates insulin signaling via subcellular redistribution of insulin receptor substrate 1 and integrates nutritional signals and metabolic signals of insulin. Mol Cell Biol. 2001;21:5050–62.
Peyrollier K, Hajduch E, Blair AS, Hyde R, Hundal HS. L-leucine availability regulates phosphatidylinositol 3-kinase, p70 S6 kinase and glycogen synthase kinase-3 activity in L6 muscle cells: evidence for the involvement of the mammalian target of rapamycin (mTOR) pathway in the L-leucine-induced up-regulation of system A amino acid transport. Biochem J. 2000;350:361–8.
Yang J, Chi Y, Burkhardt BR, Guan Y, Wolf BA. Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutr Rev. 2010;68:270–9.
Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–81.
Baum JI, O’Connor JC, Seyler JE, Anthony TG, Freund GG, Layman DK. Leucine reduces the duration of insulin-induced PI 3-kinase activity in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288:E86–91.
Tappy L, Acheson K, Normand S, Pachiaudi C, Jéquier E, Riou JP. Effects of glucose and amino acid infusion on glucose turnover in insulin-resistant obese and type II diabetic patients. Metabolism. 1994;43:428–34.
Tappy L, Acheson K, Normand S, et al. Effects of infused amino acids on glucose production and utilization in healthy human subjects. Am J Physiol. 1992;262:E826–33.
Fajans SS, Knopf RF, Floyd JC, Power L, Conn JW. The experimental induction in man of sensitivity of leucine hypoglycemia. J Clin Invest. 1963;42:216–29.
Milner RD. The stimulation of insulin release by essential amino acids from rabbit pancreas in vitro. J Endocrinol. 1970;47:347–56.
Utsugi T, Yoshida A, Kanda T, et al. Oral administration of branched chain amino acids improves virus-induced glucose intolerance in mice. Eur J Pharmacol. 2000;398:409–14.
Eizirik DL, Kettelhut IC, Migliorini RH. Administration of branched-chain amino acids reduces the diabetogenic effect of streptozotocin in rats. Braz J Med Biol Res. 1987;20:137–44.
Doi M, Yamaoka I, Fukunaga T, Nakayama M. Isoleucine, a potent plasma glucose-lowering amino acid, stimulates glucose uptake in C2C12 myotubes. Biochem Biophys Res Commun. 2003;312:1111–7.
Nuttall FQ, Schweim K, Gannon MC. Effect of orally administered isoleucine with and without glucose on insulin, glucagon and glucose concentrations in non-diabetic subjects. E Spen Eur E J Clin Nutr Metab. 2008;3:e152–8.
Stapleton D, Mitchelhill KI, Gao G, et al. Mammalian AMP-activated protein kinase subfamily. J Biol Chem. 1996;271:611–4.
Goldstein L, Newsholme EA. The formation of alanine from amino acids in diaphragm muscle of the rat. Biochem J. 1976;154:555–8.
Nishitani S, Takehana K, Fujitani S, Sonaka I. Branched-chain amino acids improve glucose metabolism in rats with liver cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1292–12300.
Kim HI, Ahn YH. Role of peroxisome proliferator-activated receptor-gamma in the glucose-sensing apparatus of liver and beta-cells. Diabetes. 2004;53 Suppl 1:S60–5.
Higuchi N, Kato M, Miyazaki M, et al. Potential role of branched-chain amino acids in glucose metabolism through the accelerated induction of the glucose-sensing apparatus in the liver. J Cell Biochem. 2011;112:30–8.
Leturque A, Brot-Laroche E, Le Gall M, Stolarczyk E, Tobin V. The role of GLUT2 in dietary sugar handling. J Physiol Biochem. 2005;61:529–37.
Iynedjian PB, Gjinovci A, Renold AE. Stimulation by insulin of glucokinase gene transcription in liver of diabetic rats. J Biol Chem. 1988;263:740–4.
Magnuson MA, Andreone TL, Printz RL, Koch S, Granner DK. Rat glucokinase gene: structure and regulation by insulin. Proc Natl Acad Sci U S A. 1989;86:4838–42.
Im SS, Kang SY, Kim SY, et al. Glucose-stimulated upregulation of GLUT2 gene is mediated by sterol response element-binding protein-1c in the hepatocytes. Diabetes. 2005;54:1684–91.
Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC. Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Invest. 1996;98:378–85.
Iynedjian PB, Hanson RW. Increase in level of functional messenger RNA coding for phosphoenolpyruvate carboxykinase (GTP) during induction by cyclic adenosine 3′: 5′-monophosphate. J Biol Chem. 1977;252:655–62.
Xu H, Yang Q, Shen M, et al. Dual specificity MAPK phosphatase 3 activates PEPCK gene transcription and increases gluconeogenesis in rat hepatoma cells. J Biol Chem. 2005;280:36013–8.
Bröer A, Klingel K, Kowalczuk S, Rasko JE, Cavanaugh J, Bröer S. Molecular cloning of mouse amino acid transport system B0, a neutral amino acid transporter related to Hartnup disorder. J Biol Chem. 2004;279:24467–76.
Bröer A, Tietze N, Kowalczuk S, et al. The orphan transporter v7-3 (slc6a15) is a Na+-dependent neutral amino acid transporter (B0AT2). Biochem J. 2006;393:421–30.
Kalogeropoulou D, LaFave L, Schweim K, Gannon MC, Nuttall FQ. Leucine, when ingested with glucose, synergistically stimulates insulin secretion and lowers blood glucose. Metabolism. 2008;57:1747–52.
Kawaguchi T, Nagao Y, Matsuoka H, Ide T, Sata M. Branched-chain amino acid-enriched supplementation improves insulin resistance in patients with chronic liver disease. Int J Mol Med. 2008;22:105–12.
Korenaga K, Korenaga M, Uchida K, Yamasaki T, Sakaida I. Effects of a late evening snack combined with alpha-glucosidase inhibitor on liver cirrhosis. Hepatol Res. 2008;38:1087–97.
Takeshita Y, Takamura T, Kita Y, et al. Beneficial effect of branched-chain amino acid supplementation on glycemic control in chronic hepatitis C patients with insulin resistance: implications for type 2 diabetes. Metabolism. 2012;61:1388–94.
Lu J, Xie G, Jia W, Jia W. Insulin resistance and the metabolism of branched-chain amino acids. Front Med. 2013;7:53–9.
Tulipano G, Sibilia V, Caroli AM, Cocchi D. Whey proteins as source of dipeptidyl dipeptidase IV (dipeptidyl peptidase-4) inhibitors. Peptides. 2011;32:835–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Yoshizawa, F. (2015). Effects of Leucine and Isoleucine on Glucose Metabolism. In: Rajendram, R., Preedy, V., Patel, V. (eds) Branched Chain Amino Acids in Clinical Nutrition. Nutrition and Health. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-1923-9_6
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1923-9_6
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-1922-2
Online ISBN: 978-1-4939-1923-9
eBook Packages: MedicineMedicine (R0)