Abstract
Oilseed crops have the potential to increase the stability and sustainability of American agriculture by replacing a portion of the fossil fuels consumed by this sector. There are several candidate oilseed species that have been identified as compatible with a dryland winter wheat-fallow rotation. Of these species, Camelina sativa has been previously identified as being a promising species for drought-prone areas of the American High Plains. This is due to its short growing season, drought tolerance, cold tolerance, and resistance to many of the insect and pest species that cause yield reductions in other Brassica oilseed species. Camelina seed oil has high concentrations (30–40 %) of linolenic fatty acid (C18:3), which is a valuable product and also improves the cold-flow properties of the feedstock oil. Camelina is a native of Europe, and breeding efforts have so far focused on optimizing the varieties to produce high yields in agricultural regions of the United States and Europe. Breeding and research efforts have created linkage maps and identified QTL for yield, agronomic characteristics, and oil characteristics. Researchers have also found success in creating transgenic varieties of camelina, which could greatly facilitate the optimization of the oil profile for use as a feedstock for industrial oils and as a biofuel.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Camelina sativa, or “gold of pleasure,” belongs to the Brassicaceae family and has been cultivated in Europe as an oilseed since the Bronze Age, which began around 4000 B.C. [1]. Numerous archeological studies have shown that camelina, flax, and other assorted cereals constituted a significant portion of the human diet in Europe and Scandinavia during the Bronze Age [1]. Cultivation of camelina waned until recent interest in low-input biofuels resulted in a reexamination of its value as an oilseed crop and as a potential source of omega-3 fatty acids for human and animal consumption [1, 2]. Interest in camelina as a biofuel feedstock stems from its drought tolerance and compatibility with existing cropping systems.
In the United States, camelina can be incorporated into a dryland winter wheat-based cropping system where it can be treated as a summer annual or a fall-seeded annual [3]. Camelina is a small-seeded crop and can be broadcast or direct seeded using existing wheat or canola planting equipment at a shallow depth of no more than 12 mm, with 6.3 mm being optimal [3]. The optimal seeding rate has been found to be 5.6–7.8 kg ha−1 depending on planting conditions such as seed bed quality, soil humidity, and pressure from weed competition [4]. No-till conditions are appropriate for camelina planting, although there are some weed-control issues that arise from this method of planting due to the lack of herbicide-resistant varieties [5].
There are two types of camelina varieties: winter varieties that are planted in the fall and allowed to overwinter in the rosette stage (fall-seeded) and those that do not have a vernalization requirement (spring-seeded) [12]. Fall-seeded varieties have a growth cycle similar to wheat in that they establish a stand and overwinter in a dormant stage. Of course, this is dependent on the presence of fall rains. Spring-seeded camelina does best when planted early [3, 6]. If camelina is planted in early March before weed emergence, it will have enough time to allow it to compete more vigorously with spring weeds [3, 5]. Camelina is a short-season crop, requiring roughly 80 days to reach maturity. Early spring planting or late winter planting will allow camelina to mature before high summer temperatures cause heat stress and lower yields [7]. The required cumulative growing degree days (GDD) for camelina are estimated to be 1,300 [8]. The optimal temperature for germination is 3.3 °C and delay of planting from March until April results in yield reductions of up to 25 % due to heat stress [6]. Dryland trials of camelina in Colorado have demonstrated superior yields compared to other oilseed crops such as canola [9, 10]. The seed oil content of camelina ranges from 30 to 45 % [1, 10, 11]. The protein content ranges from 39.2 to 47.4 %/DM, while the fiber content varies from 12.5 to 16.8 % f.f. DM [11].
Although camelina is a low-input new oilseed crop, it responds well to fertilization [12]. A general rule of thumb is that camelina needs 2–2.7 kg of N to produce 45 kg of seeds [13]. This can be applied during the growing season, or if residual nitrogen is available from previous crops, this can be utilized by the plant as well [13].
The flowers of camelina are generally autogamous and are between 5 and 7 mm in diameter [1]. These flowers become silicles which vary in number between 126 and 283 [14]. Each silicle can contain between 10 and 15 seeds [1, 14].
During growth, camelina is not susceptible to insect pressure from flea beetles (Chrysomelidae) that have been shown to negatively affect yields of canola and Brassica juncea [1]. The resistance to flea beetles is thought to be the result of defense compounds present in the leaves of camelina. A class of compounds known as quercetin glycosides has been identified as contributing to its resistance to damage from the crucifer flea beetle [15]. The presence of additional leaf compounds means that camelina is naturally resistant to some fungal infections, which is important in irrigated situations [16]. Camelina has also shown allelopathic relationships with flax, Linum usitatissimum, under controlled conditions [17, 18].
Camelina is well suited to growth in low-moisture environments. The minimum water requirement for camelina to reach its maximum yield potential has been calculated to be 333 to 422 mm in Arizona [7]. The required minimum irrigation varies with climatic conditions and evapotranspiration rate. Below this minimum, yields are negatively affected. Irrigating above the minimum does not show any positive effect on seed yields and has been shown to only raise evapotranspiration of the plant [19]. The root zone of camelina is relatively shallow compared to wheat, reaching a maximum depth of 1.4 m [19, 20].
Taxonomy and Domestication
The name camelina is derived from the Greek words chamai (dwarf) and linion (flax) [21]. The center of origin of camelina is thought to be Europe. Ghamkahar et al. [22] used amplified length fragment polymorphism (AFLP) to assess genetic diversity among accessions collected in different geographic locations. They found that Russia and Ukraine are likely a center of origin of the species due to the higher level of diversity among accessions from these areas.
The species Camelina sativa (L.) Crantz and its wild relatives, which include C. microcarpa and C. linicola, have been reported by humans since the Bronze Age (1500–400 B.C.) [1]. Wild camelina species are present throughout North America and likely arrived as contaminants of flax and other agricultural products from Europe [23]. Wild accessions of Camelina microcarpa have been found that are resistant to ALS herbicides [24]. In Montana, there are over 120 varieties found in the wild [25].
Genetic Resources
Camelina ploidy varies among accessions. Camelina has been observed to have a chromosome count of 2n = 12 to 2n = 40 [23]. The most common count has been observed to be 2n = 40 [26]. Analysis of the activity of desaturation and elongation genes has revealed Camelina sativa to be an allohexaploid [27].
The genetic diversity of existing camelina germplasm is relatively low. Vollmann et al. [28] analyzed a subset of 41 accessions of camelina using randomly amplified polymorphic DNA (RAPD) markers. They found that these accessions were also classified into four main groups based on the seed weight, oil content, and protein content, which suggested a low level of diversity among these accessions. Diversity of available camelina germplasm can be supplemented with accessions recently made available from Eastern European collections formerly inaccessible to Western researchers. Analysis of camelina accessions from Russia and Ukraine show higher levels of diversity among these accessions [22]. There is the possibility of utilizing the numerous wild relatives of camelina; however, between C. sativa and C. microcarpa, there is a barrier to cross-pollination, limiting C. microcarpa’s value as a source of new traits [21].
Major Breeding Achievements
Breeding efforts of camelina have so far succeeded in producing several widely available varieties that show high yields. Yields of trials in Western Nebraska in 2005 and 2006 were between 556 and 1,456 kg ha−1 depending on the date of planting [29]. Yields of winter camelina varieties in Minnesota from 2007 to 2008 were also reported to be within the range of 311–625 kg ha−1 [30]. Camelina in Arizona under irrigation yielded over 1,500 kg ha−1 in 2009 and 2010 [8]. Camelina yields in Chile have also been reported to vary between 420 and 2,314 kg ha−1 for 2008 and 2009 [14]. Mean yields across several environments in Germany ranged from 1,460 to 1,715 kg ha−1 [31]. Camelina is able to produce adequate yields under dryland conditions, but exposure to excessive heat during flowering negatively affects its ability to produce higher yields and affects the oil profile [32].
Public and private breeding programs for spring camelina development exist in the American Midwest, Montana, and Western Europe. In the American Midwest, the Yellow Stone variety was developed by Great Plains Oil in Ohio. High Plains Crop Development, LLC, is currently active in Torrington, WY, and is producing varieties for the High Plains Region. Blue Sun Biodiesel is currently active and has previously developed the varieties BSX G22, BSX G24, and Cheyenne. The varieties Suneson and Blaine Creek were developed in Montana at Montana State University by Dr. Duane Johnson. Dr. Johnson currently works under Clear Skies Inc. out of Big Fork, MT, developing camelina varieties. The varieties Ligena and Celine were both developed in Europe and have demonstrated high yields in Europe and in the United States under a variety of conditions. The variety Celine was developed by Limagrain and has been observed to have a lower content of glucosinolates than other varieties but is shatter-prone [33]. Sustainable Oils of Global Clean Energy Holdings, Inc. is close to releasing their proprietary high yielding varieties SO-40, SO-50, and SO-60. Due to the low rate of outcrossing in camelina, major breeding programs have developed their varieties through open-pollinated stands, using either pedigree selection or recurrent selection. Inbred camelina lines used for the production of genetic maps have been derived through single-seed descent to the F6 generation [31]. Some testing has been done to determine the combining abilities of camelina lines for use in the creation of hybrid varieties, but so far, no male sterilization methods have been commercialized [34].
Protoplast fusion has been used to create a somatic hybrid between Camelina sativa and Brassica oleracea, with the intention to transfer camelina’s resistance to the black spot leaf disease, although this was met with limited success [35].
Mutagenesis has been used as a technique to introduce new and novel traits into existing camelina germplasm. Ethyl methanesulfonate (EMS) has been used by researchers interested in developing camelina lines with resistance to residual herbicides. Walsh [36] used EMS mutagenesis to develop two camelina genotypes that demonstrated tolerance to acetolactate synthase (ALS) inhibitor herbicides. These mutant camelina lines showed increased resistance to imazethapyr and sulfosulfuron herbicides. Limited quantities of these mutant lines are available from Washington State University.
A mapping population was developed in Germany (Deutsche Saatveredelung, Lippstadt, Germany) and has been used to create linkage maps and identify QTL for favorable agronomic traits in several studies. This mapping population was derived from a cross between the European varieties “Lindo” and “Licalla.” Gehringer et al. [31] created a linkage map of camelina with 157 amplified fragment length polymorphism (AFLP) markers and 3 Brassica SSR markers and identified quantitative trait loci (QTL) for seed yield, oil content, plant height, thousand-seed weight, and fatty acid composition. Using this same mapping population, Enjalbert [32] identified 29 significant QTLs for yield, drought tolerance, and oil quality characteristics. Of these, six were found to be in common with Gehringer et al. [31].
Target Traits and Current Breeding Goals
Yield
Yield is the subject of most improvement programs. Camelina seed yield has shown a high degree of heritability at 86.5 % [37]. Oil content and thousand-seed weight also demonstrate high degrees of heritability at 95.6 % and 97.6 %, respectively [38]. Thousand-seed weight could be a characteristic of interest for breeders interested in increasing yield, as this character is easier to select than yield and has a higher heritability than yield [31]. There is evidence that higher thousand-seed weight comes at the expense of oil content and the number of seeds per plant [39].
Breeding for yield stability over environments will become more important in the future as climate change and global warming affect both absolute environmental conditions and variability of environmental conditions. Optimizing camelina growth habits to better avoid summer heat can potentially minimize environmental effects on yield. Flowering time may be a useful characteristic as a target for selection, as earlier flowering varieties can better escape heat stress and earlier flowering is positively correlated with seed yield and linolenic acid content [32].
Oil Characteristics
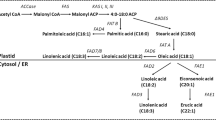
The four most important components of the fatty acid profile of camelina are C18:1, C18:2, C18:3, and C20:1, as they comprise the majority of the fatty acid profile of camelina, and environmental conditions affect the concentrations of these fatty acids [10, 32, 40]. The most economically important fatty acid, linolenic fatty acid (C18:3), has been shown to vary in concentration between 30 and 40 % [14, 31, 32]. Breeding efforts might focus on increasing the percentage of linolenic fatty acid to optimize the value of camelina press cake as an animal feed and its value as a source of polyunsaturated fatty acids. Enjalbert [31] determined the heritability of linolenic (C18:3) fatty acid to be between 0.40 and 0.83 and the heritability of oil content to vary from 0.42 to 0.87. An increase in the amount of linolenic fatty acid has been shown to improve heat and drought tolerance in some Brassica species such as canola and Arabidopsis [41–45]. Camelina seeds are composed of 30–40 % oil [1].
The high concentration of polyunsaturated fatty acids (~50 %) and protein present in the press cake is a valuable addition to feed but must be added in modest proportions. It is recommended that camelina meal comprise no more than 10 % of the feed weight due to the concentration of toxic glucosinolates that can negatively affect growth of livestock and poultry [46, 47]. Camelina meal can replace up to 5 % of broiler chicken feed without negatively impacting the quality of the meat. The incorporation of this feed increases the intramuscular concentration of omega-3 fatty acid [48]. The protein content of the press cake left over from the hexane solvent extraction process is suitable for animal consumption. It is lower in fat due to the increased efficiency of the extraction technique, but it has protein content similar to that of soybean meal. Any harmful compounds such as glucosinolates and erucic acid present in the seeds before pressing can be extracted by subsequent solvent treatment of the seed meal [49]. Vegetable consumers can recognize glucosinolates from the pungent odor that is released when cooking those in the Brassica family such as cabbages and Brussels sprouts. The leftover camelina seed meal can be heated to reduce the glucosinolate content prior to consumption, similar to these vegetables [50].
The most common, easiest, and least expensive method of oil extraction uses a mechanical oilseed crusher. This machine heats and crushes the seeds, which causes the separation of the oil from the seed meal [51]. The resulting press cake contains approximately 10 % oil by weight and is considered to have an extraction efficiency of 75 % [52]. The leftover seed meal from camelina pressing contains 40–45 % crude protein and 10 % fiber, which is lower than soybeans but comparable to rapeseed press cake [48]. With the residual fatty acids and absence of erucic acid (C22:1), the molecular profile of the leftover seed meal indicates that it could be a potentially valuable coproduct as animal feed. Future improvements and plant breeding research will need to focus on optimizing the biofuel oil profile to raise the percentage of oleic acid (18:1) and decrease concentrations of linolenic acid (18:3) [53]. This would reduce the iodine value (a measure of the degree of unsaturation of the oil) to a value that is below the acceptable limit of 120 as established by the European biodiesel standard [53].
Recent analysis of Eastern European camelina germplasm collections has identified an accession with desirable oil profile of greater than 30 % linolenic fatty acid, less than 3 % erucic acid, less than 10 % saturated fatty acids, and a ratio of linolenic to linoleic acid greater than one [22].
Breeding Strategies and Integration of New Biotechnologies
Analysis of the agronomic characteristics of recombinant inbred lines (RIL) formed from a cross between the varieties Lindo and Licalla showed that 25 % of the offspring outperformed both parents, meaning that camelina shows transgressive segregation [31]. Of these RILs, Gehringer et al. [31] identified five promising varieties as candidates for possible release. Two of these were also identified at Colorado State University as containing QTL for yield and drought tolerance and demonstrating significantly higher yields [32]. These are currently undergoing further yield evaluations with the possibility for commercial release [32]. The difference in climate between Germany and Colorado suggest that these varieties are widely adapted and show high yields in a variety of environments.
Gamma ray irradiation was used by Vollmann et al. [37] to induce mutations in camelina germplasm for improving linolenic acid content. Lines were isolated that contained higher concentrations of the fatty acid (40.8 %). In addition, some mutants were identified that contained lower concentration of erucic acid (less than 2 %).
Camelina is a suitable candidate species for transgenesis. It is widely considered a primarily self-pollinating species with a low rate of outcrossing [23]. Field experiments have estimated the outcrossing rate in camelina to vary between 0.01 and 0.28 % [54]. This is nearly equal to soybean, which is approximately 0.30 % [55]. The fact that camelina is a facultative outcrossing species means that it can be bred through recurrent selection or through the creation of hybrids by creating male sterile lines for crossing. A methodology for developing doubled haploid camelina would also greatly reduce the number of generations necessary for new lines of camelina to reach homozygosity [39].
Research looking to characterize the genome of camelina has been ongoing for some time. There have been notable efforts to identify several target genes with respect to their potential for genetic manipulation. Hutcheon et al. [27] targeted genes regulating fatty acid synthesis in Arabidopsis that were hypothesized to be homologous in camelina. These genes include fatty acid desaturase 2 (FAD2), which converts oleic acid (C18:1) to linoleic acid, and fatty acid elongase 1 (FAE1), which adds two carbons to an 18-carbon chain. It is presumed that downregulation of these genes could lead to increased production of oleic acid, a favorable fatty acid for biodiesel feedstocks. RNA sequencing techniques revealed that unlike in Arabidopsis, where only one copy of each gene is found, the camelina genome contains at least three functional copies of FAD2 and FAE1. This suggests at least three full genome duplication events occurred at some point in the evolution of the camelina genome. The polyploid nature of the camelina genome was proposed to be conducive to reverse genetic (TILLING) manipulations using protocols developed for wheat, another allohexaploid. The use of TILLING technologies would make it possible to identify individuals with mutations in the FAD1 and FAE2 genes.
Another attempt to modify the oil profile of camelina through the use of transgenics was carried out by Lu and Kang [56]. Using a seed-specific phaseolin promoter and the floral dip method, the authors were able to carry out a successful plant transformation where they inserted a castor oil gene FAHI2, which codes for a novel fatty acid (C18:1OH). The oil profiles of the resulting transgenic plants had elevated levels of oleic acids from 14.4 to 21.6 % and a resulting decrease in the polyunsaturated fatty acid percentage from 37.8 to 13.3 %.
Seed Production
Camelina can be directly harvested using existing wheat harvesting equipment with a screen of 3.6 mm installed over the lower sieves of the harvester [3, 5]. Harvesting efficiency can be improved if future varieties are selected to reduce shattering. If weeds are a problem, camelina can be swathed when the pod color is about 65 % yellow [5].
Today, camelina is being produced as a biofuel feedstock crop. Commercial camelina production in the United States centers on the Pacific Northwest region and Montana, and the majority of this production is sold to the US Air Force for its green fuel program. According to the USDA National Agricultural Statistics Service [57], about 19,500 acres of camelina were harvested in 2009 and 9,400 acres were harvested in 2010 in the United States. Camelina is currently being produced exclusively under contract; there is no open market for the crop.
Although the production areas of camelina include Montana, Oregon, and Washington, camelina testing and varietal evaluation is being conducted in several states including Colorado, Wyoming, California, Kentucky, Iowa, Florida, and Arizona. Photos of camelina stands grown in Craig, Colorado, are shown in Figs. 8.1 and 8.2.
Camelina contract price varies depending on the location and year, but in 2010, the average price was $0.16/lb [58]. Assuming the cost of meal is $0, which is not the case, the value of the camelina biodiesel would be $5.00/gallon [58]. Keske et al. [59] estimated that producing camelina for on-farm use of straight vegetable oil would have the highest probability of return when conventional diesel prices reach and exceed $1.31/L. The cost of production for camelina based on models developed at Montana State University is estimated to be $80.27/acre at 1,350 lbs/acre yields [34, 58].
Winter Varieties
Limited development of winter camelina lines exists today. High seed yield from fall-seeded winter varieties remains to be proven [30, 60]. The advantage of these varieties is to allow the camelina seedlings to be in the soil when weather conditions are optimal for emergence. Phenotypically, these varieties differ from spring varieties in leaf shape and overwintering ability.
In the case of fall-seeded, the plant establishes itself in the fall and overwinters as a rosette. The following spring, when temperatures reach 3.3 °C, growth is initiated and the plant emerges from the rosette and resumes growth [6]. The vernalization requirement for winter camelina has not been well characterized. Previous experiments have found that fall-seeded camelina has enough winter hardiness to survive the harsh winters of Minnesota, where average winter air temperatures are far lower than those found in Colorado [30]. As the plant is already established, it reaches maturity far earlier than spring-seeded camelina. Earlier maturity means that the plants are not exposed to as much of the heat and drought stress that occurs during the warmest months of summer.
In addition to the potential for increasing yields, earlier harvest allows more time for moisture recharge in the field during the summer. This could result in higher yields for wheat that is planted after fall-seeded camelina than spring-seeded camelina. This may vary based on spring temperatures and moisture conditions. Another advantage of winter camelina is that fall planting is generally drier and the seeds are already planted when spring rainfall arrives. Winter seeding of camelina would be particularly advantageous in southeastern regions of the United States, where the winters are warmer and the spring arrives earlier. In colder climates, overwintering ability is increased with snow cover [61, 62]. Aase and Siddoway [61] determined that 7 cm of snow cover is sufficient to buffer wheat seedlings from temperatures as low as −40 °C. With the increased stubble as a result of the implementation of no-till agricultural systems, there is a greater amount of snow capture on fields.
Winter camelina trials in Akron, Colorado, have encountered failures related to the presence of Ceutorhynchus cyanipennis and Ceutorhynchus americanus ([60, B. Kondratieff and G.L. Hein (2011), personal communication). These insect pests appear frequently on plant species in the Brassicaceae family, which includes camelina [63]. These insects most strongly affect winter camelina that is planted earlier in the fall, especially in August, as this is the time adult insects lay eggs [63]. Later fall planting dates have been shown to reduce the impact of these pests. Studies of winter camelina in Ireland have shown that earlier planting dates are prone to high rates of lodging [64]. This also may be due to the damage from these insects, as the larvae feed on stems at or below the soil line (G.L. Hein (2011), personal communication).
Market Challenges/Barriers to Commercialization/Opportunities
Barriers to the wide-scale adoption of camelina as a biofuel feedstock come from a variety of factors. Camelina sativa is related to weedy species that are recognizable to farmers. This might contribute to their reluctance towards planting large swaths of land with camelina. This can be overcome through education and awareness, as camelina is not very competitive as a weed species and volunteer camelina can be easily controlled with available herbicides.
The lack of an open market for camelina may dissuade some potential producers. Farmers operating outside of certain areas where contract camelina growing operations exist may have trouble selling excess product, as it is not certified for human consumption.
The main opportunity for camelina production exists in the on-farm production of biofuels. This eliminates the need for an open market, as local consumption is not subject to certification in the same way as biodiesel producers looking to sell to large blenders. Local camelina production is an opportunity for farmers to offset their costs in two ways. The first is through the utilization of the camelina oil as a diesel substitute that will offset the annual consumption of diesel fuel. The second is by utilization of leftover seed meal, or press cake, to offset consumption of animal feed.
Camelina-derived diesel fuel can be utilized directly without any fuel processing. This is known as straight vegetable oil (SVO). The SVO can be directly burned in an engine that has been modified with tank heaters to increase the viscosity of the fuel, or else, it can be blended with diesel fuel and used in an unmodified engine.
Local production of camelina is dependent on collaboration between producers. A possible model for the production of camelina-based fuel involves a community-funded crushing facility that is shared between several producers. The meal and camelina oil can be distributed among the producers along with the costs. Currently, there are locally sourced crushing facilities in three locations in Colorado. These are in Rocky Ford, Burlington, and Costilla County, Colorado [32].
More advanced producers will take advantage of the specialized oil profile of camelina and produce with the intention of selling the components of the oil such as linolenic and linoleic fatty acids, which has a higher value than vegetable oil for use as diesel fuel alternatives. Selling the components of the camelina oil is a way to increase production when fuel prices are low and ensure profits during low yielding years.
References
Zubr J. Oil-seed crop: Camelina sativa. Ind Crops Prod. 1997;6(2):113–9.
Frohlich A, Rice B. Evaluation of Camelina sativa oil as a feedstock for biodiesel production. Ind Crops Prod. 2005;21:25–31.
Enjalbert JN, Johnson JJ. Guide for producing dryland camelina in Eastern Colorado. Colorado State University extension factsheet no. 0.709. 2011. http://www.ext.colostate.edu/pubs/crops/00709.pdf. Accessed 26 June 2013.
Robinson RG. Camelina: a useful research crop and a potential oilseed crop, Station bulletin, vol. 579. St. Paul: Minnesota Agricultural Research Station; 1987.
Lafferty RM, Rife C, Foster G. Spring camelina production guide for the central high plains. Blue Sun Biodiesel special extension publication, ACRE. 2009. http://www.colorado.gov/cs/Satellite?blobcol=urldata&blobheader=application%2Fpdf&blobkey=id&blobtable= MungoBlobs&blobwhere=1251616501820&ssbinary=true. Accessed 26 June 26 2013.
Ehrensing DT, Guy SO. Oilseed crops: camelina. Oregon State University Extension Service EM 2008; 8953-E.
French AN, Hunsaker D, Thorp K, Clarke T. Evapotranspiration over camelina crop at Maricopa. Ariz Ind Crops Prod. 2009;29:289–300.
Hunsaker DJ, French AN, Thorp KR. Camelina water use and seed yield response to irrigation scheduling in an arid environment. Irrig Sc. Published online: 31 July 2012. doi: 10.1007/s00271-012-0368-7
Johnson JJ, Enjalbert N, Shay R, Heng S, Coonrod D. Investigating straight vegetable oil as a diesel fuel substitute: final report to Colorado agricultural value-added development board. Colorado: Fort Collins; 2008.
Johnson JJ, Enjalbert N, Schneekloth J, Helm A, Malhotra R, Coonrod D. Development of oilseed crops for biodiesel production under Colorado limited irrigation conditions. Completion report no. 211. Colorado Water Institute; 2009.
Zubr J. Qualitative variation of Camelina sativa seed from different locations. Ind Crops Prod. 2003;17(3):161–9.
Putnam DH, Budin JT, Field LA, Breene WM. Camelina: a promising low-input oilseed. In: Janick J, Simon JE, editors. New crops. New York: Wiley; 1993. p. 314–22.
Hulbert S, Guy S, Pan B, Paulitz T, Schillinger B, Wysocki D, Sowers K. Camelina production in the Pacific Northwest. Washington State University Extension Publication. 2011. http://css.wsu.edu/biofuels/publications/. Accessed 26 June 2013.
Berti M, Wilckens R, Fischer S, Solis A, Johnson B. Seeding date influence on camelina seed yield, yield components, and oil content in Chile. Ind Crops Prod. 2011;34(2):1358–65.
Onyilagha JC, Gruber MY, Hallet RH, Holowachuk J, Buckner A, Soroka JJ. Constitutive flavonoids deter flea beetle insect feeding in Camelina sativa. Biochem Syst Ecol. 2012;42:128–33.
Browne LM, Conn KL, Ayer WA, Tewari JP. The camelexins: new phytoalexins produced in the leaves of Camelina sativa (Cruciferae). Tetrahedron. 1991;47(24):3909–14.
Lovett JV, Jackson HF. Allelopathic activity of Camelina sativa (L.) Crantz in relation to its phyllosphere bacteria. New Phytol. 1980;86:273–7.
Lovett JV, Duffield AM. Allelochemicals of Camelina sativa. J Appl Ecol. 1981;18(1):283–90.
Hunsaker DJ, French AN, Clarke TR, El-Shikha DM. Water use, crop coefficients and irrigation management criteria for camelina production in arid regions. Irrig Sci. 2011;29:27–43.
Sabu P, Panda SN, Kumar DN. Optimal irrigation allocation: a multilevel approach. J Irrig Drain Eng. 2000;126(3):149–56.
Plessers AG, McGregor WG, Carson RB, Nakoneshny W. Species trials with oilseed plants: ii. Camelina. Can J Plant Sci. 1962;42(3):452–9.
Ghamkhar K, Croser J, Aryamanesh N, Campbell M, Kon’kova N, Francis C. Camelina (Camelina sativa (L.) Crantz) as an alternative oilseed: molecular and ecogeographic analyses. Genome. 2010;53(7):558–67.
Francis A, Warwick SI. The biology of Canadian weeds. 142. Camelina alyssum (Mill.) Thell.; C. microcarpa Andrz. ex DC.; C. sativa (L.) Crantz. Can J Plant Sci. 2009;89(4):791–810.
Hanson BD, Park KW, Mallory-Smith CA, Thrill DC. Resistance of Camelina microcarpa to acetolactate synthase inhibiting herbicides. Weed Res. 2004;44:187–94.
Shonnard DR, Williams L, Kalnes TN. Camelina-derived jet fuel and diesel: sustainable advanced biofuels. Environ Prog Sust Energy. 2010;29(3):382–92.
Warwick SI, Al-Shehbaz IA. Brassicaceae: chromosome number index and database on CD-ROM. Plant Syst Evol. 2006;259(2–4):237–48.
Hutcheon C, Ditt RF, Beilstein M, Comai L, Schroeder J, Goldstein E, Shewmaker CK, Nguyen T, De Rocher J, Kiser J. Polyploid genome of Camelina sativa revealed by isolation of fatty acid synthesis genes. BMC Plant Biol. 2010;10(1):233.
Vollman J, Grausgruber H, Stift G, Dryzhyruk V, Lelly T. Genetic diversity in camelina germplasm as revealed by seed quality characteristics and RAPD polymorphism. Plant Breed. 2005;124:446–53.
Pavlista AD, Isbell TA, Baltensperger DD, Hergert GW. Planting date and development of spring-seeded irrigated canola, brown mustard and camelina. Ind Crops Prod. 2011;33:451–6.
Gesch RW, Cermak SC. Sowing date and tillage effects on fall-seeded camelina in the northern corn belt. Agron J. 2011;103(4):980–7.
Gehringer A, Friedt W, Lühs W, Snowdon RJ. Genetic mapping of agronomic traits in false flax (Camelina sativa). Genome. 2006;49(12):1555–63.
Enjalbert JN. An integrated approach to local based biofuel development. Ph.D. Dissertation. Colorado State University Libraries. 140 p. 2011. http://hdl.handle.net/10217/46747. Accessed 26 June 2013.
Hunter J, Roth G. Camelina production and potential in Pennsylvania. Agronomy facts 72. The Pennsylvania State University. 2010. Retrieved from: http://pubs.cas.psu.edu/freepubs/pdfs/uc212.pdf. Accessed 21 June 2012.
Geschickter C, Lawrence M. Camelina aviation biofuels: market opportunity and renewable energy report. Biomass advisors report; 2010.
Hansen L. Intertribal somatic hybridization between rapid cycling Brassica oleracea L. and Camelina sativa (L.) Crantz. Euphytica. 1998;104:173–9.
Walsh DT. Selection of camelina mutants resistant to acetolactate synthase inhibitor herbicides. Master’s thesis. Washington State University; 2010. 60 p. http://www.dissertations.wsu.edu/thesis/summer2010/d_walsh_072210.pdf. Accessed 26 June 2013.
Vollmann J, Damboeck A, Baumgartner S, Ruckenbauer P. Selection of induced mutants with improved linolenic acid content in camelina. Fett/Lipid. 1997;99(10):357–61.
Vollmann J, Damboeck A, Eckl A, Schrems H, Ruckenbauer P. Improvement of Camelina sativa, an underexploited oilseed. In: Janick J, editor. Progress in new crops. Alexandria: ASHS Press; 1996. p. 357–62.
Ferrie AMR, Bethune TD. A microspore embryogenesis protocol for Camelina sativa, a multi-use crop. Plant Cell Tiss Org Cult. 2011;106(3):495–501.
Vollmann J, Moritz T, Kargl C, Baumgartner S, Wagentristl H. Agronomic evaluation of camelina genotypes selected for seed quality characteristics. Ind Crops Prod. 2007;26(3):270–7.
McConn M, Hugly S, Browse J, Somerville C. A mutation at the FAD8 locus of Arabidopsis identifies a second chloroplast omega-3 desaturase. Plant Physiol. 1994;106:1609–14.
Triboi-Blondel AM, Renard M. Effects of temperature and water stress on fatty acid composition of rapeseed oil. In: Proceedings of the 10th international rapeseed congress. Canberra; 26–30 Mar 1999.
Matsuda O, Sakamoto H, Hashimoto T, Iba K. A temperature-sensitive mechanism that regulates post-translational stability of a plastidial omega-3 fatty acid desaturase (FAD8) in Arabidopsis leaf tissues. J Biol Chem. 2005;280:3597–604.
Merrien A, Krouti M, Dechambre J, Garnon V, Evrard J. Contribution to understand the fluctuation of linolenic acid profile in winter oilseed rape grown in France. In: Proceedings of the 12th international rapeseed congress on quality, nutrition and processing, Wuhan; 26–30 Mar 2007, p. 92–5.
Mene-Saffrane L, Dubugnon L, Chetelat A, Stolz S, Gouhier-Darimont C, Farmer EE. Nonenzymatic oxidation of trienoic fatty acids contributes to reactive oxygen species management in Arabidopsis. J Biol Chem. 2009;284:1702–8.
Food and Drug Administration (FDA). Approved uses of camelina meal in feed. 2012. http://agr.mt.gov/agr/Programs/Commodities/Camelina/FeedUses.html. Accessed 26 June 2013.
Moriel P, Nayigihugu V, Cappellozza BI, Goncalves EP, Krall JM, Foulke T, Cammak KM, Hess BW. Camelina mean and crude glycerin as feed supplements for developing replacement beef heifers. J Anim Sci. 2011;89:4314–24.
Ryhanen EL, Perttla S, Tupasela T, Valaja J, Eriksson C, Larkka K. Effects of Camelina sativa expeller cake on performance and meat quality of broilers. J Sci Food Agric. 2007;87(8):1489–94.
Naczk M, Diosady LL, Rubin LJ. Functional properties of canola meals produced by a two-phase solvent extraction system. J Food Sci. 1985;50:1685–8.
Fenwick GR, Heaney RK. Glucosinolates and their breakdown products in cruciferous crops, food and feeding stuffs. Food Chem. 1983;11:249–71.
Khan LM, Hanna MA. Expression of oil from oilseeds-a review. J Agric Eng Res. 1983;28:495–503.
Boateng AA, Mullen CA, Goldberg NM. Producing stable pyrolosis liquids from the oil-seed presscakes of mustard family plants: pennycress (Thlaspi arvense L.) and camelina (Camelina sativa). Energy Fuels. 2010;24:6624–632.
Pinzi S, Garcia IL, Lopez-Gimenez FJ, Luque de Castro MD, Dorado G, Dorado MP. The ideal vegetable oil-based biodiesel composition: a review of social, economical and technical implications. Energy Fuels. 2009;23(5):2325–341.
Walsh KD, Puttick DM, Hills MJ, Yang RC, Topinka KC, Hall LM. Short communication: first report of outcrossing rates in camelina (Camelina sativa (L.) Crantz), a potential platform for bioindustrial oils. Can J Plant Sci. 2012;92(4):681–5.
Ahrent DK, Caviness CE. Natural cross-pollination of twelve soybean cultivars in Arkansas. Crop Sci. 1994;34(2):376–8.
Lu C, Kang J. Generation of transgenic plants of a potential oilseed crop Camelina sativa by Agrobacterium-mediated transformation. Plant Cell Rep. 2008;27:273–8.
United States Department of Agriculture-NASS. 2010 camelina crop. 2011. http://www.nass.usda.gov/Statistics_by_State/Montana/Publications/Press_Releases_Crops/camelina.pdf. Accessed 26 June 2013.
Stein L. Economic analysis of potential oil crop supplies in the Northwest U.S. Master’s thesis. Oregon State University; 2012. 80 p. http://ir.library.oregonstate.edu/xmlui/bitstream/handle/1957/33836/SteinLukas2012.pdf?sequence=2. Accessed 26 June 2013.
Keske CMH, Hoag DL, Brandess A, Johnson JJ. Is it economically feasible for farmers to grow their own fuel? A study of Camelina sativa produced in the Western United States as an on-farm biofuel. Biomass Bioenergy. 2013;54:89–99.
Jewett FG. Camelina variety performance for yield, yield components and oil characteristics. Master’s thesis. Colorado State University Libraries; 2013 (in press).
Aase JK, Siddoway FH. Crown-depth soil temperatures and winter protection for winter wheat survival. J Soil Sci Soc Am. 1979;43:1229–33.
Sharratt BS, Baker DG, Wall DB, Skaggs RH, Ruschy DL. Snow depth required for near steady-state soil temperatures. Agr Forest Meteorol. 1992;57:243–51.
Cripps MG, Schwarzlander M, McKenny JL, Hinz HL, Price WJ. Biogeographical comparison of the arthropod herbivore communities associated with Lepidium draba in its native, expanded and introduced ranges. J Biogeogr. 2006;33:2107–19.
Crowley JG. Evaluation of Camelina sativa as an alternative oilseed crop. End of project report no. 7. ISBN 1 84170 049 5. Oak Park: Crops Research Centre; 1998.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Jewett, F.G. (2015). Camelina sativa: For Biofuels and Bioproducts. In: Cruz, V.M.V., Dierig, D.A. (eds) Industrial Crops. Handbook of Plant Breeding, vol 9. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1447-0_8
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1447-0_8
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1446-3
Online ISBN: 978-1-4939-1447-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)