Abstract
Sorghum [Sorghum bicolor (L.) Moench] is the fifth most important cereal crop and is the dietary staple of more than 500 million people in over 90 countries, primarily in the developing world. However, sweet sorghum which is similar to grain sorghum except for accumulation of stalk sugars, is considered as a potential energy crop without impacting the food security of millions. Further, the sorghum stover is considered to be a potential lignocellulosic biofuel feedstock. Being a C4 plant, it has high photosynthetic rate, and several mechanisms are known to confer resilience that help produce higher yield in varied environmental conditions. This chapter not only discusses different breeding methodologies for improving candidate sugar and biomass traits but also the possible utilization of this smart feedstock for diverse biochemicals (lactic acid, xylitol, glycerol, etc.) and bioproducts (nanomaterials, anticancer and microbial compounds, adhesives, polymers, antidiabetic compounds, etc.) development.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The current scenario of declining fossil fuel reserves along with increased concerns on environment pollution and climate change is fundamentally responsible for greater interest in renewable energy sources globally. Sustainable availability of raw material for any economic and constant product production is one of the essential requirements. This has become more appropriate for the constant consumption products like biofuels, as the entire world economy is dependent on the availability of fuel resources. Interest in sweet sorghum (Sorghum bicolor (L.) Moench) in semiarid and rain-fed environments is increasing because of the multiple uses of this novel feedstock either for production of biofuels from stalk juice or for power generation from bagasse or for utilization in dairy industry as nutrient rich and easily digestible fodder [1, 2]. Additionally, sweet sorghum biomass is used for fiber, paper, syrup, and biopolymers. Sweet sorghum being a C4 crop has wide environmental adaptation, rapid growth, high grain and biomass productivity, suitability for marginal soils, and high concentrations of the easily fermentable sugars like sucrose, glucose, and fructose [3]. Drought and salinity are widely prevalent abiotic stresses that significantly lower the yields of various crops, and their frequency of occurrence is expected to increase due to climate change. Sweet sorghum grows in marginal areas because of its high tolerance to saline and drought conditions. Sweet sorghum has higher water-use efficiency than other summer crops under both well-watered and water-stressed conditions [4–6]. From the agronomic point of view, sweet sorghum is more environmentally friendly than maize because of its relatively low nitrogen needs and water requirements. It was reported that sorghum requires 310 kg of water to produce 1 kg of biomass, while maize consumes 23 % more water, i.e., 370 kg to produce same quantity of biomass [7]. Besides biofuel production from sweet sorghum, a plethora of food products such as beverage, cookies, syrup, sweets, chocolates [8], and bioproducts like biopolymer resin can be produced [9]. However, the commercialization of this smart feedstock primarily hinges on the national biofuel policy of respective countries besides identification of productive cultivars adapted to the targeted region owing to significant genotype × environment interaction [10].
This chapter will focus on genetic enhancement of sweet sorghum through conventional plant breeding and the production of various bioproducts based on this novel feedstock.
Food: Fuel Trade Off
It is often stated that sweet sorghum cultivars do not produce grain yield or the grain yield is very less vis-a-vis that of grain sorghum. Studies at the International Crops Research Institute for the Semiarid Tropics (ICRISAT) showed that sweet sorghum hybrids had higher stem sugar yield (11 %) and higher grain yield (5 %) compared to grain sorghum types, while sweet sorghum varieties had 54 % higher sugar yield and 9 % lower grain yield compared to non-sweet stalk varieties in the rainy season. On the other hand, both sweet sorghum hybrids and varieties had higher stalk sugar yields (50 and 89 %) and lower grain yields (25 and 2 %) in the post-rainy season. Thus, there is little trade-off between grain and stalk sugar yields in the sweet sorghum hybrids in the rainy season, while the trade-off is less in varieties in the post-rainy season [2, 3].
This is further corroborated by other published work [11] showing that there is significant soluble sugar content in the stems (79–94 %) during post-anthesis period, with the hybrids exhibiting significantly high soluble sugar content over varieties with same maturity period and effects of year, harvest time, and genotype on calculated ethanol yield (CEY) are highly significant. The experimental data on the relationship between stalk sugar traits and grain yield shows that the regression coefficient of stalk sugar yield on grain yield is not significant, thereby indicating that the grain yield is not affected when selection is done for stalk sugar yield. Hence, selection programs can aim to improve both the traits simultaneously.
Climate Change
Global warming due to climate change will affect grain and stover yields in crops, more so in tropical Africa and Asia where sorghum is a major food crop. Most climate change models predict rise in air and soil temperatures and sea levels and increased frequencies of extreme weather events leading to unprecedented changes in agricultural production in the years to come. In the Intergovernmental Panel on Climate Change (IPCC), climate models predict an increase in global average surface temperature of between 1.4 and 5.8 °C from 2001 to 2100, the range depending largely on the scale of fossil fuel burning between now and then and on the different models used. At the lower range of temperature rise (1–3 °C), global food production might actually increase, but above this range, it would probably decrease [12]. However, broad trends will be overshadowed by local differences, as the impacts of climate change are likely to be highly spatially variable. In general, the sorghum maturity period of current varieties decreases with increased temperatures. Climate change effects in terms of high temperatures and erratic rainfall may drastically reduce sorghum yields in South Asia, Southern Africa, and West Africa [13]. Climate change will cause changes in the length of the growing period (LGP) in some regions. Cooper et al. [13] showed that the extent of global semiarid tropical (SAT) areas will be changed through (1) SAT areas being “lost” from their driest margins and become arid zones due to LGPs becoming too short or (2) SAT areas being “gained” on their wetter margins from subhumid regions through the reduction in the current LGPs in those zones. It means sorghum could be grown in new areas of the currently humid tropics where sorghum is not grown at present. Therefore, development of crop cultivars with a maturity duration that suits the prevailing LGP will be one of the best options to cope with changes in LGP. ICRISAT and Indian National Agricultural Research System (NARS) have developed a wide variety of sweet sorghum female parental lines and restorers besides varieties with altered LGP that can play pivotal role in achieving the above said option.
Taxonomy
Sorghum was first described by Linnaeus in 1753 under the name Holcus. In 1974, Moench distinguished the genus Sorghum from genus Holcus [14, 15]. Subsequently, several authors have discussed the systematics, origin, and evolution of sorghum since Linnaeus [16–19]. Sorghum is classified under the family Poaceae, tribe Andropogoneae, subtribe Sorghinae, and genus Sorghum. The genus was further divided [20] into five subgenera: Sorghum, Chaetosorghum, Heterosorghum, Parasorghum, and Stiposorghum. Variation within these five subgenera except the subgenera Sorghum has been described [14]. Sorghum bicolor subsp. bicolor contains all of the cultivated sorghums. Harlan and deWet [20] have developed a simplified classification of cultivated sorghum which proved to be of real practical utility for sorghum researchers. They classified Sorghum bicolor (L.) Moench, subsp. bicolor into five basic and ten hybrid races as depicted in Table 1.1. The 15 races of cultivated sorghum can be identified by mature spikelets alone, although head type is sometimes helpful. The Biodiversity International [formerly International Plant Genetic Resources Institute (IPGRI)] advisory committee on sorghum and millet germplasm has accepted and recommended this classification to be used in describing sorghum accessions.
Sweet Sorghum Distribution and Climatic Conditions
In simple terms, wherever sorghum is currently grown, sweet sorghum can also be cultivated commercially. Thousands of hectares are grown with sweet sorghum for biofuels production in Brazil, China, and the USA, while in the Philippines, it is grown for vinegar synthesis, and considerable areas in India, the USA, Indonesia, West Asia, and North Africa go for fodder production. In Western and Southern Africa, it is widely used for chewing purposes and local beverage production. This feedstock is well adapted to the SAT and is one of the most efficient dryland crops in converting atmospheric CO2 into sugar [3]. The crop can be grown in a wide range of climatic conditions as given below.
Latitude
Sweet sorghum can be grown between 40°N and 40°S latitude on either side of the equator.
Altitude
Sorghum can be found at elevations between sea level and 1,500 m asl. Most East African sorghum is grown between the altitudes of 900–1,500 m, and cold-tolerant varieties are grown between 1,600 and 2,500 m in Mexico.
Environmental Conditions
Sweet sorghum can be grown in the temperature range of 12–37 °C. The optimum temperatures for growth and photosynthesis are 32–34 °C, day length is 10–14 h, optimum rainfall 550–800 mm, and relative humidity between 15 and 50 %. However, the lower the diurnal and nocturnal temperature differential, the less stalk sugar accumulation observed is in tropical sweet sorghums.
Soil Conditions
Alfisols (red) or vertisols (black clay loamy) with pH 6.5–7.5, organic matter >0.6 %, soil depth >80 cm, soil bulk density <1.4 gcc, water holding capacity >50 % field capacity, N ≥ 260 kg ha−1 (available), P ≥ 12 kg ha−1 (available), and K ≥ 120 kg ha−1 (available) are optimal soil conditions for sorghum growth.
Water
While sorghum will survive with a supply of less than 300 mm over the season of 100 days, sweet sorghum responds favorably with additional rainfall or irrigation water. Typically, sweet sorghum needs between 500 and 1,000 mm of water (rain and/or irrigation) to achieve good yields, i.e., 50–100 t ha−1 total aboveground biomass (fresh weight). The great advantage of this feedstock is that it can become dormant, especially in the vegetative phase, under adverse conditions and can resume growth after relatively severe drought. Early drought stops growth before panicle initiation and the plant remains vegetative; it will resume leaf production and flowering when conditions become favorable for growth again. Mid-season drought stops leaf development. Although this crop is susceptible to sustained flooding particularly at early vegetative phase, it tolerates water logging better than maize and sugarbeet [2].
Radiation
Being a C4 plant, sweet sorghum has high radiation use efficiency (RUE) (about 1.3–1.7 g MJ−1). It has been shown that taller sorghum types possess higher RUE, because of a better light penetration in the leaf canopy.
Photoperiodism
Most hybrids of sweet sorghum are relatively less photoperiod-sensitive vis-a-vis purelines. Traditional farmers, particularly in West Africa, use photoperiod-sensitive varieties. With photoperiod-sensitive types, flowering and grain maturity occurs almost during the same calendar days regardless of planting date, so that even with delayed sowing, plants mature before soil moisture is depleted at the end of rainy season.
Reproductive Biology
Breeding procedures that are used with a particular crop species are determined by its mode of reproduction. Understanding the details of phenology, i.e., floral biology, pollination, fertilization, and seed development in a crop, makes it possible to develop orderly and efficient breeding procedures.
Panicle Initiation
Sorghum blooming is hastened by short days and long nights. However, varieties differ in their photoperiod sensitivity [21]. Tropical sweet sorghum varieties initiate the reproductive stage when day lengths return to 12 h. Usually, the floral initial is 15–30 cm above the ground when the plants are about 50–75 cm tall [22]. Floral initiation marks the end of the vegetative growth due to meristematic activity. The time required for transformation from the vegetative apex to reproductive apex is largely influenced by genetic characteristics and the environment (photoperiod and temperature). The grand period of growth in sorghum follows the formation of a floral bud and consists largely of cell enlargement. Hybrids take less time to reach panicle initiation and are relatively less influenced by photoperiod and temperature [2, 3].
Panicle Emergence
During the period of rapid cell elongation, floral initials develop into an inflorescence. About 6–10 days before flowering, the boot will form as a bulge in the sheath of the flag leaf. This will occur, in a variety that flowers in 60–65 days, about 55 days from germination. Sorghum usually flowers in 55 to more than 70 days in warm climates, but flowering may range from 30 to more than 100 days. These observations are valid for tropical sweet sorghums, while temperate sorghums that mature in 5 months take 20–30 days longer for panicle emergence [2, 3].
Panicle Structure
The inflorescence is a raceme, which consists of one or several spikelets. It may be short, compact, loose, or open and composed of a central axis that bears whorls of primary branches on every node. The spikelet usually occurs in pairs, one being sessile and the second borne on a short pedicel, except the terminal sessile spikelet, which is accompanied by two pediceled spikelets. The first and second glumes of every spikelet enclose two florets: the lower one is sterile and is represented by a lemma and the upper fertile floret has a lemma and palea. Two lodicules are placed on either side of the ovary at its base. Androecium consists of one whorl of three stamens. The anthers are attached at the base of the ovule by a very fine filament and are versatile and yellowish. Gynoecium is centrally placed and consists of two pistils with one ovule from which two feathery stigmas protrude. The sessile spikelet contains a perfect flower. It varies in shape from lanceolate to almost rotund and ovate and is sometimes depressed in the middle. The pediceled spikelets, usually lanceolate in shape and possess only anthers, occasionally have a rudimentary ovary and empty glumes [9].
Anthesis and Pollination
Anthesis starts after panicle emergence from the boot leaf. Flowers begin to open 2 days after full emergence of the panicle. Floret opening or anthesis is achieved by swelling of the lodicules and is followed by the exertion of anthers on long filaments and of stigmas between the lemma and palea. Sorghum head begins to flower at its tip and flowers successively downward over a 4- or 5-day period. Flowering takes place first in the sessile spikelets from top to bottom of the inflorescence. It takes about 6 days for completion of anthesis in the panicle with maximum flowering at 3 or 4 days after anthesis begins. Flowering proceeds downwards to the base in a horizontal plane on the panicle. When flowering of the sessile spikelets is halfway down the panicle, pedicellate spikelets start to open at the top of the panicle and proceed downwards [22]. Anthesis takes place during the morning hours and frequently occurs just before or just after sunrise, but may be delayed on cloudy damp mornings. It normally starts around midnight and proceeds up to 10:00 AM depending on the cultivar, location, and weather. Maximum flowering is observed between 6:00 and 8:00 AM. The anthers dehisce when they are dry and pollen is blown into air. The pollen remains viable several hours after shedding. The flowers remain open for 30–90 min. Dehiscence of the anthers for pollen diffusion takes place through the apical pore. The pollen drifts to the stigma, where it germinates; the pollen tube, with two nuclei, grows down the style, to fertilize the egg and form a 2n nucleus [2, 3, 19].
Cytoplasmic male sterility has been found in sorghum (A1-A4 systems) and has made possible the development of a hybrid seed industry. A good male-sterile plant will not develop anthers, but in some instances dark-colored shriveled anthers with no viable pollen will appear. Partially fertile heads are also observed, and although the anthers frequently have viable pollen, the quantity is less than in normal plants. There are two types of male sterility, viz., (a) genetic male sterility (GMS) and (b) cytoplasmic nuclear male sterility (CMS), both widely used in sorghum improvement programs [4].
Genetic Male Sterility
Genetic male sterility is expressed in sorghum in many ways. Several sources of male sterility are identified. In all the cases, it was shown that a recessive allele in homozygous condition designated with a series of alleles, ms 1 , ms 2 , ms 3 , ms 4 , ms 5 , ms 6 , ms 7 , and al, confers male sterility [19, 23, 24]. The genetic male sterility genes are represented in Table 1.2.
Cytoplasmic Nuclear Male sterility
The discovery of the male sterility resulting from the interaction of cytoplasmic and nuclear genes [32] laid the foundation and revolutionized the development of hybrid cultivar and hybrid seed production technology. The milo cytoplasm was from durra race, which induced male sterility in the nuclear background of kafir race, and this is designated as A1 cytoplasm. Since then, several sources and types of male-sterile-inducing cytoplasms have been discovered and reported. In all these cytoplasms, recessive genes in the nucleus and sterile cytoplasm induce male sterility. These male-sterile cytoplasms have been differentiated based on the inheritance patterns of their fertility restoration. The inheritance of fertility restoration is not clear, as it is dependent on the specific cytoplasm and nuclear combinations. Fertility restoration is controlled by single gene in some combinations (e.g., A1) but is controlled by two or more genes when the same nuclear genotype interacts with a different cytoplasm [33]. Although diverse male-sterile cytoplasms have been identified, by far, only the milo cytoplasmic male sterility system is widely used because the hybrids based on this cytoplasm produce sufficient heterosis (20–30 %) over the best available pure lines in sweet sorghum. In spite of A2 cytoplasm being as good as A1 cytoplasm for mean performance as well as heterosis for economic traits such as stalk yield, juice yield, grain yield, days to 50 % flowering, and plant height, it is not popular as the anthers in A2 male steriles, unlike the A1 male steriles, mimic the fertile or maintainer lines and lead to difficulties in monitoring the purity of hybrid seed production, and also the restoration frequency is low. ICSSH 58 (ICSA 738 × ICSV 93046) is the first A2-based sweet sorghum hybrid in the world bred at ICRISAT and reached the farmers’ fields. Other alternate sources like A3, A4, A4M, A4VZM, A4G1, A5, A6, 9E, and KS are not useful primarily because (1) restorer frequencies are low (restorer frequency: A1 > A2 > A4 > A3) and (2) male steriles cannot be readily distinguished from male fertiles. There is a need to search for more useful form of male sterility yet different from milo (A1). Milo restorers need to be diversified in guinea background to further enhance the yield advantage in hybrid development. Restorer frequency is very low on non-milo cytoplasms. So, there is a need to identify and breed for high-yielding non-milo cytoplasm restorers [2, 34]. The high Brix% possessing (>14 %) female hybrid parents are not available in plenty on sweet sorghum breeding programs across the globe to exploit the potential heterosis for stalk yield and juice yield [2].
Breeding Sweet Sorghum
Breeding Behavior
Sorghum is basically a self-pollinating crop, but natural cross-pollination varies from 0.6 to 6 % depending on the cultivar. Sorghum has the advantage of possessing complete self-pollination due to its floral biology, cleistogamy, and genetic and cytoplasmic genetic male sterility. Breeding methods relevant to self as well as cross-pollinated crops are, therefore, applied to breed pure line varieties, hybrids, and populations in sorghum. Hand pollination should begin around 9:30 or 10:00 AM and can be extended up to 11:30 AM to 12:30 PM on a foggy morning [22].
Candidate Traits and Variability
The major characteristics which a sweet sorghum cultivar should possess are:
-
1.
High biomass productivity (75–100 t ha−1)
-
2.
High Brix% (20–23 %)
-
3.
Thick stems and juicy internodes
-
4.
Photo- and thermo-insensitivity aids to fit into diversified cropping systems
-
5.
Tolerance to shoot pests and diseases
-
6.
Good digestibility of residues when used as forage
-
7.
Tolerance to mid-season and terminal drought
-
8.
Salinity and heat tolerance
-
9.
High water, nitrogen, and radiation use efficiencies
-
10.
Juice quality and quantity sustenance during post-harvesting
-
11.
Grain yield (4.0–7.0 t ha−1)
Ayyangar [35] suggested that a single dominant gene confers the non-sweet character. Later, it was reported that stalk sugar is under the control of recessive genes with additive and dominance effects [36]. On the contrary, subsequent studies provided support for the existence of multiple genes with additive effects. Continuous variation in the amount of extractable juice was observed in juicy genotypes and inbred progeny of juicy × dry lines, suggesting multiple genes may be involved in controlling the trait [8, 37, 38]. There was also a report suggesting the involvement of several genes affecting the biofuel traits in sweet sorghum background. The evaluation of four promising sweet sorghum lines [Keller, BJ 248, Wray, and NSSH 104 (CSH 22SS) along with the check SSV 84] indicated substantial genotypic differences for extractable juice, total sugar content, fermentation efficiency, and alcohol production [39]. An analysis of 53 ICRISAT-bred elite hybrids in both the rainy and post-rainy seasons showed that the correlation and regression coefficients are significantly high for all the component traits of sugar yield (Brix%, stalk yield, juice weight, and juice volume) [2]. Knowing general (GCA) and specific (SCA) combining ability effects of genetic materials is of practical value in breeding programs. GCA effects represent the fixable component of genetic variance and are important to develop superior genotypes. SCA represents the non-fixable component of genetic variation, and it is important to provide information on hybrid performance. The line × tester analysis of 171 hybrids along with their parents in both rainy and post-rainy seasons showed that the magnitude of SCA variance was higher suggesting the importance of nonadditive gene action in inheritance of sugar yield-related traits though both additive and dominant genes controlled overall sugar yield during both rainy and post-rainy seasons in tropical sweet sorghums. Hence, selection in early generations would be ineffective and recurrent selection with periodic intercrossing is advocated. However, breeding for good combining restorer parents can produce high sugar yields in post-rainy season. There is an indication of existence of transgressive segregation for sugar yield that can be exploited [39]. The heritability for traits such as stem juice content, stem sugar concentration, total stem sugars, juice glucose, juice fructose, and juice sucrose was low [40, 41]. The predominant role of nonadditive gene action for plant height, stem girth, total soluble solids, millable stalk yield, and extractable juice yield and substantial magnitude of standard heterosis for candidate sugar traits (stem girth: up to 5.3 %, total soluble solids%: up to 7.4 %, millable stalk yield: up to 1.5 %, and extractable juice yield: up to 122.6 %) indicate the importance of heterosis breeding for improving ethanol productivity of cultivars [42]. The significant positive correlation of general combining ability (GCA) effects with per se performance of parents in sweet sorghum facilitates quicker identification and development of sugar rich, high biomass yielding hybrid parents [2, 43]. The generation mean analysis of two crosses has shown predominantly additive gene action for traits like sucrose% and Brix% of juice. However, for cane and juice yield, dominance gene action and dominance × dominance gene interaction were of higher magnitude in both the crosses. Since the traits important for high sugar content have dominance and overdominance inheritance, utilization of hybrid vigor by developing sweet sorghum hybrids is an attractive option. Also one of the parents with high sucrose content will suffice in getting good hybrids with high sugar and juice yield [44].
From these studies, it is quite evident that significant diversity exists in traits important for biofuel production and this opens up excellent opportunities for sweet sorghum improvement. Biofuel traits are governed by multiple genes and both additive and dominant components of gene action have to be exploited while breeding for high stalk sugar and juice-yielding genotypes. It was demonstrated that the improved hybrids top ranking for grain and sugar yields in rainy season are not top ranking in the post-rainy season and vice versa. It is important to breed for rainy and post-rainy seasons separately [2–4]. The selections for post-rainy season adaptation should be made in post-rainy season only, and for rainy season adaptation, selections can be made in both rainy and post-rainy seasons.
Breeding Objectives
In general, the sweet sorghum breeding programs aim to develop parents and hybrids which can address both first and second generation (lignocellulosic feedstock development) biofuel production issues. The breeding objectives are:
-
1.
To develop sweet sorghum female parents with high stalk sugar and grain yield
-
2.
To develop restorer lines/varieties with high sugar content and resistance to stem borer and shoot fly
-
3.
To develop and identify sorghum hybrids (amenable for mechanical harvesting) with high biomass suitable for use in bioethanol and bioenergy production
Breeding Methods
The most commonly used programs in sweet sorghum improvement are short-term programs (pedigree method and backcross) and long-term programs (population improvement methods). The most common approach in sweet sorghum breeding has been elite × elite crosses followed by pedigree selection. Breeding new female lines, B and R lines have increasingly become dependent on crossing elite by elite lines, B × B and in some cases such as improving for resistance B × R lines. In case of male lines (R lines) improvement, it is R × R crosses. This process progressively narrows the genetic base of breeding programs and requires new traits, especially resistances, to be brought in by pre-breeding and often backcrossing. The success of a backcrossing program depends on the precision with which the desired trait can be identified and thus introgressed into the recurrent parent through backcrossing.
Pedigree Method
Pedigree breeding method is the most commonly used method of breeding in sorghum where the selection begins in the F2 generation targeting superior plants which are expected to produce the best progenies. Hybrids between diverse parents segregate for a large number of genes, and every F2 individual is genetically different from other individuals. The population size becomes crucial for the success of recovering desirable genotypes, when several genes are involved. In this method (Fig. 1.1), superior individual plants are selected in successive segregating generations from the selected families, and a complete record of parent progeny relationship is maintained. Identifying a potentially good cross is essential since the best F1 plants produce better yielding F4 progenies. The selection in segregating generations should be based on (1) performance of the families of the selected cross on the whole and (2) the individual plants performance within the selected family. Selection for many of the per se selection criteria encompassing various traits like tallness, stem thickness, and juice yield can be rapidly applied in the first two or three segregating generations since crosses between elite lines produce a high proportion of progeny with desirable per se values. Once the promising lines have been identified, they can be test crossed onto male-sterile lines for checking fertility restoration and may be classified as B or R lines. Lines with high biomass yield and other desirable agronomic characters can be released as varieties. The pedigree method has been utilized to create new recombinants, transfer of few to many genes governing resistances to various insects, diseases, cold tolerance, etc. in sorghum. In India, the important sweet sorghum genotypes released through pedigree method of selection are SSV 74, SSV 84, CSV 19 SS, and CSV 24SS [45].
Backcross Method
This method does not offer an opportunity to provide new recombinants as hybrids are crossed back to either of the parents and thus they cannot be fixed. However, it can be utilized to incorporate brown-midrib (bmr) or specific defense-related alleles (e.g., stem borer resistance) or improve other traits like seed size, seed shape, and cold tolerance through repeated backcrosses. The backcross method has also been successfully employed in the Indian and ICRISAT breeding programs for transfer of BMR genes and genes which confer high digestibility into elite dual-purpose varieties. Several bmr lines in sweet sorghum background, stacked bmr mutants, stem borer tolerant lines, etc. have been developed through this method. Several stay-green QTLs (stgB, stg2, and stg3) are being introgressed into elite sweet sorghum cultivars by deploying this method.
Population Improvement
This method provides long-term breeding strategy to derive diverse and broad genetic-based superior varieties/hybrid parents. Therefore, a comprehensive crop improvement strategy has to combine both short- and long-term progress for continuous improvement of economic traits. The population improvement procedure involves selection of component parents with high GCA, incorporation of genetic male sterility, intercrossing and random mating among parents, and applying appropriate recurrent selection schemes. At ICRISAT-Patancheru, 24 sorghum populations encompassing characters like grain mold, good grain, photo-insensitive, and early dual purpose were developed and maintained. Recently, ICRISAT has started developing sweet sorghum population with ms 3 gene for applying recurrent selection. While population improvement programs are not the most common in sweet sorghum breeding, they are an important source of genetic variation and improved traits.
Genomics
The availability of genomic sequence for sorghum has made it possible to carry out genome-wide analyses. Whereas earlier studies on simple sequence repeat (SSR) marker development primarily utilized anonymous DNA fragments containing SSRs isolated from genomic libraries, more recent studies have used computational methods to detect SSRs in sequence data generated from genomic sequences projects. In the sorghum genome, a total of 109,039 tandem repeats were detected, of which 15,194 were microsatellite (SSR) markers [46]. In a recent studies, several major QTLs for grain and stem sugar composition and yield and their results indicated that overall energy yields could be increased by concurrent improvement for both sorghum grain and sugar traits [37, 40, 41]. Elucidating the genetic basis of stem sugar and stem juice accumulation, modifying cell wall composition so that sorghum biomass can be processed more efficiently, maximizing biomass yield for a given geographic area and production system, and understanding the different mechanisms underlying drought tolerance are the main focus areas among sorghum researchers who target bioenergy traits.
Transgenic approaches to improve stem sugar accumulation have not been attempted in sweet sorghum. However, differential expression of some genes related to sucrose metabolism has been observed between sweet sorghum and grain sorghum [47]. Further, mature internodes of sweet sorghum showed a lower expression of sucrose transporters suggesting that sucrose accumulation may result from lower transport of sucrose from sink tissues. These genes could serve as important candidate genes for transforming sorghum to achieve better stem sugar yields. However, genetic manipulation of some key enzymes involved in sucrose metabolism did not bring about greater sucrose accumulation in the mature internodes of sugarcane, suggesting their inadequacy in overcoming the osmotic limits of the sugar-storing vacuoles [48]. A microRNA miR169 was recently shown to be involved in regulating sugar levels in sweet sorghum stems suggesting epigenetic regulation of sucrose accumulation [49]. Similarly, a wide hybridization is another useful approach to transfer biotic and abiotic stress tolerance conferring gene transfer from tertiary gene pool sps to sweet sorghum cultivars exploiting iap (inhibition of alien pollen) lines like T × 3361, Nr481 [50].
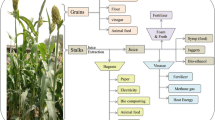
Bioproducts of Sweet Sorghum
A profile of different biomass and grain-based bioproducts derived from sweet sorghum is represented in Fig. 1.2. The following section details these bioproducts.
Beverages
Sorghum grain-based beverages are consumed in Africa [51] and known by different names across Africa, including burukuto (Nigeria), pombe (East Africa), bjala (Northern Sotho), and bil-bil (Cameroon). African sorghum grain-based beer is produced by lactic acid as well as alcoholic fermentation to achieve distinct sour taste. The souring process is initiated using yogurt, sour dough starter cultures, or by spontaneous fermentation. Opaque sorghum beers are also popular alcoholic sorghum-based beverages in Africa which is known by tchoukoutou (Benin) in West Africa, dolo (Burkina-Faso), pito (Ghana), and burukuto or otika (Nigeria) [52]. These lager beers are characterized by sour taste with relatively high dry matter content (5–13 g 100 ml−1) and low alcohol content (2–3 ml 100 ml−1) [53]. These beers are mostly prepared with Guinea corn (Sorghum bicolor) along with other cereals such as millet or maize as adjuncts or substitutes [52]. The manufacturing process consists of malting, brewing, and fermentation steps. Depending on the geographical location, variations have been observed in the production process [54].
In China, sorghum is fermented to produce distilled beverages, like baijiu (sorghum white wine), maotai (sorghum liquor), and kaoliang (sorghum wine). In USA, two sorghum-based beer products “New Grist” and “Redbridge” have been marketed since 2006, which are gluten-free and hence preferably consumed by people suffering with celiac disease and also popularized among health-conscious drinkers due to its low-carbohydrate content [54]. The nonalcoholic fermented African sorghum beverages like kunun-zaki (Nigeria), hulu-mur from sorghum malt and flour (Sudan), and motoho-oa-mabele from sorghum meal (South Africa) involve some form of lactic acid fermentation [55]. Kunun-zaki is a highly perishable product and has a short shelf life (24 h) under tropical ambient conditions; however, the shelf life may be extended under refrigerated conditions [56] or by using 0.1 % sodium benzoate or sodium metabisulfite in combination with pasteurization at 60 °C for 1 h by more than 3 weeks [57]. Hulu-mur is a traditional Sudanese nonalcoholic beverage made from a fermented mixture of unmalted flour of sorghum (Sorghum bicolor) and malt [58, 59].
Foods
Nearly 30 different fermented sorghum/sweet sorghum-based food products are consumed in Sudan. Injera is a leavened, spongy, and sour thin flat, round, staple fermented Ethiopian traditional bread prepared with flour from either of different cereals, water, and starter (ersho, a liquid saved from the previously fermented dough). Injera prepared using tef [Eragrostis tef (Zucc) Trotter], a tiny millet-like grain, is the most popular and preferred cereal ingredient, although other different types of cereals, including sorghum, tef, maize, wheat, finger millet, and barley, are used [60]. The white tannin-free sorghums are preferred due to the light injera color or because of relative brittleness and dryness of sorghum injera after storage [60]. Kisra (aseeda or aceda) is a traditional bread for Arabian Gulf, Sudan, and Iraq which is similar to injera. It is made from the fermented dough of sorghum (Sorghum bicolor) or pearl millet (Pennisetum typhodium) grains. The fermented dough is baked into thin sheets and consumed along with stew prepared from vegetables and meat. Lactobacillus sp., Acetobacter sp., and S. cerevisiae were the main microflora isolated from kisra and responsible for the fermentation process [61]. Studies on kisra preparation indicated that the fermentation of kisra enhanced riboflavin and significantly decreased thiamine, without any change in the mineral contents [62]. Sweet sorghum porridges produced by hard grain and cysteine addition to wheat flour accelerated the stress and structural relaxation [63] and reduced the mixing time by facilitating the breakdown of the wheat proteins by splitting the disulfide bonds rapidly thus aiding faster dough development [64]. Addition of l-cysteine hydrochloride (0.1 %) increased water absorption, but decreased dough development time and dough stability [65]. The Pampanga Agricultural College, Philippines, has pioneered in this work and published a compendium that enlisted a huge array of products such as cakes, cookies, biscuits, rice, porridges and beverages.
Bioethanol
Sorghum-based ethanol production is a very recent area (two decades old) which involves preprocessing steps like harvest approaches [66], juice processing techniques [67], as well as fermentation and depends on yeast strain used and yield ranges from 78 to 90 % [68, 69]. Biomass-based solid-phase fermentation and juice-based liquid batch fermentation [70] and fed-batch fermentation [71] were also been investigated. Application of immobilized yeast in a fluidized bed reactor [72], gelatin bead-packed bed reactor [73], stirred tank, and tubular bioreactors [74] as well as application of statistical approaches [72] and use of very high gravity (VHG) [75] shortened the fermentation time significantly and increased the conversion efficiency. Higher ethanol yields were reported by fermenting 30 % sulfuric acid-treated sorghum [76] and from sorghum fibers pretreated with dilute ammonia followed by enzymatic hydrolysis and fermentation [77]. Specifically, the energy yield from ethanol obtained from the above-referenced studies ranged between 6,500–8,900 kJ/kg dry and 1,400–2,700 kJ/kg fresh sorghum biomass, respectively (assuming that the energy yield from ethanol is 26,500 kJ/kg). To date, ethanol and methane are the well-known microbial-derived products from sweet sorghum [78]. The data indicates that from a metric ton of sweet sorghum having 18 % Brix, 350–450 L of juice can be realized and up on fermentation 45–55 L of transport grade ethanol can be realized [2–4]. The utilization of bagasse has a most promising future for its conversion to ethanol or butanol, while the residual solids (mainly lignin) can be incinerated to cogenerate heat and power [2]. Other byproducts of sweet sorghum ethanol value chain are vinasse and furnace oil. Vinasse can be converted into a valuable fertilizer.
Biohydrogen
Among different biofuels, hydrogen is widely acknowledged as an attractive candidate for replacement of fossil fuels as it is a clean and renewable energy carrier with high energy yield as compared to other biofuels and emits water as an end product upon consumption. Several approaches such as thermochemical (gasification, pyrolysis, supercritical conversion, etc.) and biotechnological (photo-fermentation, water–gas shift reaction or uptake hydrogenase, dark fermentation, direct and indirect biophotolysis, etc.) have been evaluated [79–82] for hydrogen production. Evaluation of different resources and available processes suggests the use of renewable energy material with high carbohydrate content which would offer better solution. Rich fermentable carbohydrate containing energy crops such as Miscanthus, sugarcane, and sweet sorghum is advantageous over other biomass resources. Thermodynamically, simple sugars are advantageous over complex carbohydrate substrates for microbial metabolism and also known to influence the biohydrogen yields. Sweet sorghum biomass has high concentration of soluble sugars 18–22 % on dry weight basis [83] predominantly sucrose with variable levels of glucose, starch, and fructose depending on genotype and has an edge over other biomasses [84]. The fermentation-based production of biofuel is mainly regulated by the structural complexity of substrate material, microbial nature, and other physiological factors [82, 85]. A significant negative correlation between lignin content and fermentative biohydrogen production was reported by Prakasham et al. [80] while working with low lignin containing brown-midrib sorghum mutants.
Multi-substrate utilizing microbial strains would offer edge over single carbohydrate metabolizing strains as conversion yields improve substantially. Rumen bacteria has such potential, and it was reported that when grown on different subparts of sweet sorghum like sorghum stalks and sorghum water extract, the biohydrogen yields were comparable [83]. The biohydrogen production was from xylose, cellobiose, arabinose, formic acid, etc. besides glucose. In a mixture of cellobiose, arabinose, xylose, and glucose, glucose was most preferred and arabinose was least for microbial metabolism and differed with microbial genetics as the initial enzymatic conversion of these carbohydrates to intermediates of glycolysis played a significant role. Glucose gets metabolized via EMP, while xylose enters only after the conversion to xylulose and subsequently to xylulose-5-phosphate by the sequential catalysis of xylose isomerase and xylulose kinase [86]. Irrespective of microbial strains and biomass material, all biohydrogen processes are regulated by hydrogen-producing enzymes [79, 87, 88] and associated with CO2 production as well as may be combined with other gases like methane and hydrogen sulfide depending on the biological source and substrate. In fact, studies on hydrogen production inhibition indicated that higher hydrogen gas concentration shifts microbial metabolic pathways to produce more lactate, ethanol, acetone, butanol, or alanine [89]. In addition, biohydrogen yield is regulated by different bioreactor conditions such as pH, microbial consortia, structural complexity of biomass, hydraulic retention time (HRT), and hydrogen gas partial pressure during anaerobic fermentation [79, 80] irrespective of the biomass used. A comparative account of biohydrogen yields using different plant biomasses is shown in Table 1.3.
Lipids
Sweet sorghum extract was also evaluated for production of lipid using Chlorella protothecoides. This microalga exhibited dry cell yield and lipid content of 5.1 g L−1 and 52.5 %, respectively, when sweet sorghum extract was used as carbon source. However, when the sorghum extract was supplemented with yeast extract, the dry cell yield and lipid productivity of the microalga reached to 1.2 g L−1 day−1 and 586.8 mg L−1 day−1, respectively [99]. Similarly, another heterotrophic thraustochytrid, Schizochytrium limacinum SR21, was explored for lipid production using sweet sorghum juice [100]. Semi-solid-state fermentation of crushed sweet sorghum has been reported to produce single cell oils (SCO) using the oleaginous fungus, Mortierella isabellina. The sugars and nitrogen present in sweet sorghum were used by the fungus for oil accumulation, and the maximum oil efficiency of 11 g/100 g dry weight of substrate was observed [101].
Nanomaterials
Sweet sorghum syrup-based facile, easy, reproducible, stable, spherical, and rapid synthesis of stable gold and silver glyconanoparticles was demonstrated at room temperature without the use any surfactants [102, 103]. Glucose and fructose present in the syrup were responsible as capping ligands along with sucrose resulting in the formation and stabilization of nanoparticles with unique H-bonding capabilities for building smart nanomaterials which find application in biomedicine as probes of carbohydrate–carbohydrate interactions and carbohydrate–protein interactions, anti-adhesive therapy, biolabels, bioamplification strategies, antimicrobial agents, and in material science for microstructure manipulation, quantum dots, and magnetic bioconjugation [102, 104].
Xylooligosaccharides
Xylooligosaccharides (XOS) have a great prebiotic prospective and their production on an industrial scale is carried out from lignocellulosic materials (LCMs) rich in xylan by chemical and enzymatic methods, and the latter is preferred in food industry because of lack of undesirable side reactions [105]. XOS seems to exert their nutritional benefits in relation to human health exhibiting excellent physiological properties including improvement in decreasing cholesterol, bowel function, calcium absorption, and lipid metabolism [106]. Furthermore, they can promote a favorable intestinal environment by selectively enhancing the growth of colonic microbiota such as Bifidobacterium and Lactobacillus [107]. In the recent years, enzymatic production of xylooligosaccharides has attracted more industries in order to make the conversion process economical and also for the effective utilization of renewable plant-based biomasses [108]. In view of this fact, sorghum grain or sorghum bagasse after pretreatment can be used as excellent sources for XOS production as its hemicellulose content varies based on the genotype of cultivar. Four types of oligosaccharides were reported from alkali-extracted sorghum glucurono-arabinoxylan by digestion with a combination of (1- > 4)-β-d-arabinoxylan arabinofurano-hydrolase (AXH) and endo-(1- > 4)-β-d-xylanase (Xyl I), both from Aspergillus awamori and were purified by size exclusion chromatography followed by preparative high-performance anion-exchange chromatography [109].
Antidiabetic Compounds
The extracts of sorghum contain various phytochemicals like tannins, phenolic acids, phytosterols, and policosanols. Phenolic extracts of some varieties of sorghum exhibited antidiabetic effects by increasing serum insulin in diabetic rats, and the effect was comparable with glibenclamide, a powerful antidiabetic drug [110]. Sorghum tea made from roasted grains is rich in procyanidins which exhibited stronger α-glucosidase and α-amylase inhibitory activities [111]. Commonly, acarbose, a commercially available drug, is used as an alpha-glucosidase inhibitor which reversibly and competitively inhibits the digestion of oligo- and disaccharides at the brush border of the small intestine and helps to keep blood sugar levels within a target range. This effect controls diabetes and also the development of obesity [112]. In this view, efforts can be made to validate the clinical role of procyanidins for the treatment of alpha-glucosidase inhibition.
Antioxidant Compounds
Antioxidant activity of various foods is significantly correlated with total phenols and tannins and based on this feature; sorghum foods were shown to possess antioxidant activity [113], hence prevents a plethora of physiological complications like cancer, early aging, diabetes, and cardiovascular diseases [114]. The quantity of antioxidant activity is based on the processing of sorghum samples and decorticated sorghum; cooking based on extrusion was shown to reduce the phenol content and accordingly the antioxidant activity [115]. To obtain the whole health benefits of sorghum, it is better to select a process which retains its total phenolic contents. Pigmented testa contains condensed tannins composed of flavan-3-ols which are excellent antioxidants [116]. The tannin sorghum contains high dietary fiber content which slows the hydrolysis of food in the GI tract and the calorific availability which may be responsible for reduced weight gain (antiobesity effect) in animals. Pigmented sweet sorghums have high concentration of 3-deoxyanthocyanins (luteolinidin and apigenidin) [117].
Antimicrobial Compounds
Studies conducted on the antimicrobial properties of sorghum extracts showed strong inhibitory activity against Escherichia coli [118]. The antimicrobial property of sweet sorghum against a specific microorganism is based on the type of cultivar as the antimicrobial property of a plant extract is based not only on the phenolic content but also on the presence of various secondary metabolites [119].
Cytotoxicity Against Cancer Cell Lines
Sorghum grain contains retrodihydrochalcones, 3-(2,4,6-trihydroxyphenyl)-1-(4-hydroxyphenyl)-propan-1-one, and 3-(2,6-dihydrox-4-methoxyphenyl)-1-(4-hydroxyphenyl)-propan-1-one which are cytotoxic in nature against various human cancer cell lines [116]. However, future studies are required to evaluate the cytotoxic effects of retrodihydrochalcones from sweet sorghum.
Polylactic Acid
Polylactic acid (PLA) is a biodegradable thermoplastic resin that can be substituted for petroleum-based thermoplastics, reducing environmental pollution and other problems associated with petroleum-based plastics [120]. Lactic acid can be produced either through chemical synthesis or through a fermentation process [121]. Agro-based materials such as cereal grains like corn, sorghum, and sweet sorghum bagasse are major potential sources to produce lactic acid through fermentation [122]. There are reports on the production of lactic acid monomer from different varieties of sorghum wherein the whole ground sorghum grain was liquefied and fermented to lactic acid using Rhizopus oryzae NRRL 395 and the efficiency of saccharification was dependent on the native glucoamylase [123]. Sweet sorghum bagasse residue after alcohol fermentation can also be used for the preparation of biodegradable PLA with a tensile strength of 49.5 M and a flexible strength of 65 MPa [124].
Protein-Based Films and Adhesives
Sorghum grain has an average protein content of 11 % and its proteins are classified as prolamins (kafirins) and non-prolamin proteins. Kafirins constitute 77–82 % of the endosperm proteins and are involved in intermolecular cross-linking. Kafirin was reported to have potential in biofilm-forming applications. Its mechanical, water-vapor barrier and color properties of free-standing films from laboratory-extracted kafirin were comparable to those of zein films of commercial importance [125]. Sorghum flour as such can be used as protein extender in phenol-formaldehyde-based plywood adhesive for sprayline coaters or foam extrusion. The sorghum-based plywood glue had a viscosity of 1,104 cP and adhesion strength of 1.37 MPa which was comparable with the industry standard glue [126].
Summary
Sweet sorghum is the only first generation feedstock that provides both food and fuel besides fodder with relatively high RUE, WUE, and NUE with greater adaptation to semiarid regions [127]. Though it has gained importance as a stable food and fodder crop, recently it is increasingly viewed as a viable feedstock for the production of various bioproducts ranging from biofuels, beverages, food, pharmaceuticals, antioxidants, antimicrobial, and antidiabetics. Hence, focused research on its production and processing is required for efficient exploitation of polymeric carbohydrates, fermentable sugars, and biomass for varied needs of the society.
References
Reddy BVS, Ramesh S, Sanjana Reddy P, Ramaiah B, Salimath PM, Rajashekar K. Sweet sorghum – a potential alternative raw material for bioethanol and bio-energy. Int Sorghum Millets Newsl. 2005;46:79–86.
Srinivasarao P, Rao SS, Seetharama N, Umakanth AV, Sanjana Reddy P, Reddy BVS, et al. Sweet sorghum for biofuel and strategies for its improvement. Information Bulletin No 77. Patancheru: International Crops research Institute for the Semi-Arid Tropics, 80 pp, ISBN: 978-92-9066-518-2, Order Code: IBE 077, 2009.
Srinivasarao P, Reddy BVS, Blümmel M, Subbarao GV, Chandraraj K, Sanjana Reddy P, et al. Sweet sorghum as a biofuel feedstock: can there be food-feed-fuel trade-offs?, ICID; 2010. Available from: http://www.corpoica.org.co/sitioWeb/Documento/JatrophaContrataciones/SWEETSORGHUMASABIOFUELSFEEDSTOCK.pdf. Accessed 8 July 2013.
Reddy BVS, Ramesh S, Ashok Kumar A, Wani SP, Ortiz R, Ceballos H, et al. Biofuel crops research for energy security and rural development in developing countries. Bioenerg Res. 2008;1:248–58.
Srinivasa Rao P, Kumar CG, Malapaka J, Kamal A, Reddy BVS. Feasibility of sustaining sugars in sweet sorghum stalks during post-harvest stage by exploring cultivars and chemicals: a desk study. Sugar Tech. 2012;14:21–5.
Srinivasa Rao P, Kumar CG, Malapaka J, Kamal A, Reddy BVS. Effect of micronutrient treatments in main and ratoon crops of sweet sorghum cultivar ICSV 93046 under tropical conditions. Sugar Tech. 2012;14:370–5.
Chapman SR, Carter LP. Crop production, principle and practices. San Francisco: W.H. Freeman; 1976. 566 pp.
Datta-Mazumdar S, Poshadri A, Srinivasa Rao P, Ravinder Reddy CH, Reddy BVS. Innovative use of sweet sorghum juice in the beverage industry. Int Food Res J. 2012;19:1361–6.
Saballos A. Development and utilization of sorghum as a bioenergy crop. In: Vermerris W, editor. Genetic improvement of bioenergy crops. New York: Springer; 2008. p. 211–48.
Srinivasarao P, Sanjana Reddy P, Rathore A, Reddy BVS, Panwar S. Application of GGE biplot and AMMI model to evaluate sweet sorghum hybrids for genotype × environment interaction and seasonal adaptation. Indian J Agric Sci. 2011;81:438–44.
Zhao YL, Dolat A, Steinberger Y, Wang X, Osman A, Xie GH. Biomass yield and changes in chemical composition of sweet sorghum cultivars grown for biofuel. Field Crops Res. 2009;111:55–64.
IPCC Chapter 11. Regional climate projections. 2007. Available from: http://www.ipcc.ch/pdf/assessment-report/ar4/wg1/ar4-wg1-chapter11.pdf. Accessed 8 July 2013.
Cooper PJM, Dimes J, Rao KPC, Shapiro B, Shiferaw B, Twomlow S. Coping better with current climate variability in the rainfed farming systems of sub-Saharan Africa: an essential first step in adapting to future climate change? Agric Ecosystems Environ. 2008;126(Suppl 1–2):24–35.
Celarier RP. Cytotaxonomy of the Andropogoneae. III. Sub-tribe Sorgheae, genus, sorghum. Cytologia. 1959;23:395–418.
Clayton WD. Proposal to conserve the generic name Sorghum Moench (Gramineae) versus Sorghum Adans (Gramineae). Taxonomy. 1961;10:242–3.
de Wet JMJ, Harlan JR. The origin and domestication of Sorghum bicolor. Econ Bot. 1971;25:128–35.
de Wet JMJ, Huckabay JP. The origin of Sorghum bicolor. II. Distribution and domestication. Evolution. 1967;211:787–802.
Dahlberg JA. Classification and characterization of sorghum. In: Smith CW, Frederiksen RA, editors. Sorghum, origin, history, technology and production, Wiley Series in Crop Science. New York: Wiley; 2000. p. 99–130.
Doggett H. Sorghum, Tropical agricultural series. 2nd ed. Essex: Longman Scientific; 1988.
Harlan JR, de Wet JMJ. A simplified classification of cultivated sorghum. Crop Sci. 1972;12:172–6.
Quinby JR, Karper RE. The effect of short photoperiod on sorghum varieties and first generation hybrids. J Agric Res. 1947;75:295–300.
House LR. A guide to sorghum breeding, vol. II. Patancheru: International Crops Research Institute for the Semi-Arid Tropics; 1985. p. 1–206.
Rooney WL. Genetics and cytogenetics. In: Smith CW, Frederiksen RA, editors. Sorghum, origin, history, technology and production, Wiley Series in Crop Science. New York: Wiley; 2000. p. 261–307.
Murty UR, Rao NGP. Sorghum. In: Bahl PN, Salimath PM, Mandal AK, editors. Genetics, cytogenetics and breeding of crop plants, vol. 2, cereal and commercial crops. New Delhi: Oxford & IBH Publishing; 1997. p. 197–239.
Ayyangar GNR, Ponnaiya BWX. The occurrence and inheritance of purple pigment on the glumes of sorghum close on emergence from the boot. Curr Sci. 1937;5:590.
Stephens JC. Male sterility in sorghum: its possible utilization in production of hybrid seed. J Am Soc Agron. 1937;29:690–6.
Webster OJ. Genetic studies in Sorghum vulgare (Pers.). Crop Sci. 1965;5:207–10.
Ayyangar GNR. The description of crop plant characters and their ranges of variation. IV. Variability of Indian sorghum. Indian J Agric Sci. 1942;12:527–63.
Barabas Z. Observation of sex differentiation in sorghum by use of induced male sterile mutants. Nature. 1962;195:257–9.
Andrews DJ, Webster OJ. A new factor for genetic male-sterility in Sorghum bicolor (L.) Moench. Crop Sci. 1971;11:308–9.
Karper RE, Stephens JC. Floral abnormalities in sorghum. J Hered. 1936;27:183–94.
Stephens JC, Holland PF. Cytoplasmic male sterility for hybrid sorghum seed production. Agron J. 1954;46:20–3.
Schertz KF. Male sterility in sorghum: its characteristics and importance. In: Witcombe JR, Duncan RR, editors. Use of molecular markers in sorghum and pearl millet breeding for developing countries, Norwich, UK: Proceedings of an ODA Plant Sciences Research Conference, Mar 29–Apr 1, 1993; 1994. p. 35–7.
Reddy BVS, Rai KN, Sarma NP, Kumar ISH, Saxena KB. Cytoplasmic-nuclear male sterility: origin, evaluation, and utilization in hybrid development. In: Jain HK, Kharkwal MC, editors. Plant breeding: Mendelian to molecular approaches. New Delhi: Narosa Publishers; 2003.
Ayyangar G, Ayyar M, Rao V, Nambiar A. Mendelian segregation for juiciness and sweetness in sorghum stalk. Madras Agric J. 1936;24:247–8.
Guiying L, Weibin G, Hicks A, Chapman KR. A training manual for sweet sorghum. Bangkok: FAO/CAAS/CAS; 2000.
Ritter KB, McIntyre CL, Godwin ID, Jordan DR, Chapman SC. An assessment of the genetic relationship between sweet and grain sorghums, within Sorghum bicolor ssp. bicolor (L.) Moench, using AFLP markers. Euphytica. 2007;157:161–76.
Kadam DE, Patil FB, Bhor TJ, Harer PN. Genetic diversity studies in sweet sorghum. J Maharashtra Agric Univ. 2001;26:140–3.
Ratnavathi CV, Dayakar Rao B, Seetharama N. Sweet sorghum stalk: a suitable raw material for fuel alcohol production. DSR/NRCS Report Number 12/2003. NATP (DSR) Series No. 1, Hyderabad: National Research Center for Sorghum (NRCS); 2003.
Reddy PS, Reddy BVS, Srinivasa Rao P. Genetic analysis of traits contributing to stalk sugar yield in Sorghum. Cereal Res Commun. 2011;39:453–64.
Murray SC, Rooney WL, Mitchell SE, Sharma A, Klein PE, Mullet JE, et al. Genetic improvement of sorghum as a biofuel feedstock: II. QTL for stem and leaf structural carbohydrates. Crop Sci. 2008;48:2180–93.
Murray SC, Sharma A, Rooney WL, Klein PE, Mullet JE, Mitchell SE, et al. Genetic improvement of sorghum as a biofuel feedstock I: QTL for stem sugar and grain nonstructural carbohydrates. Crop Sci. 2008;48:2165–79.
Sankarapandian R, Ramalingam J, Pillai MA, Vanniarajan C. Heterosis and combining ability studies for juice yield related characteristics in sweet sorghum. Ann Agric Res. 1994;15:199–204.
Selvi B, Palanisamy S. Heterosis and combining ability for grain yield in sweet sorghum. Madras Agric J. 1990;77:493–6.
AICSIP (All India Coordinated Sorghum Improvement Project) Sweet sorghum and physiology. All India Coordinated Sorghum Improvement Project Annual Progress Report for 2006–2007. AICSIP Tech. Publication No. 3, Sweet Sorghum and Physiology 2007 (Book 3 of 3-agm07 pre-meet), 102 pp, Hyderabad: National Research Centre for Sorghum; 2007.
Rooney WL, Smith CW. Techniques for developing new cultivars. In: Smith CW, Frederiksen RA, editors. Sorghum: origin, history, technology and production. New York: Wiley; 2000. p. 329–47.
Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, Gundlach H, et al. The Sorghum bicolor genome and the diversification of grasses. Nature. 2009;457:551–6.
Qazi HA, Bhargava S. Stem sugar accumulation in sweet sorghum – activity and expression of sucrose metabolizing enzymes and sucrose transporters. J Plant Physiol. 2012. doi:10.1016/j.jplph.2012.01.005.
Wu L, Birch RG. Physiological basis for enhanced sucrose accumulation in an engineered sugarcane cell line. Funct Plant Biol. 2010;37:1161–74.
Calvino M, Bruggmann R, Messing J. Characterization of the small RNA component of the transcriptome from grain and sweet sorghum stems. BMC Genomics. 2011;12:356–67.
Price HJ, Hodnett GL, Burson BL, Dillon SL, Stelly DM, Rooney WL. Genotype dependent interspecific hybridization of Sorghum bicolor. Crop Sci. 2006;46:2617–22.
Taylor JRN, Emmambux MN. Gluten-free foods and beverages from millets. In: Arendt EK, Bello FD, editors. Gluten-free cereal products and beverages. London: Academic; 2008. p. 119–48.
Kayode APP, Adegbidi A, Linnemann AR, Nout MJR, Hounhouigan DJ. Quality of farmer’s varieties of sorghum and derived foods as perceived by consumers in Benin. Ecol Food Nutr. 2005;44:271–94.
Agu RC, Palmer GH. A reassessment of sorghum for lager beer brewing. Bioresour Technol. 1998;66:253–61.
Haggblade S, Holzapfel H. Industrialization of Africa’s indigenous beer brewing. In: Steinkraus KH, editor. Industrialization of indigenous fermented foods. New York: Marcel Dekker; 1989. p. 191–283.
Anonymous Anheuser-Busch introduces first nationally available sorghum beer: Redbridge. 2006. http://www.prnewswire.com/news-releases/anheuser-busch-introduces-first-nationally-available-sorghum-beer-redbridge-57209312.html. Retrieved 1 Nov 2012.
Gaffer TC, Jideani AI, Nkuma I. Composition of Kunun – a non-alcoholic cereal beverage. Plant Food Human Nutr. 2002;57:73–81.
Gaffer TC, Jedeani IA, Nkuma I. Traditional production, consumption and storage of kunun, a non-alcoholic cereal beverage. Plant Food Human Nutr. 2002;57:82–5.
Maji AA, James O, Chigozie OE. Effects of chemical treatment and pasteurization on the shelf life of kunun zaki (sorghum and maize gruel). Eur J Food Res Rev. 2011;1:61–70.
Agab MA. Fermented food products ‘Hulu Mur’ drink made from Sorghum bicolor. Food Microbiol. 1985;2:147–55.
Gebrekidan B, GebreHiwot B. Sorghum injera preparation and quality parameters. In: Rooney LW, Murty DS, editors. Proceedings of the international symposium on sorghum grain quality. Patancheru: ICRISAT; 1982. p. 55–66.
El Tinay AH, Abdel Gadir AM, El Hidai M. Sorghum fermented kisra bread. 1. Nutritive value of kisra. J Sci Food Agric. 1979;30:859–63.
Mahgoub SEO, Ahmed BM, Ahmed MMO, El Agib El Nazeer AA. Effect of traditional Sudanese processing of kisra bread and hulu-mur drink on their thiamine, riboflavin and mineral contents. Food Chem. 1999;67:129–33.
Frater R, Hird FJ, Moss HJ. Role of disulphide exchange reactions in the relaxation of strains introduced in dough. J Sci Food Agric. 1961;12:269–73.
Babu KS. Influence of reducing agents emulsifiers on the quality of cream crackers. MSc thesis. Mysore: University of Mysore; 1995.
El-Khalifa AEO, El-Tinay AH. Effect of cysteine on bakery products from wheat–sorghum blends. Food Chem. 2002;77:133–7.
Worley JW, Cundiff JS. System analysis of sweet sorghum harvest for ethanol production in the Piedmont. Trans ASAE. 1991;34:539–47.
Weitzel TT, Cundiff JS, Vaughan DH. Optimization of sweet sorghum processing parameters. Trans ASAE. 1989;32:273–9.
Day DF, Sarkar D. Fuel alcohol from sweet sorghum: microbial aspects. Dev Ind Microbiol. 1982;23:361–6.
Bryan WL, Monroe GE, Caussanel PM. Solid-phase fermentation and juice expression systems for sweet sorghum. Trans ASAE. 1985;28:268–74.
Kundiyana DK. “Sorganol”: in-field production of ethanol from sweet sorghum. MSc thesis, 1996.
Laopaiboon L, Thanonkeo P, Jaisil P, Laopaiboon P. Ethanol production from sweet sorghum juice in batch and fed-batch fermentations by Saccharomyces cerevisiae. World J Microbiol Biotechnol. 2007;23:1497–501.
Liu R, Shen F. Impacts of main factors on bioethanol fermentation from stalk juice of sweet sorghum by immobilized Saccharomyces cerevisiae (CICC 1308). Bioresour Technol. 2008;99:847–54.
Mohite U, SivaRaman H. Continuous conversion of sweet sorghum juice to ethanol using immobilized yeast cells. Biotechnol Bioeng. 1983;26:1126–7.
Khongsay N, Laopaiboon L, Laopaiboon P. Continuous ethanol production from sweet sorghum stem juice using stirred tank and tubular bioreactors. J Biotechnol. 2008;136:S446–6.
Nuanpeng S, Laopaiboon L, Srinophakun P, Klanrit P, Jaisil P, Laopaiboon P. Ethanol production from sweet sorghum juice under very high gravity conditions: batch, repeated-batch and scale up fermentation. Electron J Biotechnol. 2011;14:1. http://dx.doi.org/10.2225/vol14-issue1-fulltext-2. Retrieved on 1 Nov 2012.
Yu J, Zhang X, Tan T. Ethanol production by solid state fermentation of sweet sorghum using thermotolerant yeast strain. Fuel Process Technol. 2008;89:1056–9.
Salvi DA, Aita GM, Robert D, Bazan V. Ethanol production from sorghum by a dilute ammonia pretreatment. J Ind Microbiol Biotechnol. 2010;37:27–34.
Mamma D, Koullas D, Fountoukidis G, Kekos D, Makris BJ, Koukios E. Bioethanol from sweet sorghum: simultaneous saccharification and fermentation of carbohydrates by a mixed microbial culture. Process Biochem. 1996;31:377–81.
Meng N, Leung DYC, Leung MKH, Sumathy K. An overview of hydrogen production from biomass. Fuel Process Technol. 2006;87:461–72.
Prakasham RS, Brahmaiah P, Nagaiah D, Srinivasa Rao P, Reddy BVS, Sreenivas Rao R, Hobbs PJ. Impact of low lignin containing brown midrib sorghum mutants to harness biohydrogen production using mixed anaerobic consortia. Int J Hydrogen Energy. 2012;37:3186–90.
Prakasham RS, Brahmaiah P, Sathish T, Sambasiva Rao KRS. Fermentative biohydrogen production by mixed anaerobic consortia: impact of glucose to xylose ratio. Int J Hydrogen Energy. 2009;34:9354–61.
Prakasham RS, Sathish T, Brahmaiah P. Biohydrogen production process optimization using anaerobic mixed consortia: a prelude study for use of agroindustrial material hydrolysate as substrate. Bioresour Technol. 2010;14:5708–11.
Ntaikou I, Gavala HN, Kornaros M, Lyberatos G. Hydrogen production from sugars and sweet sorghum biomass using Ruminococcus albus. Int J Hydrogen Energy. 2008;33:1153–63.
Billa E, Koullas DP, Monties B, Koukios EG. Structure and composition of sweet sorghum stalk components. Ind Crops Prod. 1997;6:297–302.
Nagaiah D, Srinivasa Rao P, Prakasham RS, Uma A, Radhika K, Barve Y, Umakanth AV. High biomass sorghum as a potential raw material for biohydrogen production: a preliminary evaluation. Curr Trends Biotechnol Pharm. 2012;6:183–9.
Suppmann B, Sawers G. Isolation and characterization of hypophosphite resistant mutants of Escherichia coli: identification of the FocA protein, encoded by the pfl operon, as a putative formate transporter. Mol Microbiol. 1994;11:965–82.
Thurston B, Dawson KA, Strobel HJ. Pentose utilization by the ruminal bacterium Ruminococcus albus. Appl Environ Microbiol. 1994;60:1087–92.
Koku H, Eroglu I, Gunduz U, Yucel M, Turker L. Aspects of the metabolism of hydrogen production by Rhodobacter sphaeroides. Int J Hydrogen Energy. 2002;27:1315–29.
Hojilla-Evangelista MP, Bean SR. Evaluation of sorghum flour as extender in plywood adhesives for sprayline coaters or foam extrusion. Ind Crops Prod. 2011;34:1168–72.
Datar R, Huang J, Maness PC, Mohagheghi A, Czernik S, Chornet E. Hydrogen production from the fermentation of corn stover biomass pretreated with a steam explosion process. Int J Hydrogen Energy. 2007;32:932–9.
Li CL, Fang HHP. Fermentative hydrogen production from wastewater and solid wastes by mixed cultures. J Environ Sci Technol. 2007;37:1–39.
Zhang ML, Fan YT, Xing Y, Pan CM, Zhang GS, Lay JJ. Enhanced biohydrogen production from cornstalk wastes with acidification pretreatment by mixed anaerobic cultures. J Biomass Bioenerg. 2007;3:250–4.
Pan C, Zhang S, Fan Y, Hou H. Bioconversion of corncob to hydrogen using anaerobic mixed microflora. Int J Hydrogen Energy. 2009;34:1–7.
Ivanova G, Rakhely G, Kovacs KL. Thermophilic biohydrogen production from energy plants by Caldicellulosiruptor saccharolyticus and comparison with related studies. Int J Hydrogen Energy. 2009;34:3659–70.
Nasirian N. Biological hydrogen production from acid-pretreated straw by simultaneous saccharification and fermentation. Afr J Agric Res. 2012;76:876–82.
Brown RC. Biomass-derived hydrogen from a thermally ballasted gasifier, FY 2003 Progress Report, National Renewable Energy Laboratory, 2003.
Argun H, Kargi F, Kapdan IK, Oztekin R. Biohydrogen production by dark fermentation of wheat powder solution: effects of C/N and C/P ratio on hydrogen yield and formation rate. Int J Hydrogen Energy. 2008;33:1813–9.
Niel EWJV, Claassen PAM, Stams AJM. Substrate and production inhibition of hydrogen production by the extreme thermophile Caldicellulosiruptor saccharolyticus. Biotechnol Bioeng. 2003;81:255–62.
Gao C, Zhai Y, Ding Y, Wu Q. Application of sweet sorghum for biodiesel production by heterotrophic microalga Chlorella protothecoides. Appl Energy. 2010;87:756–61.
Liang Y, Sarkany N, Cui Y, Yesuf J, Trushenski J, Blackburn JW. Use of sweet sorghum juice for lipid production by Schizochytrium limacinum SR21. Bioresour Technol. 2010;101:3623–7.
Economou CN, Makri A, Aggelis G, Pavlou S, Vayenas DV. Semi-solid state fermentation of sweet sorghum for the biotechnological production of single cell oil. Bioresour Technol. 2010;101:1385–8.
Kumar CG, Mamidyala SK, Reddy MN, Reddy BVS. Silver glyconanoparticles functionalized with sugars of sweet sorghum syrup as an antimicrobial agent. Process Biochem. 2012;47:1488–95.
Kumar CG, Mamidyala SK, Sreedhar B, Reddy BVS. Synthesis and characterization of gold glyconanoparticles functionalized with sugars of sweet sorghum syrup. Biotechnol Prog. 2011;27:1455–63.
De la Fuente JM, Penades S. Glyconanoparticles: types, synthesis and applications in glycoscience, biomedicine and material science. Biochim Biophys Acta. 2006;1760:636–51.
Aachary AA, Prapulla SG. Xylooligosaccharides (XOS) as an emerging prebiotic: microbial synthesis, utilization, structural characterization, bioactive properties, and applications. Compr Rev Food Sci Food Saf. 2010;10:1–16. doi:10.1111/j.1541-4337.2010.00135.x.
Vázquez MJ, Alonso JL, Domínguez H, Parajó JC. Xylooligosaccharides: manufacture and applications. Trends Food Sci Technol. 2000;11:387–93.
Okazaki M, Fujikawa S, Matsumoto N. Effect of xylooligosaccharide on the growth of bifidobacteria. J Jpn Soc Nutr Food Sci. 1990;43:395–401.
Suvarna Lakshmi G, Uma Maheshwari B, Prakasham RS. Biosynthesis of xylobiose: a strategic way to enrich the value of oil palm empty fruit bunch fiber. J Microbiol Biotechnol. 2012;22:1084–91.
Verbruggen MA, Spronk BA, Schols HA, Beldman G, Voragen AGJ, Thomas JR, et al. Structures of enzymically derived oligosaccharides from sorghum glucuronoarabinoxylan. Carbohydr Res. 1998;306:265–74.
Chung I-M, Kim E-H, Yeo M-A, Kim S-J, Seo M-C, Moon H-I. Antidiabetic effects of three Korean sorghum phenolic extracts in normal and streptozotocin-induced diabetic rats. Food Res Int. 2011;44:127–32.
Wu L, Huang Z, Qin P, Ren G. Effects of processing on phytochemical profiles and biological activities for production of sorghum tea. Food Res Int. 2012; http://dx.doi.org/10.1016/j.foodres.2012.07.062. Retrieved on 1 Nov 2012.
William-Olsson T. Alpha-glucosidase inhibition in obesity. Acta Med Scand Suppl. 1985;706:1–39.
Cai YZ, Sun M, Xing J, Luo Q, Corke H. Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006;78:2872–88.
Awika JM, Rooney LW. Sorghum phytochemicals and their potential impact on human health. Phytochemistry. 2004;65:1199–221.
Dlamini NR, Taylor JRN, Rooney LW. The effect of sorghum type and processing on the antioxidant properties of African sorghum-based foods. Food Chem. 2007;105:1412–9.
Dykes L, Rooney LW. Sorghum and millet phenols and antioxidants. J Cereal Sci. 2006;44:236–51.
Khalil A, Baltenweck-Guyot R, Ocampo-Torres R, Albrecht P. Retrodihydrochalcones in Sorghum species: key intermediates in the biosynthesis of 3-deoxyanthocyanidins? Phytochem Lett. 2012;5:174–6.
Kil HY, Seong ES, Ghimire BK, Chung I-M, Kwon SS, Goh EJ, et al. Antioxidant and antimicrobial activities of crude sorghum extract. Food Chem. 2009;115:1234–9.
Ćetković GS, Čanadanović-Brunet JM, Djilas SM, Tumbas VT, Markov SL, Cvetković DD. Antioxidant potential, lipid peroxidation inhibition and antimicrobial activities of Satureja montana subsp. kitaibelli extracts. Int J Mol Sci. 2007;8:1013–27.
Kharas GB, Sanchez-Riera F, Severson DK. Polymers of lactic acid. In: Mobley DP, editor. Plastics from microbes: microbial synthesis of polymers and polymer precursors. Munich: Hanser Publishers; 1994. p. 93–137.
Wee YJ, Kim JN, Ryu HW. Biotechnological production of lactic acid and its recent applications. Food Technol Biotechnol. 2006;44:163–72.
Yadav AK, Bipinraj NK, Chaudhari AB, Kothari RM. Production of L (+) lactic acid from sweet sorghum, date palm, and golden syrup as alternative carbon sources. Starch/Stärke. 2011;63:632–6.
Zhan X, Wang D, Tuinstra MR, Bean S, Seib PA, Sun XS. Ethanol and lactic acid production as affected by sorghum genotype and location. Ind Crops Prod. 2003;18:245–55.
Yu J, Zhang T, Zhong J, Zhang X, Tan T. Biorefinery of sweet sorghum stem. Biotechnol Adv. 2012;30:811–6.
Buffo RA, Weller CL, Gennadios A. Films from laboratory-extracted sorghum kafirin. Cereal Chem. 1997;74:473–5.
Srinivasa Rao P, Ganesh Kumar C, editors. Characterization of tropical sweet sorghum cultivars. Springer brief. 2013: 130 pp
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Rao, P.S., Kumar, C.G., Prakasham, R.S., Rao, A.U., Reddy, B.V.S. (2015). Sweet Sorghum: Breeding and Bioproducts. In: Cruz, V.M.V., Dierig, D.A. (eds) Industrial Crops. Handbook of Plant Breeding, vol 9. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1447-0_1
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1447-0_1
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1446-3
Online ISBN: 978-1-4939-1447-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)