Abstract
We present a review of the mathematical literature concerned with the role of feedback in bacterial quorum-sensing. We identify four principal roles: defining the shape of the system response, tuning of signal or regulator levels, filtering noise out from the system and timing of the onset of the quorum-sensing response. We conclude with simplified models of three different quorum-sensing architectures (agr, luxIR and that of Vibrio harveyi and V. cholerae), using numerical and analytical investigations of the resulting ordinary differential equations to characterise their feedback loops in terms of the roles defined above.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
9.1 Introduction
Cell-to-cell communication is now known to be a common phenomenon in the bacterial kingdom. Whether this signalling mechanism serves to unify the response of a population (from where the term quorum sensing first emerged), to detect the population density in the bacteria’s immediate environment (diffusion sensing [1]), to combine the two (efficiency sensing [2]) or even to detect (and inhibit or promote) the presence of competitive or complementary species (cross-species or cross-kingdom signalling [3]), it is clear that it plays a crucial role in the lives of these organisms. Understanding these processes, therefore, is a key challenge in the quest to unravel bacterial behaviour and exploit or modify it for our own benefit. The applications for such understanding are far-reaching and include the development of novel compounds that suppress bacterial pathogenicity, forced production of useful chemicals and mimicry of existing (or creation of new) gene regulation networks in the emergent field of synthetic biology.
Though the range of known quorum-sensing systems is ever-expanding, they fall largely into two categories: homologues of the luxIR system in Gram-negative bacteria and Gram-positive homologues of the agr system.
The luxIR system is well documented, having been the first quorum-sensing system to be discovered [4]: it was shown to regulate bioluminescence by the marine bacterium Vibrio fischeri in accordance with population size—at low cell density, light emission serves little purpose while at high cell density the bacteria are capable of producing enough light to be of use to the squid on which they live symbiotically. It is generally thought that the light, resembling moonlight on the water’s surface, camouflages the squid, thus hiding it from potential predators. The process works through the production of a signal molecule from the luxI gene, see Fig. 9.1. For Gram-negative bacteria these are, more often than not, N-acyl homoserine lactones (AHLs). The AHL can diffuse in and out of the cell, through the cell wall, thus facilitating a means by which these signal molecules can transfer (and therefore communicate) between cells of the same species. Internal AHL activates the receptor protein, LuxR, through binding (in many cases this requires oligomerisation of the receptor protein and this will be discussed further in later sections). In addition to the target genes of the quorum-sensing system which require activation or inactivation in response to population size, activated LuxR can increase transcription of the luxI gene (resulting in more signal) and, in some cases, of its own luxR gene. In short, therefore, the larger the population of bacteria, the more signal molecule there will be and, in theory at least, the faster this process will occur. Equivalently, the cells might achieve quorum-sensing upregulation as a result of entering a particularly confined environment where signal molecules will accumulate much faster due to the lack of diffusion away from the cells.
The luxIR quorum sensing circuit is depicted in (a), with its reduced version in (b): in the latter, LuxI and AHL together correspond to the signal while LuxR serves as the regulator. We use (b) to build our generic (minimal) model of Gram-negative quorum sensing in Sect. 9.3.2.1. Dashed lines illustrate processes which do not necessarily occur in all luxIR-type systems. Note that auto-repression of the signal synthase LuxI occurs via an intermediary protein (RsaL) that we neglect from the model. The shaded grey rectangle represents the cell membrane
In Gram-positive bacteria the general concept is much the same, except the difference in cell wall requires an alternative mechanism for signal secretion and detection. In this case, the quorum-sensing signal is most often a cyclic peptide, produced and secreted from the cell via the combined action of two proteins: AgrB (the exporter protein) and AgrD (the signal itself, converted into a peptide upon secretion), see Fig. 9.2. The externalised peptide, termed the autoinducing peptide (AIP), is detected by a receptor protein, AgrC, on the cell membrane. Binding of AIP to AgrC triggers a phosphorylation cascade between itself and the internal response regulator, AgrA. In its thus activated form, AgrA acts on transcription of its target genes and on the agr genes themselves (in the case of Staphylococcus aureus, in which this system was first identified [5], this applies to all four of the agr genes; in other bacteria it can be fewer).
A schematic representation of the agr quorum-sensing system. In (a) we illustrate the full network. Dashed lines illustrate processes which do not necessarily occur in all agr-type systems. The reduced network used for model building in Sect. 9.3.1.1 is given in (b), wherein the signal captures AgrB, AgrD and AIP and the regulator AgrA and AgrC. The shaded grey rectangle represents the cell membrane. We note that the abstract (minimal) version of the network, shown in (b), is a subcase of that in Fig. 9.1b, the negative feedback loop of which is missing
Homologues of these two systems form the majority of quorum-sensing mechanisms that have been discovered to this point (this widespread occurrence of the same network topologies itself being noteworthy), many of these interacting with additional gene regulation networks, including other quorum-sensing systems (as we shall note later, some bacteria are known to contain multiple quorum-sensing circuits). A small number of more complicated systems have also been uncovered, for instance in V. harveyi and V. cholerae. These will be discussed in more detail later in the chapter.
The target genes of the above quorum-sensing systems are widely varying in nature, as are their reasons for being regulated in a density-dependent manner. Common examples include pathogenicity, sporulation, bioluminescence and swarming motility [6, 7]. What appears to be both common and central to all known systems is feedback in the governing gene-regulation-network architecture. Perhaps somewhat surprising, however, is the variety of feedback architectures employed, including a role for both negative and positive feedback in different systems.
Mathematical modelling is an obvious route by which to study and investigate these networks at a systems level, their complexity rendering their representation in a computational manner particularly useful. We next provide an overview of mathematical models of quorum sensing which have a focus on the role of feedback in each network, categorising the discussion into the hypothesised roles (rather than into the usual comparison of Gram-positive and Gram-negative systems). We complement this with some simple (but in some sense generic) examples of such models, based on the quorum-sensing architectures outlined in the biology review of [8] and being of a form suggested in Figs. 9.1b and 9.2b.
9.2 The Role of Feedback in Quorum Sensing
Since feedback plays such a central role in so many different gene regulation networks, we seek here to summarise the findings of a range of mathematical investigations into its function in relation to quorum sensing. The ever-expanding literature on this subject is already vast and we cannot hope to provide a comprehensive review. Instead, we have chosen a number of mathematical studies ranging across types of bacteria (both Gram-negative and Gram-positive—though Gram-negative models are far more prevalent in the literature—and both pathogenic and non-pathogenic) and quorum-sensing systems (luxIR and agr homologues, including a hybrid of these systems used by two particular Gram-negative bacteria). We divide the discussion into hypothesised roles for feedback, a surprising number of which overlap for both positive and negative loops.
9.2.1 Defining Response Shape
Generic models of feedback in gene regulation networks have often been used to demonstrate how different feedback architectures can give rise to varying classes of response. For instance in [9], a model of interacting gene regulation and metabolic networks, it is demonstrated that rewiring the feedback can alter a system response (typically in terms of the expression level of a particular gene to the level of a signal) from monotonically increasing or decreasing, to bi- (or multi-) stable-steady behaviour through to oscillations, illustrating the potential for the exploitation of gene regulation networks in synthetic biology. From a similar viewpoint, [10] provides a review of a number of papers (both theoretical and experimental) showing that feedback in combination with nonlinearities in the system (for example, co-operative binding of two proteins in the network or, similarly, dimerisation of one type of protein) can enforce multistability in a system. The authors argue that the ability to attain a given number of distinct stable steady states should allow a population to divide into this number of subpopulations, each with their own niche in a particular environment. Given that quorum sensing is generally assumed to enforce unified behaviour amongst one population of cells, this may seem counter-intuitive in the current context. However, we shall see in Sect. 9.2.2 that the simultaneous existence of phenotypically different subpopulations could indeed have relevance to quorum sensing.
In the most common cases, however, the goal of quorum sensing can be classified more straightforwardly, namely to transition a population of cells between two states, for example to switch on or off virulence-factor production (S. aureus) or bioluminescence (V. fischeri). The ability of a quorum-sensing system to exhibit bistability, therefore, could be extremely important and has been demonstrated in a large number of cases (at least theoretically) to take place in both Gram-negative (for example, [11] and [12] concerning Pseudomonas aeruginosa) and Gram-positive ([13] and [14] on S. aureus) systems, but instances are more numerous than those listed here.
In general, low population numbers or density yield a quorum-sensing down-regulated steady state, with high numbers giving up-regulated responses. At intermediate levels three steady states may then exist: one stable and down-regulated, one stable and up-regulated, separated by one intermediary unstable state, see Fig. 9.3a. This facilitates a sharp and robust transition between the two states, alongside hysteresis when going in the opposite direction. Distinct critical population sizes switch the system from down- to up-regulated and vice versa. We shall see that this bistability has been shown to be caused directly by the presence of positive feedback onto the receptor protein (i.e., LuxR or its homologues) in Gram-negative systems, something that is not certain to occur in all bacteria possessing luxIR homologues.
A schematic illustrating the range of transition types discussed in Sect. 9.2.1. In (a) the system displays a bistable switch between responses (solid lines are stable, dotted lines unstable) and hysteresis, in (b) a monostable softer switch and in (c) a gradual transition between inactive and active (both (b) and (c) lacking hysteresis)
In [11, 15] and [16], the existence of this particular feedback loop is considered explicitly. Haseltine and Arnold [16] compares three different versions of the quorum-sensing system in V. fischeri: one contains positive feedback onto both LuxR and LuxI, one onto LuxI alone and one with no feedback at all. It is demonstrated that the transition between the two states becomes sharper and stronger with the addition of each feedback loop: with both, bistability occurs (as in Fig. 9.3a), with only LuxI feedback a rapid but monostable switch is seen (Fig. 9.3b), while with no feedback at all the response is graded (Fig. 9.3c). Feedback onto LuxR alone is not considered as it is not thought biologically realistic. Through synthetic generation of each of these operons, the authors were able to verify these results experimentally and predict that their particular strain of V. fischeri is likely to contain feedback onto LuxI but little or none onto LuxR.
This lack of bistability is consistent with the model of [17] which, rather than representing each gene in the network, considers only the signal and subpopulations of down- and up-regulated P. aeruginosa cells, thus rendering the model more amenable to parametrisation from their experimental data. Any positive feedback therefore is included only implicitly and the model predicts a graded though fairly sharp response from the system to increasing signal. It is possible that the implicit nature of the feedback in the model may be responsible for causing an intermediate response, i.e. most similar to Fig. 9.3b (representative of feedback only onto the signal in [16]).
Both [11] and [15] consider the lasIR quorum-sensing system of P. aeruginosa with and without feedback onto LasR. In this bacterium, quorum sensing plays a large role in the regulation of virulence and has accordingly been identified as a potential target for novel therapeutics. Due to the uncertainty of the presence of the LasR feedback loop, in these studies the effect of anti-quorum-sensing drugs on a circuit both with and without this loop is considered. The fact that LasR positive feedback induces bistability (as in [16]) has a knock-on effect on drug efficacy: the graded upregulation arising with no LasR feedback is best treated with a low steady dose of anti-LasR drug; conversely, the bistable switch implies one much larger dose of LasR is appropriate for successful downregulation of the quorum-sensing system.
The regulation of quorum-sensing systems by proteins that are not part of the quorum-sensing network itself is not uncommon: given that the purpose of the former is to translate a signal through a single cell or a population of cells, it is not surprising that its gene regulation network would be capable of interacting with other subcellular signalling networks. In some cases, bacteria even possess multiple interacting quorum-sensing networks. References [18] and [19] model the combined action of two quorum-sensing systems in P. aeruginosa and V. fischeri, respectively, the latter model having up to 21 steady states. Though not all of theses states will be both stable and biologically attainable, it is a fair assumption that the interaction of multiple feedback loops can cause a significant rise in the number of possible states the system can hold, in agreement with [10]. As implied above, we shall consider this further in Sect. 9.2.2.
In all of the above cases, mathematical bi- or multi-stability is caused by the presence of positive feedback loops, ensuring the cells’ response is particularly strong. Interestingly, in [20] it is a negative feedback loop which forces the quorum-sensing system of Agrobacterium tumefaciens out of always (monotonically) attaining an up-regulated state and into a bistable system. The negative feedback occurs indirectly (via the TraM protein) on the receptor (TraR), in addition to the standard positive feedback on both TraR and the signal molecule. In the presence of the two positive loops, activation is too strong to prevent upregulation without self-regulated inhibition of the receptor protein.
The results of [16] and [20], therefore, contrast: in the second, two positive feedback loops give bistability, while in the first the result is monostability. Of course, the two theoretical studies consider different bacteria (V. fischeri and A. tumefaciens), meaning different mechanisms and parameters are used, but nevertheless it is clear that the role of feedback in defining the type of switch is not as clear-cut as one might expect. Indeed, bistability has been demonstrated in the model of the agr system of S. aureus even when feedback in the system is not considered [13]. Moreover, in a model of a generic Gram-negative system in [21], it is demonstrated that a nonlinear interaction in the network (here, dimerisation of LuxR being required for it to bind the signal) is sufficient to achieve bistability in the network (though see Sect. 9.2.3 for further detail on this result). Thus, while feedback need not be the sole factor in determining the shape of the response-transition curve, it can evidently play a large part. Given the extra roles outlined below, perhaps it is the multi-tasking ability of feedback that has resulted in it being so prominent in quorum-sensing systems, with cells not relying on mechanisms like oligomerisation alone.
9.2.2 Tuning of Signal and Response Levels
We have discussed above how the presence of feedback loops can result in mixed subpopulations with varying phenotypes [10]. Such differences in phenotype might typically be explained by a difference in levels of the response regulator of a gene regulation network (in agr-homologues this is AgrA; in luxIR-homologues the response regulator is also the receptor protein, LuxR). In a model in [22] of the involvement of an agr-type quorum-sensing system in sporulation and solventogenesis by Clostridium acetobutylicum, it was shown that the absence of positive feedback into the receptor and response regulator enables a cell to tune its response more finely via response-regulator levels. Through comparisons with a model of the agr system in S. aureus [14] (where positive feedback occurs onto all elements of the agr system that controls virulence), it was clear that the reduced number of feedback loops could lower the quorum-sensing response. This ties in nicely with the purpose of quorum sensing in each of these organisms: sporulation is a survival mechanism (required in an acidic environment that is likely to occur when the population is dense) that is not needed by every cell in the population, whereas S. aureus gains no benefit by only a portion of the population becoming virulent. A population of sporulating cells can actually benefit from reserving a subpopulation in a vegetative state in case environmental conditions suddenly become more favourable to cell growth—the reversal of sporulation being both time and energy consuming.
In [22] a number of mechanisms of interaction between a quorum-sensing system and a sporulation-initiation gene regulation network were considered (either by direct phosphorylation of the response regulator of the latter, Spo0A, or via interference of the transcription of various different elements in the network). The ability of the network to tune response levels in the absence of feedback into agrA and agrC was seen only in the case of direct phosphorylation. For all other network topologies, the response was equivalent with or without these particular feedbacks, hence it was postulated that the feedback might be absent simply to prevent unnecessary overproduction of relevant elements in the network, thus saving the cells’ energy.
Similarly, [23] discusses a range of ways of inhibiting quorum sensing (background inhibition of signal production, negative feedback onto the signal and the soaking up of signal molecules through competitive binding to some other constitutively produced molecule) in a generic model of quorum sensing in Gram-negative bacteria (this work is an extension of [17], with the addition of repression producing a better model fit to their experimental data), finding that the second and third of these can result in a decreased proportion of up-regulated cells in the overall population. Furthermore, it is shown that this can prevent over-production of the signal molecule.
The level of signal molecule is a key focus of a number of studies of quorum sensing and it is often found that when the system does display bistability, moving between states unsurprisingly depends on the quantity of signal molecule. In [18], the first model to tackle two quorum-sensing systems in P. aeruginosa simultaneously, it is shown that the critical signal level required to induce this switch is dependent on the amount of RsaL protein, the protein responsible for mediating negative feedback on the LasI signal precursor: the more RsaL present, the more signal is required to induce the transition to a quorum-sensing up-regulated state. Thus feedback can be responsible for tuning both the level of response to quorum sensing within a population of cells and the quantities of signal molecule that are produced.
9.2.3 Noise Filtering
In modelling gene regulation networks, it is not uncommon to neglect certain aspects of the network to reduce its complexity and reduce the number of parameters that require estimating. In a number of quorum-sensing models this has been done by ignoring the feedback within the system. For example, [24] reduces the somewhat complicated quorum-sensing network of V. harveyi (which will be discussed further in Sect. 9.3.3) by omitting the multiple feedback loops involved. Despite the consequent lack of biological detail, the model fits luminescence data remarkably well, suggesting that perhaps the feedback is present to filter out noise and constrain the protein levels to those predicted by the model to give the observed experimental output.
In [20], the study of quorum sensing in A. tumefaciens mentioned in Sect. 9.2.1, it is postulated that the role of negative feedback in moving the system from being monostable (and always achieving upregulation) to bistable is manifested in the negative feedback dampening molecular noise in the system. Random fluctuations in receptor/regulator levels would otherwise disguise the system response, transforming the bistability into a smoother transition between states.
Interestingly, both negative and positive feedback loops appear capable of diluting the effects of noise. While we remarked that dimerisation was sufficient to induce bistability in a deterministic model of Gram-negative quorum sensing in [21], the authors also showed that bistability could be replaced with a graded response through the addition of noise, the same applying to positive feedback on LuxR without dimerisation. In combination, however, the system was shown to be able to produce bistability that was resistant to molecular noise.
The authors of [25] investigated alternative ways of dampening out noise. They hypothesised that the interaction of the lux and ain systems in V. fischeri (via the ain signal competitively binding LuxR but not activating it) could be responsible for controlling single cell variability (they had previously shown experimentally that the luxIR system alone could only maintain a stable response when averaged over the population [26]). However, even with ain in the model, a degree of variability between single cells remains. Hence it was concluded that the ain system may instead be involved in suppressing LuxR levels for as long as possible during growth and colonisation and is perhaps more likely to have a role in timing of the quorum-sensing response. Thus genetic feedback, rather than interacting systems, could be a simpler and more effective means to reduce noise within a quorum-sensing system.
9.2.4 Timing
Given that positive or negative feedback can adjust the levels of signal molecule in a system, it is natural to assume that feedback can have a role in the timing of the onset of quorum-sensing upregulation. Indeed, incorporating additional feedback into the receptor and response regulator of the quorum sensing system of C. acetobutylicum brings forward the onset of solventogenesis in [22]. However, surprisingly few mathematical models discuss the possible role for feedback in timing. While in some cases this is likely to be implicit (if feedback can, for instance, adjust the critical signal level at which a switch occurs, this ought to have a causal effect on timing of the switch in a given instance), in [23] it is actually found that, of the three types of repression investigated in the model (background inhibition, negative feedback and soaking up of signal molecule), negative feedback is the only one which does not noticeably affect timing of the quorum-sensing response.
9.3 Investigating Feedback with Mathematical Models
In order to illustrate how the role of feedback can be investigated with the aid of mathematical modelling, we now construct generic models of Gram-positive and Gram-negative circuits. These are much simplified (indeed, as simple as possible) and seek to capture, most importantly, the nature of the various feedback loops postulated; we omit details specific to individual networks as these will require more extensive work (as well as parameterisation), each being worthy of their own investigations. In the interests of being as comprehensive as possible within such a framework, we also consider the atypical quorum-sensing networks that have been proposed for V. harveyi and V. cholerae: these can be considered to be a hybrid of the generic Gram-positive and Gram-negative networks—more will be discussed on this in Sect. 9.3.3. Our approach builds on the quorum-sensing review of [8] whereby we aim to mirror the biology-based characterisation of different quorum-sensing architectures therein with our own modelling results.
In each case we build a nonlinear ordinary differential equation model where variables represent the key components of the system and the kinetic terms describe in a simple way the interactions between these components (in reality these interactions may be indirect and governed in part by variables not considered here for brevity). We subsequently demonstrate how the role of feedback can be investigated by knocking out each loop from the models (either by setting a relevant parameter to zero or by altering a particular term) and comparing the resulting time-dependent solutions and steady-state curves to the wild-type model. This process is relatively straightforward and has been used in many of the more detailed studies discussed in Sect. 9.2; moreover, it illustrates the benefits of adopting a simple modelling approach.
We restrict our study within this chapter to deterministic models and therefore do not investigate the role of feedback in a noisy system explicitly. We do consider the three remaining hypothesised roles for feedback: defining transition type, tuning of key molecules and timing of the quorum-sensing response (though we shall see that, even in the absence of noise in the system, we can also gain some insight into the role of feedback in filtering noise).
9.3.1 Gram-Positive Quorum Sensing: The agr System
We begin with the less well-studied quorum-sensing system: agr homologues. As mentioned previously, this circuitry was first discovered in the pathogenic bacterium S. aureus (where it regulates virulence factor production) and has since been discovered in a variety of other Gram-positive bacteria: in C. difficile [27], C. botulinum [28, 29], Enterococcus faecalis [30] and S. epidermidis [31], agr-like systems also have a role to play in pathogenesis, while in non-pathogenic bacteria an agr system controls sporulation in C. acetobutylicum [32] and adherence in Lactobacillus plantarum [33].
As alluded to earlier, the majority of these species appear to adopt the feedback architecture of S. aureus, but some (notably C. acetobutylicum) have reduced levels of feedback. The reasons for this were investigated in [22] and as such we do not go into detail here. Instead we consider a simplified model and link the results to the putative roles for feedback discussed in Sect. 9.2.
9.3.1.1 Model Formulation
The general agr system is depicted in Fig. 9.2a and a simplified version in Fig. 9.2b; it is from Fig. 9.2b that we derive our model.
We assume the signal, s, and regulator, r, are each produced constitutively, at rates c s and c r , respectively. Regulator is activated irreversibly in response to the presence of signal at rate α. Rates of production of signal (c s h) and regulator (c r h) above the basal levels are induced by activated regulator, r ∗. The ratio of separation to binding of the active regulator to the relevant operons is given by β; for simplicity we assume that this ratio is the same for both the signal and regulator (this is valid in S. aureus, where all the agr genes are contained within one operon, but may vary for species where this is not the case). Similarly, all molecules degrade at some rate δ (in reality this rate will vary between molecules).
The resulting (minimal) equations are
Notice that the roles for s and r in this minimal model are symmetric when feedback is present onto both. To investigate how a cell may move from quorum-sensing down-regulated to up-regulated we use
for the initial conditions.
For clarity, variable and parameter descriptions (including the default values for the latter) are given in Table 9.1. Note that we are using a specific parameter set merely to illustrate the type of behaviour that the above system can display (so that we do not even specify units). While biologically realistic parameter values are of course desirable in more detailed models, they vary between bacteria (and even between strains of the same species); we seek here instead to examine qualitative behaviour of the quorum-sensing networks.
9.3.1.2 Numerical Investigations
The system (9.1)–(9.4) can easily be solved numerically; we do so using the ode23 Runge–Kutta solver in Matlab R2013a. We also derive steady-state curves in XPPAUT Version 7.0. The simplified agr system modelled here contains two positive feedback loops (one onto the signal and one onto the regulator) and no negative feedback. The loops can be deleted from the model simply by setting c s h = 0 and/or c r h = 0.
We investigate the effect that these deletions have on the quorum-sensing behaviour by solving the system in response to various levels of signal: we can consider (9.1)–(9.4) to be representative of the quorum-sensing machinery in a single cell, hence varying c s can be seen to be equivalent to varying the amount of signal molecule (produced also by neighbouring cells) in the environment (or equivalently to increasing population size or density). Steady-state curves in response to varying c s are displayed in Fig. 9.4 and time-dependent solutions in Fig. 9.5.
Steady-state curves of the simplified agr circuitry (9.1)–(9.3) in response to changes in signal influx, c s . Stable states are given with solid lines and unstable with dotted. The x-axis in each case is c s . Column (a) represents the wild-type system, (b) when there is no feedback into signal production, (c) when there is no feedback into regulator production, and finally (d) when there is no feedback at all. The first, second and third rows represent active regulator, signal and inactive regulator levels respectively
In the wild-type model we see that the system is bistable, though much of the solution for one of the stable states lies in a biologically infeasible regime (variables should not be negative and we therefore do not plot these; we do, however, display c s < 0 for visualisation of at least one of the bifurcation points). The inactive state arising when c s = 0 is unstable and all positive values of c s inevitably result in a quorum-sensing response being activated. We see from the results of removing either or both feedback loops that this is a combined effect of both loops.
Removing feedback into signal production (c s h = 0) alters the transition shape from a sharp jump (into a quorum-sensing up-regulated state) to a gradual transition as c s increases (relating to Sect. 9.2.1). Removing feedback into the regulator (c r h) greatly lowers the overall response of the system (but retains the jump in activity) by lowering the amount of regulator which can be activated (see Sect. 9.2.2). We saw in [22] that altering the basal rate of regulator production has been found to modify (fine-tune) the response level appropriately, in agreement with results here.
Finally, if no feedback at all exists in the agr circuitry (\(c_{s}^{h} = c_{r}^{h} = 0\)), then the system, with increasing c s , moves gradually to an active state but where the response is much lower. These results are reflected in the time-dependent solutions of Fig. 9.5 where we notice also that the time it takes for the quorum-sensing system to respond to signal is not noticeably affected by removal of either of the feedback loops.
Time-dependent solutions for the agr system (9.1)–(9.4) with (a) wild-type circuitry, (b) no positive feedback into signal production, (c) no positive feedback into regulator production and (d) no feedback at all. Population size is represented indirectly via the basal rate of signal production, c s
9.3.1.3 Analytical Investigations
In addition to numerical solutions, we can also investigate the systems analytically for more insight into the system in general, though here is not the place to undertake extensive such investigations (those very limited ones that we do outline are in keeping with our goal of exploring models of minimal complexity). Here, we examine the case where the rate of activation is large. As α → ∞, two cases arise:
- Case 1. :
-
s → 0 (signal levels become negligible)
- Case 2. :
-
r → 0 (regulator levels become negligible)
Due to the symmetry in (9.1) and (9.2) noted above, we consider only Case 1. In this limit the equations become
Note that (9.7) and (9.6) can then be solved sequentially. For Case 1 to apply we require c r − c s > 0 to ensure the variables remain positive (else Case 2 arises).
In the limit as α → ∞, therefore, r ∗ (activated regulator) increases monotonically to its equilibrium state.
- Case 1a. :
-
(\(c_{r}^{h} - c_{s}^{h} > 0\)), here the production rate of r will increase with r ∗ (as a natural result of the positive feedback loop).
- Case 1b. :
-
(\(-(c_{r} - c_{s}) < c_{r}^{h} - c_{s}^{h} < 0\)) (the first inequality is needed for Case 1). This is unlikely since \(c_{r}^{h},c_{s}^{h} >> c_{r},c_{s}\) but if \(c_{r}^{h} \approx c_{s}^{h}\) we could have the counter intuitive behaviour that the increase in r ∗ leads to a decreasing production rate (and steady state value) of r.
9.3.2 Gram-Negative Quorum Sensing: The luxIR System
The luxIR system is the best characterised system and there is an abundance of literature available on the subject. In Sect. 9.2 we have already drawn attention to a number of examples of this system in a variety of bacteria and we do not echo this information here. Instead we move directly to model formulation.
9.3.2.1 Model Formulation
Figure 9.1a,b, respectively, illustrate the luxIR system and the schematic (abstract) version required for our model building in this study. Notice that when the systems are simplified, the only difference between the Gram-positive agr architecture and the luxIR one of Gram-negative species is the presence of negative feedback onto the signal in the latter. In both systems, positive feedback onto the regulator may or may not occur depending upon the species (and strain) of interest. Note that in the interests of space, we do not consider the negative feedback onto regulator production as seen in A. tumefaciens since this seems not to be widely prevalent.
The model is built in the same manner as that in Sect. 9.3.1.1, but we now require the increased signal production to be both activated and inhibited by the presence of active regulator. The model becomes
To represent inhibition of signal production being weaker than activation (since the former occurs usually indirectly via homologues of the RsaL protein) we take β 2 > β 1 (parameters and variables for this model are also provided in Table 9.1). As before, we use
9.3.2.2 Numerical Investigations
Given that when we consider only the minimal versions of the agr and luxIR networks the only difference between the two is negative feedback onto signal production, many of the results of Sect. 9.3.1 are also applicable here, see Figs. 9.6 and 9.7. Positive feedback onto signal production changes the shape of the system response from gradient to switch-like and positive feedback onto the regulator induces a much greater quorum-sensing response (that is more likely to coordinate behaviour of a whole population of cells than would a lower level of active regulator). Thus, the presence of the negative feedback loop does not appear to alter the roles of the two positive loops and the similarities between this and the agr architecture suggest that, despite the differences in mechanism between the two, any overall distinction between the two may be fairly subtle (though, naturally, a far more extensive parameter analysis than that provided here would be required to make more definitive statements).
Steady-state curves of the simplified luxIR circuitry (9.8)–(9.10) in response to changes in signal influx, c s (the x-axis in each case is c s ). Stable states are given with solid lines and unstable with dotted. Column (a) represents the wild-type system, (b) no positive feedback into signal production, (c) no negative feedback into signal production, and finally (d) no positive feedback into regulator production. To achieve (c), Eq. (9.8) is replaced by (9.1). The first, second and third rows represent active regulator, signal and inactive regulator levels, respectively
Time-dependent solutions for the luxIR system (9.8)–(9.11) with (a) wild-type circuitry, (b) no positive feedback into signal production, (c) no negative feedback into signal production, and (d) no positive feedback into regulator production. Population size is represented indirectly via the basal rate of signal production, c s
Removal of the negative feedback onto signal production in fact makes little noticeable difference in our parameter regime. At lower values of c s active regulator levels are higher in the absence of the loop while at higher values of c s there is little to distinguish between the response, meaning that this negative feedback could be in place to prevent the cells from becoming quorum-sensing active prematurely. Thus the luxIR system may adopt this negative feedback loop to play a role in filtering noise from the system.
While we have to bear in mind that these results are parameter-dependent (and we have not sought a realistic parameter set), we again see no particular position for feedback in controlling the timing of the onset of a quorum-sensing response.
9.3.2.3 Analytical Investigations
We again consider the analytical effect on the system of taking α → ∞, i.e. a fast activation rate. Two cases emerge, as follows, that need separate description since the model is no longer symmetric between s and r.
- Case 1. :
-
s → 0 (signal level becomes negligible). The model becomes:
$$\displaystyle\begin{array}{rcl} \alpha sr \sim c_{s} + \frac{c_{s}^{h}\beta _{2}r^{{\ast}}} {(r^{{\ast}} +\beta _{1})(r^{{\ast}} +\beta _{2})},& & {}\end{array}$$(9.12)$$\displaystyle\begin{array}{rcl} \frac{dr} {dt} \sim c_{r} - c_{s} +\bigg (c_{r}^{h} - c_{ s}^{h} \frac{\beta _{2}} {r^{{\ast}} +\beta _{2}}\bigg) \frac{r^{{\ast}}} {r^{{\ast}} +\beta _{1}} -\delta r,& & {}\end{array}$$(9.13)$$\displaystyle\begin{array}{rcl} \frac{dr^{{\ast}}} {dt} \sim c_{s} + \frac{c_{s}^{h}\beta _{2}r^{{\ast}}} {(r^{{\ast}} +\beta _{1})(r^{{\ast}} +\beta _{2})} -\delta r^{{\ast}}.& & {}\end{array}$$(9.14)Hence r ∗ again increases monotonically and, since for O(1) coefficients in (9.14) it never attains the regime in which the second term on the right-hand side of (9.14) \(\sim c_{s}^{h}\beta _{2}/r^{{\ast}}\), the behaviour is not qualitatively different from the previous case. If r ∗ does become sufficiently large, the c s h term becomes negligible (i.e. feedback onto the signal becomes insignificant) and the response of r becomes stronger in (9.13) relative to (9.6).
- Case 2. :
-
r → 0 (regulator levels becomes negligible). Here
$$\displaystyle\begin{array}{rcl} \alpha sr \sim c_{r} + \frac{c_{r}^{h}r^{{\ast}}} {r^{{\ast}} +\beta _{1}},& & {}\end{array}$$(9.15)$$\displaystyle\begin{array}{rcl} \frac{ds} {dt} \sim c_{s} - c_{r} +\bigg ( \frac{c_{s}^{h}\beta _{2}} {r^{{\ast}} +\beta _{2}} - c_{r}^{h}\bigg) \frac{r^{{\ast}}} {r^{{\ast}}+\beta } -\delta s,& & {}\end{array}$$(9.16)$$\displaystyle\begin{array}{rcl} \frac{dr^{{\ast}}} {dt} \sim c_{r} + \frac{c_{r}^{h}r^{{\ast}}} {r^{{\ast}}+\beta } -\delta r^{{\ast}}.& & {}\end{array}$$(9.17)In this case, the term in brackets in (9.16) becomes negative if r ∗ becomes sufficiently large, implying that signal production could effectively be switched off, giving a pulsed response rather than a sustained one.
9.3.3 The V. harveyi and V. fischeri Quorum-Sensing Systems
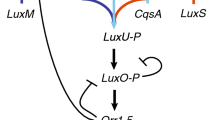
Though related to V. fischeri, the quorum-sensing systems of V. harveyi (a pathogen of marine organisms) and V. cholerae (the etiological agent of cholera) are somewhat different—[8] includes a review of these networks. These pathogens produce and secrete a signal molecule in much the same way as the luxIR system, but this signal is detected via a two-component system (akin to agr), i.e. the network is in some sense a hybrid of the two described above. Binding of the signal molecule to the receptor protein triggers a phosphorylation cascade between itself and the intracellular LuxO protein (note that in these systems, LuxO is considered active when it is in a dephosphorylated form, hence the bound receptor ultimately removes phosphates from LuxO). Activation of LuxO releases the production of multiple Qrr sRNAs that LuxO otherwise inhibits when inactive. Since Qrr sRNAs prevent production of the response regulator (LuxR) of the systems (via degradation of luxR mRNA), this releases production of LuxR, which is responsible for inducing a quorum-sensing response. We depict this process in Fig. 9.8 (including the feedback loops not addressed in the description above) but note that this is grossly simplified in several respects: intermediary molecules have been neglected where they do not have a direct role in feedback, in reality there are multiple types of Qrr sRNAs (which have different combined effects between V. harveyi and V. cholerae) and multiple signal–receptor pairs exist; see [8] for the full networks. For the purpose of this chapter, however, (i.e. to examine the role of feedback) it is satisfactory (and indeed desirable given the full system complexity) to ignore these components. In addition we neglect the receptor complex, LuxPQ, and assume LuxO dephosphorylation occurs in response to increased signal levels.
A schematic representation of the simplified V. harveyi quorum-sensing network (due to the additional components required in this minimal network compared to the agr or lux systems, we do not illustrate a version in terms of only the “signal” and “regulator”). In reality, this system contains multiple signal–receptor pairings, Qrr species and intermediary molecules that we have neglected from the model. The full system (which we omit here in the interests of space due to its complexity) can be found in [8]. We depict here the LuxPQ receptor component but we do not include this in the modelling, assuming instead that phosphorylated LuxO levels relate directly to signal level (as we did for the agr model). The homologue of LuxR in V. cholerae is HapR
It is noteworthy that there is no positive feedback present in this network, meaning that, given the results of Sects. 9.3.1 and 9.3.2, one might expect a more graded response to increasing quorum-sensing signal (we shall see in Sect. 9.3.3.2 that this is indeed the case). On the other hand, there are four negative feedback loops (see Fig. 9.8). Possible explanations behind each of these loops are discussed in [8] and we shall revisit these here with our model.
9.3.3.1 Model Formulation
Very few models relevant to V. harveyi or V. cholerae quorum sensing exist in the literature and those that do focus mostly on specific aspects of either upstream elements (for example, [24]) or the action of the Qrr sRNAs (see [34] or [35]), for the large part neglecting feedback. We instead seek to formulate the simplest model that can account for all the feedback loops in this network.
Most terms in the equations are derived in the same manner as those for the agr and luxIR systems. In addition, we require autophosphorylation of LuxO in the absence of signal (at rate ϕ), dephosphorylation (and therefore activation) of LuxO ∼ P in the presence of signal (we recall that we omit the intermediary receptor proteins from the model) at rate α and degradation of LuxO and LuxR by Qrr sRNAs at rate κ 1 and κ 2, respectively (this also results in the degradation of Qrr sRNAs; note that, since we do not consider mRNA explicitly, we treat Qrr as acting directly on the relevant proteins). All variables and parameters are provided in Table 9.2.
The model is given by
Similarly to the previous sections, we take
Note that, in contrast to the previous two models, there will always be regulator present in the model for t > 0 even when no signal is present.
9.3.3.2 Numerical Investigations
We divide this section into discussion of the four different negative feedback loops, comparing our results with the postulated roles given in the microbiology review [8].
9.3.3.2.1 LuxO Autorepression
It is suggested in [8] that LuxO autorepresses in order to constrain its own levels and consequently those of the Qrr sRNAs that are produced in response to active LuxO levels. Removal of these loops in Fig. 9.9b does incur higher levels of LuxO and LuxO ∼ P, but does not significantly alter Qrr levels in our parameter regime (though this may be due to the influence of the remaining negative feedback loops operating). Removal of this loop has very little effect on final LuxR levels. Thus the results are in agreement with [8], suggesting that this autorepression loop may be responsible for avoiding undesirable effects of noise.
Steady-state curves of the simplified V. harveyi and V. cholerae circuitry (9.18)–(9.22) in response to changes in signal influx, c s (the x-axis in each case is c s ). All steady states depicted here are stable. Column (a) represents the wild-type system, (b) when there is no negative feedback into LuxO (the first term of (9.19) is replaced by \(c_{r_{1}}\)), (c) when there is no negative feedback into LuxO via Qrr (κ 1 = 0), (d) when there is no negative feedback into LuxR via Qrr (the first term of (9.21) is replaced by \(c_{q}r_{1}^{{\ast}}/(r_{1}^{{\ast}}+\beta )\)), and finally (e) no auto-repression by LuxR (the first term of (9.22) is replaced by \(c_{r_{2}}\)). The rows represent LuxR, signal (LuxS), active LuxO, inactive LuxO ∼ P and Qrr, respectively
9.3.3.2.2 LuxO Negative Feedback via Qrr
This feedback loop can be viewed in two ways: either to constrain the levels of LuxO or those of Qrr. We see in Fig. 9.9c that Qrr levels are increased slightly at low signal concentrations on removing this feedback loop, while LuxO concentrations remain largely the same. In agreement with [36], this does have a slight consequence on LuxR by lowering its levels (see Figs. 9.9c and 9.10c). Thus this loop may both filter out noise from Qrr levels and coordinate a greater “whole population” response at low signal levels.
Time-dependent solutions for the V. harveyi and V. cholerae quorum-sensing systems (9.18)–(9.23) with (a) wild-type circuitry, (b) no negative feedback into LuxO, (c) no negative feedback into LuxO via Qrr, (d) no negative feedback into LuxR via Qrr, and (e) no auto-repression of LuxR. Population size is represented indirectly via the basal rate of signal production, c s
9.3.3.2.3 LuxR Negative Feedback via Qrr
It is hypothesised that this feedback loop should prolong the production of Qrr sRNAs and hence delay the cells attaining the levels of LuxR required for quorum-sensing activation. While it is difficult to see any difference between the wild-type steady-state curves and those where this loop has been removed (Fig. 9.9a, d) from the time-dependent solutions in Fig. 9.10d it is evident that in our parameter regime LuxR levels are actually lowered in the absence of this indirect LuxR negative feedback. This is likely to be a result of the interplay with the active loops and is worthy of further investigation to see whether this would be reproducible in reality or whether it is due to our specific parameter choice.
Timing of any quorum-sensing response again appears unaffected by this loop, but it is possible that this consequence could be lost from the model as a result of omission of intermediary species along the pathway, which would slow down the response.
9.3.3.2.4 LuxR Auto Repression
Removing LuxR autorepression has a similar effect to including positive autoinduction of the regulators in Sects. 9.3.1 and 9.3.2: LuxR levels significantly increase. This has little or no effect on the other variables in the model but the loop should prevent unnecessary production of LuxR (see Sect. 9.2.2). This agrees with the biological explanation in [8], where it is suggested that this loop will prevent runaway luxR transcription, minimising the chance of ill-timed commitment to quorum-sensing upregulation. Though it does not change the shape of the response (Sect. 9.2.1), it does alter the sensitivity of the overall system to signal levels. Furthermore, higher LuxR levels would make it trickier for the cells to switch off their quorum sensing response [36, 37].
Overall, therefore, this model implies that each individual negative feedback loop may prevent fluctuations in individual components of the network, constraining them to the desired level, whether this be to prevent unnecessary overproduction or to prevent inappropriate reactions to noisy inputs. This should ensure a coordinated response across a population of cells and matches neatly the evidence that both V. harveyi and V. cholerae are insensitive to small changes in signal level [8]. In addition, their mutual action maintains a graded response from the cells, preventing an on-off type switch [36] (though we note that in individual loop knock-outs, the response shape always remains largely the same (see Fig. 9.9), thus this may be as a result of their combined effects).
9.3.3.3 Analytical Investigations
For the V. harveyi and V. cholerae systems we consider the possibility that both the activation rate and the action of Qrr sRNAs are large: α, κ 1, κ 2 → ∞. This yields various possibilities and we focus on the case in which the signal and Qrr sRNA levels become negligible: s, q → 0. This gives
If, in addition, we take the small limit of the rate of LuxO autophosphorylation (ϕ → 0, so the cells are increasingly likely to become activated), then r 1 → 0 and we have (we note that a number of limits are required in the current case to achieve the level of simplicity attained earlier in (9.5)–(9.7) and (9.12)–(9.17): the virtue of such an approach—and the reason for including the analysis—is that the properties of the resulting system, (9.29)–(9.31), are almost completely transparent)
Note that at leading order in this scenario, response regulator levels (r 2) are governed by the negative feedback loops: the source term is monotonically decreasing in r 2 and the action of the Qrr sRNAs is present implicitly. Thus the negative feedback loops remain significant in this limit.
It is hoped that such comments indicate the potential scope and value of analytic (in particular, asymptotic) investigations.
9.4 Summary
We have provided a general review of mathematical models of quorum sensing that consider the role of feedback in bacterial cell communication. Drawing on this, four dominant roles were apparent: defining the shape of the system response (often controlled by the presence of a positive feedback loop inducing bistability), tuning of signal or regulator levels (usually either a positive feedback loop ensuring a coordinated response from a whole population of cells or the absence of a positive—or inclusion of a negative—loop either simply reducing unnecessary production of proteins or inducing population heterogeneity), filtering noise out from the system (largely via negative feedback) or in the timing of the onset of a quorum-sensing response.
Interestingly, the last of these does not frequently arise from the results of mathematical models but is often postulated in biological articles. It is possible therefore that feedback may not be as important in the timing of a quorum-sensing response as often assumed, but the modelling results could also be a consequence of the simplifying assumptions required to make a mathematical model tractable. For instance, considering transcription and translation separately (and therefore mRNA and proteins) could delay the signal transduction through a pathway in a numerical solution, providing more scope for feedback to affect timing, but would typically result in increased numbers of variables, parameters to estimate and complexity in the analysis.
The models presented of the agr, luxIR and V. harveyi/V. cholerae quorum-sensing systems were deliberately simplified for ease of analysis but were still able to identify the first three roles for feedback listed above. In the agr system, positive feedback influences the shape of the transition between quorum-sensing down- and up-regulated states and makes a coordinated response from the whole population more likely. The lux system, additionally, employs a negative feedback loop that is likely to play a role in filtering noise from the system. Similarly, the V. harveyi and V. cholerae systems adopt multiple negative feedback loops capable of constraining protein and signal levels and making the system robust to noise. Regarding this last network, it is possible to imagine that each loop dominates at the appropriate time as the signal is transmitted through the cell and this would likely best be studied using delay differential equations.
There is scope for an abundance of future work related to this review including more detailed models of specific networks (including such delay effects, as well as stochastics) and, in particular, the inclusion of experimental data for better parameter estimation (genetic manipulation can be performed experimentally to reproduce the modified network architectures discussed in this study). It will be fascinating to see if different parameter regimes are capable of displaying different goals for identical feedback architectures. Moreover, as more quorum-sensing systems and their targets are identified, it will be enlightening to see how these targets tie in with the relevant feedback architecture.
References
Redfield RJ (2002) Is quorum sensing a side effect of diffusion sensing? Trends Microbiol 10:365–370
Hense BA, Kuttler C, Müller J, Rothballer M, Hartmann A, Kreft J (2007) Does efficiency sensing unify diffusion and quorum sensing? Nature Rev Mirobiol 5:230–239
Williams P (2007) Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbology 153:3923–3938
Nealson KH, Hastings JW (1979) Bacterial bioluminescence: its control and ecological significance. Microbiol Rev 43:496–518
Ji G, Beavis RC, Novick RP (1995) Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci USA 92:12055–12059
Henke JM, Bassler BL (2004) Bacterial social engagements. Trends Cell Biol 14:648–656
Waters CM, Bassler BL (2005) Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 21:319–346
Ng W-L, Bassler BL (2009) Bacterial quorum-sensing network architectures. Annu Rev Genet 43:197–222
Oyarzún DA, Chaves M, Hoff-Hoffmeyer-Zlotnik M (2012) Multistability and oscillations in genetic control of metabolism. J Theor Biol 295:139–153
Smits WK, Kuipers OP, Veening JW (2006) Phenotypic variation in bacteria: the role of feedback regulation. Nat Rev Microbiol 4:259–271
Anguige K, King JR, Ward JP, Williams P (2004) Mathematical modelling of therapies targeted at bacterial quorum sensing. Math Biosci 192:39–83
Dockery JD, Keener JP (2001) A mathematical model for quorum sensing in P. Aeruginosa. Bull Math Biol 63:95–116
Gustafsson E, Nilsson P, Karlsson S, Arvidson S (2004) Characterizing the dynamics of the quorum-sensing system in S. aureus. J Mol Microbiol Biotechnol 8:232–242
Jabbari S, King JR, Koerber AJ, Williams P (2010) Mathematical modelling of the agr operon in S. aureus. J Math Biol 61:17–54
Anguige K, King JR, Ward JP (2005) Modelling antibiotic- and anti-quorum sensing treatment of a spatially-structured P. aeruginosa population. J Math Biol 51:557–594
Haseltine EL, Arnold FH (2008) Implications of rewiring bacterial quorum sensing. Appl Environ Microb 74:437–445
Ward JP, King JR, Koerber AJ, Williams P, Croft JM, Sockett RE (2001) Mathematical modelling of quorum sensing in bacteria. IMA J Math Appl Med 18:263–292
Fagerlind MG, Rice SA, Nilsson P, Harlén M, James S, Charlton T, Kjelleberg S (2003) The role of regulators in the expression of quorum-sensing signals in P. aeruginosa. J Mol Microbiol Biotechnol 6:88–100
Kuttler C, Hense BA (2008) Interplay of two quorum sensing regulation systems of V. fischeri. J Theor Biol 251:167–180
Goryachev AB, Toh D, Wee KB, Lee T, Zhang H, Zhang L (2005) Transition to quorum sensing in an Agrobacterium population: a stochastic model. PLoS Comp Biol 1:265–275
Goryachev AB, Toh D, Lee T (2006) Systems analysis of a quorum sensing network: design constraints imposed by the functional requirements, network topology and kinetic constants. Biosystems 83:178–187
Jabbari S, Steiner E, Heap JT, Winzer K, Minton NP, King JR (2013) The putative influence of the agr operon upon survival mechanisms used by C. acetobutylicum. Math Biosci 243: 223–239
Ward JP, King JR, Koerber AJ, Croft JM, Sockett RE, Williams P (2004) Cell-signalling repression in bacterial quorum sensing. Math Med Biol 21:169–204
Banik SK, Fenley AT, Kulkarni RV (2009) A model of signal transduction during quorum sensing in V. harveryi. Phys Biol 6:1–10
Pérez PD, Weiss JT, Hagen SJ (2011) Noise and crosstalk in two quorum-sensing inputs of V. fischeri. BMC Syst Biol 5:153
Pérez PD, Hagen SJ (2010) Heterogeneous response to a quorum-sensing signal in the luminescence of individual V. fischeri. PLoS ONE 5:e15473
Martin MJ, Clare S, Goulding D, Faulds-Pain A, Barquist L, Browne HP, Pettit L, Dougan G, Lawley TD, Wren BW (2013) The agr locus regulates virulence and colonization genes in C. difficile 027. J Bacteriol 195:3672–3681
Cooksley CM, Davis IJ, Winzer K, Chan WC, Peck MW, Minton NP (2010) Regulation of neurotoxin production and sporulation by a putative agrBD signaling system in proteolytic C. botulinum. Appl Environ Microb 76:4448–4460
Sebaihia M, Peck MW, Minton NP et al (2007) Genome sequence of a proteolytic (Group I) C. botulinum strain Hall A and comparative analysis of the clostridial genomes. Genome Res 17:1082–1092
Qin X, Singh KV, Weinstock GM, Murray BE (2001) Characterization of fsr, a regulator controlling expression of gelatinase and serine protease in E. faecalis OG1RF. J Bacteriol 183:3372–3382
Van Wamel WJB, van Rossum G, Verhoef J, Vandenbroucke-Grauls CMJE, Fluit AC (1998) Cloning and characterization of an accesory gene regulator (agr)-like locus from S. epidermidis. FEMS Microbiol Lett 163:1–9
Steiner E, Scott J, Minton NP, Winzer K (2012) An agr quorum sensing system that regulates granulose formation and sporulation in C. acetobutylicum. Appl Environ Microbiol 78:1113–1122
Sturme MHJ, Nakayama J, Molenaar D, Murakami Y, Kunugi R, Fujii T, Vaughan EE, Kleerebezem M, de Vos WM (2005) An agr-like two-component regulatory system in L. plantarum is involved in production of a novel cyclic peptide and regulation of adherence. J Bacteriol 187:5224–5235
Fenley AT, Banik SK, Kulkarni RV (2011) Computational modeling of differences in the quorum sensing induced luminescence phenotypes of V. harveyi and V. cholerae. J. Theor. Bio. 274:145–153
Mehta P, Goyal S, Wingreen NS (2008) A quantitative comparison of sRNA-based and protein-based gene regulation. Mol Syst Biol 4:221
Teng SW, Schaffer JN, Tu KC, Menta P, Lu W, Ong NP, Bassler BL, Wingreen NS (2011) Active regulation of receptor ratios controls integration of quorum-sensing signals in V. harveyi. Mol Syst Biol 7:491
Svenningsen SL, Tu KC, Bassler BL (2009) Gene dosage compensation calibrates four regulatory RNAs to control V. cholerae quorum sensing. EMBO J 28:429–439
Acknowledgements
SJ thanks the MRC for funding in the form of a Biomedical Informatics Fellowship.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Jabbari, S., King, J.R. (2015). Mathematical Insights into the Role of Feedback in Quorum-Sensing Architectures. In: Hagen, S. (eds) The Physical Basis of Bacterial Quorum Communication. Biological and Medical Physics, Biomedical Engineering. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1402-9_9
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1402-9_9
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1401-2
Online ISBN: 978-1-4939-1402-9
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)