Abstract
FMS-like tyrosine kinase 3 (FLT3) receptor and its ligand play a significant role in human hematopoiesis and the proliferation and malignant transformation of primitive hematopoietic cells. FLT3 mutations are observed in approximately 30 % of adult AML, 1–3 % of adult B-ALL and 2–5 % of MDS patients. Binding of FLT3 ligand to FLT3 receptor results in autophosphorylation and activation of downstream pathways that promote cell proliferation including the MAPK/ERK, Pi3K/AKT, signal transducer activator of transcription (STAT5), and BCL2-associated X protein (BAX) pathways. There are two major types of FLT3 mutations: The FLT3 internal tandem duplication (ITD) results from duplication and insertion of a fragment of the juxtamembrane domain coding sequence, whereas the less common tyrosine kinase domain (TKD) results from a missense point mutation within the activation loop of the second TKD. Studies suggest that patients with FLT3-ITD have significantly elevated peripheral blood white cell counts and increased bone marrow blasts at diagnosis. Furthermore, they have a significantly higher induction death rate, increased relapse risk, inferior event-free survival, and decreased overall survival. Recent studies have further indicated that FLT3-ITD mutations may be a significant prognostic indicator in patients with AML and diploid karyotype, but not in those with core-binding factor AML or AML with unfavorable cytogenetics. Also, presence of FLT3-ITD mutation may be a negative prognostic marker, not only at diagnosis but also at first relapse. Several small-molecule FLT3 inhibitors are currently in clinical trials and may improve responses for patients with FLT3-mutated AML.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Leukemogenesis results from dysregulation of pathways that regulate proliferation, differentiation, and cell death of hematopoietic cells (Stirewalt and Radich 2003). The FMS-like Tyrsoine Kinase 3 (FLT3) receptor and its ligand have been shown to play a significant role in human hematopoiesis and the survival, proliferation, and malignant transformation of primitive hematopoietic cells (Lyman et al. 1993a; Zeigler et al. 1994; Hirayama et al. 1995; Hudak et al. 1995; Piacibello et al. 1995). In normal human cells, FLT3 is predominantly expressed on early myeloid and B-lymphoid progenitors. FLT3 expression on leukemic cells reflects this expression. Subsequently, high levels of FLT3 receptor expression are seen in 70–100 % of cases of AML (Nakao et al. 1996), a vast majority of the cases of progenitor B cell acute lymphoblastic leukemia (B-ALL) , some cases of T-ALL, and chronic myeloid leukemia (CML) in lymphoid blast phase (Carow et al. 1996; Rosnet et al. 1996; Drexler 1996). Additionally, activating mutations in FLT3 are observed in approximately 30 % of adult AML patients (Odenike et al. 2011), 1–3 % of adult B-ALL patients, (Xu et al. 1999) and 2–5 % of myelodysplastic syndrome (MDS) patients (Horiike et al. 1997; Yokota et al. 1997).
2 Class II Receptor Tyrosine Kinases
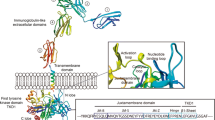
The W (dominant White spotting) locus resulting in white spots on the bellies of afflicted mice was first described in the early 1900s (Russell 1979). Subsequently, the W locus was isolated to chromosome 5, and numerous allelic variants involving multiple organ systems were identified. The W locus was found to encode a tyrosine kinase receptor known as c-kit (Geissler et al. 1988; Chabot et al. 1988). This discovery led to the identification of a new group of receptors for signaling molecules called the receptor tyrosine kinase (RTK) family of proteins. RTKs and their signaling ligands play a central role in hematopoiesis (Ullrich and Schlessinger 1990). FLT3 is a member of the class III RTK family (Rosnet et al. 1993a; Small et al. 1994). Other members of this family include receptors for colony-stimulating factor 1 (CSF-1) (Coussens et al. 1986; Rothwell and Rohrschneider 1987; Woolford et al. 1988; Sherr 1990) and steel factor (Williams et al. 1992), respectively, encoded by the FMS and KIT (Wagner and Alexander 1991) proto-oncogenes and the two receptors for platelet-derived growth factor receptors (PDGFR α and β) (Yarden et al. 1986; Matsui et al. 1989). The RTKs have significant sequence homology. Each of these receptors is approximately 1000 amino acids in length, has five immunoglobulin-like extracellular domains, a transmembrane (TM) domain, a juxtamembrane (JM) domain, and a split intracellular catalytic domain that phosphorylates tyrosine residues in specific target proteins after activation of the receptor by the ligand (Lyman and Jacobsen 1998).
2.1 FLT3 Gene
FLT3, also known as FLK-2 (fetal liver kinase-2) and STK-1 (human stem cell kinase-1) was first cloned by Mathews et al. in enriched mouse fetal liver cells in 1991 (Matthews et al. 1991). They described FLT3 as a novel RTK related to the W locus gene product C-KIT. They found the FLT3 RTK to be specifically expressed in stem cells and early uncommitted hematopoietic progenitor cells, with no expression in more mature cells. Almost simultaneously, Rosnet et al. also isolated and characterized murine FLT3. They found the gene to be expressed in placenta, in various adult tissues including gonads and brain, and in hematopoietic cells in mice (Rosnet et al. 1991). This was followed closely by the cloning and description of human FLT3 gene in 1993 (Rosnet et al. 1993a). The human FLT3 gene located on chromosome 13q12 has 85 % amino acid sequence homology with mouse FLT3 (Rosnet et al. 1993b). The FLT3 gene is expressed in precursors of both myeloid and B-lymphoid lineage(Rosnet et al. 1996;Brasel et al. 1995;Turner et al. 1996). Expression is usually restricted to early precursors, including CD34+ cells with high levels of CD117 (C-KIT) expression (Rosnet et al. 1996; Rasko et al. 1995). Cells of erythroid and megakaryocytic series do not express FLT3 gene (Gabbianelli et al. 1995; Ratajczak et al. 1996). The FLT3 gene is also expressed by placenta, liver, spleen, thymus, and gonads (deLapeyriere et al. 1995; Maroc et al. 1993).
2.2 FLT3 Ligand
The FLT3 ligand (FLT3L) was cloned by Lyman et al. in 1993. (Lyman et al. 1993a) The FLT3L is a type 1 TM protein, which contains an amino-terminal signaling peptide, four extracellular helical domains, spacer and tether regions, a TM domain, and a small cytoplasmic domain. (Lyman and Jacobsen 1998; Lyman et al. 1994b) Ligand activity resides in the extracellular domain with the intracytoplasmic domain playing minimal to no role in FLT3 activation and function. (Lyman et al. 1993b) The ligand can also be released as a soluble homodimeric protein. Both the soluble form and the TM form are capable of activating the FLT3 receptor (Hannum et al. 1994; Lyman et al. 1995). The FLT3L is expressed by hematopoietic cells (spleen, thymus, and bone marrow), placenta, lung, colon, kidney, prostate, ovary, testis, and heart. Of note, the brain does not seem to express FLT3L. In the bone marrow microenvironment, FLT3L is expressed predominantly by myeloid cells, B and T lymphoid cells, (Brasel et al. 1995) and bone marrow fibroblasts (Lisovsky et al. 1996a). FLT3L on its own is not efficient at inducing hematopoietic progenitor cell proliferation (Lyman 1995; Lyman et al. 1994a; Ebihara et al. 1997; Ray et al. 1996). However, in synergy with other growth factors, it can act as a potent stimulus for CD34+ progenitor proliferation in vitro and in vivo(Rasko et al. 1995; Rusten et al. 1996; McKenna et al. 1995; McKenna 2001; Maraskovsky et al. 2000; Broxmeyer et al. 1995; Hunte et al. 1996).
2.3 FLT3 Receptor
Unlike the c-kit receptor which is ubiquitously distributed and found in high concentration on cells of both normal and malignant tissue, the FLT3 receptor has a more restricted distribution and expression pattern. (Matthews et al. 1991; Rosnet et al. 1991; Hannum et al. 1994; Birg et al. 1992; DaSilva et al. 1994) FLT3 messenger RNA (mRNA) is found in early hematopoietic cells and in human myeloid leukemia blasts. Turner et al. first characterized FLT3 receptors on normal and leukemic human marrow cells in terms of receptor density, binding affinity, dimerization, and ligand internalization (Turner et al. 1996). In comparison to c-kit, the cells that expressed FLT3 receptors expressed relatively lower number of receptors on the cell surface. The highest expression was found in human myeloid cell lines. This is consistent with prior studies that demonstrated expression of FLT3 receptor mRNA predominantly on cells of myeloid/monocytic and pre-B cell lineage (Brasel et al. 1995; Meierhoff et al. 1995). Human erythroleukemia cells, mast cells, and megakaryocytes which strongly express c-kit receptors (Turner et al. 1995) have little to no FLT3 receptor surface expression (Ratajczak et al. 1996; Lyman et al. 1994a).
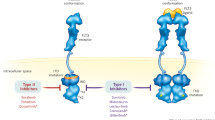
FLT3 receptors are usually found as monomers on the plasma membrane in an inactive state. Like other members of the class of proteins (Li and Stanley 1991; Blume-Jensen et al. 1991), ligand binding results in dimerization of the membrane-bound FLT3 and exposure of the TKD phosphoryl acceptor sites (Turner et al. 1996). Dimerization further stabilizes the conformational change of membrane-bound FLT3, resulting in enhanced activation of the receptor (Weiss and Schlessinger 1998). After dimerization, FLT3 dimer-phosphate complex is internalized and either degraded or recycled, whereas the FLT3L is usually degraded. Internalization usually begins at 5 min and reaches a peak at 15 min. Degradation products can be detected as early as 20 min post stimulation (Turner et al. 1996). Thus, similar to other RTKs, the entire process of activation, internalization, and degradation of FLT3 occurs within a matter of minutes.
3 FLT3 Signal Transduction
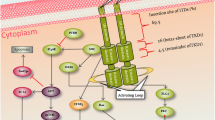
Binding of FLT3L to FLT3 receptor results in autophosphorylation of the receptor and sets off a chain of events resulting in downstream signaling of pathways that promote cell proliferation and inhibit apoptosis. These include the mitogen-activated protein kinase/extracellular signal-related kinase (MAPK/ERK), Pi3K/AKT, signal transducer activator of transcription (STAT5), and BCL2-associated X protein (BAX) (Srinivasa and Doshi 2002; Zhang and Broxmeyer 1999,2000; Zhang et al. 1999, 2000; Lisovsky et al. 1996b). Initial studies on FLT3 signal transduction were done prior to cloning of the FLT3L . These studies used a chimeric receptor containing the extracellular binding domains of human FLT3 with the intracellular catalytic domains of murine FLT3 (Dosil et al. 1993; Rottapel et al. 1994). The chimeric receptor was able to undergo independent activation in the absence of other growth factors. This activation resulted in direct association of the chimeric receptor with Src-homology 2 (SH2) domain of the p85 subunit of phosphatidylinositol 3-kinase (Pi3K), growth factor receptor-bound protein 2 (Grb2), and SH2-domain-containing inositol phosphatase (SHIP) (Marchetto et al. 1999). These associations led to phosphorylation of downstream effectors involved in the Ras-Raf-Mek-Erk pathway (Zhang et al. 1999) and other modulators of hematopoiesis such as Vav (Bustelo et al. 1992).
Cloning of the murine and human FLT3L has greatly enhanced our understanding of the signal transduction process (Lyman et al. 1993a, 1994b). Unlike chimeric FLT3 receptors, the wild-type (WT) human FLT3 did not directly associate with the p85 subunit of PI3K (Zhang and Broxmeyer 1999). Instead, it associates with numerous proteins including GRB2, GAB2, SHIP, SHP2, CBL, and CBLB (CBL-related protein) that subsequently bind to and activate p85 (Zhang and Broxmeyer 1999; Zhang et al. 1999). These proteins in association with FLT3 can stimulate the Ras-Raf-Mek-Erk pathway. Further research supports the hypothesis that the association between FLT3 and RAS pathways may play a crucial role in leukemogenesis (Lisovsky et al. 1996b; Nakagawa et al. 1992). The activated FLT3L–receptor complex can also activate STAT 5a, but not STAT 1 through 4 or STAT 5B. FLT3-mediated STAT activation occurs independent of Jannus Kinase (JAK) (Zhang et al. 2000). Thus, FLT3 plays a crucial role in differentiation, proliferation, and apoptosis of hematopoietic cells.
3.1 FLT3 in Immune Function
FLT3 seems to play an important role in immune regulation. Disruption of FLT3 gene in mice results in significant reduction in the number of dendritic cells, natural killer (NK) cells, and myeloid and B-lymphoid progenitors with resultant impairment of the immune system (McKenna et al. 2000). Conversely, FLT3L stimulation promotes dendritic-cell development resulting in the clonal expansion of dendritic cells in the bone marrow, lymph nodes, and peripheral blood (Morse et al. 2000; Manfra et al. 2003). FLT3 in synergy with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) can induce dendritic cell differentiation. Similarly, FLT3 can stimulate IL-2 resulting in proliferation of an early CD34+ NK-cell progenitor (Maraskovsky et al. 1996; Yu et al. 1998). Attempts to harness the dendritic and NK cell proliferative activity of FLT3L for antineoplastic approach have met with mixed success. In murine models, FLT3L has been used successfully to induce tumor shrinkage in murine fibrosarcoma, lymphoma, melanoma, and breast cancer models (Lynch et al. 1997; Esche et al. 1998; Chen et al. 1997). Human studies have yielded mixed results. In a phase 1 human study, 11 patients with cancer (10 breast and 1 ovarian) were vaccinated with HER2/NEU peptide followed by infusion of FLT3L alone or FLT3L in combination with GM-CSF. No significant antitumor effect was noted; however, some patients developed autoimmune manifestations possibly due to FLT3L-mediated expansion of interferon-γ T-cells (Disis et al. 2002).
3.2 FLT3 in Normal Hematopoiesis
Activated FLT3, both independently and in concert with other growth factors, plays a significant role in the proliferation and differentiation of primitive hematopoietic cells (Ray et al. 1996; Rusten et al. 1996; Veiby et al. 1996). Surprisingly, targeted disruption of FLT3 in mice resulted in healthy adult mice with relatively normal appearing hematopoietic populations (Mackarehtschian et al. 1995). However, further investigations confirmed deficiencies in pro-B cell and pre-B cell compartments. Also, transplantation experiments have shown that FLT3 nonfunctioning stem cells are unable to reconstitute myeloid and T-lymphoid cell lineages (Mackarehtschian et al. 1995). Similarly, mice with both FLT3 and C-KIT knocked out develop severe hematopoietic deficiencies (Mackarehtschian et al. 1995).
In normal human bone marrow, FLT3 is expressed predominantly on early myeloid and B-lymphoid precursors and fibroblasts (Brasel et al. 1995; Turner et al. 1996). Expression is restricted to early precursors, especially those with high CD34 expression (Rosnet et al. 1996; Rasko et al. 1995). FLT3 can promote differentiation of early hematopoietic precursors in the absence of other growth factors as shown by Gabbiani et al. (Gabbianelli et al. 1995; Rusten et al. 1996). In their experiments, FLT3 stimulation led to monocytic differentiation of hematopoietic receptors with muted proliferative response. However, FLT3-induced differentiation and proliferation of granulocyte-monocyte lineage is greatly enhanced in the presence of co-stimulatory growth factors such as IL-3, KIT-ligand, GM-CSF, G-CSF, and erythropoietin (EPO) (Gabbianelli et al. 1995; Rusten et al. 1996; Shah et al. 1996). Similarly, activated FLT3 in combination with IL-7 stimulates stromal-cell-independent expansion of human fetal pro-B cells and promotes differentiation of pro-B cells to pre-B cells (Namikawa et al. 1996). Furthermore, activated FLT3 in the presence of IL-3, IL-6, and IL-7 stimulates the growth and differentiation of early thymic progenitors of the T-cell lineage (Moore and Zlotnik 1997). As mentioned previously, FLT3 receptors are poorly expressed on erythroid and megakaryocytic precursors and FLT3 stimulation does not play a significant role in the proliferation or differentiation of these lineages (Gabbianelli et al. 1995).
3.3 FLT3 in Human Leukemia
Murine FLT3 was cloned by Matthew et al. in 1991 (Matthews et al. 1991). This was followed closely by analysis and description of FLT3 expression in human leukemias by Berg et al. in 1992 (Birg et al. 1992). They performed northern blot analysis on RNA obtained from patients with AML, ALL, biphenotypic leukemia, and CML. Forty-four AML samples were analyzed to determine FLT3 expression. FLT3 gene was expressed in 41 of 44 samples (93 %). This included eight of eight French–American–British (FAB) M1 cases, five of seven M2 cases, four of four M3 cases, nine of nine M4 cases, nine of ten M5 cases, four, two of two of the M7 subtype, and four of four of M0 (undifferentiated forms of AML of M1 subtype). Eight of eight B-ALL and four of six T-ALL samples also expressed FLT3. FLT3 was also expressed in all of eight biphenotypic (hybrid) leukemia samples. Interestingly, FLT3 transcript was detected in 0 of 11 chronic phase CML samples. In contrast, two of seven accelerated CML and twelve of sixteen blast-phase CML expressed FLT3 transcript (Birg et al. 1992).
Further studies have confirmed that FLT3 is expressed in 70–100 % of cases of AML and B-ALL (Rosnet et al. 1996; Carow et al. 1996; Meierhoff et al. 1995; Birg et al. 1994; Stacchini et al. 1996). CLL cells and a majority of chronic phase CML phase cells do not express FLT3. Similarly, FLT3 expression is uncommon in T-ALL (Uckun et al. 1997).
3.4 FLT3 Mutations in Acute Myeloid Leukemia
As previously mentioned, high levels of FLT3 receptor expression are seen in 70–100 % of cases of AML (Nakao et al. 1996) . Nakao et al. first described FLT3-ITD in AML in 1996. (Nakao et al. 1996) They observed FLT3 expression in 22 of 30 (73 %) AML and 39 of 50 (78 %) ALL samples. Five patients with AML showed unexpectedly longer transcripts on primer amplification of the TM through JM domain. On genomic amplification, these patients with abnormal FLT3 transcripts also expressed abnormal longer polymerase chain reaction (PCR) products. Sequence analysis of these abnormal reverse transcriptase-polymerase chain reaction (RT-PCR) products revealed tandem duplication of partial sequences. They concluded that ITD of the FLT3 gene is a somatic change detected preferentially in AML and may play a significant role in the pathology of AML. Subsequently, other groups of investigators have confirmed these findings (Xu et al. 1999; Horiike et al. 1997; Yokota et al. 1997; Kiyoi et al. 1997; Stirewalt et al. 2001; Meshinchi et al. 2001; Schnittger et al. 2002; Thiede et al. 2002; Iwai et al. 1997, 1999).
FLT3-ITD mutations are found in 15–35 % of patients with AML (Xu et al. 1999; Horiike et al. 1997; Yokota et al. 1997; Kiyoi et al. 1997; Stirewalt et al. 2001; Meshinchi et al. 2001; Schnittger et al. 2002; Thiede et al. 2002; Iwai et al. 1997, 1999; Kottaridis et al. 2001). Pooled data from > 5000 newly diagnosed AML patients show an overall FLT3-ITD incidence of 23 % (Levis and Small 2003). FLT3-ITD mutations have been identified in MDS and ALL, albeit at a much lower frequency (Xu et al. 1999; Horiike et al. 1997; Yokota et al. 1997; Fenaux 2001). FLT3-ITD mutations most frequently occur in exons 14 and 15 (previously known as exons 11 and 12). The FLT3-ITD results from duplication of a fragment of the JM domain coding sequence and its insertion in a direct head-to-tail orientation. The length of the ITD can vary from 3 to > 400 base pairs, but the reading frame is always preserved (Schnittger et al. 2002) .
The second most common type of FLT3 mutation is the TKD mutation, seen in 7–10 % of patients with AML (Thiede et al. 2002). This results from a missense point mutation in exon 20 (previously known as exon 17) of the TKD (Thiede et al. 2002; Spiekermann et al. 2002). These include single base substitutions, small deletions, or insertions within the activation loop of the second TKD (Thiede et al. 2002; Yamamoto et al. 2001; Abu-Duhier et al. 2001). These mutations are also called “activation loop mutations” as they cause the tyrosine kinase loop to adopt an activated configuration allowing access to the kinase. Activating mutations have been identified at amino acid position Asp816 of KIT and were subsequently identified at corresponding locations in other RTKs, including RET and MET. In FLT3, the corresponding aspartate residue is located at position 835. The most common nucleotide substitution involves substitution of aspartic acid 835 with tyrosine (D835Y). Other less frequent substitutions included Asp835Val, Asp835His, Asp835Glu, and Asp835Asn (Yamamoto et al. 2001). FLT3-TKD mutations have been identified in MDS (3 %) and ALL (3 %) (Yamamoto et al. 2001; Griffin 2001). Usually, the FLT3-ITD and FLT3-TKD mutations occur independently, although combined expression has been identified on rare occasions (Thiede et al. 2002; Meshinchi et al. 2003).
More recently, an additional class of FLT3 mutations has been identified in AML patients in which isoleucine 836 is either deleted (FLT3-Ile836del) or substituted with methionine and arginine (FLT3-Ile836Met + Arg) (Thiede et al. 2002).
Thus, approximately 25–40 % of the patients with AML will harbor FLT3 mutations (ITD or TKD), making FLT3 mutation one of the most frequent recurring molecular aberrations in this disease .
4 Incidence and Demographics of FLT3-ITD Mutations
FLT3-ITD mutations are clinically relevant in AML. The frequency of FLT3 mutations in pediatric AML is 10–15 % (Xu et al. 1999; Meshinchi et al. 2001; Iwai et al. 1999; Kondo et al. 1999), suggesting that these mutations occur more frequently in adults. The frequency seems to increase with age with a higher incidence of FLT3-ITD mutations noted in elderly populations (Stirewalt et al. 2001). FLT3 mutations are found in all FAB subtypes; however, they are found with increased frequency in FAB M5a and M5b and are relatively uncommon in M2 and M6 subtypes (Thiede et al. 2002). FLT3-ITD mutations are more frequent in patients with normal cytogenetics, and those with t(15:17) and t(6:9) (Thiede et al. 2002; Slovak et al. 2000). Thiede et al. reported that FLT3 mutations were three times more frequent in patients with normal karyotype. They noted that FLT3-ITD mutations were significantly more frequent in patients with t(15:17) and occurred in nearly 30 % of the patients harboring this translocation (Thiede et al. 2002). Similarly, other investigators have reported FLT3-ITD incidences of 20.3, 28.6, and 36 % in patients with t(15:17) (Kiyoi et al. 1997; Kottaridis et al. 2001; Schnittger et al. 2000). However, patients with core-binding factor leukemias (i.e., t(8:21) and inv(16) mutations) have a low incidence of FLT3-ITD mutations (Kottaridis et al. 2001). As mentioned previously, FLT3 mutations are occasionally identified in MDS and ALL (Xu et al. 1999; Horiike et al. 1997; Yokota et al. 1997; Fenaux 2001). As regards other hematopoietic malignancies, FLT3 mutations have been detected in a very small number of cases of CML, adult T-cell ALL, CLL, lymphoma, and multiple myeloma (Xu et al. 1999; Horiike et al. 1997; Iwai et al. 1997). FLT3 mutations have not been identified in solid tumors (Baldwin et al. 2001) or in normal hematopoietic cells (Ishii et al. 1999).
5 Biological Activity of FLT3-ITD Mutations in AML
FLT3-ITD mutations result in ligand-independent dimerization and autophosphorylation resulting in constitutive tyrosine kinase activation and activation of signaling pathways downstream of FLT3 (Hayakawa et al. 2000; Mizuki et al. 2000; Kiyoi et al. 2002). Although the length of the duplicated sequence may vary in humans, it is always in frame and results in elongation of the JM domain. Investigators have shown that FLT3-ITD-transfected murine IL-3-dependent cell lines, such as Ba/F3 and 32D, are able to proliferate independent of IL-3 when inoculated in syngeneic mice (Hayakawa et al. 2000; Zhao et al. 2002) .
The exact mechanisms by which FLT3-ITD mutations promote constitutive activation remain unknown. It is known that binding of FLT3-ligand to extracellular domain of FLT3 results in receptor dimerization and juxtaposition of intracellular cytoplasmic domains. This results in phosphorylation of tyrosine kinase residues on the JM domain and activation of downstream signal-transduction molecules such as SHC, MAP kinase, and STAT5a. In the absence of FLT3-ligand, the kinase activity is suppressed by tyrosine phosphatases that maintain the tyrosine residues in a dephosphorylated state. Investigators have recently elucidated the crystal structure of the auto-inhibited, dephosphorylated form of FLT3 (Griffith et al. 2004) and shown that, in normal resting state, the JM domain inhibits the autophosphorylation of WT FLT3 in absence of FLT3-ligand. However, in FLT3-ITD-mutated samples, dimerization can occur without the addition of ligand and can activate WT FLT3 (Kiyoi et al. 2002). Additional experiments support this hypothesis by showing that tyrosine residues within the JM domain were not essential for the signal transduction in the length-mutated FLT3 (Kiyoi et al. 2002).
FLT3-TKD mutations also result in constitutive activation of the FLT3 receptor through stabilization of the activation loop in its open binding configuration. Interestingly, in mouse transplant models, FLT3-ITD or FLT3-TKD mutations alone are not sufficient to cause overt AML. In fact, FLT3-ITD mutations alone induce a myeloproliferative (Kelly et al. 2002) state and FLT3-TKD mutations alone induce an oligoclonal lymphoproliferative state (Grundler et al. 2005). Thus, the exact mechanism by which loss of repression and constitutive activation of the FLT3 tyrosine kinase domains ultimately result in leukemogenesis remains poorly understood .
6 Clinical Relevance and Prognostic and Predictive Implications of FLT3-ITD Mutations in AML
Initial studies reported conflicting data regarding the prognostic and predictive values of FLT3-ITD mutations in AML. An initial study from Japan suggested that FLT3 mutations did not influence the complete remission (CR) rate, but adversely predicted for overall survival (OS) (Kiyoi et al. 1999). A Dutch study found that FLT3 mutations were associated with both lower CR rate and increased relapse rate (Rombouts et al. 2000). Subsequently, two large studies examined the clinical impact of FLT3-ITD mutations in a well-defined population. The first of these, by Kottaridis et al., included 854 patients, mostly 60 years of age or younger, treated in the United Kingdom Medical Research Council (UKMRC) in 10 and 12 trials (Kottaridis et al. 2001). Patients with FLT3-ITD had significantly elevated peripheral blood white cell counts and increased bone marrow blasts at diagnosis. Furthermore, they had a significantly higher induction death rate, increased relapse risk, inferior event-free survival (EFS), and decreased OS. There was a borderline association with a lower complete remission rate (P = 0.05). Thiede et al. analyzed the clinical and prognostic significance of FLT3-ITD and FLT3-TKD mutations in 979 patients treated according to the AML-96 multicenter protocol of the German Suddeutsche Hamoblastose group (SHG) (Thiede et al. 2002). Again, both ITD and TKD mutations were associated with elevated leukocyte count and bone marrow blasts. Presence of FLT3 mutations had no association with remission rates. Both ITD and TKD mutations were associated with significantly increased risk for relapse. FLT3-ITD mutations had significant association for disease-free survival (DFS) but not for OS, whereas the converse was true for TKD mutations. They also analyzed the effect of FLT3 mutant to WT ratio on outcome. Patients below the age of 60 years with diploid cytogenetics had significantly shorter OS and DFS if their FLT3 ratios were above the preselected threshold median value of 0.78. They had a relative risk of relapse of 1.6. On further sub-classifying patients on the basis of mutant to WT ratio, it was noted that patients with the highest ratio had the worst outcome with a highly significant shorter DFS and OS. Thus, in addition to the analysis for presence or absence of mutation, quantification of the mutant to WT FLT3 alleles may provide additional prognostic information. Whitman et al. noted that patients with FLT3-ITD mutation with concomitant FLT3 allelic loss had a worse prognosis than patients with WT FLT3 or FLT3 mutation without allelic loss (Whitman et al. 2001). In addition to quantification of mutant to WT burden, the presence of single versus multiple FLT3-ITD mutation variants may be of prognostic importance. Borthakur et al. noted that among patients with AML with diploid karyotype, CR duration was significantly longer in patients who had multiple FLT3-ITD mutations (P = 0.03; Borthakur et al. 2012). However, OS and EFS did not seem to be affected by the number of FLT3-ITD mutation variants .
Although FLT3-ITD mutations are associated with worse outcomes in AML in general, the precise prognostic impact of these mutations in different cytogenetic subclasses of AML remains poorly understood. In a study by Santos et al., 481 AML patients were divided into three cytogenetic subgroups: good risk/core-binding factor AML, normal karyotype AML and poor risk/unfavorable cytogenetics (Santos et al. 2011). The presence of FLT3-ITD mutation did not affect the EFS in patients with core-binding factor AML or poor risk cytogenetics. However, EFS was significantly worse for patients with normal karyotype AML who had the FLT3-ITD mutation (P < 0.001). Thus, the presence of FLT3-ITD mutations is a significant prognostic indicator in patients with AML and diploid karyotype, but not in those with core-binding factor AML or AML with unfavorable cytogenetics.
Recent data suggest that the presence of FLT3-ITD mutation may be associated with negative outcomes, not only at diagnosis but also at first relapse. Patients with FLT3-ITD mutations at relapse have a reduced likelihood of achieving second CR and a shortened survival (Ravandi et al. 2010). The feasibility of FLT3-ITD mutations at CR as a marker for minimal residual disease (MRD) has also been investigated. Nazha et al. noted that FLT3-ITD mutations were unstable and lacked consistency at follow-up (Nazha et al. 2012). Furthermore, FLT3 mutations could occur for the first time at relapse. They concluded that FLT3-ITD is not a reliable marker for MRD monitoring in AML. Prognostic impact of FLT3 mutations in the presence of other concomitant mutations also remains poorly understood. Among these, the best studied is the concurrent occurrence of FLT3-ITD and NPM1 mutations. Presence of FLT3-ITD in patients with NPM1 mutations abrogates the positive effects on CR rate and OS associated with mutated NPM1 (Schnittger et al. 2005; Gale et al. 2008).
FLT3-ITD occurs more frequently in older patients with AML but does not adversely affect outcomes. In a large study by Stirewelt et al. FLT3 mutations were identified in 34 % of evaluable elderly patients with AML (Stirewalt et al. 2001). The FLT3-ITD mutation was associated with higher absolute white cell counts, higher peripheral blast percentages, normal cytogenetics, and less disease resistance. FLT3 mutations were not associated with inferior clinical outcomes. It is possible that FLT3 mutations may not be as significant a prognostic marker in the elderly AML population as in the younger patients due to the already poor outcome of the former.
The high frequency and negative impact of FLT3 mutations, particularly the FLT3-ITD mutation, make it an ideal candidate for targeted therapy in AML patients. Several small molecule inhibitors have been developed and are currently in clinical trials. A full discussion of the mechanism of actions, efficacy, and side effects of a number of these drugs are included elsewhere in this publication. There is hope that such targeted therapies will improve responses without increasing toxicity in the treatment of patients with FLT3-mutated AML .
References
Abu-Duhier FM, Goodeve AC, Wilson GA et al (2001) Identification of novel FLT-3 Asp835 mutations in adult acute myeloid leukaemia. Br J Haematol 113:983–988
Baldwin BR, Zheng R, Small D (2001) FLT3 is not frequently mutated in solid tumors. Blood 98:156b–157b
Birg F, Courcoul M, Rosnet O et al (1992) Expression of the FMS/KIT-like gene FLT3 in human acute leukemias of the myeloid and lymphoid lineages. Blood 80:2584–2593
Birg F, Rosnet O, Carbuccia N et al (1994) The expression of FMS, KIT and FLT3 in hematopoietic malignancies. Leuk Lymphoma 13:223–227
Blume-Jensen P, Claesson-Welsh L, Siegbahn A et al (1991) Activation of the human c-kit product by ligand-induced dimerization mediates circular actin reorganization and chemotaxis. EMBO J 10:4121–4128
Borthakur G, Kantarjian H, Patel KP et al (2012) Impact of numerical variation in FMS-like tyrosine kinase receptor 3 internal tandem duplications on clinical outcome in normal karyotype acute myelogenous leukemia. Cancer 118:5819–5822. (Epub ahead of print, May 17, 2012)
Brasel K, Escobar S, Anderberg R et al (1995) Expression of the flt3 receptor and its ligand on hematopoietic cells. Leukemia 9:1212–1218
Broxmeyer HE, Lu L, Cooper S et al (1995) Flt3 ligand stimulates/costimulates the growth of myeloid stem/progenitor cells. Exp Hematol 23:1121–1129
Bustelo XR, Ledbetter JA, Barbacid M (1992) Product of vav proto-oncogene defines a new class of tyrosine protein kinase substrates. Nature 356:68–71
Carow CE, Levenstein M, Kaufmann SH et al (1996) Expression of the hematopoietic growth factor receptor FLT3 (STK-1/Flk2) in human leukemias. Blood 87:1089–1096
Chabot B, Stephenson DA, Chapman VM et al (1988) The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature 335:88–89
Chen K, Braun S, Lyman S et al (1997) Antitumor activity and immunotherapeutic properties of Flt3-ligand in a murine breast cancer model. Cancer Res 57:3511–3516
Coussens L, Van Beveren C, Smith D et al (1986) Structural alteration of viral homologue of receptor proto-oncogene fms at carboxyl terminus. Nature 320:277–280
DaSilva N, Hu ZB, Ma W et al (1994) Expression of the FLT3 gene in human leukemia-lymphoma cell lines. Leukemia 8:885–888
Disis ML, Rinn K, Knutson KL et al (2002) Flt3 ligand as a vaccine adjuvant in association with HER-2/neu peptide-based vaccines in patients with HER-2/neu-overexpressing cancers. Blood 99:2845–2850
Dosil M, Wang S, Lemischka IR (1993) Mitogenic signalling and substrate specificity of the Flk2/Flt3 receptor tyrosine kinase in fibroblasts and interleukin 3-dependent hematopoietic cells. Mol Cell Biol 13:6572–6585
Drexler HG (1996) Expression of FLT3 receptor and response to FLT3 ligand by leukemic cells. Leukemia 10:588–599
Ebihara Y, Tsuji K, Lyman SD et al (1997) Synergistic action of Flt3 and gp130 signalings in human hematopoiesis. Blood 90:4363–4368
Esche C, Subbotin VM, Maliszewski C et al (1998) FLT3 ligand administration inhibits tumor growth in murine melanoma and lymphoma. Cancer Res 58:380–383
Fenaux P (2001) Chromosome and molecular abnormalities in myelodysplastic syndromes. Int J Hematol 73:429–437
Gabbianelli M, Pelosi E, Montesoro E et al (1995) Multi-level effects of flt3 ligand on human hematopoiesis: expansion of putative stem cells and proliferation of granulomonocytic progenitors/monocytic precursors. Blood 86:1661–1670
Gale RE, Green C, Allen C et al (2008) The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood 111:2776–2784
Geissler EN, Ryan MA, Housman DE (1988) The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell 55:185–192
Griffin JD (2001) Point mutations in the FLT3 gene in AML. Blood 97:2193A–2193
Griffith J, Black J, Faerman C et al (2004) The structural basis for autoinhibition of FLT3 by the juxtamembrane domain. Mol Cell 13:169–178
Grundler R, Miething C, Thiede C et al (2005) FLT3-ITD and tyrosine kinase domain mutants induce 2 distinct phenotypes in a murine bone marrow transplantation model. Blood 105:4792–4799
Hannum C, Culpepper J, Campbell D et al (1994) Ligand for FLT3/FLK2 receptor tyrosine kinase regulates growth of haematopoietic stem cells and is encoded by variant RNAs. Nature 368:643–648
Hayakawa F, Towatari M, Kiyoi H et al (2000) Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene 19:624–631
Hirayama F, Lyman SD, Clark SC et al (1995) The flt3 ligand supports proliferation of lymphohematopoietic progenitors and early B-lymphoid progenitors. Blood 85:1762–1768
Horiike S, Yokota S, Nakao M et al (1997) Tandem duplications of the FLT3 receptor gene are associated with leukemic transformation of myelodysplasia. Leukemia 11:1442–1446
Hudak S, Hunte B, Culpepper J et al (1995) FLT3/FLK2 ligand promotes the growth of murine stem cells and the expansion of colony-forming cells and spleen colony-forming units. Blood 85:2747–2755
Hunte BE, Hudak S, Campbell D et al (1996) flk2/flt3 ligand is a potent cofactor for the growth of primitive B cell progenitors. J Immunol 156:489–496
Ishii E, Zaitsu M, Ihara K et al (1999) High expression but no internal tandem duplication of FLT3 in normal hematopoietic cells. Pediatr Hematol Oncol 16:437–441
Iwai T, Yokota S, Nakao M et al (1997) Internal tandem duplication in the juxtatransmembrane domain of the flt3 is not involved in blastic crisis of chronic myeloid leukemia. Leukemia 11:1992–1993
Iwai T, Yokota S, Nakao M et al (1999) Internal tandem duplication of the FLT3 gene and clinical evaluation in childhood acute myeloid leukemia. The Children’s Cancer and Leukemia Study Group, Japan. Leukemia 13:38–43
Kelly LM, Liu Q, Kutok JL et al (2002) FLT3 internal tandem duplication mutations associated with human acute myeloid leukemias induce myeloproliferative disease in a murine bone marrow transplant model. Blood 99:310–318
Kiyoi H, Naoe T, Yokota S et al (1997) Internal tandem duplication of FLT3 associated with leukocytosis in acute promyelocytic leukemia. Leukemia Study Group of the Ministry of Health and Welfare (Kohseisho). Leukemia 11:1447–1452
Kiyoi H, Naoe T, Nakano Y et al (1999) Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood 93:3074–3080
Kiyoi H, Ohno R, Ueda R et al (2002) Mechanism of constitutive activation of FLT3 with internal tandem duplication in the juxtamembrane domain. Oncogene 21:2555–2563
Kondo M, Horibe K, Takahashi Y et al (1999) Prognostic value of internal tandem duplication of the FLT3 gene in childhood acute myelogenous leukemia. Med Pediatr Oncol 33:525–529
Kottaridis PD, Gale RE, Frew ME et al (2001) The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood 98:1752–1759
deLapeyriere O, Naquet P, Planche J et al (1995) Expression of Flt3 tyrosine kinase receptor gene in mouse hematopoietic and nervous tissues. Differentiation 58:351–359
Levis M, Small D (2003) FLT3: ITDoes matter in leukemia. Leukemia 17:1738–1752
Li W, Stanley ER: (1991) Role of dimerization and modification of the CSF-1 receptor in its activation and internalization during the CSF-1 response. EMBO J 10:277–288
Lisovsky M, Braun SE, Ge Y et al (1996a) Flt3-ligand production by human bone marrow stromal cells. Leukemia 10:1012–1018
Lisovsky M, Estrov Z, Zhang X et al (1996b) Flt3 ligand stimulates proliferation and inhibits apoptosis of acute myeloid leukemia cells: regulation of Bcl-2 and Bax. Blood 88:3987–3997
Lyman SD (1995) Biology of flt3 ligand and receptor. Int J Hematol 62:63–73
Lyman SD, Jacobsen SE (1998) c-kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood 91:1101–1134
Lyman SD, James L, Vanden Bos T et al (1993a) Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell 75:1157–1167
Lyman SD, James L, Zappone J et al (1993b) Characterization of the protein encoded by the flt3 (flk2) receptor-like tyrosine kinase gene. Oncogene 8:815–822
Lyman SD, Brasel K, Rousseau AM et al (1994a) The flt3 ligand: a hematopoietic stem cell factor whose activities are distinct from steel factor. Stem Cells 12 Suppl 1:99–107 (discussion 108–10, 1994)
Lyman SD, James L, Johnson L et al (1994b) Cloning of the human homologue of the murine flt3 ligand: a growth factor for early hematopoietic progenitor cells. Blood 83:2795–2801
Lyman SD, Stocking K, Davison B et al (1995) Structural analysis of human and murine flt3 ligand genomic loci. Oncogene 11:1165–1172
Lynch DH, Andreasen A, Maraskovsky E et al (1997) Flt3 ligand induces tumor regression and antitumor immune responses in vivo. Nat Med 3:625–31
Mackarehtschian K, Hardin JD, Moore KA et al (1995) Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity 3:147–161
Manfra DJ, Chen SC, Jensen KK et al (2003) Conditional expression of murine Flt3 ligand leads to expansion of multiple dendritic cell subsets in peripheral blood and tissues of transgenic mice. J Immunol 170:2843–2852
Maraskovsky E, Brasel K, Teepe M et al (1996) Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med 184:1953–1962
Maraskovsky E, Daro E, Roux E et al (2000) In vivo generation of human dendritic cell subsets by Flt3 ligand. Blood 96:878–884
Marchetto S, Fournier E, Beslu N et al (1999) SHC and SHIP phosphorylation and interaction in response to activation of the FLT3 receptor. Leukemia 13:1374–1382
Maroc N, Rottapel R, Rosnet O et al (1993) Biochemical characterization and analysis of the transforming potential of the FLT3/FLK2 receptor tyrosine kinase. Oncogene 8:909–918
Matsui T, Heidaran M, Miki T et al (1989) Isolation of a novel receptor cDNA establishes the existence of two PDGF receptor genes. Science 243:800–804
Matthews W, Jordan CT, Wiegand GW et al (1991) A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell-enriched populations. Cell 65:1143–1152
McKenna HJ (2001) Role of hematopoietic growth factors/flt3 ligand in expansion and regulation of dendritic cells. Curr Opin Hematol 8:149–154
McKenna HJ, de Vries P, Brasel K et al (1995) Effect of flt3 ligand on the ex vivo expansion of human CD34+ hematopoietic progenitor cells. Blood 86:3413–3420
McKenna HJ, Stocking KL, Miller RE et al (2000) Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood 95:3489–3497
Meierhoff G, Dehmel U, Gruss HJ et al (1995) Expression of FLT3 receptor and FLT3-ligand in human leukemia-lymphoma cell lines. Leukemia 9:1368–1372
Meshinchi S, Woods WG, Stirewalt DL et al (2001) Prevalence and prognostic significance of Flt3 internal tandem duplication in pediatric acute myeloid leukemia. Blood 97:89–94
Meshinchi S, Stirewalt DL, Alonzo TA et al (2003) Activating mutations of RTK/ras signal transduction pathway in pediatric acute myeloid leukemia. Blood 102:1474–1479
Mizuki M, Fenski R, Halfter H et al (2000) Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood 96:3907–3914
Moore TA, Zlotnik A (1997) Differential effects of Flk-2/Flt-3 ligand and stem cell factor on murine thymic progenitor cells. J Immunol 158:4187–4192
Morse MA, Nair S, Fernandez-Casal M et al (2000) Preoperative mobilization of circulating dendritic cells by Flt3 ligand administration to patients with metastatic colon cancer. J Clin Oncol 18:3883–3893
Nakagawa T, Saitoh S, Imoto S et al (1992) Multiple point mutation of N-ras and K-ras oncogenes in myelodysplastic syndrome and acute myelogenous leukemia. Oncology 49:114–122
Nakao M, Yokota S, Iwai T et al (1996) Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia 10:1911–1918
Namikawa R, Muench MO, de Vries JE et al (1996) The FLK2/FLT3 ligand synergizes with interleukin-7 in promoting stromal-cell-independent expansion and differentiation of human fetal pro-B cells in vitro. Blood 87:1881–1890
Nazha A, Cortes J, Faderl S et al (2012) Activating mutations of the FMS-like tyrosine kinase-3 internaltandem duplication (FLT3-ITD) at complete response and relapsein patients with acute myeloid leukemia. Haematologica 97:1242–1245
Odenike O, Thirman MJ, Artz AS et al (2011) Gene mutations, epigenetic dysregulation, and personalized therapy in myeloid neoplasia: are we there yet? Semin Oncol 38:196–214
Piacibello W, Fubini L, Sanavio F et al (1995) Effects of human FLT3 ligand on myeloid leukemia cell growth: heterogeneity in response and synergy with other hematopoietic growth factors. Blood 86:4105–4114
Rasko JE, Metcalf D, Rossner MT et al (1995) The flt3/flk-2 ligand: receptor distribution and action on murine haemopoietic cell survival and proliferation. Leukemia 9:2058–2066
Ratajczak MZ, Ratajczak J, Ford J et al (1996) FLT3/FLK-2 (STK-1) Ligand does not stimulate human megakaryopoiesis in vitro. Stem Cells 14:146–150
Ravandi F, Kantarjian H, Faderl S et al (2010) Outcome of patients with FLT3-mutated acute myeloid leukemia in first relapse. Leuk Res 34:752–756
Ray RJ, Paige CJ, Furlonger C et al (1996) Flt3 ligand supports the differentiation of early B cell progenitors in the presence of interleukin-11 and interleukin-7. Eur J Immunol 26:1504–1510
Rombouts WJ, Blokland I, Lowenberg B et al (2000) Biological characteristics and prognosis of adult acute myeloid leukemia with internal tandem duplications in the Flt3 gene. Leukemia 14:675–683
Rosnet O, Marchetto S, deLapeyriere O et al (1991) Murine Flt3, a gene encoding a novel tyrosine kinase receptor of the PDGFR/CSF1R family. Oncogene 6:1641–1650
Rosnet O, Schiff C, Pebusque MJ et al (1993a) Human FLT3/FLK2 gene: cDNA cloning and expression in hematopoietic cells. Blood 82:1110–1119
Rosnet O, Stephenson D, Mattei MG et al (1993b) Close physical linkage of the FLT1 and FLT3 genes on chromosome 13 in man and chromosome 5 in mouse. Oncogene 8:173–179
Rosnet O, Buhring HJ, Marchetto S et al (1996) Human FLT3/FLK2 receptor tyrosine kinase is expressed at the surface of normal and malignant hematopoietic cells. Leukemia 10:238–248
Rothwell VM, Rohrschneider LR (1987) Murine c-fms cDNA: cloning, sequence analysis and retroviral expression. Oncogene Res 1:311–324
Rottapel R, Turck CW, Casteran N et al (1994) Substrate specificities and identification of a putative binding site for PI3K in the carboxy tail of the murine Flt3 receptor tyrosine kinase. Oncogene 9:1755–1765
Russell ES (1979) Hereditary anemias of the mouse: a review for geneticists. Adv Genet 20:357–459
Rusten LS, Lyman SD, Veiby OP et al (1996) The FLT3 ligand is a direct and potent stimulator of the growth of primitive and committed human CD34+ bone marrow progenitor cells in vitro. Blood 87:1317–1325
Santos FP, Jones D, Qiao W et al (2011) Prognostic value of FLT3 mutations among different cytogenetic subgroups in acute myeloid leukemia. Cancer 117:2145–2155
Schnittger S, Schoch C, Kern W et al (2000) FLT3 length mutations in AML: correlation to cytogenetics, FAB-subtype, and prognosis in 652 patients. Blood 96:826a–826a
Schnittger S, Schoch C, Dugas M et al (2002) Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood 100:59–66
Schnittger S, Schoch C, Kern W et al (2005) Nucleophosmin gene mutations are predictors of favorable prognosis in acute myelogenous leukemia with a normal karyotype. Blood 106:3733–3739
Shah AJ, Smogorzewska EM, Hannum C et al (1996) Flt3 ligand induces proliferation of quiescent human bone marrow CD34+CD38- cells and maintains progenitor cells in vitro. Blood 87:3563–3570
Sherr CJ (1990) Colony-stimulating factor-1 receptor. Blood 75:1–12
Slovak ML, Kopecky KJ, Cassileth PA et al (2000) Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 96:4075–4083
Small D, Levenstein M, Kim E et al (1994) STK-1, the human homolog of Flk-2/Flt-3, is selectively expressed in CD34+ human bone marrow cells and is involved in the proliferation of early progenitor/stem cells. Proc Natl Acad Sci U S A 91:459–463
Spiekermann K, Bagrintseva K, Schoch C et al (2002) A new and recurrent activating length mutation in exon 20 of the FLT3 gene in acute myeloid leukemia. Blood 100:3423–3425
Srinivasa SP, Doshi PD (2002) Extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways cooperate in mediating cytokine-induced proliferation of a leukemic cell line. Leukemia 16:244–253
Stacchini A, Fubini L, Severino A et al (1996) Expression of type III receptor tyrosine kinases FLT3 and KIT and responses to their ligands by acute myeloid leukemia blasts. Leukemia 10:1584–1591
Stirewalt DL, Radich JP (2003) The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer 3:650–665
Stirewalt DL, Kopecky KJ, Meshinchi S et al (2001) FLT3, RAS, and TP53 mutations in elderly patients with acute myeloid leukemia. Blood 97:3589–3595
Thiede C, Steudel C, Mohr B et al (2002) Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 99:4326–4335
Turner AM, Bennett LG, Lin NL et al (1995) Identification and characterization of a soluble c-kit receptor produced by human hematopoietic cell lines. Blood 85:2052–2058
Turner AM, Lin NL, Issarachai S et al (1996) FLT3 receptor expression on the surface of normal and malignant human hematopoietic cells. Blood 88:3383–3390
Uckun FM, Gaynon PS, Sensel MG et al (1997) Clinical features and treatment outcome of childhood T-lineage acute lymphoblastic leukemia according to the apparent maturational stage of T-lineage leukemic blasts: a Children’s Cancer Group study. J Clin Oncol 15:2214–2221
Ullrich A, Schlessinger J (1990) Signal transduction by receptors with tyrosine kinase activity. Cell 61:203–212
Veiby OP, Lyman SD, Jacobsen SE (1996) Combined signaling through interleukin-7 receptors and flt3 but not c-kit potently and selectively promotes B cell commitment and differentiation from uncommitted murine bone marrow progenitor cells. Blood 88:1256–1265
Wagner EF, Alexander WS (1991) Of kit and mouse and man. Curr Biol 1:356–358
Weiss A, Schlessinger J (1998) Switching signals on or off by receptor dimerization. Cell 94:277–280
Whitman SP, Archer KJ, Feng L et al (2001) Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res 61:7233–7239
Williams DE, de Vries P, Namen AE et al (1992) The Steel factor. Dev Biol 151:368–376
Woolford J, McAuliffe A, Rohrschneider LR (1988) Activation of the feline c-fms proto-oncogene: multiple alterations are required to generate a fully transformed phenotype. Cell 55:965–977
Xu F, Taki T, Yang HW et al (1999) Tandem duplication of the FLT3 gene is found in acute lymphoblastic leukaemia as well as acute myeloid leukaemia but not in myelodysplastic syndrome or juvenile chronic myelogenous leukaemia in children. Br J Haematol 105:155–162
Yamamoto Y, Kiyoi H, Nakano Y et al (2001) Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood 97:2434–2439
Yarden Y, Escobedo JA, Kuang WJ et al (1986) Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature 323:226–232
Yokota S, Kiyoi H, Nakao M et al (1997) Internal tandem duplication of the FLT3 gene is preferentially seen in acute myeloid leukemia and myelodysplastic syndrome among various hematological malignancies. A study on a large series of patients and cell lines. Leukemia 11:1605–1609
Yu H, Fehniger TA, Fuchshuber P et al (1998) Flt3 ligand promotes the generation of a distinct CD34(+) human natural killer cell progenitor that responds to interleukin-15. Blood 92:3647–3657
Zeigler FC, Bennett BD, Jordan CT et al (1994) Cellular and molecular characterization of the role of the flk-2/flt-3 receptor tyrosine kinase in hematopoietic stem cells. Blood 84:2422–2430
Zhang S, Broxmeyer HE (1999) p85 subunit of PI3 kinase does not bind to human Flt3 receptor, but associates with SHP2, SHIP, and a tyrosine-phosphorylated 100-kDa protein in Flt3 ligand-stimulated hematopoietic cells. Biochem Biophys Res Commun 254:440–445
Zhang S, Broxmeyer HE (2000) Flt3 ligand induces tyrosine phosphorylation of gab1 and gab2 and their association with shp-2, grb2, and PI3 kinase. Biochem Biophys Res Commun 277:195–199
Zhang S, Mantel C, Broxmeyer HE (1999) Flt3 signaling involves tyrosyl-phosphorylation of SHP-2 and SHIP and their association with Grb2 and Shc in Baf3/Flt3 cells. J Leukoc Biol 65:372–380
Zhang S, Fukuda S, Lee Y et al (2000) Essential role of signal transducer and activator of transcription (Stat)5a but not Stat5b for Flt3-dependent signaling. J Exp Med 192:719–728
Zhao M, Kiyoi H, Yamamoto Y et al (2002) In vivo treatment of mutant FLT3-transformed murine leukemia with a tyrosine kinase inhibitor. Leukemia 14:374–378
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag New York
About this chapter
Cite this chapter
Daver, N., Ravandi, F. (2015). FLT3 in AML. In: Andreeff, M. (eds) Targeted Therapy of Acute Myeloid Leukemia. Current Cancer Research. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1393-0_11
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1393-0_11
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1392-3
Online ISBN: 978-1-4939-1393-0
eBook Packages: MedicineMedicine (R0)