Abstract
Internal tandem duplications (ITD) and tyrosine-kinase domain (TKD) mutations of the FMS-like tyrosine-kinase 3 (FLT3) can be found in up to one third of patients with acute myeloid leukemia (AML) and confer a poor prognosis. First discovered 20 years ago, these mutations were identified as viable therapeutic targets, and FLT3 tyrosine-kinase inhibitors (TKIs) have been in development for the last decade with steadily increasing potency. However, FLT3-mutated AML often acquires resistance to the growing armamentarium of FLT3 inhibitors through a variety of mechanisms. In this review, we discuss the distinct clinical phenotype of FLT3-mutated AML, historically and currently available therapeutics, mechanisms of resistance, ongoing trials, and future outlook at treatment strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Essentially incurable half a century ago, durable remissions now occur in up to 40% of adult AML patients via optimization of cytotoxic chemotherapy regimens and supportive care [1•]. However, further permutations to cytotoxic chemotherapy regimens have not resulted in significantly improved outcomes [2]. Now, rather than being viewed (and treated) as a single entity, AML has been recognized as being composed of a diverse group of genetically disparate subtypes that require unique management strategies [3], with progress being made among specific subtypes (e.g., acute promyelocytic leukemia (APL)), but still with an unmet need in others.
In a model of mutational cooperativity in AML developed in 2002 [4], it was hypothesized that the development of AML requires two distinct classes of mutations working together to promote leukemogenesis. Class I mutations, in genes such as FLT3, N-RAS, and K-RAS, confer a proliferative and survival advantage to hematopoietic progenitors, but do not affect differentiation. Class II mutations, which include AML1/ETO, CBFβ/SMMHC, PML/RARα, and MLL-related fusion genes, impair differentiation but are also not sufficient alone to cause leukemia. Both of these mutations working in concert result in leukemogenesis. Recently, this model has been further refined using whole genome sequencing data of AML samples and healthy hematopoietic stem/progenitor cells, demonstrating that most mutations in AML are random events (passengers) prior to leukemogenesis, and that single driver mutations (PML-RARA or NMP1) confer a clonal survival advantage [5]. Mutations in NPM1, DNMT3A, and IDH1 or IDH2 are thought to be a part of a central AML pathway (drivers), while FLT3-ITD is the most frequently mutated gene that is thought to cooperate with these central events as a second driver and leads to developing of the founding leukemic clone detected at presentation [5]. While aberrant activation of a tyrosine kinase and/or growth factor signaling pathway may be a central feature in many AML cases, as postulated in the original model of cooperativity, whole genome sequencing studies seem to suggest that more than a third of AML cases lack such mutations [6•].
FLT3-Mutated AML
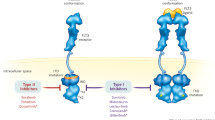
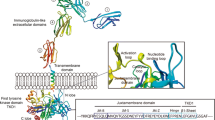
FLT3 is a member of the class III “split kinase domain” family of receptor tyrosine kinases (RTKs) which also includes PDGFR, KIT, and FMS [7]. FLT3 is composed of five extracellular domains, a transmembrane domain, a juxtamembrane domain (JM), and two tyrosine-kinase domains and is expressed on leukemic blasts in most cases of AML [8]. FLT3 binds to FLT3 ligand (FL) and then dimerizes, changing to an open conformation which allows ATP access to the binding pocket. The receptor then undergoes autophosphorylation and transduces cell growth and apoptotic inhibitory signals via Ras-GTPase activating protein, phospholipase C β, STAT5, and ERK1/2 [9].
The two most common types of FLT3 activating mutations are the so-called internal tandem duplication mutations (FLT3-ITD mutations) and point mutations within the tyrosine-kinase domain (FLT3-TKD mutations). The FLT3-ITD mutations were first discovered by Nakao et al. in 1996 [10]. FLT3 mutations are carried by up to 30% of patients with AML, most (23%) of which are FLT3-ITD, with the remainder (7%) composed of FLT3-TKD mutations primarily at residue D835 (along with some less common point mutations) [11,12,13]. Most FLT3-ITD mutations occur in or near the sequence for the JM domain of the receptor, while point mutations occur largely within the sequence for the activation loop of TKD1.
FLT3-ITD AML is a distinct clinical entity conferring a poor prognosis [14,15,16,17]. Patients with FLT3-ITD AML tend to present at a younger age on average (though overall it is less common in childhood AML) [18, 19], with higher white blood cell (WBC) counts and with normal karyotype. It is more likely to present de novo rather than as a consequence of some antecedent disorder such as MDS and to confer a significantly worse prognosis than what is seen for FLT3 wild-type patients [14,15,16,17, 20]. FLT3-TKD mutations appear to occur more frequently with high WBC, cytogenetically favorable risk, and primarily in de novo AML. FLT3-TKD mutations have been shown to be constitutively activating, though clinical outcomes are significantly better than in FLT3-ITD AML and actually trend towards superiority to FLT3-WT disease in inv.(16) AML [11, 12, 21]. In one study at 10 years, interestingly, overall survival among higher-level mutants (>25% mutant TKD) was 59%, which was superior to lower-level mutants (<25%) and FLT3-TKD-wt patients (37 and 33%, respectively) [21].
FLT3-ITD and FLT3-TKD mutations constitutively activate the receptor in different fashions. The former lesions result in in-frame duplications involving the JM which plays a role in regulating the kinase activity [22]. This is potentially mediated by two key tyrosine residues that serve as STAT5 binding sites, Y589 and Y591, which in the context of ITDs, are activating [23]. ITDs commonly vary from 2 to 42 AA (though may be more than 400 bp) and are always in frame. Specifically, arginine R595 in the JM domain is duplicated in 77% of FLT3-ITD patients and has been shown to confer factor-independent growth in cell lines and is activating of STAT5 [24]. Up to 30% of FLT3-ITD-positive patients may have activating insertions in other locations, most commonly the nucleotide binding loop (612–623) [25]. ITD location may be important with some data supporting that location of ITDs within the β1-sheet of TKD1 is an independent unfavorable prognostic factor possibly outweighing allelic ratio, and may predict nonresponsiveness to TKI therapy [26]. ITD length may be prognostically relevant as well with some support for the notion that longer ITDs confer a worse outcome [27].
TKD mutations are common at aspartate 835, and also occur at aspartate 842 and isoleucine 836. However, based on receptor data from the structurally related receptor c-KIT, these mutations may have very different mechanisms of activation resulting in different clinical and biologic effects [28].
FLT3-ITD mutations were found to occur in to 20–40% of patients APL with 6–10% having TKD mutations in two studies with nearly 500 combined APL patients [29, 30]. In this subtype, FLT3-ITD mutations are associated with higher WBC at presentation, without a clear impact on outcomes. Otherwise, FLT3-ITD mutations are uncommon in cytogenetically abnormal AML except in t(6;9) [16].
The eventual signal cascade of FLT3-ITD activation culminates in multiple pathways including phosphatidyl-inositol3-kinase (PI3K)/AKT, mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK), and signal transducer and activator of transcription 5 (STAT5) (Fig. 1) [31, 32]. FLT3-ITD expression induces independent growth in cell lines and causes a fatal myeloproliferative disorder in transgenic mouse models [33], while mice harboring the TKD mutation develop an oligoclonal lymphoid disorder with longer latency with better survival outcome [34].
Assays for FLT3 Mutations
The assay for FLT3 activating mutations is polymerase chain reaction (PCR)-based and interrogates genomic DNA from a given patient’s malignant cells from either whole blood or bone marrow. The assay is subject to dilution from nonmalignant DNA which may decrease amount of mutant alleles measured. In the most commonly used platform, PCR primers flanking the JM coding sequence amplify FLT3-ITD mutations and, simultaneously in the same assay, a second set of primers amplify the TKD domain [35]. Amplified fragments are separated by capillary gel electrophoresis. EcoRV digestion of PCR products cleaves the kinase domain unless a TKD mutation is present (D835 or I836). The assay is able to determine if a TKD mutation is present at 835 and the length of any potential ITD mutation.
Using these primers flanking the juxtamembrane domain, the wild-type FLT3 PCR fragment is 330 bases while an ITD mutation may increase this length by dozens of bases. During the PCR reaction, the shorter wild-type fragment gains a significant competitive advantage as synthesis of the shorter allele will occur more rapidly. The resultant “PCR bias” can be demonstrated even with a 1:1 mixture of mutant and wild-type gene templates. The result will be a more wild-type product than mutant (ratio less than one of mutant-to-wild type). Longer insertions lead to a greater PCR bias. This distortion can be limited by using fewer PCR cycles; however, this limits sensitivity, particularly if a low number of leukemic blasts are present in the sample being assayed [16]. Regardless, because the FLT3 mutant-to-wild-type allelic ratio derived from the PCR assay is predictive of clinical outcome (see below), the lack of a standardized assay in this field is a genuine impediment to interpreting the results of clinical studies which include this parameter. A high allelic ratio in one study may be an intermediate ratio in another.
The allelic ratio describes the amount of ITD-mutated alleles relative to the amount of wild-type alleles. It is affected by the number of leukemic cells in the sample, and amount of cells with one, both, or no allelic mutations. The dominant cell in most cases is heterozygous for the mutation. Not uncommonly, however, AML subclones may be homozygous via uniparental disomy [36], or lack the mutation altogether. Cells may also be hemizygous for the mutation and completely lack a wild-type allele (associated with a worse prognosis) [37]. In general, higher allelic ratio has been associated with worse prognosis. However, in concert with other favorable cooperating mutations (e.g., NPM1) combined with low allelic ratio (<0.5), patients may have prognosis no different than wild-type FLT3-ITD patients [38, 39]. These conclusions are all complicated by the lack of uniformity in assay method, which, as described above, influences the estimated ratio. Reference labs often use a cutoff of, for example, 10% mutant allelic burden to label a sample as “positive.” In some cases, the lab might receive a bone marrow sample from a FLT3-ITD patient in early relapse with relatively few blasts present. Based on the lab’s pre-determined cutoff, such a sample is likely labeled as “negative” for a FLT3-ITD mutation, even if this is clearly not the case. Although there are readily found examples of patients who were positive for a FLT3-ITD mutation at diagnosis and negative at relapse, this artifact in the assay has resulted in the belief that the FLT3-ITD mutation is unstable [40] and therefore of little value as a marker of minimal residual disease (MRD). More often, the FLT3-ITD burden actually increases from diagnosis to relapse [41].
Treatment
Most FLT3-ITD patients are able to achieve complete responses (CR) at similar rates to wild-type patients, with exceptions for patients with very high allelic ratio and those who are hemizygous who may often be primary refractory [37]. However, patients with FLT3-ITD AML on average relapse much sooner compared with wild type with a median time to relapse of 6–7 vs. 9–11 months. For example, in a prospective study of FLT3-mutated AML patients, the median time to relapse was 6 months [42]. The presence of a FLT3-ITD mutation is an especially poor prognosticator in first relapse resulting in very short survival [43]. The chance of achieving a second CR after relapse in less than 6 months with conventional chemotherapy was as low as 11% [42].
FLT3 Tyrosine-Kinase Inhibitors
Inhibition of FLT3 signaling via blockade of its kinase activity with small molecules has been a longstanding goal in drug development. An arsenal of small molecule TKIs has been moving through the developmental pathways for some years now. These agents compete with ATP for binding to the active pocket of the kinase, resulting in inhibition of autophosphorylation and phosphorylation of substrates [44].
The initial wave of potential FLT3 inhibitors were coopted from drugs used to treat solid tumors and were nonselective kinase inhibitors. The use of these agents often resulted in substantial toxicities. Complete, prolonged in vivo FLT3 inhibition has been difficult to achieve [44], with in vitro studies pointing towards the need for sustained blockade for efficacy [45]. Given the relatively poor potency of the older inhibitors, they have largely been used as part of combination therapy [46,47,48]. First-generation TKIs include midostaurin (PKC412), lestaurtinib (CEP-701), sunitinib (SU11248), KW2449, and sorafenib (BAY43–9006). Second-generation inhibitors include gilteritinib (ASP2215), PLX3397, quizartinib (AC220), crenolanib (CP-868596), and ponatinib (AP24534). Clinical trial data from these agents is summarized in Table 1.
Plasma Inhibitory Activity Assay
Conventional pharmacokinetic studies of TKI drug levels in patients can be often misleading due to the highly protein-bound nature of the drugs, which can even vary between patients. In general, it is often difficult to confirm that the kinase being targeted has been inhibited in vivo as target tissue (leukemia cells) may be absent from the peripheral blood. A plasma inhibitory activity assay, in which a FLT3-ITD cell line is exposed to plasma from a TKI-treated patient and then assayed for FLT3 inhibition, has been shown to be a useful surrogate for in vivo activity and outcomes [51, 71]. An immunoblot is performed which identifies the phosphorylated-FLT3 which is the active form of the receptor. This can be quantified via densitometry and compared with baseline levels. Inhibition to <15% of baseline has been used to define adequacy for inhibitors.
First-Generation FLT3 TKIs
Sunitinib
Sunitinib is an indolinone derivate, multikinase inhibitor with activity against FLT3, CSF, VEGFR, c-KIT, and PDGFR approved for use in metastatic renal cell carcinoma and gastrointestinal stromal tumors. Some in vitro evidence shows that sunitinib may be effective against TKD mutations [72]. It has been tested in the phase I setting and was shown to be an effective in vivo FLT3 inhibitor [70]. Another phase I trial with 15 patients (4 FLT3 mutations) found that all mutated patients (4/4) had a morphologic or partial response compared with 2/10 wild-type patients [69].
Midostaurin
Midostaurin is an indolocarbazole multikinase inhibitor with activity against c-KIT, PDGFR, FLT3, and VEGFR (among many others). In the phase II setting in relapsed refractory AML patients harboring FLT3 mutations, treatment with midostaurin resulted in a reduction by more than 50% in peripheral blood blasts in most patients [53]. A subsequent larger phase IIB study in relapsed and refractory patients (or those unfit for intensive chemotherapy) with 95 patients with either FLT3-ITD mutations or wild type showed greater blast reduction in the FLT3-ITD group [54]. In a phase IB combination study of midostaurin at 50 mg twice daily (BID) or 100 mg BID with daunorubicin and cytarabine for induction of 69 newly diagnosed AML patients found similar rates of overall survival at 2 years (0.62 vs. 0.52) for FLT3-ITD and wild type, respectively, with 12 of 13 FLT3-ITD patients achieving CR with induction at the 50-mg BID dose. A significant number of patients at the 100-mg BID dosing discontinued therapy due to primarily gastrointestinal side effects [48]. The subsequent phase III trial (“RATIFY”) was recently presented at the 2015 American Society of Hematology Meeting Plenary Session. Seven hundred seventeen previously untreated patients with FLT3 mutations aged 18–60 were enrolled in 17 countries in a randomized, placebo-controlled fashion. Midostaurin was combined with daunorubicin and cytarabine induction, and patients were stratified by FLT3-TKD, ITD high allelic ratio (>0.7), or low allelic ratio (0.05–0.7). The hazard ratio (HR) of midostaurin compared with placebo was 0.77 for OS and showed a benefit in TKD, as well as high and low allelic ratio ITD mutations [55•]; 402/717 patients received an allogeneic transplant (58% of those receiving midostaurin, and 54% receiving placebo) with similar median time to transplant. Interestingly, patients randomized to the midostaurin arm who then proceeded to allogeneic transplant achieved significantly longer survival than those patients who were randomized to placebo who were then transplanted. Given the importance of depth of remission in achieving success with allogeneic transplant [73], it is possible that treatment with the combination of chemotherapy and midostaurin resulted in a better quality of remission. Based on RATIFY, midostaurin was granted breakthrough status by the FDA in 2016 for newly diagnosed FLT3 mutation-positive AML. Midostaurin has also been used in combination with azacitidine in phase I–II with 54 patients that were relapsed, refractory, or unfit for intensive chemotherapy finding a response rate of 26% with longer remissions in those who had not previously undergone transplants and in those who had not previously used FLT3 inhibitors [56]. Midostaurin was studied with induction chemotherapy in 149 newly diagnosed FLT3-ITD patients with a median age of 54, followed by consolidative therapy with either allogeneic transplant or HIDAC. Overall 75% of patients achieved a completed response to one or more cycles of induction. Of 52 patients who started maintenance therapy, 40 had undergone transplant and 12 received HIDAC. Relapse at 1 year was 12% for allelic ratio <0.05 and 5% for ratio ≥0.5 for those undergoing allogeneic transplant, and 28 and 29%, respectively, after HIDAC, with only four suffering adverse events grades 3–4 attributable to midostaurin, though 55% of patients required dose-reduction or interruption of midostaurin during induction [57].
Lestaurtinib
Lestaurtinib is an indolocarbazole multikinase inhibitor and is active against FLT3, JAK2, VEGFR, and tropomyosin-related kinase A (TrKA; and even more so than midostaurin and many other kinases) [74,74,76]. In a phase I–II study of lestaurtinib in 17 relapsed or refractory AML patients with FLT3 mutations, one patient had a CR and 5/14 patients had reduction in blast counts with correlative data showing sustained inhibition of FLT3; however, all responses were of short duration ranging from 2 weeks to 3 months [51]. Another phase II trial of lestaurtinib looked at untreated elderly patients not suitable for chemotherapy, irrespective of FLT3 mutation status, and found that 3/5 FLT3-mutated patients achieved a hematologic response vs. 5/22 wild type [52]. A randomized phase III trial of FLT3 patients in first relapse compared chemotherapy alone to combination with lestaurtinib, no difference was found between the two groups and only limited in vivo inhibition was demonstrated on correlative studies [47].
Sorafenib
Sorafenib is approved for renal cell and hepatocellular carcinoma and has activity against FLT3, VEGFR, c-KIT, and RAF kinase [77]. As a single agent, and like the other multitargeted TKIs, sorafenib’s efficacy is somewhat limited by side effects at doses necessary to inhibit FLT3 in vivo. One phase I trial of 15 relapsed or refractory patients with single agent sorafenib failed to show any complete or partial responses; however, Plasma Inhibitory Activity (PIA) data showed inhibition of FLT3 in vivo at doses below the maximum tolerated dose [63]. In a phase I trial of 16 relapsed or refractory patients, sorafenib was shown to reduce leukemic blasts in the peripheral blood and the bone marrow only in those patients harboring a FLT3-ITD mutation [61]. In a somewhat larger phase 1 study, sorafenib induced complete remissions with incomplete count recovery in 10% of AML patients with a FLT3/ITD mutation [64]. Finally, a study of 13 relapsed or refractory FLT3-ITD patients with sorafenib found that 12 patients had clearance or near clearance of bone marrow myeloblasts; however, most patients relapsed after 72 days. Nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice transplanted with leukemia cells from patients prior to and after relapse recapitulated the clinical findings. Leukemia cells harboring the D835 mutation expanded at relapse providing the likely culprit for resistance [78].
Sorafenib has been combined with conventional induction chemotherapy, but with as yet uncertain results. A small single institution phase II study demonstrated suggested that the combination was feasible and potentially effective [46]. However, a large phase II randomized, placebo-controlled trial of sorafenib plus standard 7 + 3 induction in those age >60 revealed worse outcomes for the sorafenib, arm and no benefit was seen even in analysis limited to the FLT3-ITD subgroup [67]. The SORAML trial was a randomized, double-blind, placebo-controlled phase II study involving 267 patients aged 18–60, in which sorafenib was given during two cycles of 7 + 3 induction and three cycles of HIDAC consolidation. Outcomes revealed a 3-year event-free survival of 22% in placebo vs. 40% in the sorafenib arm (HR = 0.64, p = 0.013) but no improvement in the overall survival [66]. Forty-six patients harbored the FLT3-ITD mutation had a trend towards increased overall survival (not reached vs. 19 months) and relapse-free survival (18 vs. 6 months), though these were not statically significant.
There have been a number of reports of the clinical activity of sorafenib in the post-transplant setting. A retrospective review of 65 FLT3-ITD patients with relapsed or refractory disease who were treated with sorafenib monotherapy found that patients who had undergone allogeneic hematopoietic stem cell transplant (HSCT) developed resistance less frequently (38%) and significantly later (197 days) vs. those had not undergone alloHSCT (47% and 136 days) [62]. Sustained remissions were seen exclusively in the alloHSCT group. In light of this, a phase I trial of sorafenib maintenance in patients with FLT3-ITD after allogeneic HSCT found a very encouraging 1 year OS of 95% [65•].
Second-Generation TKIs
Quizartinib
Quizartinib, originally identified in a library-based small molecule inhibitor screen, has an IC50 of less than 1 nM in culture medium and an IC50 of 18 nM in plasma. This is an order of magnitude more potent than any previously studied FLT3 TKI [79]. The drug appears to completely inhibit FLT3 in vivo at doses with a minimum of off-target effects, although prolongation of the QT interval and myelosuppression from c-KIT inhibition is a problem at higher doses [58]. A phase I trial of quizartinib monotherapy in 76 relapsed or refractory patients found that 9/17 FLT3-ITD patients responded (CR + PR) compared with 5/37 wild-type patients [58].
A large phase II trial of quizartinib in 333 relapsed/refractory patients was divided into two cohorts: (1) with age >60 and (2) patients refractory to two lines of chemotherapy or who had relapsed after transplant [59, 60]. Cohort 2 revealed a composite complete remission rate of 44% compared with 34% for those without FLT3-ITD (n = 99 and 38, respectively) allowing over a third of the relapsed cohort to undergo HSCT. Cohort 1 (median age, 70) had a composite complete remission rate of 54% in those with FLT3-ITD and 32% in those who were negative (n = 92 and 41, respectively). Correlative studies revealed that quizartinib induced terminal differentiation of bone marrow blasts without changing the FLT3-ITD allelic ratio resulting in the generation of peripheral blood neutrophils that harbored the same mutations as the leukemic blasts [80]. Occasionally, there was an associated clinical differentiation syndrome, similar to that seen in acute promyelocytic leukemia [81]. Similar effects on differentiation of in vitro blasts have been shown with sorafenib and lestaurtinib [78, 82, 83]. Among those patients who responded to quizartinib, a number of them developed resistance-conferring point mutations at residues D835 and F691 [84]. While obviously troublesome from a clinical perspective, the emergence of these resistance mutations provided a clear evidence of the importance of FLT3-ITD mutations as a driver in this disease.
Crenolanib
Crenolanib was initially developed to inhibit PDGFR in solid malignancies [85]; however, it was found to have activity against FLT3-ITD and FLT3-TKD mutations [86]. In vitro studies have shown a number of possible advantages including lack of significant activity against c-KIT (avoiding myelosuppression), no QTc prolongation, low plasma protein binding, and a high degree of activity against D835 resistance mutations. The compound is in a number of single agent and combination trials for the treatment of FLT3-mutated AML [49].
Ponatinib
Ponatinib is a third-generation TKI developed to target the T315I mutation in BCR-ABL [87]. Ponatinib also inhibits the SRC, VEGFR, FGFR, and PDGFR families of receptor tyrosine kinases, including FLT3. Importantly, it has been shown in vitro to induce apoptosis in the multiresistant FLT3-F691I mutation but not against FLT3-D835 [88].
Gilteritinib
Gilteritinib is a selective inhibitor of FLT3 and AXL and has demonstrated activity against ITD and D835 mutations. The first in human study of gilteritinib, a phase I–II trial in 215 relapsed or refractory patients of which 137 had FLT3-ITD found an overall response rate of 68/114 (60%) among the ITD carriers and 4/14 (29%) in those with either D835 or D835 and ITD. Median overall survival for all FLT3+ patients was 29 weeks and was independent of prior treatment with TKIs [50]. PIA data confirmed in vivo FLT3 inhibition at doses of 80 mg day−1 and higher.
Combinations with Hypomethylating Agents
Like the proposed mechanism for TKIs in FLT3-ITD AML, DNMT inhibition also can induce terminal differentiation of myeloid blasts [89]. Ongoing work with these well-tolerated agents (azacitidine and deoxy-5-azacitidine) and FLT3 inhibitors in cell lines and patients has shown a promising synergy and may be especially useful in the elderly who have limited reserve for cytotoxic chemotherapy [68, 90]. A phase II trial in the relapsed setting, median age 64, of FLT3-ITD-mutated patients treated with 5-azactidine and sorafenib found a response rate of 46% including 27% complete responses [68].
Resistance
Resistance to FLT3 inhibition has been classified into three primary etiologies and is addressed extensively elsewhere [44]: (1) extrinsic mechanisms (drug metabolism CYP3A4 and drug interactions, plasma protein binding, FLT3 ligand), (2) receptor intrinsic mechanisms (resistance point mutations, overexpression, ITD length/site), and (3) cell intrinsic mechanisms (parallel pathways, apoptosis evasion, nonaddicted subclones).
One of the first receptor intrinsic mechanisms identified was the point mutation N676K which induced resistance in a patient treated with midostaurin [91]. Similar work identified resistance conferring TKD mutations to quizartinib [84]. Cell-based screening approaches have identified unique, nonoverlapping resistance profiles to sunitinib, midostaurin, and sorafenib, suggesting that combinatorial therapy may overcome a development of resistance [92]. Newer TKIs, such as gilteritinib, have shown an efficacy in the phase II setting regardless of prior TKI exposure [50] and crenolanib has shown in vitro efficacy against quizartinib-resistance mutations including D835 [86], as well as in the phase II setting in patients with the D835 mutation [49]. Saturation mutagenesis of FLT3-ITD followed by selection of transfected cells has been identified (F621L, A627P, F691L, and Y842C) as mutations that confer varying levels of resistance to TKIs and may be encountered in patients [93].
Alterations in key signaling pathways in FLT3 TKI-resistant cell lines and primary samples reveal other forms of resistance. These cell lines have activation of PI3k/AKT and/or Ras/MEK/MAPK pathways as well as continued expression of genes involved in FLT3-mediated cellular transformation. Inhibition of these pathways via other agents may at least partially restore sensitivity to FLT3-TKIs [94].
FL may play a key role in resistance to FLT3 inhibition as well. Gene profiling of MV4–11 cells after long-term exposure to linifanib revealed up-regulation of FL and BIRC5 (survivin). Short-hairpin RNA targeting of survivin induced apoptosis and enhanced TKI cytotoxicity [95]. Treatment of FLT3-mutated AML patients with chemotherapy leads to high levels of FL throughout recovery and consolidation, which then acts directly on the FLT3-ITD receptor maximizing its activity and promoting survival of blasts. Furthermore, this effect seems to shift the IC50 of FLT3 inhibitors upwards by 2–4-fold and can influence their inhibitory effect in vitro [96]. Given this understanding, it may be more ideal to begin FLT3 inhibition early after induction and transplant to avoid the surge in ligand levels that might blunt the effect of the inhibitors [97]. In vitro studies with a variety of FLT3 inhibitors in primary patient blast samples showed substantially more efficacy in those with high allelic burden and those in relapsed patients, possibly indicating greater addiction to FLT3 signaling [98].
Alteration of the FLT3-ITD insertion site (essentially, longer insertions that begin in the first tyrosine-kinase domain, rather than the juxtamembrane domain) may be important for resistance [26]. The N676K mutation has been shown to result in up-regulation of the anti-apoptotic protein MCL-1, which conferred resistance to midostaurin. RNAi knockdown of MCL-1 was able to rescue TKI sensitivity [99].
Leukemia cells within the bone marrow do not reliably undergo apoptosis, even with maximal FLT3 inhibition, and the bone marrow microenvironment clearly plays an important role in this phenomenon. Co-culture with bone marrow stromal cells revealed that FLT3 inhibition (via sorafenib and quizartinib) in leukemic cells results in cell cycle arrest rather than apoptosis. Persistent activation of extracellular regulated kinase (ERK) through parallel signaling pathways contributes to the protective effect. Inhibition of the upstream kinase, MEK, blocked stromal-mediated resistance [100]. In another study, stromal niche cells were shown to protect leukemic stem cells from the effects of sorafenib and sunitinib—allowing the maintenance of leukemic progenitors, even in the presence of these agents [101].
Allogeneic Bone Marrow Transplant
The role of allogeneic stem cell transplant in FLT-ITD AML had been controversial until perhaps more recently [102]. One of the first large retrospective analyses on the topic performed in 2005 out of the UK did not find a benefit for transplant [103]. However, only 35 of 68 patients with a donor actually underwent transplant and some have argued that in an as-treated analysis, there was a benefit [104]. Furthermore, the risk of relapse after allogeneic transplant was similar between FLT3-ITD-mutated and wild-type patients suggesting amelioration of the negative influence of the lesion. However, a large study of 872 adults less than 60 years of age by the German-Austrian Acute Myeloid Leukemia Study Group from 2008 revealed that among cytogenetically normal AML, only FLT3-ITD and wild-type CEBPA/NPM1 without FLT3-ITD benefitted from transplant [105]. Transplant-related mortality was only 21% (compared with 30% in the UK study), and transplant rate was 82% compared with 63%. Further studies have corroborated the finding of a benefit of allogeneic HSCT in FLT3-ITD patients [106,107,108]. Of more recent interest is the impact of allelic ratio and co-expression of mutations in NPM1 [38, 39, 109]. Due to the short duration of remissions in FLT3-ITD AML prior to relapse after an initial remission, many have argued to begin planning a transplant immediately upon detection of the mutation [97].
Conclusions
Over the past 20 years, retrospective clinical studies, in vitro and clinical studies of FLT3 inhibitors, and whole genome and whole exome studies of AML patient samples, along with a large body of basic hematopoietic laboratory research has generated a wealth of information about the disease entity we know as FLT3-ITD AML. We are now in a position to speculate about how the disease evolves during treatment, and how we might best approach it with currently available therapeutics. FLT3-ITD AML presents in polyclonal fashion, with only a relatively small subset of leukemia cells at diagnosis being dependent or addicted to FLT3-mutant signaling [98, 110•]. Following induction chemotherapy and subsequent rounds of consolidation chemotherapy, FL levels rise, conferring a protective effect on, and possibly selecting for, a subset of cells that are both chemo-resistant and FLT3 addicted [97]. These are the cells that emerge at relapse and lead to the death of the patient. To counter this, a reasonable approach might be to induce the patient with chemotherapy combined with a polyclonal TKI such as midostaurin (as in the RATIFY trial) which has broad anti-leukemic effects against FLT3, PDGFR, c-KIT, PKC, and VEGFR [55•], followed by rapid transition to an allogeneic transplant (rather than multiple rounds of chemotherapy). If necessary, rapid transplant with an alternative donor (e.g., a haplo-identical relative or cord blood) may be preferable to administering successive rounds of chemotherapy while waiting to find the “right” donor. The net beneficial effect of midostaurin in RATIFY may be due to the use of induction chemotherapy and nonselective kinase inhibition to debulk the polyclonal tumor followed by rapid consolidation with allogeneic transplant and maintenance FLT3 inhibition to prevent relapse of any residual FLT3 clones. After allogeneic transplant, a more selective FLT3 inhibitor may be an even more effective maintenance therapy [65•], since it is often the FLT3-addicted clones that are identified as the culprit at relapse, although this concept should be first studied in the context of a randomized trial. The field is moving rapidly. Several FLT3 inhibitors are now in phase III pivotal trials (see Table 1), and it seems likely that one or more will be approved over the next 5 years. How we take advantage of these new tools remains to be seen, but the prospects for our patients are hopeful.
References
References of particular interest, published recently, have been highlighted as: • Of importance
• Khwaja A, Bjorkholm M, Gale RE, Levine RL, Jordan CT, Ehninger G, et al. Acute myeloid leukaemia. Nature reviews Disease primers. 2016;2:16010. This recent review offers a comprehensive look at AML, from the molecular to the clinical.
Buchner T, Schlenk RF, Schaich M, Dohner K, Krahl R, Krauter J, et al. Acute myeloid leukemia (AML): different treatment strategies versus a common standard arm—combined prospective analysis by the German AML intergroup. J Clin Oncol. 2012;30(29):3604–10.
Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74.
Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100(5):1532–42.
Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150(2):264–78.
• Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059–74. One of the largest investigations into the genetics and epigenetic underpinnings of AML.
Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411(6835):355–65.
Carow CE, Levenstein M, Kaufmann SH, Chen J, Amin S, Rockwell P, et al. Expression of the hematopoietic growth factor receptor FLT3 (STK-1/Flk2) in human leukemias. Blood. 1996;87(3):1089–96.
Zhang S, Fukuda S, Lee Y, Hangoc G, Cooper S, Spolski R, et al. Essential role of signal transducer and activator of transcription (Stat)5a but not Stat5b for Flt3-dependent signaling. J Exp Med. 2000;192(5):719–28.
Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10(12):1911–8.
Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97(8):2434–9.
Abu-Duhier FM, Goodeve AC, Wilson GA, Care RS, Peake IR, Reilly JT. Identification of novel FLT-3 Asp835 mutations in adult acute myeloid leukaemia. Br J Haematol. 2001;113(4):983–8.
Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17(9):1738–52.
Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98(6):1752–9.
Frohling S, Schlenk RF, Breitruck J, Benner A, Kreitmeier S, Tobis K, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML study group Ulm. Blood. 2002;100(13):4372–80.
Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326–35.
Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100(1):59–66.
Xu F, Taki T, Yang HW, Hanada R, Hongo T, Ohnishi H, et al. Tandem duplication of the FLT3 gene is found in acute lymphoblastic leukaemia as well as acute myeloid leukaemia but not in myelodysplastic syndrome or juvenile chronic myelogenous leukaemia in children. Br J Haematol. 1999;105(1):155–62.
Meshinchi S, Woods WG, Stirewalt DL, Sweetser DA, Buckley JD, Tjoa TK, et al. Prevalence and prognostic significance of Flt3 internal tandem duplication in pediatric acute myeloid leukemia. Blood. 2001;97(1):89–94.
Dicker F, Haferlach C, Sundermann J, Wendland N, Weiss T, Kern W, et al. Mutation analysis for RUNX1, MLL-PTD, FLT3-ITD, NPM1 and NRAS in 269 patients with MDS or secondary AML. Leukemia. 2010;24(8):1528–32.
Mead AJ, Linch DC, Hills RK, Wheatley K, Burnett AK, Gale RE. FLT3 tyrosine kinase domain mutations are biologically distinct from and have a significantly more favorable prognosis than FLT3 internal tandem duplications in patients with acute myeloid leukemia. Blood. 2007;110(4):1262–70.
Griffith J, Black J, Faerman C, Swenson L, Wynn M, Lu F, et al. The structural basis for autoinhibition of FLT3 by the juxtamembrane domain. Mol Cell. 2004;13(2):169–78.
Rocnik JL, Okabe R, Yu JC, Lee BH, Giese N, Schenkein DP, et al. Roles of tyrosine 589 and 591 in STAT5 activation and transformation mediated by FLT3-ITD. Blood. 2006;108(4):1339–45.
Vempati S, Reindl C, Kaza SK, Kern R, Malamoussi T, Dugas M, et al. Arginine 595 is duplicated in patients with acute leukemias carrying internal tandem duplications of FLT3 and modulates its transforming potential. Blood. 2007;110(2):686–94.
Breitenbuecher F, Schnittger S, Grundler R, Markova B, Carius B, Brecht A, et al. Identification of a novel type of ITD mutations located in nonjuxtamembrane domains of the FLT3 tyrosine kinase receptor. Blood. 2009;113(17):4074–7.
Kayser S, Schlenk RF, Londono MC, Breitenbuecher F, Wittke K, Du J, et al. Insertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood. 2009;114(12):2386–92.
Stirewalt DL, Kopecky KJ, Meshinchi S, Engel JH, Pogosova-Agadjanyan EL, Linsley J, et al. Size of FLT3 internal tandem duplication has prognostic significance in patients with acute myeloid leukemia. Blood. 2006;107(9):3724–6.
Laine E, Auclair C, Tchertanov L. Allosteric communication across the native and mutated KIT receptor tyrosine kinase. PLoS Comput Biol. 2012;8(8):e1002661.
Kuchenbauer F, Schoch C, Kern W, Hiddemann W, Haferlach T, Schnittger S. Impact of FLT3 mutations and promyelocytic leukaemia-breakpoint on clinical characteristics and prognosis in acute promyelocytic leukaemia. Br J Haematol. 2005;130(2):196–202.
Barragan E, Montesinos P, Camos M, Gonzalez M, Calasanz MJ, Roman-Gomez J, et al. Prognostic value of FLT3 mutations in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline monochemotherapy. Haematologica. 2011;96(10):1470–7.
Mizuki M, Fenski R, Halfter H, Matsumura I, Schmidt R, Muller C, et al. Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood. 2000;96(12):3907–14.
Brandts CH, Sargin B, Rode M, Biermann C, Lindtner B, Schwable J, et al. Constitutive activation of Akt by Flt3 internal tandem duplications is necessary for increased survival, proliferation, and myeloid transformation. Cancer Res. 2005;65(21):9643–50.
Li L, Piloto O, Nguyen HB, Greenberg K, Takamiya K, Racke F, et al. Knock-in of an internal tandem duplication mutation into murine FLT3 confers myeloproliferative disease in a mouse model. Blood. 2008;111(7):3849–58.
Bailey E, Li L, Duffield AS, Ma HS, Huso DL, Small D. FLT3/D835Y mutation knock-in mice display less aggressive disease compared with FLT3/internal tandem duplication (ITD) mice. Proc Natl Acad Sci U S A. 2013;110(52):21113–8.
Murphy KM, Levis M, Hafez MJ, Geiger T, Cooper LC, Smith BD, et al. Detection of FLT3 internal tandem duplication and D835 mutations by a multiplex polymerase chain reaction and capillary electrophoresis assay. J Mol Diagn. 2003;5(2):96–102.
Green C, Linch DC, Gale RE. Most acute myeloid leukaemia patients with intermediate mutant FLT3/ITD levels do not have detectable bi-allelic disease, indicating that heterozygous disease alone is associated with an adverse outcome. Br J Haematol. 2008;142(3):423–6.
Whitman SP, Archer KJ, Feng L, Baldus C, Becknell B, Carlson BD, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res. 2001;61(19):7233–9.
Pratcorona M, Brunet S, Nomdedeu J, Ribera JM, Tormo M, Duarte R, et al. Favorable outcome of patients with acute myeloid leukemia harboring a low-allelic burden FLT3-ITD mutation and concomitant NPM1 mutation: relevance to post-remission therapy. Blood. 2013;121(14):2734–8.
Schlenk RF, Kayser S, Bullinger L, Kobbe G, Casper J, Ringhoffer M, et al. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood. 2014;124(23):3441–9.
Kottaridis PD, Gale RE, Langabeer SE, Frew ME, Bowen DT, Linch DC. Studies of FLT3 mutations in paired presentation and relapse samples from patients with acute myeloid leukemia: implications for the role of FLT3 mutations in leukemogenesis, minimal residual disease detection, and possible therapy with FLT3 inhibitors. Blood. 2002;100(7):2393–8.
Shih LY, Huang CF, Wu JH, Lin TL, Dunn P, Wang PN, et al. Internal tandem duplication of FLT3 in relapsed acute myeloid leukemia: a comparative analysis of bone marrow samples from 108 adult patients at diagnosis and relapse. Blood. 2002;100(7):2387–92.
Levis M, Ravandi F, Wang ES, Baer MR, Perl A, Coutre S, et al. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for FLT3 mutant AML patients in first relapse. Blood. 2009;114:788.
Chevallier P, Labopin M, Turlure P, Prebet T, Pigneux A, Hunault M, et al. A new leukemia prognostic scoring system for refractory/relapsed adult acute myelogeneous leukaemia patients: a GOELAMS study. Leukemia. 2011;25(6):939–44.
Grunwald MR, Levis MJ. FLT3 inhibitors for acute myeloid leukemia: a review of their efficacy and mechanisms of resistance. Int J Hematol. 2013;97(6):683–94.
Pratz KW, Cortes J, Roboz GJ, Rao N, Arowojolu O, Stine A, et al. A pharmacodynamic study of the FLT3 inhibitor KW-2449 yields insight into the basis for clinical response. Blood. 2009;113(17):3938–46.
Ravandi F, Cortes JE, Jones D, Faderl S, Garcia-Manero G, Konopleva MY, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol. 2010;28(11):1856–62.
Levis M, Ravandi F, Wang ES, Baer MR, Perl A, Coutre S, et al. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse. Blood. 2011;117(12):3294–301.
Stone RM, Fischer T, Paquette R, Schiller G, Schiffer CA, Ehninger G, et al. Phase IB study of the FLT3 kinase inhibitor midostaurin with chemotherapy in younger newly diagnosed adult patients with acute myeloid leukemia. Leukemia. 2012;26(9):2061–8.
Collins R, Kantarjian H, Levis M, Perl A, Ramachandran A, Ravandi F, et al. Clinical activity of crenolanib in patients with D835 mutant FLT3-positive relapsed/refractory acute myeloid leukemia (AML). J Clin Oncol. 2014;325s:7027.
Levis M, Perl A, Altman JK, Cortes J, Ritchie E, Larson RA, et al. Results of a first-in-human, phase I/II trial of ASP2215, a selective, potent inhibitor of FLT3/Axl in patients with relapsed or refractory (R/R) acute myeloid leukemia (AML). J Clin Oncol. 2015;33 suppl:7003.
Smith BD, Levis M, Beran M, Giles F, Kantarjian H, Berg K, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103(10):3669–76.
Knapper S, Burnett AK, Littlewood T, Kell WJ, Agrawal S, Chopra R, et al. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006;108(10):3262–70.
Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105(1):54–60.
Fischer T, Stone RM, Deangelo DJ, Galinsky I, Estey E, Lanza C, et al. Phase IIB trial of oral midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 2010;28(28):4339–45.
• Stone R, Mandrekar S, Sanford BL, Geyer S, Bloomfield CD, Dohner K, et al. The multi-kinase inhibitor midostaurin (M) prolongs survival compared with placebo (P) in combination with daunorubicin (D)/cytarabine (C) induction (ind), high-dose C consolidation (consol), and as maintenance (maint) therapy in newly diagnosed acute myeloid leukemia (AML) patients (pts) age 18-60 with FLT3 mutations (muts): an international prospective randomized (rand) P-controlled double-blind trial (CALGB 10603/RATIFY [alliance]). Blood. 2015;126:6. The phase III RATIFY trial of midostaurin has led to an FDA breakthrough designation of this drug for AML.
Strati P, Kantarjian H, Ravandi F, Nazha A, Borthakur G, Daver N, et al. Phase I/II trial of the combination of midostaurin (PKC412) and 5-azacytidine for patients with acute myeloid leukemia and myelodysplastic syndrome. Am J Hematol. 2015;90(4):276–81.
Schlenk RF, Dohner K, Salih H, Kundgen A, Fiedler W, Salwender H, et al. Midostaurin in combination with intensive induction and as single agent maintenance therapy after consolidation therapy with allogeneic hematopoietic stem cell transplantation or high-dose Cytarabine (NCT01477606). Blood. 2015;126:322.
Cortes JE, Kantarjian H, Foran JM, Ghirdaladze D, Zodelava M, Borthakur G, et al. Phase I study of quizartinib administered daily to patients with relapsed or refractory acute myeloid leukemia irrespective of FMS-like tyrosine kinase 3-internal tandem duplication status. J Clin Oncol. 2013;31(29):3681–7.
Levis M, Perl A, Dombret H, Dohner H, Steffen B, Rousselot P, et al. Final results of a phase 2 open-label, monotherapy efficacy and safety study of quizartinib (AC220) in patients with FLT3-ITD positive or negative relapsed/refractory acute myeloid leukemia after second-line chemotherapy or hematopoietic stem cell transplantation. Blood. 2012;120:673a.
Cortes J, Perl A, Dombret H, Kayser S, Steffen B, Rousselot P, et al. Final results of a phase 2 open-label, monotherapy efficacy and safety study of quizartinib (AC220) in patients 60 years of age with FLT3 ITD positive or negative relapsed/refractory acute myeloid leukemia. Blood. 2012;120:48a.
Zhang W, Konopleva M, Shi YX, McQueen T, Harris D, Ling X, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100(3):184–98.
Metzelder SK, Schroeder T, Finck A, Scholl S, Fey M, Gotze K, et al. High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses. Leukemia. 2012;26:2353–9.
Pratz KW, Cho E, Levis MJ, Karp JE, Gore SD, McDevitt M, et al. A pharmacodynamic study of sorafenib in patients with relapsed and refractory acute leukemias. Leukemia. 2010;24(8):1437–44.
Borthakur G, Kantarjian H, Ravandi F, Zhang W, Konopleva M, Wright JJ, et al. Phase I study of sorafenib in patients with refractory or relapsed acute leukemias. Haematologica. 2011;96(1):62–8.
• Chen YB, Li S, Lane AA, Connolly C, Del Rio C, Valles B, et al. Phase I trial of maintenance sorafenib after allogeneic hematopoietic stem cell transplantation for fms-like tyrosine kinase 3 internal tandem duplication acute myeloid leukemia. Biol Blood Marrow Transplant. 2014;20(12):2042–8. This small trial of sorafenib in the post transplant maintenence setting revealed an encouraging survival rate, providing the impetus for randomized trials of FLT3 TKIs in these patients going forward.
• Rollig C, Serve H, Huttmann A, Noppeney R, Muller-Tidow C, Krug U, et al. Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2015;16(16):1691–9. This recent large, placebo controlled study of sorafenib with chemotherapy in newly diagnosed patients with acute leukemia demonstrated a benefit in relapse-free survival, but not overall survival, for patients who received the sorafenib with induction therapy.
Serve H, Krug U, Wagner R, Sauerland MC, Heinecke A, Brunnberg U, et al. Sorafenib in combination with intensive chemotherapy in elderly patients with acute myeloid leukemia: results from a randomized, placebo-controlled trial. J Clin Oncol. 2013;31(25):3110–8.
Ravandi F, Alattar ML, Grunwald MR, Rudek MA, Rajkhowa T, Richie MA, et al. Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood. 2013;121(23):4655–62.
Fiedler W, Serve H, Dohner H, Schwittay M, Ottmann OG, O'Farrell AM, et al. A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood. 2005;105(3):986–93.
O'Farrell AM, Foran JM, Fiedler W, Serve H, Paquette RL, Cooper MA, et al. An innovative phase I clinical study demonstrates inhibition of FLT3 phosphorylation by SU11248 in acute myeloid leukemia patients. Clin Cancer Res. 2003;9(15):5465–76.
Levis M, Brown P, Smith BD, Stine A, Pham R, Stone R, et al. Plasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood. 2006;108(10):3477–83.
Kancha RK, Grundler R, Peschel C, Duyster J. Sensitivity toward sorafenib and sunitinib varies between different activating and drug-resistant FLT3-ITD mutations. Exp Hematol. 2007;35(10):1522–6.
Walter RB, Gyurkocza B, Storer BE, Godwin CD, Pagel JM, Buckley SA, et al. Comparison of minimal residual disease as outcome predictor for AML patients in first complete remission undergoing myeloablative or nonmyeloablative allogeneic hematopoietic cell transplantation. Leukemia. 2015;29(1):137–44.
Strock CJ, Park JI, Rosen M, Dionne C, Ruggeri B, Jones-Bolin S, et al. CEP-701 and CEP-751 inhibit constitutively activated RET tyrosine kinase activity and block medullary thyroid carcinoma cell growth. Cancer Res. 2003;63(17):5559–63.
Levis M, Allebach J, Tse KF, Zheng R, Baldwin BR, Smith BD, et al. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood. 2002;99(11):3885–91.
Hexner EO, Serdikoff C, Jan M, Swider CR, Robinson C, Yang S, et al. Lestaurtinib (CEP701) is a JAK2 inhibitor that suppresses JAK2/STAT5 signaling and the proliferation of primary erythroid cells from patients with myeloproliferative disorders. Blood. 2008;111(12):5663–71.
Auclair D, Miller D, Yatsula V, Pickett W, Carter C, Chang Y, et al. Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia. 2007;21(3):439–45.
Man CH, Fung TK, Ho C, Han HH, Chow HC, Ma AC, et al. Sorafenib treatment of FLT3-ITD(+) acute myeloid leukemia: favorable initial outcome and mechanisms of subsequent nonresponsiveness associated with the emergence of a D835 mutation. Blood. 2012;119(22):5133–43.
Zarrinkar PP, Gunawardane RN, Cramer MD, Gardner MF, Brigham D, Belli B, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood. 2009;114(14):2984–92.
Sexauer A, Perl A, Yang X, Borowitz M, Gocke C, Rajkhowa T, et al. Terminal myeloid differentiation in vivo is induced by FLT3 inhibition in FLT3/ITD AML. Blood. 2012;120(20):4205–14.
Fathi AT, Le L, Hasserjian RP, Sadrzadeh H, Levis M, Chen YB. FLT3 inhibitor-induced neutrophilic dermatosis. Blood. 2013;122(2):239–42.
Zheng R, Friedman AD, Levis M, Li L, Weir EG, Small D. Internal tandem duplication mutation of FLT3 blocks myeloid differentiation through suppression of C/EBP{alpha} expression. Blood. 2004;103(5):1883–90.
Radomska HS, Basseres DS, Zheng R, Zhang P, Dayaram T, Yamamoto Y, et al. Block of C/EBP alpha function by phosphorylation in acute myeloid leukemia with FLT3 activating mutations. J Exp Med. 2006;203(2):371–81.
Smith CC, Wang Q, Chin CS, Salerno S, Damon LE, Levis MJ, et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012;485(7397):260–3.
Lewis NL, Lewis LD, Eder JP, Reddy NJ, Guo F, Pierce KJ, et al. Phase I study of the safety, tolerability, and pharmacokinetics of oral CP-868,596, a highly specific platelet-derived growth factor receptor tyrosine kinase inhibitor in patients with advanced cancers. J Clin Oncol. 2009;27(31):5262–9.
Galanis A, Ma H, Rajkhowa T, Ramachandran A, Small D, Cortes J, et al. Crenolanib is a potent inhibitor of FLT3 with activity against resistance-conferring point mutants. Blood. 2014;123(1):94–100.
O'Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16(5):401–12.
Smith CC, Lasater EA, Zhu X, Lin KC, Stewart WK, Damon LE, et al. Activity of ponatinib against clinically-relevant AC220-resistant kinase domain mutants of FLT3-ITD. Blood. 2013;121(16):3165–71.
Saunthararajah Y, Triozzi P, Rini B, Singh A, Radivoyevitch T, Sekeres M, et al. p53-independent, normal stem cell sparing epigenetic differentiation therapy for myeloid and other malignancies. Semin Oncol. 2012;39(1):97–108.
Chang E, Ganguly S, Rajkhowa T, Gocke CD, Levis M, Konig H. The combination of FLT3 and DNA methyltransferase inhibition is synergistically cytotoxic to FLT3/ITD acute myeloid leukemia cells. Leukemia. 2016;30(5):1025–32.
Heidel F, Solem FK, Breitenbuecher F, Lipka DB, Kasper S, Thiede MH, et al. Clinical resistance to the kinase inhibitor PKC412 in acute myeloid leukemia by mutation of Asn-676 in the FLT3 tyrosine kinase domain. Blood. 2006;107(1):293–300.
von Bubnoff N, Engh RA, Aberg E, Sanger J, Peschel C, Duyster J. FMS-like tyrosine kinase 3-internal tandem duplication tyrosine kinase inhibitors display a nonoverlapping profile of resistance mutations in vitro. Cancer Res. 2009;69(7):3032–41.
Williams AB, Nguyen B, Li L, Brown P, Levis M, Leahy D, et al. Mutations of FLT3/ITD confer resistance to multiple tyrosine kinase inhibitors. Leukemia. 2013;27(1):48–55.
Piloto O, Wright M, Brown P, Kim KT, Levis M, Small D. Prolonged exposure to FLT3 inhibitors leads to resistance via activation of parallel signaling pathways. Blood. 2007;109(4):1643–52.
Zhou J, Bi C, Janakakumara JV, Liu SC, Chng WJ, Tay KG, et al. Enhanced activation of STAT pathways and overexpression of survivin confer resistance to FLT3 inhibitors and could be therapeutic targets in AML. Blood. 2009;113(17):4052–62.
Sato T, Yang X, Knapper S, White P, Smith BD, Galkin S, et al. FLT3 ligand impedes the efficacy of FLT3 inhibitors in vitro and in vivo. Blood. 2011;117(12):3286–93.
Levis M. FLT3/ITD AML and the law of unintended consequences. Blood. 2011;117(26):6987–90.
Pratz KW, Sato T, Murphy KM, Stine A, Rajkhowa T, Levis M. FLT3-mutant allelic burden and clinical status are predictive of response to FLT3 inhibitors in AML. Blood. 2010;115(7):1425–32.
Breitenbuecher F, Markova B, Kasper S, Carius B, Stauder T, Bohmer FD, et al. A novel molecular mechanism of primary resistance to FLT3-kinase inhibitors in AML. Blood. 2009;113(17):4063–73.
Yang X, Sexauer A, Levis M. Bone marrow stroma-mediated resistance to FLT3 inhibitors in FLT3-ITD AML is mediated by persistent activation of extracellular regulated kinase. Br J Haematol. 2013;164:61–72.
Parmar A, Marz S, Rushton S, Holzwarth C, Lind K, Kayser S, et al. Stromal niche cells protect early leukemic FLT3-ITD+ progenitor cells against first-generation FLT3 tyrosine kinase inhibitors. Cancer Res. 2011;71(13):4696–706.
Levis M. FLT3 mutations in acute myeloid leukemia: what is the best approach in 2013? Hematology Am Soc Hematol Educ Program. 2013;2013:220–6.
Gale RE, Hills R, Kottaridis PD, Srirangan S, Wheatley K, Burnett AK, et al. No evidence that FLT3 status should be considered as an indicator for transplantation in acute myeloid leukemia (AML): an analysis of 1135 patients, excluding acute promyelocytic leukemia, from the UK MRC AML10 and 12 trials. Blood. 2005;106(10):3658–65.
Meshinchi S, Arceci RJ, Sanders JE, Smith FO, Woods WB, Radich JP, et al. Role of allogeneic stem cell transplantation in FLT3/ITD-positive AML. Blood. 2006;108(1):400. author reply -1
Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358(18):1909–18.
Bornhauser M, Illmer T, Schaich M, Soucek S, Ehninger G, Thiede C. Improved outcome after stem-cell transplantation in FLT3/ITD-positive AML. Blood. 2007;109(5):2264–5. author reply 5
Dezern AE, Sung A, Kim S, Smith BD, Karp JE, Gore SD, et al. Role of allogeneic transplantation for FLT3/ITD acute myeloid leukemia: outcomes from 133 consecutive newly diagnosed patients from a single institution. Biol Blood Marrow Transplant. 2011;17(9):1404–9.
Brunet S, Labopin M, Esteve J, Cornelissen J, Socie G, Iori AP, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. J Clin Oncol. 2012;30(7):735–41.
Ho AD, Schetelig J, Bochtler T, Schaich M, Schafer-Eckart K, Hanel M, et al. Allogeneic stem cell transplantation improves survival in patients with acute myeloid leukemia characterized by a high allelic ratio of mutant FLT3-ITD. Biol Blood Marrow Transplant. 2016;22(3):462–9.
• Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC, Welch JS, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506–10. One of the first comprehensive studies into the clonal nature of AML through diagnosis and relapse.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mark B. Leick declares no potential conflicts of interest. Mark J. Levis reports grants from Novartis and Astellas and consultancies for Novartis and Daiichi-Sankyo.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Acute Myeloid Leukemias
Rights and permissions
About this article
Cite this article
Leick, M.B., Levis, M.J. The Future of Targeting FLT3 Activation in AML. Curr Hematol Malig Rep 12, 153–167 (2017). https://doi.org/10.1007/s11899-017-0381-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-017-0381-2