Abstract
This chapter gives a review of microdialysis sampling in the skin with an emphasis on dermal sampling and use in bioequivalence assessment of topical formulations. Microdialysis as a research technique is suitable for a number of different study designs for sampling in the skin or subcutaneous tissues. This versatility includes the option of conducting preclinical studies in animals as well as ex vivo human skin set-ups. The effect of even small alterations in a topical formulation can be detected by simultaneous sampling in several areas in the same person, enabling studies of bioavailability and bioequivalence in study groups of relatively few volunteers or patients. Whenever the dermis is the target tissue, dermal microdialysis can be considered the method of choice for acquisition of human in vivo data.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
In this chapter the focus is on microdialysis (MD) methodology with emphasis on cutaneous, i.e., dermal microdialysis (DMD) sampling. MD is an in vivo technique used for sampling endogenous and exogenous substances in the extracellular space, routinely used in the clinic as well as in research settings [13].
MD sampling methodology in the skin and subcutaneous tissue introduces a unique opportunity for in vivo, ex vivo, and in vitro studies of topical drug penetration, and the DMD method has gained ground in the last decade (for an in-depth review, see [20].
MD as a technique provides in vivo chronological, real-time information about the pharmacokinetics of drugs, obtained from the extracellular fluid phase at the site of action, i.e., the target tissue.
Today, there are over 14,000 publications describing the applications of MD in numerous tissues and therapeutic areas and there are over 600 publications on the applications of MD in the skin.
This chapter aims to provide a background for understanding the current position of DMD as one of several eligible methodologies for evaluation of bioequivalence of topical products, proposed by regulatory experts [29, 32].
2 The Methodology (Brief Description)
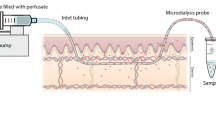
MD is a sampling technique that involves insertion of a MD probe in the tissue of interest with the aim of sampling and subsequently measuring local concentrations of the analyte in the sample.
A probe occurs in various sizes and shapes and consists of a cylindrical semipermeable membrane. The membrane is affixed on to nonpermeable tubings. The semipermeable membrane offers an exchange of solutes through the pores of the membrane. The probe is perfused with a physiological solution (called “perfusate”), which is pumped through the probe at a very slow flow-rate—typically between 1 and 5 µL/min. The slow flow rate is selected to prevent excessive periprobe drainage and/or loss of perfusate volume as well as drainage of molecules into or out of the tissue, thus minimizing any disturbance in the tissue microenvironment. The recovery (by dialysis) of a given drug or substance is independent of the concentration of the drug or substance in the tissue. The resulting solution that exits the probe is called “dialysate” and can be directly analyzed using a well validated bioanalytical method. For most probe types this dialysate is free of cells, larger molecules such as enzymes, and protein.
For a detailed review of the methodology, the probe types available, the calibration of probe efficacy, recovery evaluation, and analytical considerations, please consult Holmgaard et al. [20] for a comprehensive technical review.
3 Planning an In Vivo Bioequivalence Study
There are many choices while preparing for in vivo experimentation. The most important one is probably the study design itself, as the between-subjects variability will, for some topical applications, be much larger than the inter-subject variability. Whenever possible, a design using the opportunity for several test and reference areas in the same person should be chosen. Furthermore, the opportunity of pooling samples in collecting vials for larger sample volume as well as optimizing probe length/membrane area should be considered for low-recovery drugs.
3.1 Choice of Probe Type and Perfusate
Different types of probes for DMD are available [20] and the majority of these probes are inserted in the skin through a small guide cannula as the probes are made of fragile material. The linear probe has a unidirectional flow and compared to the other probe types has a very small diameter—down to approximately 200 μm for some structure. Linear probes will, however, need to penetrate the skin twice when inserted, since they have both an inlet and an outlet. The typical probe used in clinical studies relating to systemic drugs is the concentric probe which, like the side-by-side and loop design probes, has an inlet and outlet placed in parallel with each other. The latter probe types require guide cannulas of larger dimensions (e.g., 500 μm) than those necessary for insertion of the linear probe, which leads to a larger insertion trauma (see later)—and this is a drawback.
In cutaneous MD, the perfusate is most often an isotonic saline solution or a Ringer solution. Depending on the lipophilicity of the drug, the perfusate medium may have to be modified to allow more lipophilic substances to enter the probe. Substances such as albumin, Intralipid®, and Encapsin® have been used [22, 46]. The enhancing effect of adding binding agents such as α, β, γ-cyclodextrins to the perfusate has been evaluated by studying the recovery of several eicosanoids in vitro. Similarly, the effect of adding small organic molecules such as ethanol, propylene glycol, and dimethylsulfoxide has been studied, and the inclusion of arachidonic acid in the perfusate has been shown to increase in vitro relative recovery for hydrophilic analytes [42].
3.2 Application Site
In cutaneous MD, the most frequently used area of application is the dermis of the volar forearm [3,4 6 8, 16–18, 35, 45].
The reason for this is twofold—this is the “standard area” for investigations into noninvasive measurements of skin blood flow and barrier perturbation. Secondly, the area is easily accessible, usually not hairy and not very convex. If there is a need for shaving, this should be done preferably 3 days before the experiment to prevent an artifact by skin barrier perturbation.
Only one arm is used for cutaneous MD at a time, since that arm must be at rest and tied up during the experiment. It is uncomfortable to have both arms tied up. Blood drawn from the other arm can provide measurements of plasma drug concentrations and thus the systemic drug delivery, if relevant.
3.3 Insertion Procedure, Trauma, and Exclusion Criteria
The probes are inserted under clean or sterile procedures after gentle wash of the skin area. To minimize the discomfort during insertion, local anesthesia [18] and application of ice packs onto the skin can be used [1, 24, 45]. The probes are inserted using a 19–23 G cannula as a guide, which is inserted horizontally in the dermis or subcutis. The probe is subsequently inserted in the opposite direction through the open tip of the hollow cannula and tested to secure the functionality. Subsequently, the guide cannula is withdrawn with the probe still in place. The accuracy of placing the probe at the intended depth in the skin depends on the training and experience of the laboratory personnel, and the depth should be measured routinely by ultrasound scanning as part of the experimental protocol, see Sect. 10.6.3.
MD is a minimally invasive technique, but the skin trauma and histamine release evoked by the insertion will cause a reversible increase in the local blood flow, increased skin thickness, and hyperemia in both animals and humans. The tissue trauma needs to subside before sampling can begin and an appropriate equilibration period of minimum 60–90 min in human skin is advisable. The hyperemia reaction lasts between 90 and 135 min, but complete normalization of the skin perfusion may not occur [28]. The use of local anesthesia has been found to reduce the trauma reaction in man [18].
The presence of a MD probe will elicit an inflammatory response after approximately 12 h; infiltration of lymphocytes will begin and after 32 h scar tissue may appear [2]. Following insertion of concentric probes more extensive tissue disruption compared to a linear probe has been reported. The larger reaction could be caused by the larger diameter of the guide cannula used for implantation of concentric probes (e.g., ~ 500 μm).
The tissue reactions are generally reversible and lasting tissue damage is negligible. However, all the cytokines and cells associated with, e.g., inflammatory disease are involved in the trauma reaction [41]. In my experience the development of skin changes, visible at clinical examination after, e.g., 3 months, is very rare. One exception is if the volunteer or patient is prone to keloid formation, and for this reason we recommend screening for this as an exclusion criterion of the study protocol, thus avoiding DMD in keloid-prone individuals.
4 Bioavailability
The bioavailability of a topically applied drug is defined as “the rate and extent to which the active ingredient or active moiety is absorbed and becomes available at the target site. For drugs that are not intended to be absorbed into the bloodstream, bioavailability reflects the rate and extent to which the active ingredient becomes available at the relevant site within the skin” as per FDA “Guidance for Industry” 2002. A recent study demonstrates the use of MD to determine the bioavailability in a study comparing orally administered diclofenac with topically administered diclofenac. Thus, the bioavailability at the target site was measured by placing the MD probe in the subcutaneous tissue as well as the muscular tissue. The relative bioavailability in both muscular and subcutaneous tissue after topical application was significantly higher than after oral administration and the measured plasma levels were much lower when the drug was topically administered compared to oral administration [3, 43, 44, ]. Similarly, the diffusion of ketoprofen from transdermal patch application to the knee joint fluid has been demonstrated in rats and pigs [38]. A recent study explores, by MD sampling in subcutaneous tissues and skeletal muscle, the effect of alterations in the galenic composition of four novel topical diclofenac formulations under development. An improved drug delivery to the tissue (2.7-fold improved) over the reference product, a commercially available diclofenac gel, was demonstrated by Brunner [11].
5 Bioequivalence
FDA describes bioequivalence as a comparative test between two products using specified criteria. Bioequivalence is defined as “the absence of a significant difference in the rate and extent to which the active ingredient or active moiety in pharmaceutical equivalents or pharmaceutical alternatives becomes available at the site of drug action when administered at the same molar dose under similar conditions in an appropriately designed study.” However, the assessment of bioequivalence for locally acting and targeted delivery drugs has presented great challenges to science in the approval of generic drugs .
Kreilgaard et al. published the first human study demonstrating the potential of cutaneous MD for bioequivalence studies of topically applied drugs in 2001 [27]. The aim of that study was to evaluate the cutaneous bioequivalence of a lipophilic drug (lidocaine) applied in a novel topical microemulsion vehicle, compared to a conventional oil-in-water emulsion. Earlier, Kreilgaard had published an experimental study in rats documenting that dermal drug delivery of hydrophilic and lipophilic drugs was improved by microemulsion vehicles [26]. Subsequently, Kreilgaard proved that microemulsion vehicles can increase dermal drug delivery of lipophilic drugs in humans, and that the MD technique combined with an appropriate pharmacokinetic model provides high sensitivity in this bioequivalence study of a topically applied formulation.
When addressing the issue of DMD variability the source of variability in these studies can be extracted from data obtained in hairless rats (which are genetically inbred and of the same sex and age and thus have minimum variability). The increased variability observed in dermal sampling of the topically applied drugs can be seen to arise from interindividual variability in skin penetration kinetics/barrier function and or microcirculation , unrelated to the MD sampling methodology, which is followed by an internal calibrator simultaneously with the ongoing penetration process [40].
The comparability of dermato-pharmacokinetics, so-called tape-stripping methodology, and DMD was demonstrated in a recent study evaluating bioequivalence of lidocaine in ointment and cream [8]. The study showed agreement between the two methods, finding a 3–5 fold higher lidocaine absorption from the cream formulation over the ointment formulation. Statistical calculations from this study indicated that bioequivalence evaluation of topical formulations based on DMD sampling may be conducted using 27 subjects and applying two probes in each application site or 18 subjects using three probes in each application site with 90 % confidence interval and 80–125 % bioequivalence limits, which are the limits applicable to topical bioequivalence evaluations as per FDA protocols [38] .
A theoretical-statistical MD paper found, based on data in the literature, that an evaluation of topical bioequivalence, conducted by duplicate sampling of both formulations in the same human volunteer, could be expected to be conclusive within 80–125 % confidence limits when a population of approximately 20 subjects was to be used [30]. This number of participants was similar to what was calculated in the study on topical lidocaine formulations [8]. In the latter study, 61 % of the experimental variability could be ascribed to inter-subject variability—an important finding for planning of future studies. This result was corroborated by Tettey-Amlalo et al. who demonstrated a very low variability using the exact same probe structure for sampling drug penetration from a topical ketoprofen gel formulation [45]. However, different formulations may demonstrate different variabilities when the cutaneous penetration is studied by DMD; in a study of topical metronidazole creams the variability was higher and the number of participants for a conclusive bioequivalence evaluation would have been 34 [17]. Nevertheless, in comparison with the 6–700 subjects needed for a clinical comparative study, these numbers are small [29].
Another recent study has compared topical penetration of two commercially available tetracycline formulations and also found high variability [23], whereas a study of an aggressive ethanol solution of clobetasol propionate—a drug which it has previously not been feasible to sample by MD in the dermis—showed that using Intralipid ® as the perfusate the drug could now be reproducibly sampled [1].
Dermal MD sampling may also enable bioequivalence studies in diseased skin [6, 16, 36], which can be argued to be closer to the clinical situation than bioequivalence evaluations conducted in healthy subjects. However, variability is likely to be increased and possibly too problematic, see Sect. 10.6.1 below .
6 Sources of Variability
6.1 Skin Barrier Function
Damage of the skin barrier implies an increase in trans-epidermal water loss (TEWL) which can be quantified by measurements above the skin surface. In a number of in vivo DMD studies the impact of experimentally induced skin barrier perturbation on the cutaneous penetration of different substances has been demonstrated [8, 25, 31, 36] . Benfeldt et al. have studied the effect of different barrier disruption methods on cutaneous penetration in hairless rats as well as humans [5, 7]. The studies demonstrated highly increased drug penetration in tape stripped skin (157- and 170-fold respectively, in comparison to the penetration in unmodified skin) and in skin with irritant dermatitis (46- and 80-fold increased penetration). Since the same probe type, perfusate, flow rate, and topical drug solution were used in these studies, a direct comparison between drug penetration in hairless rats and human volunteers could be made. This showed a 46-fold increase in penetration across rat skin when compared with human skin, while increases in penetration, induced by barrier perturbation were of the same order of magnitude [3].
Other more recent studies have compared, e.g., the penetration of acyclovir and salicylic acid on disrupted skin barriers using MD in the dermis and tape stripping [25], and the penetration of a metronidazole cream formulation (1 %) applied to the forearm skin in areas of both irritant dermatitis and normal skin [36]. Furthermore, DMD sampling showed a significant threefold increased penetration of topically applied metronidazole in skin with atopic dermatitis compared with unaffected skin [16] .
6.2 Microcirculation
The bioavailability of topically applied products in skin and underlying tissues is not only dependent on the integrity of the skin barrier, but also on the local blood flow. Vasodilatation as well as vasoconstriction can be physiologically or pharmacologically induced and will have a large influence on the local blood flow. The skin concentration of a topically applied drug will increase if the blood flow is diminished, whereas an increased blood flow enhances the uptake and subsequent systemic distribution and elimination of the drug from the skin [2]. Experimental studies which induce vasoconstriction or vasodilatation have demonstrated that the bioavailability of topically as well as systemically applied test substances is highly influenced by changes in the microcirculation of the skin [9, 12, 37]—an influence much stronger than the influence of variations in probe depth.
Topically administered substances have been studied, with added noradrenaline for vasoconstriction and the nitric oxide donor glyceryltrinitrate for vasodilatation, delivered by the MD probe. The changes seen in the dialysate concentration reflected the changes in the microcirculation [10, 14].
6.3 Probe Depth in the Skin
An influence of probe depth on the drug levels sampled following topical drug application is likely from a theoretical point of view. The recommendation is to measure (normally by 20 MHz ultrasound scanning) the probe depth in three separate scans along the length of the probe in situ. With experience, probes can be inserted with great accuracy and low variability [8, 45], e.g., 0.7 ± 0.15 mm mean ± SD [36]. The preferable insertion depth is 0.6–1.0 mm in the dermis.
The original study regarding transdermal delivery of nicotine showed a correlation between depth and drug concentration, but only when the analysis included different skin layers (both dermal and subcutaneous probe placement) [33]. Following this, several studies have been unable to show a correlation between the depth of the probe and the drug concentration [8, 17, 19, 35, 36]. However, in a very recent MD study, conducted in ex vivo human donor skin, an inverse relationship between the depth of the probe in the dermis and the amount of drug sampled following topical penetration is demonstrated [21]. The result is of relevance to the in vivo situation, and it can be predicted that the differences in sampling at different probe depths will have a more significant impact in the beginning of a study or in studies of short duration.
Based on this study it can be recommended that studies of topical drug penetration using DMD sampling should include measurements of probe depth and that efforts should be made to minimize probe depth variability (e.g., to have few and similarly trained persons undertaking insertion as well as ultrasound scanning or other imaging technique for feedback of probe depth achieved) [21].
7 Advantages and Limitations
The methodological challenges that may influence in vivo experiments may typically be identified through well-planned in vitro experiments as a part of the planning phase. Founded on pre-experimental troubleshooting, conducted previous to in vivo experimentation, reproducible results with acceptable variability and validated analysis can be achieved for most drugs.
7.1 Advantages
-
MD captures the pharmacological events where they take place in the tissues, providing high-resolution real-time details.
-
There is no fluid extraction from the tissue.
-
DMD sampling allows testing of both test and reference product at the same time in the same individuals.
-
Both the drug of interest and the metabolites.
-
The method can provide protein-free samples, which is often an analytical advantage.
-
Cessation of enzyme degradation in the samples.
-
DMD is a relatively inexpensive method to use once the MD pumps have been acquired.
-
The probes allow sampling as well as delivery of substances.
-
Multiple application sites.
-
Good reproducibility.
-
DMD sampling of topical drug formulation in the bioavailability/bioequivalence setting does not depend on drug concentrations in the formulations being the same.
-
DMD sampling can be used in the presence of barrier perturbation or skin disease (unlike other methods for skin penetration assessment).
7.2 Limitations
-
Drugs with a very high lipophilicity are excellent for topical application but less favorable for sampling by MD (the tape-stripping method will often be more suitable).
-
An in vitro relative recovery of less than 4 % will most often characterize a compound as unsuitable for MD studies due to an expected even lower in vivo recovery.
-
Some topical drug formulations are of very low drug concentrations—the analysis of the dialysate will unavoidably be very challenging.
-
For protein-bound substances it is often necessary to add a protein to the perfusate to increase recovery, which will often result in more complex analytical procedures.
-
Low variability in probe insertion and probe manufacturing depends on experienced personnel.
-
The dialysate concentration will decrease with increasing flow rate and vice versa since the relative recovery of substances is flow-dependent. However, if very low flow rates are used,the time resolution can be compromised.
-
Analytical procedures may require extensive modifications prior to in vivo experiments.
-
Both drug concentrations in the tissue as well as recovery by MD sampling are known to be influenced by blood flow and this must be considered in the planning stage of an experiment.
-
The duration of a DMD experiment is in many instances limited for practical reasons, and this creates a limitation for slowly penetrating substances (this can be overcome by the use of portable pumps, which in turn typically have relatively few syringe spaces).
-
As a consequence of the above-mentioned relationships between topical dose, dermal concentration, and the ensuing concentration in the microdialysates, MD sampling in the skin will hardly ever be the right method for toxicological studies of low dose/real life skin exposures—other methods will be more relevant.
8 Future Research
A key issue in the development of a standardized protocol is the reproducible insertion of the probe at a consistent depth within the skin. Reproducible probe insertion is a skill which is only improved with practice. It is evident that implantation in either the superficial dermis or the subcutaneous tissue will affect the data collected. A thorough evaluation of this methodological issue, preferably studied under human in vivo conditions, using drugs of varying MW and lipophilicity, is needed.
Most MD studies are of limited time duration, typically less than 8 h, and histological studies of the skin response to probe implantation have not showed signs of tissue inflammation [36]. Studies with more extended observation and sampling periods have, however, demonstrated that infiltration of lymphocytes and even development of scar tissue may be observed over [36, 41].
Another concern, particularly relevant to DMD, is the use of MD in studies of inflammatory and immune-mediated diseases. Here the molecules of interest will very often be the same as those generated during the insertion trauma and subsequent wound repair. Care should be taken to incorporate the proper controls in order to confirm the correct relation between molecule, implantation trauma, and disease process [41] Studies of the exact relation between probe implantation (depth, diameter, time since implantation) and the ensuing tissue damage will be important in the further development of the MD methodology, also from an ethical point of view.
In vitro drug penetration studies using static or flow-through diffusion cells have been extensively used in the past, and there is a pertinent need to establish a correlation between results from in vitro penetration studies of topical drugs and in vivo data obtained by MD methodology.
Only few MD studies in diseased skin have been performed. An improved knowledge of the impact of the structural changes in diseased or otherwise impaired skin on topical drug penetration in vivo will improve the development of topical therapies. Furthermore, the relevance is supported by the fact that a substantial fraction of the general population suffer from different skin conditions that make the skin barrier potentially more permeable—an aspect relevant also to the occupational setting [15].
MD methodology has a place among other methodologies employed in drug research, preclinical test phases, and clinical studies as well as for use in industry for regulatory approval purposes. The potential for studies of bioequivalence of topical formulations and in evaluation of line extensions of topical is huge.
Whether the method is employed for sampling of pharmacokinetic or pharmacodynamic information, MD in the skin as a technique offers a unique opportunity for real-time chronological sampling in the target tissue.
References
Au WL, Skinner MF, Benfeldt E, Verbeeck RK, Kanfer I. Application of dermal microdialysis for the determination of bioavailability of clobetasol propionate applied to the skin of human subjects. Skin Pharmacol Physiol. 2012;25(1):17–24. doi:10.1159/000330489.
Ault JM, Riley CM, Meltzer NM, Lunte CE. Dermal microdialysis sampling in vivo. Pharm Res. 1994;11(11):1631–9.
Benfeldt E. in vivo microdialysis for the investigation of drug levels in the dermis and the effect of barrier perturbation on cutaneous drug penetration. Studies in hairless rats and human subjects. Acta Derm Venereol. 1999;206:1–59. http://www.ncbi.nlm.nih.gov/pubmed/10605601.
Benfeldt E, Groth L. Feasibility of measuring lipophilic or protein-bound drugs in the dermis by in vivo microdialysis after topical or systemic drug administration. Acta Derm Venereol. 1998;78(4):274–8. http://www.ncbi.nlm.nih.gov/pubmed/9689295.
Benfeldt E, Serup J. Effect of barrier perturbation on cutaneous penetration of salicylic acid in hairless rats: in vivo pharmacokinetics using microdialysis and non-invasive quantification of barrier function. Arch Dermatol Res. 1999;291(9):517–26. http://www.ncbi.nlm.nih.gov/pubmed/10541883.
Benfeldt E, Serup J, Menné T. Effect of barrier perturbation on cutaneous salicylic acid penetration in human skin: in vivo pharmacokinetics using microdialysis and non-invasive quantification of barrier function. Br J Dermatol. 1999a;140(4):739–48. http://www.ncbi.nlm.nih.gov/pubmed/10233334.
Benfeldt E, Serup J, Menné T. Microdialysis vs. suction blister technique for in vivo sampling of pharmacokinetics in the human dermis. Acta Derm Venereol. 1999b;79(5):338–42. http://www.ncbi.nlm.nih.gov/pubmed/10494706.
Benfeldt E, Hansen SH, Vølund A, Menné T, Shah VP. Bioequivalence of topical formulations in humans: evaluation by dermal microdialysis sampling and the dermatopharmacokinetic method. J Invest Dermatol. 2007;127(1):170–8. doi:10.1038/sj.jid.5700495.
Borg N, Götharson E, Benfeldt E, Groth L, Ståhle L. Distribution to the skin of penciclovir after oral famciclovir administration in healthy volunteers: comparison of the suction blister technique and cutaneous microdialysis. Acta Derm-Venereol. 1999;79(4):274–7. http://www.ncbi.nlm.nih.gov/pubmed/10429982.
Boutsiouki P, Thompson J, Clough G. Effects of local blood flow on the percutaneous absorption of the organophosphorus compound malathion: a microdialysis study in man. Arch Toxicol. 2001;75(6):321–8. doi:10.1007/s002040100245.
Brunner M, Davies D, Martin W, Leuratti C, Lackner E, Müller M. A new topical formulation enhances relative diclofenac bioavailability in healthy male subjects. Br J Clin Pharmacol. 2011;71(6):852–9. doi:10.1111/j.1365-2125.2011.03914.x.
Chaturvedula A, Joshi DP, Anderson C, Morris R, Sembrowich WL, Banga AK. Dermal, subdermal, and systemic concentrations of granisetron by iontophoretic delivery. Pharm Res. 2005;22(8):1313–9. doi:10.1007/s11095-005-5335-z.
Chaurasia CS, Müller M, Bashaw ED, Benfeldt E, Bolinder J, Bullock R, Bungay PM, et al. AAPS-FDA workshop white paper: microdialysis principles, application and regulatory perspectives. Pharm Res. 2007;24(5):1014–25. http://www.ncbi.nlm.nih.gov/pubmed/17458685.
Clough GF, Boutsiouki P, Church MK, Michel CC. Effects of blood flow on the in vivo recovery of a small diffusible molecule by microdialysis in human skin. J Pharmacol Exp Ther. 2002;302(2):681–6. doi:10.1124/jpet.102.035634.sampling
Fulzele SV, Babu RJ, Ahaghotu E, Singh M. Estimation of proinflammatory biomarkers of skin irritation by dermal microdialysis following exposure with irritant chemicals. Toxicol. 2007;237(1–3):77–88. http://www.ncbi.nlm.nih.gov/pubmed/17574719.
Garcia Ortiz P, Hansen SH, Shah VP, Menné T, Benfeldt E. Impact of adult atopic dermatitis on topical drug penetration: assessment by cutaneous microdialysis and tape stripping. Acta Derm Venereol. 2009;89(1):33–8. doi:10.2340/00015555-0562.
García Ortiz P, Hansen SH, Shah VP, Sonne J, Benfeldt E. Are marketed topical metronidazole creams bioequivalent? Evaluation by in vivo microdialysis sampling and tape stripping methodology. Skin Pharmacol Physiol. 2011;24(1):44–53. doi:10.1159/000320151.
Groth L, Serup J. Cutaneous microdialysis in man: effects of needle insertion trauma and anaesthesia on skin perfusion, erythema and skin thickness. Acta Derm-Venereol. 1998;78(1):5–9. http://www.ncbi.nlm.nih.gov/pubmed/9498017.
Hegemann L, Forstinger C, Partsch B. Microdialysis in cutaneous pharmacology: kinetic analysis of transdermally delivered nicotine. J Invest Dermatol. 1995;104(5):838–43. http://www.nature.com/jid/journal/v104/n5/abs/5610897a.html.
Holmgaard R, Nielsen JB, Benfeldt E. Microdialysis sampling for investigations of bioavailability and bioequivalence of topically administered drugs: current state and future perspectives. Skin Pharmacol Physiol. 2010;23(5):225–43. doi:10.1159/000314698.
Holmgaard R, Benfeldt E, Bangsgaard N, Sorensen JA, Brosen K, Nielsen F, Nielsen JB. Probe depth matters in dermal microdialysis sampling of benzoic acid after topical application: an ex vivo study in human skin. Skin Pharmacol Physiol. 2012;25(1):9–16. doi:10.1159/000330491.
Holmgaard R, Benfeldt E, Nielsen JB, Gatschelhofer C, Sorensen JA, Höfferer C, Bodenlenz M, et al. Comparison of open-flow microperfusion and microdialysis methodologies when sampling topically applied fentanyl and benzoic acid in human dermis ex vivo. Pharm Res. 2012;29(7):1808–20. doi:10.1007/s11095-012-0705-9.
Incecayir T, Agabeyoglu I, Derici U, Sindel S. Assessment of topical bioequivalence using dermal microdialysis and tape stripping methods. Pharm Res. 2011;28(9):2165–75. doi:10.1007/s11095-011-0444-3.
Kellogg DL, Zhao JL, Wu Y. Neuronal nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. J Physiol. 2008;586(3):847–57. doi:10.1113/jphysiol.2007.144642.
Klimowicz A, Farfal S, Bielecka-Grzela S. Evaluation of skin penetration of topically applied drugs in humans by cutaneous microdialysis: acyclovir vs. salicylic acid. J Clin Pharm Ther. 2007;32(2):143–8. doi:10.1111/j.1365-2710.2007.00803.x.
Kreilgaard M. Dermal pharmacokinetics of microemulsion formulations determined by in vivo microdialysis. Pharm Res. 2001;18(3):367–73. http://www.springerlink.com/index/j42w1130m307g658.pdf.
Kreilgaard M, Kemme MJ, Burggraaf J, Schoemaker RC, Cohen AF. Influence of a microemulsion vehicle on cutaneous bioequivalence of a lipophilic model drug assessed by microdialysis and pharmacodynamics. Pharm Res. 2001;18(5):593–9. http://www.ncbi.nlm.nih.gov/pubmed/11465413.
Krogstad A, Lonnroth P, Larson G, Wallin BG. Increased interstitial histamine concentration in the psoriatic plaque. J Invest Dermatol. 1997;109:632–5. http://www.nature.com/jid/journal/v109/n5/abs/5610032a.html.
Lionberger RA. FDA critical path initiatives: opportunities for generic drug development. AAPS J. 2008;10(1):103–9. doi:10.1208/s12248-008-9010-2.
McCleverty D, Lyons R, Henry B. Microdialysis sampling and the clinical determination of topical dermal bioequivalence. Int J Pharm. 2006;308(1–2):1–7. doi:10.1016/j.ijpharm.2005.09.020.
Morgan CJ, Renwick AG, Friedmann PS. The role of stratum corneum and dermal microvascular perfusion in penetration and tissue levels of water-soluble drugs investigated by microdialysis. Br J Dermatol. 2001;148(3):434–43.
Mugglestone CJ, Mariz S, Lane ME. The development and registration of topical pharmaceuticals. Int J Pharm. 2012;435(1):22–6. doi:10.1016/j.ijpharm.2012.03.052.
Müller M, Schmid R, Wagner O, Osten v. B., Shayganfar H, Eichler GH. In vivo characterization of transdermal drug transport by microdialysis. J Control Release. 1995;37:49–57
Müller M, Mascher H, Kikuta C, Schäfer S, Brunner M, Dorner G, Eichler HG. Diclofenac concentrations in defined tissue layers after topical administration. Clin Pharmacol Ther. 1997;62(3):293–9. doi:10.1016/S0009-9236(97)90032-1.
Ortiz PG, Hansen SH, Shah VP, Menné T, Benfeldt E. The effect of irritant dermatitis on cutaneous bioavailability of a metronidazole formulation, investigated by microdialysis and dermatopharmacokinetic method. Contact Dermat. 2008;59(1):23–30. doi:10.1111/j.1600-0536.2008.01348.x.
Sandberg C, Halldin CB, Ericson MB, Larkö O, Krogstad AL, Wennberg AM. Bioavailability of aminolaevulinic acid and methylaminolaevulinate in basal cell carcinomas: a perfusion study using microdialysis in vivo. Br J Dermatol. 2008;159(5):1170–6. doi:10.1111/j.1365-2133.2008.08795.x.
Seki T, Wang A, Yuan D, Saso Y, Hosoya O, Chono S, Morimoto K. Excised porcine skin experimental systems to validate quantitative microdialysis methods for determination of drugs in skin after topical application. J Control Release. 2004;100(2):181–9. doi:10.1016/j.jconrel.2004.08.016.
Shah V, Flynn G, Yacobi A. Bioequivalence of topical dermatological dosage forms-methods of evaluation of bioequivalence. Skin Pharmacol Appl Skin Physiol. 1998;11(2):117–24. http://www.karger.com/Article/Fulltext/29817.
Shinkai N, Korenaga K, Okumura Y, Mizu H, Yamauchi H. Microdialysis assessment of percutaneous penetration of ketoprofen after transdermal administration to hairless rats and domestic pigs. Eur J Pharm Biopharm. 2011;78(3):415–21. doi:10.1016/j.ejpb.2011.03.005
Simonsen L, Jørgensen A, Benfeldt E, Groth L. Differentiated in vivo skin penetration of salicylic compounds in hairless rats measured by cutaneous microdialysis. Eur J Pharm Sci. 2004;21(2–3):379–88. doi:10.1016/j.ejps.2003.11.004.
Stenken JA, Church, MK, Gill CA, Clough GF. How minimally invasive is microdialysis sampling? A cautionary note for cytokine collection in human skin and other clinical studies. AAPS J. 2010;12(1):73–8. doi:10.1208/s12248-009-9163-7.
Sun L, Stenken JA. Improving microdialysis extraction efficiency of lipophilic eicosanoids. J Pharm Biomed Anal. 2003;33(5):1059–71. doi:10.1016/S0731-7085(03)00363-7.
Tegeder I, Muth-Selbach U, Lötsch J, Rüsing G, Oelkers R, Brune K, Meller S, Kelm GR, Sörgel F, Geisslinger G. Application of microdialysis for the determination of muscle and subcutaneous tissue concentrations after oral and topical ibuprofen. Clin Pharmacol Therapeut. 1999;(65):357–68. doi:10.1016/S0009-9236(99)70128-1.
Tegeder I, Lötsch J, Kinzig-Schippers M, Sörgel F, Kelm GR, Meller ST, Geisslinger G. Comparison of tissue concentrations after intramuscular and topical administration of ketoprofen. Pharm Res. 2001;18(7):980–6. http://www.ncbi.nlm.nih.gov/pubmed/11496958.
Tettey-Amlalo RNO, Kanfer I, Skinner MF, Benfeldt E, Verbeeck RK. Application of dermal microdialysis for the evaluation of bioequivalence of a ketoprofen topical gel. Eur J Pharma Sci. 2009;36(2–3):219–25. doi:10.1016/j.ejps.2008.09.002.
Ward KW, Medina SJ, Portelli ST, Mahar Doan KM, Spengler MD, Ben MM, Lundberg D, et al. Enhancement of in vitro and in vivo microdialysis recovery of SB-265123 using Intralipid and Encapsin as perfusates. Biopharm Drug Dispos. 2003;24(1):17–25. doi:10.1002/bdd.332.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Benfeldt, E. (2014). Application of Microdialysis in Assessing Cutaneous Bioavailability. In: Shah, V., Maibach, H., Jenner, J. (eds) Topical Drug Bioavailability, Bioequivalence, and Penetration. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1289-6_10
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1289-6_10
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1288-9
Online ISBN: 978-1-4939-1289-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)