ABSTRACT
Purpose
To assess the bioequivalence of two commercial topical formulations of oxytetracycline HCl by tape stripping and microdialysis in healthy volunteers.
Methods
Tape stripping study was conducted on 12 healthy volunteers. After a 30-minute application of the formulations, adhesive tapes were used to sample stratum corneum at 0.25, 0.5, 1, 1.5, 2, 3, 4 hr. Ten of these volunteers were included in the microdialysis study with a period of 4 weeks between the experiments. Microdialysis probes were inserted into the dermis of the forearm. Following the application of the test and reference simultaneously, dialysates were collected in 30-minute sampling intervals up to 4 hr.
Results
Pharmacokinetic evaluation by microdialysis yielded that the test could not be said to be bioequivalent to the reference at 90% CI. The intersubject variability of oxytetracycline content in stratum corneum was moderate when it was compared to the dermal levels. The test was found to be bioequivalent to reference according to the dermatopharmacokinetic evaluation by tape stripping.
Conclusions
No significant correlations were found between microdialysis and tape stripping methods as regarding the topical bioequivalence of oxytetracycline HCl formulations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Assessment of bioequivalence (BE) of topical dermatological dosage forms is a challenge for pharmaceutical and regulatory scientists. As dermatological drug products are designed to target the local tissue to which they are applied, the amount of drug reaching systemic circulation is very small. Therefore, the general procedure used for BE determination of orally administered drug products is not suitable for topical products. Possible methods for the determination of BE of multisource topical drug products include clinical trials, pharmacodynamics, tape stripping (TS), dermal microdialysis (DMD), and other techniques. Among these, TS and DMD methods are declared to be the promising ones (1–3).
The TS method, which was also mentioned as the dermatopharmacokinetics (DPK) method, is attracting increasing attention as a method with which to assess the rate and extent of topical drug bioavailability (BA) in the rate-limiting barrier of skin, the stratum corneum (SC). This method is used by applying an adhesive tape to the skin surface and then removing it, thus collecting layers of cells from SC. The DPK concept involves determination of the amount of drug present in the SC as a function of time post-application and post-removal of the formulation (4–6). However, this noninvasive method has the drawback of lack of relevance to clinical safety/efficacy and not being standardized to use in diseased skin (6).
Microdialysis (MD) is an in vivo sampling method for measuring endogenous and exogenous compounds in extracellular spaces of tissues. The basic principle of MD is to mimic the passive function of a small blood vessel by perfusing a tubular semipermeable dialysis membrane implanted in the tissue. Compounds can be removed from or can be added to the extracellular space by diffusion through the dialysis membrane (7,8). Since the early 1990s, the principle has been adapted for dermatological research (9). For DMD, a small probe equipped with a semipermeable hollow fiber (the probe) is inserted superficially into dermis, parallel to the skin surface, and sampling the levels of compounds in dermis can be performed by simple diffusion. It is feasible to monitor topical drug penetration continuously with detailed real-time chronology by using DMD in dermis. The principle of MD is that a physiological solution pumped through the probe is in equilibrium with the diffusible molecules in the extracellular fluid of the surrounding tissue (10). The main feature of MD is the possibility of assessing drug levels in the target tissue directly, which is very useful to obtain the full pharmacokinetic (PK) profiles from each sampling site. However, this method needs standardization and more BA and BE studies on the field of topical drug administration (11).
The aim of the present study was to assess the BE of two commercial topical formulations of a wide spectrum antibiotic, oxytetracycline HCl (OTC), by TS and DMD in healthy volunteers and to evaluate the correlation between these methods.
MATERIALS AND METHODS
Materials
Two different OTC preparations containing 30 mg OTC per gram (Polimisin® skin ointment, Koçak Farma, serial no:1426701, date of manufacture: 03-2007, Turkey and Terramycin® skin ointment, Pfizer, serial no:120, date of manufacture: 09-2008, Turkey) were used for topical applications as the test and the reference, respectively. Pharmaceutical grade OTC, analytical grade oxalic acid and formic acid were purchased from Fluka (Sigma-Aldrich Laborchemikalien, Germany). Methanol HPLC grade was from Riedel-de Haën (Sigma-Aldrich Laborchemikalien, Germany).
Subjects
TS study was performed with 12 healthy volunteers (five females, seven males), aged 23 ± 1 years, with no known drug allergy or dermatological disease. Eleven of these subjects also volunteered to participate in the MD study. They were included in the MD study with a minimum period of 4 weeks between TS and MD experiments. One of these volunteers was further excluded from the MD study, because the implanted probes malfunctioned during the experiment.
None of the subjects used any other medications. The subjects refrained from using any topical formulations 1 week preceding each experiment. They were within 10% of their ideal body weight (68 kg and BMI, body mass index: 18.5–24.9) (12). Physical examination and biochemical tests (blood chemistry and hematology) were also carried out. Their medical history was obtained, and all were found to be suitable for the study. The study protocol was approved by the local Ethics Committee of Gazi University Hospital, Ankara, Turkey. The subjects also gave written informed consent to participate in the study.
Study Design
Dermal Microdialysis
For MD experiments, the subjects were made to lay in the supine position at room temperature with the left arm placed on an armrest. Two circular areas (i.d.: 4.3 cm), approximately 2.7 cm between the sides, were drawn on the ventral forearm. The areas were disinfected by alcohol. A CMA 66 Linear MD probe with membrane length 30 mm (CMA, Sweden) was inserted superficially into the dermis of each circular area with the help of the needle introducer, after which it was withdrawn (Fig. 1). The diameter of the dialysis membrane was 0.5 mm with a molecular cut-off of 20 kD. The entry and exit puncture sites were sealed with a drop of cyanoacrylate glue (Pattex®, Henkel) to prevent potential drug contamination from topical formulations. This implantation was done without anesthesia under aseptic conditions. The MD probes were perfused with normal saline (sterile and isotonic, 0.9% NaCl sol.) at a flow rate of 2 μl/min with the use of the MD pump (CMA 107, CMA, Sweden). After the insertion of the probes, a 30-minute equilibration period was used to diminish the insertion trauma. Then, 4 ml of test and reference formulations were applied simultaneously to the test areas of 4.3 cm diameter. Dialysates were then collected in 200 μl microvials in 30-minute sampling intervals, giving 60 μl samples each for analysis, for up to 4 h after the initial 30-minute application. The total period of the treatment applied in MD method was 4.5 hr for test and reference. The dialysate samples were kept frozen until the time of analysis, which was less than 1 week after the collection of the dialysate samples.
Relative Recovery
The relationship between the concentration of a drug in the dialysate to the dialyzed tissue or medium is termed “relative recovery” (RR) and is expressed as a percent value (13). In the present study, in vitro RR studies for OTC across the MD membrane were performed before in vivo MD studies to ensure reproducible and concentration-independent sampling of OTC during the experiment. The probe was placed into constantly stirred OTC solutions with concentrations of 0.250–50.0 μg/ml at 35°C and perfused with saline (0.9% NaCl sol.) at a flow rate of 2 μl/min up to 5 hr. Dialysate concentrations were plotted versus the respective concentration of the OTC solution. The dialysate samples were collected through 30-minute intervals for each concentration, and in vitro RR was calculated according to Eq. 1:
where Cdialysate and Cmedium are the drug concentrations in dialysate and the medium/tissue, respectively.
Drug concentration in the dialysate represents a fraction of the concentration in the dermal extracellular fluid. To obtain absolute tissue concentrations from dialysate concentrations, an in vivo calibration was carried out by the retrodialysis method (14). The principle of this method relies on the assumption that the diffusion process is quantitatively equal in both directions through the semipermeable membrane. Therefore, the compound of interest can be added to the perfusion medium, and the disappearance rate through the membrane can be taken as the in vivo RR. For in vivo RR experiments, one subject had two MD probes inserted in the dermis of the forearm. The probes were perfused at a flow rate of 2 μl/min for 4 hr with sterile and isotonic saline containing 5 μg/ml OTC. The relative loss of OTC through the membrane from the perfusate to the dermis was taken as the in vivo RR. It was calculated using Eq. 2, where Cperfusate is the drug concentration in the perfusion fluid:
Tissue concentrations were calculated according to Eq. 3:
Ultrasonography
The depth of the MD probe from the skin surface was determined on a representative subject at the end of the experiment. Three ultrasound measurements were performed over each probe in the skin by ultrasound scanning at 10 MHz, using GE LOGIQ 9 Ultrasound system with 11L wideband transducer (USA).
Tape Stripping
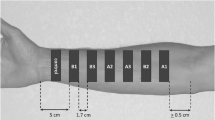
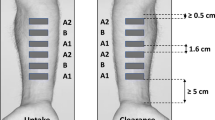
For TS sampling, both the right and left arms of the volunteers were used. One control and seven drug application sites (2.8 cm2) were assigned by putting a plastic chamber (i.d: 1.9 cm) over the skin on the right and left forearms. Hair was gently removed with a hair clipper, if it was necessary. Approximately 1 ml of test formulation was applied and spread over each area except the control one, at least 3 cm from either the wrist, or the bend of the elbow (Fig. 2). After a 30-minute treatment period, the residual formulation was removed from the drug-treated sites by gently wiping with a clean paper towel. Then, 15 consecutive adhesive tapes (Crystal 3M Scotch, USA) (1.9 × 2.0 cm) were used to remove SC of each drug-treated site at 0.25, 0.5, 1, 1.5, 2, 3 and 4 hr after the treatment removal. Tapes were applied and removed by pincers, using gentle pressure to assure good skin contact. The first two strips were discarded, because of the potential residual drug contamination at each time point. The remaining tape strips were extracted with methanol and kept at +4°C overnight before they were analyzed. Reference formulation was applied on the forearms of each volunteer, as described, after a 4-week wash-out and SC repair period. The study was accomplished over a period of 3 months.
Analytical Methods
High Performance Liquid Chromatography (HPLC)
OTC concentrations in TS samples were determined by HPLC. It was performed by using a Hewlett-Packard Series 1050 chromatography and Hewlett Packard 3396 Series II integrator system (Germany). The HPLC method was obtained from the literature (15), but worked over for better separation. Separations were carried out using an ACE-5 C18 reverse-phase column, 25 cm × 4.6 mm and 5 μm particle size (Advanced Chromatography Technologies, Scotland). Tape strips no. 3–15, applied on the same treatment area, were extracted with 10 ml of methanol overnight. The mixture was vortexed for 5 min before analysis. The supernatant was filtered through a 0.22 μm membrane filter, and 20 μl was injected onto the column. The mobile phase was methanol:0.01 M oxalic acid buffer (40:60, v/v) and delivered at a flow rate of 1 ml.min−1. The eluate was analyzed at a wavelength of 354 nm. No internal standard was used. Quality control (QC) samples were used to assess within-day precision. The retention time of OTC was 5 min. The method was linear over a concentration range of 0.500–64.0 μg/ml.
The limit of quantification (LOQ) was defined as the lowest OTC concentration that could be determined with a precision below 15% and with an accuracy between 85 and 115%. The limit of detection (LOD) was defined as the lowest concentration of the analyte that can be detected above the baseline signal. It was estimated as three times the noise level. LOQ and LOD values were 500 and 100 ng/ml, respectively. The within-day and between-day precisions expressed as coefficient of variation (CV) % were in the range of 0.237–1.25%. Blank samples of SC were extracted with metanol and spiked with known amounts of OTC for the determination of the recovery from tape strips. The recoveries ranged from 96.6 to 103%.
Ultra Performance Liquid Chromatography Tandem Mass Spectroscopy (UPLC-MS-MS)
OTC concentrations in dialysates were determined by UPLC-MS/MS and corrected for in vivo recovery. The LC-MS method obtained from the literature (16) was modified for this analysis. An Acquity™ UPLC System with Quattro Premier XE Tandem quadropole (Waters, USA) was used. MS-tuned spectra of OTC released two product ions, m/z 425.85 and m/z 442.85, used for quantification and confirmation, respectively. Separation was performed on Acquity™ BEH C18 column, 100 mm × 2.1 mm and 1.7 μm particle size (Waters, USA) in positive-electrospray ionization mode. Dialysate samples, diluted by methanol, were directly injected into UPLC-MS-MS. Mobile phase A contained 20:80 methanol:water with 8.25 mM formic acid and mobile phase B contained 95:5 methanol:water with 8.25 mM formic acid under gradient conditions with a flow rate of 0.25 ml/min. Injection volume was 10 μl with a column temperature of 35°C. No internal standard was used.Retention time of OTC was 2.3 min. Calibration curve was linear over the range of 0.5–100 ng/ml. Analytical recoveries of OTC were 96.1–105%. Intra-day precision ranged from 2.10 to 8.87% CV. LOQ and LOD values were 0.6 and 0.2 ng/ml, respectively.
Data Analysis
For TS PK data, OTC content at the treated sites was expressed as microgram OTC per square centimeter application site (μg/cm2). Peak content (Cmax) was the maximum observed OTC content of the DPK profile. Time to Cmax (tmax) was that time point at which Cmax was observed. Area under the OTC content-time profile (AUC) values were calculated over the 0 to tlast-hour time interval using the log-trapezoidal rule method (17). DPK analysis was performed based on the one-compartment open model. All PK parameters were calculated by WinNonlin® Professional V.5.2.1 (Pharsight®, USA). The AUC and Cmax were evaluated in order to assess BE of the test versus the reference. Ninety percent confidence intervals (CI) were calculated using the two one-sided test procedure, and limits of 80–125% based on log-transformed data were used as the acceptance criteria for BE.
For MD PK data, mean ± standard deviation (SD) dermis concentrations of OTC were plotted versus time. The midpoints of the dialysis intervals were used as time points. The time of drug administration is referred to as time zero. The maximum concentration in dermis (Cmax) and tmax were taken directly from the data. The area under the concentration-time profiles (AUC) were calculated using log-trapezoidal rule method (17). AUC and Cmax were evaluated in order to assess BE of the test and the reference as described for TS data.
The independent t-test was used to assess differences between means of paired PK parameters. Differences were considered statistically significant at p < 0.05. Linear regression analysis was used to evaluate the relationship between TS and MD methods.
RESULTS
Relative Recovery Measurements
In these experiments, there was a linear correlation between drug concentrations in the dialysate and bathing medium over a wide range (r 2 = 1.00). The linear regression analysis of in vitro recovery experiments is shown in Fig. 3. The slope of the regression analysis gave an in vitro RR of 63.8%. RR values during sampling periods varied between 56.7–64.6%.
In vivo RR value measured by the retrodialysis method in human skin was found to be 66.2 ± 1.8% (n = 8) for dermis and used to correct the dermis dialysate concentrations.
Pharmacokinetic Evaluation by Dermal Microdialysis
The time course of OTC levels in the dialysates of human dermis for all volunteers is shown in Fig. 4. The intersubject variability in OTC dermal concentrations ranged from 65.8 to 116% for the reference and from 88.1 to 136% for the test. The AUC ratio for test/reference was 2.25 ± 2.16 (±SD). AUC, Cmax and tmax values are given in Table I. The BE of the test versus the reference was 89–135% for AUC and 82–161% for Cmax with a 90% Cl, which is outside of 80–125% BE criteria. The test product could not be said to be bioequivalent to the reference as regarding log transformed AUC and Cmax at 90% CI.
Probe Depth Measurements
According to ultrasound measurements, the mean probe depth in the skin was found to be 1.5 ± 0.3 mm (mean ± SD, n = 6) with a CV % of 1.7.
Dermatopharmacokinetic Evaluation by Tape Stripping
The mean DPK profiles of OTC obtained for the test and the reference in human forearm SC are shown in Fig. 5. The intersubject variability in OTC contents at the individual time points in the DPK profile ranged from 38.9 to 51.0% and from 19.2 to 45.0% for the reference and the test, respectively. The AUC ratio for test/reference was found to be 0.838 ± 0.460 (±SD). AUC, Cmax and tmax values are given in Table II. The BE of the test versus the reference was 88–100% for AUC and 82–97% for Cmax with a 90% CI. The test product was found to be bioequivalent to the reference as regarding log transformed AUC and Cmax at 90% CI.
DPK analysis was performed by WinNonlin® using Eq. 4 (Table III):
where C is the amount at SC (μg/cm2), t is the time after the end of the application (hr), D is the dose (μg), ka is the absorption rate constant (hr−1), and kd is the elimination rate constant (hr−1). Vd, the volume of distribution (ml) is calculated by multiplying V, the area of distribution, by the mean SC thickness (20 μm) (18).
Correlation Between Tape Stripping and Dermal Microdialysis Methods
Correlations between the two methods were evaluated by testing PK parameters of AUC and Cmax or their logarithms from TS versus DMD results for the same subjects. There was a weak correlation between TS and DMD as regarding to AUC and Cmax values for both test and reference (Fig. 6).
DISCUSSION
Two methods, namely the TS and MD are currently under close examination for the assessment of topical BA and BE. In dermatopharmacology, TS or DPK is used to assess cutaneous drug levels in the skin after topical dermatological treatment. It follows that TS is a particularly useful method to assess the local BA of drugs whose target site is the SC itself, such as antifungals, UVA/UVB filters or antiseptics. TS method may also offer a real alternative for local BA/BE assessment of topically applied dermatological agents, whose target is the underlying viable tissues. As the SC is usually the principle resistance to the penetration of topically applied compounds, it has been argued that drug levels in the barrier should be correlated with those attained in the underlying skin layers, epidermis and dermis at which many dermatological diseases are manifest (19). The draft guidance describing the general procedures for conducting a topical BA/BE study was published by the US Food and Drug Administration (FDA) in 1998. However, it has been withdrawn because of a number of criticisms, such as interlaboratory variations and lack of correlation between drug amount in SC and clinical efficacy (3,20).
On the other hand, among the limited number of methods available for the direct assessment of drug concentrations in dermis, DMD is the only one that allows unbound analytes to be sampled (9). DMD has the unique advantage of being potentially useful in diseased skin; clearly, this is less practical with DPK (21). Hence, MD provides a PK profile, with high temporal resolution, of the drug in the target tissue. As the level of unbound drug generally determines the pharmacodynamic response, the pharmacological relevance of the method is clear (11,19). While DMD method strives to make measurements of the drug in a true “biophase” of the skin, DPK method attempts to establish the SC as the “surrogate” sampling site. Thus, the close examination of the relationship between DMD and DPK methods is a challenge with respect to the assessment of topical BA/BE, even if the sampling compartments are different in these methods.
In this study, DMD and TS methods were used to continously sample the unbound concentration of OTC in dermis and to sample the SC levels, respectively. OTC was selected as a model compound on the basis of its suitability for MD and wide commercial availability of its topical formulations. Major limitations of the MD method are the low recoveries of molecules with large molecular weights (>20 kDa) and high lipophilicity or high protein binding of some drugs (22). MD would be particularly appropriate for OTC, because it is hydrophilic and has a low molecular weight with a low protein binding of 20% (23). This was also demonstrated by in vitro recovery experiments, which showed a moderately high and concentration-independent transfer of OTC across the dialysis membrane. Furthermore, DPK of OTC in the skin has not previously been studied, and two commercial OTC topical products existing on the market would enable a comparison of DMD and TS methods for assessing the topical BE of OTC in the same healthy volunteers.
To date, numereous cutaneous drug delivery studies using MD have been published in humans (24–31). However, topical BE studies using MD in humans are limited (32–36). The first BE/BA study was the comparison of dermal absorption rate and lag time (to) of lidocaine from a microemulsion vehicle and a commercially available o/w emulsion (Xylocain 5%) by MD in eight subjects. The microemulsion vehicle was found to increase dermal drug delivery (32). Another human BA study compared tissue and plasma levels of 8-methoxypsoralen after oral administration and topical application either in bath or a cream. Concentration-time courses of 8-methoxypsolen in the skin were assessed by MD in that study (33). Benfeldt et al. investigated the BE of topical lidocaine cream and ointment in eight healthy volunteers by DMD and TS methods (34). DMD method was used for the assessment of the BA of a ketoprofen topical gel formulation, and the validity of the method was evaluated for the determination of topical BE by the authors (35). More recently, a topical BE study has been conducted to evaluate the topical BE of three marketed topical metronidazole formulations by simultaneous DMD and TS methods in humans. The result of the BE evaluation of metronidazole formulations was found to be dependent on the method employed (36).
MD data demonstrated that the measurable amounts of OTC in dialysates were determined from the first sampling points after the topical administration of the test and the reference formulations in the present study. The large variability of PK parameters following topical application, which has been demonstrated in earlier studies, was also observed for OTC (75.4% and 103% for AUC, 88.9% and 109% for Cmax). A high intersubject variability of 63% and 91% for AUC values was reported in a DMD study (32). The findings were supported by another study, where Tettey-Amlalo et al. found an intersubject variability of 68% for concentration-time profiles after administration of ketoprofen topical gel (35). Benfeldt et al. found CVs around 41% for the parameter AUC and Cmax after the topical administration of lidocain (34). A significantly lower intersubject variability was reported for steady-state salicylate tissue concentrations from 20% methylsalicylate formulation (42%) compared to 7% glycolsalicylate formulation (90%) (31). CVs of Cmax from concentration-time curves of nicotine penetration were reported to be 48% (27). Ortiz et al. also showed a very high intersubject variability of 223, 116 and 165% for metronidazole penetration of three different formulations (36). In the present study, one probe was used for the reference and one for the test application area, taking into account the invasiveness of implanting more than two probes per subject at the same time. Therefore, the intrasubject variability was not evaluated in this study. However, the variability of PK parameters from MD experiments generally appears to be larger between individuals compared to within individuals, when multiple probes are used for the assessment of cutaneous penetration from a formulation (32,34,36). Therefore, the observed variability in concentrations of OTC and consequently in the PK parameters is attributable to the interindividual difference in skin barrier function, which is the major contributor to the variability in cutaneous drug levels after topical application.
The influence of probe depth on the variability of cutaneous MD data has been a subject of discussion in the literature. However, most authors have not found a correlation between drug levels in the skin and probe depth (24,25). Ultrasound scanning confirmed that the location of MD probes inside the dermis. The probes for the test and the reference were inserted into the dermis of each volunteer on a single occasion in the present study. Caution in performing the insertion of the probes by the same investigator was taken to ensure that they were always placed in the dermis for all the subjects.
There were no significant differences in tmax of OTC with respect to test and reference obtained from MD sampling (p = 0.457). On the other hand, the AUC values for the test and the reference were significantly different, showing that the mean AUC for the test is 2.25-fold higher than that for the reference.
AUC values that are the main criteria of measuring dermal delivery were used after logarithmic transformation. There is some evidence that this transformation would be beneficial with high variability-exhibiting data (2). Therefore, AUC and Cmax parameters were log-transformed to achieve variance homogeneity. Although the test could not be said to be bioequivalent to the reference, the number of subjects used in this study should be taken into account for this result. The number of subjects necessary for an MD study to determine whether two formulations are bioequivalent depends on the degree of variability observed in the data. It was declared that there is a reasonable chance of meeting the 80–125% BE criterion with a total of 27 subjects at 90% CI when two probes are used per formulation (34). In contrast, DMD BE studies in the literature have been performed with 8 to 18 volunteers (32–36).
The insertion of the MD probe in soft tissues and the skin has been reported to be no more painful than the placement of an intravenous catheter (37). The subjects participating in this DMD study did not experience any extra discomfort during and after the insertion of the MD probes, and they found the method acceptable. Therefore, no local anaesthetic was needed for this semi-invasive procedure. There was also no sign of bruising or pain once the probe was removed from the skin. One of the limiting factors of MD is the duration of a single MD experiment, because of the requirement of resting in a supine position, which may be somewhat inconvenient to the subject. However, the duration of the present study was well tolerated by all volunteers. These findings support the feasibility of using MD for assessing percutaneous penetration of drugs.
The assessment of in vivo recovery is also an essential part of using MD to study drug cutaneous PK. In vivo RR of OTC was found to be similar to in vitro RR and time-independent during the experimental period.
The HPLC method used for the analysis of TS samples was not suitable for analysing MD samples, because of inadequate analysis sensitivity. For this reason, a highly sensitive UPLC-MS-MS method was used to measure OTC concentrations in dialysates. This confirms that the sensitivity of the analyzing technique may be a limiting factor in MD research.
Each subject served as his/her own control after a 4-week SC repair period for TS experiments, because even if stripping remains a form of enviromental insult, a homeostatic repair response in the epidermis is rapidly elicited which results in rapid restoration of barrier function (19).
TS data showed that OTC in SC can be easily detectable for both formulations. This method is considerably simple and noninvasive as declared in the literature (38). No side or allergic effects were noted in the present study. DMD was found to be comparatively more invasive than TS. The results showed that tmax was similar for the test and the reference obtained from TS (p = 0.707).
DPK modeling of TS data was performed based on the one-compartmental model. However, model fitting could not be accomplished with two subjects for the reference and with four subjects for the test because of the plateau-like level of the terminal data sets for these subjects. Therefore, the DPK parameters of these subjects were excluded from the final determinations. DPK analysis yielded 3-fold higher ka values for the reference. Half-life (t1/2), clearance (CL), kd and Vd values of OTC for the test were not significantly different from those for the reference. to values in modeling were present for both formulations. tmax was longer in SC than in dialysate.
The intersubject variability of OTC content in SC was moderate, as it was compared to the dermal drug levels. This variability coincides with the findings in literature. Pershing et al. reported the intersubject variability in tretinoin contents at the individual time points in the DPK profile. With all products, it was approximately 50% over the majority of 49 patients’ profiles (6). A similar variation was seen for triamcinolone acetonide cream formulation in 10 healthy volunteers (39). The coefficient of variation in triamcinolone acetonide uptake was subject dependent, ranging from 21 to 67%, with a mean of 38%. Ortiz et al. also found that the “two-time” point DPK method yielded data with much lower variability compared to DMD data for metronidazole (36).
Local BA may be assessed either from the combined or from the individual tape strips. An important source of variability in the TS method comes from the nonuniform removal of SC. The amount of SC in a single strip depends on several factors, such as the site of application, type of tape, mode of application (i.e. pressure force, removal rate and duration of removal) and properties of the formulations (40–42). In addition, variability is also due to intrinsic biological factors, since each anatomical site has a particular thickness of SC, number of cell layers, size and amount of corneocytes, composition and amount of lipids (43). Several methods (gravimetric, spectroscopic and microscopic) have been proposed to quantify the amount of horny layer removed on each TS (40). In this study, the controlled removal of the SC was accomplished by using the standardized 15 adhesive tapes and the mode of application performed by the same person in each case. It was shown that 90% of the drug concentration in the SC is found in the first 10 strips (44). Therefore, the number of tapes used for this study was a reasonable number to take most of the drug present in SC. The first two strips of 15 consecutive tape strips were discarded. After cleaning the skin, this fraction of dose will not be absorbed and should be discarded, especially in the case of polar drugs. However, it is still not clear in the literature if initial tape-strips should be accounted for or discarded (40). Some authors think that with a well-defined cleaning procedure, this significant fraction of drug can supply valuable information (4).
BE was assessed by log trasformed data for TS as for MD data in this study. By TS method, the test was found to be bioequivalent to the reference. Therefore, MD and TS gave different results with regards to the BE of OTC formulations. This may be explained by the reservoir role of SC, in this case for OTC. The amount of penetrated drug to the dermis seems to be dependent on the formulation.
Skin absorption varies with the physicochemical nature of the compound and the anatomical region of application and formulation influences (45). The barrier function of skin is clearly most pronounced with a hydrophilic compound, while lipophilic ones can penetrate the skin more easily (46). The role of SC in the penetration of the water-soluble drugs acyclovir and pencyclovir have been investigated by cutaneous MD, confirming SC as the major barrier to hydrophilic drug absorption (47). In general, to be well absorbed, a substance should have a molecular mass less than 0.6 kDa, adequate solubility in oil and water and a high partition coeffient (Koct ~ 1–3) (18,46). Being a hydrophilic drug, OTC is poorly absorbed through the skin, which explains the difference between the concentration profiles of OTC in dialysates and TS samples. The concentrations in SC were much higher, compared with the dialysates. It must be pointed out that the measured concentrations in the dermis are free (unbound) extracellular concentrations and not the total concentration, which is a great advantage. However, this is of no great importance for compounds with low affinity to plasma proteins, such as OTC with a protein binding of 20%.
The present study demonstrated that there has been no correlation between the data of OTC in the dermis and SC by both methods. The correlation between these methods was weak as regarding AUC and Cmax. No significant correlation was found between dermal MD levels versus relavent TS levels of OTC for the test and the reference. Such a relationship between DMD and DPK methods was first investigated by Benfeldt et al. for BE of topical lidocaine cream and ointment in eight healthy volunteers. Both methods showed higher absorption from the cream formulation, thus showing that the formulations were nonbioequivalent. Analyzing the correlations between individual MD sampling points or AUC versus DPK results, they showed a significant correlation for all time points for ointment, whereas they found weaker or no correlations for the two formulations analyzed together and cream formulation alone, respectively (34). More recently, a BE study has been conducted to evaluate the topical BE of three marketed topical metronidazole formulations by simultaneous DMD and TS methods, showing no correlation between them either (36).
CONCLUSION
Although it is accepted that the TS results may be indicative of dermis concentrations, SC concentration was found not to be predictive of OTC concentration in the dermis in the present study. Moreover, the drug levels in the living part of the skin, dermis, may be affected by the factors such as absorption, distribution, metabolism and disposition kinetics of the drug. The physicochemical properties of drugs and the formulation effects are also of great impact on dermal concentrations of drugs. TS is well known to be a useful method to assess the penetration to SC; however, MD sampling may be superior to TS for obtaining dermal PKs and BE, whenever the underlying viable tissue is the target organ. Nevertheless, DMD as a method to be routinely used in topical BE studies has to be explored extensively. It seems that more studies are needed for a wide range of topical drugs to understand whether a correlation between DMD and TS methods exists and to investigate the effect of different formulations on this relationship between the methods.
Abbreviations
- AUC:
-
area under the OTC content-time profile or area under the OTC concentration-time profile
- BA:
-
bioavailability
- BE:
-
bioequivalence
- C:
-
amount of OTC at SC
- CI:
-
confidence intervals
- CL:
-
clearance
- Cmax :
-
maximum observed OTC content of the DPK profile or maximum observed OTC concentration in dermis
- CV:
-
coefficient of variation
- D:
-
dose
- DMD:
-
dermal microdialysis
- DPK:
-
dermatopharmacokinetics
- HPLC:
-
high performance liquid chromatography
- ka :
-
absorption rate constant
- kd :
-
elimination rate constant
- LOD:
-
limit of detection
- LOQ:
-
limit of quantification
- MD:
-
microdialysis
- OTC:
-
oxytetracycline HCl
- PK:
-
pharmacokinetics
- RR:
-
relative recovery
- SC:
-
stratum corneum
- SD:
-
standard deviation
- t1/2 :
-
half life
- tmax :
-
time point at which Cmax was observed
- to :
-
lag time
- TS:
-
tape stripping
- UPLC-MS-MS:
-
ultra performance liquid chromatography tandem mass spectroscopy
- V:
-
area of distribution
- Vd :
-
volume of distribution
REFERENCES
Shah VP. Topical drug products-microdialysis: regulatory perspectives. Int J Clin Pharmacol Ther. 2004;42:379–81.
McCleverty D, Lyons R, Henry B. Microdialysis sampling and the clinical determination of topical dermal bioequivalence. Int J Pharm. 2006;308:1–7.
Narkar Y. Bioequivalence for topical products-an update. Pharm Res. 2010;27(12):2590–601.
Wiedersberg S, Leopold CS, Guy RH. Dermatopharmacokinetics of betamethasone 17-valerate: influence of formulation viscosity and skin surface cleaning procedure. Eur J Pharm Biopharm. 2009;71:362–6.
Alberti I, Kalia YN, Naik A, Bonny J-D, Guy RH. In vivo assessment of enhanced topical delivery of terbinafine to human stratum corneum. J Control Release. 2001;71:319–27.
Pershing LK, Nelson JL, Corlett JL, Shrivastava SP, Hare DB, Shah VP. Assessment of dermatopharmacokinetic approach in the bioequivalence determination of topical tretinoin gel products. J Am Acad Dermatol. 2003;48:740–51.
Plock N, Kloft C. Microdialysis-theoretical background and recent implementation in applied life-sciences. Eur J Pharm Sci. 2005;25:1–24.
Langer O, Müller M. Methods to assess tissue-specific distribution and metabolism of drugs. Curr Drug Metab. 2004;5:463–81.
Schnetz E, Fartasch M. Microdialysis for the evaluation of penetration through the human skin barrier-a promising tool for future research? Eur J Pharm Sci. 2001;12:165–74.
Kreilgaard M. Assessment of cutaneous drug delivery using microdialysis. Adv Drug Deliv Rev. 2002;54:S99–S121.
Holmgaard R, Nielsen JB, Benfeldt E. Microdialysis sampling for investigations of bioavailability and bioequivalence of topically administered drugs: current state and future perspectives. Skin Pharmacol Physiol. 2010;23(5):225–43.
Seagrave RC. Biomedical applications of heat and mass transfer. Ames: The Iowa State University Press; 1971.
Lange ECM, Boer AG, Breimer DD. Methodological issues in microdialysis sampling for pharmacokinetic studies. Adv Drug Deliv Rev. 2000;45:125–48.
Ståhle L, Arner P, Ungerstedt U. Drug distribution studies with microdialysis III: extracellular concentration of caffeine in adipose tissue in man. Life Sci. 1991;49:1853–8.
Papadoyannis IN, Samanidou VF, Kovatsi LA. A rapid high performance liquid chromatographic (HPLC) assay for the determination of oxytetracycline in commercial pharmaceuticals. J Pharm Biomed Anal. 2000;23:275–80.
Sanderson H, Ingerslev F, Brain RA, Halling-Sǿrensen B, Bestari JK, Wilson CJ, et al. Dissipation of oxytetracycline, chlortetracycline, tetracycline and doxycycline using HPLC-UV and LC/MS/MS under aquatic semi-field microcosm conditions. Chemosphere. 2005;60:619–29.
Rowland M, Tozer TN. Clinical pharmacokinetics: concept and applications. Philadelphia: Lea&Febiger; 1980.
Hadgraft J. Skin deep. Eur J Pharm Biopharm. 2004;58:291–9.
Herkenne C, Alberti I, Naik A, Kalia YN, Mathy F-X, Préat V, et al. In vivo methods for the assessment of topical drug bioavailablity. Pharm Res. 2008;25(1):87–103.
Russell LM, Guy RH. Measurement and prediction of the rate and extent of drug delivery into and through the skin. Expert Opin Drug Deliv. 2009;6(4):355–69.
Ortiz PG, Hansen SH, Shah VP, Menné T, Benfeldt E. Impact of adult atopic dermatitis on topical drug penetration: assessment by cutaneous microdialysis and tape stripping. Acta Derm Venereol. 2009;89:33–8.
Davies MI. A review of microdialysis sampling for pharmacokinetic applications. Anal Chim Acta. 1999;379:227–49.
Kunin CM, Dornbush AC, Finland M. Distribution and excretion of four tetracycline analogues in normal young men. J Clin Invest. 1959;38(11):1950–65.
Benfeldt E, Serup J, Menné T. Effect of barrier perturbation on cutaneous salicylic acid penetration in human skin: in vivo pharmacokinetics using microdialysis and non-invasive quantification of barrier function. Br J Dermatol. 1999;140:739–48.
Müller M, Mascher H, Kikuta C, Schäfer S, Brunner M, Dorner G, et al. Diclofenac concentrations in defined tissue layers after topical administration. Clin Pharmacol Ther. 1997;62:293–9.
Stagni G, O’Donnell D, Liu YJ, Kellogg DL, Morgan T, Shepherd AMM. Intradermal microdialysis: kinetics of iontophoretically delivered propranolol in forearm dermis. J Control Release. 2000;63:331–9.
Hegemann L, Forstinger C, Partsch B, Lagler I, Krotz S, Wolff K. Microdialysis in cutaneous pharmacology: kinetic analysis of transdermally delivered nicotine. J Invest Dermatol. 1995;104:839–43.
Müller M, Schmid R, Wagner O, Osten B, Shayganfar H, Eichler HG. In vivo characterization of transdermal drug transport by microdialysis. J Control Release. 1995;37:49–57.
Boelsma E, Anderson C, Karlsson AMJ, Ponec M. Microdialysis technique as a method to study the percutaneous penetration of methyl nicotinate through excised human skin, reconstructed epidermis, and human skin in vivo. Pharm Res. 2000;17(2):141–7.
Brunner M, Dehghanyar P, Seigfried B, Martin W, Menke G, Müller M. Favourable dermal penetration of diclofenac after administration to the skin using a novel spray gel formulation. Br J Clin Pharmacol. 2005;60(5):573–7.
Cross SE, Anderson C, Roberts MS. Topical penetration of commercial salicylate esters and salts using human isolated skin and clinical microdialysis studies. Br J Clin Pharmacol. 1998;46:29–35.
Kreilgaard M, Kemme MJB, Burggraaf J, Schoemaker RC, Cohen AF. Influence of a microemulsion vehicle on cutaneous bioequivalence of a lipophilic model drug assessed by microdialysis and pharmacodynamics. Pharm Res. 2001;18(5):593–9.
Tegeder I, Bräutigam L, Podda M, Meier S, Kaufmann R, Geisslinger G, et al. Time course of 8-methoxypsoralen concentrations in skin and plasma after topical (bath and cream) and oral administration of 8-methoxypsoralen. Clin Pharmacol Ther. 2002;71:153–61.
Benfeldt E, Hansen SH, Vølund A, Menné T, Shah VP. Bioequivalence of topical formulations in humans: evaluation by dermal microdialysis sampling and the dermatopharmacokinetic method. J Invest Dermatol. 2007;127:170–8.
Tettey-Amlalo RNO, Kanfer I, Skinner MF, Benfeldt E, Verbeeck RK. Application of dermal microdialysis for the evaluation of bioequivalence of a ketoprofen topical gel. Eur J Pharm Sci. 2009;36:219–25.
Ortiz PG, Hansen SH, Shah VP, Sonne J, Benfeldt E. Are marketed topical metronidazole creams bioequivalent? Evaluation by in vivo microdialysis sampling and tape stripping methodology. Skin Pharmacol Physiol. 2011;24(1):44–53.
Müller M. Science, medicine, and the future. Microdialysis BMJ. 2002;324:588–91.
Midha KK, Shah VP, Singh GJP, Patnaik R. Conference report: Bio-International 2005. J Pharm Sci. 2007;96(4):747–53.
Pershing LK, Bakhtian S, Poncelet CE, Corlett JL, Shah VP. Comparison of skin stripping, in vitro release, and skin blanching response methods to measure dose response and similarity of triamcinolone acetonide cream strengths from two manufactured sources. J Pharm Sci. 2002;91(5):1312–24.
Lademann J, Jacobi U, Surber C, Weigmann H-J, Fluhr JW. The tape stripping procedure-evaluation of some critical parameters. Eur J Pharm Biopharm. 2009;72:317–23.
Breternitz M, Flach M, Präβler J, Elsner P, Fluhr JW. Acute barrier disruption by adhesive tapes is influenced by pressure, time and anatomical location: integrity and cohesion assessed by sequential tape stripping; a randomized, controlled study. Br J Dermatol. 2007;156:231–40.
Löffler H, Dreher F, Maibach HI. Stratum corneum adhesive tape stripping: influence of anatomical site, application pressure, duration and removal. Br J Dermatol. 2004;151:746–52.
Boix-Montanes A. Relevance of equivalence assessment of topical products based on the dermatopharmacokinetics approach. Eur J Pharm Sci. 2011;42:173–9.
Caron D, Queille-Roussel C, Shah VP, Schaefer H. Correlation between the drug penetration and the blanching effect of topically applied hydrocortisone creams in human beings. J Am Acad Dermatol. 1990;23(3):458–62.
Hadgraft J, Lane ME. Skin permeation: the years of enlightenment. Int J Pharm. 2005;305:2–12.
Korting-Schäfer M, Mehnert W, Korting H-C. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv Drug Deliv Rev. 2007;59:427–43.
Morgan CJ, Renwick AG, Friedmann PS. The role of stratum corneum and dermal microvascular perfusion in penetration and tissue levels of water-soluble drugs investigated by microdialysis. Br J Dermatol. 2003;148:434–43.
ACKNOWLEDGMENTS
This study was supported by the research grant (107S177) from TUBITAK (The Turkish Scientific and Technological Research Council), which is gratefully acknowledged. The authors would like to thank Düzen-Norwest Laboratory, Ankara, Turkey, for the technical assistance in providing UPLC-MS-MS analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Incecayir, T., Agabeyoglu, I., Derici, U. et al. Assessment of Topical Bioequivalence Using Dermal Microdialysis and Tape Stripping Methods. Pharm Res 28, 2165–2175 (2011). https://doi.org/10.1007/s11095-011-0444-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-011-0444-3