Abstract
In as many as 50 % of cases, the immediate post-kidney transplant course is complicated by early kidney dysfunction most commonly related to ischemia and reperfusion injury. This acute kidney injury (AKI) is modulated by a complex interplay of multiple donor and recipient factors. Challenges to understand its mechanisms include the complex interplay of contributors derived from the donor as well as the recipient and lead to hypoxic and ischemic injury as well as altered repair mechanisms. New therapies mainly seek to suppress inflammation, limit cell death, and/or interrupt detrimental signaling of necrosis. While there are several promising novel targets and novel therapeutics available, many challenges remain in the translation from bench to bedside.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Objectives
-
To outline the major biological processes implicated in ischemia and reperfusion.
-
To recognize the importance of early graft function and its related complications.
-
To define current and new approaches aimed to limit organ injury at the time of transplantation.
Introduction
Delayed graft function (DGF) is a common early complication following deceased donor kidney transplantation. DGF is often defined as the need for dialysis in the first week after transplantation and is primarily a consequence of ischemia/reperfusion (IR) injury resulting in postischemic acute tubular necrosis (ATN) [1]. The degree of IR injury is dependent on a complex interplay of pre-transplant injury and subsequent innate and adaptive immune responses after reperfusion [2]. The consequences of developing DGF are significant. In addition to the acute complications related to renal failure and the associated costs of prolonged hospitalization, the magnitude of the association between DGF and subsequent chronic allograft dysfunction is fairly strong, but it is not clear whether DGF directly affects long-term graft survival [1]. Several new drugs show promise in animal studies in preventing or ameliorating IR injury, and clinical trials are ongoing (Tables 15.1 and 15.2). The aim of this review is to summarize the clinical risk factors and consequences and the translational science investigating the mechanism of IR injury and summarize the clinical trials regarding the prevention or management of DGF.
Important Biological Processes Implicated in Brain Death, Ischemia, and Reperfusion
It is important to differentiate in models the effects of warm versus cold ischemia and isograft versus allograft. Excellent science has been generated in animal models identifying a wide range of pathological processes contribute to hypoxic and IR-associated injury (reviewed in detail [3]). We will focus here on cell death and survival programs and innate and adaptive immune activation, as these are potentially amenable to innovative therapeutic approaches.
Cell Death, Apoptosis, and Autophagy
Ischemia and reperfusion activates various programs of cell death, which can be categorized as necrosis-, apoptosis-, or autophagy-associated cell death. Autophagy is a general term for pathways by which cytoplasmic material is delivered to lysosomes for degradation [4]. The main purposes of autophagosome formation are quality control and removal of defunct organelles, provision of an energy source during starvation, and regulation of cell survival and cell death. More recently, autophagy was identified as an important effector and regulator of innate and adaptive immunity and inflammation [4]. Several studies have reported the upregulation of autophagy in tubular cells in response to acute kidney injury caused by experimental nephrotoxic, IR, or ureteral obstruction models [4], and autophagy was identified as a protective mechanism by tubular cells during stress, suggesting it is upregulated after injury in order to selectively degrade damaged mitochondria and protein aggregates [5]. While there is huge clinical interest, the lack of validated clinical markers and the absence of selective inducers and inhibitors of autophagy are challenges for successful translational research.
Innate Immunity
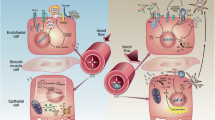
Important components and well studied in animal models of IR injury are toll-like receptors (TLR) and the complement system.
Toll-Like Receptors
TLRs are expressed on immune as well as nonimmune cells, and endogenous, cell-derived ligands (so-called damage-associated molecular patterns or DAMPs) can signal through specific TLRs. Among the list of DAMPs that have been described to be induced or upregulated after IR only, HMGB1 was so far mechanistically linked to the pathogenesis of IR injury [6]. HMGB1 is a nuclear protein that binds DNA and modulates transcription and chromatin modeling and dependent on its redox state also functions as an extracellular signaling molecule during sterile inflammation, providing a chemotactic and activation signal to inflammatory cells [6]. TLR4 was found to be upregulated, and tubular HMGB1 was detectable in deceased donor kidneys when compared with living donor kidneys. In addition, kidneys carrying the loss-of-function TLR4 variants (Asp299Gly and Thr399Ile), known to diminish ligand-receptor binding, were linked with better function immediately after transplantation [7]. Chimeric mice with deficiency in renal-associated TLR2 and TLR4 had less renal damage and dysfunction when compared with wild-type mice, and when comparing single TLR2−/− and TLR4−/− with the TLR2/4−/−, no increased protection was seen, indicating that ligands prime TLR2 and TLR4 during IR injury [6].
Complement System
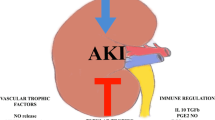
Studies in small and large animals revealed that terminal complement activation is a critical mediator in IR injury [8]. IR injury is abrogated in animals that are deficient in C3 (and factor B) but not C4 [8]. Using chimeric mice C3aR/C5aR on renal cells as well as leukocytes contributes to IR injury [9]. In kidneys retrieved from brain-dead donors compared to kidneys from living donors, systemic generation of C5a mediates renal inflammation via tubular C5a-C5aR interaction [10]. Overall, the data strongly support the model that IR injury leads to local (kidney derived) as well as immune cell-derived complement release and activation, which leads to acute organ injury. With eculizumab, a C5 inhibitor, agents are available and clinical trials in DGF are ongoing (Table 15.2), and therapeutic interventions already at the time of brain death might be needed for optimal effects on graft outcome.
Adaptive Immunity
IR injury elicits a robust adaptive immune response. Studies have shown that T cells (CD4 and CD8) accumulate during IR injury and mediate injury [3, 6]. The specific mechanisms underlying T cell activation in the absence of specific exogenous antigen remain to be elucidated, but data indicate antigen-specific and antigen-independent mechanisms of action [3].
Clinical Definitions of DGF
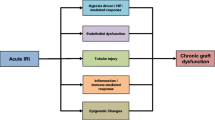
The early graft dysfunction especially using deceased donors is often classified into immediate, slow (SGF), delayed (DGF), or in the most severe cases primary nonfunction (PNF). Due to the complexity of its pathophysiology, it is complex to find one definition of early graft dysfunction explaining why currently more 18 definitions coexist [1]. The most frequent definitions are based on posttransplant dialysis requirements (most frequently one dialysis session during the first 7 days after transplantation) [1]. While useful for data reporting, this definition suffers from many pitfalls including clinical-dependent decision, dialysis required for potassium or fluid overload, residual renal function, or preemptive transplantation, which may lead to misclassification or large variations in DGF rates that were observed in multicenter trials [11]. Other definitions may rely on urine output, creatinine reduction, and analysis of urine biomarkers such as interleukin-18 (IL-18), kidney injury molecule 1 (KIM1), neutrophil gelatinase-associated lipocalin (NGAL). In order to advance in the prevention and/or treatment of DGF, it is important to isolate the diagnosis of IR-induced AKI, to dissect and evaluate the influence of factors related to the donor (age, intensive care management), to the recipient (age, quality of arteries, surgical procedure, antihuman leukocyte antigen (anti-HLA) immunization), to the transplant allocation (leading to various cold ischemia times), and finally to other causes of renal failure such as surgical complications, drug nephrotoxicity, and rejection (Fig. 15.1).
Key time points starting with the donor identification that have the potential to induce ischemic or nonischemic injury to the kidney (Adapted from Hall and Parikh [29])
Clinical Risk Factors and Consequences
The main donor factors increasing the risk of DGF are increasing donor age, donor type, and quality of pre-kidney procurement care (Fig. 15.2). The risk of DGF (and subsequent graft failure) augments from living donor kidneys to deceased donors (standard criteria donors [SCD] < expanded criteria donors [ECD] < donation after cardiac death [DCD] < ECD/DCD) [12]. This reflects mainly the positive influence of short warm and cold ischemia time in living kidney donors and the negative influence of prolonged ischemia time after brain or cardiac death. Donor serum creatinine at time of procurement is not a sensitive marker of subsequent DGF mainly because its implication is likely to be different between SCD (ischemia reperfusion) and ECD kidneys (preexisting chronic renal damage). Related to donor age are histological markers such as arteriolar hyalinosis and atherosclerosis, which may reflect an increased sensitivity to ischemia [13]. There is a clear correlation between the length of cold ischemia time and the incidence of DGF, which is very useful since it is a highly modifiable factor. Another significant modifiable factor is the choice between cold storage using varying solutions and machine perfusion, which has been reintroduced in the past years [14]. Finally, recipient factors influencing the risk of DGF include male gender, BMI greater than 30, African-American ethnicity, history of diabetes, anti-HLA immunization, and requirement for dialysis before transplantation [15] (Fig. 15.1).
The short-term clinical consequences of DGF are the need for several posttransplantation dialysis sessions leading to increased morbidity, increased length of hospitalization, and hence increased cost [16]. More interesting and still a matter of debate are long-term consequences of DGF. The classical view is that postischemic ATN leads to various repair mechanisms involving both adaptive and innate immunity [3]. May be more puzzling is the most recent viewpoint suggesting that cold ischemia time may have more subtle consequences. Kayler et al. studied the impact of cold ischemia time on graft survival among ECD kidney using a paired kidney analysis (kidneys derived from the same donor but transplanted to two different recipients) [17]. Not surprisingly, the DGF incidence was higher in pairs with greater cold ischemia time difference, but the incidence of graft loss was not different even in multivariable models adjusted for recipient factors. This first analysis was followed by a second in whom the impact of cold ischemia time-induced DGF on long-term graft loss was studied in paired kidneys when one kidney experienced DGF in one recipient but not in the second [18]. The author concluded that of course the incidence of DGF increased with increasing cold time but that graft loss was similar in both groups suggesting that cold ischemia time-induced DGF may not have deleterious long-term consequences and hence that kidneys should not be discarded because of that sole reason. Another example of the complexity in the interactions between AKI and chronic kidney disease (CKD) is the fact that patients that received the kidneys from donors without a heartbeat, which was twofold more than the incidence of DGF in matched recipients that received kidneys from donors with a heartbeat (24 %), had similar long-term graft survival [19].
Prediction and Detecting DGF
In recent years, there has been a lot of interest devoted to both prediction of DGF before transplantation and early detection at time or just after kidney transplantation. Early, noninvasive, and rapid assessment of deceased donor kidney injury could drive better allocation decisions and potentially reduce the rates of posttransplant complications. It must be stressed that the rewards of prediction and early detection require that therapeutic intervention modifies the course of DGF, which is until now far from obvious. Indeed, the few useful interventions, such as reducing drastically cold ischemia time and use of machine perfusion, are able to unselectively decrease the incidence of DGF. For example, it is a strong belief that avoiding calcineurin inhibitors (CNIs) in patients at high risk of DGF would be beneficial which has never been proven when correctly tested [20]. Even more, in the Benefit [21] and Benefit Ext studies [22], patients treated with CNIs from the time of transplantation had similar incidences of DGF as patients never exposed to CNI.
It may nevertheless be useful to predict DGF for clinical trials. Irish et al. developed a risk prediction model using a multivariable logistic regression analysis [23]. These Web-based calculators are easily accessible and helpful to assess DGF risk in a population but will not be able to assess individual DGF risk.
Interventional Trials in DGF
Prevention of Organ Injury Is Superior to Treatment
Improved donor management, namely, the use of dopamine, has been shown in a randomized trial to significantly decrease dialysis requirements and hence the length of DGF but without a difference in graft failure at 3 years [24]. Moers et al. demonstrated that the use of hypothermic machine perfusion instead of cold storage was able to reduce the incidence and duration of DGF as well as improve 1-year graft survival [25]. At 3 years, the benefit was still present especially in ECD kidneys [25]. It is interesting to note that despite the lower DGF incidence in kidneys recovered after cardiac death, there was no improvement in graft survival. A recent meta-analysis concluded that hypothermic machine perfusion reduces DGF rate but does not modify primary nonfunction, acute rejection, and patient and graft survival [14]. Data on the effect of the preservation solutions (two most commonly used are histidine-tryptophan-ketoglutarate [HTK] and University of Wisconsin [UW] solution) have been inconsistent and were summarized in detail elsewhere; however, prospective adequately powered trials in high-risk kidneys are needed [12].
Published and Ongoing Interventional Trials
Most interventional strategies are recipient directed and target cell death and inflammation. These trials differ in terms of endpoint definition and donor/recipient selection (Tables 15.1 and 15.2). Recombinant P-selectin glycoprotein ligand IgG fusion protein, rPSGL-Ig, efficiently binds P- and E- selectin and prevents polymorphonuclear neutrophil (PMN) adhesion and sequestration to the site of injury. A multicenter phase 2 study found that while there were no differences in the DGF rate, fewer patients receiving the drug had serum creatinine >6.0 mg/dl on the postoperative day 5 (26 % vs 55 %, p = 0.04) [11]. No effect on the DGF incidence (hemodialysis [HD] requirement within 1 week) of high doses of erythropoietin, with its potential antiapoptotic and regenerative effects, was seen in two trials when compared to placebo. One relatively small study applied 40,000 U of epoetin [EPO]-alpha (Procrit) as a single dose at the time of reperfusion into the ipsilateral artery proximal to the graft anastomosis [26]. Martinez and colleagues used 30,000 U EPO-beta before and three subcutaneous injections after transplantation (12 h, 7 and 14 days) of ECD kidneys [27]. It is not known whether earlier EPO administration (e.g., during cold storage) would achieve the desired effects. Based on strong preclinical data on the role of complement in IR injury, eculizumab is now being tested in a clinical pilot trial in 24 patients at high risk for DGF (NCT01919346). Another interesting target is TLR2. OPN-305 is a monoclonal antibody that blocks TLR2 and is currently tested in a multicenter trial in the USA and Europe including DCD, ECD, and SCD kidneys with a cold ischemia time greater than 18 h (NCT01794663).
Conclusion
DGF is a clinic description of a series of complex events that start during donor management and progress during organ procurement, transport, implantation, and reperfusion (Fig. 15.1). While there is ample of excellent science using animal models of IR injury, many challenges in translation from bench to bedside remain. Animal models are important to test basic pathophysiological mechanisms, but there is no reliable animal model available that mimics human AKI with or without transplantation. Indeed, the poor correlation of murine models with human inflammatory diseases supports for priority for translational medical research addressing complex human conditions [28].
Interventions that limit the short-term and long-term effects of peri-transplant injury in humans are urgently needed. Reduction of the discard rate of procured organs is also an important area for the development of new therapeutics. In the past the main focus was to dampen the injury, and more recently strategies that enhance tissue regeneration are increasing, including highly effective tools to manipulate microRNAs. Once our understanding of how microRNAs affect gene expression in hypoxic and injured tissues involves these tools are likely being integrated into clinical practice.
For clinical trial design clear defined endpoints are critical. In addition, the logistics and ethics in deceased donor intervention need to be addressed with the help of academia, transplant societies, and government. There are promising novel therapeutics in the pipeline, but any DGF intervention will be measured whether it provides improved long-term outcomes.
Key Messages
-
DGF is often defined as the need for dialysis in the first week after transplantation and is primarily a consequence of ischemia/reperfusion injury resulting in postischemic ATN.
-
The main donor factors increasing the risk of DGF are increasing donor age, donor type, quality of pre-kidney procurement care, and length of cold ischemia time.
-
Early, noninvasive, and rapid assessment of deceased donor kidney injury could drive better allocation decisions and potentially reduce the rates of posttransplant complications.
-
Most interventional strategies are recipient directed and target cell death and inflammation.
References
Yarlagadda SG, Coca SG, Formica Jr RN, Poggio ED, Parikh CR. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant. 2009;24:1039–47.
Cheung KP, Kasimsetty SG, McKay DB. Innate immunity in donor procurement. Curr Opin Organ Transplant. 2013;18:154–60.
Eltzschig HK, Eckle T. Ischemia and reperfusion – from mechanism to translation. Nat Med. 2011;17:1391–401.
Huber TB, Edelstein CL, Hartleben B, Inoki K, Dong Z, Koya D, Kume S, Lieberthal W, Pallet N, Quiroga A, Ravichandran K, Susztak K, Yoshida S, Dong Z. Emerging role of autophagy in kidney function, diseases and aging. Autophagy. 2012;8:1009–31.
Liu S, Hartleben B, Kretz O, Wiech T, Igarashi P, Mizushima N, Walz G, Huber TB. Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy. 2012;8:826–37.
Leventhal JS, Schroppel B. Toll-like receptors in transplantation: sensing and reacting to injury. Kidney Int. 2012;81:826–32.
Kruger B, Krick S, Dhillon N, Lerner SM, Ames S, Bromberg JS, Lin M, Walsh L, Vella J, Fischereder M, Kramer BK, Colvin RB, Heeger PS, Murphy BT, Schroppel B. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci U S A. 2009;106:3390–5.
Damman J, Daha MR, van Son WJ, Leuvenink HG, Ploeg RJ, Seelen MA. Crosstalk between complement and Toll-like receptor activation in relation to donor brain death and renal ischemia-reperfusion injury. Am J Transplant. 2011;11:660–9.
Peng Q, Li K, Smyth LA, Xing G, Wang N, Meader L, Lu B, Sacks SH, Zhou W. C3a and C5a promote renal ischemia-reperfusion injury. J Am Soc Nephrol. 2012;23:1474–85.
van Werkhoven MB, Damman J, van Dijk MC, Daha MR, de Jong IJ, Leliveld A, Krikke C, Leuvenink HG, van Goor H, van Son WJ, Olinga P, Hillebrands JL, Seelen MA. Complement mediated renal inflammation induced by donor brain death: role of renal C5a-C5aR interaction. Am J Transplant. 2013;13:875–82.
Gaber AO, Mulgaonkar S, Kahan BD, Woodle ES, Alloway R, Bajjoka I, Jensik S, Klintmalm GB, Patton PR, Wiseman A, Lipshutz G, Kupiec-Weglinski J, Gaber LW, Katz E, Irish W, Squiers EC, Hemmerich S. YSPSL (rPSGL-Ig) for improvement of early renal allograft function: a double-blind, placebo-controlled, multi-center Phase IIa study. Clin Transplant. 2011;25:523–33.
Cavaille-Coll M, Bala S, Velidedeoglu E, Hernandez A, Archdeacon P, Gonzalez G, Neuland C, Meyer J, Albrecht R. Summary of FDA workshop on ischemia reperfusion injury in kidney transplantation. Am J Transplant. 2013;13:1134–48.
Matignon M, Desvaux D, Noel LH, Roudot-Thoraval F, Thervet E, Audard V, Dahan K, Lang P, Grimbert P. Arteriolar hyalinization predicts delayed graft function in deceased donor renal transplantation. Transplantation. 2008;86:1002–5.
O'Callaghan JM, Knight SR, Morgan RD, Morris PJ. Preservation solutions for static cold storage of kidney allografts: a systematic review and meta-analysis. Am J Transplant. 2012;12:896–906.
Doshi MD, Garg N, Reese PP, Parikh CR. Recipient risk factors associated with delayed graft function: a paired kidney analysis. Transplantation. 2011;91:666–71.
Schnitzler MA, Gheorghian A, Axelrod D, L'Italien G, Lentine KL. The cost implications of first anniversary renal function after living, standard criteria deceased and expanded criteria deceased donor kidney transplantation. J Med Econ. 2013;16:75–84.
Kayler LK, Magliocca J, Zendejas I, Srinivas TR, Schold JD. Impact of cold ischemia time on graft survival among ECD transplant recipients: a paired kidney analysis. Am J Transplant. 2011;11:2647–56.
Kayler LK, Srinivas TR, Schold JD. Influence of CIT-induced DGF on kidney transplant outcomes. Am J Transplant. 2011;11:2657–64.
Weber M, Dindo D, Demartines N, Ambuhl PM, Clavien PA. Kidney transplantation from donors without a heartbeat. N Engl J Med. 2002;347:248–55.
Kamar N, Garrigue V, Karras A, Mourad G, Lefrancois N, Charpentier B, Legendre C, Rostaing L. Impact of early or delayed cyclosporine on delayed graft function in renal transplant recipients: a randomized, multicenter study. Am J Transplant. 2006;6:1042–8.
Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, Massari P, Mondragon-Ramirez GA, Agarwal M, Di Russo G, Lin CS, Garg P, Larsen CP. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010;10:535–46.
Durrbach A, Pestana JM, Pearson T, Vincenti F, Garcia VD, Campistol J, Rial Mdel C, Florman S, Block A, Di Russo G, Xing J, Garg P, Grinyo J. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study). Am J Transplant. 2010;10:547–57.
Irish WD, Ilsley JN, Schnitzler MA, Feng S, Brennan DC. A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am J Transplant. 2010;10:2279–86.
Schnuelle P, Gottmann U, Hoeger S, Boesebeck D, Lauchart W, Weiss C, Fischereder M, Jauch KW, Heemann U, Zeier M, Hugo C, Pisarski P, Kramer BK, Lopau K, Rahmel A, Benck U, Birck R, Yard BA. Effects of donor pretreatment with dopamine on graft function after kidney transplantation: a randomized controlled trial. JAMA. 2009;302:1067–75.
Moers C, Smits JM, Maathuis MH, Treckmann J, van Gelder F, Napieralski BP, van Kasterop-Kutz M, van der Heide JJ, Squifflet JP, van Heurn E, Kirste GR, Rahmel A, Leuvenink HG, Paul A, Pirenne J, Ploeg RJ. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009;360:7–19.
Sureshkumar KK, Hussain SM, Ko TY, Thai NL, Marcus RJ. Effect of high-dose erythropoietin on graft function after kidney transplantation: a randomized, double-blind clinical trial. Clin J Am Soc Nephrol. 2012;7:1498–506.
Martinez F, Kamar N, Pallet N, Lang P, Durrbach A, Lebranchu Y, Adem A, Barbier S, Cassuto-Viguier E, Glowaki F, Le Meur Y, Rostaing L, Legendre C, Hermine O, Choukroun G. High dose epoetin beta in the first weeks following renal transplantation and delayed graft function: results of the Neo-PDGF Study. Am J Transplant. 2010;10:1695–700.
Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–12.
Hall IE, Parikh CR. Human models to evaluate urinary biomarkers of kidney injury. Clin J Am Soc Nephrol. 2010;5(12):2141–3.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Schröppel, B., Legendre, C. (2015). Acute Allograft Injury After Kidney Transplantation. In: Thakar, C., Parikh, C. (eds) Perioperative Kidney Injury. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1273-5_15
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1273-5_15
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1272-8
Online ISBN: 978-1-4939-1273-5
eBook Packages: MedicineMedicine (R0)