Abstract
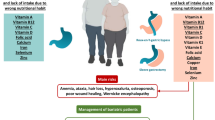
Bariatric surgery is associated with development of several micronutrient deficiencies that are predictable based on the surgically altered anatomy and the imposed dietary changes. The three restrictive-malabsorptive procedures—Roux-en-Y gastric bypass, biliopancreatic diversion, and biliopancreatic diversion with duodenal switch, pose a greater risk for micronutrient malabsorption and deficiency than the purely restrictive laparoscopic adjustable gastric banding. The newer laparoscopic gastric sleeve (LGS) procedure poses a unique risk due to partial resection of the stomach. Metabolic and clinical deficiencies of two minerals (iron and calcium) and four vitamins (thiamine, folate vitamin B12, and vitamin D) have been well described in the literature. Deficiency of vitamin A and copper are reported less often. This chapter reviews the pathophysiology, clinical presentation, screening tests, and treatment for each micronutrient deficiency. With careful monitoring and adequate supplementation, these deficiencies are largely avoidable and treatable.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Micronutrient Deficiencies
- Bariatric Surgical Patients
- Biliopancreatic Diversion With Duodenal Switch (BPDDS)
- Bariatric Surgery
- Laparoscopic Gastric Sleeve (LGS)
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Bariatric surgery has been endorsed as an acceptable weight loss option for patients with severe (also called extreme, morbid or class III) obesity or those with moderate obesity who have comorbid conditions by several authoritative guidelines and conferences [1–6]. The exponential growth in procedures is due to several factors including improved surgical techniques, reduction in the postoperative mortality rate, significant improvement in obesity-related comorbid conditions [7], increased media attention, and profitability. The upsurge in surgical procedures also reflects the increasing prevalence of severe obesity in the United States. Approximately 6 % of adult Americans are considered severely obese (body mass index ≥40 kg/m2) with prevalence figures reaching 18 % for non-Hispanic black women [8]. It is therefore likely that healthcare professionals from all disciplines will encounter patients who have undergone a bariatric surgical procedure. Similarly, primary care physicians and specialists will be expected to monitor and manage their patients on a long-term basis. Although physicians are trained to manage chronic diseases commonly associated with severe obesity, such as type 2 diabetes, obstructive sleep apnea, hypertension, mixed hyperlipidemia, and arthritis among others, nutritional management following bariatric surgery is not routinely taught. The combined restrictive-malabsorptive surgical procedures—Roux-en-Y gastric bypass (RYGB), biliopancreatic diversion (BPD), and biliopancreatic diversion with duodenal switch (BPDDS), place patients at high risk for development of both macro- and micronutrient deficiencies unless they are properly counseled and supplemented. Since most of the deficiencies can be identified early at a preclinical stage, early treatment will prevent or reduce symptoms and deficiency syndromes. This chapter will review the identification and management of the most common micronutrient deficiencies that may occur following restrictive-malabsorptive bariatric surgeries.
Bariatric Surgery-Related Micronutrient Deficiencies
By definition, micronutrients are essential nutrients that are required in only small quantities (mg or micrograms) such as minerals, trace elements, and vitamins. Deficiencies of micronutrients following bariatric surgery can arise from several mechanisms that include (1) preoperative deficiency, (2) reduced dietary intake, (3) malabsorption, and (4) inadequate supplementation. Bariatric surgery is unique in that the RYGB, LGS, BPD, and BPDDS procedures surgically alter the gastrointestinal anatomy in known ways. By bypassing the stomach, duodenum, and varying portions of the jejunum and ileum, malabsorption of four vitamins (thiamine, folate, vitamin B12,, and vitamin D) and two minerals (calcium and iron) may occur. Although less common, deficiencies of other vitamins and minerals have also been described, including vitamin A and copper. In general, the greater the malabsorption, the higher the risk for developing nutritional deficiencies. The prevalence of these deficiencies varies widely in the literature due to differences in surgical technique, patient population, definition of deficiency, supplementation protocols, and length and completion of patient follow-up. For example, iron deficiency is reported to range from 20 to 49 % and vitamin B12 deficiency from 26 to 70 % [9–17]. In the following section, ‘at-risk’ micronutrients will be each reviewed considering pathophysiology, clinical presentation, screening tests, and treatment. Table 18.1 provides a summary of the assessment and treatment of micronutrient deficiencies. Other recent review articles address the general topic of nutritional and metabolic problems following bariatric surgery [18–24].
Micronutrient Deficiency
Thiamine
Thiamine (vitamin B1) is a coenzyme for the essential enzymes transketolase, pyruvate dehydrogenase, and pyruvate carboxylase, in the early stages of the tricarboxylic acid cycle and in the pentose phosphate pathway [25]. Thiamine is mainly absorbed in the jejunum by both active and passive diffusion. Since the biological half-life of the vitamin is rather short (in the range of 9–18 days) and only a small percentage of a high dose is absorbed [26], patients are at risk of developing deficiency syndromes after bariatric surgery. Over the past 3 decades, numerous case reports of thiamine deficiency have been reported following both restrictive and restrictive-malabsorptive surgeries [27–44]. An acute deficiency of thiamine associated with rapidly progressing clinical symptoms appears to most commonly result from a combination of restricted food intake and persistent intractable vomiting. Symptoms commonly occur 1–3 months postoperatively although may occur later. The clinical presentation varies but three conditions have been reported. Classical Wernicke’s encephalopathy is the most common presentation and consists of double vision, nystagmus, ataxia, and a global confusion manifested by apathy, impaired awareness of the immediate situation, disorientation, inattention, and an inability to concentrate. Dry beriberi presents as bilateral, symmetric, lower extremity paresthesia, while wet beriberi manifests as high output congestive heart failure, edema, and metabolic acidosis.
Several recent reviews of neurologic complications following bariatric surgery have been published [45–50]. These authors describe a constellation of symptoms including mono- and polyneuropathy with weakness and/or paresthesias, burning feet syndrome, and hyporeflexia. Chang et al. [51] coined the acronym APGARS (Acute post-gastric reduction surgery neuropathy) to describe conditions with features of weakness, hyporeflexia, and vomiting. Since all symptoms did not improve with thiamine treatment, the authors suggest that additional nutritional deficiencies may be involved in the etiology of this syndrome.
Thiamine status is best assessed by determining erythrocyte transketolase activity. Magnetic resonance imaging (MRI) is useful in confirming the diagnosis of acute Wernicke’s encephalopathy with a sensitivity of 53 % and specificity of 93 % [52]. With this test, increased T2 signal of paraventricular regions of the thalamus and increased T2 signal of periaqueductal regions of the midbrain are seen. However, treatment should not be delayed if a thiamine deficiency syndrome is suspected. Treatment with thiamine 100 mg IV every 8 h for 7–14 days followed by 50–100 mg po daily is recommended for these syndromes until the patient fully recovers. To avoid deficiency, patients should be routinely discharged from the hospital receiving a chewable multiple vitamin-mineral supplement that contains between 1.5 and 1.8 mg thiamine.
Vitamin B12
Vitamin B12 (cobalamin) is a cofactor in the biosynthesis of succinyl-coenzyme A and methionine and is important for the functioning of hemopoetic and neural cells [25]. Vitamin B12 absorption requires a complex sequence of orchestrated metabolic steps within the gastrointestinal tract (Fig. 18.1). In the stomach, food-bound B12 is first dissociated from animal proteins by acid and peptic hydrolysis to liberate free vitamin B12. Once released, the vitamin is avidly bound to R proteins, which are glycoproteins secreted by the salivary glands and the gastric mucosa. In the intestine, pancreatic proteases then degrade R proteins and permit vitamin B12 to associate with intrinsic factor (IF), a glycoprotein that the parietal cells of the stomach secrete after being stimulated by food. The resulting IF-vitamin B12 complex is then bound to specific receptors in the distal ileum, where absorption occurs [26].
The restrictive-malabsorptive procedures disrupt several of these key steps. Vitamin B12 deficiency may occur due to decreased acid and pepsin digestion of protein-bound cobalamins from food, incomplete release of vitamin B12 from R proteins, and decreased availability of IF to form IF-vitamin B12 complexes. Because the parietal cells which secrete acid and IF, and chief cells which secrete pepsinogen, are located primarily in the fundus and body of the stomach, the LGS and RYGB procedures essentially excludes food from the normal gastric digestive process. Acid secretion has been demonstrated to be virtually absent in the small pouch constructed from the gastric cardia [53, 54]. Consequently, cobalamins are not liberated from protein and are not available for intestinal absorption. In all three restrictive-malabsorptive procedures, pancreatic secretions are diverted distally to mix with nutrients in a shortened common channel, thus affecting the vitamin’s binding to IF and subsequent attachment to ileal IF-vitamin B12 receptors.
Although B12 deficiency is predictable, onset of signs and symptoms are typically delayed for months to years due to prolonged hepatic storage of the vitamin. When they do occur, clinical effects of deficiency are similar to those of pernicious anemia—hematological and neurological. Hypersegmented polymorphonuclear leukocytes and macrocytic erythrocytes can be seen on peripheral blood smear along with a macrocytic anemia. Neurological manifestations include sensory disturbances in the lower extremities (tingling and numbness); motor disturbances including abnormalities in gait; and cognitive changes ranging from loss of concentration to memory loss and disorientation [26].
Vitamin B12 status is most commonly and easily assessed by serum or plasma vitamin levels. The concentration of B12 in the serum or plasma reflects both the B12 intake and stores. The lower limit is considered to be approximately 120–180 pmol/L (170–250 pg/mL). However, a more sensitive biochemical indicator of deficiency is elevation of serum homocysteine and methylmalonic acid (MMA), levels which rise when the supply of B12 is low and virtually confirms the diagnosis.
All patients who undergo restrictive-malabsorptive procedures should receive prophylactic vitamin B12 supplementation to prevent deficiency. In contrast to the disruption of food-bound B12 absorption, crystalline vitamin B12 (the form found in vitamin supplements) can be absorbed in the surgical patient since approximately 1 % of orally administered crystalline cobalamin is absorbed by passive diffusion [55, 56]. An oral dose of at least 200 times the recommended dietary allowance (RDA) was shown to normalize mild vitamin B12 deficiency in older people assessed by reduction in plasma MMA concentration [57]. Oral treatment has also been effective in patients with pernicious anemia [58]. As a practical matter, patients should receive at least 500 μg B12 daily as a dietary supplement delivered orally as a tablet or liquid or sublingually; as a once weekly nasal spray 500 μg cyanocobalamin gel (Nascobal®), or by intramuscular injection (100 μg monthly to 3,000 μg every 6 months). The route of delivery is based on patient preference and monitoring of vitamin B12 status.
Folate
Folate deficiency occurs with lower frequency than vitamin B12 deficiency; however, it should be considered when evaluating a patient who develops anemia. Folate is a cofactor in the biosynthesis of methionine, thymidine, and purine nucleotides, and for the synthesis of the coenzyme tetrahydrofolate [25]. Folate is absorbed primarily from the proximal third of the small intestine after food folate polyglutamates are hydrolyzed to monoglutamates by intestinal brush border conjugases. Folate deficiency presents with many features similar to vitamin B12 deficiency, including hypersegmentation of the neutrophils, increased mean cell volume (MCV), and macrocytic anemia. Inadequate folate intake first leads to a decrease in serum folate concentration, then a decrease in erythrocyte folate concentration, a rise in homocysteine concentration, followed by clinical hematological changes as mentioned above [26]. A serum folate concentration of less than 7 nmol/L (3 ng/mL) indicates negative folate balance. All patients undergoing a restrictive-malabsorptive bariatric operation should receive supplemental doses of folate to prevent deficiency. Supplements of folic acid are nearly 100 % bioavailable. Typically, the amount of folate present in a general (400 μg) or prenatal multivitamin supplement (800–1,000 μg) is adequate to prevent deficiency.
Iron
Patients who undergo restrictive-malabsorptive procedures are at particular risk for developing iron deficiency and iron deficiency anemia (IDA) due to reduced iron absorption, decreased iron intake, and for menstruating women, increased iron losses. Surgical bypass of the duodenum and proximal jejunum decreases total iron uptake because the majority of iron absorption occurs across the apical and basolateral membrane of duodenal enterocytes [59]. Furthermore, acid secretion is nearly absent in the small gastric pouch [53, 54] or remnant gastric sleeve due to the paucity of parietal cells. Hypoacidity exacerbates iron deficiency because both heme (found only in animal products) and nonheme (found in cereals and green leafy vegetables) iron depend upon the acidic environment of the stomach for efficient absorption [60]. Specifically, nonheme iron requires an acidic pH to reduce it from the ferric (Fe3+) to the ferrous (Fe2+) state, before it can be transported across the brush border membrane by divalent metal ion transporter 1 (DMT 1) in the alkaline duodenum. Although heme iron is more soluble and readily absorbed than nonheme iron, it must be released from its protein structure by the acid and proteases present in gastric juice before absorption can occur [61]. If iron is required by the body, it will cross the basolateral membrane through iron export protein ferroportin and enter the circulation in which is binds to plasma transferrin [62]. Iron absorption from the diet or from supplementation has been shown to decrease significantly after 6 months following RYGB to 33 % and 40 % of their initial values, respectively [63].
In addition to decreased iron absorption, bariatric surgical patients typically consume less heme iron due to an intolerance of meat products (which is full of hemoglobin and myoglobin) [64]. Women with menorrhagia are particularly prone to develop iron deficiency and IDA due to excessive menstrual blood loss. Menstrual iron losses range from 1.5 to 2.1 mg/day, bringing the RDA for females between 19 and 50 years old to 18 mg/day compared to 8 mg/day for males [65]. Due to the combination of these factors, IDA occurs postoperatively in 33–50 % of patients, with a higher incidence in menstruating women [66–68].
Iron deficiency may also be exacerbated in these patients as a result of a nutrient–nutrient inhibitory absorptive interaction between iron and calcium, another mineral that is routinely supplemented during the postoperative period. Most [69–74] but not all [75, 76] studies show that nonheme- and heme-iron absorption is inhibited up to 50–60 % when consumed in the presence of calcium supplements or with dairy products. Calcium at doses of 300–600 mg has a direct dose-related inhibiting effect on iron absorption. This has been seen with calcium carbonate, calcium citrate, and calcium phosphate. Studies by Hallberg et al. [70, 72] suggest that the inhibitory effect is situated within the intestinal mucosal cells. These observations are particularly important for bariatric surgical patients who are routinely prescribed calcium supplements and advised to consume dairy foods high in calcium, such as milk, cheese, and yogurt. In these patients, it appears prudent to recommend that iron and calcium supplementation be separated by several hours to avoid inhibitory interaction.
Early functional symptoms of iron deficiency include fatigue, poor exercise tolerance, and decreased work performance [77]. Signs on physical examination include pale conjunctiva and spoon nails. Serum ferritin is the most sensitive indicator of iron status (normal values usually fall in the range of 20–300 μg/L) and is recommended for diagnosing early iron deficiency [78]. The concentration of serum ferritin reflects the size of the storage iron compartment, with each μg/L representing 8–10 mg of storage iron [60]. However, caution is needed in interpreting ferritin concentration levels in the presence of acute and chronic inflammation since ferritin is also an acute phase reactant. Thus, serum ferritin concentrations may fall within normal range in individuals who have diminished iron stores. The concentration of liver-derived peptide hepcidin reflects body iron requirements and may be useful in the future as a biomarker for monitoring iron status [62]. Hepcidin regulates iron homeostasis by regulating ferroportin-mediated release of iron from enterocytes and macrophages. After the iron storage pool is depleted, there is an increase in total iron-binding capacity (TIBC), decreased serum transferrin saturation (serum iron concentration divided by TIBC × 100), followed by microcytosis (reduced mean corpuscular volume or MCV), hypochromia (reduced mean corpuscular hemoglobin concentration or MCHC), and anemia.
An unusual and fascinating symptom that is particularly associated with IDA is ice eating, or pagophagia, one of the most commonly reported forms of pica. Pica has been previously reported to occur with IDA of pregnancy [79, 80], gastrointestinal blood loss [81], and sickle cell disease [82]. Our group previously reported the first five cases of pagophagia associated with RYGB surgery [83, 84]. All patients were women between 34 and 45 years old with menorrhagia. Onset of pica symptoms ranged from 1 to 23 months post-surgery. Three of the patients described symptoms suggestive of pica when they were children and one during a previous pregnancy.
In order to prevent iron deficiency, all patients undergoing restrictive-malabsorptive surgeries should be prescribed a daily multivitamin–mineral supplement containing elemental iron. Supplementation with one prenatal vitamin and mineral tablet, which typically contains 28–40 mg elemental iron, is often sufficient. High-risk individuals, for example, those who have preoperative iron deficiency or excessive blood loss or those who develop iron deficiency or any degree of anemia, require additional supplementation with an iron salt preparation. Only ferrous iron formulations should be used such as ferrous sulfate, gluconate, or fumarate, since they are more readily absorbed [85]. However, it is important to note that a tablet of the sulfate salt contains twice the amount of elemental iron (65 mg) as a tablet of the other two salts. Therefore, twice as many ferrous gluconate or ferrous fumarate tablets are required to provide the amount of elemental iron in ferrous sulfate tablets [86]. Typical dosing of iron therapy is 150–200 mg/day po given in 2–3 divided doses for several months or until the serum ferritin reaches 50 μg/L. Patients who are responsive to treatment should develop reticulocytosis followed by an increase in hemoglobin. Co-administration with ascorbic acid (vitamin C), the best known reducing agent, is recommended to increase iron absorption [87]. In the presence of ascorbic acid, ferrous iron forms a soluble iron-ascorbic acid complex. Patients who were anemic may require long-term daily supplementation in addition to their other nutritional supplements. In patients with profound iron deficits and severe anemia unresponsive to oral iron supplementation, intravenous administration of iron dextran (InFed), ferric gluconate (Ferrlecit), ferric sucrose (Venofer), or ferumoxytol (Feraheme) will be required.
Calcium and Vitamin D
Calcium and vitamin D are considered together since deficiency of both nutrients may result in metabolic bone disease and their metabolism is interrelated. A negative calcium balance may result from limited intake of calcium- and vitamin D-containing dairy products, reduced fractional intestinal absorption due to surgical bypass of the absorptive sites, and vitamin D deficiency. The latter factor is important since calcium is absorbed by an active transport process dependent on the action of 1,25-dihydroxyvitamin D (1,25(OH)2D) which binds with high affinity to the vitamin D receptor (VDR) to enhance calcium absorption primarily in the duodenum and jejunum [88] although most of the absorption occurs in the lower segment of the small intestine, the ileum [89]. Calcium is also absorbed by passive diffusion across the intestinal mucosa which becomes important at high calcium intakes such as supplemental calcium [90]. In a prospective study of women who underwent RYGB, fractional radiolabeled calcium absorption in milk was reduced 33 % 6 months after surgery [91].
Vitamin D deficiency may occur for the same reasons listed above for calcium deficiency, that is, reduced intake of vitamin D fortified dairy products and malabsorption of vitamin D due to mismixing of pancreatic and biliary secretions in the distal small intestine. Since vitamin D is fat soluble, it must be incorporated into the intestinal micelle along with bile salts for absorption. However, the major source of vitamin D for most people comes from casual exposure to sunlight. Unlike any other vitamin, vitamin D3, or cholecalciferol is photosynthesized by the skin by UVB irradiation, converting 7-dehydrocholesterol (provitamin D) to previtamin D3 in the plasma membrane of skin keratinocytes. Once formed in the skin, previtamin D3 is rapidly converted to vitamin D3 [90]. In the liver, vitamin D undergoes hydroxylation at the 25-carbon position to form 25-hydroxy vitamin D (25(OH)D) and subsequently transported to the kidney for additional hydroxylation at the 1-carbon position to form 1,25(OH)2D, the biological active form of the vitamin. Several factors will impede the initial photosynthetic process including living at northern latitudes, wearing sunscreen lotion, limited sun exposure, dark skin pigmentation [92], aging, and obesity itself [93–96]. Melanin skin pigmentation is an effective natural sunscreen that greatly reduces UVB-mediated cutaneous synthesis of vitamin D3. Thus dark-skinned individuals need longer UVB exposure to generate the same 25(OH)D stores compared with fair-skinned individuals. Several studies have demonstrated an inverse correlation between vitamin D concentrations and BMI or body fat percentage, suggesting decreased bioavailability of skin-derived vitamin D in obese individuals [97]. Thus, severely obese individuals are predisposed to vitamin D insufficiency or deficiency prior to undergoing bariatric surgery.
Clinical deficiency of calcium or vitamin D due to bariatric surgery cannot be detected on a routine chemistry panel, although an elevated alkaline phosphatase level and low calcium or phosphorus level may be seen. Symptoms of vitamin D deficiency are commonly nonspecific such fatigue or easy tiring, muscular weakness predominantly of the proximal limb muscles, and chronic musculoskeletal pain [98]. Unless specifically monitored, the first indication of deficiency is likely to be a vertebral or wrist fracture secondary to development of osteoporosis or osteomalacia. Physiologically, chronic calcium deficiency causes the circulating ionized calcium concentration to decline, which triggers an increase in parathyroid hormone (PTH) synthesis and release. In turn, PTH acts on three organs to restore the circulating calcium concentration to normal. At the kidney, PTH promotes the reabsorption of calcium in the distal tubule. PTH affects the intestine indirectly by stimulating the production of 1,25(OH)2D. PTH also induces bone resorption, thereby releasing calcium into the blood [88]. Chronic vitamin D deficiency can result in secondary hyperparathyroidism, increased bone turnover, enhanced bone loss, and increased risk of fragility fracture [99]. Secondary hyperparathyroidism is diagnosed by an elevated PTH level in the setting of low or normal serum calcium [92]. Therefore, detection of subclinical calcium and/or vitamin D deficiency requires measurement of several nutrients, hormone levels, and biochemical markers of bone turnover that are not routinely assessed.
Serum 25(OH) vitamin D is the best indicator for determining adequacy of vitamin D intake since it represents the combination of cutaneous production of vitamin D and the oral ingestion of both vitamin D2 (ergocalciferol or plant-based vitamin D) and vitamin D3. 25(OH)D is not only the transport form of the vitamin D but a direct measure of stores [100]. In the current literature, severe vitamin D deficiency is identified as a 25(OH)D level of less than 5–8 ng/mL (12.5–20 nmol/mL) and mild deficiency or insufficiency as a serum level less than 20 ng/mL (50 nmol/mL) [101]. However, there is debate about the exact cutoff values defining ‘deficiency’ and ‘insufficiency’ since these are static rather than functional definitions. When elevated PTH levels (secondary hyperparathyroidism) are used as a functional indicator of vitamin D deficiency, circulating levels of 25(OH)D of at least 30 ng/mL appear optimal [102, 103]. Biochemical monitoring of bone turnover includes measurement of bone formation markers—serum osteocalcin and bone-specific alkaline phosphatase, and the bone resorption marker—serum and urine peptide-bound N-telopeptide crosslinks of type 1 collagen (NTX) [104]. Assessment of bone mineral density (BMD) and bone mineral content (BMC) by dual energy X-ray absorptiometry (DXA) remains the gold standard for the diagnosis of osteoporosis [105].
Abnormalities in vitamin D and bone metabolism among patients undergoing restrictive-malabsorptive bariatric operations have been reported in numerous case series, case reports, and recent reviews [106–118]. Although the studies are primarily observational, contain few patients, and uncontrolled for diet and vitamin mineral supplementation, most studies document the occurrence of hypovitaminosis D and elevated PTH over the first 1–3 postoperative years, with a prevalence ranging from 30 [98] to 80 % [119]. Many of the studies also show a corresponding elevation in alkaline phosphatase levels and biochemical markers of bone turnover. There are multiple factors involved in postsurgical bone mass loss that includes nutritional deficiencies, rapid weight loss, and possibly changes in adipokines and gut-derived appetite regulatory hormones [118]. In a prospective study of 73 patients who underwent either a restrictive or restrictive-malabsorptive bariatric surgical procedure, both urinary NTX and osteocalcin increased significantly by 3 months after surgery and remained significantly elevated through 18 months [120]. Median serum PTH levels at 9 and 12 months were higher in patients who underwent a restrictive-malabsorptive procedure compared to those who underwent LAGB. PTH levels were associated with increased serum 1,25(OH)2D.
It is important to prospectively monitor patients preoperatively and postoperatively since many obese patients present to surgical centers with underlying vitamin and mineral deficiencies, including D deficiency and some with secondary hyperparathyroidism [121–125]. Several cases of severe secondary hyperparathyroidism with osteomalacia have been reported to occur from 9 to 17 years post-surgery [112, 126]. However, since weight reduction itself is associated with reduced BMD and BMC [127, 128], it is important to distinguish between the weight loss and malabsorptive effects of bariatric surgery. Pugnale et al. [129] showed that BMD of the cortical bone decreased significantly among 31 women who underwent a restrictive banding procedure without evidence of secondary hyperparathyroidism. In a 1-year prospective study among 23 obese men and women who underwent RYGB, there was a significant decrease in BMD at the femoral neck and total hip by 9.2 % and 8 %, respectively, associated with a significant increase in urinary NTX and serum osteocalcin [130]. Similarly, Guney et al. demonstrated that weight reduction causes bone loss among both diet treated patients and those who underwent a restrictive vertical banded gastroplasty without a significant change in PTH levels [131]. In another study, six obese control patients were compared to four patients who underwent an RYGB and nine patients who received gastric banding [111]. The RYGB operation resulted in significant net loss of bone mass in comparison to the banding and obese control group. In the study by El-Kadre et al. [109], 10 % of patient had elevated PTH levels preoperatively, whereas the prevalence was 22 % and 25 % in the series by Johnson et al. [114] and Hamoui et al. [110], respectively.
Inclusion of calcium- and vitamin D-containing dairy products in the postoperative diet is important. One serving of milk contains approximately 300 mg calcium. However, many patients will avoid or limit dairy foods due to lactose intolerance or lack of an acquired taste. Choosing lactaid milk or adding lactase to dairy products will address the former problem. To avoid deficiency and supplement the diet, all patients should receive calcium supplements of at least 1,200–2,000 mg/day in divided doses, depending on the adequacy of dietary calcium. Postmenopausal, lactating or pregnant women may require higher ranges due to increased needs. Calcium citrate + vitamin D is the preferred preparation because it is more soluble than calcium carbonate in the absence of gastric acid production [132]. The optimal dose for vitamin D is more controversial since some studies have shown continued deficiency despite high dose supplementation [116, 120, 121]. General guidelines recommend 3,000–5,000 IU per day. Since multivitamin–mineral tablet typically contains 400 IU and calcium + Vitamin D tablets typically contain 500 IU, many patients will require additional vitamin D.
If vitamin D deficiency is detected, measurement of PTH should be obtained to provide a functional assessment. Treatment may involve recommending higher supplemental doses of calcium and vitamin D and reassessing in about 3 months. In patients with severe vitamin D deficiency, initial repletion of stores should be treated with vitamin D 50,000 IU 1–3 times weekly for 8 weeks, then checking 25(OH)D levels [111]. Monitoring of the alkaline phosphatase level and serum and urinary calcium should also be performed.
Other Deficiencies
Micronutrient deficiencies of vitamin A and copper have been reported following bariatric surgery, although less often that the aforementioned nutrients. They will briefly be reviewed.
Vitamin A
Vitamin A, whether ingested as preformed vitamin A (retinyl esters) or as provitamin A carotenoids, requires processing in the intestine to release the nutrients in an absorbable form [25]. Since vitamin A is a fat-soluble vitamin, any condition that interferes with emulsification is likely to reduce intestinal absorption of retinol. Thus vitamin A dietary compounds are more likely to be malabsorbed with the BPD and BPDDS procedures which limit the exposure of food with biliopancreatic digestive secretions within a shortened common channel. Subsequently, deficiency of vitamin A has been more frequently reported among patients who have undergone a BPD or BPDSS procedure compared to RYGB [16], although deficiency has been reported following RYGB as well [133–135]. Slater et al. [136] observed an incidence of vitamin A deficiency of 52 % at 1 year which increased to 69 % by year 4. Similarly elevated incidence rates of vitamin A deficiency were seen by Dolan et al. [137] at 12–18 months after BPD or BPDDS. In a retrospective chart review among 122 patients who underwent RYGB, 35 % and 18 % of patients were vitamin A deficient at 6 weeks and 1 year, respectively [135]. Several cases of symptomatic vitamin A deficiency have been reported following bariatric surgery, occurring 18 and 24 months postoperatively [138, 139]. Patients presented with night blindness (nyctalopia) while one developed diffuse conjunctival xerosis with a Bitot’s spot, and diffuse punctuate keratitis of both corneas. In another study of 64 RYGB patients who completed a postoperative survey, reported ocular symptoms potentially related to vitamin A deficiency included xerosis (38 %), night vision changes (68 %), and eye pain/foreign body sensation (23 %) [133].
Preformed vitamin A is found in foods from animal sources, including dairy products, fish, and meat (especially liver). By far the most important provitamin A carotenoid is beta-carotene. Good sources of carotenoids include spinach, sweet potato, carrots, and cantaloupe. Routine screening for vitamin A is recommended for the BPD and BPDDS malabsorptive procedures. To prevent deficiency, a daily dose of at least 10,000 IU is recommended. For patients who undergo RYGB, a prenatal multiple vitamin-mineral supplement containing at least 5,000 IU vitamin A should be provided on a daily basis
Copper
Copper deficiency has emerged as a cause of an array of neurological symptoms that may occur among patients who have undergone bariatric surgery. Reported symptoms include unsteady gait, extremity numbness, paresthesias, or paralysis occurring as long as 10 years following the procedure [140–142]. Other accompanying presentations may include anemia, neutropenia, and pancytopenia. Copper is absorbed primarily in the small intestine with a small amount absorbed in the stomach through a saturable, active transport process [25]. Iron and zinc have been shown to interfere with copper absorption, a particularly important fact since bariatric surgical patients are often asked to take multiple mineral supplements. Copper is required for the formation and maintenance of myelin and in iron metabolism. Most of the copper in blood is bound to ceruloplasmin which is the most reliable index of copper status. The incidence of copper deficiency is uncertain since the micronutrient is not commonly measured.
Copper administration of 2 mg/day should be included as part of routine multivitamin–mineral supplementation. Routine copper screening is not recommended for bariatric surgery patients. However, patients presenting with signs and symptoms of myelopathy or myeloneuropathy should have serum copper and ceruloplasmin values measured. In severe deficiency, treatment can be initiated with IV copper (2–4 mg/day) for 6 days, followed by oral administration [23].
Prophylactic Management and Monitoring for Nutritional Deficiencies
Nutritional management of the bariatric surgical patient must include prophylactic administration of vitamins and minerals to avoid deficiencies. As a practical manner, all patients should be discharged from the hospital receiving a chewable multiple vitamin-mineral supplement. After the first postoperative month, patients can be switched to a prescribed or over-the-counter supplement. Examples of products with nutrient content are shown in Table 18.2. Since the calcium, vitamin D, and vitamin B12 contents of the supplements are inadequate to meet postsurgical needs, all patients should receive additional calcium citrate 1,200–2,000 mg daily depending on dairy calcium along with at least 500 μg vitamin B12.
Monitoring of nutritional status should begin preoperatively. Table 18.3 displays a list of routine laboratory and micronutrient tests and procedures. Once detected, deficiencies should be treated and monitored carefully. Patients at particularly high risk, such as women with menorrhagia, will likely require additional supplementation of selected nutrients.
Conclusion
The restrictive-malabsorptive bariatric surgeries are associated with an increased risk of developing several micronutrient deficiencies. With judicious monitoring and adequate supplementation, these deficiencies are largely avoidable and treatable. However, the long-term sequela of calcium and vitamin D malabsorption and development of metabolic bone disease remains a major concern. It is recommended that patients be screened preoperatively and at periodic intervals postoperatively. Identification of micronutrient deficiencies should be aggressively treated.
References
Gastrointestinal surgery for severe obesity. NIH Consens Dev Conf Consens Statement. Am J Clin Nutr. 1992;55:615S–9S
National Heart, Lung, and Blood Institute (NHLBI) and National Institute for Diabetes and Digestive and Kidney Diseases (NIDDKD). Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. The evidence report. Obes Res. 1998;6 Suppl 2:51S–210S.
National Heart, Lung, and Blood Institute (NHLBI)/North American Association for the Study of Obesity (NAASO). Practical guide on the identification, evaluation, and treatment of overweight and obesity in adults. Bethesda, MD: National Institutes of Health; 2000. NIH Publication Number 00-4084.
Jones DB, Provost DA, De Maria EJ, Smith CD, Morgenstern L, Schirmer B. Optimal management of the morbidly obese patient. SAGES appropriateness conference statement. Surg Endosc. 2004;18:1029–37.
Buchwald H. Bariatric surgery for morbid obesity: health implications for patients, health professionals, and third-party payers. J Am Coll Surg. 2005;200:593–604.
2013 AHA/ACC/TOS Guideline for the management of overweight and obesity in adults. http://circ.ahajournals.org/content/early/2013/11/11/01.cir.0000437739.71477.ee
Replace with SOS study Steinbrook R. Surgery for severe obesity. N Engl J Med. 2004;350:1075–9.
Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–7.
Avinoah E, Ovnat A, Charuzi I. Nutritional status seven years after Roux-en-Y gastric bypass surgery. Surgery. 1992;111:137–42.
Brolin RE, Gorman JH, Gorman RC, Petschenik AJ, Bradley LJ, Kenler HA, Cody RP. Are vitamin B12 and folate deficiency clinically important after Roux-en-Y gastric bypass? J Gastrointest Surg. 1998;2:436–42.
Brolin RE, Leung M. Survey of vitamin and mineral supplementation after gastric bypass and biliopancreatic diversion for morbid obesity. Obes Surg. 1999;9:150–4.
Skroubis G, Sakellaropoulos G, Pouggouras K, Mead N, Nikiforidis G, Kalfarentzos F. Comparison of nutritional deficiencies after Roux-en-Y gastric bypass and after biliopancreatic diversion with Roux-en-Y gastric bypass. Obes Surg. 2002;12:551–8.
Blume CA, Boni CC, Casagrande DS, Rizzolli J, Padoin AV, Mottin CC. Nutritional profile of patients before and after Roux-en-Y gastric bypass: 3-year follow-up. Obes Surg. 2012;22:1676–85.
Dalcanale L, Oliveira CPMS, Faintuch J, Nogueira MA, et al. Long-term nutritional outcome after gastric bypass. Obes Surg. 2010;20:181–7.
Gehrer S, Kern B, Peters T, Christoffel-Courtin C, Peterli R. Fewer nutrient deficiencies after laparoscopic sleeve gastroctomy (LSG) than after laparoscopic roux-y-gastric bypass (LRYGB)-a prospective study. Obes Surg. 2010;20:447–53.
Aasheim ET, Bjorkman S, Sovik TT, Engstrom M, Hanvold SE, Mala T, Olbers T, Bohmer T. Vitamin status after bariatric surgery: a randomized study of gastric bypass and duodenal switch. Am J Clin Nutr. 2009;90:15–22.
Aasheim ET, Johnson LK, Hofso D, Bohmer T, Hjelmesaeth J. Vitamin status after gastric bypass and lifestyle intervention: a comparative prospective study. Surg Obes Relat Dis. 2012;8:169–75.
Aills L, Blankenship J, Buffington C, Furtado M, Parott J. Bariatric nutrition: Suggestions for the surgical weight loss patient. Surg Obes Relat Dis. 2008;4(4S):1–36.
Mechanick JI, Kushner RF, Sugerman HJ, et al. American Association of Clinical Endocrinologists, The Obesity Society, and the American Society for Metabolic & Bariatric Surgery. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Obesity. 2009;17 Suppl 1:S1–S70.
Heber D, Greenway FL, Kaplan LM, Livingston E, Salvador J, Still C. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4823–43.
Bal BS, Finelli FC, Shope TR, Koch TR. Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol. 2012;8:544–56.
Saltzman E, Karl JP. Nutritional deficiencies after gastric bypass surgery. Annu Rev Nutr. 2013;33:183–203.
Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient – 2013 update: American Association of Clinical Endocrinologists, The Obesity Society, and the American Society for Metabolic & Bariatric Surgery. Obesity. 2013;21:S1–27.
Gletsu-Miller N, Wright BN. Mineral malnutrition following bariatric surgery. Adv Nutr. 2013;4:506–17.
Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern nutrition in health and disease. 10th ed. Philadelphia: Lippincott Williams & Wilkins; 2006.
Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board (FNB), Institute of Medicine. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: Institute of Medicine, National Academy Press; 1998.
MacLean JB. Wernicke’s encephalopathy after gastric placation. JAMA. 1982;248:1311.
Feit H, Glasberg M, Ireton C, et al. Peripheral neuropathy and starvation after gastric partitioning for morbid obesity. Ann Intern Med. 1982;96:453–5.
Fawcett C, Young B, Holliday RL. Wernicke’s encephalopathy after gastric partitioning for morbid obesity. Can J Surg. 1984;27:169–70.
Viller HV, Ranne RD. Neurologic deficit following gastric partitioning: possible role of thiamine. JPEN J Parenter Enteral Nutr. 1984;8:575–8.
Somer H, Bergstrom L, Mustajoki P, et al. Morbid obesity, gastric placation and a severe neurological deficit. Acta Med Scand. 1985;217:575–6.
Paulson GW, Martin EW, Mojzisik C, et al. Neurologic complications of gastric partitioning. Arch Neurol. 1985;42:675–7.
Oczkowski WJ, Kertesz A. Wernicke’s encephalopathy after gastroplasty for morbid obesity. Neurology. 1985;35:99–101.
Abarbanel JM, Berginer VM, Osimani A, Solomon H, Charuzi I. Neurologic complications after gastric restriction surgery for morbid obesity. Neurology. 1987;37:196–200.
Seehra H, MacDermott N, Lascelles RG, Taylor TV. Wernicke’s encephalopathy after vertical banded gastroplasty for morbid obesity. BMJ. 1996;312:434.
Mason EE. Starvation injury after gastric reduction for obesity. World J Surg. 1998;22:1002–7.
Cirignotta F, Manconi M, Mondini S, et al. Wernicke-Korsakoff encephalopathy and polyneuropathy after gastroplasty for morbid obesity. Arch Neurol. 2000;57:1356–9.
Bozbora A, Coskun H, Ozarmagan S, et al. A rare complication of adjustable gastric banding: Wernicke’s encephalopathy. Obes Surg. 2000;10:274–5.
Toth C, Volt C. Wernicke’s encephalopathy following gastroplasty for morbid obesity. Can J Neurol Sci. 2001;28:89–92.
Chaves LC, Faintuch J, Kahwage S, Alencare A. A cluster of polyneuropathy and Wernecke-Korsakoff syndrome in a bariatric unit. Obes Surg. 2002;12:328–34.
Sola E, Morllas C, Garzon S, et al. Rapid onset of Wernicke’s encephalopathy following gastric restrictive surgery. Obes Res. 2003;13:661–2.
Loh T, Watson WD, Verman A, et al. Acute wernicke’s encephalopathy following bariatric surgery; clinical course and MRI correlation. Obes Surg. 2004;14:129–32.
Nautiyal A, Singh S, Alaimo DJ. Wernicke’s encephalopathy—an emerging trend after bariatric surgery. Am J Med. 2004;117:804–5.
Towbin A, Inge TH, Garcia VF, Roehrig HR, Clements RH, Harmon CM, Daniels SR. Beriberi after gastric bypass surgery in adolescence. J Pediatr. 2004;145:263–7.
Koffman BM, Greenfield LJ, Ali II, Pirzada NA. Neurologic complications after surgery for obesity. Muscle Nerve. 2006;33:166–76.
Singh S, Kumer A. Wernicke encephalopathy after obesity surgery. A systematic review. Neurology. 2007;68:807–11.
Rudnicki SA. Prevention and treatment of peripheral neuropathy after bariatric surgery. Curr Treat Options Neurol. 2010;12:29–36.
Kumar N. Neurologic presentations of nutritional deficiencies. Neurol Clin. 2010;28(1):107–70.
Kazemi A, Frazier T, Cave M. Micronutrient-related neurologic complications following bariatric surgery. Curr Gastroenterol Rep. 2010;12(4):288–9.
Frantz DJ. Neurologic complications of bariatric surgery: involvement of central, peripheral, and enteric nervous systems. Curr Gastroenterol Rep. 2012;14:367–72.
Chang CG, Adams-Huet B, Provost DA. Acute post-gastric reduction surgery (APGARS) neuropathy. Obes Surg. 2004;14:182–9.
Antunwz E, Estruch R, Cardenal C, Nicolas JM, Fernandez-Sola J, Urbano-Marquezz A. Usefulness of CT and MR imaging in the diagnosis of acute Wernicke’s encephalopathy. AJR Am J Roentegenol. 1998;171:1131–7.
Smith CD, Herkes SB, Behrns KE, et al. Gastric acid secretion and vitamin B12 absorption after vertical Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 1993;218:9–96.
Behrns KE, Smith CD, Sarr MG. Prospective evaluation of gastric acid secretion and cobalamin absorption following gastric bypass for clinically severe obesity. Dig Dis Sci. 1994;39:315–20.
Balk HW, Russell RM. Vitamin B12 deficiency in the elderly. Annu Rev Nutr. 1999;19:357–77.
Rhode BM, Arseneau P, Cooper BA, Katz M, Gilfix BM, MacLean LD. Vitamin B12 deficiency after gastric surgery for obesity. Am J Clin Nutr. 1996;63:103–9.
Eussen SJPM, De Groot LCPM, Clarke R, Schneede J, Ureland PM, Hoefnagels WHL, van Staveren WA. Oral cyanocobalamin supplementation in older people with vitamin B12 deficiency. A dose-finding trial. Arch Intern Med. 2005;165:1167–72.
Kuzminski AM, Del Giacco EJ, Allen RH, Stabler SP, Lindenbaum J. Effective treatment of cobalamin deficiency with oral cobalamin. Blood. 1998;92:1191–8.
Conrad ME, Umbreit JN. Iron absorption and transport—an update. Am J Hematol. 2000;64:287–98.
Beard JL, Dawson H, Pinero DJ. Iron metabolism: A comprehensive review. Nutr Rev. 1996;54:295–317.
Lash A, Saleem A. Iron metabolism and its regulation. A review. Ann Clin Lab Sci. 1995;25:20–30.
Anderson GJ, Frazer DM, McLaren GD. Iron absorption and metabolism. Curr Opin Gastroenterol. 2009;25:129–35.
Ruz M, Carrasco F, Rojas P, et al. Iron absorption and iron status are reduced after Roux-en-Y gastric bypass. Am J Clin Nutr. 2009;90:527–32.
Brolin RE, Robertson LB, Kenler HA, et al. Weight loss and dietary intake after vertical banded gastroplasty and Roux-en-Y gastric bypass. Ann Surg. 1994;220:782–90.
Food and Nutrition Board (FNB), Institute of Medicine. Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. A report of the panel on micronutrients, Dietary Reference Intakes. Washington, DC: Institute of Medicine, National Academy Press; 2001.
Crowley LV, Seay J, Mullen G. Late effects of gastric bypass for obesity. Am J Gastroenterol. 1984;79:850–60.
Halverson JD. Micronutrient deficiencies after gastric bypass for morbid obesity. Am Surg. 1985;52:594–8.
Amaral JE, Thompson WR, Caldwell MD, et al. Prospective hematologic evaluation of gastric exclusion surgery for morbid obesity. Ann Surg. 1985;201:186–93.
Monsen ER, Cook JD. Food iron absorption in human subjects IV. The effects of calcium and phosphate salts on the absorption of nonheme iron. Am J Clin Nutr. 1976;29:1142–8.
Hallberg L, Brune M, Erlandsson M, et al. Calcium: effect of different amounts on nonheme-and heme-iron absorption in humans. Am J Clin Nutr. 1991;53:112–9.
Cook JD, Dassenko SA, Whittaker P. Calcium supplementation: effect on iron absorption. Am J Clin Nutr. 1991;53:106–11.
Hallberg L, Rossander-Hulthen L, Brune M, et al. Calcium and iron absorption: mechanism of action and nutritional importance. Eur J Clin Nutr. 1992;46:317–27.
Gleerup A, Rossander-Hulthen L, Gramatkovski E, et al. Iron absorption from the whole diet: comparison of the effect of two different distributions of daily calcium intake. Am J Clin Nutr. 1995;61:97–104.
Minihane AM, Fairweather-Tait SJ. Effect of calcium supplementation on daily nonheme-iron absorption and long-term iron status. Am J Clin Nutr. 1998;68:96–102.
Reddy MB, Cook JD. Effect of calcium intake on nonheme-iron absorption from a complete diet. Am J Clin Nutr. 1997;65:1820–5.
Grinder-Pederson L, Bukhave K, Jensen M, et al. Calcium from milk or calcium-fortified foods does not inhibit nonheme-iron absorption from a whole diet consumed over a 4-day period. J Clin Nutr. 2004;80:404–9.
Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341:1986–95.
Ross EM. Evaluation and treatment of iron deficiency in adults. Nutr Clin Care. 2002;5:220–4.
Rainville AJ. Pica practices of pregnant women are associated with lower maternal hemoglobin level at delivery. J Am Diet Assoc. 1998;98:293–6.
Simpson E, Mull JD, Longley E, et al. Pica during pregnancy in low-income women born in Mexico. West J Med. 2000;173:20–4.
Rector WG. Pica: Its frequency and significance in patients with iron-deficiency anemia due to chronic gastrointestinal blood loss. J Gen Intern Med. 1989;4:512–3.
Ivascu NS, Sarnaik S, McCrae J, et al. Characterization of pica prevalence among patients with sickle cell disease. Arch Pediatr Adolesc Med. 2001;155:1243–7.
Kushner RF, Gleason B, Shanta-Retelny V. Reemergence of pica following gastric bypass surgery for obesity: a new presentation of an old problem. J Am Diet Assoc. 2004;104:1393–7.
Kushner RF, Shanta-Retelny V. Emergence of pica (ingestion of non-food substances) accompanying iron deficiency anemia after gastric bypass surgery. Obes Surg. 2005;15:1491–5.
Brolin RE, Gorman JH, Gorman RC, Petschenik AJ, Bradley LB, Kenler HA, Cody RP. Prophylatic iron supplementation after Roux-en-Y gastric bypass. A prospective, double-blind, randomized study. Arch Surg. 1998;133:740–4.
Alleyne M, Horne MK, Miller JL. Individualized treatment for iron-deficiency anemia in adults. Am J Med. 2008;121:943–8.
Rhode BM, Shustik C, Christou NV, MacLean LD. Iron absorption and therapy after gastric bypass. Obes Surg. 1999;9:17–21.
Wasserman RH. Vitamin D, and the dual processes of intestinal calcium absorption. J Nutr. 2004;134:3137–9.
Charles P. Calcium absorption and calcium bioavailability. J Intern Med. 1992;231:161–8.
Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board (FNB), Institute of Medicine (IOM). Dietary Reference Intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: Institute of Medicine, National Academy Press; 1997.
Riedt CS, Brolin RE, Sherrell RM, Field MP, Shapses SA. True fractional calcium absorption is decreased after Roux-en-Y gastric bypass surgery. Obesity. 2006;14:1940–8.
Looker AC. Body fat and vitamin D status in black versus white women. J Clin Endocrinol Metab. 2005;90:635–40.
Wortsman J, Matsuika LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–3.
Parikh SJ, Edelman M, Uwaifo GI, Freedman RJ, Semega-Janneh M, Reynolds J, Yanovski JA. The relationship between obesity and serum 1.12-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004;89:1196–9.
Kamycheva E, Sundsfjord J, Jorde R. Serum parathyroid hormone level is associated with body mass index. The 5th Tromso study. Eur J Endocrinol. 2004;151:167–72.
Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab. 2003;88:157–61.
Pramyothin P, Biancuzzo RM, Lu Z, Hess DT, Apovian CM, Holick MF. Vitamin D adipose tissue and serum 25-hydroxyvitamin D after Roux-en-Y -gastric bypass. Obesity. 2011;19:2228–34.
Cannell JJ, Hollis BW, Heaney RP. Diagnosis and treatment of vitamin D deficiency. Expert Opin Pharmacother. 2008;9:1–12.
Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–73.
Whiting SJ, Calvo MS. Dietary recommendations for vitamin D: a critical need for functional end points to establish an estimated average requirement. J Nutr. 2005;135:304–9.
Hickey L, Gordon CM. Vitamin D deficiency: new perspectives on an old disease. Curr Opin Endocrinol Diabetes. 2004;11:18–25.
Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–22.
Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–43.
Rosen HN. Biochemical markers of bone turnover: clinical utility. Curr Opin Endocrinol Diabetes. 2003;10:387–93.
Burke MS. Current roles and realities of noninvasive assessment of osteoporosis. Curr Opin Endocrinol Diabetes. 2004;11:330–7.
Ott MT, Fanti P, Malluche HH, Yun Ryo U, Whaley FS, Strodel WE, Colacchio TA. Biochemical evidence of metabolic bone disease in women following Roux-Y gastric bypass for morbid obesity. Obes Surg. 1992;2:341–8.
Newbury L, Dolan K, Hatzifotis M, Low N, Fielding G. Calcium and vitamin D depletion and elevated parathyroid hormone following biliopancreatic diversion. Obes Surg. 2003;13:893–5.
Diniz MFHS, Diniz MTC, Sanches SRA. de Almeida Salgado PPC, Valadao MMA, Araujo FC, Martins DS, Rocha ALS. Elevates serum parahormone after Roux-en-Y gastric bypass. Obes Surg. 2004;14:1222–6.
El-Kadre LJ, Rocha PR, de Almeida Tinoco AC, Tinoco RC. Calcium metabolism in pre- and postmenopausal morbidly obese women at baseline and after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2004;14:1062–6.
Hamoui N, Anthone G, Crookes PF. Calcium metabolism in the morbidly obese. Obes Surg. 2004;14:9–12.
von Mach MA, Stoeckli R, Bilz S, Kraenzlin M, Langer I, Keller U. Changes in bone mineral content after surgical treatment of morbid obesity. Metabolism. 2004;53:918–21.
Prisco CD, Levine SN. Metabolic bone disease after gastric bypass surgery for obesity. Am J Med Sci. 2005;329:57–61.
Coates PS, Fernstrom JD, Fernstrom MH, Schauer PR, Greenspan SL. Gastric surgery for morbid obesity leasds to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004;89:1061–5.
Johnson JM, Maher JW, Samuel I, Heitshusen D, Doherty C, Downs RW. Effects of gastric bypass procedures on bone mineral density, calcium, parathyroid hormone, and vitamin D. J Gastrointest Surg. 2005;9:1106–11.
Soleymani T, Tejavanija S, Morgan S. Obesity, bariatric surgery, and bone. Curr Opin Rheumatol. 2011;23:395–405.
Compher CW, Badellino KO, Boullata JL. Vitamin D and the bariatric surgical patient: a review. Obes Surg. 2008;18:220–4.
Folli F, Sabowitz BN, Schwesinger W, Fanti P, Guardada-Mendoza R, Muscogiuri G. Bariatric surgery and bone disease: from clinical perspective to molecular insights. Int J Obes (Lond). 2012;36:1373–9.
Brzozowska MM, Sainbury A, Eisman JA, Baldock PA, Center JR. Bariatric surgery, bone loss, obesity and possible mechanism. Obes Rev. 2013;14:52–67.
Ybarra J, Sanchez-Hernandez J, Gich I, De Leiva A, Rius X, Rodriguez-Espinosa J, Perez A. Unchanged hypovitaminosis D and secondary hyperparathyroidism in morbid obesity after bariatric surgery. Obes Surg. 2005;15:330–5.
Sinha N, Shieh A, Stein EM, et al. Increased PTH and 1.25(OH)3D levels associated with increased markers of bone turnover following bariatric surgery. Obesity. 2011;19:2388–93.
Mahlay NF, Verka LG, Thomsen K, Merugu S, Salomone M. Vitamin D status before Roux-en-Y and efficacy of prophylactic and therapeutic doses o vitamin D in patients after Roux-en-Y gastric bypass surgery. Obes Surg. 2009;19:590–4.
Gemmei K, Santry HP, Prachand VN, Alverdy JC. Vitamin D deficiency in preoperative bariatric surgery patients. Surg Obes Relat Dis. 2009;5:54–9.
de Luis DA, Pacheco D, Izaola O. Micronutrient status in morbidly obese women before bariatric surgery. Surg Obes Relat Dis. 2013;9:323–7.
Damms-Machado A, Friedich A, Kramer KM, et al. Pre- and postoperative nutritional deficiencies in obese patients undergoing laparoscopic sleeve gastrectomy. Obes Surg. 2012;22:881–9.
Ernst B, Thurnheer M, Schmid SM, Schultes B. Evidence for the necessity to systematically assess micronutrient status prior to bariatric surgery. Obes Surg. 2009;19:66–73.
Goldner WS, O’Dorisio TM, Dillon JS, Mason EE. Severe metabolic bone disease as a long-term complication of obesity surgery. Obes Surg. 2002;12:685–92.
van Loan MD, Johnson HL, Barbieri TF. Effect of weight loss on bone mineral content and bone mineral density in obese women. Am J Clin Nutr. 1998;67:734–8.
Shapses SA, Sukumar D. Bone metabolism in obesity and weight loss. Annu Rev Nutr. 2012;32:287–309.
Pugnale N, Giusti V, Suter M, Zysset E, Heraief E, Gaillard RC, Burckhardt P. Bone metabolism and risk of secondary hyperparathyroidism 12 months after gastric banding in obese pre-menopausal women. Int J Obes Relat Metab Disord. 2003;27:110–6.
Fieischer J, Stein EM, Bessier M, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93:3735–40.
Guney E, Kisakol G, Ozgen G, Yilmaz C, Yilmaz R, Kabalak T. Effect of weight loss on bone metabolism: comparison of vertical banded gastroplasty and medical intervention. Obes Surg. 2003;13:383–8.
Tondapu P, Provost D, Adams-Huet B, Sims T, Chang C, Sakhaee K. Comparison of the absorption of calcium carbonate and calcium citrate after Roux-en-Y gastric bypass. Obes Surg. 2009;19:1256–61.
Eckert MJ, Perry JT, Sohn VY, et al. Incidence of low vitamin A levels and ocular symptoms after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2010;6:653–7.
Pereira S, Saboya C, Chaves G, Ramalho A. Class III obesity and its relationship with the nutritional status of vitamin A in pre- and postoperative gastric bypass. Obes Surg. 2009;19:738–44.
Zalesin KC, Miller WM, Franklin B, et al. Vitamin A deficiency after gastric bypass surgery: an Underreported postoperative complication. J Obes. 2011:760695
Slater GH, Ren CF, Siegel N, Williams T, Wolfe B, Dolan K, Fielding GA. Serum fat-soluble vitamin D deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg. 2004;8:48–55.
Dolan K, Hatzfotis M, Newbury L, Lowe N, Fielding G. A clinical and nutritional comparison of biliopancreatic diversion with and without duodenal switch. Ann Surg. 2004;240:51–6.
Hatzifotis M, Dolan K, Newbury L, Fielding G. Symptomatic vitamin A deficiency following biliopancreatic diversion. Obes Surg. 2003;13:655–7.
Lee WB, Hamilton SM, Harris JP, Schwab IR. Ocular complications of hypovitaminosis A after bariatric surgery. Ophthalmology. 2005;112:1031–4.
Kumar N, Crum B, Petersen RC, et al. Copper deficiency myelopathy. Arch Neurol. 2004;61:762–6.
Griffith DP, Liff DA, Ziegler TR, Esper GJ, Winton EF. Acquired copper deficiency: A potentially serious and preventable complication following gastric bypass surgery. Obesity. 2009;17:827–31.
Gletsu-Miller N, Broderius M, Frediani JK, et al. Incidence and prevalence of copper deficiency following Roux-en-Y gastric bypass surgery. Int J Obes (Lond). 2012;36:328–35.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Kushner, R.F. (2014). Managing Micronutrient Deficiencies in the Bariatric Surgical Patient. In: Kushner, R., Bessesen, D. (eds) Treatment of the Obese Patient. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1203-2_18
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1203-2_18
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1202-5
Online ISBN: 978-1-4939-1203-2
eBook Packages: MedicineMedicine (R0)