Abstract
This chapter describes astaxanthin and other xanthophylls, which are a class of carotenoids with oxygenated groups in their structure and characteristic vivid colors. Taking astaxanthin as a key example, the presented concepts focus on the functional aspects and applications of xanthophylls, such as their nutritional value, potential for protection against free radicals, benefits for human and animal health and economic importance. The metabolic pathway leading to the biosynthesis of astaxanthin is described in two steps: (1) the formation of β(beta)-carotene and (2) the formation of astaxanthin from β(beta)-carotene. In describing these steps, a comparative analysis is made of the intermediates, enzymes, and genes involved in several organisms. In addition, different strategies for genetic improvements that would enhance astaxanthin production in the basidiomycetous yeast Xanthophyllomyces dendrorhous are discussed. Finally, an overview of the complexity of the molecular mechanisms regulating the biosynthesis of astaxanthin in this yeast is presented.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Carotenoid Production
- Isoprenoid Biosynthesis
- Astaxanthin Production
- Mucor Circinelloides
- Lycopene Cyclase
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
In 1837, the Swedish chemist Jöns Jacob Berzelius described the yellow pigments extracted from autumn leaves, which he named xanthophylls (from the Greek xanthos: yellow and phyllon: leaf). Later, the Russian-Italian botanist M.S. Tswett found that these pigments were a complex mixture of “polychromes” and, using adsorption chromatography, isolated and purified xanthophylls and carotenes, which he named carotenoids in 1911. These yellow, orange, or red pigments play important physiological roles in all living organisms, but their synthesis is circumscribed to photosynthetic organisms, some fungi and bacteria. Animals must obtain these essential molecules from food, as they are not able to synthesize carotenoids de novo [1]. Since H.W.F. Wackenroder isolated the first carotenoid from the cells of carrot roots in 1831 [2], more than 750 different chemical structures of natural carotenoids have been described [3]. The annual production of carotenoids is estimated to be more than 100 million tons [4].
The molecular structure of carotenoids consists of a hydrocarbon backbone of forty carbon atoms (C40) usually composed of eight isoprene units joined such that the two methyl groups nearest the center of the molecule are in a 1,6-positional relationship and the remaining nonterminal methyl groups are in a 1,5-positional relationship (Nomenclature of Carotenoids, IUPAC and IUPAC-IUB, rules approved in 1974). All carotenoids derived from the acyclic C40H56 structure have a long central chain of conjugated double bonds (that constitutes the chromophoric system of the carotenoids) that may have some chemical modifications such as hydrogenation, the incorporation of oxygen-containing functional groups and the cyclization of one or both ends, resulting in monocyclic or bicyclic carotenoids [5]. The oxygenated carotenoids with a hydroxy, epoxy, and/or oxo group form a separate subclass named the xanthophylls [6], while the non-oxygenated carotenoids are named carotenes. The oxygenation in these molecules contributes to the enhanced solubility of xanthophylls and is the reason why they are more polar than the purely hydrocarbon carotenes, thus allowing the separation of xanthophylls from carotenes by chromatography.
Xanthophylls: Functions and Applications
Xanthophylls are synthesized by several organisms in which they fulfill important biological roles. For example, in photosynthetic organisms they work as accessory light harvesting pigments and are involved in the protection against photo-oxidative damage, such as the peroxidation of lipid membranes by reactive radicals [7]. Their photoprotective properties are attributed to their strong light absorption in the 400–500 nm range of the visible spectrum. In recent decades, there have been an increasing number of reports confirming the beneficial effects of xanthophylls to animal and human health, which has positioned these metabolites as a very promising group of phytonutrients. In this sense, the first functional role recognized for carotenoid pigments was as a vitamin A precursor in animals. Because of the coloring properties of xanthophylls, there is interest in their economic impact on the production of animal feed; for example, the poultry industry uses xanthophylls to contribute to chicken and egg yolk pigmentation [8, 9]. For consumers, appearance is one of the most important factors affecting the decision to purchase a food product, and the color together with the freshness are ranked as the main criteria for selection [10]. Furthermore, the outstanding antioxidant properties of xanthophylls have been linked to their capacity to protect animal cells from free radicals. Cumulative reports refer to their positive influence on human health and their anti-disease effects in cancer and obesity. For these reasons, the use of xanthophylls has been explored in several industries over the past 30 years, and they have been used as active ingredients in medicinal pharmaceuticals, as cosmetics ingredients and as colorants and additives in the food industry [11]. In particular, there has been a significant increase in their use in the Functional Food field. Currently, the global market for astaxanthin (see below) is similar to that for β(beta)-carotene and is followed by lutein and canthaxanthin in economic importance [12, 13].
Even though efforts to develop commercial methods for the extraction and purification of carotenes and xanthophylls date back to the middle of the twentieth century [14], chemical synthesis remains the main method for the production of xanthophylls and remains in high demand. However, the modern world’s penchant for natural products has increased the search of naturally occurring xanthophylls. In this regard, advances in fermentation processes have been driven by a strong demand for microbial sources of carotenoids for the food industry. Moreover, the development of recombinant DNA technologies and metabolic engineering protocols has contributed to advances in the microbial production of some xanthophylls, even in non-carotenogenic organisms [15, 16], which can provide competitive alternatives to chemical synthesis (Table 9.1).
Some commercially relevant xanthophylls are described as follows:
Astaxanthin [3,3′-Dihydroxy-β(Beta),β(Beta)-Carotene-4,4′-Dione]
Astaxanthin is a red-orange pigment naturally synthesized by a number of bacteria, microalgae, and yeasts. The commercial production of this pigment has traditionally been performed by chemical synthesis, but the yeast Xanthophyllomyces dendrorhous (e.g., Phaffia rhodozyma) and the microalga Haematococcus pluvialis appear to be the most promising sources for its industrial biological production. Astaxanthin has strong antioxidant properties, shown to be better than those of β(beta)-carotene or even α(alpha)-tocopherol [17]. There are an increasing number of reports on the potential benefits of astaxanthin on human health, including benefits on cardiovascular diseases [18], the prevention of Helicobacter pylori infection in mice [19], the enhancement of the immune response in humans [20], and the inhibition of carcinogenesis in mice [21]. Furthermore, astaxanthin is a very important pigment worldwide. It is used in aquaculture for the pigmentation of salmonid flesh, which is desired by the consumers, thus having considerable economic impact on this industry. In the same way, astaxanthin is used to enrich the nutritional value of egg yolks and to enhance the health and fertility of layer hens [17]. Consequently, the global market for astaxanthin was US$234 million in 2004 [22], which was approximately a quarter of the total global market for carotenoids.
β(Beta)-Cryptoxanthin [β(Beta),β(Beta)-Caroten-3-ol]
This xanthophyll is mainly found in fruits such as papaya, tangerine, orange, and watermelon, and has the potential to act as a provitamin A [23, 24]. The main medical application reported for β(beta)-cryptoxanthin is in bone homeostasis. It has a stimulatory effect on bone calcification (demonstrated in vitro) and in periodontitis, preventing bone resorption most likely by inhibiting the interleukin production induced by bacterial pathogens and mechanical stress [23, 25]. Cancer-preventative effects of β(beta)-cryptoxanthin have been reported as well. For example, β(beta)-cryptoxanthin protects HeLa and Caco-2 cells from H2O2 and visible light damage and induces DNA repair [26]. Moreover, in combination with hesperidin, β(beta)-cryptoxanthin has inhibitory effects on chemically induced tumorigenesis in several rat and mouse tissues [27]. The commercial production of β(beta)-cryptoxanthin is based on natural sources such as citrus and capsicums, but new methods have been developed for its production by the conversion of lutein or lutein esters [28]. There are very few reports on microbial sources of β(beta)-cryptoxanthin. Bacteria transformed with the β(beta)-carotene hydroxylase gene from Arabidopsis thaliana were able to produce β(beta)-cryptoxanthin as the principal carotenoid [29]. In addition, although produced in low yields, β(beta)-cryptoxanthin production has been described in Brevibacterium linens [30] and in Flavobacterium lutescens [31].
Canthaxanthin [β(Beta),β(Beta)-Carotene-4,4′-Dione]
Canthaxanthin has an orange-red color and is naturally produced by some plants, fungi, microalgae, Archaea, and bacteria [32]. Together with other carotenoids, it was reported that canthaxanthin induces gap junction communication in murine fibroblasts and, therefore, intercellular communication. Moreover, it has important effects on the immune response. Due to its antioxidant properties, it has been noted that canthaxanthin is the most potent methyl linoleate inhibitor, providing a model for lipid peroxidation in vivo [33]. Together with astaxanthin, canthaxanthin is the most important pigment used in aquaculture for salmonid flesh coloration and is also used for chicken skin and egg yolk coloring [8, 34]. The main microbial source of commercial canthaxanthin is the alga Haematococcus lacustris [35]. Nevertheless, there are laboratory-scale reports of canthaxanthin production by several microorganisms with the potential for use at larger industrial scales for organisms such as Aspergillus carbonarius [36], Dietzia natronolimnaea [37], and the microalga Chlorella zofingiensis [38, 39], to name a few.
Capsanthin [3,3′-Dihydroxy-β(Beta),κ(Kappa)-Caroten-6′-One]
Capsanthin is the major xanthophyll in peppers and in Lolium lancifolium “Splendens” flowers (tiger lily) [40]. This pigment is not produced by chemical synthesis and is mainly extracted from red peppers to be used for pigmentation of poultry feed [34]. Recently it was described that paprika pigments contain large amounts of capsanthin and capsorubin and reduce adipocyte chronic inflammation caused by obesity [41]. In addition, epidemiological studies suggested that capsanthin has a strong inhibitory effect on colon carcinogenesis [42].
Fucoxanthin [5,6-Epoxy-3′-Ethanoyloxy-3,5′-Dihydroxy-6′,7′-Didehydro-5,6,7,8,5′,6′-Hexahydro-β(Beta), β(Beta)-Caroten-8-One]
The fucoxanthin pigment is found in different types of comestible seaweeds and is responsible for their brown or olive-green color [43]. Seaweeds are the main sources of this pigment [44] because chemical synthesis is still very expensive. Fucoxanthin is considered to be an anticarcinogenic compound and was recently demonstrated to have apoptosis-inducing effects, most likely through the down-regulation of STAT3/EGFR signaling [45]. Furthermore, anti-obesity and antidiabetic roles have been described for fucoxanthin [43].
Lutein [β(Beta),ε(Epsilon)-Carotene-3,3′-Diol]
Together with zeaxanthin, lutein forms the macular pigment, which is the yellow spot at the center of the human retina. The adequate intake of lutein might help to prevent or ameliorate age-related macular degeneration and other degenerative human diseases [46–48]. Studies on the effects of lutein on the immune response have been performed in several animal species, and its immune-modulatory effect on macrophages was recently reported in both murine and primary-cultured peritoneal macrophages [49]. Lutein is the major xanthophyll present in green leafy vegetables. Currently, lutein is extracted from marigold petals [50], mainly in the esterified form. The market for lutein in the USA is estimated at $150 million [51]. Because of the assumption that esterification diminishes the bioavailability of lutein, a preceding saponification step is performed in commercial formulations to remove esters; however, it has been demonstrated that this modification does not significantly affect lutein bioavailability, which mainly depends on its solubilization [52, 53]. Several studies have been performed to develop carriers to enhance lutein bioavailability, for example, by using solubilized lutein in mixed micelles containing lysophosphatidylcholine [54] and water-soluble, low molecular weight chitosan [55]. There is a constant search for alternative sources of lutein besides plants, and it has been mainly reported that algae and microalgae might become real economic alternatives for the production of lutein. This is the case for Chlorococcum humicola [56] and Coccomyxa acidophila (which also accumulate significant amounts of β-carotene) [57], C. zofingiensis (which also accumulates astaxanthin) [58], and Dunaliella salina [59] and Chlorella protothecoides (which also contain significant amounts of canthaxanthin, echinenone, and astaxanthin) [60].
Neoxanthin [5′,6′-Epoxy,6,7-Didehydro-5,6,5′,6′-Tetrahydro-β (Beta), β(Beta)-Carotene-3,5,3′-Triol]
Neoxanthin is a precursor of the plant growth regulator abscisic acid [61] in green leafy vegetables, including common edible vegetables [62]. It has been demonstrated that neoxanthin affects the proliferation of human prostate cancer cells, most likely by caspase induction [63, 64].
Violaxanthin [5,6:5′,6′-Diepoxy-5,6,5′,6′-Tetrahydro-β(Beta),β(Beta)-Carotene-3,3′-Diol]
Violaxanthin is a xanthophyll of orange color synthesized by a variety of plants, including the well-known pansies. Significant amounts of violaxanthin have also been reported in orange juices and peels. Together with neoxanthin, violaxanthin is commercially attractive because it is also one of the abscisic acid precursors—the plant hormone indispensable for plant adaptation with important roles in dormancy and embryo development [65]. Recently, low amounts of violaxanthin were reported in intracellular extracts from microalga Scenedesmus obliquus strain M2-1 [66].
Zeaxanthin [β(Beta),β(Beta)-Carotene-3,3′-Diol]
Zeaxanthin is a yellow pigment found in vegetables and fruits. By far the main reported role for zeaxanthin in human health is in ocular health, where, together with lutein, zeaxanthin provides protection against age-related macular degeneration [67, 68]. In addition, potential antitumor properties have been described for zeaxanthin [69]. Furthermore, it was recently found that meso-zeaxanthin has an inhibitory effect on the mutagenicity of five mutagenic agents, including nitro-o-phenylenediamine and N-methyl- N′-nitro-N-nitrosoguanidine [70]. At present, the commercial production of zeaxanthin is mainly based on the extraction from plant tissues such as marigold flowers [71] and its use in the generation of new functional foods has been successfully explored [72]. A recent study indicated that spirulina is a rich dietary source of zeaxanthin, as the administration of spirulina increased the zeaxanthin concentration in human serum [73]. No commercial microbial sources for the production of zeaxanthin have been established yet; however, microorganisms that produce zeaxanthin are continuously being described and some of them are promising sources for satisfying the zeaxanthin demands of the future market. Examples include a marine bacterium belonging to the genus Algibacter [74], Flavobacterium sp. [75], novel bacterial species belonging to the Sphingobacteriaceae and Sphingomonadaceae families [76] and a new Chlorella saccharophila strain that has the potential to be used for biofuel and carotenoid co-production [77].
Biosynthesis of Xanthophylls
The biosynthesis of xanthophylls derives from the synthesis of carotenoids (Fig. 9.1). Although the carotenoid compounds found in nature are enormous in structural diversity, all of them are synthesized through the universally conserved isoprenoid biosynthesis pathway. The biosynthesis of isoprenoids originates from a basic C5 isoprene unit to which prenyl transferase enzymes sequentially add three other isoprenic units [78] resulting in the formation of C20 geranylgeranyl-pyrophosphate (GGPP). The active forms of the isoprene unit are the isopentenyl-pyrophosphate (IPP) and its allylic isomer dimethylallyl-pyrophosphate (DMAPP). In most eukaryotes, IPP derives from the mevalonate pathway [79], while in prokaryotes and in plant plastids, it is synthesized via the 2-C-methyl-d-erythritol-4-phosphate (MEP) pathway, which is also known as the non-mevalonate pathway [80]. In the first step of isoprenoid biosynthesis, one IPP molecule is isomerized to DMAPP by the isopentenyl-pyrophosphate isomerase and then both molecules are joined together generating C10-geranyl pyrophosphate (GPP), the precursor of monoterpenes [81]. The addition of a second molecule of IPP to GPP by prenyl transferases gives the precursor of sesquiterpenes, C15-farnesyl pyrophosphate (FPP), which is converted to GGPP (the precursor of diterpenes) by the further addition of IPP by the GGPP synthase enzyme. Next, phytoene synthase condenses two molecules of GGPP in a tail-to-tail manner, yielding phytoene [79]. This is the first carotenoid synthesized in the pathway, which is colorless as it has a symmetrical carotenoid skeleton with only three conjugated double bonds. The huge structural diversity of carotenoids is generated by further modifications such as desaturations, cyclizations, isomerizations, and oxygenations [82].
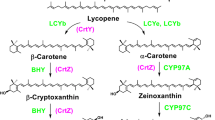
Biosynthetic pathway of xanthophylls. Systematic illustration of the metabolic pathways leading to the synthesis of the xanthophylls described in the text (adapted from [147, 148]). The biosynthesis of astaxanthin is enclosed in a box indicating the proposed genes that control each step in X. dendrorhous (in squares, adapted from [114]), H. pluvialis (in circles, adapted from [149]), bacteria (no special mark, adapted from [150]), and in A. aestivalis (in dotted box, adapted from [99]). Abbreviations: MVA mevalonate, IPP isopentenyl-pyrophosphate, DMAPP dimethylallyl-pyrophosphate, GGPP geranylgeranyl-pyrophosphate. Metabolite structures were confirmed according to [151]
The phytoene synthase enzyme is well conserved among carotenogenic organisms. It is encoded by the crtB gene in bacteria and by the PSY gene in plants, algae, and cyanobacteria [11]. Fungi have a bifunctional enzyme, named phytoene β(beta)-carotene synthase (PBS) because it has both phytoene synthase and lycopene cyclase activities, which gives rise to β(beta)-carotene. In this particular enzyme, encoded by the crtYB gene, the phytoene synthase and lycopene cyclase activities are restricted to the C-terminal and the N-terminal functional domains, respectively. It is likely that such a bifunctional enzyme was acquired early in the evolution of fungi [83] because genes encoding this unique enzyme have been reported in ascomycetes, zygomycetes, and basidiomycetes such as Neurospora crassa [84], Mucor circinelloides [85], Phycomyces blakesleeanus [86], and Xanthophyllomyces dendrorhous [87].
Next, phytoene is desaturated by the incorporation of two, three, four, or five double bonds producing the colored carotenoids ζ(zeta)-carotene (yellow, synthesized by some plants, cyanobacteria, and algae), neurosporene (yellow, accumulates in Rhodobacter capsulatus and R. sphaeroides), lycopene (red, found in most eubacteria and fungi), or 3,4-didehydrolycopene (found in N. crassa) [79], respectively. In photosynthetic organisms, the formation of ζ(zeta)-carotene by the sequential insertion of two double bonds in phytoene is generally performed by a phytoene desaturase, encoded by PDS in plants and algae or by crtP in cyanobacteria [11]. Next, the ζ(zeta)-carotene is converted into lycopene by the introduction of two additional double bonds, which is catalyzed by a ζ(zeta)-carotene desaturase, encoded by ZDS in plants and algae or by crtQ in cyanobacteria [11]. In non-photosynthetic carotenogenic organisms, such as fungi and eubacteria, the desaturation of phytoene leading to lycopene is controlled by only one gene, crtI [11].
Although there are acyclic carotenoids, the cyclization of lycopene is a frequent step in the biosynthesis of carotenoids, forming three types of ionone rings: β(beta)-, ε(epsilon)-, and γ(gamma)-rings [81]. The β(beta)-ring is the most common form; the ε(epsilon)-type is found in plants and in some algae, and the γ(gamma)-ring is the rarest. Several non-phylogenetically related lycopene β(beta)-cyclases have been described, which are encoded by the crtL gene (also known as LCY) in plants, cyanobacteria, and algae, and by crtY in eubacteria, which produces β(beta)-carotene when a β(beta)-ring is introduced at both ends of lycopene [88]. Another type of lycopene cyclase was described in the actinomycete bacterium B. linens, in which a heterodimeric enzyme formed by polypeptides encoded by the crtYc and crtYd genes (unrelated to crtY or crtL) is responsible for the conversion of lycopene into β(beta)-carotene [83]. In fungi, the domain of the bifunctional enzyme PBS that exhibits lycopene cyclase activity seems to be related to the crtYc and crtYd genes of B. linens, which led to the hypothesis that PBS developed from a recombination of these two genes and a phytoene synthase gene [83]. Nevertheless, the existence of other types of lycopene cyclases is still expected because no lycopene cyclase genes have been found in the completely sequenced and available genomes of the cyanobacteria Synechocystis sp. and Anabaena sp., both of which are β(beta)-carotene-producers [89].
The synthesis of xanthophylls involves the oxidation of post-phytoene carotenoid molecules, mainly from α(alpha)- and β(beta)-carotenes, resulting in oxygenated products with hydroxyl-, epoxy-, and oxo-functional groups.
Astaxanthin Biosynthesis in X. dendrorhous and in Other Organisms
In the late nineteenth century, Ludwig described a red yeast-like organism responsible for the color of the sap of deciduous trees and named it Rhodomyces dendrorhous [90]. In the late 1960s, Herman Phaff and coworkers isolated a red fermenting yeast from natural slime fluxes and exudates on wounded trees from mountainous regions of Japan and Alaska. It was originally designated as Rhodozyma montanae nov. gen. et sp, but in 1976 it was renamed Phaffia rhodozyma because it has a basidiomycetous origin [91]. Subsequently, many strains were isolated from the European part of Russia, where it was noticed that P. rhodozyma was the predominant yeast in the red exudates of trees, suggesting that it corresponded to Rhodomyces dendrorhous, as originally described by Ludwig [90]. In 1995, Golubev described the life cycle of this yeast, which was unknown in the basidiosporogenous yeasts, indicating that it was a new teleomorphic genus, and the name Xanthophyllomyces was proposed [90]. Currently, the anamorphic strains are designated as P. rhodozyma and the teleomorphic strains as X. dendrorhous. In 1976, Andrewes and coworkers reported that astaxanthin was the principal carotenoid pigment produced by this yeast and one of the first models for the biosynthesis of astaxanthin in X. dendrorhous was suggested [92].
The biosynthesis of astaxanthin is limited to a few organisms such as the microalgae H. pluvialis [93], some marine bacteria such as Paracoccus haeundaensis [94] and Brevudimonas sp. [95], the basidiomycete yeast X. dendrorhous [92] and the plant Adonis, which accumulates astaxanthin in the petals of the flower [96].
The formation of astaxanthin from β(beta)-carotene involves the introduction of a hydroxyl and a keto group at carbons 3 and 4, respectively, for each of the β(beta)-ionone rings via eight possible intermediate xanthophylls, depending on the producing organism. In the bacterial, plant, and algal systems, these reactions are catalyzed by hydroxylases and ketolases. Ketolases have been described in several organisms that do not produce astaxanthin, but produce other keto-xanthophylls such as echinenone. The bacterial ketolases, encoded by the crtW gene, can use a non-substituted β(beta)-ionone ring as well as 3-hydroxylated β(beta)-ionone rings as a substrate [97]. Two paralogous genes with significant identity to crtW, bkt1 and bkt2, encode β(beta)-carotene ketolases (BKT) and were described in H. pluvialis. The microalga BKTs can only accept a non-substituted β(beta)-ionone ring as a substrate, so it is unlikely that the astaxanthin synthesis from β(beta)-carotene begins with a hydroxylation step in H. pluvialis [93]. During the green vegetative phase of H. pluvialis, a hydroxylase (CHY) incorporates a hydroxyl group onto the carbon at position 3 of both β(beta)-ionone rings of the β(beta)-carotene substrate, producing zeaxanthin. However, under stress conditions, β(beta)-carotene is converted into astaxanthin primarily via echinenone, canthaxanthin, and phoenicoxanthin, being β(beta)-carotene and echinenone substrates of BKT, while canthaxanthin and phoenicoxanthin are the substrates of CHY. In this way, astaxanthin accumulates as a secondary carotenoid under stress conditions [98]. Further introduction of fatty acids to the hydroxyl groups by esterification leads to the production of mono- and di-esterified astaxanthin in H. pluvialis [93].
It has been suggested that in Adonis plants, the synthesis of a 3-hydroxy-4-keto-β(beta)-ionone ring from the β(beta)-ionone ring substrate is controlled by two genes and occurs in three steps [99]. First, a 4-hydroxy-β(beta)-ring is formed by carotenoid-β(beta)-ring-4-dehydrogenase (CBFD); second, 4-hydroxy-β(beta)-ring-4-dehydrogenase (HBFD) continues with the further dehydrogenation of carbon 4 giving a keto group at this position; and third, CBFD introduces a hydroxyl group at carbon 3 of the 4-keto-β(beta)-ring to form the 3-hydroxy-4-keto-β(beta)-ring.
There are two major groups of β(beta)-carotene hydroxylases: the non-heme di-iron (NH-di-iron) hydroxylases and the cytochrome P450 monooxygenases (reviewed in [100]). The NH-di-iron hydroxylases are related to fatty acid desaturases, and based on their primary structure, are classified into three groups corresponding to (1) non-photosynthetic eubacteria, (2) plants and green algae, and (3) cyanobacteria. These enzymes require molecular oxygen, iron, ferredoxin, and ferredoxin oxido-reductase for their function; and even though they share low protein identity, carry out the same reaction and conserve iron-coordinating histidines essential for enzyme activity [101]. The bacterial β(beta)-carotene hydroxylases, encoded by crtZ, can convert non-substituted and 4-ketolated β(beta)-ionone rings into the respective 3-hydroxylated forms [97]. A cytochrome P450 monooxygenase involved in the β(beta)-carotene hydroxylation was first described in the thermophilic bacterium Thermus thermophilus [102]. By functional complementation in an Escherichia coli strain carrying the Erwinia uredovora carotenoid biosynthetic genes [103], it was demonstrated that this enzyme could introduce hydroxyl groups to both β(beta)-rings of β(beta)-carotene producing zeaxanthin [102].
Cytochrome P450s (P450s) are a large superfamily of heme-containing monooxygenases that have been described in organisms from all domains of life [104, 105], playing significant roles in the oxidative metabolism of a wide range of exogenous and endogenous substrates [106]. They are involved in the metabolism of many physiologically important compounds such as sterols, fatty acids, and vitamins [107]; secondary metabolites [108]; and in the activation and detoxification of many xenobiotics, such as drugs, carcinogens, and environment-polluting chemicals [109]. These enzymes act as a terminal electron acceptor in multicomponent P450-dependent monooxygenation systems (P450 systems) that lead to the reductive activation of molecular oxygen followed by the insertion of one oxygen atom into the substrate molecule and the reduction of the other to water [110]. The two electrons required for cytochrome P450 activity are transferred primarily from NADPH via a redox partner [111], but the specificity of a particular reaction is given by the P450 enzymatic properties and substrate specificity. In the eukaryotic microsomal P450 system, the general P450 redox partner is a cytochrome P450 reductase, CPR [104, 110, 112]. Although in most organisms there are several genes encoding different P450 enzymes, in most species there is only one CPR-encoding gene, with few exceptions [113].
In this regard, X. dendrorhous has a single astaxanthin synthase (CrtS, encoded by the crtS gene), belonging to the cytochrome P450 protein family, which catalyzes the hydroxylation and ketolation of β(beta)-carotene to produce astaxanthin [114, 115]. To the best of our knowledge, the synthesis of astaxanthin from β(beta)-carotene through a P450 system has only been reported in X. dendrorhous, demonstrating that only in this yeast a unique P450 system evolved and specialized for the synthesis of astaxanthin.
An X. dendrorhous mutant strain missing the crtR wild-type gene, which encodes a CPR-type enzyme (CrtR), accumulates β(beta)-carotene and is unable to synthesize astaxanthin, demonstrating that CrtR is essential for the synthesis of astaxanthin [116]. It is important to highlight the fact that the disruption of crtR was not lethal because the mutant strain was able to grow normally under the studied conditions. Additionally, Ukibe and coworkers [15] stated that CrtS has a high specificity for its own CPR. As in metabolically engineered S. cerevisiae strains with the X. dendrorhous carotenogenic genes, astaxanthin production was only achieved when CrtS was co-expressed with CrtR. This result indicates that the S. cerevisiae endogenous CPR was not able to reduce the X. dendrorhous CrtS, even though the heterologous expression of several cytochrome P450s in this yeast has been functionally successful [110]. The introduction of crtR was crucial for the functional expression of CrtS and astaxanthin production in S. cerevisiae, suggesting “that the X. dendrorhous CrtS is a unique cytochrome P450 protein that has high specificity for its own P450 reductase” [15].
Genetic Improvement of Astaxanthin Production in X. dendrorhous
The specific production of astaxanthin in natural X. dendrorhous isolates is too low (200–400 μg/g of dry weight of yeast, ppm) to provide an economically competitive natural source of this xanthophyll [117]. Therefore, several efforts have been made to improve the production of astaxanthin in this yeast (reviewed in [118]). There is a complex interaction between nutritional factors, such as carbon or nitrogen sources and vitamins [119, 120], and physical factors such as oxygen levels [119, 121], pH [122], and light intensities [123], that influence the cell growth and carotenogenesis in X. dendrorhous. Moreover, different natural isolates and astaxanthin-hyperproducing mutant X. dendrorhous strains may respond differently in their carotenoid production when cultivated under the same conditions, hindering the process of optimization of culture parameters.
In contrast, classical random mutagenesis methods have been applied to generate mutants with increased astaxanthin production [124–126]. N-Methyl-N′-nitro-N-nitrosoguanidine (NTG) has proven to be an effective chemical mutagen for X. dendrorhous, although the achieved astaxanthin levels are still not very attractive from an industrial point of view.
A promising strategy to increase the astaxanthin yield in X. dendrorhous is metabolic engineering, and several attempts have been made, including the overexpression of genes involved in carotenoid synthesis. Although the overexpression of the crtYB gene (which encodes the phytoene-lycopene synthase) led to an increase in the overall carotenoid synthesis, it was mainly due to higher amounts of β(beta)-carotene and echinenone with an unaffected (or even lower) astaxanthin content observed [127]. In contrast, the overexpression of the crtI gene (phytoene desaturase encoding gene) decreased the total carotenoid production and varied its composition. The carbon flux was diverted to the synthesis of monocyclic carotenoids such as 3-hydroxy-3′,4′-didehydro-β(beta)-φ(phi)-caroten-4-one (HDCO), torulene and hydroxy-ketotorulene, while the astaxanthin proportion was reduced to half [127].
An interesting alternative genetic modification is to increase the metabolic flow towards the synthesis of the precursor molecules of a specific pathway. However, when the isopentenyl-isomerase encoding gene (idi) was overexpressed in X. dendrorhous, the amount of total carotenoids decreased [128]. In contrast, the overexpression of the geranylgeranyl-pyrophosphate synthase encoding gene (crtE) resulted in a strain with slightly higher carotenoid levels, which was improved when the strain was cultured under additional air supply and permanent illumination [129]. More recently, a combinatorial approach fused conventional mutagenesis, metabolic pathway engineering (including the simultaneous overexpression of crtYB and crtS) and different culture medium analysis, and resulted in the highest astaxanthin content for X. dendrorhous reported to this day (9,700 ppm) [130]. Although these studies represent a great contribution, the achieved astaxanthin levels are still not industrially sufficient.
Improved astaxanthin yields have been achieved by the addition of the carotenogenesis precursors such as MVA to the culture medium [117]. Many of the regulatory aspects of isoprenoid biosynthesis involve elements of the MVA pathway, which are well conserved throughout evolution. The limiting step in the metabolic flux control of the MVA pathway is catalyzed by the enzyme 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase [131]. For example, the overexpression of the catalytic domain of a HMGR from S. cerevisiae (HMG1 gene) increased the heterologous production of carotenoids in Candida utilis [132]. In the same way, additional HMGR gene copies in N. crassa also resulted in an increase in the amount of carotenoids produced [133]. In X. dendrorhous, only one HMGR gene has been detected and results by Miao and coworkers [134] indicated that its transcript levels were increased in an astaxanthin overproducing X. dendrorhous strain obtained by random mutagenesis. This mutant also showed lower ergosterol content, suggesting that ergosterol might regulate the HMGR gene expression in X. dendrorhous and, in turn, affect the carotenoid biosynthesis. Recently, the X. dendrorhous CYP61 gene involved in the ergosterol biosynthesis was identified and characterized [135]. The disruption of this gene abolished ergosterol production and the carotenoid content, including astaxanthin, was almost doubled relative to the parental strain. Moreover, it was shown that the transcript levels of HMGR were significantly increased in the cyp61 − mutant strains. This background suggests that engineering the steps involved in the MVA pathway could be a good approach for the improvement of astaxanthin production in X. dendrorhous.
Conclusion
Future Perspectives
As described in this chapter, the biosynthesis of astaxanthin in X. dendrorhous is a complex process. Although the structural genes that control this metabolic pathway are known, knowledge of the mechanisms regulating the synthesis of carotenoids in this yeast is still rather limited. Currently, it is known that carotenogenesis is affected by numerous internal and external factors. Among the external factors, the carbon source plays a fundamental role in the regulation of the synthesis of astaxanthin in this yeast. Specifically, when X. dendrorhous is cultivated with glucose as the sole carbon source, carotenogenesis is only induced after this sugar is completely depleted. This point (the late exponential - early stationary phase of growth) also coincides with the maximum ethanol concentration reached, which is produced by the fermentative metabolism of glucose. Along the same lines, when the yeast is cultivated with a non-fermentable carbon source, such as succinate, the synthesis of astaxanthin starts at the beginning of the growth and the achieved carotenoid levels are higher than those reached when the cells are grown in glucose [136]. Furthermore, there are astaxanthin-overproducing strains, obtained by random mutagenesis, in which carotenogenesis starts at the beginning of the culture even in the presence of glucose, indicating that they might be deregulated [137]. Moreover, glucose reduces the mRNA levels of at least three carotenogenic genes (crtYB, crtI and crtS) [138]. All these findings suggest that carotenogenesis in X. dendrorhous is regulated by catabolite repression. Similarly, it is known that the carbon to nitrogen (C/N) ratio and the levels of oxidative stress are also relevant factors that can alter the production of carotenoids in this yeast, and may also participate in the mechanisms that govern the carotenogenesis. Among the internal factors that might regulate carotenogenesis in X. dendrorhous, we highlight the fact that alternative carotenogenic mRNAs are produced for at least two genes: crtYB and crtI [139].
Based on the aforementioned observations, understanding the regulatory mechanisms of the astaxanthin biosynthetic pathway in X. dendrorhous will have a key impact on the comprehension of the complexity of this biological process. Similarly, the knowledge of the interactions between carotenogenesis and other metabolic processes, whether at the genetic level of the structural and regulatory genes or at the level of their gene products, will be useful in developing new astaxanthin-overproducing strains important for the industrial production of this xanthophyll. Thus, molecular genetic studies leading to the identification of regulatory genes, their targets, and the genetic interactions involved in carotenogenesis will aid in the design of new genetic improvement strategies for the production of astaxanthin in X. dendrorhous. Similarly, this knowledge could be applied to develop strains in which the metabolic flux towards intermediary metabolites of carotenogenesis could be diverted to favor the production of new biotechnological products, such as drugs, vitamins, nutrients, or other carotenoids, to name a few.
Finally, the use of metabolic engineering—for example, by modifying the metabolic flux of pathways that interact with carotenogenesis at different levels—will not only generate a greater knowledge of the process itself, but will also create new tools that can be applied in the construction of attractive strains for carotenoid production processes.
References
Goodwin TW. Carotenoid-protein complexes. The biochemistry of the carotenoids, vol. 2. Dordrecht: Springer; 1984. p. 1–21.
Sourkes TL. The discovery and early history of carotene. Bull Hist Chem. 2009;34:33.
Takaichi S. Carotenoids in algae: distributions, biosynthesis and functions. Mar Drugs. 2011;9:1101–18.
Fraser PD, Bramley PM. The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res. 2004;43:228–65.
Britton G. Structure and properties of carotenoids in relation to function. FASEB J. 1995;9: 1551–8.
Bhosale P, Bernstein PS. Microbial xanthophylls. Appl Microbiol Biotechnol. 2005;68: 445–55.
Strzałka K, Kostecka-Gugała A, Latowski D. Carotenoids and environmental stress in plants: significance of carotenoid-mediated modulation of membrane physical properties. Russ J Plant Physiol. 2003;50:168–73.
Zhang W, Zhang KY, Ding XM, Bai SP, Hernandez JM, Yao B, Zhu Q. Influence of canthaxanthin on broiler breeder reproduction, chick quality, and performance. Poult Sci. 2011;90:1516–22.
Perez-Vendrell AM, Hernandez JM, Llaurado L, Schierle J, Brufau J. Influence of source and ratio of xanthophyll pigments on broiler chicken pigmentation and performance. Poult Sci. 2001;80:320–6.
Baker R, Günther C. The role of carotenoids in consumer choice and the likely benefits from their inclusion into products for human consumption. Trends Food Sci Tech. 2004;15:484–8.
Sieiro C, Poza M, de Miguel T, Villa TG. Genetic basis of microbial carotenogenesis. Int Microbiol. 2003;6:11–6.
Vergari F, Tibuzzi A, Basile G. An overview of the functional food market: from marketing issues and commercial players to future demand from life in space. In: Maria Teresa Giardi, Giuseppina Rea and Bruno Berra (eds.). Bio-farms for nutraceuticals. New York: Springer; 2010:308–21.
Breithaupt DE. Modern application of xanthophylls in animal feeding—a review. Trends Food Sci Tech. 2007;18:501–6.
Burdick EM. Extraction and utilization of carotenes and xanthophylls. Econ Bot. 1956;10: 267–79.
Ukibe K, Hashida K, Yoshida N, Takagi H. Metabolic engineering of Saccharomyces cerevisiae for astaxanthin production and oxidative stress tolerance. Appl Environ Microbiol. 2009;75: 7205–11.
Shao Z, Zhao H, Zhao H. DNA assembler, an in vivo genetic method for rapid construction of biochemical pathways. Nucleic Acids Res. 2009;37:e16.
Higuera-Ciapara I, Felix-Valenzuela L, Goycoolea FM. Astaxanthin: a review of its chemistry and applications. Crit Rev Food Sci Nutr. 2006;46:185–96.
Fassett RG, Coombes JS. Astaxanthin in cardiovascular health and disease. Molecules. 2012;17:2030–48.
Wang X, Willen R, Wadstrom T. Astaxanthin-rich algal meal and vitamin C inhibit Helicobacter pylori infection in BALB/cA mice. Antimicrob Agents Chemother. 2000;44: 2452–7.
Park JS, Chyun JH, Kim YK, Line LL, Chew BP. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab. 2010;7:18.
Yasui Y, Hosokawa M, Mikami N, Miyashita K, Tanaka T. Dietary astaxanthin inhibits colitis and colitis-associated colon carcinogenesis in mice via modulation of the inflammatory cytokines. Chem Biol Interact. 2011;193:79–87.
Mortensen A. Supplements. In: Britton G, Liaaen-Jensen S and Pfander H (eds.). Carotenoids. New York: Springer; 2009:67–82.
Nishigaki M, Yamamoto T, Ichioka H, Honjo K, Yamamoto K, Oseko F, Kita M, Mazda O, Kanamura N. Beta-cryptoxanthin regulates bone resorption related-cytokine production in human periodontal ligament cells. Arch Oral Biol. 2013;58:880–6.
Irwig MS, El-Sohemy A, Baylin A, Rifai N, Campos H. Frequent intake of tropical fruits that are rich in β-cryptoxanthin is associated with higher plasma β-cryptoxanthin concentrations in Costa Rican adolescents. J Nutr. 2002;132:3161–7.
Yamaguchi M. Role of carotenoid β-cryptoxanthin in bone homeostasis. J Biomed Sci. 2012;19:1–13.
Lorenzo Y, Azqueta A, Luna L, Bonilla F, Dominguez G, Collins AR. The carotenoid beta-cryptoxanthin stimulates the repair of DNA oxidation damage in addition to acting as an antioxidant in human cells. Carcinogenesis. 2009;30:308–14.
Tanaka T, Tanaka T, Tanaka M, Kuno T. Cancer chemoprevention by citrus pulp and juices containing high amounts of beta-cryptoxanthin and hesperidin. J Biomed Biotechnol. 2012;2012:516981.
Khachik F, Liu Y, Showalter H. Process for the preparation of β-and α-cryptoxanthin. European Patent Application EP1678111 A1. 2012.
Louie MTM, Fuerst EJ. Biosynthesis of beta-cryptoxanthin in microbial hosts using an Arabidopsis thaliana beta-carotene hydroxylase gene. US Patent Application US20080124755 A1. 2006.
Guyomarc’h F, Binet A, Dufosse L. Production of carotenoids by Brevibacterium linens: variation among strains, kinetic aspects and HPLC profiles. J Ind Microbiol Biotechnol. 2000;24:64–70.
Serrato‐Joya O, Jiménez‐Islas H, Botello‐Álvarez E, Rico‐Martínez R, Navarrete‐Bolaños JL. Production of β‐cryptoxanthin, a provitamin‐A precursor, by Flavobacterium lutescens. J Food Sci. 2006;71:E314–9.
Gharibzahedi SMT, Razavi SH. Microbial canthaxanthin: perspectives on biochemistry and biotechnological production. Eng Life Sci. 2013;00:1–10.
Kadian SS, Garg M. Pharmacological effects of carotenoids: a review. Int J Pharm Sci Res. 2012;3:42–8.
Breithaupt DR. Xanthophylls in poultry feeding. In: Carotenoids. New York: Springer; 2008, pp. 255–64.
Aberoumand A. A review article on edible pigments properties and sources as natural biocolorants in foodstuff and food industry. World J Dairy Food Sci. 2011;6(1):71–8.
Krupa D, Nakkeeran E, Kumaresan N, Vijayalakshmi G, Subramanian R. Extraction, purification and concentration of partially saturated canthaxanthin from Aspergillus carbonarius. Bioresour Technol. 2010;101:7598–604.
Nasri Nasrabadi MR, Razavi SH. Use of response surface methodology in a fed-batch process for optimization of tricarboxylic acid cycle intermediates to achieve high levels of canthaxanthin from Dietzia natronolimnaea HS-1. J Biosci Bioeng. 2010;109:361–8.
Li H, Fan K, Chen F. Isolation and purification of canthaxanthin from the microalga Chlorella zofingiensis by high‐speed counter‐current chromatography. J Sep Sci. 2006;29(5):699–703.
Pelah D, Sintov A, Cohen E. The effect of salt stress on the production of canthaxanthin and astaxanthin by Chlorella zofingiensis grown under limited light intensity. World J Microbiol Biotechnol. 2004;20:483–6.
Jeknić Z, Morré JT, Jeknić S, Jevremović S, Subotić A, Chen THH. Cloning and functional characterization of a gene for capsanthin-capsorubin synthase from tiger lily (Lilium lancifolium thunb. ‘Splendens’). Plant Cell Physiol. 2012;53:1899–912.
Maeda H, Saito S, Nakamura N, Maoka T. Paprika pigments attenuate obesity-induced inflammation in 3T3-L1 adipocytes. ISRN Inflamm. 2013;2013:1–9.
Kim S, Ha TY, Hwang IK. Analysis, bioavailability, and potential healthy effects of capsanthin, natural red pigment from Capsicum spp. Food Rev Int. 2009;25:198–213.
Miyashita K, Nishikawa S, Beppu F, Tsukui T, Abe M, Hosokawa M. The allenic carotenoid fucoxanthin, a novel marine nutraceutical from brown seaweeds. J Sci Food Agric. 2011;91:1166–74.
Li Y, Li L. Method for producing fucoxanthin. US Patent Application US20100152286 A1. 2009.
Wang J, Chen S, Xu S, Yu X, Ma D, Hu X, Cao X. In vivo induction of apoptosis by fucoxanthin, a marine carotenoid, associated with down-regulating STAT3/EGFR signaling in sarcoma 180 (S180) xenografts-bearing mice. Mar Drugs. 2012;10:2055–68.
Kijlstra A, Tian Y, Kelly ER, Berendschot TT. Lutein: more than just a filter for blue light. Prog Retin Eye Res. 2012;31:303–15.
Calvo MM. Lutein: a valuable ingredient of fruit and vegetables. Crit Rev Food Sci Nutr. 2005;45:671–96.
Shegokar R, Mitri K. Carotenoid lutein: a promising candidate for pharmaceutical and nutraceutical applications. J Diet Suppl. 2012;9:183–210.
Lo HM, Chen CL, Yang CM, Wu PH, Tsou CJ, Chiang KW, Wu WB. The carotenoid lutein enhances matrix metalloproteinase-9 production and phagocytosis through intracellular ROS generation and ERK1/2, p38 MAPK, and RAR beta activation in murine macrophages. J Leukoc Biol. 2013;93:723–35.
Puzio P, Blau A, Plesch G, Kamlage B, Looser R, Schmitz O, Wendel B. Process for the production of lutein. European Patent Application EP2096177 A2. 2009.
Fernandez-Sevilla JM, Acien Fernandez FG, Molina GE. Biotechnological production of lutein and its applications. Appl Microbiol Biotechnol. 2010;86:27–40.
Bowen PE, Herbst-Espinosa SM, Hussain EA, Stacewicz-Sapuntzakis M. Esterification does not impair lutein bioavailability in humans. J Nutr. 2002;132:3668–73.
Yonekura L, Nagao A. Intestinal absorption of dietary carotenoids. Mol Nutr Food Res. 2007;51:107–15.
Lakshminarayana R, Raju M, Krishnakantha TP, Baskaran V. Enhanced lutein bioavailability by lyso-phosphatidylcholine in rats. Mol Cell Biochem. 2006;281:103–10.
Arunkumar R, Harish P, Veerappa K, Baskaran V. Promising interaction between nanoencapsulated lutein with low molecular weight chitosan: characterization and bioavailability of lutein in vitro and in vivo. Food Chem. 2013;141:327–37.
Sivathanu B, Palaniswamy S. Purification and characterization of carotenoids from green algae Chlorococcum humicola by HPLC-NMR and LC-MS-APCI. Biomed Prev Nutr. 2012;2:276–82.
Casal C, Cuaresma M, Vega JM, Vilchez C. Enhanced productivity of a lutein-enriched novel acidophile microalga grown on urea. Mar Drugs. 2011;9:29–42.
Del Campo JA, Rodriguez H, Moreno J, Vargas MA, Rivas J, Guerrero MG. Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta). Appl Microbiol Biotechnol. 2004;64:848–54.
Garcia-Gonzalez M, Moreno J, Manzano JC, Florencio FJ, Guerrero MG. Production of Dunaliella salina biomass rich in 9-cis-beta-carotene and lutein in a closed tubular photobioreactor. J Biotechnol. 2005;115:81–90.
Campenni L, Nobre BP, Santos CA, Oliveira AC, Aires-Barros MR, Palavra AMF, Gouveia L. Carotenoid and lipid production by the autotrophic microalga Chlorella protothecoides under nutritional, salinity, and luminosity stress conditions. Appl Microbiol Biotechnol. 2013;97:1383–93.
Hartung W. The evolution of abscisic acid (ABA) and ABA function in lower plants, fungi and lichen. Funct Plant Biol. 2010;37:806–12.
Garcia-Herrera P, Sanchez-Mata MC, Camara M, Tardio J, Olmedilla-Alonso B. Carotenoid content of wild edible young shoots traditionally consumed in Spain (Asparagus acutifolius L. Humulus lupulus L. Bryonia dioica Jacq. and Tamus communis L.). J Sci Food Agric. 2013;93:1692–8.
Kotake-Nara E, Kushiro M, Zhang H, Sugawara T, Miyashita K, Nagao A. Carotenoids affect proliferation of human prostate cancer cells. J Nutr. 2001;131:3303–6.
Kotake-Nara E, Asai A, Nagao A. Neoxanthin and fucoxanthin induce apoptosis in PC-3 human prostate cancer cells. Cancer Lett. 2005;220:75–84.
Meléndez-Martínez AJ, Vicario IM, Heredia FJ. Geometrical isomers of violaxanthin in orange juice. Food Chem. 2007;104:169–75.
Guedes A, Gião MS, Seabra R, Ferreira AC. Evaluation of the antioxidant activity of cell extracts from microalgae. Mar Drugs. 2013;11(4):1256–70.
Abdel-Aal E-SM, Akhtar H, Zaheer K, Ali R. Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients. 2013;5:1169–85.
SanGiovanni JP, Neuringer M. The putative role of lutein and zeaxanthin as protective agents against age-related macular degeneration: promise of molecular genetics for guiding mechanistic and translational research in the field. Am J Clin Nutr. 2012;96:1223S–33.
Wu NL, Chiang YC, Huang CC, Fang JY, Chen DF, Hung CF. Zeaxanthin inhibits PDGF-BB-induced migration in human dermal fibroblasts. Exp Dermatol. 2010;19:e173–81.
Firdous AP, Sindhu ER, Ramnath V, Kuttan R. Anti-mutagenic and anti-carcinogenic potential of the carotenoid meso-zeaxanthin. Asian Pac J Cancer Prev. 2010;11:1795–800.
Khachik F. Process for extraction and purification of lutein, zeaxanthin and rare carotenoids from marigold flowers and plants. US Patent Application US6262284 B1. 2007.
Zuorro A, Lavecchia R. New functional food products containing lutein and zeaxanthin from marigold (Tagetes erecta L.) flowers. J Biotechnol. 2010;150:296.
Yu B, Wang J, Suter PM, Russell RM, Grusak MA, Wang Y, Wang Z, Yin S, Tang G. Spirulina is an effective dietary source of zeaxanthin to humans. Br J Nutr. 2012;108:611.
Issouf M, Mearns SA, Fraser K, Hodgson R. Biological production of zeaxanthin and carotenoid biosynthesis control. World Intellectual Property Organization (WIPO) Patent Application WO2006120400 A1. 2012.
Chavez-Parga M, Munguia-Franco A, Aguilar-Torres M, Escamilla-Silva EM. Optimization of Zeaxanthin production by immobilized Flavobacterium sp. cells in fluidized bed bioreactor. Adv Microbiol. 2012;2:598–604.
Asker D, Awad TS, Beppu T, Ueda K. Novel zeaxanthin-producing bacteria isolated from a radioactive hot spring water. Methods Mol Biol. 2012;892:99–131.
Singh D, Puri M, Wilkins S, Mathur AS, Tuli DK, Barrow CJ. Characterization of a new zeaxanthin producing strain of Chlorella saccharophila isolated from New Zealand marine waters. Bioresour Technol. 2013;143:308–14.
Liang P-H, Ko T-P, Wang AH-J. Structure, mechanism and function of prenyltransferases. Eur J Biochem. 2002;269:3339–54.
Lee PC, Schmidt-Dannert C. Metabolic engineering towards biotechnological production of carotenoids in microorganisms. Appl Microbiol Biotechnol. 2002;60:1–11.
Misawa N. Pathway engineering for functional isoprenoids. Curr Opin Biotechnol. 2011;22: 627–33.
Britton G. Overview of carotenoid biosynthesis. Carotenoids. 1998;3:13–147.
Ajikumar PK, Tyo K, Carlsen S, Mucha O, Phon TH, Stephanopoulos G. Terpenoids: opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol Pharm. 2008;5:167–90.
Krubasik P, Sandmann G. Molecular evolution of lycopene cyclases involved in the formation of carotenoids with ionone end groups. Biochem Soc Trans. 2000;28:806–9.
Schmidhauser TJ, Lauter F-R, Schumacher M, Zhou W, Russo VE, Yanofsky C. Characterization of al-2, the phytoene synthase gene of Neurospora crassa. Cloning, sequence analysis, and photoregulation. J Biol Chem. 1994;269:12060–6.
Velayos A, Eslava AP, Iturriaga EA. A bifunctional enzyme with lycopene cyclase and phytoene synthase activities is encoded by the carRP gene of Mucor circinelloides. Eur J Biochem. 2000;267:5509–19.
Arrach N, Fernández-Martín R, Cerdá-Olmedo E, Avalos J. A single gene for lycopene cyclase, phytoene synthase, and regulation of carotene biosynthesis in Phycomyces. Proc Natl Acad Sci. 2001;98:1687–92.
Verdoes JC, Krubasik KP, Sandmann G, van Ooyen AJ. Isolation and functional characterisation of a novel type of carotenoid biosynthetic gene from Xanthophyllomyces dendrorhous. Mol Gen Genet. 1999;262:453–61.
Cheng Q. Structural diversity and functional novelty of new carotenoid biosynthesis genes. J Ind Microbiol Biotechnol. 2006;33:552–9.
Mochimaru M, Masukawa H, Maoka T, Mohamed HE, Vermaas WF, Takaichi S. Substrate specificities and availability of fucosyltransferase and beta-carotene hydroxylase for myxol 2′-fucoside synthesis in Anabaena sp. strain PCC 7120 compared with Synechocystis sp. strain PCC 6803. J Bacteriol. 2008;190:6726–33.
Golubev WI. Perfect state of Rhodomyces dendrorhous (Phaffia rhodozyma). Yeast. 1995;11:101–10.
Johnson EA. Phaffia rhodozyma: colorful odyssey. Int Microbiol. 2003;6:169–74.
Andrewes AG, Starr MP. (3R, 3′R)-astaxanthin from the yeast Phaffia rhodozyma. Phytochemistry. 1976;15:1009–11.
Lemoine Y, Schoefs B. Secondary ketocarotenoid astaxanthin biosynthesis in algae: a multifunctional response to stress. Photosynth Res. 2010;106:155–77.
Lee JH, Kim YT. Cloning and characterization of the astaxanthin biosynthesis gene cluster from the marine bacterium Paracoccus haeundaensis. Gene. 2006;370:86–95.
Yokoyama A, Miki W. Composition and presumed biosynthetic pathway of carotenoids in the astaxanthin‐producing bacterium Agrobacterium aurantiacum. FEMS Microbiol Lett. 1995;128:139–44.
Renstrøm B, Berger H, Liaaen-Jensen S. Esterified, optical pure (3S, 3′S)-astaxanthin from flowers of Adonis annua. Biochem Syst Ecol. 1981;9:249–50.
Misawa N, Satomi Y, Kondo K, Yokoyama A, Kajiwara S, Saito T, Ohtani T, Miki W. Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene cluster and astaxanthin biosynthetic pathway proposed at the gene level. J Bacteriol. 1995;177:6575–84.
Vidhyavathi R, Venkatachalam L, Sarada R, Ravishankar GA. Regulation of carotenoid biosynthetic genes expression and carotenoid accumulation in the green alga Haematococcus pluvialis under nutrient stress conditions. J Exp Bot. 2008;59:1409–18.
Cunningham FXJ, Gantt E. Elucidation of the pathway to astaxanthin in the flowers of Adonis aestivalis. Plant Cell. 2011;23:3055–69.
Martín J, Gudiña E, Barredo J. Conversion of β-carotene into astaxanthin: two separate enzymes or a bifunctional hydroxylase-ketolase protein? Microb Cell Fact. 2008;7:3.
Tian L, DellaPenna D. Progress in understanding the origin and functions of carotenoid hydroxylases in plants. Arch Biochem Biophys. 2004;430:22–9.
Blasco F, Kauffmann I, Schmid RD. CYP175A1 from Thermus thermophilus HB27, the first beta-carotene hydroxylase of the P450 superfamily. Appl Microbiol Biotechnol. 2004;64: 671–4.
Misawa N, Nakagawa M, Kobayashi K, Yamano S, Izawa Y, Nakamura K, Harashima K. Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expressed in Escherichia coli. J Bacteriol. 1990;172:6704–12.
McLean KJ, Sabri M, Marshall KR, Lawson RJ, Lewis DG, Clift D, Balding PR, Dunford AJ, Warman AJ, McVey JP. Biodiversity of cytochrome P450 redox systems. Biochem Soc Trans. 2005;33:796.
Zhang H, Im SC, Waskell L. Cytochrome b5 increases the rate of product formation by cytochrome P450 2B4 and competes with cytochrome P450 reductase for a binding site on cytochrome P450 2B4. J Biol Chem. 2007;282:29766–76.
Degtyarenko KN, Archakov AI. Molecular evolution of P450 superfamily and P450-containing monooxygenase systems. FEBS Lett. 1993;332:1–8.
Bernhardt R. Cytochromes P450 as versatile biocatalysts. J Biotechnol. 2006;124:128–45.
Estabrook RW. A passion for P450s (remembrances of the early history of research on cytochrome P450). Drug Metab Dispos. 2003;31:1461–73.
Porter TD, Coon MJ. Cytochrome P-450. Multiplicity of isoforms, substrates, and catalytic and regulatory mechanisms. J Biol Chem. 1991;266:13469–72.
van den Brink H, van Gorcom RFM, van den Hondel CAMJJ, Punt PJ. Cytochrome P450 enzyme systems in fungi. Fungal Genet Biol. 1998;23:1–17.
Sevrioukova IF, Li H, Zhang H, Peterson JA, Poulos TL. Structure of a cytochrome P450—redox partner electron-transfer complex. Proc Natl Acad Sci U S A. 1999;96:1863–8.
Munro AW, Girvan HM, McLean KJ. Cytochrome P450—redox partner fusion enzymes. Biochim Biophys Acta. 2007;1770:345–59.
Lah L, Krasevec N, Trontelj P, Komel R. High diversity and complex evolution of fungal cytochrome P450 reductase: cytochrome P450 systems. Fungal Genet Biol. 2008;45: 446–58.
Ojima K, Breitenbach J, Visser H, Setoguchi Y, Tabata K, Hoshino T, van den Berg J, Sandmann G. Cloning of the astaxanthin synthase gene from Xanthophyllomyces dendrorhous (Phaffia rhodozyma) and its assignment as a beta-carotene 3-hydroxylase/4-ketolase. Mol Genet Genomics. 2006;275:148–58.
Alvarez V, Rodriguez-Saiz M, de la Fuente JL, Gudina EJ, Godio RP, Martin JF, Barredo JL. The crtS gene of Xanthophyllomyces dendrorhous encodes a novel cytochrome-P450 hydroxylase involved in the conversion of beta-carotene into astaxanthin and other xanthophylls. Fungal Genet Biol. 2006;43:261–72.
Alcaino J, Barahona S, Carmona M, Lozano C, Marcoleta A, Niklitschek M, Sepulveda D, Baeza M, Cifuentes V. Cloning of the cytochrome p450 reductase (crtR) gene and its involvement in the astaxanthin biosynthesis of Xanthophyllomyces dendrorhous. BMC Microbiol. 2008;8:169.
Calo P, Gonzalez T. The yeast Phaffia rhodozyma as an industrial source of astaxanthin. Microbiologia. 1995;11:386–8.
Rodriguez-Saiz M, de la Fuente JL, Barredo JL. Xanthophyllomyces dendrorhous for the industrial production of astaxanthin. Appl Microbiol Biotechnol. 2010;88:645–58.
Yamane Y, Higashida K, Nakashimada Y, Kakizono T, Nishio N. Influence of oxygen and glucose on primary metabolism and astaxanthin production by Phaffia rhodozyma in batch and fed-batch cultures: kinetic and stoichiometric analysis. Appl Environ Microbiol. 1997;63: 4471–8.
Flores-Cotera LB, Martín R, Sánchez S. Citrate, a possible precursor of astaxanthin in Phaffia rhodozyma: influence of varying levels of ammonium, phosphate and citrate in a chemically defined medium. Appl Microbiol Biotechnol. 2001;55:341–7.
Liu YS, Wu JY. Hydrogen peroxide-induced astaxanthin biosynthesis and catalase activity in Xanthophyllomyces dendrorhous. Appl Microbiol Biotechnol. 2006;73:663–8.
Hu Z-C, Zheng Y-G, Wang Z, Shen Y-C. pH control strategy in astaxanthin fermentation bioprocess by Xanthophyllomyces dendrorhous. Enzyme Microb Technol. 2006;39:586–90.
An G-H, Johnson EA. Influence of light on growth and pigmentation of the yeast Phaffia rhodozyma. Antonie Van Leeuwenhoek. 1990;57:191–203.
An G-H, Schuman DB, Johnson EA. Isolation of Phaffia rhodozyma mutants with increased astaxanthin content. Appl Environ Microbiol. 1989;55:116–24.
Retamales P, León RUBEN, Martinez C, Hermosilla G, Pincheira G, Cifuentes V. Complementation analysis with new genetic markers in Phaffia rhodozyma. Antonie Van Leeuwenhoek. 1998;73:229–36.
Ukibe K, Katsuragi T, Tani Y, Takagi H. Efficient screening for astaxanthin-overproducing mutants of the yeast Xanthophyllomyces dendrorhous by flow cytometry. FEMS Microbiol Lett. 2008;286:241–8.
Verdoes JC, Sandmann G, Visser H, Diaz M, van Mossel M, van Ooyen AJJ. Metabolic engineering of the carotenoid biosynthetic pathway in the yeast Xanthophyllomyces dendrorhous (Phaffia rhodozyma). Appl Environ Microbiol. 2003;69:3728–38.
Visser H, Sandmann G, Verdoes JC. Xanthophylls in fungi. In: Microbial processes and products. Edited by José Luis Barredo. New York: Springer; 2005, pp. 257–72.
Breitenbach J, Visser H, Verdoes JC, van Ooyen AJ, Sandmann G. Engineering of geranylgeranyl pyrophosphate synthase levels and physiological conditions for enhanced carotenoid and astaxanthin synthesis in Xanthophyllomyces dendrorhous. Biotechnol Lett. 2011;33:755–61.
Gassel S, Schewe H, Schmidt I, Schrader J, Sandmann G. Multiple improvement of astaxanthin biosynthesis in Xanthophyllomyces dendrorhous by a combination of conventional mutagenesis and metabolic pathway engineering. Biotechnol Lett. 2013;35:565–9.
Miziorko HM. Enzymes of the mevalonate pathway of isoprenoid biosynthesis. Arch Biochem Biophys. 2011;505:131–43.
Shimada H, Kondo K, Fraser PD, Miura Y, Saito T, Misawa N. Increased carotenoid production by the food yeast Candida utilis through metabolic engineering of the isoprenoid pathway. Appl Environ Microbiol. 1998;64:2676–80.
Wang G. Amplification of HMG-CoA reductase production enhances carotenoid accumulation in Neurospora crassa. Metab Eng. 2002;4:193–201.
Miao L, Chi S, Tang Y, Su Z, Yin T, Guan G, Li Y. Astaxanthin biosynthesis is enhanced by high carotenogenic gene expression and decrease of fatty acids and ergosterol in a Phaffia rhodozyma mutant strain. FEMS Yeast Res. 2011;11:192–201.
Loto I, Gutiérrez MS, Barahona S, Sepúlveda D, Martínez-Moya P, Baeza M, Cifuentes V, Alcaíno J. Enhancement of carotenoid production by disrupting the C22-sterol desaturase gene (CYP61) in Xanthophyllomyces dendrorhous. BMC Microbiol. 2012;12:235.
Lodato P, Alcaino J, Barahona S, Niklitschek M, Carmona M, Wozniak A, Baeza M, Jimenez A, Cifuentes V. Expression of the carotenoid biosynthesis genes in Xanthophyllomyces dendrorhous. Biol Res. 2007;40:73–84.
Wozniak A, Lozano C, Barahona S, Niklitschek M, Marcoleta A, Alcaino J, Sepulveda D, Baeza M, Cifuentes V. Differential carotenoid production and gene expression in Xanthophyllomyces dendrorhous grown in a nonfermentable carbon source. FEMS Yeast Res. 2011;11:252–62.
Marcoleta A, Niklitschek M, Wozniak A, Lozano C, Alcaíno J, Baeza M, Cifuentes V. Glucose and ethanol-dependent transcriptional regulation of the astaxanthin biosynthesis pathway in Xanthophyllomyces dendrorhous. BMC Microbiol. 2011;11:1–11.
Lodato P, Alcaino J, Barahona S, Retamales P, Cifuentes V. Alternative splicing of transcripts from crtI and crtYB genes of Xanthophyllomyces dendrorhous. Appl Environ Microbiol. 2003;69:4676–82.
Dragoş N, Bercea V, Bica A, Drugă B, Nicoară A, Coman C. Astaxanthin production from a new strain of Haematococcus pluvialis grown in batch culture. Ann Rom Soc Cell Biol. 2010;15:353–61.
Gharibzahedi SMT, Razavi SH, Mousavi SM, Moayedi V. High efficiency canthaxanthin production by a novel mutant isolated from Dietzia natronolimnaea HS-1 using central composite design analysis. Ind Crop Prod. 2012;40:345–54.
Papp T, Csernetics Á, Nagy G, Bencsik O, Iturriaga EA, Eslava AP, Vágvölgyi C. Canthaxanthin production with modified Mucor circinelloides strains. Appl Microbiol Biotechnol. 2013;97:4937–50.
Cordero BF, Obraztsova I, Couso I, Leon R, Vargas MA, Rodriguez H. Enhancement of lutein production in Chlorella sorokiniana (Chorophyta) by improvement of culture conditions and random mutagenesis. Mar Drugs. 2011;9:1607–24.
Sánchez JF, Fernández JM, Acién FG, Rueda A, Pérez-Parra J, Molina E. Influence of culture conditions on the productivity and lutein content of the new strain Scenedesmus almeriensis. Process Biochem. 2008;43:398–405.
Blanco AM, Moreno J, Del Campo JA, Rivas J, Guerrero MG. Outdoor cultivation of lutein-rich cells of Muriellopsis sp. in open ponds. Appl Microbiol Biotechnol. 2007;73:1259–66.
Scoma A, Krawietz D, Faraloni C, Giannelli L, Happe T, Torzillo G. Sustained H2 production in a Chlamydomonas reinhardtii D1 protein mutant. J Biotechnol. 2012;157:613–9.
Lohr M, Wilhelm C. Algae displaying the diadinoxanthin cycle also possess the violaxanthin cycle. Proc Natl Acad Sci U S A. 1999;96:8784–9.
Dharmapuri S, Giuliano G. Plant metabolic engineering: requirements for success. Br Food J. 2001;103:764–9.
Huang J, Zhong Y, Sandmann G, Liu J, Chen F. Cloning and selection of carotenoid ketolase genes for the engineering of high-yield astaxanthin in plants. Planta. 2012;236:691–9.
Tao L, Wilczek J, Odom JM, Cheng Q. Engineering a β-carotene ketolase for astaxanthin production. Metab Eng. 2006;8:523–31.
Britton G, Liaaen-Jensen S, Pfander H (ed.). Carotenoids. Handbook. – Birkhäuser Verlag, Basel – Boston – Berlin; 2004.
Acknowledgements
This work was supported by projects: Fondecyt 11121200 and INACH RG_07-12 to JA, Fondecyt 1130333 to MB and Fondecyt 1100324 to VC.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Alcaino, J., Baeza, M., Cifuentes, V. (2014). Astaxanthin and Related Xanthophylls. In: Martín, JF., García-Estrada, C., Zeilinger, S. (eds) Biosynthesis and Molecular Genetics of Fungal Secondary Metabolites. Fungal Biology. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1191-2_9
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1191-2_9
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1190-5
Online ISBN: 978-1-4939-1191-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)