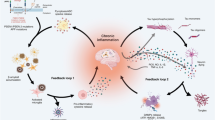
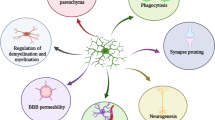
Abstract
The neuropathology of Alzheimer’s disease (AD) is still only partly understood. Beyond doubt neuroinflammation plays a key role in pathophysiology of the disease. Still it has not been fully understood when and how inflammation arises in the course of AD. Whether inflammation is an underlying cause or a resulting condition in AD remains unresolved. Mounting evidence indicates that microglia activation contributes to neuronal damage in neurodegenerative diseases. However, also beneficial aspects of microglia activation have been identified. The purpose of this review is to highlight new insights into the detrimental and beneficial role of neuroinflammation in AD. In regard to this, we discuss the limitations and the advantages of anti-inflammatory treatment options and identify what future implications might result from this underlying neuroinflammation for AD therapy. Here we put a special focus on the therapy with COX-1 and COX-2 Inhibitors as well as anti-Aß antibodies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Alzheimer’s disease (AD) is the most common form of dementia, amongst others, that humans are at risk of as they age. The most achievable long-term aim is to diagnose AD in an earlier stage, thus starting treatment before most clinical symptoms are present [1]. This is possible with new diagnostic imaging, e.g. amyloid-PET imaging, which can show the amyloid burden in the brain [2]. Anyway the pathophysiology of AD is not yet clearly identified. Still a lot of issues related to what causes AD are still unclear, limiting the identification of effective disease-modifying therapies. The main neuropathologic hallmarks of AD are peptide deposition (senile plaques), extracellular ß-amyloid (Ae) and intracellular neurofibrillary tangles containing hyperphosphorylated tau protein [3]. Today it is possible to demonstrate neuroinflammation induced by Aß burden in the brain in vivo. Apart from the disease’s distinct pathological markers, the neurodegenerative features are characterized by chronic neuroinflammatory processes. Yet, those inflammatory markers are not exclusively associated with AD. Also, brains of ‘healthy aged’ individuals show concentrations of serum markers related to inflammation, such as elevated homocysteine and altered cholesterol homeostasis associated with cognitive functioning in the nondemented healthy ageing population [4]. In AD pathology, these ageing-related inflammatory processes are increased.
The suggestion that inflammation may participate in AD first appeared more than two decades ago. As several clinical trials have shown a beneficial effect for nonsteroidal anti-inflammatory drugs for the occurrence and course of AD, the inflammatory hypothesis in AD gained a lot of attention. In regard to treatment and prevention of AD, several classes of medications have emerged on the market, which improve the cognitive symptoms of this disorder (e.g. the cholinesterase inhibitors). But the relief that these drugs provide remains symptomatic—so it is a major goal for the future to develop effective disease-modifying therapy.
Different substantial efforts have been made to identify potential strategies to ameliorate or prevent AD pathology, with data stemming from basic research as well as from animal and epidemiological studies. Because many investigators have concluded that neuroinflammation contributes to neuronal damage in the brain during AD [5, 6], the use of anti-inflammatory drugs as a possible treatment option has been widely investigated [7–9]. Anti-inflammatory therapy has therefore been credited as a strategy for reducing the risk or slowing the progression of AD. However, the results of these studies remain inconsistent [10]. Until now, many questions regarding the inflammatory response are still unresolved. Discussion continues whether neuroinflammation is an underlying cause or a resulting condition in AD [11]. There are several studies showing that an intact immune response including intact T cell immunity is a prerequisite for cognitive function. T-cell-deficient mice show impaired learning abilities, which can be reversed with T cell substitution [12, 13].
Regarding the fact that the T cellular immune response declines with age, starting from about the age of 55 years—‘immunosenescence’—such immunodeficiency in aged patients may also explain cognitive deficits. The “Maastricht Aging Study” followed this approach. Nearly 100 healthy people, mean age 57 years, were followed up in a longitudinal analysis with regard to inflammatory markers and cognitive tests. It could be shown that high levels of haptoglobin (an acute phase protein) correlated significantly negatively with cognitive abilities, measured by the Stroop test and the auditory verbal learning test. High levels of the inflammatory marker C-reactive protein (CRP) also correlated negatively with the auditory verbal learning test after 3 and 6 years follow-up. Lower cognitive abilities were associated with higher concentrations of CRP and haptoglobin [14]. In a similar design, a prospective cohort study in 4,200 healthy persons examined CRP and interleukin (IL)-6 as inflammatory markers combined with cognitive tests at 7 years and 12 years of follow-up. It could be shown that CRP and IL-6 were significantly associated with cognitive performance, in particular in men. Higher levels of pro-inflammatory markers during midlife correlated significantly with lower cognitive abilities and weakly with the decline of cognitive abilities. Interestingly, in animal experiments it was shown that an increased secretion of IL-6 leads to deficits in learning and memory, while IL-6 knockout (KO) mice were less prone to forget learned skills and exhibited a better cognitive performance compared to wild-type mice. Accordingly, the intraventricular administration of anti-IL-6 antibodies resulted in improved memory function.
Inflammation in the brain is characterized by activation of glial cells (mainly microglia and astrocytes) and expression of key inflammatory mediators as well as neurotoxic free radicals. It has been suggested that neuroinflammation is associated with neurodegenerative disorders—both acute (e.g. stroke, injury) and chronic (e.g. multiple sclerosis, AD). In this context, microglial cells play a crucial role, and therefore, microglia and cytokines have been extensively studied in these conditions. In the central nervous system, microglia are the resident phagocytes of the innate immune system. Microglia are found in a highly activated state in close anatomical proximity to senile plaques within the AD brain. In this activated state, microglia produce various pro-inflammatory cytokines and other immune mediators that create a neurotoxic milieu leading to disease progression [6, 15].
It is our intention in this chapter to focus on newer controversies in the field of microglia activation and its role in AD pathology. For this purpose, we asked ourselves several questions: Are neuroinflammatory alterations neuroprotective—or are they rather an underlying cause of AD? And what strategies result from this underlying neuroinflammation for future treatment options?
2 Characteristics of Neuroinflammation in AD
The relevance of neuroinflammation to AD pathology has been established by multiple lines of direct and indirect evidence. One argument is that increased microglia activation has been shown in regions associated with Aβ deposition [16]. Upregulated inflammatory mechanisms co-localize in the AD brain with those regions that exhibit high levels of AD pathology (e.g. frontal and limbic cortex) and are minimal in brain regions with low AD pathologic susceptibility (e.g. cerebellum) [17].
As a second point, many of the inflammatory mechanisms that have been uncovered in the AD brain are established to be cytotoxic in the periphery of the body. Therefore, it seems likely that they are also cytotoxic in the brain, an organ that is sensitive to inflammation (e.g. in meningitis and encephalitis). However, inflammation in the brain is different from inflammation in the peripheral body. AD brains lack the classical hallmarks of inflammation such as neutrophil infiltration and perivascular mononuclear cuffing. As for other neurodegenerative diseases, a local inflammatory reaction is sustained by activated microglia and reactive astrocytes [11]. This is indicated by the presence of antigens associated with microglia activation and inflammatory mediators, such as factors of the complement system, cytokines and free radicals [18].
Only modest elevations of inflammatory markers are found in the autopsy of patients lacking a clinical presentation of dementia but who exhibit sufficient Aβ and neurofibrillary tangles to otherwise qualify for the diagnosis of AD. Their level of inflammatory markers is significantly greater than levels of nondemented patients, but dramatically less than AD patients [19]. These findings further strengthen that inflammation is a necessity for clinical symptoms of AD.
For AD a huge variety of pro-inflammatory markers have been identified, whereas this was not the case for other forms of dementia. A relevant reduction of monocyte chemotactic protein-1 levels in the grey matter in dementia patients has been shown. For IL-6 and related markers of this pro-inflammatory cytokine system, decreases were observed in the brain and cerebrospinal fluid of demented patients [20, 21]. It is unclear, however, whether this decrease is related to further psychopathological symptoms such as depression [21]. On the other hand, IL-6 has also neuroprotective properties and decreased IL-6 might be associated with decreased neuroprotection [22].
There also is direct evidence of inflammatory toxicity in the AD brain. For instance, complement fixation and lysis of neurites could be demonstrated ultrastructurally in AD cortex, but in contrast it was only very weakly detected in nondemented elderly cortex under the same conditions [23].
Finally, many clinical and animal studies have strongly suggested that especially nonsteroidal anti-inflammatory drugs (NSAIDs) could be used as preventive or treatment strategies in AD. This aspect is further discussed in a later section of this chapter, where we focus on anti-inflammatory treatment.
Even though there are many indicators that neuroinflammation plays a key role in AD pathology, this does not answer which of these inflammatory activities are causing disease progression. The question remains: do some of these processes help to fight against the disease? In order to address this question, the role of microglia seems important, because these cells are known for neuroprotective and—degenerative functions.
2.1 Are Activated Microglia Neuroprotective or Neurodegenerative in Brain of AD Patients?
There are three glial cell types in the central nervous system (CNS), one of which is microglia. Since the 1970s there has been wide recognition that microglia are immune effectors in the CNS that respond to pathological conditions and participate in initiation and progression of neurological disorders (including AD) by releasing potentially cytotoxic molecules such as pro-inflammatory cytokines, reactive oxygen intermediates, proteinases and complement proteins [24]. This means that their phagocytic function can be beneficial while their inflammation-related functions might be detrimental.
Several studies give evidence for an increased number of morphologically reactive microglia in AD brains compared to nondemented individuals [25, 26]. The location of these reactive microglia has been indentified directly around plaques [27]. This finding has been verified in a recent imaging study using a specific ligand for positron emission tomography (PET), which showed increased microglia activation in regions associated with amyloid deposition [16]. Up to now, the exact timing of this association could not be identified. Microgliosis might be an early component of the disease process and not necessarily dependent upon Aβ plaque interaction as a stimulus. What is known so far is that activation of microglia by Aβ fibrils is associated with a chemotactic response and extensive clustering of microglia around Aβ plaques in the AD brain [28]. These findings indicate the prominent role of microglia cells in AD. Nonetheless, it remains unclear, whether their functions are beneficial or detrimental.
The following section explains the checkered role of activated microglia in AD pathology.
2.1.1 Neuroprotective Properties of Microglia in AD
Perhaps activated microglial cells are beneficial in neurodegenerative diseases. For the useful role of microglia is that neuroprotection results from the microglia glutamate removal. Glutamate has been identified as a relevant neurotoxic substance that acts through N-methyl-d-aspartic acid (NMDA) receptors on neurons and can lead to increased neuronal cell death. Microglial cells can increase their capacity to take up glutamate upon stimulation with lipopolysaccharide (LPS) via a mechanism that is tumour necrosis factor (TNF)α dependent [11, 29] . In AD this microglia function could be relevant because memantine (the NMDA receptor antagonist) has been shown to improve cognition, function (activities of daily living), agitation and delusions in AD patients [30]. Taken together, microglial cells are important for the control of glutamate levels and might therefore contribute to neuronal survival. There is also evidence that microglia are capable of secreting neurotrophic or neuron survival factors (e.g. nerve growth factor and neurotrophin 3) upon activation via inflammation or injury [31].
Furthermore, it has been suggested that newly recruited microglia have different phagocytotic properties than intrinsic microglia, which is important for Aβ elimination. Lysosomes from the macrophage cell line are more acidic than those of microglia lysosomes [32]. This indicates that microglia derived from the periphery might be more efficient in eliminating Aβ than brain microglia. Furthermore, phagocytic activity of microglia is dampened by pro-inflammatory cytokines like TNF-α [33]. These findings show that microglia that are committed to an inflammatory response may have a lower phagocytotic capacity than newly recruited microglia. In mouse models of AD, it could be demonstrated drugs with anti-inflammatory properties like minocycline improve cognitive function and reduce the activation of microglial cells but do not alter Aβ plaque deposition and distribution [34]. Seabrook et al. showed in amyloid precursor protein transgenic mice an age-dependent effect of minocycline: in young animals the drug increased the amyloid load indicating a beneficial effect of microglia in clearing amyloid [35]. Minocycline has been investigated not only as a potential treatment for AD but also in schizophrenia as an adjunctive therapy where it appeared to be effective in cognitive performance and reducing a broad range of psychotic symptoms [36]. Another mechanism that might help microglial cells with elimination of Aβ involves transforming growth factor-β 1 which has been demonstrated to promote microglia Aβ clearance and reduce plaque burden [37]. This supports the idea that microglia activation is useful in the clearance of Aβ.
A recent review explains that microglia—when they are challenged—may adapt to different stimulatory contexts and pass through a sequence of reactive profiles. This is in line with the finding that microglia are not just ‘resting’ but have active sensor and versatile functions [11, 38].
Are most microglia cells functions beneficial in AD? Several studies suggest an overbalance of the detrimental microglia properties.
2.1.2 Neurodegenerative Aspects of Microglia
In order to address this question, it is important to focus on timing: One must investigate when microglia activity begins during the time course of the disease. An increase in microglia activation has been observed in very early stages of AD. This increase surprisingly disappeared over time [39]. The suggestion of Vehmas et al. strengthens the assumption that microglia activation begins early in disease progression [39]. Microglia initially try to eliminate Aβ, but over time of the disease microglia fail and therefore decrease their activity. Alternatively, the microglia role in AD could be detrimental and they initiate the underlying AD pathology.
In order to further evaluate this issue, a closer look needs to be taken on what causes the microglia activation in AD, and it seems important to distinguish between acute and chronic stimulation of microglial cells. While an acute insult may trigger oxidative and nitrosative stress, it is typically short-lived and unlikely to be harmful to long-term neuronal survival. Therefore, it is believed that an acute neuroinflammatory response is generally beneficial to the CNS, since it tends to minimize further injury and contributes to repair of damaged tissue. The opposite is the case for a chronic stimulation. Chronic neuroinflammation is most often detrimental and damaging to nervous tissue. Thus, whether neuroinflammation has beneficial or harmful outcomes in the brain may depend critically on the duration of the inflammatory response. The progressive deposition of Aβ in AD disease might provide a chronic stimulus to microglial cells. Also, the chemotactic functions of Aβ to attract microglia contribute further to the ongoing inflammatory process [28]. The ratio of the pro-inflammatory cytokine IL-1β to the anti-inflammatory cytokine IL-10 is drastically elevated in the serum of AD patients, giving these patients a definite long-term pro-inflammatory profile [40], indicating a chronic neuroinflammatory state of the CNS. In addition, the accumulating loss of neurons that characterizes AD further contributes to generation of debris and keeps microglia activated indefinitely maintaining microglia in an activated state long term. These data indicate that in AD the inflammation might be chronic, therefore contributing to disease progression [11].
There is also the emerging idea that an inflamed CNS environment may influence the ability of microglia to contribute to plaque deposition rather than plaque removal [33]. This strongly suggests that the microenvironment of the brain can influence whether microglia perform beneficial or deleterious functions in pathophysiological states. This means that microglial cells functionally adapt to their environment [38]. Recent studies show that in response to certain environmental toxins and endogenous proteins, microglia can enter an overactivated state and release reactive oxygen species (ROS) that cause neurotoxicity [41]. Overactivated microglia can be detected using imaging techniques and therefore this knowledge offers an opportunity not only for early diagnosis but eventually also for the development of targeted anti-inflammatory therapies that might diminish the progression of the disease [24].
In addition, activated microglia release the excitotoxin quinolinic acid [42] and microglia activated by AD plaques produce an apparently novel amine that evokes fulminant excitotoxicity [43]. One interesting implication of an excitotoxic contribution to inflammatory mechanisms is the potential for limited damage to functional cellular compartments. Because excitatory amino acid receptors are restricted to synapses and dendrites, these subcellular compartments are preferentially vulnerable.
As a result, microglia-produced excitotoxins may lead to cognitive impairment that is not necessarily correlated with neuronal cell loss [5]. However, activated microglia not only produce neurotoxic metabolites: Some of their products like 3-hydroxyanthralinic acid (which is—like quinolinic acid—one of the downstream products of the tryptophan metabolism) exert antioxidant and anti-inflammatory functions [44, 45].
Since tryptophan/kynurenine metabolism—i.e. the degradation of tryptophan to the partly neuroprotective, partly neurotoxic metabolites of the degradation to quinolinic acid—is driven by the enzyme indoleamine 2,3 dioxygenase (IDO), immune mechanisms are key players in this system. IDO is activated by pro-inflammatory cytokines such as interferon-gamma or IL-2. Immune activation is associated with an increased degradation of tryptophan and kynurenine. In an interesting study it was investigated whether an imbalance between neurotoxic and neuroprotective kynurenine metabolites could be detected in patients with AD. Serum levels of tryptophan, kynurenic acid, 3-hydroxykynurenine (HK), picolinic acid and quinolinic acid were measured in patients with AD, and it was found that serum levels of 3-HK were markedly increased in AD patients compared to the comparison groups (p < .0001), while serum levels of the other KP metabolites were not significantly different between groups. In contrast to its downstream metabolites, quinolinic acid and picolinic acid, 3-HK can cross the blood-brain barrier via an active transport process. These data therefore indicate an enhanced availability of 3-hydroxykynurenine in the brain of AD patients, which may be related to the previously reported higher production of quinolinic acid in AD brains [46].
Therefore, the balance of these products that result from activated microglia is important for the inflammatory process.
Finally up the results from microglia studies, clear evidence that exists for an important role of neuroinflammation contributing to disease progression in AD was found. However, some aspects of microglia activation might also be beneficial during the course of AD. As explained above, neuroinflammation is a critical event in AD. It has been suggested that anti-inflammatory therapy could be beneficial in delaying the onset or slowing the progression of AD. Cyclooxygenase (COX) is a unique enzyme. First, it exhibits two catalytic activities, a bis-oxygenase activity, which catalyses prostaglandin G2 (PG) formation from arachidonic acid, and a peroxidase activity, which reduces PG G2 to PG H2. The peroxidase activity also results in the production of free radicals, which are in part utilized by COX itself [47]. Although nonsteroidal inflammatory drugs (NSAIDs) may have other effects as well, it is generally assumed that their primary mechanism of action is by competitive inhibition of COX activity, thereby reducing the production of inflammatory prostaglandins from membrane-derived arachidonate. COX not only helps mediate production of prostaglandins and other inflammatory factors; it is itself upregulated by pro-inflammatory mediators [11, 47].
In AD, Aβ neurotoxicity may result from several mechanisms, most likely in combination. These mechanisms include oxidative damage, direct cytotoxicity and induction of destructive inflammatory mechanisms; efforts have been directed at the control of each of these processes [48].
3 Possible Mechanisms of Action of NSAIDs in AD
The treatment of AD with NSAIDs is one of the most promising approaches. If NSAIDs are beneficial in AD, the presumed mechanism would be inhibition of COX expressed in the brain. Both COX-1 and COX-2 are expressed there and COX-2 plays a unique role in the brain compared to the periphery: Only in the brain is COX-2 expressed constitutively, whereas elsewhere the expression is activation dependent. Although in vivo the majority of COX-2 appears to be made in neurons, COX-2 was also seen in rat astrocytes and microglia [49]. It has been demonstrated that COX-inhibiting NSAIDs reduce microglia activation following infusion of Aβ in rats [50]. Neuronal stress, such as ischaemia and excitotoxicity, is associated with strong upregulation of neuronal COX-2 expression. This suggests that COX-2 is involved in neurotoxic mechanisms and may therefore represent a target for drug therapy in the treatment of AD [51, 52].
Several epidemiological studies provide the background for possible mechanisms of action of NSAIDs in AD. In most studies COX-2 inhibitors are used, because neuronal COX-2 is upregulated in response to exposure to Aβ [53], and focal increases in COX-2 have been shown in the region of amyloid plaques in double transgenic mice carrying genes that encode both mutant APP and mutant presenilin 1 [54]. Many studies seem to show that COX-2 inhibition confers neuroprotection [55–58]. Some studies have revealed an upregulation of neuronal COX-2 in the brains of patients with AD [59, 60], though this has not been a universal finding [61, 62]. One explanation for the variation of COX expression is the short half-life of COX-2 transcripts or individual variability of inflammatory-related processes.
COX-1 is also localized in microglia and is actively involved in brain injury induced by pro-inflammatory stimuli including Aß, LPS and interleukins. A study with 20-month-old triple transgenic AD mice showed that their memory function increases, when treated with COX-1 inhibitors. In addition, amyloid deposits and tau hyperphosphorylation in hippocampus decrease [63]. Triflusal, a platelet anti-aggregant and irreversible COX-1 inhibitor, could protect against cognitive deficits by reducing the dense-core amyloid plaque load, associated glial cell activation and pro-inflammatory cytokine levels in a transgenic mouse model [64]. Unfortunately, this could only be clearly demonstrated in animal experiments.
Another principle of how NSAIDs could act comes from the finding that prostaglandin E2 levels are elevated in patients with AD, especially in early stages of the disease [65]. Therefore, NSAIDs blocking prostaglandin E2 synthesis might be beneficial. This issue is further strengthened by glial culture studies indicating that prostaglandins, particularly prostaglandin E, alter the production of several inflammation-related molecules, including IL-6, chemokines and APP [66–68].
In addition to the more traditional inflammatory mechanisms associated with COX, unique functions of COX-mediated damage may also occur in the AD brain. For example, several of the prostanoid products of arachidonate metabolism potentiate glutamate excitotoxicity, and COX-2 overexpressing transgenic mice exhibit increased neuronal susceptibility to excitotoxic insult [69].
Some of the previously mentioned studies of COX in ischaemia also suggest that intraneuronal COX-2 levels may contribute to neuronal death by production of free radicals [70]. In addition, increased COX-2 levels in AD neurons may directly damage neurons or increase their vulnerability to other detrimental processes occurring in AD brain [70]. Resulting, the inhibitory action of NSAIDs on COX-mediated production of apoptotic factors by neurons could be one of the mechanisms by which these anti-inflammatory drugs cause beneficial effects in AD.
Another non-COX-dependent mechanism of NSAIDs is to attenuate inflammatory processes in a manner by directly activating the peroxisome proliferator-activated receptor gamma (PPARγ), a receptor and nuclear transcription factor [71–73]. PPARγ is a member of the orphan nuclear receptor family. In cells of the monocytic lineage, including microglia, PPARγ acts to suppress the expression of a broad range of proinflammatory genes [71, 73]. Some NSAIDs act as PPARγ agonists, directly binding to it and initiating its transcriptional activity. Activation of PPARγ inhibits the Aβ-stimulated activation of microglia and monocytes and their secretion of proinflammatory and neurotoxic products. For example, PPARγ agonists act to inhibit the Aβ-stimulated expression of IL-6 and TNF-alpha [74], by microglia and monocytes, and to prevent Aβ-mediated conversion of microglia into an activated phenotype [11, 75].
A further underlying mechanism of AD pathology is oxidative stress [76, 77]. Activated microglial cells are known to release ROS, which might possibly cause this oxidative stress. However, glial cells can also exhibit antioxidative functions by releasing hemeoxygenase-1 (HO-1) triggered by accumulation of 3-hydroxyanthrallinic acid (3-HAA), a downstream product of the tryptophan metabolism. The association of neuronal injury in AD and oxidative stress has been demonstrated by overexpression of immunoreactive HO-1 protein in neurons and astrocytes of the cerebral cortex and hippocampus. HO-1 was found to be co-localized to senile plaques, neurofibrillary tangles and corpora amylacea [78]. It is widely accepted that a moderate activation of heme catabolism is neuroprotective and contributes to degradation of neurotoxic protein aggregates. Regulatory interactions between HO-1 and COX pathways have also been reported [79]. However, experimental observations indicate that the extent of HO-1 induction may be critical because excessive heme degradation may result in toxic levels of carbon monoxide, bilirubin and iron. Pharmacological modulation of HO-1 levels in the brain shows promising results in models of AD and Parkinson’s disease [80].
Referring to the oxidative stress underlying AD pathology, one further aspect of these ROS includes activation of COX-1/2, which is blocked by NSAIDs. It has been shown that daily doses of NSAIDs increase circulating levels of antioxidants [81]. In a rat model of AD, it was suggested that treatment with a COX-2 inhibitor reduces oxidative stress and might therefore be beneficial for the course of AD [82].
Another neuroprotective mechanism has been suggested for NSAIDs whereby these drugs directly affect amyloid pathology in the brain by reducing Aβ-42 peptide levels over the gamma-secretase activity independently of COX activity [83]. Weggen et al. reported that the NSAIDs ibuprofen, indomethacin and sulindac sulphide preferentially decrease the highly amyloidogenic Aβ-42 peptide produced from a variety of cultured cells by as much as 80 % [84]. However, for some NSAIDs the lowering effect of Aβ-42 could not be shown; instead, an increase in Aβ-42 levels was observed [85]. The underlying mechanism of how NSAIDs decrease Aβ-42 was clarified by Lleo et al., who demonstrated that Aβ-42 by lowering NSAIDs specifically affects the proximity between APP and presenilin 1 and alters a novel allosteric mechanism of action [86].
4 Anti-inflammatory Treatment Studies in AD
In recent years it has become widely accepted that inflammatory processes are an underlying condition of AD. Therefore, a number of clinical trials investigating different anti-inflammatory treatment regimens have been performed. In the following paragraph, we summarize the most import findings in regard to first mainly COX-2 dominant and second COX-1 inhibitors.
4.1 COX-1 and COX-2 Inhibitors
A prospective cohort study with 6,989 subjects showed that long-term use of NSAIDs protects against AD but not against vascular dementia [7]. More recently, Szekely et al. provided very similar findings. They concluded that NSAID use reduced the risk of preferentially AD versus vascular dementia but mainly in those individuals having an apolipoprotein E (APO) epsilon 4 allele. This study was done with over 3,000 subjects aged 65 years and older [8]. Not only selective COX-2 inhibitors were shown to be associated with decreased risk of AD; a reduced occurrence of AD could also be demonstrated for the use of the mixed COX-1/COX-2 inhibitor aspirin [9]. A meta-analysis of 17 epidemiological studies yielded strong, generally consistent, statistical evidence that NSAID and steroid use is associated with reduced risk of AD [87]. Vlad et al. investigated 49,349 patients with AD and 196,850 controls: long-term (>5 years) nonsteroidal anti-inflammatory drug use was shown to be protective against AD. These findings were clearest for ibuprofen, but did not appear for other NSAIDs [88].
Naproxen, which is slightly more selective for COX-1 than COX-2, cuts the risk of developing AD in 117 patients with MCI from whom CSF was collected 21–41 months after treatment was terminated. The tau to Aß42 ratio was reduced by more than 40 % in the group treated with naproxen [89]. Also, another NSAID with preferential COX-1 selectivity such as indomethacin reduced amyloid burden in transgenic mice [90].
To conclude there are at least ten studies showing beneficial effects of NSAIDs on amyloid burden and inflammation in mice.
In humans however, not all studies showed a positive outcome for COX-inhibitors. The failure of selective COX-2 inhibition (rofecoxib) over placebo was found in a 1-year randomized controlled study. The authors argued that their results could indicate that the disease process was too advanced to be modified, as the goal of the study was slowing the progression of dementia in patients with already established AD [10]. For another COX-2 inhibitor, celecoxib, no beneficial effect on the occurrence of AD could be demonstrated in an age group over 70 years [91]. Also, Wolfson et al. looked retrospectively at a case-control population and found no support for a beneficial effect for NSAIDs in the AD subjects [92]. However, this negative result may have been caused by an insufficient period of data collection before disease onset.
4.2 Passive and Active Anti-Aß Immunotherapies
In the last few years, most of the efforts of the pharmaceutical industry were directed against the production and accumulation of Aß. The most revolutionary development in the last several years consists in the removal of brain ß-amyloid via anti-Aß antibodies. Both passive and active anti-Aß immunotherapies can clear Aß deposits from the brain of the AD patients. AN1792, which was used in AD patients, showed some clues of clinical efficacy but was associated with aseptic meningoencephalitis in about 6 % of patients. So this medication has been abandoned. The next generation of active and passive vaccines has been developed in the past few years and is currently under clinical investigation. The aim of these vaccines is to clear the brain from Aß deposits and to stop the progression of AD.
Bapineuzumab, composed of humanized anti-Aß monoclonal antibodies, is the most advanced product. It has been tested in two phase II trials and Aß burden was reduced in the brain of AD patients. Some patients experienced vasogenic edema especially apolipoprotein E4 carriers. This limits its clinical use, especially in higher doses (2 mg/kg). The proposed remedy is to treat AD patients with lower doses, particularly in APOE4 carriers. A large phase III trial with bapineuzumab is ongoing. This study will tell us if passive anti-Aß immunization is able to reduce progression of the disease [93]. Of course, improvements in vaccine design are needed to improve the safety and the efficacy of anti-Aß immunotherapy. Unfortunately, at this point we cannot definitely identify individuals in the preclinical stages of AD; therefore, passive immunotherapy is indicated in patients that are diagnosed with AD, i.e. have clinical symptoms. At that point patients have already accumulated substantial neuropathology in affected regions of the brain [94].
Perhaps amyloid-PET imaging combined with genetic markers and last but not least with clinical symptoms such as mild cognitive impairment will potentially detect individuals with a higher risk to develop an AD [95]. Development of valid biomarkers for AD should be a high priority aim of research on AD in the next few years.
5 Conclusion
Neuroinflammation plays a key role in the pathophysiology of the AD. Mechanisms that parallel those encountered in inflammatory diseases involving other organ systems are readily identified, along with detailed pathways for how the mechanisms interact. Although still controversial, on balance, it is likely that AD neuroinflammation exacerbates AD pathogenesis.
A general treatment principle in neurology and psychiatry that an intervention as early as possible leads to the best outcome seems to be especially true for AD. However, lack of appropriate biomarkers such as genetic risk factors or neuroimaging techniques is a problem that needs to be solved in the next few years. Until then, the treatment cannot start early enough.
Many lines of evidence show that Aβ-induced neuroinflammation is an early event in neurodegeneration of AD [96], as increases in microglia activation have been observed in very early stages of AD and disappeared over time [39]. The fact that neuroinflammation occurs very early in AD could explain why anti-inflammatory treatment seems to be most efficient as preventive or early treatment.
There are several reasons why early use of NSAIDs is superior to a late treatment. COX expression in the brain decreases over time in AD brains [97]. And the CSF PG E2 levels in patients with AD are high when their short-term memory scores were just below those of controls, but were low in later stages of the disease. These findings further support that inflammatory processes predominate early in AD [98] and therefore require early intervention with anti-inflammatory agents.
This might also explain that the failure of some prospective clinical trials of selective COX-2 inhibitors delayed the onset of treatment. Lack of clinical efficacy could also be due to drug selection (regarding different effects of COX-1 and COX-2) or to dose and duration of treatment. The drug selection seems essential as some NSAIDs have recently been shown to increase Aβ-42 levels. It also has to be noted that the protective effects of NSAIDs may be via non-COX-inhibitory mechanisms, such as lowering of Aβ levels and activation of the peroxisome proliferator-activated receptor [gamma] [99]. These non-COX-dependent mechanisms might be differentially distributed amongst different COX-inhibitors.
While the harmful inflammatory processes seem to dominate in AD pathology, there are also some beneficial functions for inflammatory subsets. If AD neuroinflammation is approached with realistic expectations and rational drug design, AD patients could significantly benefit from anti-inflammatory treatment, especially with NSAIDs.
Another aspect could be to not only utilize the efficient treatment properties of NSAIDs in early AD but also make use of the neuroprotective aspects of neuroinflammation with combination therapy that maximizes the potential of glial activation. This would include treatment with NSAIDs and drugs that enforce anti-inflammatory and antioxidative properties (e.g. with 3-HAA and HO-1 enhancement).
Finally, a promising therapeutic approach using passive and active immunization against amyloid-beta has emerged. If in the next few years we are able to detect AD much earlier, starting treatment with immunization before the clinical symptoms appear could prove effective.
References
Sperling RA, Aisen PS, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–92.
Rosenberg PB et al. Cognition and amyloid load in Alzheimer disease imaged with Florbetapir F 18(AV-45) positron emission tomography. Am J Geriatr Psychiatry. 2013;21(3):272–8.
Panza F et al. Immunotherapy for Alzheimer’s disease: from anti-beta-amyloid to tau-based immunization strategies. Immunotherapy. 2012;4(2):213–38.
Teunissen CE et al. [Serum markers in relation to cognitive functioning in an aging population: results of the Maastricht Aging Study (MAAS)]. Tijdschr Gerontol Geriatr. 2003;34(1):6–12.
McGeer EG, McGeer PL. Neuroinflammation in Alzheimer’s disease and mild cognitive impairment: a field in its infancy. J Alzheimers Dis. 2010;19(1):355–61.
Akiyama H et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21(3):383–421.
in t’ Veld BA, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N Engl J Med. 2001;345(21):1515–21.
Szekely CA et al. NSAID use and dementia risk in the Cardiovascular Health Study: role of APOE and NSAID type. Neurology. 2008;70(1):17–24.
Anthony JC et al. Reduced prevalence of AD in users of NSAIDs and H2 receptor antagonists: the Cache County study. Neurology. 2000;54(11):2066–71.
Reines SA et al. Rofecoxib: no effect on Alzheimer’s disease in a 1-year, randomized, blinded, controlled study. Neurology. 2004;62(1):66–71.
Dl K. Muller N, MN. Neuroinflammation, microglia and implications for anti-inflammatory treatment in Alzheimer’s disease. Int. J Alzheimers Dis. 2010;14(732806):732806. 10.4061/2010/732806 [doi] 732806 [pii].
Kipnis J et al. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci U S A. 2004;101(21):8180–5.
Ziv Y et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9(2):268–75.
Teunissen CE et al. Inflammation markers in relation to cognition in a healthy aging population. J Neuroimmunol. 2003;134(1–2):142–50.
Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12(9):1005–15.
Edison P et al. Microglia, amyloid, and cognition in Alzheimer’s disease: an [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol Dis. 2008;32(3):412–9.
Rogers J, Shen Y. A perspective on inflammation in Alzheimer’s disease. Ann N Y Acad Sci. 2000;924:132–5.
Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4(2):103–12.
Lue LF et al. Inflammation, a beta deposition, and neurofibrillary tangle formation as correlates of Alzheimer’s disease neurodegeneration. J Neuropathol Exp Neurol. 1996;55(10):1083–8.
Mulugeta E et al. Inflammatory mediators in the frontal lobe of patients with mixed and vascular dementia. Dement Geriatr Cogn Disord. 2008;25(3):278–86.
Stubner S et al. Interleukin-6 and the soluble IL-6 receptor are decreased in cerebrospinal fluid of geriatric patients with major depression: no alteration of soluble gp130. Neurosci Lett. 1999;259(3):145–8.
Wang XQ et al. Neuroprotection of interleukin-6 against NMDA attack and its signal transduction by JAK and MAPK. Neurosci Lett. 2009;450(2):122–6.
Webster S et al. Molecular and cellular characterization of the membrane attack complex, C5b-9, in Alzheimer’s disease. Neurobiol Aging. 1997;18(4):415–21.
Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69.
Cras P et al. Neuronal and microglial involvement in beta-amyloid protein deposition in Alzheimer’s disease. Am J Pathol. 1990;137(2):241–6.
Styren SD, Civin WH, Rogers J. Molecular, cellular, and pathologic characterization of HLA-DR immunoreactivity in normal elderly and Alzheimer’s disease brain. Exp Neurol. 1990;110(1):93–104.
Perlmutter LS, Barron E, Chui HC. Morphologic association between microglia and senile plaque amyloid in Alzheimer’s disease. Neurosci Lett. 1990;119(1):32–6.
Lue LF et al. Inflammatory repertoire of Alzheimer’s disease and nondemented elderly microglia in vitro. Glia. 2001;35(1):72–9.
Persson M et al. Lipopolysaccharide increases microglial GLT-1 expression and glutamate uptake capacity in vitro by a mechanism dependent on TNF-alpha. Glia. 2005;51(2):111–20.
Francis PT. Altered glutamate neurotransmission and behaviour in dementia: evidence from studies of memantine. Curr Mol Pharmacol. 2009;2(1):77–82.
Kim SU, de Vellis J. Microglia in health and disease. J Neurosci Res. 2005;81(3):302–13.
Majumdar A et al. Activation of microglia acidifies lysosomes and leads to degradation of Alzheimer amyloid fibrils. Mol Biol Cell. 2007;18(4):1490–6.
Koenigsknecht-Talboo J, Landreth GE. Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J Neurosci. 2005;25(36):8240–9.
Fan R et al. Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J Neurosci. 2007;27(12):3057–63.
Seabrook TJ et al. Minocycline affects microglia activation, A beta deposition, and behavior in APP-tg mice. Glia. 2006;53(7):776–82.
Chaves C et al. Glutamate-N-methyl-D-aspartate receptor modulation and minocycline for the treatment of patients with schizophrenia: an update. Braz J Med Biol Res. 2009;42(11):1002–14.
Wyss-Coray T et al. TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat Med. 2001;7(5):612–8.
Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387–94.
Vehmas AK et al. Immune reactive cells in senile plaques and cognitive decline in Alzheimer’s disease. Neurobiol Aging. 2003;24(2):321–31.
Remarque EJ et al. Patients with Alzheimer’s disease display a pro-inflammatory phenotype. Exp Gerontol. 2001;36(1):171–6.
Innamorato NG, Lastres-Becker I, Cuadrado A. Role of microglial redox balance in modulation of neuroinflammation. Curr Opin Neurol. 2009;22(3):308–14.
Espey MG et al. Activated human microglia produce the excitotoxin quinolinic acid. Neuroreport. 1997;8(2):431–4.
Giulian D et al. Senile plaques stimulate microglia to release a neurotoxin found in Alzheimer brain. Neurochem Int. 1995;27(1):119–37.
Leipnitz G et al. In vitro evidence for an antioxidant role of 3-hydroxykynurenine and 3-hydroxyanthranilic acid in the brain. Neurochem Int. 2007;50(1):83–94.
Thomas SR, Witting PK, Stocker R. 3-Hydroxyanthranilic acid is an efficient, cell-derived co-antioxidant for alpha-tocopherol, inhibiting human low density lipoprotein and plasma lipid peroxidation. J Biol Chem. 1996;271(51):32714–21.
Schwarz MJ, Guillemin GJ, et al. Increased 3-Hydroxykynurenine serum concentrations differentiate Alzheimer’s disease patients from controls. Eur Arch Psychiatry Clin Neurosci. 2012;29:29.
Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271(52):33157–60.
Aisen PS, Davis KL. The search for disease-modifying treatment for Alzheimer’s disease. Neurology. 1997;48(5 Suppl 6):S35–41.
Hirst WD et al. Expression of COX-2 by normal and reactive astrocytes in the adult rat central nervous system. Mol Cell Neurosci. 1999;13(1):57–68.
Hauss-Wegrzyniak B, Vraniak P, Wenk GL. The effects of a novel NSAID on chronic neuroinflammation are age dependent. Neurobiol Aging. 1999;20(3):305–13.
Planas AM et al. Induction of cyclooxygenase-2 mRNA and protein following transient focal ischemia in the rat brain. Neurosci Lett. 1995;200(3):187–90.
Tocco G et al. Maturational regulation and regional induction of cyclooxygenase-2 in rat brain: implications for Alzheimer’s disease. Exp Neurol. 1997;144(2):339–49.
Pasinetti GM, Aisen PS. Cyclooxygenase-2 expression is increased in frontal cortex of Alzheimer’s disease brain. Neuroscience. 1998;87(2):319–24.
Matsuoka Y et al. Inflammatory responses to amyloidosis in a transgenic mouse model of Alzheimer’s disease. Am J Pathol. 2001;158(4):1345–54.
Hewett SJ et al. Cyclooxygenase-2 contributes to N-methyl-D-aspartate-mediated neuronal cell death in primary cortical cell culture. J Pharmacol Exp Ther. 2000;293(2):417–25.
Willard LB et al. The cytotoxicity of chronic neuroinflammation upon basal forebrain cholinergic neurons of rats can be attenuated by glutamatergic antagonism or cyclooxygenase-2 inhibition. Exp Brain Res. 2000;134(1):58–65.
Kunz T, Oliw EH. The selective cyclooxygenase-2 inhibitor rofecoxib reduces kainate-induced cell death in the rat hippocampus. Eur J Neurosci. 2001;13(3):569–75.
Araki E et al. Cyclooxygenase-2 inhibitor ns-398 protects neuronal cultures from lipopolysaccharide-induced neurotoxicity. Stroke. 2001;32(10):2370–5.
Yasojima K et al. Distribution of cyclooxygenase-1 and cyclooxygenase-2 mRNAs and proteins in human brain and peripheral organs. Brain Res. 1999;830(2):226–36.
Ho L et al. Neuronal cyclooxygenase 2 expression in the hippocampal formation as a function of the clinical progression of Alzheimer disease. Arch Neurol. 2001;58(3):487–92.
Lukiw WJ, Bazan NG. Cyclooxygenase 2 RNA message abundance, stability, and hypervariability in sporadic Alzheimer neocortex. J Neurosci Res. 1997;50(6):937–45.
Chang JW, Coleman PD, O‘Banion MK. Prostaglandin G/H synthase-2 (cyclooxygenase-2) mRNA expression is decreased in Alzheimer’s disease. Neurobiol Aging. 1996;17(5):801–8.
Choi SH et al. Cyclooxygenase-1 inhibition reduces amyloid pathology and improves memory deficits in a mouse model of Alzheimer’s disease. J Neurochem. 2013;124(1):59–68.
Coma M, Sereno L, et al. Triflusal reduces dense-core plaque load, associated axonal alterations and inflammatory changes, and rescues cognition in a transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2010;38(3):482–91.
Montine TJ et al. Elevated CSF prostaglandin E2 levels in patients with probable AD. Neurology. 1999;53(7):1495–8.
Lee RK, Knapp S, Wurtman RJ. Prostaglandin E2 stimulates amyloid precursor protein gene expression: inhibition by immunosuppressants. J Neurosci. 1999;19(3):940–7.
Blom MA et al. NSAIDS inhibit the IL-1 beta-induced IL-6 release from human post-mortem astrocytes: the involvement of prostaglandin E2. Brain Res. 1997;777(1–2):210–8.
Fiebich BL et al. Prostaglandin E2 induces interleukin-6 synthesis in human astrocytoma cells. J Neurochem. 1997;68(2):704–9.
Kelley KA et al. Potentiation of excitotoxicity in transgenic mice overexpressing neuronal cyclooxygenase-2. Am J Pathol. 1999;155(3):995–1004.
Pasinetti GM. Cyclooxygenase and inflammation in Alzheimer’s disease: experimental approaches and clinical interventions. J Neurosci Res. 1998;54(1):1–6.
Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391(6662):82–6.
Lehmann JM et al. Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1997;272(6):3406–10.
Ricote M et al. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82.
Combs CK et al. Identification of microglial signal transduction pathways mediating a neurotoxic response to amyloidogenic fragments of beta-amyloid and prion proteins. J Neurosci. 1999;19(3):928–39.
Combs CK et al. Inflammatory mechanisms in Alzheimer’s disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. J Neurosci. 2000;20(2):558–67.
Ansari MA, Scheff SW. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol. 2010;69(2):155–67.
Smith MA et al. Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. J Alzheimers Dis. 2010;19(1):363–72.
Schipper HM et al. Heme oxygenase-1 and neurodegeneration: expanding frontiers of engagement. J Neurochem. 2009;110(2):469–85.
Alcaraz MJ, Fernandez P, Guillen MI. Anti-inflammatory actions of the heme oxygenase-1 pathway. Curr Pharm Des. 2003;9(30):2541–51.
Cuadrado A, Rojo AI. Heme oxygenase-1 as a therapeutic target in neurodegenerative diseases and brain infections. Curr Pharm Des. 2008;14(5):429–42.
Kimura K. Mechanisms of active oxygen species reduction by non-steroidal anti-inflammatory drugs. Int J Biochem Cell Biol. 1997;29(3):437–46.
Nivsarkar M, Banerjee A, Padh H. Cyclooxygenase inhibitors: a novel direction for Alzheimer’s management. Pharmacol Rep. 2008;60(5):692–8.
Guardia-Laguarta C, Pera M, Lleo A. A gamma-Secretase as a therapeutic target in Alzheimer‘s disease. Curr Drug Targets. 2010;11(4):506–17.
Weggen S et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414(6860):212–6.
Kukar T et al. Diverse compounds mimic Alzheimer disease-causing mutations by augmenting Abeta42 production. Nat Med. 2005;11(5):545–50.
Lleo A et al. Nonsteroidal anti-inflammatory drugs lower Abeta42 and change presenilin 1 conformation. Nat Med. 2004;10(10):1065–6.
McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: a review of 17 epidemiologic studies. Neurology. 1996;47(2):425–32.
Vlad SC et al. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology. 2008;70(19):1672–7.
Breitner JC, Baker LD, et al. Extended results of the Alzheimer’s disease anti-inflammatory prevention trial. Alzheimers Dement. 2011;7(4):402–11.
Jantzen PT, Connor KE, et al. Microglial activation and beta -amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. J Neurosci. 2002;22(6):246–54.
Martin BK et al. Cognitive function over time in the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008;65(7):896–905.
Wolfson C et al. A case-control analysis of nonsteroidal anti-inflammatory drugs and Alzheimer’s disease: are they protective? Neuroepidemiology. 2002;21(2):81–6.
Panza F, Frisardi V, et al. Anti-beta-amyloid immunotherapy for Alzheimer’s disease: focus on bapineuzumab. Curr Alzheimer Res. 2011;8(8):808–17.
DH C. Abeta DNA vaccination for Alzheimer’s disease: focus on disease prevention. CNS Neurol Disord Drug Targets. 2010;9(2):207–16.
Fleisher AS, Chen K, et al. Florbetapir PET analysis of amyloid-beta deposition in the presenilin 1 E280A autosomal dominant Alzheimer’s disease kindred: a cross-sectional study. Lancet Neurol. 2012;11(12):1057–65.
Craft JM, Watterson DM, Van Eldik LJ. Human amyloid beta-induced neuroinflammation is an early event in neurodegeneration. Glia. 2006;53(5):484–90.
Yermakova AV, O‘Banion MK. Downregulation of neuronal cyclooxygenase-2 expression in end stage Alzheimer’s disease. Neurobiol Aging. 2001;22(6):823–36.
Combrinck M et al. Levels of CSF prostaglandin E2, cognitive decline, and survival in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006;77(1):85–8.
Aisen PS. The potential of anti-inflammatory drugs for the treatment of Alzheimer’s disease. Lancet Neurol. 2002;1(5):279–84.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Reinisch, V.M., Krause, D.L., Müller, N. (2014). Neuroinflammation in Alzheimer’s Disease. In: Peterson, P., Toborek, M. (eds) Neuroinflammation and Neurodegeneration. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1071-7_9
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1071-7_9
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1070-0
Online ISBN: 978-1-4939-1071-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)