Abstract
Astrocytes, the most abundant cell in the central nervous system, are essential for brain function and homeostasis. This chapter focuses on the immunological role of astrocytes in the pathology of major neurodegenerative diseases. Astrocyte activation, or astrogliosis, has been observed in many neurodegenerative diseases. Factors associated with neurodegeneration including extracellular oligomerized proteins such as amyloid β and α-synuclein as well as inflammatory cytokines and chemokines can influence the functionality of astrocytes. In response to such stimuli, astrocytes produce a multitude of soluble factors including cytokines, chemokines, reactive oxygen/nitrogen species, and growth factors. This astrocytic response is initially protective, limiting damage and promoting functional recovery. However, the prolonged and progressive nature of neurodegenerative diseases establishes an environment in which astrogliosis may be aberrantly sustained, and the ongoing production of astrocyte-derived molecules contributes to the non-resolving inflammatory and neurotoxic landscape associated with neurodegeneration.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Astrocytes are intriguing and remarkable cells controlling virtually every facet of central nervous system (CNS) functions. Astrocytes work together with neurons, microglia, oligodendrocytes, endothelial cells, and other cells to ensure harmonious function within the unique environment of the CNS. For example, astrocytes form the tripartite synapse where they take up glutamate as well as synthesize and release glutamine for use by neurons for conversion to glutamate, together ensuring proper neurotransmission. Additionally, astrocytes release trophic factors including brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor (GDNF), and others. Astrocyte end-feet interact with the neurovasculature and influence blood–brain barrier (BBB) function. The many diverse functions of astrocytes are too complex and numerous to describe in detail here; however, there are a number of excellent reviews describing the phenotypic and functional characteristics of astrocytes [1–5].
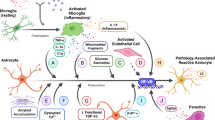
The focus of this chapter is to describe the role of astrocytes in neuroinflammation in the context of neurodegenerative diseases. Microglia are typically thought of as the main innate immunity effector cell in the CNS because of their macrophage-like phenotype, robust inflammatory responses, and ability to present antigen via major histocompatibility complex (MHC) class II. It is now appreciated that astrocytes have important innate immune functions as well [6]. In response to injury, infection, disease, or any disturbance, astrocytes undergo a phenotypic change known as astrogliosis. Widely characterized as the increased expression of the intermediate filament protein glial fibrillary acidic protein (GFAP), astrogliosis involves a host of transcriptional, translational, and phenotypic changes aimed at resolving and limiting damage to the CNS [2]. GFAP is expressed at variable levels in unstimulated astrocytes and forms a fibrous network typical of cytoskeletal proteins (Fig. 1). Additionally, astrocytes express pattern recognition receptors (PRR), although their repertoire is more restricted than that of microglia. Toll-like receptors (TLRs) recognize pathogen-associated molecular patterns and astrocytes express TLR2, TLR3, TLR4, TLR5, and TLR9. TLR3, which recognizes double-stranded RNA, appears to be the most abundant TLR expressed by astrocytes [6]. In addition, astrocytes express nucleotide-binding oligomerization domain (NOD) proteins that recognize bacterial components [7]. Astrocytes can also sense and respond to damage-associated molecular patterns (DAMPs) such as ATP through purinergic receptors and the multi-protein NLRP2 inflammasome [8, 9]. Astrocytes respond to interferons (IFN) and a wide array of cytokines and chemokines. When stimulated, astrocytes in turn produce many cytokines and chemokines including IL-1, IL-6, LIF, CNTF, IL-8, IL-10, IFN-α, IFN-β, M-CSF, GM-CSF, TNF-α, TGF-β, CCL2, CCL3, CCL4, CCL5, CCL20, CXCL10, and CXCL12 [10–12]. In addition, inflammatory stimulation of astrocytes can lead to the production of the free radical nitric oxide (NO) which is toxic to neurons and oligodendrocytes and may promote neurodegeneration [13]. While astrocytes contribute to the local inflammatory response, they are also essential to limit and resolve CNS inflammation. Following traumatic brain injury (TBI), as well as other insults, astrocytes proliferate and form a glial scar around the injury [2]. The selective ablation of proliferating astrocytes following TBI in mice results in a prolonged inflammatory response and increased neuronal degeneration [14]. In acute conditions such as injury or infection, the astrocytic response is paramount to reestablish homeostasis in the CNS. However, in chronic conditions such as neurodegenerative diseases, astrocytes may eventually contribute to pathology.
2 Astrocytes in Multiple Sclerosis
Multiple sclerosis (MS) is a debilitating T cell-mediated autoimmune disease in which leukocytes (T cells, macrophages, neutrophils, and others) invade the CNS, leading to demyelination and axonal degeneration, eventually resulting in permanent disability. The etiology of MS is complex, involving genetic, environmental, and geographic factors, and usually develops in young adults (20–40 years of age) with a bias toward females [15]. MS initially manifests as highly variable transient episodes disrupting sensory and/or motor function, followed by full or partial recovery and disease remission (relapsing-remitting MS). In conjunction with symptoms, inflammatory lesions are also observed in the brain and spinal cord. MS lesions are areas of demyelination and inflammation involving invading peripheral leukocytes as well as resident glial cells. Cytokines and chemokines are key players in this inflammatory attack. Cytokines including IFN-γ, IL-17, and IL-6 are elevated in MS lesions as are the C-C chemokines CCL2, CCL3, CCL4, CCL5, CCL7, and CXCL12 [16–18]. MS patients have multiple attacks causing incremental damage to the CNS, and many patients progress to secondary progressive MS, where remission and recovery are reduced [19, 20]. Additionally, cognitive impairment is observed in at least 50 % of MS patients, contributing to disability and reduced quality of life [21]. Treatments for MS including IFN-β, glatiramer acetate, fingolimod, and others have greatly improved the quality of life for many MS patients; however, not all patients respond to or can tolerate these treatments [22, 23]. As such, new therapeutic targets for the treatment of MS are greatly needed.
The animal model of MS, experimental autoimmune encephalomyelitis (EAE), has greatly facilitated understanding the immunological interactions with the CNS. Although EAE is by no means a perfect replica of human MS, it shares many similar pathological features. EAE can be induced in a number of animals including nonhuman primates, rabbits, guinea pigs, hamsters, rats, and mice with an array of protocols and CNS antigens [24, 25]. Most current research utilizes the murine model. EAE, like MS, is a demyelinating disease involving perivascular infiltration of peripheral immune cells and axonal degeneration, manifesting with physical symptoms in a relapsing-remitting and/or progressive fashion. T helper (Th) cells, specifically IFN-γ-producing Th1 cells and IL-17-producing Th17 cells, are the main effector cells in the initiation of EAE [26].
In postmortem studies of MS lesions, markers of Th1 and Th17 cells have been described, among other cell types [27]. Coincident with infiltration of leukocytes, astrocyte damage and hypertrophy have been observed in MS lesions [28]. Moreover, astrogliosis is present in the CNS of MS patients [1]. These examples highlight an abundance of data that suggest astrocytes are important players in the pathogenesis of MS. This has been supported by studies in EAE. Astrocyte activation, as measured by GFAP expression, correlates with or precedes the onset of clinical symptoms [29–31]. Additionally, there is astrocyte proliferation within the white matter of the spinal cord [32]. Astrocytes in MS and EAE produce the potent leukocyte-attracting chemokines CCL2 [33, 34] and CCL20 [12, 35] among others, and disruption of either of the receptors for these chemokines, CCR2 and CCR6, respectively, results in amelioration of EAE [36, 37]. While astrocytes produce chemoattractants, they also form a barrier around perivascular lesions in EAE to block further leukocyte infiltration into the healthy parenchyma [38]. IL-6 is a multifaceted proinflammatory cytokine that is elevated in the CNS following injury or in diseases including MS [39]. Astrocytes are a major source of endogenous IL-6 in the CNS, and IL-6 drives its own expression through autocrine signaling in conjunction with the soluble IL-6 receptor (trans-signaling) [40, 41]. Transgenic mice expressing IL-6 under the control of the GFAP promoter alters EAE disease such that inflammatory leukocytes invade mainly the cerebellum rather than the spinal cord [42]. Disruption of gp130, the common signal-transducing receptor for the IL-6 family of cytokines, in astrocytes leads to exacerbated EAE, indicating that astrocytes also have a key role in limiting disease [43]. Additionally, the importance of astrocytes in EAE was further established in a recent study which demonstrated that intact IL-17 signaling in astrocytes is required for induction of disease [44]. Moreover, IL-17 enhances IL-6-induced IL-6 and CCL20 expression in astrocytes [45, 46]. This likely reflects the cooperative actions of the IL-6-induced transcription factor STAT3 and the IL-17-induced transcription factor NF-κB [47, 48]. Disruption of NF-κB activity in astrocytes ameliorates CNS inflammation and EAE disease severity [49, 50]. In MS and EAE, Th1 cells and Th17 cells contribute to the pathogenesis of disease. However, IL-4-producing Th2 cells and T regulatory cells (Tregs) are protective in EAE models [51, 52]. Thus, the repertoire of T cells interacting with the CNS is critical to the outcome of disease, and astrocytes influence this through production of chemoattractant molecules. For example, during EAE, astrocytes produce CXCL10 which recruits T cells, the monocyte chemoattractant CCL2, as well as CCL20 that can recruit both Th17 cells and Tregs [34, 35, 53, 54]. Additionally, as nonprofessional antigen-presenting cells, astrocytes, in an IFN-γ-inducible fashion, can express major histocompatibility complex (MHC class II) and present myelin-derived autoantigens to encephalitogenic T cells [55–57], potentially providing a stimulus for reactivation of T cells in the CNS. Collectively, these studies indicate that astrocytes are active participants in MS and EAE pathology and are potential therapeutic targets.
3 Astrocytes in Alzheimer’s Disease
Alzheimer’s disease (AD) is a progressive neurodegenerative disease that robs individuals of their memory and reduces cognitive function. Extracellular amyloid β (Aβ) deposition and tau-containing neurofibrillary tangles (NFTs) are hallmarks of AD pathology [58]. However, beginning with Alois Alzheimer’s initial description of AD more than 100 years ago, alterations in glial cells have also been appreciated [59]. Upon postmortem analysis, brains from patients with AD display clear astrogliosis, and the levels of GFAP inversely correlate with cognitive function [60, 61]. Astrocytes influence several features of AD. Astrocytes are thought to phagocytosis Aβ [62], and blockade of astrocyte activation in a transgenic AD mouse model increases Aβ plaque burden [63]. The exact mechanisms leading to gliosis in AD is not well understood. Fibrillar Aβ1-42 can stimulate pattern recognition receptors, including the lipopolysaccharide (LPS) coreceptor CD14 and the NOD-like receptor NALP3, leading to microglial activation and production of IL-1β [64, 65]. Similarly, astrocytes express TLRs and NLRs which may engage Aβ1-42 and promote astrogliosis, but this has not yet been formally demonstrated. However, several molecules have been implicated in mediating various astrocytic responses to Aβ including low-density lipoprotein receptors, aquaporin-4, adenosine A2A receptor, as well as the scavenger receptors CD36 and CD47 [66–69]. More recently, Aβ was shown to interact with the α7 nicotinic acetylcholine receptor and promote astrocytic glutamate release [70, 71]. While astrogliosis is initially beneficial, the long-term production of cytokines and chemokines may be deleterious and promote AD pathology.
Glial-derived IL-1 and IL-6 are important proinflammatory cytokines elevated in the brain of patients with AD [72, 73]. From animal studies, we have learned that these cytokines may be active participants in AD pathology. Transgenic AD mice (Tg2576) that express an APP mutant associated with early onset familial AD have increased IL-6 in the brain that precedes detectable Aβ plaques [74]. Moreover, IL-6 expression, in the same transgenic mouse model, persists into the established disease state with IL-6-producing astrocytes observed near Aβ deposits [75]. Mice overexpressing IL-6 in astrocytes have learning defects, suggesting that IL-6 may exacerbate cognitive decline [76]. Moreover, IL-1β directs astrocytes to produce IL-6 [77]. As mentioned previously, astrocytes are a potent source of chemokines that likely help to recruit and direct the peripheral monocytes observed in the AD brain [78]. Direct injection of IL-1β into the rat forebrain leads to prolonged astrocyte activation with concomitant increases in GABA and glutamate [79]. Elevated glutamate may be associated with the ability of Aβ1-42 to reduce astrocyte-dependent glutamate clearance [80]. Astrocytes stimulated with IL-1β also secrete S100B [81]. Secreted S100B has cytokine-like functions and at low concentrations is neurotrophic. However, extracellular S100B is elevated in neurological disorders including AD, and at higher concentrations S100B can promote neuronal cell death [82]. Further, antibody-mediated blockade of IL-1β in 3 × Tg-AD mice, which express mutants of APP, presenilin, and tau, reduces S100B expression, tau pathology, and disease pathology [83, 84]. Nitric oxide may also play an important role in AD. Mixed glial cultures respond to Aβ peptides with increased production of IL-1β and TNF-α that leads to increased expression of iNOS and synthesis of nitric oxide [85]. In AD astrocytes appear to be the main source of nitric oxide [86]. Nitric oxide is neurotoxic and may facilitate neurodegeneration in AD [87, 88]. Moreover, stimulation with the microglial- and astrocyte-derived cytokines IL-β, TNF-α, and IFN-γ can also stimulate nitric oxide production with subsequent neurotoxicity from astrocytes [89]. Astrocytes can also modulate microglial function through the production of soluble cytokines and chemokines. Astrocyte-produced S100B can stimulate activation of microglia that includes the production of IL-1β [90], potentially reinforcing or promoting astrogliosis. Additionally, inflammatory cytokines, as well as Aβ fibrils, can also stimulate astrocyte- and neuron-dependent APP expression and Aβ production [91–93]. Ultimately, the interactions between cytokines (particularly IL-1) with neurons, microglia, and astrocytes drive a cycle of inflammation and Aβ production that culminates in neurological dysfunction and cognitive decline [94].
4 Astrocytes in Parkinson’s Disease
Parkinson’s disease (PD) is characterized by the selective loss of dopaminergic neurons in the substantia nigra (SN) and the associated physical manifestations. In addition to dopaminergic neurodegeneration, neuropathology includes the accumulation of α-synuclein-containing Lewy bodies, activated microglia, infiltrating CD4+ and CD8+ T cells, and increased numbers of astrocytes surrounding dopaminergic neurons [95–97]. Elevated levels of cytokines including TNF-α, TGF-β1, IL-1β, IL-6, IL-2, IFN-γ, and reactive oxygen/nitrogen species are also observed in brains from PD patients [98]. These findings (and many others) indicate an ongoing, non-resolving inflammatory reaction in the brain of PD patients.
Several animal models suggest that inflammation is important in the pathogenesis of PD. Mice expressing human α-synuclein driven by the thy1 promoter display activated microglia and elevated TNF-α as early as 1 month of age in the striatum [99]. Importantly, the striatum contains axon terminals emanating from the SN as part of the nigrostriatal pathway. These findings support the idea that inflammation maybe a key participant in neurodegeneration and not just a consequence of tissue damage [99]. In toxin-induced models, including MPTP and 6-OHDA, inflammatory cytokines and activated microglia are present [100]. MPTP intoxication leads to prolonged (years) glial activation, suggesting glial cells are involved in the pathological outcome. Direct injection of LPS is toxic to dopaminergic neurons [101], indicating that inflammation, even in the absence of disease, can recapitulate the cell death seen in PD. Additionally, LPS can synergize with MPTP to induce dopaminergic neuronal cell death in neuron-glia cocultures [102]. LPS-induced neuronal death is likely indirect. In support of this are in vitro studies demonstrating that microglia and astrocytes work in concert to drive neurotoxicity in response to LPS [103].
Astrocyte accumulation of α-synuclein is observed in the PD brain [104], and recent findings suggest that α-synuclein can be transmitted from neurons to surrounding cells [105]. Indeed, astrocytes can take up α-synuclein via endocytosis. Not only do astrocytes take up α-synuclein, but an inflammatory reaction is stimulated that includes production of IL-6 and TNF-α as well as chemokines and matrix metalloproteinases (MMPs) [105]. Moreover, transgenic mice expressing a mutant α-synuclein associated with familial PD, A53T α-synuclein, in astrocytes display paralysis and mortality. This is associated with widespread gliosis and increased expression of TNF-α, IL-1β, and IL-6 in the brainstem. Conditioned media from the A53T α-synuclein-expressing astrocytes stimulated IL-1β and Cox1 expression in microglia [106]. These findings suggest that the effects of α-synuclein on astrocytes may contribute to the pathology of PD.
Astrocytes can have both protective and neurotoxic effects. Alpha-synuclein can enhance IL-1β-induced CXCL10 expression in astroglial cultures through mRNA stabilization [107]. CXCL10 is toxic to neurons; this has been demonstrated in the cholinergic LAN-2 cell line and in mixed human fetal neurons [108, 109]. While the dark pigment found in the SN, neuromelanin, attenuates astrocyte-derived CXCL10 [107], the direct influence of CXCL10 on dopaminergic neurons has not been examined. Astrocyte expression of the antioxidant transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) in the Thy1-hSYNA53T mice protects motor neurons, reduces synuclein aggregates in the brain and spinal cord, and enhances overall survival. Additionally, Nrf2 expression reduces gliosis [110].
The exact mechanisms responsible for activation of the glial reaction in PD are unknown. Gliosis is observed in PD patients and most animal models and once active a self-perpetuating inflammatory reaction may result. In adult macaques injected with MPTP, persistent astrogliosis is observed as well as elevated IFN-γ and TNF-α in the SN. Mice lacking either IFN-γ or TNF-α have attenuated gliosis following MPTP treatment [111].
Some mutations associated with familial forms of PD have been shown to alter inflammatory responses. Cortical slices from PINK1−/− mice have increased production of TNF-α, IL-1β, and IL-6 [112]. The cytokines produced suggest activation of microglia and astrocytes, although it should be noted that PINK−/− astrocytes are dysfunctional in their proliferative capacity and do not have elevated GFAP expression [113]. Astrocytes deficient for DJ-1 are more sensitive to LPS-induced inflammatory gene expression [114]. Mutations in Nurr1 are associated with a rare form of PD. Nurr1 suppresses inflammation in microglia and astrocytes through repression of NF-κB, and loss of Nurr1 enhances astrocyte-derived neurotoxic molecules [103]. This indicates that astrocytes are involved in both sporadic and familial PD.
5 Astrocytes in Huntington’s Disease
In contrast to most neurodegenerative diseases in which the etiology is unknown, we know that Huntington’s disease (HD) is caused by a CAG expansion (poly Q) in the huntingtin gene, leading to neuronal loss in the striatum and cortex [115, 116]. As with other neurodegenerative diseases, inflammation is likely a key player in HD. Immune activation is detectable in the periphery and CNS of HD patients. Elevated plasma levels of IL-6, IL-8, IL-4, IL-10, TNF-α, and IL-5 have been shown in HD patients compared to healthy controls. Similarly, IL-6 and IL-8 levels are elevated in the CSF and in striatal tissue [117]. In addition to elevated cytokines, cellular alterations including microglial activation and astrogliosis are present in HD [118].
The role of astrocytes in HD is multifaceted, involving the production of inflammatory mediators as well as the potential loss of neuronal support. The expression of mutant huntingtin is not restricted to neurons; it is also expressed in astrocytes and peripherally. The targeted expression of mutant htt (160Q) in astrocytes leads to neurological and motor dysfunction [119], suggesting that astrocytes can directly influence HD pathology. A key function of astrocytes is to support neurons through the secretion of neurotrophins and buffering of extracellular glutamate. Evidence from HD mouse models suggests that astrocyte dysfunction may be an important aspect of the disease. Astrocytes produce BDNF, and this was found to be impaired in astrocytes expressing a mutant huntingtin fragment (htt552-100Q) [120]. Others have shown that astrocyte-produced BDNF provides therapeutic benefit. Astrocyte-targeted overexpression of BDNF attenuated quinolinate-induced lesions [121]. Additionally, viral delivery of BDNF driven by the GFAP promoter delayed disease progression in the R6/2 HD mouse model which expresses the 5′end of human huntingtin with 115-150 CAG repeats [122, 123]. Similarly, delivery of GDNF protects neurons and reduces disease severity [124, 125]. In vitro, astrocyte-conditioned media protect a striatal neuronal cell line expressing huntingtin Q111 from oxidative and excitotoxic cell death [126].
Evidence suggests that excitotoxic injury is an underlying mechanism of striatal neuronal loss in HD [127]. The uptake of glutamate, the main excitatory neurotransmitter, is impaired in the prefrontal cortex of HD patients [128]. Expression of mutant huntingtin in astrocytes reduces glutamate transporter expression and impairs the ability of astrocytes to take up glutamate. Moreover, in a coculture system, mutant htt-expressing astrocytes were less efficient at protecting neurons from glutamate-induced excitotoxicity [129]. In an in vivo model in which striatal astrocytes express mutant htt, reduced expression of glutamate transporters GLAST and GLT-1 as well as impaired glutamate uptake was observed. In addition, these mice displayed astrogliosis and neuronal dysfunction [130]. The ability of mutant htt to impair astrocyte-dependent glutamate handling may potentiate neuronal death in HD.
Despite evidence of microglial, astrocytic, and complement activation in the brains of HD patients, few studies have examined the contribution of glial cells to inflammation in HD [131]. In line with previous studies that mutant htt impairs astrocyte function, astrocytes from R6/2 mice express and secrete less CCL5 (RANTES). Impaired secretion results in aberrant accumulation of CCL5 in astrocytes and is observed in HD mouse models and in HD patients [132]. CCL5 has neurotrophic effects and its reduction may contribute to HD pathogenesis [132]. A recent study has examined the inflammatory responses in HD mice and astrocytes. In Hdh150Q mice, acute LPS treatment leads to enhanced TNF-α and IL-1β production in the cortex, striatum, and periphery [133]. Not only is the initial inflammatory reaction greater in the mutant htt mice, it is also prolonged. The enhanced inflammation was associated with excessive NF-κB activation in astrocytes. A single injection of LPS resulted in chronic inflammation and accelerated disease in the R6/2 mice. In addition, isolated R6/2 astrocytes stimulated with LPS produced higher levels of nitric oxide and were more toxic to isolated neurons [133].
6 Astrocytes in ALS
Amyotrophic lateral sclerosis (ALS) is caused by the selective degeneration of motor neurons resulting in progressive paralysis and premature death. In most cases, ALS is sporadic with unknown etiology. In a small number of cases, ALS is caused by mutations in the gene encoding superoxide dismutase 1 (SOD1). Through the use of SOD1 mutant mice, the mechanisms and cells involved in pathogenesis have been examined, and the non-cell autonomous processes involved in ALS have gained attention. Collectively, it appears that disease onset is determined by motor neurons, most likely through mutant SOD1-dependent damage; however, other cells including microglia and astrocytes are important in overall disease progression [134]. Consistently, ALS patients display activated microglia and astrocytes and increased expression of proinflammatory cytokines [135].
Glial activation is observed in the postmortem analysis of patients with ALS and in ALS animal models. It is likely that astrocytes and microglia work together, along with other cells types such as peripheral leukocytes, to modulate disease pathology. Using a SOD1G37R mouse model in which mutant SOD1 could be deleted from astrocytes, Yamanaka and colleagues demonstrated that disease onset was unaffected, but disease progression was greatly attenuated [136]. Although astrogliosis, based on GFAP expression, was not reduced by astrocyte-selective ablation of SOD1G37R, microgliosis was diminished. Concomitant with reduced microglial activation was a reduction in the expression of iNOS. These studies indicate that mutant SOD1-expressing astrocytes can influence disease progression in part through modulation of microglia [136]. Expression of SOD1G37R in astrocytes elicits an inflammatory response and toxicity toward motor neurons in coculture. This includes elevated expression of iNOS and NOX2 with increased production of nitric oxide and reactive oxygen, respectively. The antioxidant apocynin attenuated astrocyte-produced ROS and motor neuron toxicity [137]. Accordingly, motor neurons are sensitive to NO-induced cell death, most likely through reaction with superoxide to form highly reactive peroxynitrite [138]. Additionally, astrocytes derived from both familial and sporadic ALS patients are toxic to motor neurons. This toxicity was associated with upregulation of a number of astrocyte-produced inflammatory molecules including several C-C and C-X-C chemokines, TNF and IL-8 [139]. Similarly, mutant SOD1-expressing mouse astrocytes are toxic to primary motor neurons in coculture [140]. Expression of SOD1G93A alters inflammatory gene expression in astrocytes leading to upregulation of CCL8, CXCL7, and CCL5 [141]. In addition the prostaglandin D2 (PGD2) receptor was markedly increased. While these chemokines do not mediate the astrocyte-dependent toxicity toward motor neurons, blockade of the PGD2 receptor attenuated cell death, suggesting that prostaglandins may have role in motor neuron death [141].
IFN-γ has also been implicated in the demise of motor neurons. SOD1G93A-expressing astrocytes produce IFN-γ, and antibody-mediated neutralization of IFN-γ blocks astrocyte-dependent toxicity toward motor neurons in this model [142]. The toxic effects of IFN-γ are mediated in part through stimulation of the TNF family member, LIGHT (TNFSF14), from motor neurons which binds the lymphotoxin-β receptor (LT-βR) in an autocrine fashion, activating a pro-death signaling cascade. Consistent with a role for astrocytes in driving disease progression, deletion of LIGHT delays disease progress but not onset [142]. Type I IFNs may also have a role through stimulation of interferon-stimulated genes (ISGs) in astrocytes. ISG15 was reported to be elevated in human ALS and mouse spinal cords. Deletion of IFNAR1 delayed disease progress but not onset in SOD1G93A mice [143]. Thus, astrocyte-dependent production and responses to IFNs may have important roles in the progression of ALS.
7 Conclusions
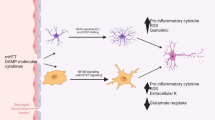
Astrocytes have a key role in controlling inflammatory responses in the CNS (Fig. 2). Here, we have focused on astrocytes in only the most prevalent neurodegenerative diseases. It is worth noting that activated astrocytes and increased inflammatory cytokines are observed in many other neurodegenerative diseases including prion diseases [144] and lysosomal storage diseases [145]. While astrocytes have numerous beneficial functions [1–4, 6, 146–148], it seems that long-term perpetual stimulation, as likely occurs in neurodegenerative diseases, may exacerbate disease. Thus, we must continue to define the physiological and pathological functions of astrocytes as they may hold the key to new therapies.
Astrocytes orchestrate CNS inflammation. In neurodegenerative diseases, astrocytes respond to soluble factors including protein/peptide oligomers produced by neurons and inflammatory cytokines and chemokines produced by endogenous microglia and invading peripheral leukocytes. In response, astrocytes activate transcription factors such as NF-κB and STATs that leads to the production of a plethora of molecules which dictate the behavior and/or recruitment of the surrounding cells. The astrocyte-directed response may be beneficial through release of anti-inflammatory mediators and growth factors, or it may promote neurodegeneration through production of ROS and proinflammatory mediators
Abbreviations
- 6-OHDA:
-
6-hydroxydopamine
- AD:
-
Alzheimer’s disease
- ALS:
-
Amyotrophic lateral sclerosis
- Aβ:
-
Amyloid β
- BBB:
-
Blood–brain barrier
- BDNF:
-
Brain-derived neurotrophic factor
- CNS:
-
Central nervous system
- DAMP:
-
Damage-associated molecular pattern
- GDNF:
-
Glial-derived neurotrophic factor
- GFAP:
-
Glial fibrillary acidic protein
- HD:
-
Huntington’s disease
- IFN:
-
Interferon
- ISG:
-
Interferon-stimulated genes
- MHC:
-
Major histocompatibility complex
- MMP:
-
Matrix metalloproteinase
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MS:
-
Multiple sclerosis
- NFT:
-
Neurofibrillary tangles
- NF-κB:
-
Nuclear factor-kappa B
- NOD:
-
Nucleotide-binding oligomerization domain
- PGD2:
-
Prostaglandin D2
- PRR:
-
Pattern recognition receptor
- STAT:
-
Signal transducer and activator of transcription
- SN:
-
Substantia nigra
- SOD1:
-
Superoxide dismutase 1
- TBI:
-
Traumatic brain injury
- Th:
-
T helper
- TLR:
-
Toll-like receptor
References
Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. PubMed PMID: 20012068, Pubmed Central PMCID: 2799634.
Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32(12):638–47. PubMed PMID: 19782411, Pubmed Central PMCID: 2787735.
Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22(5):208–15. PubMed PMID: 10322493.
Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, et al. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55(12):1251–62. PubMed PMID: 17659524.
Sofroniew MV. Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist. 2014;20(2):160–72. PubMed PMID: 24106265, Pubmed Central PMCID: 24106265.
Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28(3):138–45. PubMed PMID: 17276138.
Sterka Jr D, Rati DM, Marriott I. Functional expression of NOD2, a novel pattern recognition receptor for bacterial motifs, in primary murine astrocytes. Glia. 2006;53(3):322–30. PubMed PMID: 16265673.
Minkiewicz J, de Rivero Vaccari JP, Keane RW. Human astrocytes express a novel NLRP2 inflammasome. Glia. 2013;61(7):1113–21. PubMed PMID: 23625868.
Franke H, Verkhratsky A, Burnstock G, Illes P. Pathophysiology of astroglial purinergic signalling. Purinergic Signal. 2012;8(3):629–57. PubMed PMID: 22544529, Pubmed Central PMCID: 22544529.
Oh JW, Schwiebert LM, Benveniste EN. Cytokine regulation of CC and CXC chemokine expression by human astrocytes. J Neurovirol. 1999;5(1):82–94. PubMed PMID: 10190694.
Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36(2):180–90. PubMed PMID: 11596126, Pubmed Central PMCID: 11596126.
Ambrosini E, Remoli ME, Giacomini E, Rosicarelli B, Serafini B, Lande R, et al. Astrocytes produce dendritic cell-attracting chemokines in vitro and in multiple sclerosis lesions. J Neuropathol Exp Neurol. 2005;64(8):706–15. PubMed PMID: 16106219.
Brown GC. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem Soc Trans. 2007;35(Pt 5):1119–21. PubMed PMID: 17956292, Pubmed Central PMCID: 17956292.
Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23(2):297–308. PubMed PMID: 10399936.
Hauser SL, Oksenberg JR. The neurobiology of multiple sclerosis: genes, inflammation, and neurodegeneration. Neuron. 2006;52(1):61–76. PubMed PMID: 17015227, Pubmed Central PMCID: 17015227.
Steinman L. Nuanced roles of cytokines in three major human brain disorders. J Clin Invest. 2008;118(11):3557–63. PubMed PMID: 18982162, Pubmed Central PMCID: 2575716.
Ubogu EE, Cossoy MB, Ransohoff RM. The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol Sci. 2006;27(1):48–55. PubMed PMID: 16310865, Pubmed Central PMCID: 16310865.
Holman DW, Klein RS, Ransohoff RM. The blood-brain barrier, chemokines and multiple sclerosis. Biochim Biophys Acta. 2011;1812(2):220–30. PubMed PMID: 20692338, Pubmed Central PMCID: 20692338.
Steinman L. Multiple sclerosis: a two-stage disease. Nat Immunol. 2001;2(9):762–4. PubMed PMID: 11526378.
Frohman EM, Racke MK, Raine CS. Multiple sclerosis – the plaque and its pathogenesis. N Engl J Med. 2006;354(9):942–55. PubMed PMID: 16510748.
Amato MP, Portaccio E, Goretti B, Zipoli V, Hakiki B, Giannini M, et al. Cognitive impairment in early stages of multiple sclerosis. Neurol Sci. 2010;31 Suppl 2:S211–4. PubMed PMID: 20640466.
Miller AE, Rhoades RW. Treatment of relapsing-remitting multiple sclerosis: current approaches and unmet needs. Curr Opin Neurol. 2012;25(Suppl):S4–10. PubMed PMID: 22398662, Pubmed Central PMCID: 22398662.
Gasperini C, Ruggieri S. Development of oral agent in the treatment of multiple sclerosis: how the first available oral therapy, fingolimod will change therapeutic paradigm approach. Drug Des Devel Ther. 2012;6:175–86. PubMed PMID: 22888218, Pubmed Central PMCID: 22888218.
Baxter AG. The origin and application of experimental autoimmune encephalomyelitis. Nat Rev Immunol. 2007;7(11):904–12. PubMed PMID: 17917672.
Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1(4):1810–9. PubMed PMID: 17487163.
Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162(1):1–11. PubMed PMID: 20682002, Pubmed Central PMCID: 2990924.
Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8(5):500–8. PubMed PMID: 11984595.
Brosnan CF, Raine CS. The astrocyte in multiple sclerosis revisited. Glia. 2013;61(4):453–65. PubMed PMID: 23322421, Pubmed Central PMCID: 23322421.
Aquino DA, Shafit-Zagardo B, Brosnan CF, Norton WT. Expression of glial fibrillary acidic protein and neurofilament mRNA in gliosis induced by experimental autoimmune encephalomyelitis. J Neurochem. 1990;54(4):1398–404. PubMed PMID: 1690269, Pubmed Central PMCID: 1690269.
Tani M, Glabinski AR, Tuohy VK, Stoler MH, Estes ML, Ransohoff RM. In situ hybridization analysis of glial fibrillary acidic protein mRNA reveals evidence of biphasic astrocyte activation during acute experimental autoimmune encephalomyelitis. Am J Pathol. 1996;148(3):889–96. PubMed PMID: 8774143, Pubmed Central PMCID: 8774143.
Luo J, Ho P, Steinman L, Wyss-Coray T. Bioluminescence in vivo imaging of autoimmune encephalomyelitis predicts disease. J Neuroinflammation. 2008;5:6. PubMed PMID: 18237444, Pubmed Central PMCID: 18237444.
Guo F, Maeda Y, Ma J, Delgado M, Sohn J, Miers L, et al. Macroglial plasticity and the origins of reactive astroglia in experimental autoimmune encephalomyelitis. J Neurosci. 2011;31(33):11914–28. PubMed PMID: 21849552, Pubmed Central PMCID: 21849552.
Van Der Voorn P, Tekstra J, Beelen RH, Tensen CP, Van Der Valk P, De Groot CJ. Expression of MCP-1 by reactive astrocytes in demyelinating multiple sclerosis lesions. Am J Pathol. 1999;154(1):45–51. PubMed PMID: 9916917.
Ransohoff RM, Hamilton TA, Tani M, Stoler MH, Shick HE, Major JA, et al. Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB J. 1993;7(6):592–600. PubMed PMID: 8472896.
Ambrosini E, Columba-Cabezas S, Serafini B, Muscella A, Aloisi F. Astrocytes are the major intracerebral source of macrophage inflammatory protein-3alpha/CCL20 in relapsing experimental autoimmune encephalomyelitis and in vitro. Glia. 2003;41(3):290–300. PubMed PMID: 12528183.
Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J Exp Med. 2000;192(7):1075–80. PubMed PMID: 11015448, Pubmed Central PMCID: PMC2193310.
Liston A, Kohler RE, Townley S, Haylock-Jacobs S, Comerford I, Caon AC, et al. Inhibition of CCR6 function reduces the severity of experimental autoimmune encephalomyelitis via effects on the priming phase of the immune response. J Immunol. 2009;182(5):3121–30. PubMed PMID: 19234209.
Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LBJ, Tiwari-Woodruff S, et al. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci. 2009;29(37):11511–22. PubMed PMID: 19759299, Pubmed Central PMCID: 19759299.
Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8(9):1254–66. PubMed PMID: 23136554, Pubmed Central PMCID: 23136554.
Van Wagoner NJ, Oh JW, Repovic P, Benveniste EN. Interleukin-6 (IL-6) production by astrocytes: autocrine regulation by IL-6 and the soluble IL-6 receptor. J Neurosci. 1999;19(13):5236–44. PubMed PMID: 10377335.
Mackiewicz A, Schooltink H, Heinrich PC, Rose-John S. Complex of soluble human IL-6-receptor/IL-6 up-regulates expression of acute-phase proteins. J Immunol. 1992;149(6):2021–7. PubMed PMID: 1381393.
Quintana A, Müller M, Frausto RF, Ramos R, Getts DR, Sanz E, et al. Site-specific production of IL-6 in the central nervous system retargets and enhances the inflammatory response in experimental autoimmune encephalomyelitis. J Immunol. 2009;183(3):2079–88. PubMed PMID: 19597000.
Haroon F, Drogemuller K, Handel U, Brunn A, Reinhold D, Nishanth G, et al. Gp130-dependent astrocytic survival is critical for the control of autoimmune central nervous system inflammation. J Immunol. 2011;186(11):6521–31. PubMed PMID: 21515788.
Kang Z, Altuntas CZ, Gulen MF, Liu C, Giltiay N, Qin H, et al. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity. 2010;32(3):414–25. PubMed PMID: 20303295.
Ma X, Reynolds SL, Baker BJ, Li X, Benveniste EN, Qin H. IL-17 enhancement of the IL-6 signaling cascade in astrocytes. J Immunol. 2010;184(9):4898–906. PubMed PMID: 20351184.
Meares GP, Ma X, Qin H, Benveniste EN. Regulation of CCL20 expression in astrocytes by IL-6 and IL-17. Glia. 2012;60(5):771–81. PubMed PMID: 22319003, Pubmed Central PMCID: 22319003.
Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9(8):556–67. PubMed PMID: 19575028.
Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21(1):11–9. PubMed PMID: 20018552, Pubmed Central PMCID: 20018552.
Brambilla R, Persaud T, Hu X, Karmally S, Shestopalov VI, Dvoriantchikova G, et al. Transgenic inhibition of astroglial NF-kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J Immunol. 2009;182(5):2628–40. PubMed PMID: 19234157.
van Loo G, De Lorenzi R, Schmidt H, Huth M, Mildner A, Schmidt-Supprian M, et al. Inhibition of transcription factor NF-kappaB in the central nervous system ameliorates autoimmune encephalomyelitis in mice. Nat Immunol. 2006;7(9):954–61. PubMed PMID: 16892069.
Chitnis T, Khoury SJ. Cytokine shifts and tolerance in experimental autoimmune encephalomyelitis. Immunol Res. 2003;28(3):223–39. PubMed PMID: 14713716.
Zozulya AL, Wiendl H. The role of regulatory T cells in multiple sclerosis. Nat Clin Pract Neurol. 2008;4(7):384–98. PubMed PMID: 18578001.
Carter SL, Müller M, Manders PM, Campbell IL. Induction of the genes for Cxcl9 and Cxcl10 is dependent on IFN-gamma but shows differential cellular expression in experimental autoimmune encephalomyelitis and by astrocytes and microglia in vitro. Glia. 2007;55(16):1728–39. PubMed PMID: 17902170, Pubmed Central PMCID: 17902170.
Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B, et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol. 2008;181(12):8391–401. PubMed PMID: 19050256.
Fontana A, Fierz W, Wekerle H. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature. 1984;307(5948):273–6. PubMed PMID: 6198590.
Soos JM, Morrow J, Ashley TA, Szente BE, Bikoff EK, Zamvil SS. Astrocytes express elements of the class II endocytic pathway and process central nervous system autoantigen for presentation to encephalitogenic T cells. J Immunol. 1998;161(11):5959–66. PubMed PMID: 9834077.
Tan L, Gordon KB, Mueller JP, Matis LA, Miller SD. Presentation of proteolipid protein epitopes and B7-1-dependent activation of encephalitogenic T cells by IFN-gamma-activated SJL/J astrocytes. J Immunol. 1998;160(9):4271–9. PubMed PMID: 9574529.
Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314(5800):777–81. PubMed PMID: 17082447, Pubmed Central PMCID: 17082447.
Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin Anat. 1995;8(6):429–31. PubMed PMID: 8713166.
Kashon ML, Ross GW, O’Callaghan JP, Miller DB, Petrovitch H, Burchfiel CM, et al. Associations of cortical astrogliosis with cognitive performance and dementia status. J Alzheimers Dis. 2004;6(6):595–604. discussion 73–81, PubMed PMID: 15665400.
Simpson JE, Ince PG, Lace G, Forster G, Shaw PJ, Matthews F, et al. Astrocyte phenotype in relation to Alzheimer-type pathology in the ageing brain. Neurobiol Aging. 2010;31(4):578–90. PubMed PMID: 18586353.
Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, et al. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003;9(4):453–7. PubMed PMID: 12612547.
Kraft AW, Hu X, Yoon H, Yan P, Xiao Q, Wang Y, et al. Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice. FASEB J. 2013;27(1):187–98. PubMed PMID: 23038755, Pubmed Central PMCID: 3528309.
Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9(8):857–65. PubMed PMID: 18604209, Pubmed Central PMCID: 3101478.
Fassbender K, Walter S, Kühl S, Landmann R, Ishii K, Bertsch T, et al. The LPS receptor (CD14) links innate immunity with Alzheimer’s disease. FASEB J. 2004;18(1):203–5. PubMed PMID: 14597556, Pubmed Central PMCID: 14597556.
Basak JM, Verghese PB, Yoon H, Kim J, Holtzman DM. Low-density lipoprotein receptor represents an apolipoprotein E-independent pathway of Aβ uptake and degradation by astrocytes. J Biol Chem. 2012;287(17):13959–71. PubMed PMID: 22383525, Pubmed Central PMCID: 22383525.
Yang W, Wu Q, Yuan C, Gao J, Xiao M, Gu M, et al. Aquaporin-4 mediates astrocyte response to β-amyloid. Mol Cell Neurosci. 2012;49(4):406–14. PubMed PMID: 22365952, Pubmed Central PMCID: 22365952.
Matos M, Augusto E, Machado NJ, dos Santos-Rodrigues A, Cunha RA, Agostinho P. Astrocytic adenosine A2A receptors control the amyloid-β peptide-induced decrease of glutamate uptake. J Alzheimers Dis. 2012;31(3):555–67. PubMed PMID: 22647260, Pubmed Central PMCID: 22647260.
Jones RS, Minogue AM, Connor TJ, Lynch MA. Amyloid-β-induced astrocytic phagocytosis is mediated by CD36, CD47 and RAGE. J Neuroimmune Pharmacol. 2013;8(1):301–11. PubMed PMID: 23238794, Pubmed Central PMCID: 23238794.
Talantova M, Sanz-Blasco S, Zhang X, Xia P, Akhtar MW, Okamoto S-I, et al. Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci U S A. 2013;110(27):E2518. PubMed PMID: 23776240, Pubmed Central PMCID: 23776240.
Wang HY, Lee DH, Davis CB, Shank RP. Amyloid peptide Abeta(1-42) binds selectively and with picomolar affinity to alpha7 nicotinic acetylcholine receptors. J Neurochem. 2000;75(3):1155–61. PubMed PMID: 10936198, Pubmed Central PMCID: 10936198.
Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86(19):7611–5. PubMed PMID: 2529544, Pubmed Central PMCID: 2529544.
Strauss S, Bauer J, Ganter U, Jonas U, Berger M, Volk B. Detection of interleukin-6 and alpha 2-macroglobulin immunoreactivity in cortex and hippocampus of Alzheimer’s disease patients. Lab Invest. 1992;66(2):223–30. PubMed PMID: 1370967, Pubmed Central PMCID: 1370967.
Tehranian R, Hasanvan H, Iverfeldt K, Post C, Schultzberg M. Early induction of interleukin-6 mRNA in the hippocampus and cortex of APPsw transgenic mice Tg2576. Neurosci Lett. 2001;301(1):54–8. PubMed PMID: 11239715.
Benzing WC, Wujek JR, Ward EK, Shaffer D, Ashe KH, Younkin SG, et al. Evidence for glial-mediated inflammation in aged APP(SW) transgenic mice. Neurobiol Aging. 1999;20(6):581–9. PubMed PMID: 10674423.
Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc Natl Acad Sci U S A. 1997;94(4):1500–5. PubMed PMID: 9037082, Pubmed Central PMCID: 19820.
Benveniste EN, Sparacio SM, Norris JG, Grenett HE, Fuller GM. Induction and regulation of interleukin-6 gene expression in rat astrocytes. J Neuroimmunol. 1990;30(2–3):201–12. PubMed PMID: 2121800.
Rezai-Zadeh K, Gate D, Town T. CNS infiltration of peripheral immune cells: D-Day for neurodegenerative disease? J Neuroimmune Pharmacol. 2009;4(4):462–75. PubMed PMID: 19669892, Pubmed Central PMCID: 2773117.
Casamenti F, Prosperi C, Scali C, Giovannelli L, Colivicchi MA, Faussone-Pellegrini MS, et al. Interleukin-1beta activates forebrain glial cells and increases nitric oxide production and cortical glutamate and GABA release in vivo: implications for Alzheimer’s disease. Neuroscience. 1999;91(3):831–42. PubMed PMID: 10391466.
Scimemi A, Meabon JS, Woltjer RL, Sullivan JM, Diamond JS, Cook DG. Amyloid-beta1-42 slows clearance of synaptically released glutamate by mislocalizing astrocytic GLT-1. J Neurosci. 2013;33(12):5312–8. PubMed PMID: 23516295.
de Souza DF, Leite MC, Quincozes-Santos A, Nardin P, Tortorelli LS, Rigo MM, et al. S100B secretion is stimulated by IL-1beta in glial cultures and hippocampal slices of rats: likely involvement of MAPK pathway. J Neuroimmunol. 2009;206(1–2):52–7. PubMed PMID: 19042033.
Sen J, Belli A. S100B in neuropathologic states: the CRP of the brain? J Neurosci Res. 2007;85(7):1373–80. PubMed PMID: 17348038, Pubmed Central PMCID: 17348038.
Kitazawa M, Cheng D, Tsukamoto MR, Koike MA, Wes PD, Vasilevko V, et al. Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal beta-catenin pathway function in an Alzheimer’s disease model. J Immunol. 2011;187(12):6539–49. PubMed PMID: 22095718.
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39(3):409–21. PubMed PMID: 12895417, Pubmed Central PMCID: 12895417.
Akama KT, Van Eldik LJ. Beta-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1 beta- and tumor necrosis factor-alpha (TNF alpha)-dependent, and involves a TNF alpha receptor-associated factor- and NF kappa B-inducing kinase-dependent signaling mechanism. J Biol Chem. 2000;275(11):7918–24. PubMed PMID: WOS:000085913300068.
Meda L, Baron P, Scarlato G. Glial activation in Alzheimer’s disease: the role of Abeta and its associated proteins. Neurobiol Aging. 2001;22(6):885–93. PubMed PMID: 11754995.
Steinert JR, Chernova T, Forsythe ID. Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist. 2010;16(4):435–52. PubMed PMID: 20817920.
Abramov AY, Canevari L, Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci. 2004;24(2):565–75. PubMed PMID: 14724257.
Brown GC, Bal-Price A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol Neurobiol. 2003;27(3):325–55. PubMed PMID: 12845153.
Liu L, Li Y, Van Eldik LJ, Griffin WST, Barger SW. S100B-induced microglial and neuronal IL-1 expression is mediated by cell type-specific transcription factors. J Neurochem. 2005;92(3):546–53. PubMed PMID: 15659225, Pubmed Central PMCID: 15659225.
Brugg B, Dubreuil YL, Huber G, Wollman EE, Delhaye-Bouchaud N, Mariani J. Inflammatory processes induce beta-amyloid precursor protein changes in mouse brain. Proc Natl Acad Sci U S A. 1995;92(7):3032–5. PubMed PMID: 7708769, Pubmed Central PMCID: 7708769.
Blasko I, Veerhuis R, Stampfer-Kountchev M, Saurwein-Teissl M, Eikelenboom P, Grubeck-Loebenstein B. Costimulatory effects of interferon-gamma and interleukin-1beta or tumor necrosis factor alpha on the synthesis of Abeta1-40 and Abeta1-42 by human astrocytes. Neurobiol Dis. 2000;7(6 Pt B):682–9. PubMed PMID: 11114266, Pubmed Central PMCID: 11114266.
Zhao J, O’Connor T, Vassar R. The contribution of activated astrocytes to Aβ production: implications for Alzheimer’s disease pathogenesis. J Neuroinflammation. 2011;8:150. PubMed PMID: 22047170, Pubmed Central PMCID: 22047170.
Griffin WS, Sheng JG, Royston MC, Gentleman SM, McKenzie JE, Graham DI, et al. Glial-neuronal interactions in Alzheimer’s disease: the potential role of a ‘cytokine cycle’ in disease progression. Brain Pathol. 1998;8(1):65–72. PubMed PMID: 9458167, Pubmed Central PMCID: 9458167.
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. PubMed PMID: 12498954.
Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119(1):182–92. PubMed PMID: 19104149, Pubmed Central PMCID: 2613467.
Damier P, Hirsch EC, Zhang P, Agid Y, Javoy-Agid F. Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience. 1993;52(1):1–6. PubMed PMID: 8433802.
Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8(4):382–97. PubMed PMID: 19296921.
Watson MB, Richter F, Lee SK, Gabby L, Wu J, Masliah E, et al. Regionally-specific microglial activation in young mice over-expressing human wildtype alpha-synuclein. Exp Neurol. 2012;237(2):318–34. PubMed PMID: 22750327, Pubmed Central PMCID: 3443323.
Barnum CJ, Tansey MG. Modeling neuroinflammatory pathogenesis of Parkinson’s disease. Prog Brain Res. 2010;184:113–32. PubMed PMID: 20887872.
Herrera AJ, Castaño A, Venero JL, Cano J, Machado A. The single intranigral injection of LPS as a new model for studying the selective effects of inflammatory reactions on dopaminergic system. Neurobiol Dis. 2000;7(4):429–47. PubMed PMID: 10964613, Pubmed Central PMCID: 10964613.
Gao H-M, Liu B, Zhang W, Hong J-S. Synergistic dopaminergic neurotoxicity of MPTP and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson’s disease. FASEB J. 2003;17(13):1957–9. PubMed PMID: 12923073, Pubmed Central PMCID: 12923073.
Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, et al. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137(1):47–59. PubMed PMID: 19345186, Pubmed Central PMCID: 2754279.
Braak H, Sastre M, Del Tredici K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol. 2007;114(3):231–41. PubMed PMID: 17576580.
Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, et al. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285(12):9262–72. PubMed PMID: 20071342, Pubmed Central PMCID: 2838344.
Gu XL, Long CX, Sun L, Xie C, Lin X, Cai H. Astrocytic expression of Parkinson’s disease-related A53T alpha-synuclein causes neurodegeneration in mice. Mol Brain. 2010;3:12. PubMed PMID: 20409326, Pubmed Central PMCID: 2873589.
Tousi NS, Buck DJ, Curtis JT, Davis RL. alpha-Synuclein potentiates interleukin-1beta-induced CXCL10 expression in human A172 astrocytoma cells. Neurosci Lett. 2012;507(2):133–6. PubMed PMID: 22178859, Pubmed Central PMCID: 3259703.
van Marle G, Henry S, Todoruk T, Sullivan A, Silva C, Rourke SB, et al. Human immunodeficiency virus type 1 Nef protein mediates neural cell death: a neurotoxic role for IP-10. Virology. 2004;329(2):302–18. PubMed PMID: 15518810.
Mehla R, Bivalkar-Mehla S, Nagarkatti M, Chauhan A. Programming of neurotoxic cofactor CXCL-10 in HIV-1-associated dementia: abrogation of CXCL-10-induced neuro-glial toxicity in vitro by PKC activator. J Neuroinflammation. 2012;9:239. PubMed PMID: 23078780, Pubmed Central PMCID: 3533742.
Gan L, Vargas MR, Johnson DA, Johnson JA. Astrocyte-specific overexpression of Nrf2 delays motor pathology and synuclein aggregation throughout the CNS in the alpha-synuclein mutant (A53T) mouse model. J Neurosci. 2012;32(49):17775–87. PubMed PMID: 23223297, Pubmed Central PMCID: 3539799.
Barcia C, Ros CM, Annese V, Gomez A, Ros-Bernal F, Aguado-Yera D, et al. IFN-gamma signaling, with the synergistic contribution of TNF-alpha, mediates cell specific microglial and astroglial activation in experimental models of Parkinson’s disease. Cell Death Dis. 2011;2:e142. PubMed PMID: 21472005, Pubmed Central PMCID: 3122054.
Kim J, Byun JW, Choi I, Kim B, Jeong HK, Jou I, et al. PINK1 deficiency enhances inflammatory cytokine release from acutely prepared brain slices. Exp Neurobiol. 2013;22(1):38–44. PubMed PMID: 23585721, Pubmed Central PMCID: 3620457.
Choi I, Kim J, Jeong HK, Kim B, Jou I, Park SM, et al. PINK1 deficiency attenuates astrocyte proliferation through mitochondrial dysfunction, reduced AKT and increased p38 MAPK activation, and downregulation of EGFR. Glia. 2013;61(5):800–12. PubMed PMID: 23440919.
Waak J, Weber SS, Waldenmaier A, Gorner K, Alunni-Fabbroni M, Schell H, et al. Regulation of astrocyte inflammatory responses by the Parkinson’s disease-associated gene DJ-1. FASEB J. 2009;23(8):2478–89. PubMed PMID: 19276172.
A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72(6):971–83. PubMed PMID: 8458085. Pubmed Central PMCID: 8458085.
Ross CA, Tabrizi SJ. Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10(1):83–98. PubMed PMID: 21163446, Pubmed Central PMCID: 21163446.
Bjorkqvist M, Wild EJ, Thiele J, Silvestroni A, Andre R, Lahiri N, et al. A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington’s disease. J Exp Med. 2008;205(8):1869–77. PubMed PMID: 18625748, Pubmed Central PMCID: 2525598.
Hsiao HY, Chern Y. Targeting glial cells to elucidate the pathogenesis of Huntington’s disease. Mol Neurobiol. 2010;41(2–3):248–55. PubMed PMID: 20107928.
Bradford J, Shin J-Y, Roberts M, Wang C-E, Li X-J, Li S. Expression of mutant huntingtin in mouse brain astrocytes causes age-dependent neurological symptoms. Proc Natl Acad Sci U S A. 2009;106(52):22480–5. PubMed PMID: 20018729, Pubmed Central PMCID: 20018729.
Wang L, Lin F, Wang J, Wu J, Han R, Zhu L, et al. Truncated N-terminal huntingtin fragment with expanded-polyglutamine (htt552-100Q) suppresses brain-derived neurotrophic factor transcription in astrocytes. Acta Biochim Biophys Sinica. 2012;44(3):249–58. PubMed PMID: 22234237.
Giralt A, Friedman HC, Caneda-Ferron B, Urban N, Moreno E, Rubio N, et al. BDNF regulation under GFAP promoter provides engineered astrocytes as a new approach for long-term protection in Huntington’s disease. Gene Ther. 2010;17(10):1294–308. PubMed PMID: 20463759.
Arregui L, Benitez JA, Razgado LF, Vergara P, Segovia J. Adenoviral astrocyte-specific expression of BDNF in the striata of mice transgenic for Huntington’s disease delays the onset of the motor phenotype. Cell Mol Neurobiol. 2011;31(8):1229–43. PubMed PMID: 21681558.
Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87(3):493–506. PubMed PMID: 8898202, Pubmed Central PMCID: 8898202.
Alberch J, Perez-Navarro E, Canals JM. Neuroprotection by neurotrophins and GDNF family members in the excitotoxic model of Huntington’s disease. Brain Res Bull. 2002;57(6):817–22. PubMed PMID: 12031278.
Ebert AD, Barber AE, Heins BM, Svendsen CN. Ex vivo delivery of GDNF maintains motor function and prevents neuronal loss in a transgenic mouse model of Huntington’s disease. Exp Neurol. 2010;224(1):155–62. PubMed PMID: 20227407.
Ruiz C, Casarejos MJ, Gomez A, Solano R, de Yebenes JG, Mena MA. Protection by glia-conditioned medium in a cell model of Huntington disease. PLoS Curr. 2012;4:e4fbca54a2028b. PubMed PMID: 22919565, Pubmed Central PMCID: 22919565.
Raymond LA, Andre VM, Cepeda C, Gladding CM, Milnerwood AJ, Levine MS. Pathophysiology of Huntington’s disease: time-dependent alterations in synaptic and receptor function. Neuroscience. 2011;198:252–73. PubMed PMID: 21907762, Pubmed Central PMCID: 3221774.
Hassel B, Tessler S, Faull RL, Emson PC. Glutamate uptake is reduced in prefrontal cortex in Huntington’s disease. Neurochem Res. 2008;33(2):232–7. PubMed PMID: 17726644.
Shin JY, Fang ZH, Yu ZX, Wang CE, Li SH, Li XJ. Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J Cell Biol. 2005;171(6):1001–12. PubMed PMID: 16365166, Pubmed Central PMCID: 2171327.
Faideau M, Kim J, Cormier K, Gilmore R, Welch M, Auregan G, et al. In vivo expression of polyglutamine-expanded huntingtin by mouse striatal astrocytes impairs glutamate transport: a correlation with Huntington’s disease subjects. Hum Mol Genet. 2010;19(15):3053–67. PubMed PMID: 20494921, Pubmed Central PMCID: 2901144.
Soulet D, Cicchetti F. The role of immunity in Huntington’s disease. Mol Psychiatr. 2011;16(9):889–902. PubMed PMID: 21519341.
Chou SY, Weng JY, Lai HL, Liao F, Sun SH, Tu PH, et al. Expanded-polyglutamine huntingtin protein suppresses the secretion and production of a chemokine (CCL5/RANTES) by astrocytes. J Neurosci. 2008;28(13):3277–90. PubMed PMID: 18367595.
Hsiao HY, Chen YC, Chen HM, Tu PH, Chern Y. A critical role of astrocyte-mediated nuclear factor-kappaB-dependent inflammation in Huntington’s disease. Hum Mol Genet. 2013;22(9):1826–42. PubMed PMID: 23372043.
Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187(6):761–72. PubMed PMID: 19951898, Pubmed Central PMCID: 2806318.
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–34. PubMed PMID: 20303880.
Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11(3):251–3. PubMed PMID: 18246065, Pubmed Central PMCID: 3137510.
Marchetto MC, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3(6):649–57. PubMed PMID: 19041781.
Estevez AG, Spear N, Manuel SM, Radi R, Henderson CE, Barbeito L, et al. Nitric oxide and superoxide contribute to motor neuron apoptosis induced by trophic factor deprivation. J Neurosci. 1998;18(3):923–31. PubMed PMID: 9437014.
Haidet-Phillips AM, Hester ME, Miranda CJ, Meyer K, Braun L, Frakes A, et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol. 2011;29(9):824–8. PubMed PMID: 21832997, Pubmed Central PMCID: 3170425.
Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10(5):615–22. PubMed PMID: 17435755.
Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3(6):637–48. PubMed PMID: 19041780.
Aebischer J, Cassina P, Otsmane B, Moumen A, Seilhean D, Meininger V, et al. IFNγ triggers a LIGHT-dependent selective death of motoneurons contributing to the non-cell-autonomous effects of mutant SOD1. Cell Death Differ. 2011;18(5):754–68. PubMed PMID: 21072055, Pubmed Central PMCID: 21072055.
Wang R, Yang B, Zhang D. Activation of interferon signaling pathways in spinal cord astrocytes from an ALS mouse model. Glia. 2011;59(6):946–58. PubMed PMID: 21446050, Pubmed Central PMCID: 21446050.
Van Everbroeck B, Dewulf E, Pals P, Lübke U, Martin J-J, Cras P. The role of cytokines, astrocytes, microglia and apoptosis in Creutzfeldt-Jakob disease. Neurobiol Aging. 2002;23(1):59–64. PubMed PMID: 11755020, Pubmed Central PMCID: 11755020.
Myerowitz R, Lawson D, Mizukami H, Mi Y, Tifft CJ, Proia RL. Molecular pathophysiology in Tay-Sachs and Sandhoff diseases as revealed by gene expression profiling. Hum Mol Gen. 2002;11(11):1343–50. PubMed PMID: 12019216, Pubmed Central PMCID: 12019216.
Ransom BR, Ransom CB. Astrocytes: multitalented stars of the central nervous system. Methods Mol Biol. 2012;814:3–7. PubMed PMID: 22144296, Pubmed Central PMCID: 22144296.
Liberto CM, Albrecht PJ, Herx LM, Yong VW, Levison SW. Pro-regenerative properties of cytokine-activated astrocytes. J Neurochem. 2004;89(5):1092–100. PubMed PMID: 15147501, Pubmed Central PMCID: 15147501.
Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11(5):400–7. PubMed PMID: 16151042, Pubmed Central PMCID: 16151042.
Acknowledgments
This work was supported in part by grants from the National Multiple Sclerosis Society (NMSS), CA-1059A-13 and RG-4885-A-14 to E.N.B. and TA-3050-A-1 to G.P.M., and NIH grants NS45290 and NS57563 (E.N.B.).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Meares, G.P., Benveniste, E.N. (2014). Inflammation and the Pathophysiology of Astrocytes in Neurodegenerative Diseases. In: Peterson, P., Toborek, M. (eds) Neuroinflammation and Neurodegeneration. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1071-7_4
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1071-7_4
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1070-0
Online ISBN: 978-1-4939-1071-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)