Abstract
This chapter provides several examples of epigenetic deregulation in autoimmune diseases, a heterogeneous group of human conditions characterized by a deregulated immune response against the body own organs and tissues. Early studies based on the candidate gene approach have been flanked by genome-wide screenings in the last few years, revealing global changes in DNA methylation or histone tail modifications, as well as deregulated methylation and/or expression of hundreds of genes and microRNAs in cells from patients affected by those disorders. This chapter will focus on epigenetic deregulations observed in systemic lupus erythematosus, rheumatoid arthritis, Sjögren’s syndrome, psoriasis, multiple sclerosis, systemic sclerosis, and autoimmune thyroid diseases, even though epigenetic modifications are increasingly being observed in many other autoimmune diseases. By contrast, only a few environmental factors have been shown or suspected to induce the observed epigenetic changes. Epigenetic drugs and RNA silencing experiments have often reversed autoimmune disease-like phenotypes in rodents or cell cultures, leading researchers to debate on their potential use in the treatment of these human conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Epigenetics
- Autoimmune diseases
- Systemic lupus erythematosus
- Rheumatoid arthritis
- Sjögren’s syndrome
- Psoriasis
- Multiple sclerosis
- Systemic sclerosis
6.1 Introduction
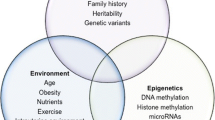
Autoimmune diseases include over 100 conditions characterized by an inappropriate immune response against the body own tissues and falling into two general types: systemic autoimmune diseases that damage many organs and systems such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), Sjögren’s syndrome (SjS), and systemic sclerosis or scleroderma (SSc) among others, and diseases characterized by an autoimmune response against a specific organ or tissue such as psoriasis, Hashimoto’s thyroiditis (HT), Graves’ disease (GD), and many others (Selmi 2012). It is now clear that while many of these pathologies can be influenced by heritable factors, also environmental factors such as drugs, ultraviolet light, infectious agents, and diet play a role, and recent genome-wide association studies revealed that genomics alone cannot fully explain the individual susceptibility to those disorders (Selmi 2012). Increasing evidence points to additional mechanisms linking the individual susceptibility with environmental factors, and epigenetics is increasingly recognized as one of the most promising missing links (De Santis and Selmi 2012). Indeed, since epigenetic mechanisms are sensitive to external stimuli, several environmental effects on immune responses could be mediated by epigenetic changes (Costenbader et al. 2012).
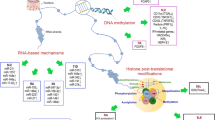
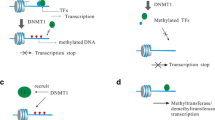
The term epigenetics comprises heritable and reversible modifications that alter gene expression without resulting from direct changes in the primary DNA sequence. Several epigenetic mechanisms are known, including DNA methylation, covalent modifications of histone tails, and nucleosome positioning, all interacting to determine chromatin folding and the relative accessibility of a given genetic locus to activating and suppressing transcription factors (Martín-Subero 2011). Noncoding RNAs affecting gene expression are also largely recognized as epigenetic mechanisms (Esteller 2011). All those mechanisms are exhaustively described in Chap. 1 of this book.
Most of the studies performed so far have focused on epigenetic modifications in SLE and rheumatoid arthritis (Quintero-Ronderos and Montoya-Ortiz 2012), but there is increasing evidence of epigenetic changes in other autoimmune disorders such as SjS, multiple sclerosis, systemic sclerosis, psoriasis, autoimmune thyroid diseases (AITDs), and others (Meda et al. 2011; Zhang et al. 2011; Quintero-Ronderos and Montoya-Ortiz 2012). This chapter will provide a summary of the most relevant evidence of epigenetic deregulation in autoimmune disorders.
6.2 Systemic Lupus Erythematosus
SLE is a systemic autoimmune disorder characterized by the production of auto-antibodies directed against nuclear self-antigens. The disease can target many organs, including the skin and joints, but also the heart, the kidneys, the nervous system, and others. It afflicts both sexes but occurs more frequently in women. Genetic association studies, genome-wide association studies, and discordance in disease inheritance in twins, have revealed that genetics alone does not completely account for disease heritability (Deapen et al. 1992; Cunninghame Graham 2009). Early studies of SLE epigenetics showed that CD4+ T cells treated with the DNA methylation inhibitor 5-azacytidine respond to the presentation of self antigens (Richardson 1986), and their injection in mice caused a lupus-like syndrome (Quddus et al. 1993). Those studies suggested that impairments of DNA methylation might be involved in autoimmunity.
6.2.1 DNA Methylation in SLE
DNA methylation represents one of the most studied epigenetic mechanisms for gene regulation and consists of the addition of a methyl group to the 5′ position of the cytosine pyrimidine ring (5-methylcytosine) mediated by DNA methyltransferase enzymes (DNMTs) using S-adenosylmethione (SAM) as the methyl donor compound (Jones 2012). There are multiple families of DNMTs in mammals. Among them DNMT1 is primarily involved in the maintenance of DNA methylation patterns during development and cell division, whereas DNMT3a and DNMT3b are the de novo methyltransferases and establish DNA methylation patterns during early development (Jones and Liang 2009). Methylated DNA can be specifically recognized by a set of proteins called methyl-CpG binding proteins (MBPs), including MECP2 (methyl-CpG binding protein 2) and MBD proteins (methyl-CpG binding domain proteins), that contain a transcription repression domain to interact with other proteins and enhance DNA methylation-mediated transcriptional repression (Fournier et al. 2012).
Following the observation that inhibitors of DNA methylation were able to induce autoimmune reactions and lupus-like symptoms in animals (Richardson 1986, Quddus et al. 1993), several investigators analyzed DNA methylation levels in SLE patients (Table 6.1). One of the most replicated findings is a global DNA hypomethylation observed in the CD4+ T cells of these patients (Balada et al. 2007a). DNA hypomethylation has been often linked to a reduction of DNMT1 mRNA levels in those cells (Zhu et al. 2011; Qin et al. 2013), but data on DNMT1, DNMT3a, and DNMT3b expression levels are still conflicting (Balada et al. 2008; Liu et al. 2011; Zhu et al. 2011). Also an increased expression of MBD2 and an inverse correlation between MBD2 expression and DNA methylation levels was often observed in CD4+ T cells of SLE patients (Balada et al. 2007b; Qin et al. 2013).
Apart from studies on global DNA methylation levels, the analysis of gene-specific promoter methylation by means of candidate gene approaches led researchers to identify several genes that are hypomethylated and therefore over-expressed in CD4+ T cells of SLE patients, some examples are ITGAL, CD40LG, PRF1, TNFSF7(CD70), and KIR family genes (reviewed in Hughes and Sawalha 2011). Many of those genes encode proteins involved in immune function and inflammation, and it is believed that their overexpression leads to lupus T cell autoreactivity and subsequent induction of autoreactive B cell immunoglobulin production (Hughes and Sawalha 2011). Recently, Balada and coworkers determined the expression levels of ITGAL, PRF1, KIR2DL4, TNFSF7(CD70), and CD40LG genes in CD4+ T cells of patients with SLE and performed correlations with the global DNA methylation status and the levels of DNMT and MBD proteins. SLE patients had significantly elevated transcript levels of ITGAL, PRF1, and TNFSF7(CD70), and those levels correlated with global DNA hypomethylation as well as with the expression of most of the DNA methylation-related genes, reinforcing the hypothesis of an epigenetic deregulated network in SLE (Balada et al. 2012).
The analysis of methylation of repetitive elements in SLE patients revealed hypomethylation of LINE-1 but not Alu in CD4+ T lymphocytes, CD8+ T lymphocytes, and B lymphocytes (Nakkuntod et al. 2011).
The candidate gene approach analysis has been paralleled in recent years by whole-genome methylation studies that are revealing hundreds of genes differentially methylated and expressed in SLE patients with respect to controls. One of such approaches performed on monozygotic twins discordant for SLE featured widespread changes in the DNA methylation status of a significant number of genes associated with immune function that occurred in parallel with a global decrease in the 5-methylcytosine content in the affected twins (Javierre et al. 2010). A subsequent genome-wide DNA methylation analysis in CD4+ T cells from SLE patients revealed 236 hypomethylated and 105 hypermethylated CpG sites (representing 232 and 104 genes, respectively). Hypomethylated genes in lupus T cells included, among others, many involved in autoimmunity, while genes involved in folate biosynthesis, required for SAM production and DNA methylation, resulted hypermethylated (Jeffries et al. 2011). Another whole-genome methylation analysis of SLE revealed that hypomethylation of interleukin IL10 and interleukin receptor IL1R2 promoters is associated with disease activity (Lin et al. 2012).
6.2.2 Histone Tail Modifications in SLE
The chromatin state represents another important modulator of gene expression profiles. Histone tail acetylation on lysine residues is mediated by histone acetyltransferases (HATs) and represents one of the most studied modifications associated with chromatin relaxation and transcriptional activation (Berger 2007). Another frequently studied modification of histone tails is methylation on either lysine or arginine residues, mediated by protein methyltransferases. Methylation of histone tails can be associated with either condensation or relaxation of the chromatin structure, since several sites for methylation are present on each tail thus allowing many combinations (Martin and Zhang 2005). Most of our current knowledge on histone tail modifications in SLE derives from studies based upon in vitro cell cultures and in vivo studies in murine models of lupus demonstrating that histone deacetylase inhibitors (HDACi) reversed the expression of multiple genes involved in autoimmunity and SLE pathogenesis (reviewed in Reilly et al. 2011). For example a global hypoacetylation of histone H3 and H4 was observed in a mouse model of lupus (MRL/lpr mice) compared to control mice, and the administration of the HDACi trichostatin A reversed histone hypoacetylation with improvement of disease phenotype (Garcia et al. 2005). Others observed an aberrant expression pattern of HATs and histone deacetylases (the enzymes responsible for histone deacetylation) in CD4+ T cells of MRL/lpr mice, among which the overexpression of histone deacetylase SIRT1 was implicated in lupus pathogenesis (Hu et al. 2009). Suppression of SIRT1 expression by means of RNA silencing in the animals resulted in an increase of global histone H3 and H4 acetylation levels and mitigated the disease-related phenotype (Hu et al. 2009).
Studies in humans revealed global histone H3 and H4 hypoacetylation and increased SIRT1 mRNA levels in active lupus CD4+ T cells of SLE patients compared with controls, as well as global histone H3K9 hypomethylation in both active and inactive lupus CD4+ T cells (Hu et al. 2008). There is also evidence that aberrant histone modifications within the TNFSF7(CD70) promoter may contribute to the development of lupus by increasing CD70 expression in CD4+ T cells (Zhou et al. 2011). More than 100 different auto-antibodies to nuclear antigens were found in patients with SLE, some of which recognize insoluble nuclear antigens (chromatin, DNA, histones, and RNA), leading researchers to formulate the hypothesis of a potential relationship between auto-antibodies production in SLE with changes in epigenetic patterns, such as DNA methylation and histone tail modifications (Thabet et al. 2012).
6.2.3 RNA-Mediated Epigenetic Mechanisms in SLE
Among noncoding RNAs, microRNA (miRNAs) are a group of small noncoding RNAs of about 22 nucleotides in length that bind to the 3′ untranslated region (3′-UTR) of target mRNAs and mediate their posttranscriptional regulation leading to either degradation or translational inhibition, depending on the degree of sequence complementarities. MiRNAs target about 60 % of all genes (Sato et al. 2011), and a complex network of interactions exists among miRNAs and other epigenetic mechanisms, such as DNA methylation and histone modification processes, to organize the whole gene expression profile.
In 2007 Dai and coworkers published the first report of a difference in miRNA expression between SLE patients and healthy controls (Dai et al. 2007). Since then studies profiling miRNA expression in blood cells, body fluids, and target tissues from SLE patients revealed unique miRNA signatures when compared with healthy individuals or those with other autoimmune diseases (Amarilyo and La Cava 2012; Shen et al. 2012). Among over-expressed miRNAs in lupus CD4+ T cells, miR-21, miR-148a, and miR-126 lead to DNMT1 downregulation by directly targeting its transcript (miR-148a and miR-126) or transcripts of genes that operate in the Ras–MAPK pathway upstream of DNMT1 (miR-21), and resulting in DNMT1 inhibition, DNA hypomethylation, and altered expression of genes involved in both pro-inflammatory and anti-inflammatory processes (Amarilyo and La Cava 2012). Other miRNAs were found to be up-regulated or down-regulated in cells of SLE patients; some examples include miR-142-3p and miR-142-5p that are down-regulated in SLE CD4+ T cells, causing T cell over-activation and B cell hyperstimulation (Ding et al. 2012). By contrast miR-146a, a negative regulator in immune and inflammatory responses, is down-regulated in SLE patients (Chan et al. 2012). A recent study profiled the expression of 270 human miRNAs in T cells from five SLE patients and five healthy controls, and identified under-expressed miR-145 and over-expressed miR-224 as well as down-regulated expression of their target genes linked to accelerated T cell activation-induced cell death (Lu et al. 2013).
6.2.4 Environmental Factors and Their Potential Epigenetic Properties in SLE
The demethylating agent 5-azacytidine was the first drug to be identified to cause lupus-like symptoms in rodents by altering DNA methylation levels (Richardson 1986; Quddus et al. 1993). Since then procainamide and hydralazine also have been suspected of causing SLE by inhibiting DNA methylation and inducing T cells autoreactivity. Procainamide is a competitive inhibitor of DNMT1 enzymatic activity and hydralazine inhibits T and B cell ERK pathway (Cornacchia et al. 1988). Other chemicals, such as air pollutants, have been suspected to act on DNA methylation and other epigenetic mechanisms in SLE (De Santis and Selmi 2012). Among physical agents, it has been suggested that UV light might induce the overexpression of autoimmunity-related genes through aberrant T cell DNA demethylation (Li et al. 2010). Additional factors suspected to epigenetically contribute to the incidence of autoimmune diseases are increasing age and infectious agents (De Santis and Selmi 2012).
6.3 Rheumatoid Arthritis
RA is a systemic autoimmune disease primarily characterized by chronic inflammation of the joints and ultimately leading to joint destruction. Both genetic and environmental factors are involved in disease pathogenesis, but increasing evidence (Klein et al. 2012) supports a role for epigenetic modifications (Table 6.2). RA synovial fibroblasts (RASFs) play a major role in the initiation and perpetuation of the disease; they are the most common cell type at the site of invasion and active contributors in joint damage due to their ability to secrete cytokines, chemokines, and joint-damaging enzymes. Moreover, RASFs show tumoral behavior including invasiveness and resistance to apoptosis. Epigenetic mechanisms have been largely investigated as contributors of RASFs aggressiveness and those cells are the best-characterized ones for epigenetic alterations in RA (Klein et al. 2012; Nakano et al. 2013).
6.3.1 DNA Methylation in RA
In 1991, Corvetta and coworkers observed reduced global DNA methylation in peripheral blood, synovial mononuclear cells, and synovial tissues from RA patients (Corvetta et al. 1991). Others observed hypomethylation and overexpression of LINE 1 retrotransposable elements in RASFs, affecting the expression of other genes likely contributing to cell activation (Neidhart et al. 2000; Kuchen et al. 2004). A subsequent study confirmed those previous findings and revealed that proliferating RASFs were deficient in DNMT1, and that the demethylating agent 5-azacytidine reproduced the activated phenotype of RASFs in normal synovial fibroblasts, with upregulation of over 100 genes including growth factors and receptors, extracellular matrix proteins, adhesion molecules, and matrix-degrading enzymes (Karouzakis et al. 2009). The search for specific genes regulated by DNA methylation in RASFs revealed other demethylated and over-expressed ones, such as for example CXCL12 that contributes to the expression of matrix metalloproteinases (Karouzakis et al. 2011). Other genes were found to be hypermethylated in those cells, including the promoter of the death receptor 3 (DR3) gene, whose downregulation in RASFs was linked to resistance to apoptosis (Takami et al. 2006). Impairments of DNA methylation were also observed in peripheral blood mononuclear cells of RA patients, some examples are demethylation of CpG sites in interleukin-6 (IL-6) and interleukin-10 (IL-10) gene promoters (Nile et al. 2008; Fu et al. 2011). The incidence of both RA and SLE is higher in females than in males, and the CD40LG gene on the X chromosome was found to be demethylated and over-expressed in CD4+ T cells from female RA and SLE patients (Lian et al. 2012; Liao et al. 2012).
A recent genome-wide approach in RASFs revealed 207 hypermethylated or hypomethylated genes, with hypomethylation increased in multiple pathways related to cell migration (Nakano et al. 2013), and recent studies in RA animal models showed an increased expression of MeCP2 in synovium and fibroblast-like synoviocytes, suggesting that MeCP2 could participate in RA pathogenesis through silencing of certain genes (Miao et al. 2013).
6.3.2 Histone Tail Modifications in RA
Several investigators observed increased overexpression and activity of histone deacetylases, and particularly of HDAC1, in RASFs and peripheral blood mononuclear cells of RA patients, suggesting a role for histone tail modifications in disease pathogenesis (Horiuchi et al. 2009; Gillespie et al. 2012). Moreover, HDACi, such as for example trichostatin A, were potent inhibitors of tumor necrosis factor and IL-6 production in those cells (Gillespie et al. 2012; Grabiec et al. 2012). There is also evidence from studies in vitro and in animal models that HDACi have the potential to suppress bone destruction in chronic inflammatory diseases such as RA (Cantley et al. 2012). These are only some of many examples showing anti-inflammatory properties of HDACi in RA models, whose beneficial effects are exerted through reduced production of cytokines, chemokines, and related receptors (De Santis and Selmi 2012).
6.3.3 RNA-Mediated Epigenetic Mechanisms in RA
MiRNAs have been largely investigated in the pathogenesis of RA, and some of them, such as for example miR-146a, miR-155, and miR-223, are of particular interest in disease pathogenesis (Ammari et al. 2013). Mir-146a is a negative regulator in immune and inflammatory responses up-regulated in several tissues of RA patients, including RASFs and peripheral blood mononuclear cells, and associated with tumor necrosis factor alpha production and disease activity (Xu et al. 2012). MiR-155 is up-regulated in synovial membrane and synovial fluid macrophages from RA patients (Kurowska-Stolarska et al. 2011), and has a powerful regulatory potential in a wide variety of immune cells through targeting specific mRNAs (Leng et al. 2011). MiR-223 is intensely expressed in RA synovium, and its overexpression suppresses osteoclastogenesis in vitro (Shibuya et al. 2013). Those miRNAs are currently investigated as potential therapeutic targets in RA, and recent integrated analyses of DNA methylation and miRNA expression profiling in RASFs are revealing novel markers of DNA methylation and sets of miRNAs that are controlled by DNA methylation, as well as genes that are regulated by DNA methylation and are targeted by miRNAs with a potential use as clinical markers (de la Rica et al. 2013).
6.3.4 Environmental Factors and Their Potential Epigenetic Properties in RA
Among environmental factors, cigarette smoke condensate was shown to up-regulate gene and protein expression of pro-inflammatory cytokines in human fibroblast-like synoviocytes (Shizu et al. 2008), and tobacco smoke is recognized among environmental RA risk factors (Karlson and Deane 2012). However, an epigenetic effect of tobacco smoke in RA is at present only speculative (De Santis and Selmi 2012).
6.4 Other Autoimmune Diseases
Epigenetic studies in autoimmune diseases other than SLE and RA are increasing in recent years (Tables 6.3, 6.4, 6.5, 6.6, and 6.7). Within this paragraph we describe some of the most recent examples.
6.4.1 Epigenetics of SjS
SjS is a systemic autoimmune disease characterized by chronic inflammation leading to reduced secretion of the exocrine salivary and lacrimal glands. Epigenetic studies in SjS are still in their infancy (Table 6.3); however, hypomethylation and overexpression of TNFSF7(CD70) were observed in CD4+ T cells of SjS patients (Yin et al. 2010), and hypermethylation of BP230, coding for a protein involved in the anchorage of salivary gland cells, was observed in labial salivary glands in SjS (González et al. 2011). The methylation profile of the gene coding for the interferon regulatory factor 5 (IRF5) was investigated in CD4+ T cells, B lymphocytes, and monocytes from patients with SjS, but the observed methylation levels were similar to those observed in cells from controls (Gestermann et al. 2012).
Abnormal distribution of aquaporin 5 (AQP5) in salivary gland acini is likely to contribute to the deficiency of fluid secretion in SjS, and the tumor necrosis factor alpha plays an important role in the destruction of acinar structures in exocrine glands, and inhibits AQP5 gene expression in human salivary gland acinar cells by suppression of acetylation of histone H4 in the promoter region (Yamamura et al. 2012).
Also some miRNAs were found to be deregulated in SjS salivary glands (miR-547 and miR-768-3p) and/or in peripheral mononuclear cells (miR-146a and miR-146b) (Alevizos et al. 2011; Pauley et al. 2011; Zilahi et al. 2012). Moreover, a recent study has shown that the SjS antigen B is a pre-miRNA-binding protein that regulates miRNA processing in vitro (Liang et al. 2013).
6.4.2 Epigenetics of Psoriasis
Psoriasis is an organ-specific autoimmune disease triggered by an active immune system causing cells to build up rapidly on the surface of the skin, resulting in thick, white, silvery, or red patches that are sometimes painful. The pathology of psoriasis is complex, involving both genetic and environmental components (Zhang et al. 2012), and increasing evidence supports a role for epigenetic modifications (Table 6.4). Early studies on DNA methylation revealed SHP-1 promoter methylation in normal epithelial tissues and demethylation in psoriasis. SHP-1 is a tyrosine phosphatase and has been proposed as a candidate tumor suppressor gene in lymphoma, leukemia, and other cancers, as it functions as an antagonist to the growth-promoting and oncogenic potentials of tyrosine kinases (Ruchusatsawat et al. 2006). A reduced proliferative activity has been detected in the hematopoietic cells from patients with psoriasis and linked to hypomethylation of the genes coding for p16, p21, and p53 (Zhang et al. 2007, 2009). More recent genome-wide approaches are revealing hundreds of novel methylation markers of the disease, thereby strengthening the contribution of epigenetics in psoriasis. The methylation levels at 27,578 CpG sites in skin samples from individuals with psoriasis and unaffected individuals revealed different methylation of 1,108 sites (Roberson et al. 2012). Similarly, differences in DNA methylation were found in CD4+ T cells of monozygotic twins discordant for psoriasis (Gervin et al. 2012). A genome-wide DNA methylation profiling of naïve CD4+ T cells showed distinct hypomethylation in 26 regions of the genome ranging in size from 10 to 70 kb (most of them pericentromeric) in patients with psoriasis with respect to healthy controls. Conversely, the promoter regions of 121 genes, and particularly of immune-related genes, on the X chromosome were hypermethylated in psoriasis patient T cells compared to those from healthy controls (Han et al. 2012).
Concerning histone tail modifications, the HDAC-1 mRNA resulted over-expressed in psoriatic skin samples compared with skin specimens from healthy subjects (Tovar-Castillo et al. 2007). Moreover, global histone H4 hypoacetylation was observed in peripheral blood mononuclear cells from psoriasis patients, and there was a negative correlation between the degree of histone H4 acetylation and disease activity (Zhang et al. 2011).
A comprehensive analysis of the normal and psoriatic skin miRNAome with next-generation sequencing revealed 80 known and 18 novel miRNAs that were differentially expressed in psoriatic skin. Of particular significance was the 2.7-fold upregulation of a novel miRNA derived from the antisense strand of the miR-203 locus, which plays a role in epithelial differentiation. Other differentially expressed miRNAs included hematopoietic-specific miRNAs such as miR-142-3p and miR-223/223*, and angiogenic miRNAs such as miR-21, miR-378, miR-100, and miR-31, which was the most highly up-regulated miRNA in psoriatic skin (Joyce et al. 2011). Subsequent functional studies of those miRNAs revealed that miR-21 suppresses apoptosis in activated T cells, and thus, overexpression of miR-21 may contribute to T cell-derived psoriatic skin inflammation (Meisgen et al. 2012), while miR-31 modulates inflammatory cytokine and chemokine production in keratinocytes via targeting serine/threonine kinase 40 (Xu et al. 2013). Moreover, the analysis of more than 670 million qualified reads from 67 small RNA libraries, revealed 21 novel, noncanonical miRNAs (3 small nuclear RNA-derived, 2 tRNA-derived miRNAs, and 16 miRtrons) and 39 novel endo-siRNAs that were expressed in skin, and 15 of them were significantly differentially expressed in psoriatic versus normal skin (Xia et al. 2013).
6.4.3 Epigenetics of Multiple Sclerosis
Multiple sclerosis is an autoimmune demyelinating disease and a common cause of neurodegeneration and disability in young adults. Disease discordance in monozygotic twins indicates environmental importance in its pathogenesis, but a genome-wide DNA methylation study in CD4+ lymphocytes of monozygotic twins discordant for MS failed to find significant differences, thereby dampening research expectations (Baranzini et al. 2010). However, the promoter of the peptidyl argininedeiminase 2 (PAD2) gene was hypomethylated in the white matter from MS patients, resulting in increased synthesis of PAD2 protein that is responsible for the increased amount of citrullinated myelin basic protein, which in turn results in loss of myelin stability in MS brain (Mastronardi et al. 2007). Similar results were observed in peripheral blood mononuclear cells of MS patients, where PAD2 overexpression was associated with promoter demethylation (Calabrese et al. 2012).
If data on DNA methylation alterations are scarce in MS, an increased histone H3 acetylation associated with increased levels of transcriptional inhibitors of oligodendrocyte differentiation was observed in the white matter of patients with chronic MS (Pedre et al. 2011), and a number of miRNAs have been found to be dysregulated in blood cells from MS patients, in brain lesions, as well as in biological fluids such as serum and plasma (Table 6.5). Some examples are miR-326 that was found to be up-regulated in MS blood and promoted T-helper CD4+ cells differentiation, miR-21, miR-146a and miR-146b up-regulated in peripheral blood mononuclear cells of MS patients as compared with controls, and miR-155, miR-326, and miR-34a that were found to be up-regulated in active MS brain lesions and targeted CD47, a regulatory membrane protein (reviewed in Fenoglio et al. 2012). These are only some of several examples of miRNAs deregulation in MS tissues, and recent large-scale studies are revealing dozens of novel markers, such as for example an expression profiling of 1,059 miRNAs in B lymphocytes that revealed 49 miRNAs down-regulated in untreated MS patients compared with healthy controls (Sievers et al. 2012). A recent integration of miRNAs databases revealed that the miRNAs associated with MS according to different studies are able or predicted to target about 1,500 different genes many of which play a role in T cell activation and signaling, or have transcription factor activity (Angerstein et al. 2012).
Among environmental factors considerable evidence has linked past Epstein–Barr virus (EBV) infection to an increased risk of MS, and, since a complete silencing of the EBV genome in memory B cells is under epigenetic control via DNA methylation and histone tail modifications, some authors have suggested that an epigenetic dysregulation of the EBV latency might contribute to the development of MS and other autoimmune diseases (Niller et al. 2011).
6.4.4 Epigenetics of Systemic Sclerosis
SSc is a systemic autoimmune disease characterized by deposition of collagen in the skin and, less commonly, in other tissues with progressive vasculopathy. Early studies on DNA methylation (Table 6.6) revealed association between enhanced type I collagen expression and epigenetic repression (hypermethylation) of the FLI1 gene in scleroderma fibroblasts (Wang et al. 2006). Subsequent studies revealed that CD4+ T cell DNA from patients with SSc was significantly hypomethylated relative to controls, and DNMT1, MBD3, and MBD4 mRNAs were significantly decreased in the SSc group (Lei et al. 2009). Demethylation of TNFSF7(CD70) was observed to contribute to CD70 overexpression in CD4+ T cells from patients with SSc (Jiang et al. 2012). Moreover, SSc occurs more frequently in females than males, suggesting that epigenetic modifications of genes on the X chromosome might be involved. Particularly, demethylation of CD40LG regulatory elements on the inactive X chromosome contributed to CD40L overexpression in CD4+ T cells from female patients with SSc, but no significant difference was observed in the expression of CD40L between male patients with SSc and male control subjects (Lian et al. 2012). A recent methylation profile of all X chromosome genes in peripheral blood mononuclear cells from monozygotic twins discordant for SSc revealed sites with an elevated probability to be consistently hypermethylated (n = 18) or hypomethylated (n = 25) in affected twins. Identified genes include transcription factors and surface antigens, and pathway analysis suggests their involvement in cell proliferation, apoptosis, inflammation, and oxidative stress (Selmi et al. 2012).
Increasing evidence suggests the involvement of histone tail modifications in fibrosis (Table 6.6), the hallmark of SSc, characterized by a persistent fibroblast activation triggered by transforming growth factor-β (TGF-β). Indeed, it was observed that the expression of the HAT p300 is markedly elevated in SSc skin biopsies and is induced by TGF-β in explanted normal skin fibroblasts. Moreover, TGF-β enhanced both p300 recruitment and histone H4 acetylation at the COL1A2 (collagen, type I, α2) locus, suggesting that p300-mediated histone acetylation could represent a fundamental epigenetic mechanism in fibrogenesis (Ghosh et al. 2013). Similarly, inhibition of trimethylation of histone H3 on lysine 27 (H3K27me3), induced by treatment with 3-deazaneplanocin A, stimulated the release of collagen in cultured fibroblasts in a time and dose-dependent manner and was sufficient to induce fibrosis, suggesting that trimethylation of histone H3 on lysine 27 acts as a negative regulator of fibroblast activation (Krämer et al. 2012).
An increasing number of miRNAs was found to be deregulated in SSc samples (Table 6.6). For example, a miRNA array analysis in skin tissues from SSc patients and healthy controls revealed 24 miRNAs that were differentially expressed in patients with SSc and six miRNAs that may be correlated with the pathogenesis of SSc. Particularly, miR-23b and let-7 were up-regulated, while miR-125b, miR-133a, miR-206, and miR-140-5p were down-regulated (Li et al. 2012). Others observed that in comparison with the normal skin tissues, miRNAs were aberrantly expressed in limited cutaneous scleroderma and diffuse cutaneous scleroderma skin tissues, and identified six miRNAs whose expressions were correlated with SSc fibrosis: miR-21, miR-31, miR-146, miR-503, miR-145, and miR-29b. Particularly, the study confirmed that miR-21 was increased whereas miR-145 and miR-29b were decreased both in the skin tissues and in fibroblasts. As predicted target genes, SMAD7, SAMD3, and COL1A1 were regulated by these tree miRNAs (Zhu et al. 2012). Previous results had shown that miR-29a was strongly down-regulated in SSc fibroblasts and skin sections as compared with the healthy controls, and that this miRNA acts as a key regulator of collagen expression in SSc (Maurer et al. 2010). Overall, miRNA-29 is a recently discovered class of miRNAs which is related to fibrotic disease and a potential therapeutic target for systemic sclerosis (Peng et al. 2012). More recently, it was found that miR-150 downregulation contributes to the constitutive type I collagen overexpression in SSc dermal fibroblasts via the induction of integrin β3 (Honda et al. 2013).
6.4.5 Epigenetics of AITDs
AITDs comprise Graves’ disease and Hashimoto’s thyroiditis, both organ-specific autoimmune diseases characterized by female preponderance, and in which the autoimmune attack of the thyroid takes place by infiltration of lymphocytes of the glandule. A possible role of skewed X chromosome inactivation, mediated by epigenetic mechanisms, has been suggested in the etiology of AITD to partially explain the female preponderance (Brix et al. 2005; Chabchoub et al. 2009).
A few studies have been performed to clarify the association between factors regulating DNA methylation and the prognosis of AITDs (Table 6.7). Particularly, those studies focused on polymorphisms in genes encoding DNMTs, methylenetetrahydrofolate reductase (MTHFR), and methionine synthase reductase (MTRR), which are all enzymes essential for DNA methylation reactions. The MTHFR C677T polymorphism was associated with reduced GD risk in women (Mao et al. 2010), while the DNMT1 32204GG genotype was correlated with DNA hypomethylation and with the intractability of GD, and the MTRR 66AA genotype with the severity of HD (Arakawa et al. 2012). Albeit in their infancy, those studies suggest that those genes might account for AITD susceptibility, severity, and response to treatment, partially mediated by changes in DNA methylation (Mao et al. 2010; Arakawa et al. 2012).
Also the few available studies on miRNA profiling in AITD tissues (Table 6.7) suggest deregulated networks in those disorders. Liu and coworkers showed that the expression of miR-154*, miR-376b, and miR-431* was suppressed in peripheral blood mononuclear cells from initial GD patients, and that their expression levels were recovered in GD patients in remission (Liu et al. 2012). Another group showed that miR-146a1 was significantly decreased in the thyroid tissue of GD patients, in comparison with the control group (Bernecker et al. 2012). Similarly, miR-155_2 was significantly decreased and miR-200a1 was significantly increased in the thyroid of HT patients, with respect to the control tissues (Bernecker et al. 2012). Albeit preliminary, those studies suggest a potential role of miRNA deregulations in AITDs that warrants further research.
6.5 Concluding Remarks
In the present chapter we described some examples of epigenetic deregulation in human autoimmune diseases. This field of research has gained tremendous attention in the last 2–3 years and it is now emerging that epigenetic modifications play a role, or are supposed to do it, in several autoimmune disorders, including but not limited to those detailed in this chapter. Indeed, evidence of an epigenetic contribution is increasing also in inflammatory bowel diseases (Jenke and Zilbauer 2012), type 1 diabetes (Dang et al. 2013), immune thrombocytopenic purpura (Khorshied and El-Ghamrawy 2012), and many other inflammatory and/or autoimmune diseases. Despite this, only few environmental factors have been suggested to epigenetically contribute to those disorders, some examples are drugs, air pollutants, ultraviolet light, cigarette smoke, and microbial infections, but for most of them the epigenetic link is still only speculative (De Santis and Selmi 2012). Several authors have suggested that epigenetic deregulations of genes on the X chromosome might account for gender differences, i.e., female predominance, in the incidence of many autoimmune diseases (Lian et al. 2012; Liao et al. 2012), and age-related epigenetic changes might also be of interest (De Santis and Selmi 2012).
Early epigenetic studies in autoimmune diseases, based on the candidate gene approach, have been paralleled and/or replaced in recent years by whole-genome approaches, that are revealing dozens, or even hundreds of genes or miRNAs that are deregulated in the affected tissues as well as in peripheral tissues of the patients (Tables 6.1–6.7). This is leading to a better understanding of the networks involved in disease pathogenesis, thereby opening the way for potential diagnostic and prognostic tools, as well as for epigenetic interventions based on miRNA silencing or chromatin remodeling agents, such as HDAi (Garchow et al. 2011; Reilly et al. 2011).
References
Alevizos I, Alexander S, Turner RJ, Illei GG (2011) MicroRNA expression profiles as biomarkers of minor salivary gland inflammation and dysfunction in Sjögren’s syndrome. Arthritis Rheum 63:535–544
Amarilyo G, La Cava A (2012) miRNA in systemic lupus erythematosus. Clin Immunol 144:26–31
Ammari M, Jorgensen C, Apparailly F (2013) Impact of microRNAs on the understanding and treatment of rheumatoid arthritis. Curr Opin Rheumatol 25:225–233
Angerstein C, Hecker M, Paap BK, Koczan D, Thamilarasan M, Thiesen HJ, Zettl UK (2012) Integration of microRNA databases to study microRNAs associated with multiple sclerosis. Mol Neurobiol 45:520–535
Arakawa Y, Watanabe M, Inoue N, Sarumaru M, Hidaka Y, Iwatani Y (2012) Association of polymorphisms in DNMT1, DNMT3A, DNMT3B, MTHFR and MTRR genes with global DNA methylation levels and prognosis of autoimmune thyroid disease. Clin Exp Immunol 170:194–201
Balada E, Ordi-Ros J, Vilardell-Tarrés M (2007a) DNA methylation and systemic lupus erythematosus. Ann N Y Acad Sci 1108:127–136
Balada E, Ordi-Ros J, Serrano-Acedo S, Martinez-Lostao L, Vilardell-Tarrés M (2007b) Transcript overexpression of the MBD2 and MBD4 genes in CD4+ T cells from systemic lupus erythematosus patients. J Leukoc Biol 81:1609–1616
Balada E, Ordi-Ros J, Serrano-Acedo S, Martinez-Lostao L, Rosa-Leyva M, Vilardell-Tarrés M (2008) Transcript levels of DNA methyltransferases DNMT1, DNMT3A and DNMT3B in CD4+ T cells from patients with systemic lupus erythematosus. Immunology 124:339–347
Balada E, Castro-Marrero J, Felip L, Ordi-Ros J, Vilardell-Tarrés M (2012) Associations between the expression of epigenetically regulated genes and the expression of DNMTs and MBDs in systemic lupus erythematosus. PLoS One 7:e45897
Baranzini SE, Mudge J, Van Velkinburgh JC, Khankhanian P, Khrebtukova I, Miller NA, Zhang L, Farmer AD, Bell CJ, Kim RW, May GD, Woodward JE, Caillier SJ, Mcelroy JP, Gomez R, Pando MJ, Clendenen LE, Ganusova EE, Schilkey FD, Ramaraj T, Khan OA, Huntley JJ, Luo S, Kwok PY, Wu TD, Schroth GP, Oksenberg JR, Hauser SL, Kingsmore SF (2010) Genome, epigenome and RNA sequences of monozygotic twins discordant for multiple sclerosis. Nature 464:1351–1356
Berger SL (2007) The complex language of chromatin regulation during transcription. Nature 447:407–412
Bernecker C, Lenz L, Ostapczuk MS, Schinner S, Willenberg H, Ehlers M, Vordenbäumen S, Feldkamp J, Schott M (2012) MicroRNAs miR-146a1, miR-155_2, and miR-200a1 are regulated in autoimmune thyroid diseases. Thyroid 22:1294–1295
Brix TH, Knudsen GP, Kristiansen M, Kyvik KO, Orstavik KH, Hegedüs L (2005) High frequency of skewed X-chromosome inactivation in females with autoimmune thyroid disease: a possible explanation for the female predisposition to thyroid autoimmunity. J Clin Endocrinol Metab 90:5949–5953
Calabrese R, Zampieri M, Mechelli R, Annibali V, Guastafierro T, Ciccarone F, Coarelli G, Umeton R, Salvetti M, Caiafa P (2012) Methylation-dependent PAD2 upregulation in multiple sclerosis peripheral blood. Mult Scler 18:299–304
Cantley MD, Bartold PM, Fairlie DP, Rainsford KD, Haynes DR (2012) Histone deacetylase inhibitors as suppressors of bone destruction in inflammatory diseases. J Pharm Pharmacol 64:763–774
Chabchoub G, Uz E, Maalej A, Mustafa CA, Rebai A, Mnif M, Bahloul Z, Farid NR, Ozcelik T, Ayadi H (2009) Analysis of skewed X-chromosome inactivation in females with rheumatoid arthritis and autoimmune thyroid diseases. Arthritis Res Ther 11:R106
Chan EK, Ceribelli A, Satoh M (2012) MicroRNA-146a in autoimmunity and innate immune responses. Ann Rheum Dis 72(Suppl 2):S90–S95
Cornacchia E, Golbus J, Maybaum J, Strahler J, Hanash S, Richardson B (1988) Hydralazine and procainamide inhibit T cell DNA methylation and induce autoreactivity. J Immunol 140:2197–2200
Corvetta A, Della Bitta R, Luchetti MM, Pomponio G (1991) 5-Methylcytosine content of DNA in blood, synovial mononuclear cells and synovial tissue from patients affected by autoimmune rheumatic diseases. J Chromatogr 566:481–491
Costenbader KH, Gay S, Alarcón-Riquelme ME, Iaccarino L, Doria A (2012) Genes, epigenetic regulation and environmental factors: which is the most relevant in developing autoimmune diseases? Autoimmun Rev 11:604–609
Cunninghame Graham DS (2009) Genome-wide association studies in systemic lupus erythematosus: a perspective. Arthritis Res Ther 11:119
Dai Y, Huang YS, Tang M, Lv TY, Hu CX, Tan YH, Xu ZM, Yin YB (2007) Microarray analysis of microRNA expression in peripheral blood cells of systemic lupus erythematosus patients. Lupus 16:939–946
Dang MN, Buzzetti R, Pozzilli P (2013) Epigenetics in autoimmune diseases with focus on type 1 diabetes. Diabetes Metab Res Rev 29:8–18
De La Rica L, Urquiza JM, Gómez-Cabrero D, Islam AB, López-Bigas N, Tegnér J, Toes RE, Ballestar E (2013) Identification of novel markers in rheumatoid arthritis through integrated analysis of DNA methylation and microRNA expression. J Autoimmun 41:6–16
De Santis M, Selmi C (2012) The therapeutic potential of epigenetics in autoimmune diseases. Clin Rev Allergy Immunol 42:92–101
Deapen D, Escalante A, Weinrib L, Horwitz D, Bachman B, Roy-Burman P, Walker A, Mack TM (1992) A revised estimate of twin concordance in systemic lupus erythematosus. Arthritis Rheum 35:311–318
Ding S, Liang Y, Zhao M, Liang G, Long H, Zhao S, Wang Y, Yin H, Zhang P, Zhang Q, Lu Q (2012) Decreased microRNA-142-3p/5p expression causes CD4+ T cell activation and B cell hyperstimulation in systemic lupus erythematosus. Arthritis Rheum 64:2953–2963
Esteller M (2011) Non-coding RNAs in human disease. Nat Rev Genet 12:861–874
Fenoglio C, Ridolfi E, Galimberti D, Scarpini E (2012) MicroRNAs as active players in the pathogenesis of multiple sclerosis. Int J Mol Sci 13:13227–13239
Fournier A, Sasai N, Nakao M, Defossez PA (2012) Role of methyl-binding proteins in chromatin organization and epigenome maintenance. Brief Funct Genomics 11:251–264
Fu LH, Ma CL, Cong B, Li SJ, Chen HY, Zhang JG (2011) Hypomethylation of proximal CpG motif of interleukin-10 promoter regulates its expression in human rheumatoid arthritis. Acta Pharmacol Sin 32:1373–1380
Garchow BG, Bartulos Encinas O, Leung YT, Tsao PY, Eisenberg RA, Caricchio R, Obad S, Petri A, Kauppinen S, Kiriakidou M (2011) Silencing of microRNA-21 in vivo ameliorates autoimmune splenomegaly in lupus mice. EMBO Mol Med 3:605–615
Garcia BA, Busby SA, Shabanowitz J, Hunt DF, Mishra N (2005) Resetting the epigenetic histone code in the MRL-lpr/lpr mouse model of lupus by histone deacetylase inhibition. J Proteome Res 6:2032–2042
Gervin K, Vigeland MD, Mattingsdal M, Hammerø M, Nygård H, Olsen AO, Brandt I, Harris JR, Undlien DE, Lyle R (2012) DNA methylation and gene expression changes in monozygotic twins discordant for psoriasis: identification of epigenetically dysregulated genes. PLoS Genet 8:e1002454
Gestermann N, Koutero M, Belkhir R, Tost J, Mariette X, Miceli-Richard C (2012) Methylation profile of the promoter region of IRF5 in primary Sjögren’s syndrome. Eur Cytokine Netw 23:166–172
Ghosh AK, Bhattacharyya S, Lafyatis R, Farina G, Yu J, Thimmapaya B, Wei J, Varga J (2013) p300 is elevated in systemic sclerosis and its expression is positively regulated by TGF-β: epigenetic feed-forward amplification of fibrosis. J Invest Dermatol 133:1302–1310
Gillespie J, Savic S, Wong C, Hempshall A, Inman M, Emery P, Grigg R, McDermott MF (2012) Histone deacetylases are dysregulated in rheumatoid arthritis and a novel histone deacetylase 3-selective inhibitor reduces interleukin-6 production by peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Rheum 64:418–422
González S, Aguilera S, Alliende C, Urzúa U, Quest AF, Herrera L, Molina C, Hermoso M, Ewert P, Brito M, Romo R, Leyton C, Pérez P, González MJ (2011) Alterations in type I hemidesmosome components suggestive of epigenetic control in the salivary glands of patients with Sjögren’s syndrome. Arthritis Rheum 63:1106–1115
Grabiec AM, Korchynskyi O, Tak PP, Reedquist KA (2012) Histone deacetylase inhibitors suppress rheumatoid arthritis fibroblast-like synoviocyte and macrophage IL-6 production by accelerating mRNA decay. Ann Rheum Dis 71:424–431
Han J, Park SG, Bae JB, Choi J, Lyu JM, Park SH, Kim HS, Kim YJ, Kim S, Kim TY (2012) The characteristics of genome-wide DNA methylation in naïve CD4+ T cells of patients with psoriasisor atopic dermatitis. Biochem Biophys Res Commun 422:157–163
Honda N, Jinnin M, Kira-Etoh T, Makino K, Kajihara I, Makino T, Fukushima S, Inoue Y, Okamoto Y, Hasegawa M, Fujimoto M, Ihn H (2013) miR-150 down-regulation contributes to the constitutive type I collagen overexpression in scleroderma dermal fibroblasts via the induction of integrin β3. Am J Pathol 182:206–216
Horiuchi M, Morinobu A, Chin T, Sakai Y, Kurosaka M, Kumagai S (2009) Expression and function of histone deacetylases in rheumatoid arthritis synovial fibroblasts. J Rheumatol 36:1580–1589
Hu N, Qiu X, Luo Y, Yuan J, Li Y, Lei W, Zhang G, Zhou Y, Su Y, Lu Q (2008) Abnormal histone modification patterns in lupus CD4+ T cells. J Rheumatol 35:804–810
Hu N, Long H, Zhao M, Yin H, Lu Q (2009) Aberrant expression pattern of histone acetylation modifiers and mitigation of lupus by SIRT1-siRNA in MRL/lpr mice. Scand J Rheumatol 38:464–471
Hughes T, Sawalha AH (2011) The role of epigenetic variation in the pathogenesis of systemic lupus erythematosus. Arthritis Res Ther 13:245
Javierre BM, Fernandez AF, Richter J, Al-Shahrour F, Martin-Subero JI, Rodriguez-Ubreva J, Berdasco M, Fraga MF, O’hanlon TP, Rider LG, Jacinto FV, Lopez-Longo FJ, Dopazo J, Forn M, Peinado MA, Carreño L, Sawalha AH, Harley JB, Siebert R, Esteller M, Miller FW, Ballestar E (2010) Changes in the pattern of DNA methylation associate with twin discordance in systemic lupus erythematosus. Genome Res 20:170–179
Jeffries MA, Dozmorov M, Tang Y, Merrill JT, Wren JD, Sawalha AH (2011) Genome-wide DNA methylation patterns in CD4+ T cells from patients with systemic lupus erythematosus. Epigenetics 6:593–601
Jenke AC, Zilbauer M (2012) Epigenetics in inflammatory bowel disease. Curr Opin Gastroenterol 28:577–584
Jiang H, Xiao R, Lian X, Kanekura T, Luo Y, Yin Y, Zhang G, Yang Y, Wang Y, Zhao M, Lu Q (2012) Demethylation of TNFSF7 contributes to CD70 overexpression in CD4+ T cells from patients with systemic sclerosis. Clin Immunol 143:39–44
Jones PA, Liang G (2009) Rethinking how DNA methylation patterns are maintained. Nat Rev Genet 10:805–811
Jones PA (2012) Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet 13:484–492
Joyce CE, Zhou X, Xia J, Ryan C, Thrash B, Menter A, Zhang W, Bowcock AM (2011) Deep sequencing of small RNAs from human skin reveals major alterations in the psoriasis miRNAome. Hum Mol Genet 20:4025–4040
Karlson EW, Deane K (2012) Environmental and gene-environment interactions and risk of rheumatoid arthritis. Rheum Dis Clin North Am 38:405–426
Karouzakis E, Gay RE, Michel BA, Gay S, Neidhart M (2009) DNA hypomethylation in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum 60:3613–3622
Karouzakis E, Rengel Y, Jüngel A, Kolling C, Gay RE, Michel BA, Tak PP, Gay S, Neidhart M, Ospelt C (2011) DNA methylation regulates the expression of CXCL12 in rheumatoid arthritis synovial fibroblasts. Genes Immun 12:643–652
Khorshied MM, El-Ghamrawy MK (2012) DNA methyltransferase 3B (DNMT3B–579G > T) promotor polymorphism and the susceptibility to pediatric immune thrombocytopenic purpura in Egypt. Gene 511:34–37
Klein K, Ospelt C, Gay S (2012) Epigenetic contributions in the development of rheumatoid arthritis. Arthritis Res Ther 14:227
Krämer M, Dees C, Huang J, Schlottmann I, Palumbo-Zerr K, Zerr P, Gelse K, Beyer C, Distler A, Marquez VE, Distler O, Schett G, Distler JH (2012) Inhibition of H3K27 histone trimethylation activates fibroblasts and induces fibrosis. Ann Rheum Dis 72:614–620
Kuchen S, Seemayer CA, Rethage J, Von Knoch R, Kuenzler P, Beat AM, Gay RE, Gay S, Neidhart M (2004) The L1 retroelement-related p40 protein induces p38delta MAP kinase. Autoimmunity 37:57–65
Kurowska-Stolarska M, Alivernini S, Ballantine LE, Asquith DL, Millar NL, Gilchrist DS, Reilly J, Ierna M, Fraser AR, Stolarski B, McSharry C, Hueber AJ, Baxter D, Hunter J, Gay S, Liew FY, Mcinnes IB (2011) MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc Natl Acad Sci U S A 108:11193–11198
Lei W, Luo Y, Lei W, Luo Y, Yan K, Zhao S, Li Y, Qiu X, Zhou Y, Long H, Zhao M, Liang Y, Su Y, Lu Q (2009) Abnormal DNA methylation in CD4+ T cells from patients with systemic lupus erythematosus, systemic sclerosis, and dermatomyositis. Scand J Rheumatol 38:369–374
Leng RX, Pan HF, Qin WZ, Chen GM, Ye DQ (2011) Role of microRNA-155 in autoimmunity. Cytokine Growth Factor Rev 22:141–147
Li H, Yang R, Fan X, Gu T, Zhao Z, Chang D, Wang W (2012) MicroRNA array analysis of microRNAs related to systemic scleroderma. Rheumatol Int 32:307–313
Li Y, Zhao M, Yin H, Gao F, Wu X, Luo Y, Zhao S, Zhang X, Su Y, Hu N, Long H, Richardson B, Lu Q (2010) Overexpression of the growth arrest and DNA damage-induced 45alpha gene contributes to autoimmunity by promoting DNA demethylation in lupus T cells. Arthritis Rheum 62:1438–1447
Lian X, Xiao R, Hu X, Kanekura T, Jiang H, Li Y, Wang Y, Yang Y, Zhao M, Lu Q (2012) DNA demethylation of CD40l in CD4+ T cells from women with systemic sclerosis: a possible explanation for female susceptibility. Arthritis Rheum 64:2338–2345
Liang C, Xiong K, Szulwach KE, Zhang Y, Wang Z, Peng J, Fu M, Jin P, Suzuki HI, Liu Q (2013) Sjogren syndrome antigen B (SSB)/La promotes global microRNA expression by binding microRNA precursors through stem-loop recognition. J Biol Chem 288:723–736
Liao J, Liang G, Xie S, Zhao H, Zuo X, Li F, Chen J, Zhao M, Chan TM, Lu Q (2012) CD40L demethylation in CD4(+) T cells from women with rheumatoid arthritis. Clin Immunol 145:13–18
Lin SY, Hsieh SC, Lin YC, Lee CN, Tsai MH, Lai LC, Chuang EY, Chen PC, Hung CC, Chen LY, Hsieh WS, Niu DM, Su YN, Ho HN (2012) A whole genome methylation analysis of systemic lupus erythematosus: hypomethylation of the IL10 and IL1R2 promoters is associated with disease activity. Genes Immun 13:214–220
Liu CC, Ou TT, Wu CC, Li RN, Lin YC, Lin CH, Tsai WC, Liu HW, Yen JH (2011) Global DNA methylation, DNMT1, and MBD2 in patients with systemic lupus erythematosus. Lupus 20:131–136
Liu R, Ma X, Xu L, Wang D, Jiang X, Zhu W, Cui B, Ning G, Lin D, Wang S (2012) Differential microRNA expression in peripheral blood mononuclear cells from Graves’ disease patients. J Clin Endocrinol Metab 97:E968–E972
Lu MC, Lai NS, Chen HC, Yu HC, Huang KY, Tung CH, Huang HB, Yu CL (2013) Decreased microRNA(miR)-145 and increased miR-224 expression in T cells from patients with systemic lupuserythematosus involved in lupusimmunopathogenesis. Clin Exp Immunol 171:91–99
Mao R, Fan Y, Zuo L, Geng D, Meng F, Zhu J, Li Q, Qiao H, Jin Y, Bai J, Fu S (2010) Association study between methylenetetrahydrofolate reductase gene polymorphisms and Graves’ disease. Cell Biochem Funct 28:585–590
Martin C, Zhang Y (2005) The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol 6:838–849
Martín-Subero JI (2011) How epigenomics brings phenotype into being. Pediatr Endocrinol Rev 9:506–510
Mastronardi FG, Noor A, Wood DD, Paton T, Moscarello MA (2007) Peptidyl argininedeiminase 2 CpG island in multiple sclerosis white matter is hypomethylated. J Neurosci Res 85:2006–2016
Maurer B, Stanczyk J, Jüngel A, Akhmetshina A, Trenkmann M, Brock M, Kowal-Bielecka O, Gay RE, Michel BA, Distler JH, Gay S, Distler O (2010) MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum 62:1733–1743
Meda F, Folci M, Baccarelli A, Selmi C (2011) The epigenetics of autoimmunity. Cell Mol Immunol 8:226–236
Meisgen F, Xu N, Wei T, Janson PC, Obad S, Broom O, Nagy N, Kauppinen S, Kemény L, Ståhle M, Pivarcsi A, Sonkoly E (2012) MiR-21 is up-regulated in psoriasis and suppresses T cell apoptosis. Exp Dermatol 21:312–314
Miao CG, Yang YY, He X, Li J (2013) New advances of DNA methylation and histone modifications in rheumatoid arthritis, with special emphasis on MeCP2. Cell Signal 25:1118–1125
Nakano K, Boyle DL, Firestein GS (2013) Regulation of DNA methylation in rheumatoid arthritis synoviocytes. J Immunol 190:1297–1303
Nakkuntod J, Avihingsanon Y, Mutirangura A, Hirankarn N (2011) Hypomethylation of LINE-1 but not Alu in lymphocyte subsets of systemic lupus erythematosus patients. Clin Chim Acta 412:1457–1461
Neidhart M, Rethage J, Kuchen S, Künzler P, Crowl RM, Billingham ME, Gay RE, Gay S (2000) Retrotransposable L1 elements expressed in rheumatoid arthritis synovial tissue: association with genomic DNA hypomethylation and influence on gene expression. Arthritis Rheum 43:2634–2647
Nile CJ, Read RC, Akil M, Duff GW, Wilson AG (2008) Methylationstatus of a single CpG site in the IL6 promoter is related to IL6 messenger RNA levels and rheumatoid arthritis. Arthritis Rheum 58:2686–2693
Niller HH, Wolf H, Ay E, Minarovits J (2011) Epigenetic dysregulation of epstein-barr virus latency and development of autoimmune disease. Adv Exp Med Biol 711:82–102
Pauley KM, Stewart CM, Gauna AE, Dupre LC, Kuklani R, Chan AL, Pauley BA, Reeves WH, Chan EK, Cha S (2011) Altered miR-146a expression in Sjögren’s syndrome and its functional role in innate immunity. Eur J Immunol 41:2029–2039
Pedre X, Mastronardi F, Bruck W, López-Rodas G, Kuhlmann T, Casaccia P (2011) Changed histone acetylation patterns in normal-appearing white matter and early multiple sclerosis lesions. J Neurosci 31:3435–3445
Peng WJ, Tao JH, Mei B, Chen B, Li BZ, Yang GJ, Zhang Q, Yao H, Wang BX, He Q, Wang J (2012) MicroRNA-29: a potential therapeutic target for systemic sclerosis. Expert Opin Ther Targets 16:875–879
Qin HH, Zhu XH, Liang J, Yang YS, Wang SS, Shi WM, Xu JH (2013) Associations between aberrant DNA methylation and transcript levels of DNMT1 and MBD2 in CD4+ T cells from patients with systemic lupus erythematosus. Australas J Dermatol 54:90–95
Quddus J, Johnson KJ, Gavalchin J, Amento EP, Chrisp CR, Young RL, Richardson BC (1993) Treating activated CD4+ T cells with either of two distinct DNA methyltransferase inhibitors, 5-azacytidine or procainamide, is sufficient to cause a lupus-like disease in syngeneic mice. J Clin Invest 92:38–53
Quintero-Ronderos P, Montoya-Ortiz G (2012) Epigenetics and autoimmune diseases. Autoimmune Dis 2012:593720
Reilly CM, Regna N, Mishra N (2011) HDAC inhibition in lupus models. Mol Med 17:417–425
Richardson B (1986) Effect of an inhibitor of DNA methylation on T cells. II. 5-azacytidine induces selfreactivity in antigen-specific T4+ cells. Hum Immunol 17:456–470
Roberson ED, Liu Y, Ryan C, Joyce CE, Duan S, Cao L, Martin A, Liao W, Menter A, Bowcock AM (2012) A subset of methylated CpG sites differentiate psoriatic from normal skin. J Invest Dermatol 132:583–592
Ruchusatsawat K, Wongpiyabovorn J, Shuangshoti S, Hirankarn N, Mutirangura A (2006) SHP-1 promoter 2 methylation in normal epithelial tissues and demethylation in psoriasis. J Mol Med 84:175–182
Sato F, Tsuchiya S, Meltzer SJ, Shimizu K (2011) MicroRNAs and epigenetics. FEBS J 278:1598–1609
Selmi C (2012) Autoimmunity in 2011. Clin Rev Allergy Immunol 43:194–206
Selmi C, Feghali-Bostwick CA, Lleo A, Lombardi SA, De Santis M, Cavaciocchi F, Zammataro L, Mitchell MM, Lasalle JM, Medsger TJR, Gershwin ME (2012) X chromosome gene methylation in peripheral lymphocytes from monozygotic twins discordant for scleroderma. Clin Exp Immunol 169:253–262
Shen N, Liang D, Tang Y, De Vries N, Tak PP (2012) MicroRNAs-novel regulators of systemic lupus erythematosus pathogenesis. Nat Rev Rheumatol 8:701–709
Shibuya H, Nakasa T, Adachi N, Nagata Y, Ishikawa M, Deie M, Suzuki O, Ochi M (2013) Overexpression of microRNA-223 in rheumatoid arthritis synovium controls osteoclast differentiation. Mod Rheumatol 23:674–685
Shizu M, Itoh Y, Sunahara R, Chujo S, Hayashi H, Ide Y, Takii T, Koshiko M, Chung SW, Hayakawa K, Miyazawa K, Hirose K, Onozaki K (2008) Cigarette smoke condensate upregulates the gene and protein expression of proinflammatory cytokines in human fibroblast-like synoviocyte line. J Interferon Cytokine Res 28:509–521
Sievers C, Meira M, Hoffmann F, Fontoura P, Kappos L, Lindberg RL (2012) Altered microRNA expression in B lymphocytes in multiple sclerosis: towards a better understanding of treatment effects. Clin Immunol 144:70–79
Takami N, Osawa K, Miura Y, Komai K, Taniguchi M, Shiraishi M, Sato K, Iguchi T, Shiozawa K, Hashiramoto A, Shiozawa S (2006) Hypermethylated promoter region of DR3, the death receptor 3 gene, in rheumatoid arthritis synovial cells. Arthritis Rheum 54:779–787
Thabet Y, Cañas F, Ghedira I, Youinou P, Mageed RA, Renaudineau Y (2012) Altered patterns of epigenetic changes in systemic lupus erythematosus and auto-antibody production: is there a link? J Autoimmun 39:154–160
Tovar-Castillo LE, Cancino-Díaz JC, García-Vázquez F, Cancino-Gómez FG, León-Dorantes G, Blancas-González F, Jiménez-Zamudio L, García-Latorre E, Cancino-Díaz ME (2007) Under-expression of VHL and over-expression of HDAC-1, HIF-1alpha, LL-37, and IAP-2 in affected skin biopsies of patients with psoriasis. Int J Dermatol 46:239–246
Wang Y, Fan PS, Kahaleh B (2006) Association between enhanced type I collagen expression and epigenetic repression of the FLI1 gene in scleroderma fibroblasts. Arthritis Rheum 54:2271–2279
Xia J, Joyce CE, Bowcock AM, Zhang W (2013) Noncanonical microRNAs and endogenous siRNAs in normal and psoriatic human skin. Hum Mol Genet 22:737–748
Xu N, Meisgen F, Butler LM, Han G, Wang XJ, Söderberg-Nauclér C, Ståhle M, Pivarcsi A, Sonkoly E (2013) MicroRNA-31 is overexpressed in psoriasis and modulates inflammatory cytokine and chemokine production in keratinocytes via targeting serine/threonine kinase 40. J Immunol 190:678–688
Xu WD, Lu MM, Pan HF, Ye DQ (2012) Association of microRNA-146a with autoimmune diseases. Inflammation 35:1525–1529
Yamamura Y, Motegi K, Kani K, Takano H, Momota Y, Aota K, Yamanoi T, Azuma M (2012) TNF-α inhibits aquaporin 5 expression in human salivary gland acinar cells via suppression of histone H4 acetylation. J Cell Mol Med 16:1766–1775
Yin H, Zhao M, Wu X, Gao F, Luo Y, Ma L, Liu S, Zhang G, Chen J, Li F, Zuo X, Lu Q (2010) Hypomethylation and overexpression of CD70 (TNFSF7) in CD4+ T cells of patients with primary Sjögren’s syndrome. J Dermatol Sci 59:198–203
Zhang K, Zhang R, Li X, Yin G, Niu X, Hou R (2007) The mRNA expression and promoter methylation status of the p16 gene in colony-forming cells with high proliferative potential in patients with psoriasis. Clin Exp Dermatol 32:702–708
Zhang K, Zhang R, Li X, Yin G, Niu X (2009) Promoter methylationstatus of p15 and p21 genes in HPP-CFCs of bone marrow of patients with psoriasis. Eur J Dermatol 19:141–146
Zhang P, Su Y, Zhao M, Huang W, Lu Q (2011) Abnormal histonemodifications in PBMCs from patients with psoriasis vulgaris. Eur J Dermatol 21:527–552
Zhang P, Su Y, Lu Q (2012) Epigenetics and psoriasis. J Eur Acad Dermatol Venereol 26:399–403
Zhou Y, Qiu X, Luo Y, Yuan J, Li Y, Zhong Q, Zhao M, Lu Q (2011) Histone modifications and methyl-CpG-binding domain protein levels at the TNFSF7 (CD70) promoter in SLE CD4+ T cells. Lupus 20:1365–1371
Zhu H, Li Y, Qu S, Luo H, Zhou Y, Wang Y, Zhao H, You Y, Xiao X, Zuo X (2012) MicroRNA expression abnormalities in limited cutaneous scleroderma and diffuse cutaneous scleroderma. J Clin Immunol 32:514–522
Zhu X, Liang J, Li F, Yang Y, Xiang L, Xu J (2011) Analysis of associations between the patterns of global DNA hypomethylation and expression of DNA methyltransferase in patients with systemic lupus erythematosus. Int J Dermatol 50:697–704
Zilahi E, Tarr T, Papp G, Griger Z, Sipka S, Zeher M (2012) Increased microRNA-146a/b, TRAF6 gene and decreased IRAK1 gene expressions in the peripheral mononuclear cells of patients with Sjögren’s syndrome. Immunol Lett 141:165–168
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Coppedè, F., Migliore, L. (2014). Epigenetics of Autoimmune Diseases. In: Maulik, N., Karagiannis, T. (eds) Molecular mechanisms and physiology of disease. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-0706-9_6
Download citation
DOI: https://doi.org/10.1007/978-1-4939-0706-9_6
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-0705-2
Online ISBN: 978-1-4939-0706-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)