Abstract
The yellow-tailed woolly monkey (Oreonax flavicauda) is Critically Endangered and endemic to a small area of the Andean forest in northern Peru. I collected data on the home ranges, daily path lengths, diet and habitat use of two groups of O. flavicauda. Group follows took place at La Esperanza, Amazonas department, for 15 months between October 2009 and February 2011. The study site comprised a matrix of disturbed primary and regenerating secondary cloud forest. Home ranges were between 95 and 147 ha using 95 % minimum convex polygons, and home range overlap between the two groups was 1.6 ha. The range used by both groups varied between the wet and dry seasons. Daily path lengths were between 1.03 and 1.2 km. Fruit was the most commonly consumed dietary item followed by leaves and insects; a total of 16 plant resources were identified. There was a significant increase in consumption of leaves and insects during the dry seasons. Both groups used a variety of habitats but were only occasionally observed to use areas of white-sand forest. Home range and daily path length estimates are similar to results from studies of other woolly monkeys (Lagothrix spp.), although home ranges were among the smallest recorded for woolly monkeys. O. flavicauda at La Esperanza are less frugivorous than Lagothrix spp., and the estimates here are lower than those from the previous preliminary work at this site. My results suggest that O. flavicauda are able to survive in disturbed habitat with small home ranges and at high group densities. More research is urgently needed at other sites with different ecological conditions to enable proper conservation planning and actions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Ecology Lagothrix flavicauda
- Ecology yellow-tailed woolly monkey
- Minimum convex polygon
- Home pange overlap
- Conservation
1 Introduction
The Peruvian yellow-tailed woolly monkey (Oreonax flavicauda) is one of the rarest and least studied of all primate taxa. This species is endemic to a small area of pre-montane and montane Andean cloud forest in the Peruvian departments of Amazonas and San Martin (Shanee 2011) as well as border areas of the neighbouring departments of Huánuco, La Libertad and Loreto (Shanee 2011; Graves and O’Neil 1980; Parker and Barkley 1981; Mittermeier et al. 1975). O. flavicauda habitat is characterized by rugged terrain of steep mountains, high ridges and deep river valleys (Shanee 2011). This area of Peru is also the centre of many sociopolitical problems caused by drug trafficking and terrorism (Shanee 2011; Young 1996; Shanee and Shanee this volume). Because of these difficulties, few previous field studies exist on this species.
Thought extinct until the mid-1970s (Mittermeier et al. 1975), O. flavicauda was ‘rediscovered’ in the department of Amazonas (Mittermeier et al. 1975; Macedo-Ruiz and Mittermeier 1979); following this, more localities and basic ecological data were published (Graves and O’Neil 1980; Parker and Barkley 1981; Leo Luna 1980; Butchart et al. 1995) along with many conservation recommendations (DeLuycker 2007; Leo Luna 1980; Rios and Ponce del Prado 1983; Shanee et al. 2008; Shanee et al. 2007). Leo-Luna (1980, 1984, 1987, 1989) carried out the first field studies on this species collecting valuable data on the species distribution, conservation, habitat preferences and diet.
Listed as Critically Endangered (CR; IUCN category A4c), O. flavicauda has been considered one of the 25 most endangered primate species by the International Primatological Society three times (Mittermeier et al. 2012). This species is also considered endangered under Peruvian law (Decreto Supremo 34-2004-AG) and listed in appendix I of Convention on International Trade in Endangered Species (CITES 2005). There is currently discussion as to this species’ taxonomic status (Oreonax vs. Lagothrix, see Matthews and Rosenberger 2008).
Similar to common woolly monkey species (Genus = Lagothrix), O. flavicauda are large-bodied diurnal primates that live in large multi-male, multi-female groups of up to 23 individuals (Shanee and Shanee 2011a, DeLuycker 2007). O. flavicauda is restricted to high elevation forests between 1,500 and 2,700 m.a.s.l. and is not found in lowland Amazonian rain forests (Shanee 2011), which is rare for woolly monkeys (but see Cifuentes et al. this volume).
Habitat at high elevations is generally characterized by lower primary production levels (Lawes 1992; Smith and Killeen 1998; Costa 2006; Bendix et al. 2008; Shanee and Peck 2008). Lowered production is a function of decreased temperature, changes in soil pH and precipitation levels, thin soils and increased exposure to solar radiation and wind (Marshall et al. 2005). Conversely, primary production levels in secondary and disturbed forests can be higher than those of the primary forests (Brown and Lugo 1990). High elevation forests, even near the Equator, are also subject to stronger seasonal fluctuations in temperatures and rainfall than lowland forests. However, some sub-Andean forests have comparable fruit production levels as lowland forests (Cifuentes et al. this volume). Species found at high elevations often show ecological adaptations to better cope with the harsher conditions. These may include changes in diet, home ranges as well as daily path lengths and seasonal ranges, group sizes and habitat use (Durham 1975; Caldecott 1980; Marshall et al. 2005; Shanee 2009). The constraints of high elevation habitats are especially important for large-bodied frugivorous primates such as woolly monkeys. The ecology of common woolly monkeys is highly variable depending on forest type and primary production levels (Di Fiore and Campbell 2010; Defler 1987; Di Fiore 2003; Stevenson 2001, 2006; Defler and Defler 1996; Stevenson and Castellanos 2000).
Home ranges are defined as the area traversed by an animal or a group during its normal activities, but occasional sallies outside the area should not be included (Burt 1943). Daily path lengths can be defined as the distance travelled by an animal or a group of animals during their active period in a 24-h cycle. Both home ranges and daily path lengths are highly dependent on habitat quality, diet, resource availability and temporal fluctuations in resource availability, particularly in seasonal environments (Milton and May 1976). Body size, group size and defensibility also play roles in determining home range sizes and daily path lengths (Milton and May 1976; Mitani and Rodman 1979; Chapman 1988; Janson and Goldsmith 1995).
There exist many previous studies on the ranging behaviour and habitat use of common woolly monkeys (Di Fiore and Campbell 2010). However, no studies as yet exist on O. flavicauda ranging and only basic data are available on this species’ habitat use and preferences (Clark 2009, Shanee and Shanee 2011a, Leo Luna 1980, 1984; Butchart et al. 1995).
I collected data on habituated groups of O. flavicauda at La Esperanza, Amazonas, Peru. This site has been the focus of several studies on this and other primate species (Shanee and Shanee 2009,2011a, b). I collected data on ranging behaviour using a geographic positioning system (GPS) during group follows, as well as data on dietary preferences, habitat types and habitat use by the monkeys. This investigation forms a part of a larger conservation initiative for the yellow-tailed woolly monkey, its habitat and sympatric species (Shanee and Shanee 2009).
2 Methods
2.1 Study Site
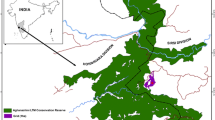
La Esperanza is located in the Comunidad Campesina de Yambrasbamba on the eastern slopes of the Andes in northeastern Peru. Two permanent camps were used (Fig. 10.1) in areas known locally as Peroles (S 5°40′9.04″, W 77°54′14.44″) and El Toro (S 5°39′11.60″, W 77°54′55.79″). The study site encompasses approximately 700 ha of disturbed primary forest and regenerating secondary forest interspersed with pasture. The study site is bounded to the south, east and west by fragmented forests, pasture and agricultural lands. To the north, contiguous forest reaches to the Río Maranon (approximately 100 km). This site lies in a natural forest corridor between five protected areas where the species’ presence has been recorded: Zona Reservada Rio Nieva, Santuario Nacional Cordillera Colan, Bosque de Proteccion Alto Mayo, Area de Conservacion Privada Abra Patricia-Alta Nieva and the Area de Conservacion Privada Pamapa del Burro (Fig. 10.1). Peroles is located about 4 km north of the village of La Esperanza; El Toro is a further 2 km north. Both areas cover an altitudinal range of between 1,800 and 2,600 m.a.s.l. Average monthly rainfall is approximately 1,500 mm, with a dry season from August to December. Average temperature for the area is 14 °C (± 5.7). Humidity is high year-round.
The terrain is very rugged with high ridges and deep valleys. Habitat in the area is characterized by Ficus spp.-dominated primary pre-montane and montane cloud forest, with a thick mid-storey and understorey (Daoudi 2011; Clark 2009). The area has seen low intensity logging over the past 30 years which has removed many mature timber species.
Study Groups
Data were collected on three groups of O. flavicauda at the two camps, resulting in 281 h of group follows. Average group size in the area was 10.7 individuals (range 3–19), including all full counts. Because of an uneven distribution of data across the three groups, I only calculated home ranges and seasonal ranges for the two groups (group A and group B) that presented the most data. Group A consisted of 15 individuals (three adult male; three adult female; six sub-adult/juvenile and three infants) and group B of 12 individuals (two adult male; five adult female; one sub-adult/juvenile and two infants).
2.2 Data Collection
2.2.1 Ranging and Path Lengths
I collected data for 15 months between October 2009 and June 2010 and between August 2010 and February 2011. Prior to this study, the area had been used for investigation on O. flavicauda, the sympatric Peruvian night monkey (Aotus miconax) and habitat characterization (Shanee and Shanee 2011a, b; Clark 2009). Therefore, clearance of new trails and other potentially disturbing preparatory work was kept to a minimum. The two study areas had been heavily logged and hunted during the 1980s and 1990s. However, these practices were not recorded during this study and were much reduced during the previous work (Shanee and Shanee 2011a, b), and primate populations in the area had previously recovered. No systematic habituation process was used on any of the study groups, as preparatory investigation suggested this was unnecessary because of the focal groups’ familiarity with human presence (Shanee and Shanee 2011b). Bimonthly 5-day field trips were made throughout the study period. Data on ranging were collected during group follows using handheld GPS units (Garmin Etrex and Garmin GPSMap 60CSx). Fieldwork began at sunrise (approximately 0630 h) and continued until just after sunset (approximately 1900 h). Group follows were conducted by teams of two to three observers including a local field guide. When groups were encountered, their location was recorded as a GPS point. During group follows, I recorded additional GPS points whenever the group stopped at a new location (i.e. they were no longer moving as a group in a single direction). GPS points were recorded at the approximate centre of the group. Whenever possible, we tracked groups to a sleeping site at sunset and began the subsequent days’ follow from the sleeping site prior to their waking.
2.2.2 Diet and Habitat Use
During group follows, I recorded data on food sources used by individuals. I recorded the type of food consumed by focal individuals for the duration of feeding bouts, recording food type every 2 min . Food types were divided into the following categories: fruit, leaf, flower, bud, insect and moss. When possible field identification was made of the plant species consumed; alternatively, when field identification was not possible, samples were taken and stored in a field press to be identified later. Identification of plants was made using previously established keys. When identification was not possible, I took samples to local botanists from the Universidad Nacional Toribio Rodriguez de Mendoza and the Servicio Nacional de Areas Naturales Protegidas. Plant samples were also deposited at the Herbarium of the Universidad Nacional Mayor de San Marcos in Lima .
I made a habitat map (Fig. 10.2) of the study area using data from previous work in the area (Clark 2009, unpublished data) and information from the Zoneficacion Ecologica Economica of Amazonas department (IIAP 2008). These maps defined areas of forest based on elevation, soil, dominant vegetation type and topographical features such as valley, slope and ridge top. I recorded the elevation in metres above sea level of the approximate centre of the group every time I took a GPS point .
2.2.3 Data Analysis
Ranging and Path Lengths
I entered GPS point data into a geographic information system (arcGIS 9.3, ESRI 2008) and analysed it using the Home Range Tools extension (Version 1.1, Rodgers and Kie 2011). I separated points into focal groups and by season prior to estimating home range sizes, seasonal ranges and daily path length. I used two common methods to estimate home and seasonal ranges: percentage minimum convex polygons (MCPs; Michener 1979) and kernel density estimation (KDE; Worton 1989). I fit various models to find the most appropriate for the data. Before I began the Analysis, I cheked the data for duplicate points. I calculated MCPs using the fixed mean method selecting 90, 95 and 99 % of points.
For KDE, I checked the grouped points for autocorrelation and calculated the bandwidth (h). Using different models and proportions of the reference bandwidth, I calculated home ranges and seasonal ranges at 90 and 95 %. When range estimates from MCPs or KDE overlapped with deforested areas, I clipped the resulting maps to exclude these areas (Grueter et al. 2009). I also calculated territorial overlap between the groups for both home and seasonal ranges.
I calculated daily path lengths by converting point data to polylines in arcGIS using the X-Tools Pro extension (Version 8.1). I then measured the lengths of the polylines from full-day and half-day follows. I included distances travelled in half-day follows to increase the sample size (Fashing and Cords 2000; van Schaik et al. 1983). Half-day follows were evenly distributed between morning and afternoon follows to avoid possible bias (van Schaik et al. 1983). I calculated average daily path lengths for each group over the whole study period and seasonally.
Diet and Habitat Use
I evaluated habitat use of the groups by overlaying home and seasonal range polygons on top of habitat maps to evaluate the use of the different forest types in the study area. This map was used to clip areas of unused habitat types from range estimates to improve accuracy (Grueter et al. 2009). Similarly, dietary data were collected during the same full- and half-day follows as ranging data using instantaneous sampling on focal animals every 2 min, recording the food type consumed (Shanee and Shanee 2011b). I analysed seasonal dietary preferences shown by focal animals during follows, looking for differences in consumption of food types and sources.
3 Results
3.1 Ranging
I analysed the data without rescaling for variance using adaptive kernels with a simple Gaussian (bivariate) model. Home ranges using MCPs at 95 % were 147 ha for group A and 95 ha for group B. Home range overlap between the two groups at 95 % coverage was 1.6 ha (Fig. 10.3). Seasonal ranges for the two groups were 120 ha for group A and 58 ha for group B during the dry season, and 82 ha for group A and 92 ha for group B during the wet season, giving an average home range of 121 ha and average seasonal ranges of 89 ha in the dry season and 87 ha in the wet season. No significant difference was found between home range estimates at 95 %.
Home ranges using KDE at 95 % were 236 ha for group A and 200 ha for group B, and home range overlap was 30 ha (Fig. 10.4). Seasonal home ranges at 95 % were 175 ha for group A and 164 ha for group B in the dry season, and 165 ha for group A and 163 ha for group B in the wet season. Seasonal range overlap at 95 % was 13 ha in the dry season and 22 ha in the wet season. No significant difference was observed between estimated home ranges at 95 %.
Results from MCP analysis were similar to estimates from previous density surveys and ad-lib observations (Shanee and Shanee 2011a ); however, results from KDE were ~ 200 % larger. Complete results for both groups at 90, 95 and 99 % are given in Table 10.1.
3.2 Daily Path Lengths
Average daily path lengths were 1.03 km (SD 0.6) and 1.2 km (SD 0.9) for group A and B, respectively. Average seasonal daily path lengths were 1.7 km (SD 0.6) and 1.3 km (SD 0.9) in the dry season and 0.8 km (SD 0.4) and 0.6 km (SD 0.4) in the wet season, giving an average daily path length of 1.0 km (SD 0.8) and average daily path lengths of 1.4 km (SD 0.9) in the dry season and 0.8 km (SD 0.4) in the wet season.
Significant differences were found between daily path lengths in the dry and wet seasons for both groups (χ 2 = 16.01, P = 0.0001 for group A and χ 2 = 12.60, P = 0.0004 for group B). Differences in average path lengths including both groups were not significant (χ 2 = 0.371, P = 0.542).
3.3 Diet and Habitat Use
Diet
The most common dietary item was fruit (46.3 % of recorded items) followed by leaves (23.3 %) and insects (19.1 %). Moss (6.6 %), buds (2.8 %) and flowers (1.8 %) were also consumed. I recorded a large difference in the consumption of fruits between the wet and dry seasons, 48.6 and 40.2 %, as well as leaves, 20.4 and 35.9 %, and insects, 20.2 and 7.2 %. There was also an increase in consumption of all non-fruit food items during the dry season. Differences in leaf consumption (χ 2 = 21.21, P = 0.0001) and insect consumption (χ 2 = 31.68, P = 0.0001) were found to be significant.
I identified 16 different plant food sources used by O. flavicauda. By far the most common food source was Ficus spp., followed by Cecropia montanta, C. utcubambana, Styloceras laurifolium and Chrysophyllum venezuelanense (Table 10.2). In most cases, multiple plant parts were consumed from each food source.
Habitat Use
The study site consisted of a matrix of Ficus spp.-dominated disturbed primary cloud forest, white-sand forest, bamboo-dominated forest, ridge-top forest and deforested areas (Fig. 10.2). During follows, O. flavicauda did not enter areas of white-sand forest but did occasionally use this forest type during ad-lib observations; similarly, the groups only occasionally entered areas of bamboo-dominated forest and ridge-top forest, usually when passing between areas of Ficus spp.-dominated forest. The groups used all elevations in the study area, average 2,138 m.a.s.l. (min. 1,836 m, max. 2,429 m).
4 Discussion
Ninety-five percent MCPs gave the best results when compared to estimates of range sizes and group densities from transect surveys (Table 10.1). This is similar to findings from work on northern bearded sakis (Chiropotes satanas chiropotes; Boyle et al. 2009). Also, MCPs have been found to be more robust when sample sizes are small (Grueter et al. 2009). Home and seasonal range methods from KDE were much larger than those from MCPs and around 200 % greater than range sizes and densities from transect surveys (Table 10.1); although other studies have shown that KDEs can give accurate results (Grueter et al. 2009; Seaman and Powell 1996; Worton 1995), in this case, they seem to significantly overestimate home ranges.
MCPs can overestimate ranges with the inclusion of outlying points and if areas of un-used habitat are not removed (Grueter et al. 2009). By creating a habitat map and removing areas of unsuitable habitat, I have minimized this problem (Grueter et al. 2009); similarly, by choosing polygons at 95 % inclusion, I have reduced the effect the outlying points may have had, for example MCPs for group B at 99 % (Fig. 10.3) extended the estimated home range by ~ 25 % (35 ha) with the inclusion of a single point.
Home ranges of other woolly monkey species are highly variable between species and sites (Table 10.3). Unfortunately, no other home range estimates exist from other studies of O. flavicauda, so comparison between sites and methods is impossible. Compared to home range estimates from previous studies of other woolly monkey species, the results presented here show much smaller home range estimates than from most studies (Table 10.4) but are similar to estimates for Lagothrix poepigii at the Yasuni National Park, Ecuador, and L. lagothricha at the Tinigua National Park, Colombia, using quadrat methods and focal animal sampling, respectively (Di Fiore 2003; Stevenson 2006). The variation in Lagothrix spp. range sizes is possibly due to differences in group sizes, with larger groups necessitating larger areas to forage (Milton and May 1976; Dunbar 1988), although habitat quality and resource availability have also been shown to determine differences in some populations (Gonzalez and Stevenson 2009). In the case of O. flavicauda, reduced access to resources at elevation could necessitate reduced group sizes, as increasing home range sizes would unsustainably increase energetic demands, which may increase with elevation.
Again, no estimates exist for O. flavicauda path lengths from other studies. Daily path lengths found in this study are well within the limits of those found for other woolly monkey species, as are maximum path lengths (Table 10.3).
Defler (1996) found large monthly differences in areas of home range used by a group of L. lagothricha at Caparu, Colombia. Stevenson (2006) found a correlation between the increased use of flooded forest during the rainy season, when certain fruits are more abundant, in a group of L. lagothricha at Tinigua National Park, Colombia. In the same study, significant relationships were found between path length and seasonal fruit availability, habitat quality and group sizes. Daily path lengths for both groups in this study were significantly larger, and average home range increased during the dry season which coincides with reduced availability of fruits.
No study has looked at soil fertility at this or other sites with O. flavicauda, but the naturally lower primary production levels, a characteristic of high elevation forests (Lawes 1992; Smith and Killeen 1998; Costa 2006; Bendix et al. 2008; Shanee and Peck 2008), would suggest that this study site would be a suboptimal habitat for frugivorous primates. Habitat quality may also be low because of previous anthropogenic activities, such as logging. However, this will be at least partially offset by the higher production levels that can be found in secondary and regenerating areas (Brown and Lugo 1990). In such cases, logged forests can support high densities of Ateline primates when hunting pressure is low (Aldana et al. 2008). The densities calculated from range estimates indicate a healthy O. flavicauda population at La Esperanza and provide further evidence that this species is able to survive even in disturbed habitat when hunting pressure is low (Shanee and Shanee 2011a). However, more studies are needed to determine densities and ranges at undisturbed sites before conclusions can be drawn.
Leo Luna (1980) gives some details of O. flavicauda diets, finding that fruits are the major food source for this species and that leaves are rarely consumed, with Ficus spp. and Cecropia spp. being important food sources. Both are important food sources for O. flavicauda at La Esperanza. The diet of yellow-tailed woolly monkeys at La Esperanza is much less frugivorous than that of Lagothrix spp. (Defler and Defler 1996; Stevenson 1992; Stevenson et al. 1994; Soini 1990; Di Fiore 1997, Dew 2001) and previous preliminary estimates for O. flavicauda of 71 % (Table 10.4; Shanee and Shanee 2011a). Ripe fruits are replaced in the diet of this species with a higher consumption of leaves and other food items during the dry season when fruits are less available, lessening the need for larger home ranges.
Patterns of habitat use found were fairly simple, with the majority of available habitat being used year-round. The avoidance of white-sand forest will certainly be because of the lack of large fruiting trees in this forest type, which also has a much lower canopy height and thicker mid-storey and understorey (unpublished data). Although this species has been recorded in white-sand forest occasionally, it is not clear to what extent it is used. Similarly, areas of bamboo-dominated forest and ridge tops were only occasionally used, generally to gain access to other areas of forest. Stevenson (2006) found that common woolly monkeys preferred mature forests to open or degraded areas in Tinigua National Park, Colombia. Soils in white-sand forests are much more acidic (Escobedo Torres 2007), in many cases prohibiting the growth of many of O. flavicauda’s preferred food sources. Increased exposure to wind, solar radiation, thinner soils and decreased temperatures on ridge tops also causes differences in forest structure with reduced presence of large fruiting trees. This is in addition to reduced productivity of O. flavicauda’s preferred food source (Ficus spp.) with increasing elevation (Shanee and Peck 2008).
My results suggest that O. flavicauda is able to survive in highly disturbed habitats, with restricted ranges, allowing the species to live at comparatively high group densities. However, more research is urgently needed on this species’ habitat requirements and ecology at more sites. Without data from other sites, a proper understanding of O. flavicauda’s requirements is impossible and informed conservation actions cannot be planned or implemented.
References
Aldana AM, Beltran M, Torres-Neira J, Stevenson PR (2008) Habitat characterization and population density of brown spider monkeys (Ateles hybridus) in Magdalena Valley, Colombia. Neotrop Primates 15:46–50
Bendix J, Rollenbeck R, Richter M, Fabian P, Emck P (2008) Climate. In: Beck E, Bendix J, Kottke I, Makeschin F, Mosandl R (eds) Gradients in a tropical mountain ecosystem of Ecuador. Springer-Verlag, Berlin, p 63–74
Boyle SA, Lourenco WC, da Silva LR, Smith AT (2009) Home range estimates vary with simple size and methods. Folia Primatol 80:33–42
Brown S, Lugo AE (1990) Tropical secondary forests. J Trop Ecol 6:1–32
Burt WH (1943) Territoriality and home range concepts as applied to mammals. J Mammal 24:346–352
Butchart SHM, Barnes R, Davies CWN, Fernandez M, Seddon N (1995) Observations of two threatened primates in the Peruvian Andes. Primate Conserv 16:15–19
Caldecott JO (1980) Habitat quality and populations of two sympatric gibbons (Hylobatidae) on a mountain in Malaya. Folia Primatol 33:291–309
Chapman C (1988) Patterns of foraging and range use by three species of Neotropical primates. Primates 29:177–194
CITES (2005) Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES). http://www.cites.org/eng/app/appendices.shtml. Accessed 20 Nov 2009
Clark JC (2009) A comparison of two habitats used by the critically endangered yellow-tailed woolly monkey (Oreonax flavicauda). MSc Dissertation, Oxford Brookes University, Oxford
Costa FRC (2006) Mesoscale gradients of herb richness and abundance in central amazonia. Biotropica 38:711–717
Defler TR (1989) Recorrido y uso de del espacio en un grupo de Lagothrix lagothricha (Primates: Cebidae) mono lanudo churuco en la Amazonia Colombiana. Trianea 3:183–205
Defler TR (1987) Ranging and the use of space in a group of woolly monkeys (Lagothrix lagothricha) in the NW Amazon of Colombia. Int J Primatol 8:420–420
Defler TR (1996) Aspects of the ranging pattern in a group of wild woolly monkeys (Lagothrix lagothricha). Am J Primatol 38:289–302
Defler TR, Defler SB (1996) Diet of a group of Lagothrix lagothricha lagothricha in southeastern Colombia. Int J Primatol 16:107–120
DeLuycker AM (2007) Notes on the yellow-tailed woolly monkey (Oreonax flavicauda) and its status in the protected forest of Alto Mayo, northern Peru. Primate Conserv 22:41–47
Dew JL (2001) Synecological and seed dispersal patterns in woolly monkeys (Lagothrix lagothricha peoppigii) and spider monkeys (Ateles belzebuth belzebuth) in Perque Nacional Yasuni, Ecuador. PhD Dissertation, Univeristy of California, Davis
Di Fiore A (1997) Ecology and behaviour of lowland woolly monkeys (Lagothrix lagothricha peoppigii, Atelinae) in eastern Ecuador. PhD Dissertation, University of California, Davis
Di Fiore A (2003) Ranging behavior and foraging ecology of lowland woolly monkeys (Lagothrix lagothricha poeppgii) in Yasuni national park, Ecuador. Am J Primatol 59:47–56
Di Fiore A (2004) Diet and feeding ecology of woolly monkeys in a western Amazonian rainforest. Int J Primatol 24:767–801
Di Fiore A, Campbell CJ (2010) The atelines: variation in ecology, behavior and social organization. In: Campbell CJ, Fuentes A, Mackinnon KC, Bearder SK, Stumpf RM (eds) Primates in perspective, 2nd edn. Oxford University Press, Oxford, p 156–185
Daoudi S (2011) Using detection/non-detection surveys for modelling occupancy of the Critically Endangered yellow-tailed woolly monkey (Oreonax flavicauda) in El Torro and Cordillera Colan, Peru. MSc Dissertation, Oxford Brookes University, Oxford
Dunbar RIM (1988) Primate social systems. Cornell University Press, Ithaca
Durham NM (1975) Some ecological, distributional and group behavioural features of Atelinae in southern Peru: with comments on interspecific relations. In: Tuttle RT (ed) Socioecology and psychology of primates. Mouton & Co, Netherlands, p 87–102
Escobedo Torres R (2007) Suelo y capacidad de uso mayor de la tierra. Zonificacion Ecologica Economica del Departamento de Amazonas. Instituto de Investigacionde la Amazonia Peruana, Iquitos
ESRI (2008) GIS for wildlife conservation, GIS best practices. Environmental Systems Research Institute (ESRI). Redlands, CA. http://www.esri.com/library/bestpractices/wildlife-conservation.pdf. Accessed 18 Jan 2008
Fashing PJ, Cords M (2000) Diurnal primate densities and biomass in the Kakamega forest: an evaluation of census methods and a comparison with other forests. Am J Primatol 50:139–152
Gonzalez M, Stevenson PR (2009) Patterns of daily movement, activities and diet in woolly monkeys (genus Lagothrix): a comparison between sites and methodologies. In: Potoki E, Krasinski J (eds) Primatology: theories, methods and research. Nova Science Publishers Inc, New York, p 171–186
Graves GR, O’Neill JP (1980) Notes on the yellow tailed woolly monkey (Oreonax flavicauda) of Peru. J Mammal 61:345–347
Grueter CC, Li D, Ren B, Wei F (2009) Choice of analytical method can have dramatic effects on primate home range estimates. Primates 50:81–84
IIAP (2008) Propuesta de zonificacion ecologica y economica del departamento de Amazonas/IIAP/Peru. Instituto de Investigaciones de la Amazonía Peruana (IIAP), Iquitos, Loreto, Peru. http://www.iiap.org.pe/IIAPinfo.aspx Accessed 18 Jan 2008
Janson CH, Goldsmith ML (1995) Predicting group size in primates: foraging costs and predation risks. Beahvioral Ecology 6:326–336
Krebs CJ (1999) Ecological methodology. Benjamin Cummings, Menlo Park
Lawes MJ (1992) Estimates of population density and correlates of the status of the samango monkey Cercopithecus mitis in Natal, South Africa. Biolog Conserv 60:197–210
Leo Luna M (1980) First field study of the yellow-tailed woolly monkey. Oryx 15:386–389
Leo Luna M (1984) The effect of hunting, sellective logging and clear cutting on the conservation of the yellow-tailed woolly monkey (Oreonax flavicauda). Masters Dissertation, University of Florida, FL
Leo Luna M (1987) Primate conservation in Peru: a case study of the yellow-tailed woolly monkey. Primate Conserv 8:122–123
Leo Luna M (1989) Biología y conservación del mono choro de cola amarilla (Oreonax flavicauda), especie en peligro de extinción. In: Saavedra CJ, Mittermeier RA, Santos IB (eds) La Primatologia en Latinoamérica. World Wildlife Fund-US, Washington, DC, p 23–30
Marshall AR, Topp-Jorgensen JE, Brink H, Fanning E (2005) Monkey abundance and social structure in two high-elevation forest reserves in the Udzungwa mountains of Tanzania. Int J Primatol 26:127–145
Mathews LJ, Rosenberger AL (2008) Oreonax: not a genus. Neotrop Primates 15:8–12
Macedo Ruiz H, Mittermeier RH (1979) Rediscubrimiento del primate Peruano Oreonax flavicauda (Humboldt 1912) y primeras observaciones sobre su biología. Rev Ciencia 71:70–92
Michener GR (1979) Spatial relationships and social organization of adult Richardsons ground squirrels. Can J Zool 57:125–139
Milton K, May ML (1976) Body weight, diet and home range area in primates. Nature 259:459–462
Mitani JC, Rodman PS (1979) Territoriality: the relationship of ranging pattern and home range size to defendability, with an analysis of territoriality among primate species. Behav Ecol Sociobiol 5:241–251
Mittermeier RA, de Macedo RH, Luscombe A (1975) A woolly monkey rediscovered in Peru. Oryx 13:41–46
Mittermeier RA, Rylands AB, Schwitzer C, Taylor LA, Chiozza F, Williamson EA (2012) Primates in Peril: the world’s 25 most endangered primates 2010–2012. IUCN/SSC Primate Specialist Group (PSG), International Primatological Society (IPS), and Conservation International (CI), Arlington, 40 p.
Nishimura A (1990) A sociological and behavioral study of woolly monkeys, Lagothrix lagothricha, in the upper Amazon. Sci Eng Rev Doshisha U 31:87–121
Parker TA, Barkley LJ (1981) New locality for the yellow-tailed woolly monkey. Oryx 26:71–72
Peres CA (1996) Use of space, spatial group structure, and foraging group size of grey woolly monkeys (Lagothrix lagothricha cana) at Urucu, Brazil. In: Norconk MA, Rosenberger AL, Garber PA (eds) Adaptive radiations of neotropical primates. Plenum press, New York, p 467–488
Peres CA (1994) Diet and feeding ecology of gray woolly monkeys (Lagothrix lagotricha cana) in Central Amazonia: comparisons with other Atelines. int J Primatol 15:333–372
Ramirez M (1980) Grouping patterns of the woolly monkeys, Lagothrix lagothricha, at the Manu National Park, Peru. Am J Phys Anthropol 52:269
Rios M, Ponce del Prado CF (1983) El status de las áreas de conservación propuestas para choro de cola amarilla (Lagothrix flavicauda): una investigación sobre la planificación regional de áreas naturales protegidas. In: Saavedra CJ, Mittermeier RA, Santos IB (eds) La primatologia en Latinoamerica. World Wildlife Fund–US, Washington, DC, pp 31–65
Rodgers AR, Kie JG (2011) HRT: home range tools for ArcGIS. Draft 2011. Center for Ecosystem Research, Ontario Museum of Natural History, Ontario
Seaman DE, Powell RA (1996) An evaluation of the accuracy of kernel density estimators for home range analysis. Ecol 77:2075–2085
Shanee S (2009) Modelling spider monkeys Ateles spp. Gray, 1825: ecological responses and conservation implications to increased elevation. J Threatened Taxa 1:450–456
Shanee S (2011) Distribution survey and threat assessment of the yellow tailed woolly monkey (Oreonax flavicauda; Humboldt 1812), north eastern Peru. Int J Primatol 32:691–707
Shanee S, Peck MR (2008) Elevational changes in a neotropical fig (Ficus spp.) community in north western Ecuador. iForest 1:104–106
Shanee S, Shanee N (2009) A new conservation NGO, neotropical primate conservation: project experiences in Peru. Int NGO J 4:329–332
Shanee S, Shanee N (2011a) Population density estimates for the critically endangered yellow tailed woolly monkey (Oreonax flavicauda) at La Esperanza, northeastern Peru. Int J Primatol 32:691–180
Shanee S, Shanee N (2011b) Activity budget and behavioural patterns of free-ranging yellow-tailed woolly monkeys Oreonax flavicauda (Mammalia: Primates), at La Esperanza, northeastern Peru. Cont to Zool 80:269–277
Shanee N, Shanee S, Maldonado AM (2007) Conservation assessment and planning for the yellow-tailed woolly monkey in Peru. Wildl Biol Prac 3:73–82
Shanee S, Shanee N, Maldonado AM (2008) Distribution and conservation status of the yellow-tailed woolly monkey (Oreonax flavicauda [Humboldt 1812]) in Amazonas and San Martín, Peru. Neotrop Primates 14:115–119
Smith ND, Killeen TJ (1998) A comparison of the structure and composition of montane and lowland tropical forest in the serranía Pilón Lajas, Beni, Bolivia. In: Dallmeier F, Comiskey JA (eds) Forest biodiversity in north, central and South America, and the Caribbean. Man Biosph 21:681–700
Soini P (1986) A synecological study of a primate community in the Pacaya-Samiria National reserve, Peru. Primate Conserv 7:63–71
Soini P (1990) Ecologia y dinamica poblacional del “Choro” (Lagothrix lagotricha, Primates) en el Rio Pacaya, Peru. In: La Primatologia en el Peru. Proyecto Peruano de Primatologia, Lima, p 382–396
Stevenson PR (1992) Diet of woolly monkeys (Lagothrix lagotricha) at La Macarena, Colombia. Field Studies of New World Monkeys, La Macarena. Colombia 6:3–14
Stevenson PR (2001) The relationship between fruit production and primate abundance in Neotropical forests. Biol J Linn Soc 72:161–178
Stevenson PR (2002) Frugivory and seed dispersal by woolly monkeys at Tinigua National Park, Colombia. Phd Dissertation. State University of New York, Stony Brook
Stevenson PR (2006) Activity and ranging patterns of Colombian woolly monkeys in north-western Amazonia. Primates 47:239–247
Stevenson PR, Castellanos MC (2000) Feeding rates and daily path range of the Colombian woolly monkeys as evidence for between- and within-group competition. Folia Primatol 71:399–408
Stevenson PR, Quinones MJ, Ahumada JA (1994) Ecological strategies of woolly monkeys (Lagothrix lagothricha) at Tinigua National Park, Colombia. Am J Primatol 32:123–140
van Schaik VP, van Noordwijk MA, de Boer RJ, den Tonkelaar I (1983) The effect of group size on time budgets and social behaviour in wild long-tailed macaques (Macaca fascicularis). Behav Ecol Sociobiol 13:173–181
Worton BJ (1989) Kernal methods for estimating the utilization distribution in home-range studies. Ecol 70:164–168
Worton JB (1995) Using monte carlo simulation to evaluate kernal-based home range estimators. J Wildl Manage 59:794–800
Young KR (1996) Threats to biological diversity caused by coca/cocaine deforestation in Peru. Environ Conserv 23:7–15
Acknowledgments
I wish to thank Noga Shanee, Nestor Allgas Marchena, Keefe Keely, Jermaine Clark, Josefin Sundberg, Linda Romero, Karla Ramirez and many other researchers for their help in the field and with analysis. Also, Leyda Rimerachin and Oscar Gammara Torres for help in identifying botanical samples. This work was funded by the Neotropical Primate Conservation, thanks to grants from the International Primate Protection League—UK, Wild Futures, Apenheul Primate Conservation Trust, La Vallee des Singes, International Primate Protection League—US, Primate Conservation Inc., Community Conservation and the Margot Marsh Biodiversity Foundation. I would also like to thank the Instituto Nacional de Recursos Naturales/Ministerio de Agricultura and Dirección General de Flora y Fauna Silvestre/Ministerio de Agricultura for permission to carry out this investigation (Autorización N 122-2008-INRENA-IFFS-DCB, N 102-2009-AG-DGFFS-DGEFFS and N 384-2010-AG-DGFFS-DGEFFS), the Universidad Nacional Torribeo Rodriguez de Mendoza, Servicio Nacional de Areas Naturales Protegidas, Instituto de Investigación de la Amazonia Peruana, Sociedad Peruana de Derecho Ambiental and the Asociación Peruana para la Conservación de la Naturaleza. Finally, I thank the countless local authorities and campesinos for all their help and guidance during fieldwork.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer New York
About this chapter
Cite this chapter
Shanee, S. (2014). Ranging Behaviour, Daily Path Lengths, Diet and Habitat Use of Yellow-Tailed Woolly Monkeys (Lagothrix flavicauda) at La Esperanza, Peru. In: Defler, T., Stevenson, P. (eds) The Woolly Monkey. Developments in Primatology: Progress and Prospects, vol 39. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-0697-0_10
Download citation
DOI: https://doi.org/10.1007/978-1-4939-0697-0_10
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-0696-3
Online ISBN: 978-1-4939-0697-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)