Abstract
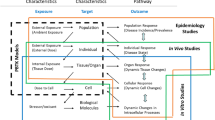
In the new paradigm of toxicology, risk assessment of chemicals needs to be based on a proper interpretation of the compound’s mechanism of action. When these assessments are based on in silico and in vitro prediction models, the quantification of the toxicodynamic as well as biokinetic parameters becomes essential. For this purpose, well-designed in vitro toxicity tests need to be used that are relevant for the risk situation to be evaluated. Furthermore, in vitro data may be applied in constructing physiologically based biokinetic models. Parameters that can be derived either from in vitro data or from in silico predictions include estimates of absorption, partitioning, metabolism, and excretion. The combination of in vitro toxicodynamics and biokinetic modelling in integrated testing strategies has proven to be a useful concept that can be applied in the process of human risk assessments.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
HCN 2001: Health Council of the Netherlands (2001) Toxicity testing: a more efficient approach. The Hague: Health Council of the Netherlands, 2001, The Netherlands; publication no. 2001/24E. ISBN 90-5549-415-1
NRC: National Research Council (2007) Toxicity testing in the 21st century: a vision and a strategy. National Academy Press, Washington, DC
Jennings P (2013) Stress response pathways, toxicity pathways and adverse outcome pathways. Arch Toxicol 87(1):13–14
Murk AJ, Rijntjes E, Blaauboer BJ, Clewell R, Crofton KM, Dingemans MML, David Furlow J, Kavlock R, Köhrle J, Opitz R, Traas T, Visser TJ, Xia M, Gutleb AC (2013) Mechanism-based testing strategy using in vitro approaches for identification of thyroid hormone disrupting chemicals. Toxicol In Vitro 27(4):1320–1346
Blaauboer BJ, Boekelheide K, Clewell HJ, Daneshian M, Dingemans MML, Goldberg AM, Heneweer M, Jaworska J, Kramer NI, Leist M, Seibert H, Testai E, Vandebriel RJ, Yager JD, Zurlo J (2012) The use of biomarkers of toxicity for integrating in vitro hazard estimates into risk assessment for humans. ALTEX 29(4):411–425
Eisenbrand G, Pool-Zobel B, Baker V, Balls M, Blaauboer BJ, Boobis A, Carere A, Kevekordes S, Lhuguenot JC, Pieters R, Kleiner J (2002) Methods of in vitro toxicology. Food Chem Toxicol 40(2–3):193–236
Punt A, Schiffelers MJWA, Jean Horbach G, van de Sandt JJM, Groothuis GMM, Rietjens IMCM, Blaauboer BJ (2011) Evaluation of research activities and research needs to increase the impact and applicability of alternative testing strategies in risk assessment practice. Regul Toxicol Pharmacol 61(1):105–114
Vermeire T, Munns WR Jr, Sekizawa J, Suter G, Van Der Kraak G (2007) An assessment of integrated risk assessment. Hum Ecol Risk Assess 13(2):339–354
Kramer NI, Van Eijkeren JCH, Hermens JLM (2007) Influence of albumin on sorption kinetics in solid-phase microextraction: consequences for chemical analyses and uptake processes. Anal Chem 79(18):6941–6948
Kramer NI, Hermens JLM, Schirmer K (2009) The influence of modes of action and physicochemical properties of chemicals on the correlation between in vitro and acute fish toxicity data. Toxicol In Vitro 23(7):1372–1379
Kramer NI, Krismartina M, Rico-Rico A, Blaauboer BJ, Hermens JLM (2012) Quantifying processes determining the free concentration of phenanthrene in basal cytotoxicity assays. Chem Res Toxicol 25(2):436–445
Groothuis FA, Heringa MB, Nicol B, Hermens JLM, Blaauboer BJ, Kramer NI (2014) Dose metric considerations in in vitro assays to improve quantitative in vitro-in vivo dose extrapolations. Toxicology. doi:10.1016/j.tox.2013.08.012
Blaauboer BJ (2010) Biokinetic modeling and in vitro-in vivo extrapolations. J Toxicol Environ Health B Crit Rev 13(2–4):242–252
Yoon M, Campbell JL, Andersen ME, Clewell HJ (2012) Quantitative in vitro to in vivo extrapolation of cell-based toxicity assay results. Crit Rev Toxicol 42(8):633–652
Broeders JJW, Blaauboer BJ, Hermens JLM (2013) In vitro biokinetics of chlorpromazine and the influence of different dose metrics on effect concentrations for cytotoxicity in Balb/c 3T3, Caco-2 and HepaRG cell cultures. Toxicol In Vitro 27(3):1057–1064
van Midwoud PM, Merema MT, Verpoorte E, Groothuis GMM (2011) Microfluidics enables small-scale tissue-based drug metabolism studies with scarce human tissue. J Lab Autom 16(6):468–476
Sivagnanam V, Gijs MAM (2013) Exploring living multicellular organisms, organs, and tissues using microfluidic systems. Chem Rev 113(5):3214–3247
Coecke S, Ahr H, Blaauboer BJ, Bremer S, Casati S, Castell J, Combes R, Corvi R, Crespi CL, Cunningham ML, Elaut G, Eletti B, Freidig A, Gennari A, Ghersi-Egea JF, Guillouzo A, Hartung T, Hoet P, Ingelman-Sundberg M, Munn S, Janssens W, Ladstetter B, Leahy D, Long A, Meneguz A, Monshouwer M, Morath S, Nagelkerke F, Pelkonen O, Ponti J, Prieto P, Richert L, Sabbioni E, Schaack B, Steiling W, Testai E, Vericat JA, Worth A (2006) Metabolism: a bottleneck in in vitro toxicological test development. Altern Lab Anim 34(1):49–84
Coecke S, Pelkonen O, Leite SB, Bernauer U, Bessems JGM, Bois FY, Gundert-Remy U, Loizou G, Testai E, Zaldívar JM (2013) Toxicokinetics as a key to the integrated toxicity risk assessment based primarily on non-animal approaches. Toxicol In Vitro 27(5):1570–1577
Bouvier d’Yvoire M, Prieto P, Blaauboer BJ, Bois FY, Boobis A, Brochot C, Coecke S, Freidig A, Gundert-Remy U, Hartung T, Jacobs MN, Lavé T, Leahy DE, Lennernäs H, Loizou GD, Meek B, Pease C, Rowland M, Spendiff M, Yang J, Zeilmaker M (2007) Physiologically-based kinetic modelling (PBK modelling): meeting the 3Rs agenda. The report and recommendations of ECVAM workshop 63. Altern Lab Anim 35:661–671
Bois FY, Jamei M, Clewell HJ (2010) PBPK modelling of inter-individual variability in the pharmacokinetics of environmental chemicals. Toxicology 278(3):256–267
Prieto P, Blaauboer BJ, De Boer AG, Boveri M, Cecchelli R, Clemedson C, Coecke S, Forsby A, Galla HJ, Garberg P, Greenwood J, Price A, Tähti H (2004) Blood-brain barrier in vitro models and their application in toxicology: the report and recommendations of ECVAM workshop 49. Altern Lab Anim 32(1):37–50
Kell DB, Dobson PD, Bilsland E, Oliver SG (2013) The promiscuous binding of pharmaceutical drugs and their transporter-mediated uptake into cells: what we (need to) know and how we can do so. Drug Discov Today 18(5–6):218–239
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP (1997) Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health 13(4):407–484
Fiserova-Bergerova V, Diaz ML (1986) Determination and prediction of tissue-gas partition coefficients. Int Arch Occup Environ Health 58:75–87
Poulin P, Krishnan K (1995) An algorithm for predicting tissue: blood partition coefficients of organic chemicals from n-octanol: water partition coefficient data. J Toxicol Environ Health 46(1):117–129
DeJongh J, Verhaar HJM, Hermens JLM (1997) A quantitative property-property relationship (QPPR) approach to estimate in vitro tissue blood partition coefficients of organic chemicals in rats and humans. Arch Toxicol 72(1):17–25
Schmitt W (2008) General approach for the calculation of tissue to plasma partition coefficients. Toxicol In Vitro 22(2):457–467
Poulin P, Ekins S, Theil FP (2011) A hybrid approach to advancing quantitative prediction of tissue distribution of basic drugs in human. Toxicol Appl Pharmacol 250(2):194–212
Broeders JJW, Van Eijkeren JCH, Blaauboer BJ, Hermens JLM (2012) Transport of chlorpromazine in the Caco-2 cell permeability assay: a kinetic study. Chem Res Toxicol 25(7):1442–1451
Hubatsch I, Ragnarsson EGE, Artursson P (2007) Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat Protoc 2(9):2111–2119
Dehouck M-P, Vandenhaute E, Dehouck L, Sevin E, Lenfant A-M, Delplace Y, Hallier-Vanuxeem D, Culot M, Cecchelli R (2011) Modelling the blood-brain barrier. Neuromethods 56:145–160
Cartwright L, Poulsen MS, Nielsen HM, Pojana G, Knudsen LE, Saunders M, Rytting E (2012) In vitro placental model optimization for nanoparticle transport studies. Int J Nanomed 7:497–510
Testa B, Balmat AL, Long A, Judson P (2005) Predicting drug metabolism—an evaluation of the expert system METEOR. Chem Biodivers 2:872–885
Marchant CA, Briggs KA, Long A (2008) In silico tools for sharing data and knowledge on toxicity and metabolism: derek for windows, meteor, and vitic. Toxicol Mech Methods 18(2–3):177–187
van Leeuwen K, Schultz TW, Henry T, Diderich B, Veith GD (2009) Using chemical categories to fill data gaps in hazard assessment. SAR QSAR Environ Res 20(3–4):207–220
Ellison CM, Enoch SJ, Cronin MT (2011) A review of the use of in silico methods to predict the chemistry of molecular initiating events related to drug toxicity. Expert Opin Drug Metab Toxicol 7:1481–1495
Treijtel N, Barendregt A, Freidig AP, Blaauboer BJ, Van Eijkeren JCH (2004) Modeling the in vitro intrinsic clearance of the slowly metabolized compound tolbutamide determined in sandwich-cultured rat hepatocytes. Drug Metab Dispos 32(8):884–891
Godoy P, Hewitt NJ, Albrecht U, Andersen ME, Ansari N, Bhattacharya S, Bode JG, Bolleyn J, Borner C, Böttger J, Braeuning A, Budinsky RA, Burkhardt B, Cameron NR, Camussi G, Cho CS, Choi YJ, Craig Rowlands J, Dahmen U, Damm G, Dirsch O, Donato MT, Dong J, Dooley S, Drasdo D, Eakins R, Ferreira KS, Fonsato V, Fraczek J, Gebhardt R, Gibson A, Glanemann M, Goldring CEP, Gómez-Lechón MJ, Groothuis GMM, Gustavsson L, Guyot C, Hallifax D, Hammad S, Hayward A, Häussinger D, Hellerbrand C, Hewitt P, Hoehme S, Holzhütter HG, Houston JB, Hrach J, Ito K, Jaeschke H, Keitel V, Kelm JM, Kevin Park B, Kordes C, Kullak-Ublick GA, Lecluyse EL, Lu P, Luebke-Wheeler J, Lutz A, Maltman DJ, Matz-Soja M, McMullen P, Merfort I, Messner S, Meyer C, Mwinyi J, Naisbitt DJ, Nussler AK, Olinga P, Pampaloni F, Pi J, Pluta L, Przyborski SA, Ramachandran A, Rogiers V, Rowe C, Schelcher C, Schmich K, Schwarz M, Singh B, Stelzer EHK, Stieger B, Stöber R, Sugiyama Y, Tetta C, Thasler WE, Vanhaecke T, Vinken M, Weiss TS, Widera A, Woods CG, Xu JJ, Yarborough KM, Hengstler JG (2013) Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol 87(8):1315–1530
Houston JB, Carlile DJ (1997) Incorporation of in vitro drug metabolism data into physiologically- based pharmacokinetic models. Toxicol In Vitro 11(5):473–478
Prieto P, Baird AW, Blaauboer BJ, Ripoll JVC, Corvi R, Dekant W, Dietl P, Gennari A, Gribaldo L, Griffin JL, Hartung T, Heindel JJ, Hoet P, Jennings P, Marocchio L, Noraberg J, Pazos P, Westmorland C, Wolf A, Wright J, Pfaller W (2006) The assessment of repeated dose toxicity in vitro: a proposed approach. ATLA Altern Lab Anim 34(3):315–341
Limonciel A, Aschauer L, Wilmes A, Prajczer S, Leonard MO, Pfaller W, Jennings P (2011) Lactate is an ideal non-invasive marker for evaluating temporal alterations in cell stress and toxicity in repeat dose testing regimes. Toxicol In Vitro 25(8):1855–1862
Wilmes A, Limonciel A, Aschauer L, Moenks K, Bielow C, Leonard MO, Hamon J, Carpi D, Ruzek S, Handler A, Schmal O, Herrgen K, Bellwon P, Burek C, Truisi GL, Hewitt P, Di Consiglio E, Testai E, Blaauboer BJ, Guillou C, Huber CG, Lukas A, Pfaller W, Mueller SO, Bois FY, Dekant W, Jennings P (2013) Application of integrated transcriptomic proteomic and metabolomic profiling for the delineation of mechanisms of drug induced cell stress. J Proteome 79:180–194
DeWoskin RS, Thompson CM (2008) Renal clearance parameters for PBPK model analysis of early life stage differences in the disposition of environmental toxicants. Regul Toxicol Pharmacol 51(1):66–86
Hudachek SF, Gustafson DL (2013) Incorporation of ABCB1-mediated transport into a physiologically-based pharmacokinetic model of docetaxel in mice. J Pharmacokinet Pharmacodyn 40(4):437–449
Blaauboer BJ, Barratt MD, Houston JB (1999) The integrated use of alternative methods in toxicological risk evaluation: ECVAM integrated testing strategies task force report I. ATLA Altern Lab Anim 27(2):229–237
DeJongh J, Nordin-Andersson M, Ploeger BA, Forsby A (1999) Estimation of systemic toxicity of acrylamide by integration of in vitro toxicity data with kinetic simulations. Toxicol Appl Pharmacol 158(3):261–268
Forsby A, Blaauboer B (2007) Integration of in vitro neurotoxicity data with biokinetic modelling for the estimation of in vivo neurotoxicity. Hum Exp Toxicol 26(4):333–338
Gubbels-Van Hal WMLG, Blaauboer BJ, Barentsen HM, Hoitink MA, Meerts IATM, Van Der Hoeven JCM (2005) An alternative approach for the safety evaluation of new and existing chemicals, an exercise in integrated testing. Regul Toxicol Pharmacol 42(3):284–295
Louisse J, de Jong E, van de Sandt JJM, Blaauboer BJ, Woutersen RA, Piersma AH, Rietjens IMCM, Verwei M (2010) The use of in vitro toxicity data and physiologically based kinetic modeling to predict dose–response curves for in vivo developmental toxicity of glycol ethers in rat and man. Toxicol Sci 118(2):470–484
Blaauboer BJ (2008) The contribution of in vitro toxicity data in hazard and risk assessment: current limitations and future perspectives. Toxicol Lett 180(2):81–84
Blaauboer BJ, Andersen ME (2007) The need for a new toxicity testing and risk analysis paradigm to implement REACH or any other large scale testing initiative [2]. Arch Toxicol 81(5):385–387
Clewell HJ III, Andersen ME, Blaauboer BJ (2008) On the incorporation of chemical-specific information in risk assessment. Toxicol Lett 180(2):100–109
Basketter DA, Clewell H, Kimber I, Rossi A, Blaauboer B, Burrier R, Daneshian M, Eskes C, Goldberg A, Hasiwa N, Hoffmann S, Jaworska J, Knudsen TB, Landsiedel R, Leist M, Locke P, Maxwell G, McKim J, McVey EA, Ouédraogo G, Patlewicz G, Pelkonen O, Roggen E, Rovida C, Ruhdel I, Schwarz M, Schepky A, Schoeters G, Skinner N, Trentz K, Turner M, Vanparys P, Yager J, Zurlo J, Hartung T (2012) A roadmap for the development of alternative (non-animal) methods for systemic toxicity testing—t4 report. ALTEX 29(1):3–91
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this protocol
Cite this protocol
Blaauboer, B.J. (2014). In Vitro Approaches to Predictive Biokinetics. In: Bal-Price, A., Jennings, P. (eds) In Vitro Toxicology Systems. Methods in Pharmacology and Toxicology. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-0521-8_23
Download citation
DOI: https://doi.org/10.1007/978-1-4939-0521-8_23
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-0520-1
Online ISBN: 978-1-4939-0521-8
eBook Packages: Springer Protocols