Abstract
Salicylic acid (SA) is a simple phenolic compound distributed in a wide range of plant taxa. Depending on the plant species, developmental stage, and growth conditions, it can be synthesized from cinnamic acid produced by phenylalanine ammonia-lyase in the cytosol or from isochorismic acid generated by isochorismate synthase in chloroplasts. However, a fully defined SA biosynthetic pathway is still unavailable in plants. Besides its role in regulating various aspects of plant growth and development, SA is a plant immune signal essential for both local defense response and systemic acquired resistance. Significant progress has been made recently in understanding SA-mediated defense signaling networks including identification of SA receptors and elucidation of the crucial role of NPR1 (nonexpressor of pathogenesis-related genes 1) in SA signal execution. Understanding of SA-mediated plant defense has facilitated the development of disease-resistant crops through genetic manipulation of the SA signaling pathway. Although the use of NPR1 and its orthologs in developing broad-spectrum transgenic disease resistance has been successfully extended to a variety of crop species, commercial application of these transgenic crops has been hampered by ethical concerns. In this regard, cisgenesis may hold the potential for application of bioengineered disease-resistant crops in agriculture.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Salicylic acid (SA)
- Systemic acquired resistance (SAR)
- NPR1 (nonexpressor of pathogenesis-related genes1)
- SA receptor
- Overexpression
- Fitness penalty
Introduction
Salicylic acid (SA, 2-hydroxy benzoic acid) is a small phenolic compound synthesized by a wide range of prokaryotic and eukaryotic organisms. It has a broad distribution in the plant kingdom as free phenolic acid and/or conjugated forms generated by glucosylation, methylation, amino acid conjugation, sulfonation, or hydroxylation (Pridham 1965; Pierpoint 1994; Vlot et al. 2009; Dempsey et al. 2011). Among these natural SA derivatives, salicin (β-glucoside salicylic alcohol) is the best known one. It accumulates to high levels in several willow species including Salix alba, S. purpurea, S. daphnoides, and S. fragilis whereby the name of salicylic acid was derived from (Raskin 1992; Foster and Tyler 1999). However, the highest levels of total SA were found in inflorescence of thermogenic plants and in spice herbs (Raskin et al. 1990). Under optimal conditions, rice, crabgrass, green foxtail, barley, and soybean have SA levels in excess of 1 μg g−1 fresh weight (FW) (Raskin et al. 1990). In the model plant Arabidopsis thaliana, basal levels of total SA range from 0.25 μg to 1 μg g−1 FW (Nawrath and Métraux 1999; Wildermuth et al. 2001; Brodersen et al. 2005). However, basal SA levels differ widely among species (up to 100-fold differences), even among members of the same family (Yalpani et al. 1991; Malamy et al. 1992; Navarre and Mayo 2004). As ubiquitous distributed secondary metabolites, salicylates (the general name of SA and its derivatives) have been known to possess medicinal properties since the fifth century bc when Hippocrates prescribed salicylate-rich willow leaf and bark for pain relief during childbirth (Weissman 1991). It eventually led to the development of aspirin, one of the world’s most widely used drugs, in the 1890s (Raskin 1992). Recently, SA has been established as a distinct class of plant hormone because of its important regulatory roles in seed germination (Rajou et al. 2006), seedling establishment (Alonso-Ramírez et al. 2009), cell growth (Rate et al. 1999; Vanacker et al. 2001), trichome development (Traw and Bergelson 2003), flowering (Cleland 1974; Cleland and Ajami 1974; Martínez et al. 2004), thermogenesis (Raskin et al. 1987), nodulation (Stacey et al. 2006), respiration (Norman et al. 2004), stomatal responses (Manthe et al. 1992; Lee 1998), senescence (Morris et al. 2000; Rao and Davis 2001; Rao et al. 2002), and responses to biotic and abiotic stresses (Janda et al. 2007; Vlot et al. 2009).
The best-established role for SA is as a signal molecule functioning in plant immune responses (Enyedi et al. 1992; Alvarez 2000; Nishimura and Dangl 2010). Due to sessile nature and lacking specialized immune cells, plants have developed the capability to sense pathogen and mount immune response through individual cells. Recognition of pathogen-associated molecular patterns (PAMPs) leads to PAMP-triggered immunity (PTI) that prevents pathogen colonization. While PTI is sufficient to prevent further colonization by many microbes, some pathogens have evolved effectors to dampen PAMP-triggered signals. In turn, host plants have evolved resistance (R) proteins to detect the presence of pathogen effectors and induce effector-triggered immunity (ETI) including hypersensitive response (HR) (Jones and Dangl 2006). Activation of defense signaling pathways (PTI or ETI) results in the generation of a mobile signal(s) that moves from local infected tissue to distal tissues to induce systemic acquired resistance (SAR), which is a long-lasting immunity against a broad spectrum of pathogens (Fu and Dong 2013). SA-mediated immune responses are important parts of PTI and ETI and also essential for the activation of SAR (Durrant and Dong 2004). Efforts to elucidate the crucial role of SA in immune responses have uncovered that pathogen infection leads to SA accumulation not only in the local infected tissue but also in systemic tissues that develop SAR (Malamy et al. 1990; Métraux et al. 1990) and that SA accumulation usually parallels or precedes the increase in expression of pathogenesis-related (PR) genes and development of SAR. Consistently, exogenous application of SA and its functional analogs induces PR gene expression and resistance against viral, bacterial, oomycete, and fungal pathogens in both dicotyledonous and monocotyledonous plants (Malamy and Klessig 1992; Wasternack et al. 1994; Gorlach et al. 1996; Ryals et al. 1996; Morris et al. 1998; Shah and Klessig 1999; Pasquer et al. 2005; Makandar et al. 2006). Conversely, blocking SA accumulation through expression of a bacterial naphthalene (nah)-catabolic gene nahG, which encodes a salicylate hydroxylase that converts SA to catechol, in transgenic tobacco and Arabidopsis plants compromises both HR and SAR (Gaffney et al. 1993; Delaney et al. 1994). Similarly, mutations of genes involved in SA biosynthesis and inhibition of SA biosynthesis have been shown to enhance susceptibility to pathogens, yet the resistance can be restored through exogenous SA application (Mauch-Mani and Slusarenko 1996; Nawrath and Métraux 1999; Wildermuth et al. 2001; Nawrath et al. 2002). Therefore, SA is an important endogenous marker and determinant of plant disease resistance.
In the past two decades, intensive studies have revealed a complex network of SA biosynthesis and signaling in plant immunity. Increasing knowledge of SA-mediated immunity in model systems has led to translational research on developing disease-resistant crop cultivars through transgenic approaches. Genetic screens, transcriptomics, proteomics, and protein interaction studies predominantly in Arabidopsis have provided a large number of candidate genes for biotechnological manipulation in crops. At the same time, outcomes of genetic engineering have enhanced our understanding of the SA-mediated immune responses in different plant species. Here, we describe the recent progresses in our understanding of SA biosynthesis, signal perception and execution, and their biotechnological applications in improvement of crop disease resistance.
Salicylic Acid Biosynthesis
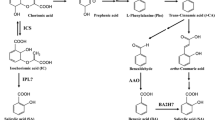
Studies of SA biosynthesis in plants have discovered two distinct and differentially compartmentalized pathways: the phenylalanine ammonia-lyase (PAL) pathway starting in the cytosol and the isochorismate synthase (ICS) pathway operative in chloroplasts (Fig. 1). Both pathways require the primary metabolite chorismate. However, to date neither biosynthetic route has been fully resolved.
Salicylic acid biosynthetic pathways in Arabidopsis thaliana. AAO Arabidopsis aldehyde oxidase, BZL benzoyl-CoA ligase, BA2H benzoic acid-2-hydroxylase, 4CL 4-coumaroyl:CoA ligase, ICS isochorismate synthase, IPL isochorismate pyruvate lyase, PAL phenylalanine ammonia-lyase. Enzymes that have not been identified so far are marked with a question marker
The PAL Pathway
PAL (EC 4.3.1.5) is the first enzyme in the phenylpropanoid pathway, which catalyzes phenylalanine (Phe) to trans-cinnamic acid (t-CA) and NH3 via a non-oxidative deamination reaction (Raes et al. 2003; Rohde et al. 2004). Early radiolabeling studies with Phe, t-CA, or benzoic acid (BA) suggested that SA is synthesized from Phe via t-CA, which is then converted to SA through two possible routes depending on the plant species and growing conditions (Klämbt 1962; El-Basyouni et al. 1964; Chadha and Brown 1974).
-
1.
Hydroxylation of t-CA to ortho-coumaric acid followed by its decarboxylation to SA (Fig. 1). Feeding of 14C-labled Phe and t-CA to young Primula acaulis and Gaultheria procumbens leaf segments leads to accumulation of ortho-coumaric acid and SA, indicating the function of ortho-coumaric acid pathway in SA biosynthesis (Griesebach and Vollmer 1963; El-Basyouni et al. 1964). Similarly, upon Agrobacterium tumefaciens infection, young tomato seedlings synthesize SA through hydroxylation of t-CA to ortho-coumaric acid (Chadha and Brown 1974). Although the conversion of t-CA to ortho-coumaric acid is believed to be catalyzed by trans-cinnamate-4-hydroxylase in multiple species (Russel and Conn 1967; Alibert and Ranjeva 1971, 1972; Gabriace et al. 1991), the activity of 2-hydroxylation of t-CA to form ortho-coumaric acid was only detected in the suspension of chloroplasts instead of the cytosol of the sweet clover (Melilotus alba Desr.) (Gestetner and Conn 1974). Nevertheless, the enzyme(s) that catalyzes the conversion of ortho-coumaric acid to SA has not yet been identified.
-
2.
Decarboxylation of the side chains of t-CA to generate BA followed by hydroxylation at C2 position (Fig. 1). A growing body of evidence indicates that plants can potentially develop three biosynthetic subroutes to BA, including an β-oxidative route from cinnamoyl Co-A, a non-oxidative route from cinnamoyl Co-A, and a non-oxidative route from t-CA to BA (Wildermuth 2006). Radiolabeling studies using Phe or putative pathway intermediates performed in tobacco mosaic virus (TMV)-infected tobacco, smoke-treated coyote tobacco, or cucumber detected incorporation of radiolabeled carbon into BA and SA but not benzaldehyde, suggesting that SA is synthesized through the cinnamoyl-Co-A β-oxidative subroute (Ribnicky et al. 1998; Jarvis et al. 2000). Similar studies have not been performed in Arabidopsis to probe downstream components of SA biosynthesis via PAL pathway. However, a study of BA production in developing seeds identified an Arabidopsis aldehyde oxidase4 (AAO4) that catalyzes the conversion of benzaldehyde to BA, which is then incorporated into benzoyl glucosinolates (Ibdah et al. 2009). Additionally, the formation of [14C]BA from [14C]Phe through [14C]t-CA was observed in Tsuga canadensis, young Gaultheria procumbens tissue, and uninfected tomato seedlings (Zenk and Muller 1964; Ellis and Amrhein 1971; Chadha and Brown 1974). Furthermore, 14C-tracer studies with tobacco cell suspensions or TMV-inoculated leaves indicated that the label moves from t-CA to SA via BA (Yalpani et al. 1993). Similarly, rice shoots can convert both [14C]t-CA and [14C]BA to SA (Silverman et al. 1995).
The direct conversion of [14C]BA to [14C]SA discovered in etiolated Helianthus annuus hypocotyls, Solanum tuberosum tubers, Pisum sativum internodes, and infected cucumber plants was proposed to be catalyzed by an inducible BA 2-hydroxylase (BA2H) (Klämbt 1962; Meuwly et al. 1995). BA2H activity was further detected in ozone-exposed tobacco leaves, heat-treated pea plants, and salt-stressed rice seedlings (León et al. 1995; Ogawa et al. 2005; Sawada et al. 2006; Pan et al. 2006). Biochemical characterization indicated that tobacco BA2H is a soluble P450 oxygenase that specifically hydroxylates the ortho position of BA (León et al. 1995). Although there has been no subsequent report describing a BA2H-encoding gene in plants, similar activity has been observed in Arabidopsis, which converts neonicotinoid metabolite 6-chloropyridinyl-3-carboxylic acid to the SA mimic 6-chloro-2-hydroxypyridinyl-3-carboxylic acid in planta (Ford et al. 2010). Studies conducted in poplar and tobacco indicated that it might also be possible that the glucose-conjugated ester of BA acts as an intermediate for the synthesis of the SA glucose ester and SA (Chong et al. 2001; Ruuhola and Julkunen-Tiitto 2003).
The preference of SA biosynthetic route in the PAL pathway depends on plant species and growth conditions. Isotope-feeding experiments revealed that SA is mainly synthesized from BA in some plant species such as tobacco, rice, potato, cucumber, sunflower, and pea (Klämbt 1962; Yalpani et al. 1993; León et al. 1995; Silverman et al. 1995; Sticher et al. 1997), while other plant species can form SA through the route of ortho-coumaric acid (Yalpani et al. 1993; León et al. 1995; Silverman et al. 1995). However, feeding of 14C-labeled Phe, ortho-coumaric acid, and BA to young Primula acaulis and G. procumbens leaf segments all leads to SA, suggesting that both routes are probably utilized in SA biosynthesis (El-Basyouni et al. 1964). Similarly, SA is formed mostly via BA in young tomato seedlings, but after infection with A. tumefaciens, SA biosynthesis is shifted to the route of hydroxylation of cinnamate to ortho-coumaric acid (Chadha and Brown 1974).
Elucidation of the above PAL pathway largely relied on isotope feeding of the perspective SA biosynthetic precursors to suspension cells or plant segments. Since isotope feeding is not an accurate reflection of in planta metabolism, the results might be misleading. Further supports to the PAL pathway in SA biosynthesis came from the evidence that pathogen-resistant tobacco and Arabidopsis show increased PAL expression and SA levels (Pellegrini et al. 1994; Mauch-Mani and Slusarenko 1996; Dempsey et al. 1999). Additionally, loss of PAL activity, due to sense suppression or treatment with the PAL inhibitor 2-aminoindan-2-phosphonic acid (AIP), reduces pathogen-induced SA accumulation in tobacco, cucumber, and Arabidopsis, and the defense phenotypes of PAL-inhibited plants can be complemented by exogenous SA application (Meuwly et al. 1995; Mauch-Mani and Slusarenko 1996; Pallas et al. 1996). Moreover, increases in BA2H activity parallel or precede SA accumulation induced by TMV infection, UV exposure, or treatment with BA or hydrogen peroxide in tobacco (Léon et al. 1993; Yalpani et al. 1993; León et al. 1995). Similarly, salinity induces BA2H activity and SA biosynthesis in rice seedlings, and the induced SA accumulation can be inhibited by uniconazole, a BA2H inhibitor, suggesting that inhibition of BA2H can prevent salinity-induced SA accumulation (Sawada et al. 2006). Importantly, genetic analysis of the pal quadruple mutant (pal1 pal2 pal3 pal4) revealed a ~75 % reduction in the basal level of total SA as compared with wild-type plants and a ~50 % reduction in total SA levels following avirulent bacterial pathogen infection (Huang et al. 2010). Therefore, it is generally believed that SA can be synthesized through the PAL pathway (Raskin 1992; Lee et al. 1995; Coquoz et al. 1998; Dempsey et al. 2011).
The ICS Pathway
Although early studies suggested that plants might synthesize SA through the PAL pathway, there have been accumulating data questioning its role in the overall SA biosynthesis. In some of the radiolabeling studies described above, the incorporation rate of labeled precursor into SA is lower than expected, particular under infection/induction conditions (Chadha and Brown 1974; Yalpani et al. 1993; Coquoz et al. 1998). Inhibiting PAL activity by AIP can only reduce chemical- or pathogen-induced SA accumulation by several folds in potato or Arabidopsis, respectively (Mauch-Mani and Slusarenko 1996; Coquoz et al. 1998). These pieces of evidence indicated that there might be another pathway in plants leading to SA biosynthesis (Fig. 1).
Bacteria in several genera have been shown to synthesize SA in the production of iron-chelating siderophores (Garcion and Métraux 2006). In the bacterial pathway, chorismate is converted to SA through an isochorismate (IC) intermediate (Verberne et al. 1999). In some bacterial species, like Pseudomonas aeruginosa and P. fluorescens, chorismate is first converted to IC by isochorismate synthase (ICS, EC 5.4.4.2) and followed by conversion to SA and pyruvate by another unifunctional enzyme, isochorismate pyruvate lyase (IPL, EC 4.2.99.21) (Serino et al. 1995; Mercado-Blanco et al. 2001). In contrast, SA synthesis in Yersinia enterocolitica and Mycobacterium tuberculosis is achieved through a sole, bifunctional enzyme named SA synthase (SAS) that directly converts chorismate to SA via an isochorismate intermediate (Pelludat et al. 2003; Kerbarh et al. 2005; Harrison et al. 2006). Structurally, ICS and SAS are similar and contain conserved active sites (Harrison et al. 2006; Kerbarh et al. 2005; Kolappan et al. 2007; Parsons et al. 2008). Functionally, both enzymes begin with nucleophilic attack at C2 of chorismate, with water as the nucleophile, concomitant with displacement of the C4 hydroxyl group in an SN2 reaction (He et al. 2004); however, reactions on SAS is followed by elimination of pyruvate and release of SA.
In plants, chorismate is synthesized in the plastid (Poulsen and Verpoorte 1991; Schmid and Amrhein 1995). Considering the fact that many plastid-localized pathways are derived from prokaryotic endosymbionts, it is possible that plants may also utilize a similar ICS pathway for SA biosynthesis (Verberne et al. 1999; Wildermuth et al. 2001). To assess whether plants contain an endogenous pathway to synthesize SA through IC, Wildermuth et al. (2001) identified two putative ICS genes in the Arabidopsis genome. ICS1 (At1g74710) and ICS2 (At1g18870) share 78 % identity at the amino acid level and ICS1 is 57 % identical to a Catharanthus roseus ICS, whose activity has been confirmed biochemically (van Tegelen et al. 1999; Garcion et al. 2008). However, only ICS1 transcript is accumulated in leaves infected with fungal (Golovinomyces orontii) and bacterial (P. syringae pv. maculicola) pathogens (Wildermuth et al. 2001). ICS1 expression correlates with SA accumulation and expression of the SA-inducible PR1 gene. Subsequent analyses indicated that ICS1 transcripts also accumulate in response to a variety of biotic or abiotic stresses, including UV light, ozone, PAMPs, (hemi)biotrophic pathogens, and exogenous SA treatment (Ogawa et al. 2005; Killian et al. 2007; Nobuta et al. 2007; Postel et al. 2010; Dempsey et al. 2011; Harrower and Wildermuth 2011). Two Arabidopsis mutants, sid2-1 (salicylic acid induction-deficient2-1) and eds16-1 (enhanced disease susceptibility16-1) (Nawrath and Métraux 1999; Dewdney et al. 2000), which can accumulate only 5–10 % of the wild-type level of SA following infection of virulent or avirulent pathogens, were found to contain lesions in the ICS1 gene (Wildermuth et al. 2001). Exogenous SA application can complement their enhanced disease susceptibility phenotype (Wildermuth et al. 2001).
Biochemical and molecular analyses provided further evidence supporting the role of ICS1 in SA biosynthesis. As expected, ICS1 contains a putative plastid transit sequence and a cleavage site (Wildermuth et al. 2001). The high affinity of ICS1 for chorismate allows ICS1 to compete successfully with other pathogen-induced enzymes that use chorismate as their substrate, such as anthranilate synthase (Strawn et al. 2007; Ziebart and Toney 2010). Unlike the bifunctional SAS, the recombinant ICS1 only converts chorismate to IC, since no SA was detected in the products of this reaction (Strawn et al. 2007). Additional analyses revealed that proper function of ICS1 requires Mg2+. However, ICS1 displays maximal activity over a broad range of pH and temperature, which is suitable for the light-mediated changes in the stromal environment.
Similarly to ICS1, ICS2 encodes a functional ICS enzyme that can be imported into the chloroplast stroma (Strawn et al. 2007; Garcion et al. 2008). The fact that null ics1 mutant still accumulates some SA suggests a likely role for ICS2 in SA biosynthesis. Comparison of SA accumulation in ics1 and the double mutant ics1 ics2 demonstrated that ICS2 indeed participates in the biosynthesis of SA. Upon UV exposure, ics1 and ics1 ics2 accumulate roughly 10 and 4 % of total SA compared to wild type, respectively. Therefore, the majority of SA (about 95 %) is synthesized from the ICS pathway in UV-treated Arabidopsis plants with the remaining through an alternative pathway (Garcion et al. 2008).
ICS homologs have also been identified in a wide variety of plant species (van Tegelen et al. 1999; Ogawa et al. 2005; Uppalapati et al. 2007; Yuan et al. 2007; Catinot et al. 2008). Given their role in phylloquinone synthesis, it is very likely that ICS homologs are present in all plant species. However, identification of an ICS gene in a given plant species is not sufficient to confirm its role in SA biosynthesis. Nevertheless, isotope-feeding experiment, with the intension to reflect in planta metabolism, revealed that most SA is synthesized via the ICS pathway in Pythium aphanidermatum-elicitated C. roseus cells. In addition, virus-induced gene silencing of ICS expression in N. benthamiana or tomato suppresses UV- and/or pathogen-induced SA accumulation (Uppalapati et al. 2007; Catinot et al. 2008).
Although it is becoming clear that SA is synthesized via the ICS pathway in various plant species, how isochorismate, the product of ICS, is converted to SA is still unclear. This conversion should be accomplished by an enzymatic reaction since nonenzymatic synthesis of SA from IC is negligible when the reactants are incubated under conditions consistent with chloroplast stroma (Strawn et al. 2007). In addition, it is expected that the enzyme(s) involved in SA synthesis from IC is plastid localized, as transgenic Arabidopsis expressing nahG fused to a chloroplast localization sequence fails to accumulate SA upon pathogen infection or UV treatment (Fragnière et al. 2011). However, no plant genes encoding IPL activity have been reported (Chen et al. 2009). Thus, whether plants contain IPLs that are structurally unrelated to or highly divergent from the bacterial counterparts or use a metabolic pathway distinct from that in bacteria and, consequently, catalyzed by enzymes unrelated to IPL merits further investigation.
Signal Perception and Execution of Salicylic Acid-Induced Responses
Over the past more than two decades, many genetic screens have been conducted to identify genes that are involved in SA biosynthesis/metabolism, perception, and signal transduction in Arabidopsis. These screens have yielded numerous mutants with genetic lesions either upstream or downstream of SA biosynthesis. Furthermore, recent studies have revealed the involvement of epigenetic factors in SA-mediated plant defense signaling. All these have sketched an integrated model for regulation of SA accumulation and a finely tuned SA-mediated defense signaling network. Here, we focus on SA perception and downstream signal execution. For regulation of SA accumulation, readers are referred to the recent review in The Arabidopsis Book (Dempsey et al. 2011).
SA Receptors
Although SA plays a pivotal role in galvanizing immune responses, until very recently it was unclear how plant cells perceived SA. There have been serious efforts to identify SA receptors using biochemical purification of SA-binding proteins (SABPs). To date, four types of SABPs have been identified including a catalase, a methyl salicylate esterase, a cytoplasmic ascorbate peroxidase, and a chloroplastic carbonic anhydrase (Du and Klessig 1997; Slaymaker et al. 2002; Kumar and Klessig 2003; Park et al. 2007; Vlot et al. 2008, 2009). Although these SABPs are involved in mediating some aspects of SA metabolism or action, genetic analyses suggested that none of them fulfill the criteria for a bonafide SA receptor, because these molecules do not have functional roles in plant immune signaling. Using different ligand-receptor binding methods, two research groups recently reported that NPR1 (nonexpressor of pathogenesis-related genes1) and NPR1-related proteins, NPR3 and NPR4, are the long-sought-after SA receptors in Arabidopsis (Fu et al. 2012; Wu et al. 2012). NPR1, NPR3, and NPR4 are all characterized by a conserved N-terminal BTB/POZ (broad complex, tramtrack, and bric-à-brac/poxvirus, zinc finger) domain and an ankyrin repeat in the middle of the proteins (Cao et al. 1997; Kinkema et al. 2000; Liu et al. 2005).
Using a special equilibrium dialysis ligand binding method, Wu et al. (2012) demonstrated that NPR1 binds to SA when NPR1 and SA are in equilibrium. SA binds strongly to a C-terminal transactivation (TA) domain of NPR1 through Cys521 and Cys529 via the transition metal copper (Rochon et al. 2006; Wu et al. 2012). Mutations of cysteines to serines or metal chelation abolish the binding of SA by NPR1. In the absence of SA, the NPR1 TA domain is inhibited by the BTB domain and thus fails to activate the expression of SA response genes. However, increased SA concentration upon pathogen infection facilitates binding of SA to Cys521 and Cys529 through coordinated copper. Thus, the direct binding of NPR1 to SA and the functional importance of this interaction in plant immunity indicate NPR1 may be an SA receptor in Arabidopsis.
The presence of a BTB domain in NPR1 suggests that, like other BTB domain-containing proteins, NPR1 may interact with Cullin 3 (CUL3) E3 ligase and mediate substrate degradation. Even though the substrate for NPR1 has yet to be identified, NPR1 protein itself can be degraded by the proteasome both before and after SAR induction (Spoel et al. 2009). NPR1 paralogs NPR3 and NPR4 are adaptor proteins for the CUL3 E3 ligase that specifically targets NPR1 for degradation in an SA concentration-dependent manner (Fu et al. 2012). NPR1 and NPR4 interact with one another in the absence of SA; SA disrupts this interaction and promotes interaction between NPR1 and NPR3 instead. Using conventional ligand-receptor binding assays, Fu and colleagues (2012) found that the NPR1 protein does not have considerable SA-binding activity under different conditions but two NPR1-related proteins, NPR3 and NPR4, bind to SA with different affinity. Since NPR4 has high affinity for SA (nanomolar range) while NPR3 has low affinity for SA (micromolar range), low SA levels should reduce NPR1 degradation, whereas high SA levels should enhance it. According to the proposed model, in the absence of pathogen infection, NPR4 constantly removes most of the NPR1 protein through CUL3-NPR4-mediated degradation, and basal SA disrupts some of the NPR1–NPR4 interactions, allowing some NPR1 to escape degradation, which is required for keeping basal immunity (PTI). Following pathogen infection, recognition of pathogen effectors by plant resistance proteins induces a high level of SA in local infected tissue, which promotes interaction between NPR1 and NPR3, triggering CUL3-NPR3-mediated NPR1 degradation. As NPR1 is likely a negative regulator of programmed cell death (PCD) during ETI, degradation of NPR1 allows PCD to occur at the site of infection. In systemic tissues, on the other hand, an intermediate level of SA is insufficient to bring about NPR1–NPR3 interaction but high enough to disrupt NPR1–NPR4 interaction and, consequently, enables NPR1 to accumulation, leading to SAR activation. Thus, as SA receptors, NPR3 and NPR4 appear to regulate the homeostasis of NPR1, thus modulating the function of NPR1 in basal immunity, ETI, and SAR.
The seemingly conflicting results on the identification of SA receptors can be attributed to the different experimental approaches used to test the direct binding of SA to NPR1. Crystal structure analysis of NPR1, NPR3, and NPR4 will be the next crucial step to further unravel the binding sites and the exact SA-sensing mechanisms of these receptors. NPR3 and NPR4 may not be the merely SA-binding proteins that facilitate SA-mediated degradation of NPR1 and additional proteins are yet to be discovered (Kaltdorf and Naseem 2013). Alternatively, SA could be perceived by both NPR1 and NPR3/NPR4, resembling the multireceptor sensing of other phytohormones like abscisic acid (Spartz and Gray 2008). Given the fact of the existence of SA-dependent but NPR1-independent defense signaling pathway, in which NPR3/NPR4 may not participate, additional SA perception mechanisms may be present. Furthermore, it has now been well established that SA is also a prominent regulator of plant growth, development, and response to abiotic stresses (Vicente and Plasencia 2011), suggesting the possible existence of additional SA receptors in plants. Regardless, identification of NPR1, NPR3, and NPR4 as SA receptors represents a great step forward in elucidation of SA immune signaling and is expected to have a long-lasting impact on future research in plant immunity.
NPR1-Dependent SA Signaling
As a central transcription coactivator, NPR1 is responsible for controlling approximately 95 % of SA-dependent genes, thus represents a key node in signaling downstream from SA (Dong 2004; Durrant and Dong 2004; Pieterse and van Loon 2004). The NPR1 gene promoter contains W-box sequences, which are binding sites of WRKY transcription factors. Mutations in the W-box region of the NPR1 gene affect its expression, suggesting that WRKY transcription factor(s) is crucial in mediating SA-induced NPR1 expression (Yu et al. 2001). SA treatment or pathogen inoculation enhances NPR1 expression. SA also promotes the translocation of NPR1 from cytoplasm to the nucleus. SA-induced changes in cellular redox state lead to reduction of disulfide bonds formed among conserved cysteine residues such as Cys82 and Cys216 likely though the function of TRX-H5 (thioredoxin-H5) and/or TRX-H3 (Mou et al. 2003; Tada et al. 2008). SA binding to the NPR1 protein appears to also play a role in this oligomer-to-monomer transition (Wu et al. 2012). Nevertheless, mutation of either Cys82 or Cys216 elevates the level of monomeric, nuclear localized NPR1, and consequently upregulates PR1 gene expression (Mou et al. 2003). Since the NPR1 protein does not have DNA-binding capability, relaying NPR1-mediated signaling requires other transcription factors. Indeed, genome-wide expression profiling analysis indicated that several members of the WRKY transcription factor family act downstream of NPR1 (Wang et al. 2006), and protein–protein interaction assays revealed that NPR1 interacts with at least seven TGA (TGACG motif-binding factor) transcription factors (Zhang et al. 1999; Després et al. 2000; Zhou et al. 2000; Subramaniam et al. 2001; Song et al. 2011) and three structurally related NIMIN (noninducible immunity1 (NIM1)-interacting) proteins (Weigel et al. 2001, 2005).
The TGA transcription factors can directly interact with PR1 gene promoter through binding to the activator sequence-1 (as-1) element in the promoter (Lebel et al. 1998). In planta analyses showed that the interaction between NPR1 and TGA1 and/or TGA4 needs the presence of SA (Després et al. 2000) and that the ability of TGA2 and TGA3 to activate transcription of downstream genes requires both SA and NPR1 (Johnson et al. 2003). In another study, however, interaction between NPR1 and TGA2 was detected in the absence of SA, but the interaction is weaker than in the presence of SA (Fan and Dong 2002). More recent studies suggested that the repressor activity of TGA2 is transformed into an activator activity by its incorporation into a transactivation complex with NPR1 (Rochon et al. 2006; Boyle et al. 2009). All these results indicate that SA and NPR1 likely enhance the DNA-binding activity of certain TGA factors and thus affect the transcription of PR genes (Durrant and Dong 2004). Indeed, mutant characterization confirmed that TGA2, TGA5, and TGA6 function redundantly in SA signaling and SAR and that TGA3 and TGA7 are required for SA-mediated basal immunity (Zhang et al. 2003; Kesarwani et al. 2007; Song et al. 2011).
The NIMIN proteins appear to regulate SA/NPR1 signaling in a negative manner. While NIMIN3 is expressed constitutively at a low level, both NIMIN1 and NIMIN2 are responsive to SA treatment (Weigel et al. 2001; Hermann et al. 2013). Overexpression of NIMIN1 compromises ETI and SAR, whereas reducing its expression enhances SA-induced PR1 gene expression (Weigel et al. 2005). NIMIN3 appears to also suppress SA-induced PR1 gene expression, though to a lesser extent than NIMIN1 (Hermann et al. 2013). It was proposed that the NIMIN proteins act in a strictly consecutive and SA-regulated manner on NPR1 to repress the PR1 gene at the onset of SAR (Hermann et al. 2013).
In a genetic screen for suppressors of npr1, a mutant named sni1 (suppressor of npr1-1, inducible1) was identified (Li et al. 1999). The sni1 mutation restores SA inducibility of PR genes and resistance to npr1-1 and renders plants with a wild-type copy of the NPR1 gene more sensitive to SAR signals. SNI1 is a nuclear protein with limited similarity to the mouse retinoblastoma protein, a negative transcription regulator, suggesting that SNI1 is likely a negative regulator of SAR (Mosher et al. 2006). Further genetic screens for suppressors of the sni1 mutation identified a group of proteins including RAD51D (RAS associated with diabetes51d), BRCA2A (breast cancer2a), and SSN2 (suppressor of SNI1,2) that are required for SA-mediated defense gene transcription (Durrant et al. 2007; Wang et al. 2010; Song et al. 2011). Since RAD51D, BRCA2A, and SSN2 are all involved in homologous recombination or DNA repair, these results demonstrated that proteins from homologous recombination or DNA repair pathways play important roles in SA- and NPR1-mediated defense signaling (Moore et al. 2011).
Recent progresses have defined the function of a number of plant Mediator (MED) subunits in SA-mediated plant immune responses. As a conserved multiprotein cofactor of RNA polymerase II (RNAPII), the Mediator complex is recognized as an important player to fine-tune gene-specific and pathway-specific transcriptional reprogramming by acting as an adaptor/coregulator between sequence-specific transcription factor and RNAPII. Mutations in genes encoding the Mediator subunits MED14, MED15, and MED16 all affect SA-induced PR gene expression, compromise basal resistance against biotrophic bacterial pathogens, and block biological induction of SAR (Canet et al. 2012; Wathugala et al. 2012; Zhang et al. 2012b, 2013a). However, only med15 causes SA hyperaccumulation and reduced SA tolerance like npr1 (Canet et al. 2012). MED16 and NPR1 function largely independently of each other in basal immunity, whereas MED14 and NPR1 have significant overlapping functions in regulating basal immunity. Unlike the med16 mutation, which differentially affects expression of several SAR positive and negative regulators, med14 inhibits induction of a large group of defense genes including both SAR positive and negative regulators (Zhang et al. 2012b, 2013a). Both MED14 and MED15 appear to function downstream of NPR1 and do not affect NPR1 nuclear localization and/or stability (Canet et al. 2012; Zhang et al. 2013a), whereas MED16 positively contributes to NPR1 protein accumulation (Zhang et al. 2012b). Interestingly, although the med8 mutant displays enhanced susceptibility to bacterial pathogens, it has no significant defects in biological induction of SAR (Kidd et al. 2009; Zhang et al. 2012b). Furthermore, mutations in MED25 attenuate the induction of SA-responsive genes but have no significant effects on resistance to biotrophic bacterial pathogens and biological induction of SAR (Kidd et al. 2009; Zhang et al. 2012b). Thus, these Mediator subunits employ distinct mechanisms to regulate SA-mediated defense gene expression and pathogen resistance.
NPR1-Independent SA Signaling
In Arabidopsis, ETI is suppressed by expression of the nahG gene, but not by the npr1 mutation, suggesting the presence of NPR1-independent SA signaling in plant immunity (Raridan and Delaney 2002; Kachroo et al. 2001; Takahashi et al. 2002). The existence of NPR1-independent SA signaling is further supported by the results from characterization of a group of Arabidopsis mutants that either display SA inducibility of PR genes or constitutively accumulate SA and PR gene transcripts in the absence of a functional NPR1 gene. The sni1 mutation confers SA inducibility of PR genes to the npr1-1 mutant, suggesting an NPR1-independent mechanism (Li et al. 1999). More components in the NPR1-independent SA signaling pathway were identified through screening for suppressors of the npr1-5 mutant. The ssi (suppressor of SA insensitivity) npr1 double mutants ssi1 npr1, ssi2 npr1, and ssi4 npr1 constitutively accumulate SA and exhibit heightened resistance to a variety of pathogens (Shah et al. 1999, 2001; Shirano et al. 2002). The ssi1 and ssi2 single mutants accumulate higher levels of PR1 gene transcripts than the ssi1 npr1 and ssi2 npr1 double mutants, respectively, indicating an NPR1-independent pathway functioning additively with the NPR1-dependent pathway (Shah et al. 1999, 2001). Another npr1 suppressor, snc1 (suppressor of npr1-1 constitutive1), displays constitutive SA-dependent, NPR1-independent resistance owning to a mutation in a Toll-interleukin-1 receptor-nucleotide binding site-leucine-rich repeat type R gene. The gain-of-function snc1 mutation leads to constitutive activation of the R protein and downstream immune responses without the presence of pathogens. The snc1 mutant also accumulates high levels of SA, constitutively expresses PR genes, and displays enhanced resistance to pathogens (Li et al. 2001). Further genetic screens for suppressors of snc1 identified a series of mos (modifier of snc1) mutations affecting signal transduction downstream of snc1 (Zhang and Li 2005). New members of the snc mutants such as snc2-1D (suppressor of npr1-1, constitutive 2-1D) and snc4-1D have been identified and characterized (Bi et al. 2010; Zhang et al. 2010b). Moreover, a set of genes that may be involved in SA-regulated, NPR1-independent signaling pathway encode WHIRLY (WHY) and MYB transcription factors. The single-stranded DNA-binding activity of WHY1 is stimulated by SA treatment in both wild-type and npr1 mutant plants (Desveaux et al. 2002, 2004), indicating its important role in NPR1-independent PR1 expression and resistance against pathogens. The Arabidopsis MYB30 (myeloblastosis30) gene positively regulates the HR in an SA-dependent, NPR1-independent manner (Raffaele et al. 2006). Additionally, the cpr5 (constitutive expressor of PR genes5), cpr6, and hrl1 (hypersensitive response-like lesions1) mutants exhibit NPR1-independent and SA-dependent immune phenotypes (Clarke et al. 2000; Devadas et al. 2002). Interestingly, the cpr5, cpr6, and hrl1 mutations also activate jasmonic acid (JA)- and ethylene (ET)-mediated immune responses, indicating that the SA-dependent, NPR1-independent signaling may function synergistically with the JA/ET-mediated defense pathways (Clarke et al. 2000; Devadas et al. 2002).
In a genetic screen for suppressors of the npr1 mutant based on its intolerance to SA, an elp2 (Elongator subunit2) mutant allele was isolated (DeFraia et al. 2010). ELP2 is one of the six subunits of the Elongator complex, which interacts with elongating RNAPII to facilitate transcription (Winkler et al. 2002; Close et al. 2006). Despite the structural diversity of the Elongator subunits, loss of any Elongator subunit generally compromises its integrity and renders the complex inactive (Versées et al. 2010; Glatt et al. 2012). The Elongator catalytic subunit ELP3/ELO3 (ELONGATA3) harbors a C-terminal histone acetyltransferase (HAT) domain and an N-terminal cysteine-rich motif that resembles an iron-sulfur radical S-adenosylmethionine (SAM) domain (Chinenov 2002; Winkler et al. 2002; Nelissen et al. 2005). Both the HAT and SAM domains are required for Elongator’s function in plant immunity (DeFraia et al. 2013). Mutations in ELP2 and ELP3 restore SA tolerance to npr1, suppress npr1-mediated hyperaccumulation of SA, and delay the induction of SA accumulation and defense gene expression (DeFraia et al. 2010, 2013). Although Elongator regulates the NPR1 transcriptional cascade, Elongator and NPR1 appear to function largely independently of each other in ETI, and mutations in ELP2 and ELP3 do not affect SAR (DeFraia et al. 2010, 2013). Further mutant characterization revealed that ELP2 is an epigenetic regulator required for P. syringae-induced rapid transcriptome reprogramming likely through maintaining histone acetylation levels in defense genes, modulating genomic DNA methylation landscape, and influencing pathogen-induced dynamic DNA methylation changes (Wang et al. 2013). Such chromatin modification has recently been described as an additional layer of regulation on plant immunity. Several reports have shown that the state of histone acetylation or DNA methylation is associated with SA-mediated defense responses (Mosher et al. 2006; Butterbrodt et al. 2006; Koornneef et al. 2008; van den Burg and Takken 2009; Choi et al. 2012; Luna et al. 2012). Compared with other epigenetic regulators, Elongator is unique in that it regulates both histone acetylation and DNA methylation status of defense-related genes (Winkler et al. 2002; Nugent et al. 2010; Xu et al. 2012). The NPR1 transcriptional cascade exemplifies a signal cascade where Elongator modulates the chromatin structure of both the key transcription regulator and its target genes, forming a transcriptional feed-forward loop and determining the kinetics of the transcription. However, the mechanism of the cooperative interaction between the specific transcription regulator NPR1 and the chromatin modulator Elongator in regulating gene transcription during immune responses is still unclear.
Biotechnological Manipulation of Salicylic Acid Signaling and Biosynthesis in Agriculture
Disease is a major threat to the yield and quality of crop plants worldwide. One major goal in plant science is the production of crops with increased and durable resistance to a spectrum of pathogens. Compared with other approaches employed to develop disease-resistant crops, genetic engineering is faster and allows transference of individual traits into crops in a calculated manner. Strategies for developing transgenic disease resistance have been evolved from overexpression of a single or combination of a small number of genes, which suffer from either incomplete efficacy or durability, to modification of existing innate signaling pathways, which can activate a battery of defense responses (Collinge et al. 2010). The accumulating knowledge of SA-mediated defense signaling pathways provides new opportunities for manipulating plant disease resistance. Several genes have received attention with respect to possible exploitation for developing transgenic disease-resistant crops. Among them NPR1 is the most promising gene for generating broad-spectrum disease-resistant crop plants.
The NPR1 gene was originally discovered in several independent genetic screens performed in Arabidopsis. The npr1 (also known as nim1 and sai1 (salicylic acid-insensitive1 )) mutants are unable to either mount a SAR response or accumulate PR transcripts and are hypersusceptible to biotrophic pathogens (Cao et al. 1994; Delaney et al. 1994; Shah et al. 1997). The original study in Arabidopsis using NPR1 showed that overexpression of this gene increases resistance to two diverse biotrophic pathogens, the bacterium P. syringae pv. maculicola and the oomycete Hyaloperonospora arabidopsidis (Cao et al. 1998; Table 1). Since then transgenic studies using NPR1 or its orthologs from other species have been extended to a large group of crop plants for resistance against pathogens with either biotrophic or necrotrophic lifestyle (Tables 1 and 2). In addition, overexpression of NPR1 seems to enhance resistance to insect and root-knot nematode in tobacco plants (Meur et al. 2008; Priya et al. 2011). Interestingly, the majority of the transgenic plants display little or no constitutive expression of PR genes; rather, the transgenic plants exhibit a “primed” phenotype where induction of PR genes is faster, at higher intensity, and for a longer duration, resulting in a heightened capacity to undergo SAR when challenged with pathogens or treated with SA analogs. However, transgenic rice expressing either NPR1 or the rice ortholog OsNH1 (Oryza sativa NPR1 HOMOLOGUES1) is different, which exhibits constitutive expression of PR genes (Fitzgerald et al. 2004; Quilis et al. 2008).
Another avenue for boosting SA-mediated plant immunity is to manipulate SA biosynthesis. Tobacco plants overexpressing heterologous PAL transgenes display enhanced resistance to the fungal pathogen Cercospora nicotianae and the oomycete Phytophthora parasittica pv. nicotianae (Felton et al. 1999; Way et al. 2002). However, based on comparison of PAL-overexpressing plants and PAL-overexpressing plants harboring a nahG gene, which compromises SA accumulation, it has been suggested that the accumulation of phenylpropanoid intermediates such as chlorogenic acid is primarily responsible for the enhanced resistance to C. nicotianae in PAL-overexpressing plants, whereas SA accumulation has limited contributions (Shadle et al. 2003). Nevertheless, targeting the bacterial SA biosynthesis enzymes ICS and IPL to chloroplasts in transgenic tobacco plants increases SA and SA glucoside accumulation, leading to constitutive expression of defense genes and resistance to viral and fungal infection (Verberne et al. 2000). Importantly, overaccumulation of SA in transgenic tobacco plants does not affect plant growth, which is crucial for engineering disease-resistant crops. However, targeting a functional fusion enzyme of the bacterial ICS and IPL to chloroplasts in Arabidopsis strongly inhibits plant growth and significantly reduces seed production (Mauch et al. 2001).
As an increasing number of important SA signaling components are discovered, the list of candidate genes for genetic manipulation grows. Interestingly, many of the SA signaling components also plays important roles in nonhost resistance, which is the most common form of resistance exhibited by plants against a wide variety of microbial pathogens (An and Mou 2011). Therefore, manipulating these genes in crop species hold the potential to boost both host and nonhost resistance. However, limited investigations have been conducted on utilizing nonhost resistance to develop disease-resistant crops. Furthermore, manipulating SA-mediated immune responses through suppression of negative regulators or activation of positive regulators represents an attractive strategy for engineering disease resistance (Gurr and Rushton 2005b; Salomon and Sessa 2012). Thus far, the function of many defense regulators in manipulating disease resistance has been tested in Arabidopsis, but the efforts of translating these technologies to crops still lag behind.
It should be noted that because of the involvement of SA in diverse physiological processes other than plant immunity, increasing SA biosynthesis or signaling might lead to fitness penalties. Although little evidence for fitness penalties has been found for overexpression of NPR1 in the laboratory, one study using controlled environments suggested that there seem to be fitness penalties for overexpression of NPR1 under high nutrient conditions (Heidel and Dong 2006). To minimize the cost of defense activation on plant growth, pathogen- or chemical-inducible and tissue-specific promoters may be useful as they limit the cost of resistance by controlling temporal and spatial expression of the defense genes (Gurr and Rushton 2005a).
Although our understanding of the role of SA in plant defense against pathogens has increased considerably over the last two decades, much still remains to be elucidated. Among them, SA biosynthesis in plants is still not fully understood and the central signaling components, such as NPR1, still require more in-depth studies. Additionally, SA-mediated defense signaling pathways and other defense pathways are not isolated but rather interconnected to form a well-regulated network. Elucidating genetic components, especially those connecting multiple defense pathways, will continue to be a major task of the research community. On the other hand, understanding of SA-mediated plant defense has facilitated development of more effective ways for controlling important crop diseases. While gene efficacy in transgenic plants has often been good, field trials of transgenic disease-resistant crops have been hampered by ethical concerns. In this regard, the recently developed cisgenic approach (Schouten et al. 2006), which utilizes target crop-derived genes and regulatory elements (promoters) together with improved transformation methods that do not rely on or subsequently eliminate selective marker genes, has the potential to develop resistant cultivars more acceptable to consumers.
References
Alibert G, Ranjeva R (1971) Recharches sur les enzymes catalysant la biosynthese des acides phenoliques chez Quarcus pedunculata (Ehrn): I—formation des series cinnamique et benzoique. FEBS Lett 19:11–14
Alibert G, Ranjeva R (1972) Recharches sur les enzyme catalysant la biosyntheses des acid phenoliques chez Quarcus pedunculata (Ehrn): II—localization intercellulaire de la phenylalanine mmonique-lyase, de la cinnamate 4-hydroxylase, et de la “benzoate synthase”. Biochim Biophys Acta 279:282–289
Alonso-Ramírez A, Rodríguez D, Reyes D, Jiménez JA, Nicolás G, López-Climent M, Gómez-Cadenas A, Nicolás C (2009) Cross-talk between gibberellins and salicylic acid in early stress responses in Arabidopsis thaliana seeds. Plant Signal Behav 4:750–751
Alvarez ME (2000) Salicylic acid in the machinery of hypersensitive cell and death and disease resistance. Plant Mol Biol 44:429–442
An C, Mou Z (2011) Salicylic acid and its function in plant immunity. J Integr Plant Biol 53:412–428
Bergeault K, Bertsch C, Merdinoglu D, Walter B (2010) Low level of polymorphism in two putative NPR1 homologs in the Vitaceae family. Biol Direct 5:9
Bi D, Cheng Y, Li X, Zhang Y (2010) Activation of plant immune responses by a gain-of-function mutation in an atypical receptor-like kinase. Plant Physiol 153:1771–1779
Boyle P, Le Su E, Rochon A, Shearer HL, Murmu J, Chu JY, Fobert PR, Després C (2009) The BTB/POZ domain of the Arabidopsis disease resistance protein NPR1 interacts with the repression domain of TGA2 to negate its function. Plant Cell 21:3700–3713
Brodersen P, Malinovsky FG, Hematy K, Newman MA, Mundy J (2005) The role of salicylic acid in the induction of cell death in Arabidopsis acd11. Plant Physiol 138:1037–1045
Butterbrodt T, Thurow C, Gatz C (2006) Chromatin immunoprecipitation analysis of the tobacco PR-1a- and the truncated CaMV35S promoter reveals differences in salicylic acid-dependent TGA factor binging and histone acetylation. Plant Mol Biol 61:665–674
Canet JV, Dobón A, Tornero P (2012) Non-Recognition-of-BTH4, an Arabidopsis Mediator subunit homolog, is necessary for development and response to salicylic acid. Plant Cell 24:1–16
Cao H, Bowling SA, Gordon AS, Dong X (1994) Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6:1583–1592
Cao H, Glazebrook J, Clarke JD, Volko S, Dong X (1997) The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88:57–63
Cao H, Li X, Dong X (1998) Generation of broad-spectrum disease resistance by overexpression of an essential regulatory gene in systemic acquired resistance. Proc Natl Acad Sci U S A 95:6531–6536
Catinot J, Buchala A, Abou-Mansour E, Métraux JP (2008) Salicylic acid production in response to biotic and abiotic stress depends on isochorismate in Nicotiana benthamiana. FEBS Lett 582:473–478
Chadha KC, Brown SA (1974) Biosynthesis of phenolic acids in tomato plants infected with Agrobacterium tumefaciens. Can J Bot 52:2041–2047
Chen Z, Zheng Z, Huang J, Lai Z, Fan B (2009) Biosynthesis of salicylic acid in plants. Plant Signal Behav 4:493–496
Chen XK, Zhang JY, Zhang Z, Du XL, Du BB, Qu SC (2012) Overexpressing MhNPR1 in transgenic Fuji apples enhances resistance to apple powdery mildew. Mol Biol Rep 39:8083–8089
Chern MS, Fitzgerald HA, Canlas PE, Navarre DA, Ronald PC (2005) Overexpression of a rice NPR1 homolog leads to constitutive activation of defense response and hypersensitivity to light. Mol Plant Microbe Interact 18:511–520
Chinenov Y (2002) A second catalytic domain in the Elp3 histone acetyltransferases: a candidate for histone demethylase activity? Trends Biochem Sci 27:115–117
Choi SM, Song HR, Han SK, Han M, Kim CY, Park J, Lee YH, Jeon JS, Noh YS, Noh B (2012) HAD19 is required for the repression of salicylic acid biosynthesis and salicylic acid-mediated defense responses in Arabidopsis. Plant J 71:135–146
Chong J, Pierrel MA, Atanassova R, Werck-Reichhart D, Fritig B, Saindrenan P (2001) Free and conjugated benzoic acid in tobacco plants and cell cultures. Induced accumulation upon elicitation of defense responses and role as salicylic acid precursors. Plant Physiol 125:318–328
Clarke JD, Volko SM, Ledford H, Ausubel FM, Dong X (2000) Role of salicylic acid, jasmonic acid, and ethylene in cpr-induced resistance in Arabidopsis. Plant Cell 12:2175–2190
Cleland CF (1974) Isolation of flower-inducing and flower-inhibitory factors from aphid honeydew. Plant Physiol 54:899–903
Cleland CF, Ajami A (1974) Identification of the flower-inducing factor isolated from aphid honeydew as being salicylic acid. Plant Physiol 54:904–906
Close P, Hawkes N, Cornez I, Creppe C, Lambert CA, Rogister B, Siebenlist U, Merville MP, Slaugenhaupt SA, Bours V, Svejstrup JQ, Chariot A (2006) Transcription impairment and cell migration defects in Elongator-depleted cells: implication for familial dysautonomia. Mol Cell 22:521–531
Collinge DB, Jørgensen HJL, Lund OS, Lyngkjær MF (2010) Engineering pathogen resistance in crop plants: current trends and future prospects. Annu Rev Phytopathol 48:269–291
Coquoz JL, Buchala A, Metraux JP (1998) The biosynthesis of salicylic acid in potato plants. Plant Physiol 117:1095–1101
Dang FF, Liu JH, Chen CC, Cheng YP, Huang GD, Guan DY, He SL (2012) Overexpression of CaNPR1 enhances resistance to Ralstonia solanacearum infection in tobacco. Plant Sci J 30:494–500
DeFraia CT, Zhang X, Mou Z (2010) Elongator subunits 2 is an accelerator of immune responses in Arabidopsis thaliana. Plant J 64:511–523
DeFraia CT, Wang Y, Yao J, Mou Z (2013) Elongator subunits 3 positively regulates plant immunity through its histone acetyltransferase and radical S-adenosylmethionine domains. BMC Plant Biol 13:102
Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E, Ryals J (1994) A central role of salicylic acid in plant disease resistance. Science 266:1247–1250
Dempsey DA, Shah J, Klessig DF (1999) Salicylic acid and disease resistance in plants. Crit Rev Plant Sci 18:547–575
Dempsey DA, Vlot AC, Wildermuth MC, Klessig DF (2011) Salicylic acid biosynthesis and metabolism. The Arabidopsis Book 9:e0156
Després C, DeLong C, Glaze S, Liu E, Fobert PR (2000) The Arabidopsis NPR1/NIM1 protein enhances the DNA binding activity of a subgroup of the TGA family of bZIP transcription factors. Plant Cell 12:279–290
Desveaux D, Allard J, Brisson N, Sygusch J (2002) A new family of plant transcription factors displays a novel ssDNA-binding surface. Nat Struct Biol 9:512–517
Desveaux D, Subramaniam R, Després C, Mess JN, Lévesque CL, Fobert PR, Dangl JL, Brisson N (2004) A “whirly” transcription factor is required for salicylic acid-dependent disease resistance in Arabidopsis. Dev Cell 6:229–240
Devadas SK, Enyedi A, Raina R (2002) The Arabidopsis hrl1 mutation reveals novel overlapping roles for salicylic acid, jasmonic acid and ethylene signaling in cell death and defence against pathogens. Plant J 30:467–480
Dewdney J, Reuber TL, Wildermuth MC, Devoto A, Cui J, Stutius LM, Drummond EP, Ausubel FM (2000) Three unique mutants of Arabidopsis identify eds loci required for limiting growth of a biotrophic fungal pathogen. Plant J 24:205–218
Dong X (2004) NPR1, all things considered. Curr Opin Plant Biol 7:547–552
Du H, Klessig DF (1997) Identification of a soluble, high-affinity salicylic acid-binding protein in tobacco. Plant Physiol 113:1319–1327
Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42:185–209
Durrant WE, Wang S, Dong X (2007) Arabidopsis SNI1 and RAD51D regulate both gene transcription and DNA recombination during the defense response. Proc Natl Acad Sci U S A 104:4223–4227
El Oirdi M, El Rahman TA, Rigano L, El Hadrami A, Rodriguez MC, Daayf F, Vojnov A, Bouarab K (2011) Botrytis cinerea manipulates the antagonistic effects between immune pathways to promote disease development in tomato. Plant Cell 23:2405–2421
El-Basyouni SZ, Chen D, Ibrahim RK, Neish AC, Towers GHN (1964) The biosynthesis of hydroxybenzoic acids in higher plants. Phytochemistry 3:485–492
Ellis BE, Amrhein N (1971) NIH-shift during aromatic orthodihydroxylation in higher plants. Phytochemistry 10:3069
Enyedi AJ, Yalpani N, Silverman P, Raskin I (1992) Localization, conjugation, and function of salicylic acid in tobacco during the hypersensitive response reaction to tobacco mosaic-virus. Proc Natl Acad Sci U S A 89:2480–2484
Fan W, Dong X (2002) In vivo interaction between NPR1 and transcription factor TGA2 leads to salicylic acid-mediated gene activation in Arabidopsis. Plant Cell 14:1377–1389
Felton GW, Korth KL, Bi JL, Wesley SV, Huhman DV, Mathews MC, Murphy JB, Lamb C, Dixon RA (1999) Inverse relationship between systemic resistance of plants to microorganisms and to insect herbivory. Curr Biol 9:317–320
Feng JX, Cao L, Li J, Duan CJ, Luo XM, Le N, Wei HH, Liang SJ, Chu CC, Pan QH, Tang JL (2011) Involvement of OsNPR1/NH1 in rice basal resistance to blast fungus Magnaporthe oryzae. Eur J Plant Pathol 131:221–235
Fitzgerald HA, Chern MS, Navarre R, Ronald PC (2004) Overexpression of (At)NPR1 in rice leads to a BTH- and environment-induced lesion-mimic/cell death phenotype. Mol Plant Microbe Interact 17:140–151
Ford KA, Casida JE, Chandran D, Gulevich AG, Okrent RA, Durkin KA, Sarpong R, Bunnelle EM, Wildermuth MC (2010) Neonicotinoid insecticides induced salicylate-associated plant defense responses. Proc Natl Acad Sci U S A 107:17527–17532
Foster S, Tyler VE (1999) Tyler’s honest herbal, 4th edn. Haworth Herbal, Binghamton
Fragnière C, Serrano M, Abou-Mansour E, Métraux JP, L’Haridon F (2011) Salicylic acid and its location in response to biotic and abiotic stress. FEBS Lett 585:1847–1852
Friedrich L, Lawton K, Dietrich R, Willits M, Cade R, Ryals J (2001) NIM1 overexpression in Arabidopsis potentiates plant disease resistance and results in enhanced effectiveness of fungicides. Mol Plant Microbe Interact 14:1114–1124
Fu ZQ, Dong X (2013) Systemic acquired resistance: turning local infection into global defense. Annu Rev Plant Biol 64:839–863
Fu ZQ, Yan S, Saleh A, Wang W, Ruble J, Oka N, Mohan R, Spoel SH, Tada Y, Zheng N, Dong X (2012) NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486:228–232
Gabriace B, Werck-Reichhart D, Teutsch H, Durst F (1991) Purification and immunocharacterization of a plant cytochrome P450: the cinnamic acid 4-hydrozylase. Arch Biochem Biophys 288:302–309
Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J (1993) Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261:754–756
Garcion C, Métraux JP (2006) Salicylic acid. In: Hedden P, Thomas SG (eds) Plant hormone signaling. Blackwell, Oxford
Garcion C, Lohmann A, Lamodière E, Catinot J, Buchala A, Doermann P, Métraux JP (2008) Characterization and biological function of the Isochorismate Synthase2 gene of Arabidopsis. Plant Physiol 147:1279–1287
Gestetner B, Conn EE (1974) The 2-hydroxylation of transcimannic acid by chloroplasts from Melilotus alba Desr. Arch Biochem Biophys 163:617–624
Glatt S, Séraphin B, Müller CW (2012) Elongator: transcriptional or translational regulator? Transcription 3:273–276
Gorlach J, Volrath S, Knauf-Beiter G, Hengy G, Beckhove U, Kogel KH, Oostendorop M, Staub T, Ward E, Kessmann H, Ryals J (1996) Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant Cell 8:629–643
Griesebach H, Vollmer KO (1963) Untersuchungen zur biosyntheses des salicylsauremethylesters in Gaultheria procumbens L. Z Naturforsch B 18:753
Gurr SJ, Rushton PJ (2005a) Engineering plants with increased disease resistance: how are we going to express it? Trends Biotechnol 23:283–290
Gurr SJ, Rushton PJ (2005b) Engineering plants with increased disease resistance: what are we going to express it? Trends Biotechnol 23:275–282
Harrison AJ, Yu M, Gårdenborg T, Middleditch M, Ramsay RJ, Baker EN, Lott JS (2006) The structure of Mbtl from Mycobacterium tuberculosis, the first enzyme in the biosynthesis of the siderophore mycobactin, reveals it to be a salicylate synthase. J Bacteriol 188:6081–6091
Harrower J, Wildermuth MC (2011) Exogenous salicylic acid treatment of Arabidopsis thaliana Col-0. NCBI Gene Expression Omnibus Accession No: GSE33402
He Z, Stigers Lavoie KD, Bartlett PA, Toney MD (2004) Conservation of mechanism in three chorismate-utilizing enzymes. J Am Chem Soc 126:2378–2385
Heidel AJ, Dong X (2006) Fitness benefits of systemic acquired resistance during Hyaloperonospora parasitica infection in Arabidopsis thaliana. Genetics 173:1621–1628
Hermann M, Maier F, Masroor A, Hirth S, Pfitzner AJP, Pfitzner UM (2013) The Arabidopsis NIMIN proteins affect NPR1 differentially. Front Plant Sci 4:88
Huang J, Gu M, Lai Z, Fan B, Shi K, Zhou YH, Yu JQ, Chen Z (2010) Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol 153:1526–1538
Ibdah M, Chen YT, Wilkerson CG, Pichersky E (2009) An aldehyde oxidase in developing seeds of Arabidopsis converts benzaldehyde to benzoic acid. Plant Physiol 150:416–423
Janda T, Horvath E, Szalai C, Palide E (2007) Role of salicylic acid in the induction of abiotic stress tolerance. In: Hayat S, Ahmad A (eds) Salicylic acid: a plant hormone. Springer, Dordrecht
Jarvis AP, Schaaf O, Oldham NJ (2000) 3-Hydroxy-3-phenylpropanoic acid is an intermediate in the biosynthesis of benzoic acid and salicylic acid but benzaldehyde is not. Planta 212:119–126
Johnson C, Boden E, Arias J (2003) Salicylic acid and NPR1 induce the recruitment of trans-activating TGA factors to a defense gene promoter in Arabidopsis. Plant Cell 15:1846–1858
Jones JD, Dangl JL (2006) The plant immune system. Nature 444:323–329
Kachroo P, Shanklin J, Shah J, Whittle EJ, Klessig DF (2001) A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proc Natl Acad Sci U S A 98:9448–9453
Kaltdorf M, Naseem M (2013) How many salicylic acid receptors does a plant cell need? Sci Signal 6(279):jc3
Kerbarh O, Ciulli A, Howard NI, Abell C (2005) Salicylate biosynthesis: overexpression, purification and characterization of Irp9, a bifunctional salicylate synthase from Yersinia enterocolitica. J Bacteriol 187:5061–5066
Kesarwani M, Yoo J, Dong X (2007) Genetic interactions of TGA transcription factors in the regulation of pathogenesis-related genes and disease resistance in Arabidopsis. Plant Physiol 144:336–346
Kidd BN, Edgar CI, Kumar KK, Aitken EA, Schenk PM, Manners JM, Kazan K (2009) The Mediator complex subunit PFT1 is a key regulator of jasmonate-dependent defense in Arabidopsis. Plant Cell 21:2237–2252
Killian J, Whitehead D, Horak J, Wanke D, Weinl S, Batistic O, D’Angelo C, Bornberg-Bauer E, Kudla J, Harter K (2007) The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J 50:347–363
Kinkema M, Fan W, Dong X (2000) Nuclear localization of NPR1 is required for activation of PR gene expression. Plant Cell 12:2339–2350
Klämbt HD (1962) Conversion in plants of benzoic acid to salicylic acid and its β-D-glucoside. Nature 196:491
Kolappan S, Zwahlen J, Zhou R, Truglio JJ, Tonge PJ, Kisker C (2007) Lysine 190 is the catalytic base in MenF, the menaquinone-specific isochorismate synthase from Escherichia coli: implications for an enzyme family. Biochemistry 46:946–953
Koornneef A, Rindermann K, Gatz C, Pieterse CMJ (2008) Histone modification do not play a major role in salicylate mediated suppression of jasmonate-induced PDF1.2 gene expression. Commun Integr Biol 1:143–145
Kumar D, Klessig DF (2003) High-affinity salicylic acid-binding protein 2 is required for plant innate immunity and has salicylic acid-stimulated lipase activity. Proc Natl Acad Sci U S A 100:16101–16106
Kumar V, Joshi SG, Bell AA, Rathore KS (2013) Enhanced resistance against Thielaviopsis basicola in transgenic cotton plants expressing Arabidopsis NPR1 gene. Transgenic Res 22:359–368
Le Henanff G, Farine S, Kieffer-Mazet F, Miclot AS, Heitz T, Mestre P, Bertsch C, Chong J (2011) Vitis vinifera VvNPR1.1 is the functional ortholog of AtNPR1 and its overexpression in grapevine triggers constitutive activation of PR genes and enhanced resistance to powdery mildew. Planta 234:405–417
Lebel E, Heifetz P, Thorne L, Uknes S, Ryals J, Ward E (1998) Functional analysis of regulatory sequences controlling PR1 gene expression in Arabidopsis. Plant J 16:223–233
Lee JS (1998) The mechanism of stomatal closing by salicylic acid in Commelina communis L. J Plant Biol 41:97–102
Lee HI, León J, Raskin I (1995) Biosynthesis and metabolism of salicylic acid. Proc Natl Acad Sci U S A 92:4076–4079
Léon J, Yalpani N, Raskin I, Lawton MA (1993) Induction of benzoic acid 2-hydroxylase in virus-inoculated tobacco. Plant Physiol 103:323–328
León J, Shulaev V, Yalpani N, Lawton MA, Raskin I (1995) Benzoic acid 2-hydroxylase, a soluble oxygenase from tobacco, catalyzes salicylic acid biosynthesis. Proc Natl Acad Sci U S A 92:10413–10417
Li X, Zhang Y, Clarke JD, Li Y, Dong X (1999) Identification and cloning of a negative regulator of systemic acquired resistance, SNI1, through a screen for suppressors of npr1-1. Cell 98:329–339
Li X, Clarke JD, Zhang Y, Dong X (2001) Activation of an EDS1-mediated R-gene pathway in the snc1 mutant leads to constitutive, NPR1-independent pathogen resistance. Mol Plant Microbe Interact 14:1131–1139
Lin WC, Lu CF, Wu JW, Cheng ML, Lin YM, Yang NS, Black L, Green SK, Wang JF, Cheng CP (2004) Transgenic tomato plants expressing the Arabidopsis NPR1 gene display enhanced resistance to a spectrum of fungal and bacterial diseases. Transgenic Res 13:567–581
Liu G, Holub EB, Alonso JM, Ecker JR, Fobert PR (2005) An Arabidopsis NPR1-like gene, NPR4, is required for disease resistance. Plant J 41:304–318
Luna E, Bruce TJA, Roberts MR, Flors V, Ton J (2012) Next-generation systemic acquired resistance. Plant Physiol 158:844–853
Makandar R, Essig JS, Schapaugh MA, Trick HN, Shah J (2006) Genetically engineered resistance to Fusarium head blight in wheat by expression of Arabidopsis NPR1. Mol Plant Microbe Interact 19:123–129
Malamy J, Klessig DF (1992) Salicylic acid and plant disease resistance. Plant J 2:643–654
Malamy J, Carr JP, Klessig DF, Raskin I (1990) Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science 250:1002–1004
Malamy J, Hennig J, Klessig DF (1992) Temperature-dependent induction of salicylic acid and its conjugates during the resistance response to tobacco mosaic virus infection. Plant Cell 4:359–366
Malnoy M, Jin Q, Borejsza-Wysocka EE, He SY, Aldwinckle HS (2007) Overexpression of the apple MpNPR1 gene confers increased disease resistance in Malus x domestica. Mol Plant Microbe Interact 19:123–129
Manthe B, Schulz M, Schnabl H (1992) Effects of salicylic acid on growth and stomatal movement of Vicia faba L.: evidence for salicylic acid metabolization. J Chem Ecol 18:1525–1539
Martínez C, Pons E, Prats G, Leon J (2004) Salicylic acid regulates flowering time and links defence responses and reproductive development. Plant J 37:209–217
Mauch F, Mauch-Mani B, Gaille C, Kull B, Haas D, Reimmann C (2001) Manipulation of salicylate content in Arabidopsis thaliana by the expression of an engineered bacterial salicylate synthase. Plant J 25:67–77
Mauch-Mani B, Slusarenko AJ (1996) Production of salicylic acid precursors is a major function of phenylalanine ammonia-lyase in the resistance of Arabidopsis to Peronospora parasitica. Plant Cell 8:203–212
Mercado-Blanco J, van der Drift KMGM, Olsson PE, Thomas-Oates JE, van Loon LC, Bakker PAHM (2001) Analysis of the pmsCEAB gene cluster involved in biosynthesis of salicylic acid and the siderophore Pseudomonine in the biocontrol strain Pseudomonas fluorescens WCS374. J Bacteriol 183:1909–1920
Métraux JP, Signer H, Ryals JA, Ward E, Wyss-Benz M, Gaudin J, Raschdorf K, Schmid E, Blum W, Inverardi B (1990) Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science 250:1004–1006
Meur G, Budatha M, Srinivasan T, Rajesh Kumar KR, Dutta Gupta A, Kirti PB (2008) Constitutive expression of Arabidopsis NPR1 confers enhanced resistance to the early instars of Spodoptera litura in transgenic tobacco. Physiol Plant 133:765–775
Meuwly P, Mölders W, Buchala A, Métraux JP (1995) Local and systemic biosynthesis of salicylic acid in infected cucumber plants. Plant Physiol 109:1107–1114
Moore JW, Loake GJ, Spoel SH (2011) Transcription dynamics in plant immunity. Plant Cell 23:2809–2820
Morris SW, Vernooij B, Titatarn S, Starrett M, Thomas S, Wiltse CC, Frederiksen RA, Bhandhufalck A, Hulbert S, Ukness S (1998) Induced resistance response in maize. Mol Plant Microbe Interact 11:643–658
Morris K, MacKerness SA, Page T, John CF, Murphy AM, Carr JP, Buchanan-Wollaston V (2000) Salicylic acid has a role in regulating gene expression during leaf senescence. Plant J 23:677–685
Mosher RA, Durrant WE, Wang D, Song J, Dong X (2006) A comprehensive structure-function analysis of Arabidopsis SNI1 defines essential regions and transcriptional repressor activity. Plant Cell 18:1750–1765
Mou Z, Fan W, Dong X (2003) Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 113:935–944
Navarre DA, Mayo D (2004) Differential characteristics of salicylic acid-mediated signaling in potato. Physiol Mol Plant Pathol 64:179–188
Nawrath C, Métraux JP (1999) Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11:1393–1404
Nawrath C, Heck S, Parinthawong N, Métraux JP (2002) EDS5, an essential component of salicylic acid–dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 14:275–286
Nelissen H, Fleury D, Bruno L, Robles P, De Veylder L, Traas J, Micol JL, Van Montagu M, Inze D, Van Lijsebettens M (2005) The elongata mutants identify a functional Elongator complex in plants with a role in cell proliferation during organ growth. Proc Natl Acad Sci U S A 102:7754–7759
Nishimura MT, Dangl JL (2010) Arabidopsis and the plant immune system. Plant J 61:1053–1066
Nobuta K, Okrent RA, Stoutemyer M, Rodibaugh N, Kempema L, Wildermuth MC, Innes RW (2007) The GH3 acyl adenylase family member PBS3 regulates salicylic acid-dependent defense responses in Arabidopsis. Plant Physiol 144:1144–1156
Norman C, Howell KA, Millar AH, Whelan JM, Day DA (2004) Salicylic acid is an uncoupler and inhibitor of mitochondrial electron transport. Plant Physiol 134:492–501
Nugent RL, Johnsson A, Fleharty B, Gogol M, Xue-Franzén Y, Seidel C, Wright AP, Forsburg SL (2010) Expression profiling of S. pombe acetyltransferase mutants identifies redundant pathways of gene regulation. BMC Genomics 11:59
Ogawa D, Nakajima N, Sano T, Tamaoki M, Aono M, Kubo A, Kanna M, Ioki M, Kamada H, Saji H (2005) Salicylic acid accumulation under O3 exposure is regulated by ethylene in tobacco plants. Plant Cell Physiol 46:1062–1072
Pallas JA, Paiva NL, Lamb C, Dixon RA (1996) Tobacco plants epigenetically suppressed in phenylalanine ammonia-lyase expression do not develop systemic acquired resistance in response to infection by tobacco mosaic virus. Plant J 10:281–293
Pan Q, Zhan J, Liu H, Zhang J, Chen J, Wen P, Huang W (2006) Salicylic acid synthesis by benzoic acid 2-hydroxylase participates in the development of thermo tolerance in pea plants. Plant Sci 171:226–233
Park SW, Kaimoyo E, Kumar D, Mosher S, Klessig DF (2007) Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318:113–116
Parkhi V, Kumar V, Campbell LM, Bell AA, Rathore KS (2010a) Expression of Arabidopsis NPR1 in transgenic cotton confers resistance to non-defoliating isolates of Verticillium dahliae but not the defoliating isolate. J Phytopathol 158:822–825
Parkhi V, Kumar V, Campbell LM, Bell AA, Shah J, Rathore KS (2010b) Resistance against various fungal pathogens and reniform nematode in transgenic cotton plants expressing Arabidopsis NPR1. Transgenic Res 19:959–975
Parsons JF, Shi KM, Ladner JE (2008) Structure of isochorismate synthase in complex with magnesium. Acta Crystallogr D64:607–610
Pasquer F, Isidore E, Zarn J, Keller B (2005) Specific patterns of changes in wheat gene expression after treatment with three antifungal compounds. Plant Mol Biol 57:693–707
Pellegrini L, Rohfritsch O, Fritig B, Legrand M (1994) Phenylalanine ammonia-lyase in tobacco. Molecular cloning and gene expression during the hypersensitive reaction to tobacco mosaic virus and the response to a fungal elicitor. Plant Physiol 106:877–886
Pelludat C, Brem D, Heesemann J (2003) Irp9, encoded by the high-pathogenicity island of Yersinia enterocolitica, is able to convert chorismate into salicylate, the precursor of the siderophore yersiniabactin. J Bacteriol 185:5648–5653
Pierpoint WS (1994) Salicylic acid and its derivatives in plants: medicines, metabolites and messenger molecules. Adv Bot Res 20:163–235
Pieterse CMJ, van Loon LC (2004) NPR1: the spider in the web of induced resistance signaling pathways. Curr Opin Plant Biol 7:456–464
Postel S, Küfner I, Beuter C, Mazzotta S, Schwedt A, Borlotti A, Halter T, Kemmerling B, Nürnberger T (2010) The multifunctional leucine-rich repeat receptor kinase BAK1 is implicated in Arabidopsis development and immunity. Eur J Cell Biol 89:169–174
Potlakayala SD, Reed DW, Covello PS, Fobert PR (2007) Systemic acquired resistance in canola is linked with pathogenesis-related gene expression and requires salicylic acid. Phytopathology 97:794–802
Poulsen C, Verpoorte R (1991) Roles of chorismate mutase, isochorismate synthase and anthranilate synthase in plants. Phytochemistry 30:377–386
Pridham JB (1965) Low molecular weight phenols in higher plants. Annu Rev Plant Physiol 6:13–36
Priya B, Somasekhar N, Prasad JS, Kirti PB (2011) Transgenic tobacco plants constitutively expressing Arabidopsis NPR1 show enhanced resistance to root-knot nematode, Meloidogyne incognita. BMC Res Notes 4:231
Quilis J, Penas G, Messeguer J, Brugidou C, Segundo BS (2008) The Arabidopsis AtNPR1 inversely modulates defense responses against fungal, bacterial, or viral pathogens while conferring hypersensitivity to abiotic stresses in transgenic rice. Mol Plant Microbe Interact 21:1215–1231
Raes J, Rhode A, Christensen JH, van de Peer Y, Boerjan W (2003) Genome-wide characterization of the lignification toolbox in Arabidopsis. Plant Physiol 133:1051–1071
Raffaele S, Rivas S, Roby D (2006) An essential role for salicylic acid in AtMYB30-mediated control of the hypersensitive cell death program in Arabidopsis. FEBS Lett 580:3498–3504
Rajou L, Belghazi M, Huguet R, Robin C, Moreau A, Job C, Job D (2006) Proteomic investigation of the effect of salicylic acid on Arabidopsis seed germination and establishment of early defense mechanisms. Plant Physiol 141:910–923
Rao MV, Davis KR (2001) The physiology of ozone-induced cell death. Planta 213:682–690
Rao MV, Lee HI, Davis KR (2002) Ozone-induced ethylene production is dependent on salicylic acid, and both salicylic acid and ethylene act in concert to regulate ozone-induced cell death. Plant J 32:447–456
Raridan GJ, Delaney TP (2002) Role of salicylic acid and NIM1/NPR1 in race-specific resistance in Arabidopsis. Genetics 29:439–451
Raskin I (1992) Role of salicylic acid in plants. Annu Rev Plant Physiol 43:438–463
Raskin I, Ehmann A, Melander WR, Meeuse BJD (1987) Salicylic acid: a natural induced of heat production in Arum lilies. Science 237:1601–1602
Raskin I, Skubatz H, Tang W, Meeuse BJD (1990) Salicylic acid levels in thermogenic and nonthermogenic plants. Ann Bot 66:369–373
Rate DN, Cuenca JV, Bowman GR, Guttman DS, Greenberg JT (1999) The gain-of-function Arabidopsis acd6 mutant reveals novel regulation and function of the salicylic acid signaling pathway in controlling cell death, defense, and cell growth. Plant Cell 11:1695–1708
Ribnicky DM, Shulaev V, Raskin I (1998) Intermediates of salicylic acid biosynthesis in tobacco. Plant Physiol 118:565–572
Rochon A, Boyle P, Wignes T, Fobert PR, Despré C (2006) The coactivator function of Arabidopsis NPR1 requires the core of its BTB/POZ domain and the oxidation of C-terminal cysteines. Plant Cell 18:3670–3685
Rohde A, Morreel K, Ralph J, Goeminne G, Hostyn G, De Rycke R, Kushnir S, van Doorsselaere J, Joseleau JP, Vuylsteke M, van Driessche G, van Beeumen J, Messens E, Boerjan W (2004) Molecular phenotyping of the pal1 and pal2 mutants of Arabidopsis thaliana reveals far-reaching consequences on phenylpropanoid, amino acid, and carbohydrate metabolism. Plant Cell 16:2749–2771
Russel DW, Conn EE (1967) The cinnamic acid 4-hydroxylase of pea seedlings. Arch Biochem Biophys 122:256–258
Ruuhola T, Julkunen-Tiitto R (2003) Trade-off between synthesis of salicylates and growth of micropropagated Salix pentandra. J Chem Ecol 29:1565–1588
Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8:1809–1819
Salomon D, Sessa G (2012) Biotechnological strategies for engineering plants with durable resistance to fungal and bacterial pathogens. In: Altman A, Hasegawa PM (eds) Plant biotechnology and agriculture: prospects for the 21st century. Elsevier, Waltham
Sandhu D, Tasma IM, Frasch R, Bhattacharyya MK (2009) Systemic acquired resistance in soybean is regulated by two proteins, orthologous to Arabidopsis NPR1. BMC Plant Biol 9:105
Sawada H, Shim IS, Usui K (2006) Induction of benzoic acid 2-hydroxylase and salicylic acid biosynthesis-modulation by salt stress in rice seedlings. Plant Sci 171:263–270
Schmid J, Amrhein N (1995) Molecular organization of the shikimate pathway in higher plants. Phytochemistry 39:737–749
Schouten HJ, Krens FA, Jacobsen E (2006) Cisgenic plants are similar to traditionally bred plants: international regulations for genetically modified organisms should be altered to exempt cisgenesis. EMBO Rep 7:750–753
Serino L, Reimmann C, Baur H, Beyeler M, Visca P, Haas D (1995) Structure genes for salicylate biosynthesis from chorismate in Pseudomonas aeruginosa. Mol Gen Genet 249:217–228
Shadle GL, Wesley SV, Korth KL, Chen F, Lamb C, Dixon RA (2003) Phenylpropanoid compounds and disease resistance in transgenic tobacco with altered expression of L-phenylalanine ammonia-lyase. Phytochemistry 64:153–161
Shah J, Klessig DF (1999) Salicylic acid: signal perception and transduction. In: Hooykaas PPJ, Hall MA, Libbega KR (eds) Biochemistry and molecular biology of plant hormones. Elsevier, Amsterdam
Shah J, Tsui F, Klessig DF (1997) Characterization of a salicylic acid-insensitive mutant (sail1) of Arabidopsis thaliana, identified in a selective screen utilizing the SA-inducible expression of the tms2 gene. Mol Plant Microbe Interact 10:69–78
Shah J, Kachroo P, Klessig DF (1999) The Arabidopsis ssi1 mutation restores pathogenesis-related gene expression in npr1 plants and renders defensin gene expression salicylic acid dependent. Plant Cell 11:191–206
Shah J, Kachroo P, Nandi A, Klessig DF (2001) A recessive mutation in the Arabidopsis SSI2 gene confers SA- and NPR1-independent expression of PR genes and resistance against bacterial and oomycete pathogens. Plant J 25:563–574
Shi Z, Maximova SN, Liu Y, Verica J, Guiltinan MJ (2010) Functional analysis of the Theobroma cacao NPR1 gene in Arabidopsis. BMC Plant Biol 10:248
Shirano Y, Kachroo P, Shah J, Klessig DF (2002) A gain-of-function mutation in an Arabidopsis toll interleukiin1 receptor-nucleotide binding site-leucine-rich repeat type R gene triggers defense responses and results in enhanced disease resistance. Plant Cell 14:3149–3162
Silverman P, Seskar M, Kanter D, Schweizer P, Metraux JP, Raskin I (1995) Salicylic acid in rice: biosynthesis, conjugation and possible role. Plant Physiol 108:633–639
Slaymaker DH, Navarre DA, Clark D, Del Pozo O, Martin GB, Klessig DF (2002) The tobacco salicylic acid-binding protein 3 (SABP3) is the chloroplast carbonic anhydrase, which exhibits antioxidant activity and plays a role in the hypersensitive defense response. Proc Natl Acad Sci U S A 99:11640–11645
Song J, Durrant WE, Wang S, Yan S, Tan EH, Dong X (2011) DNA repair proteins are directly involved in regulation of gene expression during plant immune response. Cell Host Microbe 9:115–124
Spartz A, Gray WM (2008) Plant hormone receptors: new perceptions. Genes Dev 22:2139–2148
Spoel SH, Mou Z, Tada Y, Spivey NW, Genschik P, Dong X (2009) Proteasome-mediated turnover of the transcription coactivator NPR1 plays dual roles in regulating plant immunity. Cell 137:860–872
Srinivasan T, Kumar KRR, Meur G, Kirti PB (2009) Heterologous expression of Arabidopsis NPR1 (AtNPR1) enhances oxidative stress tolerance in transgenic tobacco plants. Biotechnol Lett 31:1343–1351
Stacey G, McAlvin CB, Kim SY, Olivares J, Soto MJ (2006) Effects of endogenous salicylic acid on nodulation in the model legumes Lotus japonicas and Medicago truncatula. Plant Physiol 141:1473–1481
Sticher L, Mauch-Mani B, Métraux JP (1997) Systemic acquired resistance. Annu Rev Phytopathol 35:235–270
Strawn MA, Marr SK, Inoule K, Inada N, Zubieta C, Wildermuth MC (2007) Arabidopsis isochorismate synthase functional in pathogen-induced salicylate biosynthesis exhibits properties consistent with a role in diverse stress responses. J Biol Chem 282:5919–5933
Subramaniam R, Desveaux D, Spickler C, Michnick SW, Brisson N (2001) Direct visualization of protein interactions in plant cells. Nat Biotechnol 19:769–772
Tada Y, Spoel SH, Pajerowska-Mukhtar K, Mou Z, Song J, Dong X (2008) Plant immunity requires conformational changes of NPR1 via S-nitrosylation and thioredoxins. Science 321:952–956
Takahashi H, Miller J, Nozaki Y, Sukamoto, Takeda M, Shah J, Hase S, Ikegami M, Ehara Y, Dinesh-Kumar SP (2002) RCY1, an Arabidopsis thaliana RPP8/HRT family resistance gene, conferring resistance to cucumber mosaic virus requires salicylic acid, ethylene and a novel signal transduction mechanism. Plant J 32:655–667
Traw MB, Bergelson J (2003) Interactive effects of jasmonic acid, salicylic acid, and gibberellin on induction of trichomes in Arabidopsis. Plant Physiol 133:1367–1375
Uppalapati SR, Ishiga Y, Wangdi T, Kunkel BN, Anand A, Mysore KS, Bender CL (2007) The phytotoxin coronatine contributes to pathogen fitness and is required for suppression of salicylic acid accumulation in tomato inoculated with Pseudomonas syringae pv. Tomato DC3000. Mol Plant Microbe Interact 20:955–965
van den Burg HA, Takken FLW (2009) Does chromatin remodeling mark systemic acquired resistance? Trends Plant Sci 14:286–294
van Tegelen LJP, Moreno PRH, Croes AF, Verpoorte R, Wullems GJ (1999) Purification and cDNA cloning of isochorismate synthase from elicited cell cultures of Catharanthus roseus. Plant Physiol 119:705–712
Vanacker H, Lu H, Rate DN, Greenberg JT (2001) A role for salicylic acid and NPR1 in regulating cell growth in Arabidopsis. Plant J 28:209–216
Verberne MC, Budi Muljono RA, Verpoorte R (1999) Salicylic acid biosynthesis. In: Hall PPJ, Libbenga KR (eds) Biochemistry and molecular biology of plant hormones. Elsevier Science BV, Amsterdam
Verberne MC, Verpoorte R, Bol JF, Mercado-Blanco J, Linthorst HJM (2000) Overproduction of salicylic acid in plants by bacterial transgene enhances pathogen resistance. Nat Biotechnol 18:779–783
Versées W, De Groeve S, van Lijsebettens M (2010) Elongator, a conserved multitasking complex? Mol Microbiol 76:1065–1069
Vicente MRS, Plasencia J (2011) Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62:3321–3338
Vijayan S, Kirti P (2012) Mungbean plants expressing BjNPR1 exhibit enhanced resistance against the seedling rot pathogen Rhizoctonia solani. Transgenic Res 21:193–200
Vlot AC, Liu PP, Cameron RK, Park SW, Yang Y, Kumar D, Zhou F, Padukkavidana T, Gustafsson C, Pichersky E, Klessig DF (2008) Identification of likely orthologs of tobacco salicylic acid-binding protein 2 and their role in systemic acquired resistance in Arabidopsis thaliana. Plant J 56:445–456
Vlot AC, Dempsey DA, Klessig DF (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47:177–206
Wally O, Jayaraj J, Punja Z (2009) Broad-spectrum disease resistance to necrotrophic and biotrophic pathogens in transgenic carrots (Daucus carota L.) expressing an Arabidopsis NPR1 gene. Planta 231:131–141
Wang D, Amornisripanitch N, Dong X (2006) A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog 2:e123
Wang S, Durrant WE, Song J, Spivey NW, Dong X (2010) Arabidopsis BRCA2 and RAD51 proteins are specifically involved in defense gene transcription during plant immune responses. Proc Natl Acad Sci U S A 107:22716–22721
Wang Y, An C, Zhang X, Yao J, Zhang Y, Sun Y, Yu F, Amador DM, Mou Z (2013) The Arabidopsis Elongator complex subunit2 epigenetically regulates plant immune responses. Plant Cell 25:762–776
Wasternack C, Atzorn R, Jarosch B, Kogel KH (1994) Induction of a thionin, the jasmonate-induced 6 kDa protein of barley by 2,6-dichloroisonicotinic acid. J Phytopathology 140:280–284
Wathugala DL, Hemsley PA, Moffat CS, Cremelie P, Knight MR, Knight H (2012) The Mediator subunit SFR6/MED16 controls defense gene expression mediated by salicylic acid and jasmonate responsive pathways. New Phytol 195:217–230
Way HM, Kazan K, Mitter N, Goulter KG, Birch RG, Manners JM (2002) Constitutive expression of a phenylalanine ammonia-lyase gene from Stylosanthes humilis in transgenic tobacco leads to enhanced disease resistance but impaired plant growth. Physiol Mol Plant Pathol 60:275–282
Weigel RR, Bäuscher C, Pfitzner AJP, Pritzner UM (2001) NIMIN-1, NIMIN-2 and NIMIN-3, members of a novel family of proteins from Arabidopsis that interact with NPR1/NIM1, a key regulator of systemic acquired resistance in plants. Plant Mol Biol 46:143–160
Weigel RR, Pfitzner UM, Gatz C (2005) Interaction of NIMIN1 with NPR1 modulates PR gene expression in Arabidopsis. Plant Cell 17:1279–1291
Weissman G (1991) Aspirin. Sci Am 264:84–90
Wildermuth MC (2006) Variations on a theme: synthesis and modification of plant benzoic acids. Curr Opin Plant Biol 9:288–296
Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defense. Nature 414:562–565
Winkler GS, Kristjuhan A, Erdjument-Bromage H, Tempst P, Svejstrup JQ (2002) Elongator is a histone H3 and H4 acetyltransferase important for normal histone acetylation levels in vivo. Proc Natl Acad Sci U S A 99:3517–3522
Wu Y, Zhang D, Chu JY, Boyle P, Wang Y, Brindle ID, De Luca V, Després C (2012) The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep 1:639–647
Xu D, Huang W, Li Y, Wang H, Huang H, Cui X (2012) Elongator complex is critical for cell cycle progression and leaf patterning in Arabidopsis. Plant J 69:792–808
Yalpani N, Silverman P, Wilson TMA, Kleier DA, Raskin I (1991) Salicylic acid is a systemic signal and an inducer of pathogenesis-related proteins in virus-infected tobacco. Plant Cell 3:809–818
Yalpani N, León J, Lawton MA, Raskin I (1993) Pathway of salicylic acid biosynthesis in healthy and virus-inoculated tobacco. Plant Physiol 103:315–321
Yu D, Chen C, Chen Z (2001) Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. Plant Cell 13:1527–1540
Yuan Y, Zhong S, Zhu Z, Lou Y, Wang J, Wang M, Li Q, Yang D, He Z (2007) Functional analysis of rice NPR1-like genes reveals the OsNPR1/NH1 is the rice orthologue conferring disease resistance with enhanced herbivore susceptibility. Plant Biotechnol J 5:313–324
Zenk MH, Muller G (1964) Biosynthesis von p-hydroxybenzoesaure und anderer benzoesauren in hoheren Pflanzen. Z Naturforsch B 19:398
Zhang Y, Li X (2005) A putative nucleoporin 96, is required for both basal defense and constitutive resistance responses mediated by snc1. Plant Cell 17:1306–1316
Zhang Y, Fan W, Kinkema M, Li X, Dong X (1999) Interaction of NPR1 with basic leucine zipper protein transcription factors that bind sequences required for salicylic acid induction of the PR-1 gene. Proc Natl Acad Sci U S A 96:6523–6528
Zhang Y, Tessaro MJ, Lassner M, Li X (2003) Knockout analysis of Arabidopsis transcription factor TGA2, TGA5, and TGA6 reveals their redundant and essential roles in systemic acquired resistance. Plant Cell 15:2647–2653
Zhang X, Francis MI, Dawson WO, Graham JH, Orbović V, Triplett EW, Mou Z (2010a) Overexpression of the Arabidopsis NPR1 gene in citrus increase resistance to citrus canker. Eur J Plant Pathol 128:91–100
Zhang Y, Yang Y, Fang B, Gannon P, Ding P, Li X, Zhang Y (2010b) Arabidopsis snc2-1D activates receptor like protein-mediated immunity transduced through WRKY70. Plant Cell 22:3153–3163
Zhang JY, Qiao YS, Lv D, Gao ZH, Qu SC, Zhang Z (2012a) Malus hupehensis NPR1 induces pathogenesis-related protein gene expression in transgenic tobacco. Plant Biol (Stuttg) 14:46–56
Zhang X, Wang C, Zhang Y, Su Y, Mou Z (2012b) The Arabidopsis Mediator complex subunit16 positively regulates salicylate-mediated systemic acquired resistance and jasmonate/ethylene-induced defense pathways. Plant Cell 24:4294–4309
Zhang X, Yao J, Zhang Y, Sun Y, Mou Z (2013a) The Arabidopsis Mediator complex subunits MED14/SWP and MED16/SFR16/IEN1 differentially regulate defense gene expression in plant immune responses. Plant J 75:484–497
Zhang Y, Ni X, Ma H, Qiu W (2013b) Characterization of NPR1 genes from Norton and Cabernet sauvignon grapevine. J Integr Agric 12:1152–1161
Zhou JM, Trifa Y, Silva H, Pontier D, Lam E, Shah J, Klessig DF (2000) NPR1 differentially interacts with members of the TGA/OBF family of transcription factors that bind an element of the PR-1 gene required for induction by salicylic acid. Mol Plant Microbe Interact 13:191–202
Ziebart KT, Toney MD (2010) Nucleophile specificity in anthranilate synthase, amidodeoxychorismate synthase, and salicylate synthase. Biochemistry 49:2851–2859
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
An, C., Mou, Z. (2014). Salicylic Acid and Defense Responses in Plants. In: Tran, LS., Pal, S. (eds) Phytohormones: A Window to Metabolism, Signaling and Biotechnological Applications. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-0491-4_7
Download citation
DOI: https://doi.org/10.1007/978-1-4939-0491-4_7
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-0490-7
Online ISBN: 978-1-4939-0491-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)