Abstract
Cancer is increasingly recognized as not solely a disease of the genes and chromosomes but as a systemic disease that affects numerous components of the host including blood vessel formation, immune cell function, and nutrient recycling. This review summarizes a variety of time-dependent mathematical models that focus on the consequences of tumor growth within an evolving microenvironment, represented by a dynamic carrying capacity. Transcending the specifics of each model, their overview reveals that the key to tumor control really lies in controlling the support furnished the tumor by its microenvironment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Tumor microenvironment
- Mathematical modeling
- Cancer systems biology
- Tumor-promoting inflammation
- Metronomic chemotherapy
1 Introduction
Despite the increase in targeted efforts that followed the National Cancer Act of 1971, where President Nixon declared a “war on cancer,” the cure for cancer remains elusive. In the following 40 years, significant advances have been made for certain genetically simple cancers, such as acute lymphoblastic leukemia (ALL), a rare case where the cancerous clone can be therapeutically eradicated [1]. More genetically complex cancers, such as those of the breast, prostate, pancreas, and lung, however, are still not responsive to the majority of available treatments [1]. The limited therapeutic success of these cancers may be due to the fact that genetically complex cancers continually engage their microenvironment, creating a niche within the host, where abnormal cells have a competitive advantage over normal cells of the surrounding tissue [2].
Tumor microenvironments are dynamic heterogeneous systems influenced by many factors, including space and nutrient availability. Additionally, continuous interactions between somatic cells and immune cells influence the environment through both pro- and antitumor actions, depending on their context [3]. In this review we focus on three main biological processes that affect tumor growth: angiogenesis, tumor-promoting inflammation, and nutrient availability. Tumor growth is always accompanied by new blood vessel growth, or angiogenesis [4]. Since this discovery, angiogenesis has become a target for anticancer therapies [5, 6]. Inflammatory responses also accompany tumor growth and can promote tumor development from initiation to progression and metastasis [3, 7]. In light of these developments, anti-inflammatory drugs have been proposed as anticancer agents [8]. And finally, competition for nutrients such as phosphorus—used in ribosome synthesis—can modulate tumor growth and development [9, 10], and thus dialysis has been proposed after cytotoxic therapy to aid tumor control [11].
In order to successfully treat a cancerous lesion, the tumor’s influence over its microenvironment needs to be understood, requiring thorough investigation of the constituent parts, both individually and as a whole. Mathematical modeling provides a useful conceptual tool to evaluate the relative importance of various components of the microenvironment in tumor growth dynamics, which can then be translated into more targeted and fiscally responsible experimental, and eventually clinical, investigations.
A frequent criticism of the mathematical modeling toolkit is that it results in little more than an intellectual exercise, lacking practical use since it cannot compare to the weight and importance of wet-lab experiments. One must remember, however, that the purpose of mathematical modeling is not to replace such experiments, but rather to extend them. A carefully constructed conceptual model can aid in evaluating the relative importance of major players within the experimentally observed system. Not only can modeling provide a more financially responsible framework to validate theories and design experiments, but it can also provide valuable negative results. Since the basic assumptions underlying a model are predetermined, if predicted results qualitatively contradict experimental observations, then the assumptions must be reevaluated. Such is usually easier to address in silico rather than in vivo or even in vitro, which makes mathematical modeling an important and powerful complement to experimental work, and it should be treated as such.
Mathematical models of tumor growth and cancer-immune interactions provide a framework within which the complex biological system can be analyzed. Several approaches have been applied to quantify this system, with perhaps the most common approach being ordinary differential equations [12–20]. Such time-dependent models provide valuable insight into the complex dynamics of the system and can be modified to consider stochastic effects [21, 22] and the evolutionary nature of the system [23, 24]. Other approaches allow incorporation of spatial effects with either partial differential equations [25, 26] or agent-based simulations [27, 28].
Here, we review several mathematical models of tumor growth, selected because of their ability to describe the continuous evolution of the tumor microenvironment. In these models, the microenvironment is represented as a dynamic carrying capacity that supports, and ultimately limits, tumor growth. Noticeably, rather than discuss specific details of each model, we present the general functional forms originally proposed by the corresponding authors and instead focus our discussions on the major features and conclusions derived from each framework. We then use these frameworks to explore the significance of various components of the tumor microenvironment, in particular, of angiogenic factors, tumor-promoting inflammation, and nutrient availability. To conclude, we discuss how these results could be used to influence and augment current approaches to cancer therapy.
2 A Model of Angiogenesis-Dependent Tumor Growth
We start by discussing a model of tumor growth by Hahnfeldt et al. [6], where the effective vascular support of the tumor microenvironment, or carrying capacity, grows in a time-dependent manner with the tumor mass. The tumor-associated vasculature is controlled by the production of angiogenic stimulators and inhibitors, in a similar fashion to healing wounds or organogenesis. That is, the growing mass of cancer and stromal cells produce both pro- and antiangiogenic factors that initially stimulate angiogenesis and ultimately limit the angiogenic capacity of the tumor environment.
To study this process quantitatively, they proposed a mathematical framework composed of two compartments: the tumor volume V (t) and the carrying capacity of the tumor environment K(t). The two compartments are coupled in that the tumor cannot exceed the size allowed by the capacity and the capacity is controlled by the tumor volume.
Mathematically, the tumor volume is assumed to grow according to a generalized logistic law. That is,
This general law captures both of the most commonly assumed tumor growth models: logistic growth with \(P(t) =\lambda \Big (1 -\tfrac{V (t)} {K(t)}\Big)\) if α = 1 and Gompertzian growth with \(P(t) = -\lambda _{1}\ln \Big(\tfrac{V (t)} {K(t)}\Big)\) in the limit as α → 0.
The rate of change of the variable carrying capacity, \(\tfrac{\mathrm{d}K} {\mathrm{d}t}\), should depend on the current level of environmental support, K(t), the current tumor volume, V (t), and time, t. Four proposed factors that may influence the capacity are an intrinsic loss rate, stimulatory and inhibitory angiogenic signals produced by cancer-stroma interactions, and inhibition due to antiangiogenic treatments. Mathematically, these can be interpreted as the four terms written below on the right-hand side:
Under the assumption that angiogenic stimulators have fast clearance rates while angiogenic inhibitors have slow clearance rates, the final functional form of
was proposed. While the exact form of the stimulator (bV ) and inhibitor \((dKV ^{\frac{2} {3} })\) terms can vary, the main conclusion of the work was that the ratio of the two terms should be proportional to the volume raised to the power \(\frac{2} {3}\). Note that both tumor volume and the carrying capacity have units of volume. Details of the derivation can be found in [6].
The effects of antiangiogenic drugs on tumor growth are well characterized by this model because it specifically describes the angiogenic support of the tumor environment. As such, model validation by a series of experiments involving Lewis lung carcinoma cells in C57BL/6 mice with antiangiogenic treatments of TNP-470, angiostatin, or endostatin was performed. Assuming a Gompertzian growth law, tumor growth parameters (λ 1, λ 2, b, d, and K(0)) as well as treatment-specific parameters (e and g(t)) for the three drugs were estimated from experimental data.
The predictive power of this model was demonstrated by the excellent agreement between simulated growth dynamics and experimental data for drug dosages and combination therapies not used in the parameter estimation process (see Fig. 1). This quantitative theory may be useful for clinical determination of optimal drug and dosing protocols. Additionally, it has led to many theoretical investigations including generalizations of the theory for antiangiogenic therapy [29, 30], prediction of optimal antiangiogenic [31, 32] and combination of antiangiogenic-cytotoxic [33, 34] treatment protocols, spatial analyses for tumors with varying chemotherapeutic sensitivities [35], predictions of metastatic spread including angiogenesis [36], and investigations of the environmental regulatory effects on tumor growth and cancer-immune dynamics [37]. Overall, the concept of a dynamic tumor microenvironment, as captured by this model, has led to advances in our understanding of tumor growth dynamics and the consequences of cancer treatment.
A demonstration of the Hahnfeldt et al. [6] model for tumor growth in a dynamically growing carrying capacity. In (a), the carrying capacity grows with the tumor mass in the control (untreated) condition. In (b), parameters for the antiangiogenic drug endostatin were estimated from the experimental data at the dosage of 20 mg/kg/day. In (c) these same parameter values were used to predict the outcome of an additional experiment where the dosage was changed to 4 mg/kg/day. Excellent agreement of the model prediction to the experimental data is observed
3 Immune Predation in the Dynamic Tumor Microenvironment
The microenvironment of a tumor provides sustainability signals, such as nutrient and space availability, to the growing neoplasm. Cancer cells, however, can have varying sensitivities to these signals [38], especially across cancer type. Recent work by Wilkie and Hahnfeldt [37] demonstrates how varying sensitivities to these environmental regulatory signals can affect tumor dynamics and cancer-immune interactions, as well as result in significant variation in therapeutic outcome.
To do so, generalized logisitic growth is used in the mathematical formulation instead of Gompertzian or logistic growth. This introduces another parameter that represents the strength of the connection between the growing tumor and the carrying capacity. Additionally, they consider immune predation of cancer cells, resulting in the three-compartment model described below:
Here, C(t) represents the cancer population, I(t) the immune population, K I the constant carrying capacity of the immune population, and K C (t) the carrying capacity of the cancer population, which is considered to be either constant or dynamic (according to the Hahnfeldt et al. [6] model) in their analyses [37]. Immune predation of cancer cells occurs through the growth-modulating function Ψ(I, C) < 0.
With a constant cancer carrying capacity, parameter fitting to experimental data determines a fixed parameter set specifying the level of sensitivity to the regulatory signals provided by the environment. With a dynamic cancer carrying capacity, however, which has the ability to stimulate support at various rates, several sets of parameters were found specifying varying degrees of sensitivity, yet all fit the growth data equally well.
Environmental growth regulatory signals typically grow and evolve with, and in response to, the growing neoplasm. This process requires a conversion of the microenvironment from a normal state to a tumor-supporting niche. To expedite this conversion process, Hu et al. [39] co-injected cancer cells with either normal fibroblasts, tumor-associated fibroblasts, or pro-inflammatory arthritis-associated fibroblasts. They found that tumor weight may be enhanced by the tumor- or arthritis-associated fibroblasts compared to the normal fibroblasts or control, suggesting that inflammatory pro-tumor stromal cells may accelerate the conversion process within the microenvironment. In other words, the addition of pro-tumor inflammatory fibroblasts altered the growth regulatory signals produced by the environment and sensed by the cancer cells, resulting in accelerated growth.
Another example of dysregulation in environmental regulatory signaling can occur after chemotherapy or radiation therapy. Following such treatments, cancer regrowth can occur at rates up to 15–20 times faster than the rate observed pre-treatment [40, 41]. This accelerated regrowth may be partially due to tumor mass de-bulking without simultaneously targeting the environment, essentially leaving the pro-tumor behaviors of the stroma unaltered. Hence, residual cancer cells do not have to face the challenge and initial resistance associated with converting the environment to a tumor-supporting niche and thus can flourish in the already existing tumor-supporting environment.
With the above mentioned mathematical model, the phenomenon of accelerated regrowth was demonstrated by disrupting the environmental regulatory signals from those that grow with the cancer (a dynamic carrying capacity) to those that are already highly tumor supporting (a constant capacity equal to the maximum value for each parameter set). Interestingly, the sensitivity of each cancer (or parameter set) determined how the tumor would regrow following this disruption. Some sets predicted accelerated regrowth while others closely matched the original growth rate.
In terms of cancer-immune interactions, the immune-induced dormant state was shown to be essentially eliminated for cancers (or parameter sets) associated with high sensitivity to environmental regulation, and simulated immunotherapy treatments were shown to result in different outcomes depending on this sensitivity. The same therapy was predicted to result in the elimination for low-sensitivity cancers but growth and escape for high-sensitivity cancers. Disruption of the regulatory signals further altered the predicted outcomes of simulated immunotherapy, causing all cancers to grow large but at rates dependent on their level of sensitivity.
Important implications of this work are the potential explanation for why the same treatment may work for some patients but not others (cancer cell sensitivity to environmental regulation), and that primary versus follow-up treatments should not be expected to achieve the same outcomes (due to disruption of environmental signals). This work further supports the idea that to control a cancer, both the tumor and the surrounding environment should be targeted, simultaneously, to remove both the cancerous cells and the tumor-supporting environment.
4 Immune-Mediated Tumor Stimulation via the Dynamic Tumor Microenvironment
It is now well accepted that various immune cell types can stimulate cancer at every step from initiation to progression to metastasis [7, 42–45]. Immune cells are recruited to the tumor site by cytokines and danger signals that initiate an inflammatory response and thus can promote angiogenesis and tumor growth [3, 7, 46]. These dichotomous behaviors that immune cells exhibit confound the already complex system of cellular interplay that evolves in the tumor microenvironment.
A first attempt to mathematically investigate the dichotomous roles of immune cells within the tumor environment was recently undertaken by Wilkie and Hahnfeldt [47]. Based on the model for dynamic carrying capacity described above, the new framework incorporates both the cytotoxic effects and the proangiogenic effects of immune cells in a tumor microenvironment. The polarization of the grouped action for all immune cell types within the microenvironment is classified as either pro-tumor or antitumor, referring to the relative production of factors controlling angiogenesis, immunosuppression, and cancer cell predation.
The model modifies the Wilkie and Hahnfeldt framework described above to allow both immune and cancer population growth with dynamic carrying capacities [37]. The model equations below describe the dynamics of the four compartments: cancer cells C(t), immune cells I(t), the cancer carrying capacity K C (t), and the immune carrying capacity K I (t):
Notice that the cancer carrying capacity now considers the angiogenic actions of immune cells, that is, K C (t) = f(K C , C, I, t), and the cytotoxic actions are incorporated into the growth-modulating function Ψ(I, C).
Parameters a and b control the immune polarization as discussed above. Pro-tumor immunity polarization is described by a > b so that more weight is placed on the pro-angiogenic actions of immune cells than the antiangiogenic actions. Similarly, antitumor immunity polarization is described by a < b. In the immune carrying capacity, equal weight is placed on the immune and cancer cell actions to control the environmental recruitment signals for the immune response.
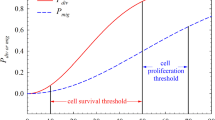
The antitumor and pro-tumor immunity polarizations predict different outcomes based solely on the level of angiogenesis-promoting inflammation. This is easily seen in the simulations without immune predation (Ψ = 0) in Fig. 2. With more weight placed on antiangiogenic functions in antitumor immunity, the final tumor burden is reduced compared to the control case where no immune cells are present. Conversely, with more weight placed on proangiogenic functions in pro-tumor immunity, the final tumor burden is enhanced compared to the control case. In both situations, however, there is an early period of growth where the presence of inflammation stimulates the tumor to grow faster than the control, regardless of whether the final tumor burden is reduced or enhanced.
A demonstration of the effect that antitumor (a) and pro-tumor (b) immunity can have on tumor growth. When present at constant levels, antitumor immunity causes a decrease in final tumor burden whereas pro-tumor immunity causes an increase in final tumor burden. Before these final states are achieved, however, both polarizations cause a period where immune presence increases tumor burden compared to the control (zero-immune) case. These results highlight the importance of time dynamics in the evolution of cancer-immune interactions as antitumor immunity may appear to enhance tumor growth in the short term while actually suppressing it in the long term
This work has great clinical importance as it demonstrates how stimulating the immune response can result in an early period of enhanced tumor growth even if a later-stage reduction in growth is obtained. Such contradictory results have been observed in clinical trials for immunotherapies [48] where responses were classified into four distinct patterns: shrinkage of the tumor, stable disease, response after initial increase in burden, and response in the presence of new lesions. With chemotherapeutic treatments, shrinkage of the tumor is expected, differing substantially from the response patterns described above for immunotherapies. As such, different evaluation criteria are required for immunotherapies versus chemotherapies, which have only recently been established. The predictions of this model suggest that a response after initial increase should not be a surprising consequence of immunotherapy, but rather an expected outcome of stimulating an antitumor immune response. Furthermore, the results suggest that targeting the polarization of the microenvironment to transform a pro-tumor environment into an antitumor environment will lead to improved tumor suppression. Insights into cancer-immune dynamics gained through mathematical modeling of both tumor-promoting and tumor-inhibiting immune effects will lead to a greater understanding of the disease, of treating the disease, and of how therapeutic success should be evaluated.
5 Nutrient Availability in the Tumor Microenvironment
The dynamic carrying capacity can also be analyzed from the point of view of nutrient availability. Within such a paradigm, tumors are similar to ecological systems where heterogeneous populations of cells, such as can be found in most solid tumors, compete with somatic cells and with each other not only for space but also for nutrients. Moreover, in the context of limited nutrient availability, it is the appropriate allocation of the limited nutrients that may prove to be a crucial factor in whether the tumor will progress to malignancy or not.
Based on experimental observations, Elser [9, 10] has proposed what has become known as the growth rate hypothesis (GRH), which suggests that variations not in absolute amounts of carbon, phosphorus, and nitrogen in the cell’s, or organism’s microenvironment, but their ratios may affect the growth rate of the organism. Specifically, the GRH predicts that increased phosphorus availability can select in favor of more rapidly growing phenotypes, since additional nutrients can be available for phosphorus-rich ribosomal RNA, which is a requirement for rapid growth. This prediction was verified experimentally [49], as mice that were fed a phosphorus-rich diet had more advanced lung tumor progression and growth compared to mice kept on a phosphorus-poor diet.
The GRH also predicts that highly proliferative cells, such as actively growing tumor cells, should be characterized by a low intracellular carbon:phosphorus ratio, since most of the phosphorus would be allocated in favor of replicative machinery and specifically ribosomes. Interestingly, these predictions were confirmed experimentally [50] for cancers of the colon and the lung but not in the kidney or liver. These results raised the possibility that variations in microenvironmental conditions can provide a selective force towards either highly replicative cell clones in some cases, or slower-growing but perhaps more apoptosis-resistant cell clones in other cases.
In this case, phosphorus becomes a dynamic resource that may determine whether or not a more proliferative cancer clone comes to dominate the population. Phosphorus, much like many other nutrients that are consumed by growing cells, can be recycled through cell death, suggesting that tumors with high cell mortality rates may create conditions that naturally favor high proliferation. Moreover, it is also possible that changing phosphorus availability in the tumor microenvironment may be an additional factor that can drive cells out of the dormant state. Therefore, the implications of this hypothesis need to be thoroughly investigated, as they can have pivotal implications for determining the aggressiveness of a growing tumor.
Several models have been introduced to look at the effects of phosphorus as a dynamic resource on tumor dynamics. Kuang and colleagues [51] introduced a model where they study the dynamic interactions of a population of healthy cells x(t) within the organ, tumor cells y i (t), where i represents the parenchyma cell type, tumor microvessels z(t), particularly mature vascular endothelial cells, extracellular phosphorus \(P_{e}(t) = g(P(t),x(t),y_{i}(t),z(t))\) and total phosphorus P(t). The details of the derivation and full explanation and analysis of this particular complex and thorough model can be found in [51], however, the functional forms can be summarized as follows:
Similar to the previously discussed models, the model proposed by Kuang et al. considers a situation where the support of the tumor is dynamic. Unlike other models, however, the dynamic carrying capacity depends not only on the dynamics of the tumor cells, the stroma, and the angiogenic support but also on fluctuating nutrient availability, specifically phosphorus.
Through a number of simulations, the authors observed that, interestingly, the ultimate size of the tumor is insensitive to its predetermined carrying capacity but instead depends primarily on phosphorus supply. The authors emphasize the importance of stoichiometric constrains imposed by limiting nutrients by demonstrating a common feature of a variety of model variations that they explored: phosphorus supply plays a key role in affecting tumor growth dynamics and final size, even compared to cell birth or death rates, which, incidentally, are the primary focus of most therapeutic intervention strategies. Moreover, the authors demonstrate that over time, it is the slower-growing tumors with lower phosphorus demands that come to dominate rather than faster-growing tumors, lending further support to the idea of focusing less on cytotoxic treatments and more on targeting the microenvironment in which the tumor cells exist. The authors also raised the possibility that excessive phosphorous that may accumulate in the organ after any cytotoxic treatment could explain a phenomenon known as “tumor lysis syndrome,” where the concentration of plasma phosphorus concentration can become toxic to the patient, in addition to providing remaining tumor cells with ribosome-building materials.
6 Nutrient Availability and Tumor Heterogeneity
The effects of phosphorus availability on the growth dynamics of heterogeneous tumors were further explored by Kareva [52]. More specifically, the analysis focused on the effects of changes in nutrient availability in the microenvironment and initial composition of the tumor on overall tumor dynamics. In this particular model, the author looked at the dynamics of tumor cells x α (t), where each cell is characterized by a value of α from some initial distribution and where α represents a choice of strategy in terms of resource allocation. Other variables included were intracellular phosphorus P in (t) and extracellular phosphorus P ex (t), both of which directly influence cell growth and death rates. The functional form of the full system is given by
This modeling framework focused on distinguishing the microenvironmental conditions that can lead to selection of corresponding alternative resource allocation strategies, expanding on the hypothesis proposed by Elser et al. [50]. Specifically, the analysis focuses on whether significant changes in phosphorus availability can influence selection towards or away from a more proliferative cell phenotype. Numerical simulations predict that tumor composition can evolve to be different depending on the initial state of the microenvironment and which might not be reflected in final tumor size. This suggests that tumor size is not necessarily a good predictor of final tumor composition and hence potential aggressiveness. It was also shown that modification of parameters pertaining to nutrient uptake rates did not affect tumor composition as much as expected, suggesting that blocking nutrient transporters might not be an effective therapeutic intervention strategy. Finally, sensitivity analysis revealed two important conclusions: phosphorus availability is a major factor in determining tumor growth dynamics, and different parameters gain relative importance at different stages of tumor growth. Specifically, while growth and nutrient consumption rates were of highest importance in the initial stages of tumor growth, cell death rates gain the highest importance at later stages of growth, potentially due to increased cell turnover and increased phosphorous availability through nutrient recycling.
While approaching the question of phosphorus availability in the tumor microenvironment from different viewpoints, together, these two models highlight the importance of nutrient availability as a major factor influencing tumor growth. It is particularly important that this type of dynamic resource is contained within the cells themselves and gets replenished through cell death, making tumors a self-sustainable ecosystem. To address this issue, the administration of dialysis after cytotoxic therapy was proposed [11] as a means to reduce an otherwise self-renewing carrying capacity.
All of these models, regardless of the manner in which they describe the dynamic tumor microenvironment (whether it be a space-, nutrient-, and/or angiogenesis-dependent viewpoint), suggest that extensive cell death may not be the optimal way to achieve long-term remission. Rather, they suggest that such strategies may select for more dangerous or aggressive cancers. In light of this collection of work, a rational conclusion is that the best chance of cancer elimination is through treatments simultaneously targeting both the cancer cells and the surrounding microenvironment.
7 Therapeutic Implications
Despite the large amounts of financial and intellectual resources that have been employed in the area of cancer research, and despite the large number of therapeutic agents that are currently available on the market as a result of these efforts, with a few notable exceptions, cancer mortality rates have not significantly decreased over the past 40 years [53]. One possible explanation for these results is a lack of recognition of the fact that cancer is a systemic disease, which cannot be successfully managed without considering its nature not solely as a disease of the genes but also of the microenvironment and host. Such an approach has been confirmed theoretically with several mathematical models, some of which were discussed above. Interestingly, these conclusions are supported by a variety of mathematical models, both descriptive and conceptual, regardless of the level of their complexity. These models suggest that improved clinical outcome requires particular attention be paid to not only the cancer cells but to the different components of the tumor microenvironment.
One approach, initially proposed by Folkman [4], is to target the tumor microenvironment via antiangiogenic therapy, which attempts to block the formation of new blood vessels in the hopes of starving the tumor. Unfortunately, patient response to such treatments has been more modest than anticipated [54]. Importantly, while antiangiogenic therapy has proven to be a relatively safe treatment, it has not demonstrated an increase in patient survival [54] despite promising results in preclinical testing. One possible explanation for these unexpected results is that deactivating the angiogenic factors by blocking the molecular receptors does not remove the ultimate source of the factors, namely, the tumor cells and associated stroma. Indeed, a combination of antiangiogenic therapy with chemotherapy has been shown to improve treatment response [55], possibly due to the simultaneous targeting of both the angiogenic signals and the source of the signals (the cancer cells themselves).
The most promising therapeutic approach proposed to date, however, is to alter the dosage and timing of standard chemotherapeutics to target the tumor-associated endothelial cells, which effectively determine the tumor carrying capacity. Such an approach has been termed “metronomic chemotherapy” and is characterized by more frequent administration of lower doses of cytotoxic agents. Hahnfeldt et al. [56] evaluated the predicted effectiveness of metronomic chemotherapy compared to the standard maximum tolerated dose protocol and demonstrated that, indeed, frequent administration of lower doses of cytotoxic drugs is the most efficient approach to achieve a smaller tumor burden. Specifically, metronomic chemotherapy reduces the emergence of therapeutic resistance since all the subpopulations within the tumor, whether initially sensitive to chemotherapy or not, are equally affected by the diminishing carrying capacity. Furthermore, they suggest that continuous administration of chemotherapy is superior to other forms of dose administration, an idea that has recently been challenged.
According to Doloff and Waxman [57], continuous administration of chemotherapy, while effective against the tumor, may damage the host’s natural cytotoxic immune response and thus potentially diminish overall treatment effectiveness. Their experiments demonstrate that a 6-day cycle may yield the highest overall therapeutic effectiveness, first by achieving a slower but longer-lasting reduction in tumor volume due to the frequent administration of cytotoxic agents and second by preserving antitumor immunity due to an appropriate period of recovery between doses.
Finally, the work presented by Elser and colleagues [11, 50, 51] focuses on nutrient availability in the tumor microenvironment, specifically the amount of phosphorus required by growing cells to construct molecular machinery such as ribosomes. They suggest that cytotoxic therapies should be accompanied by treatments such as dialysis. This approach may serve the dual purpose of avoiding tumor lysis syndrome, where concentrations of liberated intracellular phosphorus may reach toxic levels, and removing recycled nutrients that may otherwise become available to remaining cancer cells.
One commonality amongst the studies discussed here is that cancer should be thought of as a systemic disease that becomes increasingly difficult to manage due to its ability to engage both the tumor microenvironment, via endothelial and other stromal cells, and the host (via the immune response). The microenvironment, which ultimately transforms to support tumor growth, can be polarized into a pro-tumor niche in a variety of ways, including pro-tumor inflammation, nutrient recycling, and recruitment of angiogenic stimulators. Therefore, the successful management of cancer, a complex and systemic disease, requires a systemic multifaceted treatment approach. It may be that only by addressing the changes that occur in the microenvironment and host as a result of cancer presence can one hope for improved tumor suppression and positive clinical outcomes.
Grant Support
This work was financially supported by the National Cancer Institute under Award Number U54CA149233 (L. Hlatky) and by the Office of Science (BER), US Department of Energy, under Award Number DE-SC0001434 (P. Hahnfeldt).
References
V.T. DeVita Jr., T.S. Lawrence, S.A. Rosenberg (eds.), DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology, 9th edn. (Lippincott, Williams & Wilkins, Philadelphia, 2011)
I. Kareva, What can ecology teach us about cancer? Transl. Oncol. 4(5), 266–270 (2011)
K.E. deVisser, A. Eichten, L.M. Coussens, Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 6(1), 24–37 (2006)
J. Folkman, Tumor angiogenesis: therapeutic implications. New Engl. J. Med. 285(21), 1182–1186 (1971)
M.S. O’Reilly, T. Boehm, Y. Shing, N. Fukai, G. Vasios, W.S. Lane et al., Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88, 277–285 (1997)
P. Hahnfeldt, D. Panigraphy, J. Folkman, L. Hlatky, Tumor development under angiogenic signaling: a dynamical theory of tumor growth, treatment response, and postvascular dormancy. Cancer Res. 59(19), 4770–4775 (1999)
S.I. Grivennikov, F.R. Greten, M. Karin, Immunity, inflammation, and cancer. Cell 140(6), 883–899 (2010)
M.J. Thun, S.J. Henley, C. Patrono, Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J. Natl. Cancer Inst. 94(4), 252–266 (2002)
M. Boersma, J.J. Elser, Too much of a good thing: on stoichiometrically balanced diets and maximal growth. Ecology 87(5), 1325–1330 (2006)
J. Elser, Biological stoichiometry: a chemical bridge between ecosystem ecology and evolutionary biology. Am. Nat. 168(Suppl 6), S25–S35 (2006)
J.J. Elser, J.D. Nagy, Y. Kuang, Biological stoichiometry: an ecological perspective on tumor dynamics. Bioscience 53(11), 1112–1120 (2003)
A.K. Laird, Dynamics of tumor growth. Br. J. Cancer 18(3), 490–502 (1964)
C. DeLisi, A. Rescigno, Immune surveillance and neoplasia–1 a minimal mathematical model. Bull. Math. Biol. 39(2), 201–221 (1977)
V.A. Kuznetsov, I.A. Makalkin, M.A. Taylor, A.S. Perelson, Nonlinear dynamics of immunogenic tumors: parameter estimation and global bifurcation analysis. Bull. Math. Biol. 56(2), 295–321 (1994)
D. Kirschner, J.C. Panetta, Modeling immunotherapy of the tumor-immune interaction. J. Math. Biol. 37(3), 235–252 (1998)
L.G. de Pillis, A.E. Radunskaya, C.L. Wiseman, A validated mathematical model of cell-mediated immune response to tumor growth. Cancer Res. 65(17), 7950–7958 (2005)
A. d’Onofrio, A general framework for modeling tumor-immune system competition and immunotherapy: mathematical analysis and biomedical inferences. Physica D 208(3–4), 220–235 (2005)
A. Cappuccio, M. Elishmereni, Z. Agur, Cancer immunotherapy by interleukin-21: potential treatment strategies evaluated in a mathematical model. Cancer Res. 66(14), 7293–7300 (2006)
R. Eftimie, J.L. Bramson, D.J. Earn, Interactions between the immune system and cancer: a brief review of non-spatial mathematical models. Bull. Math. Biol. 73(1), 2–32 (2011)
K.P. Wilkie, A review of mathematical models of cancer-immune interactions in the context of tumor dormancy. Adv. Exp. Med. Biol. 734, 201–234 (2013). doi:10.1007/978-1-4614-1445-2_10
R. Lefever, W. Horsthemke, Bistability in fluctuating environments. implications in tumor immunology. Bull. Math. Biol. 41, 469–490 (1979)
A. d’Onofrio, Bounded-noise-induced transitions in a tumor-immune system interplay. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 81(2), 021923, 1–7 (2010)
A. d’Onofrio, A. Ciancio, Simple biophysical model of tumor evasion from immune system control. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 84(3), 031910 (2011). doi:10.1103/PhysRevE.84.031910
K.P. Wilkie, P. Hahnfeldt, Mathematical models of immune-induced cancer dormancy and the emergence of immune evasion. Interface Focus 3, 20130010 (2013)
A. Matzavinos, M.A.J. Chaplain, V.A. Kuznetsov, Mathematical modelling of the spatio-temporal response of cytotoxic T-lymphocytes to a solid tumour. Math. Med. Biol. 21(1), 1–34 (2004)
T. Roose, S.J. Chapman, R.K. Maini Mathematical models of avascular tumor growth. SIAM Rev. 49(2), 179–208 (2007)
H. Enderling, L. Hlatky, P. Hahnfeldt, Immunoediting: evidence of the multifaceted role of the immune system in self-metastatic tumor growth. Theor. Biol. Med. Model. 9, 31 (2012). doi:10.1186/1742-4682-9-31
T. Takayanagi, H. Kawamura, A. Ohuchi, Cellular automaton model of a tumor tissue consisting of tumor cells, cytotoxic T lymphocytes (CTLs), and cytokine produced by CTLs. IPSJ Trans. Math. Model. Appl. 47(1),61–67 (2006). doi:10.2197/ipsjdc.2.138
A. d’Onofrio, A. Gandolfi, Tumor eradication by antiangiogenic therapy: analysis and extensions of the model by Hahnfeldt et al. (1999). Bath Biosci. 191(2), 159–184 (2004)
A. d’Onofrio, A. Gandolfi, A family of models of angiogenesis and antiangiogenesis anti-cancer therapy. Math. Med. Biol. 26(1), 63–95 (2009)
U. Ledzewicz, H. Schättler, Antiangiogenic therapy in cancer treatment as an optimal control problem. SIAM J. Control Optim. 46(3), 1052–1079 (2007)
U. Ledzewicz, H. Schättler, Optimal and suboptimal protocols for a class of mathematical models of tumor anti-angiogenesis. J. Theor. Biol. 252(2), 295–312 (2008)
U. Ledzewicz, A. d’Onofrio, H. Schättler, Tumor development under combination treatments with anti-angiogenic therapies, in Mathematical Methods and Models in Biomedicine, ed. by U. Ledzewicz, H. Schättler, A. Friedman, E. Kashdan. Lecture Notes on Mathematical Modeling in the Life Sciences (Springer, Heidelberg, 2012), pp. 301–327
S. Benzekry, G. Chapuisat, J. Ciccolini, A. Erlinger, F. Hubert, A new mathematical model for optimizing the combination between antiangiogenic and cytotoxic drugs in oncology. C. R. Math. Acad. Sci. Paris 350(1–2), 23–28 (2012)
T.L. Jackson, Vascular tumor growth and treatment: consequences of polyclonality, competition and dynamic vascular support. J. Math. Biol. 44(3), 201–226 (2002)
S. Benzekr, Mathematical analysis of a two-dimensional population model of metastatic growth including angiogenesis. J. Evol. Equat. 11(1), 187–213 (2010)
K.P. Wilkie, P. Hahfeldt, Tumor-immune dynamics regulated in the microenvironment inform the transient nature of immune-induced tumor dormancy. Cancer Res. 73(12), 3534–3544 (2013)
G.N. Naumov, E. Bender, D. Zurakowski, S.Y. Kang, D. Sampson, E. Flynn et al., A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype. J. Natl. Cancer Inst. 98(5), 316–325 (2006)
M. Hu, J. Yao, D.K. Carroll, S. Weremowicz, H. Chen, D. Carrasco et al., Regulation of in situ to invasive breast carcinoma transition. Cancer Cell 13(5), 394–406 (2008)
H. Withers, Treatment-induced accelerated human tumor growth. Semin. Radiat. Oncol. 3(2), 135–143 (1993)
S.Y. El Sharouni, H.B. Kal, J.J. Battermann, Accelerated regrowth of non-small-cell lung tumours after induction chemotherapy. Br. J. Cancer 89(12), 2184–2189 (2003)
S. Kraus, N. Arber, Inflammation and colorectal cancer. Curr. Opin. Pharmacol. 9(4), 405–410 (2009)
A. Mantovani, P. Romero, A.K. Palucka, F.M. Marincola, Tumour immunity: effector response to tumour and role of the microenvironment. Lancet 371(9614), 771–783 (2008)
F.R. Balkwill, L.M. Coussens, Cancer: an inflammatory link. Nature 431(7007), 405–406 (2004)
J. Condeelis, J.W. Pollard, Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124(2), 263–266 (2006)
D. Nelson, R. Ganss, Tumor growth or regression: powered by inflammation. J. Leukocyte Biol. 80(4), 685–690 (2006)
K.P. Wilkie, P. Hahnfeldt, Modeling the dichotomy of the immune response to cancer: cytotoxic effects and tumor-promoting inflammation (2013). ArXiv:1305.3634
J.D. Wolchok, A. Hoos, S. O’Day, J.S. Weber, O. Hamid, C. Lebbé et al., Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 15(23), 7412–7420 (2009)
H. Jin, C. Xu, H. Lim, S. Park, J. Shin, Y. Chung et al., High dietary inorganic phosphate increases lung tumorigenesis and alters AKT signaling. Am. J. Respir. Crit. Care Med. 179(1), 59–68 (2009)
J.J. Elser, M.M. Kyle, M.S. Smith, J.D. Nagy, Biological stoichiometry in human cancer. PLoS ONE 2(10), e1028 (2007). doi:10.1371/journal.pone.0001028
Y. Kuang, J.D. Nagy, J.J. Elser, Biological stoichiometry of tumor dynamics: mathematical models and analysis. Discrete Contin. Dyn. B 4(1), 221–240 (2004)
I. Kareva, Biological stoichiometry in tumor micro-environments. PLoS ONE 8(1), e51844 (2013)
S.M. Gapstur, M.J. Thun, Progress in the war on cancer. J. Am. Med. Assoc. 303(11), 1084–1085 (2010)
J.M.L. Ebos, R.S. Kerbel, Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat. Rev. Clin. Oncol. 8(4), 210–221 (2011)
L. Bello, G. Carrabba, C. Giussani, V. Lucini, F. Ceruutti, F. Scaglione et al., Low-dose chemotherapy combined with an antiangiogenic drug reduces human glioma growth in vivo. Cancer Res. 61(20), 7501–7506 (2001)
P. Hahnfeldt, J. Folkman, L.R. Hlatky, Minimizing long-term tumor burden: the logic for metronomic chemotherapeutic dosing and its antiangiogenic basis. J. Theor. Biol. 220, 545–554 (2003)
J.C. Doloff, D.J. Waxman, VEGF receptor inhibitors block the ability of metronomically dosed cyclophosphamide to activate innate immunity-induced tumor regression. Cancer Res. 72(5), 1103–1115 (2012)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Kareva, I., Wilkie, K.P., Hahnfeldt, P. (2014). The Power of the Tumor Microenvironment: A Systemic Approach for a Systemic Disease. In: d'Onofrio, A., Gandolfi, A. (eds) Mathematical Oncology 2013. Modeling and Simulation in Science, Engineering and Technology. Birkhäuser, New York, NY. https://doi.org/10.1007/978-1-4939-0458-7_6
Download citation
DOI: https://doi.org/10.1007/978-1-4939-0458-7_6
Published:
Publisher Name: Birkhäuser, New York, NY
Print ISBN: 978-1-4939-0457-0
Online ISBN: 978-1-4939-0458-7
eBook Packages: Mathematics and StatisticsMathematics and Statistics (R0)