Abstract
Macrophages are malleable cells that can adapt varying phenotypes and functions. They are critical components of the innate immune system that have the potential to eliminate intracellular pathogens, to facilitate wound healing, and to activate T cells and natural killer (NK) cells. Their specific phenotype and functions are regulated by the cytokines and chemokines in their microenvironment. Although macrophages have the potential to be tumoricidal, the inflammatory milieu of the tumor microenvironment co-opts macrophages and renders them tumor promoting. Tumor-associated macrophages (TAMs) are present in most solid tumors where they facilitate tumor progression by enhancing angiogenesis, promoting tumor cell invasion and metastasis, protecting tumor cells from chemotherapy, and inhibiting antitumor immunity. Macrophage infiltrates in solid tumors have long been recognized as indicators of poor prognosis. However, current studies are exploring novel strategies for reprogramming TAMs and converting them into cells that facilitate tumor rejection.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- M1
- M2
- Tumor-associated macrophages
- Immune suppression
- Angiogenesis
- Tumor invasion and metastasis
- Polarization
- Inflammation
1 Introduction
Macrophages are an exceptionally diverse population of cells. They are critical players in both innate and adaptive immunity and their functions impact responses to pathogens, to self-antigens (autoimmunity), and to cancer cells. Macrophages were first identified in the late 1800s by Elie Metchnikoff, a Ukrainian pathologist. He observed that if starfish were injected with dye particles, cells within the starfish would engulf the particles. He called these cells “phagocytes” based on the Greek words macros (large) and phagein (to eat). He subsequently observed similar cells in human blood, and in 1892 he proposed his “cellular (phagocytic) theory of immunity,” stating that white blood cells are the key elements of the immune system that protect against pathogens [1], [2]. At that time, this theory was both groundbreaking and controversial because it contradicted the prevailing concept that humoral immunity, mediated by soluble proteins (antitoxins) and serum, was responsible for immune protection. It was not until the 1940s that immunologists appreciated the dual role of humoral and cellular immunity, despite the recognition of both cellular and humoral immunity by the awarding of a shared Nobel Prize to Metchnikoff and Paul Ehrlich, the discoverer of antitoxins (antibodies), in 1908.
The phagocytic cells seen by Metchnikoff were actually a mixture of multiple cell types, including what we now know as dendritic cells (DC), neutrophils, and macrophages. In addition to phagocytosis, macrophages also mediate their effects through their production of soluble factors including cytokines and chemokines, and by direct cell-to-cell contact with their cellular targets. Macrophages are now recognized as central players in the immune system and as having extensive plasticity. Their role and function depend largely on their anatomical location and microenvironment since their plasticity is largely driven by factors produced by surrounding cells. In healthy individuals, macrophages play an important role in facilitating wound healing, in regulating adaptive immunity, in eliminating infectious agents, and in regulating metabolism (reviewed in [3]–[6]). Macrophages have been categorized as “M1-like” and “M2-like” to reflect this apparent dichotomy of function (Fig. 6.1). M1-like macrophages typically reside in healthy tissue or at sites of acute inflammation . They eliminate intracellular pathogens, activate type 1 cluster of differentiation 4 (CD4+) and CD8+ T cells by functioning as antigen-presenting cells (APC), and may be cytotoxic for tumor cells. In contrast, M2-like macrophages (also referred to as “alternatively activated macrophages”) mediate wound healing, promote angiogenesis , and drive type 2 immunity. The conditions and factors that drive the development of M1 and M2 macrophages are described in Sect. 5. In individuals with cancer, macrophages are usually co-opted by tumor-secreted factors and facilitate the development and progression of tumors. These tumor-associated macrophages (TAMs) are immune suppressive and share many characteristics with M2-like macrophages .
2 Macrophages Promote Tumor Progression Through Multiple Mechanisms
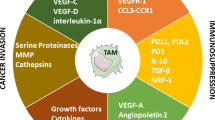
It is well established that macrophages localize to tumor sites and many studies demonstrate a strong correlation between quantity of macrophages and poor prognosis [7], [8]. TAMs are recruited to solid tumors because of the hypoxic and inflammatory tumor microenvironment. These conditions retain macrophages within tumors while additional factors modulate macrophage phenotype and enable them to promote tumor progression [9], [10]. TAMs facilitate tumor progression through diverse mechanisms. Figure 6.2 summarizes these mechanisms which are described in the following sections.
2.1 TAMs Drive Angiogenesis and Neovascularization
As solid tumors enlarge, they become too large to obtain their nutrients and oxygen through simple diffusion and must induce neovascularization and establish a vascular network. This process, known as the “angiogenic switch,” as well as continued neovascularization of growing tumors, is mediated by macrophages through their production of vascular endothelial growth factor A (VEGF-A) and other pro-angiogenesis factors [11]–[14]. Macrophages expressing Tie2, the receptor for angiopoietin-2 (ANG2), are particularly associated with neovascularization [12]. Induction of VEGF in macrphages is partially regulated by hypoxia [15], [16]. Poorly vascularized regions of solid tumors become hypoxic and chemoattract macrophages [17]. The hypoxic environment turns on the transcription factors, hypoxia-inducible factors (HIF) 1α and 2α, which in turn upregulate VEGF synthesis. VEGF then chemoattracts more macrophages [18], [19]. Macrophages also produce matrix metalloproteinases (MMP) and other proteases that degrade the extracellular matrix (ECM) causing the release of additional pro-angiogenic factors [20].
Inflammation through the action of the pro-inflammatory cytokine interleukin-1 beta (IL-1β) also induces VEGF synthesis by stabilizing HIF1α. This upregulation occurs in both normoxic [21] and hypoxic [22] conditions and involves nuclear factor kappa B (NF-κB) upregulation of cyclooxygenase-2 [21]. Other factors also contribute to macrophage-induced angiogenesis . Urokinase-type plasminogen activator and its receptor, molecules that increase vascularization, are upregulated on TAMs [23]. Macrophage-induced angiogenesis has also been attributed to fibroblast growth factor-2, angiopoietin, chemokine (C–X–C motif) ligand 1 (CXCL8 or IL-8), and leptin [24], as well as more than 30 other genes that are upregulated by hypoxia [25].
2.2 TAMs Promote Tumor Cell Invasion and Metastasis
Tumor cell invasion and metastasis are complex processes that require tumor cells to leave their primary site, invade their surrounding normal tissue and ECM, intravasate into and traffic via either the circulatory or lymphatic system, extravasate at a distant location, and acquire nutrients and oxygen at their final destination. Macrophages facilitate several of these steps. Early experiments using knockout mice deficient for colony-stimulating factor 1 (CSF-1), a factor required for macrophage differentiation, demonstrated that macrophages in the local microenvironment are essential for tumor cell invasion and metastasis [26]. Elegant multiphoton microscopy studies demonstrated that TAMs facilitate tumor cell intravasation into surrounding normal tissue and blood vessels [27]. In vivo studies demonstrated that resistance to established murine mammary carcinoma metastatic disease requires macrophage depletion or polarization of macrophages towards a tumor-rejecting M1-like phenotype [28], [29]. Several factors produced by macrophages contribute to these processes. In addition to CSF-1 serving as a differentiation factor, it also stimulates macrophage production of epidermal growth factor (EGF) which is a chemoattractant for tumor cells and promotes tumor cell invasiveness [30]. The release of macrophage inhibitory factor (MIF) also promotes tumor cell trafficking [31]. TAMs also synthesize and secrete multiple MMP (MMP-9, -2, and -7) [32], [33], cathepsins [34], [35], urokinase [36], and other proteases [37] which degrade the ECM and basement membrane, thereby allowing tumor cells to migrate to distant sites . Macrophages also produce platelet-derived growth factor, placental growth factor (PIGF), and hepatocyte growth factor which directly support tumor cell proliferation [25]. Recent studies in multiple mouse tumor systems and in immune-deficient mice with human tumors definitively identified CD11b+ F4/80+ macrophages as essential for promoting tumor cell extravasation and for “conditioning” a metastatic niche prior to arrival of migrating tumor cells [38]. Subsequent studies revealed that the progenitors of these macrophages are Gr1+ inflammatory monocytes that express C–C chemokine receptor type 2 (CCR2) and are chemoattracted by tumor and stromal cells producing chemokine (C–C motif) ligand 2 (CCL2), and that extravasation involves monocyte and macrophage production of VEGF. These studies demonstrate the link between inflammation and cancer in the metastatic process and provide a mechanistic explanation for the poor prognosis of patients with high levels of CCL2 and tumor-infiltrating macrophages [39].
2.3 TAMs Inhibit Antitumor Immunity
TAMs inhibit both innate and adaptive antitumor immunity and are themselves impacted by the immune system of tumor-bearing individuals. Unlike M1-like macrophages which have high levels of major histocompatibility complex (MHC) class II molecules and are effective APC, TAMs are poor APC because they have reduced levels of MHC II molecules as well as low levels of the costimulatory molecules CD80 and CD86. Hypoxia contributes to these decreased levels [40], [41], as does interaction with myeloid-derived suppressor cells (MDSC) [42].
TAMs are characterized by their high production of IL-10 and their minimal production of IL-12. IL-10 polarizes immunity towards a type 2 response whereas IL-12 polarizes towards a type 1 response. Type 1 responses favor tumor rejection while type 2 responses promote tumor progression. IL-10 production drives CD4+ Th2 cells at the expense of CD4+ Th1. Optimal activation of potentially tumoricidal CD8+ T cells requires help from CD4+ Th1 cells, so the absence of Th1 cells usually results in sub-optimally activated cytotoxic CD8+ T cells. IL-10 also inhibits CD8+ T-cell function by inducing the expansion of natural T regulatory cells (Tregs) and the development of induced Tregs. The Tregs, in turn, directly inhibit CD8+ cytotoxic activity [43]. Since IL-12 contributes to the activation and efficacy of both natural killer (NK) cells [44] and DC [45], TAMs also impact antitumor immunity by diminishing NK-mediated cytotoxicity and antigen presentation by DC.
TAMs directly impact T-cell activation and proliferation through several mechanisms. They produce high levels of arginase 1, an enzyme that degrades arginine. Arginine degradation not only deprives T cells of L-arginine needed for protein synthesis but also results in the generation of toxic catabolic products including oxygen and nitrogen radicals [46], [47].
TAMs also suppress T-cell activation through their expression of immune suppressive co-inhibitory molecules . B7-H4 is a member of the B7 family of genes and is induced in macrophages by IL-6 and IL-10. A subset of TAMs from ovarian cancer patients express B7-H4. When B7-H4 interacts with its receptor on T cells, T cells fail to progress through the cell cycle and therefore neither proliferate nor produce the cytokines essential for tumor cell killing [48]. Some TAMs also express the co-inhibitory molecule programmed cell death ligand-1 (PD-L1, also called B7-H1). PD-L1 causes T-cell apoptosis when it binds to its receptor, PD-1, which is expressed on activated T cells. IL-10 and tumor necrosis factor alpha (TNFα) in the tumor microenvironment of hepatocellular carcinoma patients induce expression of cell surface PD-L1 in TAMs , and these macrophages suppress antitumor immunity [49].
The enzyme indoleamine 2,3-dioxygenase (IDO) is an immune suppressive molecule produced by macrophages and other cells of myeloid origin . IDO degrades tryptophan and therefore depletes the microenvironment of tryptophan making surrounding cells unable to progress through the cell cycle [137]. Tumor cells themselves secrete IDO which to some extent reduces tumor growth. However, DC and macrophages also produce IDO, and this IDO suppresses T-cell activation and outweighs the direct effects of IDO on tumor cells. As a result, lymphocytes in the lymph nodes draining solid tumors are tolerized or nonresponsive to tumor antigens, thereby eliminating adaptive antitumor immunity [138], [139]. Recent studies in mouse models have established that the immune suppressive effects of IDO significantly contribute to the progression of both primary and metastatic lung and mammary tumors [50].
2.4 TAMs Protect Tumor Cells from Chemotherapy
Macrophages also promote tumor growth by interfering with chemotherapy [51]. In patients with multiple myeloma, large quantities of macrophages are present in the bone marrow. Chemotherapy of multiple myeloma with dexamethasone and melphalan induces apoptosis of tumor cells by cleaving and activating caspase 3. Cell-to-cell contact between macrophages and myeloma cells prevents caspase 3 activation and therefore protects tumor cells from apoptosis [52]. Interactions between myeloma cells and macrophages depend on binding of myeloma cell-expressed P-selectin glycoprotein-1 and ICAM-1/CD18 to their respective partners on macrophages (selectins and CD11b, respectively). Binding of these ligand–receptor pairs activates the c-myc pathway and kinases Src and Erk1/2 in myeloma cells, rendering them resistant to caspase activation [53].
3 Origin and Identification of Macrophages
Macrophages are a type of white blood cell (leukocyte) that reside in both lymphoid and non-lymphoid tissues. In the mouse, macrophages are identified by their expression of the cell surface markers CD11b (a subunit of Mac-1) and F4/80, a G protein-coupled receptor. CD11b is also expressed by macrophages in humans, along with the cell surface protein CD68. Mouse and human macrophages are also characterized by their expression of other markers which are acquired in response to their microenvironment (see the following sections on macrophage polarization and inflammation) .
Macrophages may have specialized names depending on their location. For example, macrophages within the central nervous system are called microglia while those in the lung are termed alveolar macrophages. Most macrophages are derived from pluripotent hematopoietic stem cells (HSC) that originate in the bone marrow (Fig. 6.3). HSC give rise to a common myeloid progenitor (CMP) that in turn gives rise to a granulocyte/macrophage progenitor (GMP) which serves as the progenitor for multiple myeloid cells including monocytes that circulate in the blood, bone marrow, and spleen [54]. Circulating monocytes become macrophages when they migrate into and become resident in tissues. Recent studies have demonstrated that some tissue-resident macrophages are present and proliferate in the absence of HSC and have identified a second lineage of macrophages that arises from the yolk sac in 9.5–10.5-day-old mouse embryos. The transcription factor Myb was found to be essential for the development of classical hematopoietic-derived macrophages, but was not necessary for the development of microglia or macrophages in the liver [55]. Although studies have not specifically been conducted on the origin of tumor-infiltrating macrophages, it is likely that these cells are of hematopoietic origin since they are derived from circulating monocytes.
Macrophages differentiate from hematopoietic stem cells in bone marrow or from yolk sac. Hematopoietic stem cells (HSC) in the bone marrow give rise to a common myeloid progenitor (CMP) which gives rise to a granulocyte/macrophage progenitor (GMP). The GMP differentiates into monocytes, neutrophils, and monocytic and granulocytic myeloid-derived suppressor cells (MDSC) which circulate in the blood. When monocytes move into tissue or tumor, they become macrophages. Tissue macrophages also differentiate from a progenitor cell that arises in the yolk sac during embryogenesis. Cell surface markers for neutrophils, granulocytic MDSC (Gr-MDSC), and monocytic MDSC (MO-MDSC) are for murine cells
4 Inflammation Drives Malignant Transformation and Tumor Progression and Recruits TAMs
Inflammation is now appreciated as one of the hallmarks of cancer [56]–[58]. Inflammation in the tumor microenvironment facilitates the growth of established tumors, and approximately one quarter of cancers are thought to be the result of chronic inflammatory conditions [59]–[64]. The tumor microenvironment is a highly complex milieu of pro-inflammatory cytokines, chemokines, and bioactive lipids that are produced by tumor cells themselves, as well as by infiltrating host cells, including macrophages [65]. Pro-inflammatory cytokines such as IL-6 and TNFα produced by many tumor cells are key drivers of inflammation in the tumor microenvironment [9], [63], [66]–[68]. In addition to their inherent inflammatory properties, TNFα and IL-6 stimulate production of pro-inflammatory chemokines such as CCL2, CXCL1, CXCL8, and CXCL12, as well as MMP [65]. These cytokines and chemokines establish a setting that not only maintains inflammation but also recruits macrophages, other immune cells, and stromal cells that produce inflammatory mediators. The result is a network of autocrine factors that sustain an inflammatory tumor microenvironment.
The transcription factor NF-κB is the major intracellular regulator of the inflammatory program and is activated in macrophages and in tumor cells through a Toll-like receptor-myeloid differentiation primary response gene (88) (TLR-MyD88) pathway. Ligands include bacterial products, cellular debris, IL-1β, and TNFα [56]. In tumor cells, it induces a more aggressive phenotype and regulates the production of chemokines and cytokines that chemoattract macrophages that in turn promote tumor growth [69]. The role of NF-κB in macrophages is less well defined. In agreement with its pro-inflammatory role in malignant cells, inhibition of NF-κB in macrophages of mice that spontaneously develop tumors results in delayed tumor onset [70], as well as in mice with chronic inflammation that progresses to malignancy [62], [64], [71]. However, in late stage tumors, TAMs have defective NF-κB and restoring NF-κB activity in these TAMs delays tumor growth [72]. Therefore, whether activated NF-κB is pro-tumor or antitumor may depend on the stage of disease and the type of cancer.
5 Macrophage Polarization and Phenotype: M1-like Macrophages have Antitumor Activity While M2-like Macrophages Facilitate Tumor Progression
Depending on environmental cues, macrophages can either facilitate the destruction of tumor cells or promote the progression of tumor growth. This dichotomy of function is the result of the extreme plasticity of macrophages and their phenotypic adaptation to their environment. Similar to the Th1 vs. Th2 paradigm for CD4+ T lymphocytes, Mills proposed that macrophages also polarize into two categories based on their phenotype and function [73]. This nomenclature was further developed by Mantovani and colleagues and has now become the accepted jargon to describe macrophages [3], [74]–[76]. M1-like macrophages (also known as “classically activated” macrophages) are activated by bacterial products such as lipopolysaccharides (LPS) in combination with interferon gamma (IFNγ). M1 polarization has also been attributed to TNFα. These cells produce high levels of IL-12 and low levels of IL-10 and are able to lyse tumor cells. They also synthesize and contain inducible nitric oxide synthase (iNOS or NOS2) and typically express high levels of cell surface CD86 and MHC class II molecules. In contrast, M2-like macrophages are activated by IL-4, IL-13, IL-10, and glucocorticoid hormones. They produce low levels of IL-12, high levels of IL-10, and support tumor growth. They also synthesize and contain high levels of arginase 1 and express low levels of MHC class II and high levels of the cell surface molecules FIZZ1 and Ym1. Their high level of expression of IL-4Rα facilitates their polarization towards an M2 phenotype by the binding of IL-4 and IL-13 [6].
M1 macrophages eliminate intracellular pathogens and mediate tumor cell destruction by their production of a variety of reactive intermediate species that result from the production of nitric oxide (NO) and its reaction with molecular oxygen [77]. M1 macrophages also contribute to tumor destruction through their skewing of immunity towards a type 1 response that activates tumor-reactive T cells. Their production of the chemokines Mig (CXCL9) and IP-10 (CXCL10) chemoattract Th1 cells [65], [78]. Type 1 immunity is further facilitated by their production of pro-inflammatory cytokines including IL-1β, IL-6, IL-23, and TNFα combined with their ability to function as APC to activate Th1 and Tc1 CD4+ and CD8+ T cells, respectively [79].
In contrast, M2 macrophages are predominately anti-inflammatory, although they secrete some pro-inflammatory molecules. They promote type 2 immunity that facilitates the elimination of parasites, promotes wound healing, and drives tumor progression. TAMs are polarized towards an M2 phenotype. The production of CCL22 by M2 macrophages chemoattracts Tregs which inhibit T-cell activation [80]. M2 macrophages also secrete prostaglandin E2 and transforming growth factor beta (TGFβ), both of which are immunosuppressive [81], and they express PD-L1 which binds to its receptor PD-1 on activated T cells and causes T-cell apoptosis [49]. Their production of IL-10 and their lack of IL-1β production due to their high expression of IL-4 receptor antagonist and decoy type II receptor promote their anti-inflammatory effects [82]. M2 macrophages also stimulate angiogenesis which facilitates tumor growth [74] and they produce high levels of arginase 1 (Arg1) which prevents T-cell proliferation by depriving T cells of arginine [83], [84].
The metabolism of the amino acid arginine by M1 vs. M2 macrophages is a major distinguishing characteristic of the two macrophage phenotypes. M1 macrophages predominantly metabolize arginine via the NO pathway, while M2 macrophages use the arginase pathway [85]. iNOS or NOS2 is upregulated in M1 macrophages by IFNγ and TNFα. The NO that is produced is cytotoxic and destroys target cells by inhibiting cell proliferation [86], blocking mitochondrial respiration [87], and inducing apoptosis [88], [89]. NO also reacts with superoxide (O2 −) to produce peroxynitrite (ONOO−) [88], [90]. In contrast, Arg1 is upregulated in M2 macrophages by IL-4, IL-13, TGFβ, and IL-10 [91], [92]. Arg1 converts arginine to ornithine which is subsequently converted to polyamines. Ornithine is a precursor for proline, a major constituent of collagen which is necessary for tumor infrastructure. Polyamines promote tumor cell proliferation because they are required for deoxyribonucleic acid (DNA) replication [93]. Figure 6.4 schematically illustrates the two pathways. MDSC metabolize arginase through the same two pathways with monocytic MDSC (MO-MDSC) using the iNOS pathway while granulocytic MDSC use the Arg1 pathway [47]. Interestingly, MDSC production of iNOS does not result in tumoricidal activity as it does for M1 macrophages .
The preceding description of macrophage polarization applies to murine macrophages. Human macrophages are more enigmatic in terms of polarization and distinct classes of macrophages have not been conclusively identified. Whether this is due to a lack of adequate markers or a different program of differentiation is unclear at present.
Macrophages are characterized by their expression of the specific markers discussed above. However, some of these markers are also expressed by other cell types and not all macrophages express all of these markers. For example, murine macrophages are often characterized by their expression of CD11b; however, CD11b is also expressed by other myeloid cells. Likewise, MHC II serves as a marker for macrophages; however, DC also express MHC II. Similarly, F4/80 is expressed by macrophages as well as by eosinophils and Langerhans cells. Expression of functional molecules such as iNOS and Arg1 is also not restricted to macrophages in that subpopulations of MDSC also express these molecules. There are many fewer cell surface markers for human macrophages. CD68 expression is typically used, although it is also expressed by some fibroblasts. Therefore, macrophage identification by marker expression is not always unambiguous and straightforward, although a combination of cell surface markers and functional markers can typically give a reliable identification.
Figure 6.5 summarizes the phenotypes and functions of M1- and M2-polarized macrophages.
Macrophages display a continuum of phenotypes and functions, with M1-like and M2-like cells representing the extremes. M2-like and M1-like macrophages have distinct phenotypes (shown in yellow boxes) and functions (shown in blue boxes). M2-like macrophages are anti-inflammatory and facilitate tumor progression, while M1-like macrophages are pro-inflammatory and facilitate tumor rejection
6 Macrophages are a Heterogeneous Mixture of Myeloid Cells
The M1–M2 paradigm is a convenient nomenclature for categorizing macrophages. However, most macrophages do not conveniently fit into one or the other of these categories, and the M1 and M2 states represent the extremes of a continuum of macrophage phenotypes that are governed by the local environment . Since tumors include a diversity of microenvironments, it is not unusual that different regions of solid tumors will have macrophages with differing and intermediate phenotypes. Since the microenvironments within solid tumors are generated by factors secreted by tumor cells, and different types of cancers produce different factors, macrophage populations between tumors can vary significantly. Likewise, as solid tumors evolve in individuals through the process of immunoediting and selection, tumor-secreted factors are likely to change such that macrophage populations will also evolve with tumor progression.
Macrophages also differ significantly within a single tumor. Movahedi and colleagues have identified seven distinct subsets of macrophages in mammary carcinoma and lung adenocarcinoma [94]. These subsets are distinguished by their level of expression of MHC II, the monocyte marker Ly6C, the homing receptor L-selectin (CD62L), and the chemokine receptors CX3CR1 and CCR2. The different subsets had varying half-lives as well as different differentiation kinetics. Monocytes with a Ly6Chi phenotype were observed to be precursors of all TAMs. Gene expression profiles have been performed on macrophages from multiple different types of tumors [95]–[97]. These studies revealed that overall TAMs are predominantly of an M2 phenotype. Additional profiling studies on macrophage subsets from three different mouse tumors revealed that macrophages with an MHC IIlow phenotype were the dominant TAM population and that these cells express high levels of M2 genes including Arg1, IL4Rα, and Il10. MHC IIlow TAMs were also present, but at a lower level and expressed high levels of M1 genes including Cox2 and IL-1β. RNA levels roughly correlated with protein levels for most genes; however, NOS2 mRNA was highest in MHC IIlow macrophages, while NOS2 protein was highest in MHC IIhigh macrophages [94]. These studies also noted that MHC IIlow macrophages were most frequently present in hypoxic regions of the tumor, while MHC IIhigh cells localized to normoxic areas. Hypoxia has also been shown to drive expression of the angiopoietin receptor Tie2 on a subset of macrophages (TEMs) that are pro-angiogenic [98]. Another subset of TAMs identified by their motility, invasiveness, and wound-healing properties was characterized by their high content of molecules associated with the Wnt signaling pathway [99].
7 The Tumor Microenvironment Regulates Macrophage Polarization
In addition to TAMs and tumor cells, the tumor microenvironment includes a complex milieu of host cells such as CD4+ and CD8+ T lymphocytes, Tregs, B lymphocytes, DC, mast cells, MDSC, NK cells, natural killer T (NKT) cells, neutrophils, and cancer-associated fibroblasts (CAFs). Many of these host cells are induced by tumor-secreted products to secrete factors that drive the polarization of macrophages towards an M2 phenotype. For example, both solid and ascites human ovarian cancer cells produce soluble mediators that upregulate macrophage production of IL-10 and CSF-1. These cells also produce many chemokines characteristic of the M2 phenotype. TNFα, a cytokine produced in abundance by ovarian cancer cells, upregulates hemoglobin scavenger receptor A (CD163), a marker characteristic of TAMs [100].
CD4+ T cells also impact macrophages polarization . In vitro coculture studies demonstrated that CD4+ CD25+ Foxp3+ Tregs divert macrophages towards an M2 phenotype by increasing macrophage expression of CD163 and CCL18, and by increasing macrophage phagocytic activity through their production of IL-10. IL-4, IL-13, and IL-10 produced by Tregs downregulate macrophage production of pro-inflammatory mediators produced in response to LPS. Tregs also reduced macrophage expression of MHC class II molecules [101]. Studies in transgenic mice that spontaneously develop mammary carcinoma (PyMT mice) confirmed an in vivo role for CD4+ T cells in polarizing macrophages and further demonstrated that the altered macrophages promote metastatic disease by activating EGF receptor signaling in the mammary epithelial cells. These latter effects were due to CD4+ T effector cells and not Tregs, indicating that CD4+ T cells alter macrophage phenotype through diverse mechanisms [102].
Macrophages are also impacted by B lymphocytes and by CAFs. Coculture and in vivo experiments using B1 lymphocytes (B220lowIgMhighCD11b+) increased macrophage production of IL-10 and decreased macrophage production of M1-type molecules including TNFα, IL-1β, and CCL3. M2 markers FIZZ1 and Ym1 were also increased [103]. In a chemically induced two-stage skin carcinogenesis system, large quantities of fibroblasts accumulate at the site of carcinogen application. The fibroblasts produce high levels of monocyte chemotactic protein 1 (MCP-1) which chemoattracts macrophages to the carcinogenic locale and promotes papilloma progression [104].
Crosstalk with MDSC also drives macrophage phenotype. In the presence of MDSC, macrophage production of IL-12 and IL-6 and expression of MHC II are reduced. Macrophages, in turn, increase MDSC production of IL-10 via an IL-6-dependent mechanism [42], [105]. This crosstalk is regulated by signaling through the TLR4 pathway in MDSC, involves upregulation of CD14 on MDSC, and is exacerbated by inflammation [106].
Figure 6.6 summarizes how tumor-secreted factors and host cells impacted by tumor-secreted factors drive macrophages towards a tumor-promoting phenotype.
Tumors produce multiple factors that condition their environment and surrounding cells and mold macrophage phenotype. Tumor-produced factors polarize immunity towards a type 2 response and induce B cells, CD4 + Th2 cells, T regulatory cells (Tregs), and myeloid-derived suppressor cells (MDSC) to produce cytokines and chemokines that polarize macrophages towards an M2 tumor-promoting phenotype. Tumor-secreted factors also induce an inflammatory and hypoxic environment that favors the development of TAMs. Cytokines and inflammatory mediators (in yellow boxes) are produced by tumor cells, the indicated B cells, CD4 + Th2 cells, Tregs, MDSC, inflammation, and/or hypoxia and induce specific markers and molecules in TAMs (in gray boxes). TAMs may express all or a subset of the markers/molecules shown in the green box
8 Multiple Regulatory Elements Control Macrophage Polarization
Macrophage polarization has been attributed to multiple genes and regulatory elements. Deletion of the suppressor of cytokine signaling 3 (SOCS3) gene in myeloid cells generated M2-like macrophages, while deletion of the SOCS2 gene yielded phenotypically M1 macrophages . Targeted deletion of SOCS in macrophages also altered macrophage function. SOCS3-deficient macrophages were more potent recruiters of Tregs, while SOCS2-deficient macrophages were not. Polarization towards an M2 phenotype yielded increased activation via signal transducer and activator of transcription 6 (STAT6), while polarization towards an M1 phenotype increased activation through STAT1 [107]. These changes in signal transduction preference correlate with the known role of STAT1 in transmitting signals from IFNγ, a known inducer of M1 phenotype cells, and STAT6 in transmitting signals from IL-4 and IL-13, known inducers of M2 macrophages .
MicroRNAs (miRNA) have also been shown to regulate macrophage polarization. The mannose receptor (CD206), encoded by the MRC1 gene, is a marker of TAMs and facilitates macrophage phagocytosis of microbial and host glycoproteins [108]. The MRC1 gene encodes miR-511-3p. This miRNA is constitutively expressed at high levels in TAMs; however, increasing expression inhibits tumor growth and reduces the pro-tumor phenotype of TAMs. Therefore, miR-511-3p levels appear to be important for maintaining TAM phenotype, but overexpression skews macrophages away from an M2 state [109].
Tumor antagonizing/malignancy suppressor genes (TAG/MSG) are a family of genes that suppress tumorigenicity in vivo but have no apparent in vitro effects. RNASET2, an extracellular RNAse, is a member of this family. Ovarian cancer cells expressing wild type or catalytically inactive RNASET2 grow much more slowly than cells containing mutated RNASET2. Reduced tumor progression is due to the infiltration of iNOS+ M1 macrophages since supernatants of cancer cells containing wild-type RNASET2 polarized macrophages towards an M1 phenotype [110]. Thus, RNASET2 is another gene that regulates macrophage polarization and its ability to polarize is independent of its catalytic RNAse activity.
Histone-rich glycoprotein (HRG) is an anti-angiogenic and immunomodulatory factor present in serum and produced by platelets that regulates blood vessel formation. The tumor microenvironment typically contains less HRG than tumor-free corresponding normal tissue. Overexpression of HRG in several mouse tumor cell lines prevented the development of disorganized blood vessel formation associated with wild-type tumor, and delayed tumor progression. HRG overexpression reduced the number of M2-like TAMs within tumors by half and increased the number of M1-like cells. These were direct effects of HRG on macrophages since similar skewing was observed when macrophages were cultured in the presence of HRG. HRG mediates these effects by downregulating PIGF, a known driver of angiogenesis and homolog of VEGF-A. Depletion of TAMs from HRG overexpressing tumors restored blood vessel abnormalities and increased tumor growth, indicating that HRG regulates tumor progression via macrophage polarization [111].
Macrophage polarization is also regulated epigenetically by chromosome remodeling. Proteins containing a Jumonji-C (JmjC) domain, including Jmjd3, are histone demethylases. Many genes that are activated by LPS are targets for Jmjd3. TLR stimulation activates Jmjd3 in macrophages via an NF-κB-dependent mechanism that controls the expression of the Bmp2 and Hox genes. In a parasite model, macrophages attain an M1 phenotype in the absence of Jmjd3 activation, whereas Jmjd3 activation is essential for the generation of M2 macrophages [112], [113]. Jmjd3 is also activated in macrophages by IL-4 through a STAT6-dependent mechanism. Activated STAT6 increases transcription of Jmjd3 which subsequently demethylates M2 marker genes, polarizing macrophages towards an M2 phenotype [114].
9 Macrophages as Prognostic Indicators of Tumor Progression
Macrophage infiltrates in solid tumors have long been recognized as indicators of poor prognosis. A recent clinical study in Hodgkin’s lymphoma patients cemented this correlation. Gene profiling and immunohistochemistry revealed that high levels of CD68+ macrophages predicted poor outcome after primary and secondary therapy. In contrast, low levels of tumor-associated CD68+ macrophages were associated with a subgroup of patients that had 100 % long-term disease-free survival [115]. In contrast to most cancers, levels of peritumoral CD68+ macrophages correlate with a good prognosis in colorectal cancer patients [116], [117]. This apparent inconsistency is because most macrophages in colorectal cancer are M1-like macrophages . As for other cancers, high levels of M2-like macrophages correlate with poor prognosis [118]. Expression of CD40 by HLA-DR+ CD80+ CD86+ M1-like macrophages has also been identified as an indicator of favorable prognosis in colorectal cancer patients [119]. Experimental studies in mice indicate that activation of macrophages by antibodies to CD40 in combination with IL-2 therapy induces macrophage production of NO and reduces metastatic disease [120]. High levels of peritumoral macrophages also correlate with good prognosis for patients with high-grade osteosarcoma. These tumors contain both M1-like and M2-like macrophages ; however, there was no correlation between macrophage phenotype and patient outcome [121].
These studies suggest that although most tumors induce tumor-promoting M2-like macrophages, some tumors induce M1-like macrophages which may contribute to tumor regression. A better understanding of the conditions that drive M1 vs. M2 polarization is essential to eliminate pro-tumor M2-like macrophages and induce antitumor M1-like macrophages .
10 TAMs Can be Reprogrammed Toward an M1-like Phenotype
Given the potent antitumor activity of M1 macrophages, considerable attention has been devoted to strategies to repolarize TAMs. Several studies have identified IL-12 as a key molecule for converting M2 macrophages to an M1 phenotype. Treatment with IL-12 decreases TAM expression of the tumor-promoting factors IL-10, MCP-1, and TGF-β and induces expression of TNFα, IL-15, and IL-18, factors that facilitate tumor rejection [122]. TAMs are also repolarized by IL-12 through treatment with a chimeric antigen receptor (CAR) that redirects cytotoxic T lymphocytes (CTL) to release IL-12 at the tumor site [123]. Delivery of IL-12 by engineered tumor-specific CD8+ T cells reprograms much of the myelomonocytic compartment of tumor stoma by converting immune suppressive macrophages , DC and MDSC into cells that sustain T-cell activation and promote debulking of large tumors [124]. This latter effect involves IFNγ and is consistent with previous findings that IFNγ repolarizes TAMs from an immune suppressive M2 phenotype to an antitumor M1 phenotype [125].
Other conditions also reeducate TAMs. Administration of IL-2 in combination with antibodies to CD40 converts TAMs to an M1 phenotype of high NO production [120]. In the inflammatory tumor microenvironment, macrophages acquire an M2 phenotype by signaling through the IL-1R and activating NF-κB. Inhibition of NF-κB signaling “reeducates” TAMs to an MHC IIhigh, IL-12high, ARGlow phenotype. Inhibition of NF-κB also renders them tumoricidal and generates antitumor activity through the recruitment of IL-12-dependent NK cells [126]. Whether inhibition of NF-κB will be generally applicable, however, is unclear because microarray studies demonstrated that TAMs induced by some tumors are constitutively defective in NF-κB signaling [95]. The Notch pathway has also been invoked in macrophage polarization . In mouse tumor models, TAMs have lower levels of Notch pathway activation compared to M1 macrophages . Macrophages with defective Notch signaling or TAMs treated with Notch signaling inhibitors acquire an M2 phenotype [127].
Additional cytokines and growth factors have also been identified as potential reagents for reprogramming TAMs. Restoration of host-produced histidine-rich protein (HRP) converts TAMs to M1 macrophages by downregulating PIGF [111]. TAMs, as well as DC, are repolarized by treatment with anti-IL-10R antibodies plus the TLR9 ligand, CpG [128]. In another study, IL-4 and IL-10 were identified as critical factors for driving macrophage polarization towards an M2 phentoype, and inhibition of the IL-4Rα in mice with VEGF-induced skin carcinogenesis prevented M2 polarization of macrophages [129], [130].
Macrophage polarization is also regulated by the transcription factor STAT6 since IL-4 and IL-13 drive M2 polarization by binding to the IL-4Rα and signaling through the JAK2/STAT6 pathway. As a result, macrophages in STAT6-deficient mice default develop into M1 cells and these cells contribute to the rejection of established metastatic mammary carcinoma [28]. The nonclassical MHC class I CD1d gene also regulates macrophage polarization. CD1−/− mice are IL-13-deficient because they lack NKT cells which produce IL-13. The absence of IL-13 causes macrophages to default to an M1 phenotype. These mice also reject established metastatic mammary carcinoma [29] and are resistant to recurring fibrosarcomas [131].
These reprogramming studies have been done in mouse tumor models and have resulted in innate antitumor immunity and delayed tumor progression and/or reduction of metastasis. Given the heterogeneity of TAMs , and the lack of well-defined markers for M1 and M2 phenotypes in human cancer, it remains to be demonstrated whether the findings in mice will be applicable to human systems. Table 6.1 summarizes the therapeutic approaches that have been developed to reprogram M2 to M1 macrophages . Several recent reviews include additional information [9], [79], [132], [133].
11 Conclusions
Macrophages can either enhance tumor progression or facilitate tumor rejection. Their ultimate phenotype and function are determined by their tissue microenvironment. Virtually all solid tumors chemoattract monocytes and polarize them to M2-like tumor-promoting macrophages. These TAMs are a major contributor to disease progression through their direct promotion of tumor cell growth, and their indirect effects of suppressing antitumor immunity, promoting angiogenesis, and facilitating tumor cell invasion and metastasis. Strategies for eliminating TAMs are being actively pursued in animal models. However, given the extreme plasticity of macrophages and the tumoricidal properties of M1-like macrophages, therapies that repolarize TAMs towards a tumor-rejecting M1 phenotype may be more beneficial.
References
Metchnikoff E (1905) Immunity in infective disease (trans: Binnie FG). Cambridge University Press, Cambridge
Tauber AI, Chernyak L (1991) Metchnikoff and the origins of immunology: from metaphor to theory. Oxford University Press, New York
Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M (2012) Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. doi:10.1002/path.4133
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8(12):958–969. doi:10.1038/nri2448
Murray PJ, Wynn TA (2011) Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11(11):723–737. doi:10.1038/nri3073
Gordon S, Martinez FO (2010) Alternative activation of macrophages: mechanism and functions. Immunity 32(5):593–604. doi:10.1016/j.immuni.2010.05.007
Mantovani A, Sica A (2010) Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol 22(2):231–237. doi:10.1016/j.coi.2010.01.009
Qian BZ, Pollard JW (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141(1):39–51. doi:10.1016/j.cell.2010.03.014
Allavena P, Mantovani A (2012) Immunology in the clinic review series; focus on cancer: tumour-associated macrophages: undisputed stars of the inflammatory tumour microenvironment. Clin Exp Immunol 167(2):195–205. doi:10.1111/j.1365–2249.2011.04515.x
Riboldi E, Porta C, Morlacchi S, Viola A, Mantovani A, Sica A (2012) Hypoxia-mediated regulation of macrophage functions in pathophysiology. Int Immunol. doi:dxs110 [pii]10.1093/intimm/dxs110
Bingle L, Lewis CE, Corke KP, Reed MW, Brown NJ (2006) Macrophages promote angiogenesis in human breast tumour spheroids in vivo. Br J Cancer 94(1):101–107
De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L (2005) Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8(3):211–226. doi:10.1016/j.ccr.2005.08.002
Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW (2006) Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res 66(23):11238–11246
Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA (2006) Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer 95(3):272–281. doi:10.1038/sj.bjc.6603240
Murdoch C, Lewis CE (2005) Macrophage migration and gene expression in response to tumor hypoxia. Int J Cancer 117(5):701–708
Murdoch C, Muthana M, Lewis CE (2005) Hypoxia regulates macrophage functions in inflammation. J Immunol 175(10):6257–6263
Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL (1996) Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 56(20):4625–4629
Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL (2000) The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol 157(2):411–421
Squadrito ML, De Palma M (2011) Macrophage regulation of tumor angiogenesis: implications for cancer therapy. Mol Aspects Med 32(2):123–145. doi:10.1016/j.mam.2011.04.005S0098-2997(11)00020-3 [pii]
Murdoch C, Muthana M, Coffelt SB, Lewis CE (2008) The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 8(8):618–631. doi:10.1038/nrc2444
Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L (2003) IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. Faseb J 17(14):2115–2117
Carmi Y, Voronov E, Dotan S, Lahat N, Rahat MA, Fogel M, Huszar M, White MR, Dinarello CA, Apte RN (2009) The role of macrophage-derived IL-1 in induction and maintenance of angiogenesis. J Immunol 183(7):4705–4714. doi:10.4049/jimmunol.0901511jimmunol.0901511 [pii]
Hildenbrand R, Dilger I, Horlin A, Stutte HJ (1995) Urokinase and macrophages in tumour angiogenesis. Br J Cancer 72(4):818–823
Lewis C, Murdoch C (2005) Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am J Pathol 167(3):627–635
White JR, Harris RA, Lee SR, Craigon MH, Binley K, Price T, Beard GL, Mundy CR, Naylor S (2004) Genetic amplification of the transcriptional response to hypoxia as a novel means of identifying regulators of angiogenesis. Genomics 83(1):1–8
Lin EY, Nguyen AV, Russell RG, Pollard JW (2001) Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 193(6):727–740
Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J (2007) Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res 67(6):2649–2656
Sinha P, Clements VK, Ostrand-Rosenberg S (2005) Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol 174(2):636–645
Sinha P, Clements VK, Ostrand-Rosenberg S (2005) Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res 65(24):11743–11751
Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, Stanley ER, Segall JE, Condeelis JS (2005) Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res 65(12):5278–5283
Sun B, Nishihira J, Yoshiki T, Kondo M, Sato Y, Sasaki F, Todo S (2005) Macrophage migration inhibitory factor promotes tumor invasion and metastasis via the Rho-dependent pathway. Clin Cancer Res 11(3):1050–1058
Hagemann T, Robinson SC, Schulz M, Trumper L, Balkwill FR, Binder C (2004) Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up-regulation of matrix metalloproteases. Carcinogenesis 25(8):1543–1549
Kessenbrock K, Plaks V, Werb Z (2010) Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141(1):52–67. doi:10.1016/j.cell.2010.03.015
Vasiljeva O, Papazoglou A, Kruger A, Brodoefel H, Korovin M, Deussing J, Augustin N, Nielsen BS, Almholt K, Bogyo M, Peters C, Reinheckel T (2006) Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res 66(10):5242–5250. doi:10.1158/0008-5472.CAN-05-4463
Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, Berman T, Joyce JA (2010) IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Develop 24(3):241–255. doi:10.1101/gad.1874010
Almholt K, Lund LR, Rygaard J, Nielsen BS, Dano K, Romer J, Johnsen M (2005) Reduced metastasis of transgenic mammary cancer in urokinase-deficient mice. Int J Cancer 113(4):525–532. doi:10.1002/ijc.20631
Mason SD, Joyce JA (2011) Proteolytic networks in cancer. Trends Cell Biol 21(4):228–237. doi:10.1016/j.tcb.2010.12.002
Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, Lang RA, Pollard JW (2009) A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One 4(8):e6562. doi:10.1371/journal.pone.0006562
Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW (2011) CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475(7355):222–225. doi:10.1038/nature10138nature10138 [pii]
Elgert KD, Alleva DG, Mullins DW (1998) Tumor-induced immune dysfunction: the macrophage connection. J Leukoc Biol 64(3):275–290
Lahat N, Rahat MA, Ballan M, Weiss-Cerem L, Engelmayer M, Bitterman H (2003) Hypoxia reduces CD80 expression on monocytes but enhances their LPS-stimulated TNF-alpha secretion. J Leukoc Biol 74(2):197–205
Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK (2012) Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol 22(4):275–281. doi:10.1016/j.semcancer.2012.01.011S1044-579X(12)00013-2 [pii]
Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M (2009) Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol 10(11):1178–1184. doi:10.1038/ni.1791ni.1791 [pii]
Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L (2012) Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol 12(4):239–252. doi:10.1038/nri3174nri3174 [pii]
Shurin GV, Ouellette CE, Shurin MR (2012) Regulatory dendritic cells in the tumor immunoenvironment. Cancer Immunol Immunother 61(2):223–230. doi:10.1007/s00262-011-1138-8
Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P (2003) L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol 24(6):302–306
Bronte V, Zanovello P (2005) Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol 5(8):641–654
Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, Lackner A, Alvarez X, Ochoa A, Chen L, Zou W (2006) B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med 203(4):871–881. doi:10.1084/jem.20050930
Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L (2009) Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med 206(6):1327–1337. doi:10.1084/jem.20082173jem.20082173 [pii]
Smith C, Chang MY, Parker KH, Beury DW, DuHadaway JB, Flick HE, Boulden J, Sutanto-Ward E, Soler AP, Laury-Kleintop LD, Mandik-Nayak L, Metz R, Ostrand-Rosenberg S, Prendergast GC, Muller AJ (2012) IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Disc 2(8):722–735. doi:10.1158/2159-8290.CD-12-0014
De Palma M, Lewis CE (2011) Cancer: Macrophages limit chemotherapy. Nature 472(7343):303–304. doi:10.1038/472303a
Zheng Y, Cai Z, Wang S, Zhang X, Qian J, Hong S, Li H, Wang M, Yang J, Yi Q (2009) Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood 114(17):3625–3628. doi:10.1182/blood-2009-05-220285
Zheng Y, Yang J, Qian J, Qiu P, Hanabuchi S, Lu Y, Wang Z, Liu Z, Li H, He J, Lin P, Weber D, Davis RE, Kwak L, Cai Z, Yi Q (2012) PSGL-1/selectin and ICAM-1/CD18 interactions are involved in macrophage-induced drug resistance in myeloma. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. doi:10.1038/leu.2012.272
Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K (2010) Development of monocytes, macrophages, and dendritic cells. Science 327(5966):656–661. doi:10.1126/science.1178331
Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F (2012) A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336(6077):86–90. doi:10.1126/science.1219179
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A (2009) Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30(7):1073–1081. doi:10.1093/carcin/bgp127
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674. doi:10.1016/j.cell.2011.02.013
Mantovani A (2009) Cancer: inflaming metastasis. Nature 457(7225):36–37. doi:10.1038/457036b
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917):860–867
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357(9255):539–545
Karin M, Greten FR (2005) NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5(10):749–759
Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M (2004) IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118(3):285–296
Balkwill F, Charles KA, Mantovani A (2005) Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7(3):211–217
Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y (2004) NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 431(7007):461–466
Balkwill FR (2012) The chemokine system and cancer. J Pathol 226(2):148–157. doi:10.1002/path.3029
Mantovani A (2005) Cancer: inflammation by remote control. Nature 435(7043):752–753
Pollard JW (2004) Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 4(1):71–78
Balkwill FR, Mantovani A (2012) Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol 22(1):33–40. doi:10.1016/j.semcancer.2011.12.005
Karin M, Lin A (2002) NF-kappaB at the crossroads of life and death. Nat Immunol 3(3):221–227
Colombo MP, Mantovani A (2005) Targeting myelomonocytyic cells to revert inflammatin-dependent cancer promotion. Cancer Res 65:9113–9116
Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M (2009) IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15(2):103–113. doi:10.1016/j.ccr.2009.01.001
Saccani A, Schioppa T, Porta C, Biswas SK, Nebuloni M, Vago L, Bottazzi B, Colombo MP, Mantovani A, Sica A (2006) p50 nuclear factor-kappaB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res 66(23):11432–11440
Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM (2000) M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 164(12):6166–6173
Biswas SK, Mantovani A (2010) Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 11(10):889–896. doi:10.1038/ni.1937ni.1937 [pii]
Mantovani A, Sica A, Locati M (2005) Macrophage polarization comes of age. Immunity 23(4):344–346
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23(11):549–555
Weigert A, Brune B (2008) Nitric oxide, apoptosis and macrophage polarization during tumor progression. Nitric Oxide 19(2):95–102. doi:10.1016/j.niox.2008.04.021S1089-8603(08)00073-6 [pii]
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454(7203):436–444. doi:10.1038/nature07205nature07205 [pii]
Sica A, Porta C, Morlacchi S, Banfi S, Strauss L, Rimoldi M, Totaro MG, Riboldi E (2012) Origin and functions of tumor-associated myeloid cells (TAMCs). Cancer Microenviron 5(2):133–149. doi:10.1007/s12307-011-0091-6
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10(9):942–949. doi:10.1038/nm1093nm1093 [pii]
Torroella-Kouri M, Silvera R, Rodriguez D, Caso R, Shatry A, Opiela S, Ilkovitch D, Schwendener RA, Iragavarapu-Charyulu V, Cardentey Y, Strbo N, Lopez DM (2009) Identification of a subpopulation of macrophages in mammary tumor-bearing mice that are neither M1 nor M2 and are less differentiated. Cancer Res 69(11):4800–4809. doi:10.1158/0008-5472.CAN-08-34270008-5472.CAN-08-3427 [pii]
Song X, Krelin Y, Dvorkin T, Bjorkdahl O, Segal S, Dinarello CA, Voronov E, Apte RN (2005) CD11b + /Gr-1 + immature myeloid cells mediate suppression of T cells in mice bearing tumors of IL-1beta-secreting cells. J Immunol 175(12):8200–8208
Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC (2005) Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med 202(7):931–939
Rodriguez PC, Ochoa AC (2006) T cell dysfunction in cancer: role of myeloid cells and tumor cells regulating amino acid availability and oxidative stress. Semin Cancer Biol 16(1):66–72
Mills CD (2001) Macrophage arginine metabolism to ornithine/urea or nitric oxide/citrulline: a life or death issue. Crit Rev Immunol 21(5):399–425
MacMicking J, Xie QW, Nathan C (1997) Nitric oxide and macrophage function. Annu Rev Immunol 15:323–350
Nathan CF, Hibbs JB, Jr. (1991) Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol 3(1):65–70
Albina JE, Cui S, Mateo RB, Reichner JS (1993) Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol 150(11):5080–5085
Saio M, Radoja S, Marino M, Frey AB (2001) Tumor-infiltrating macrophages induce apoptosis in activated CD8( + ) T cells by a mechanism requiring cell contact and mediated by both the cell-associated form of TNF and nitric oxide. J Immunol 167(10):5583–5593
Koppenol WH, Moreno JJ, Pryor WA, Ischiropoulos H, Beckman JS (1992) Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol 5(6):834–842
Morris SM, Jr., Kepka-Lenhart D, Chen LC (1998) Differential regulation of arginases and inducible nitric oxide synthase in murine macrophage cells. Am J Physiol 275(5 Pt 1):E740–747
Wu G, Morris SM, Jr. (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336(Pt 1):1–17
Pegg AE (1988) Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res 48(4):759–774
Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, Mack M, Pipeleers D, In’t Veld P, De Baetselier P, Van Ginderachter JA (2010) Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res 70(14):5728–5739. doi:10.1158/0008-5472.CAN-09-46720008-5472.CAN-09-4672 [pii]
Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, Bottazzi B, Doni A, Vincenzo B, Pasqualini F, Vago L, Nebuloni M, Mantovani A, Sica A (2006) A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood 107(5):2112–2122
Ojalvo LS, King W, Cox D, Pollard JW (2009) High-density gene expression analysis of tumor-associated macrophages from mouse mammary tumors. Am J Pathol 174(3):1048–1064. doi:10.2353/ajpath.2009.080676S0002-9440(10)60963-7 [pii]
Schmieder A, Michel J, Schonhaar K, Goerdt S, Schledzewski K (2012) Differentiation and gene expression profile of tumor-associated macrophages. Semin Cancer Biol 22 (4):289–297. doi:10.1016/j.semcancer.2012.02.002S1044-579X(12)00025-9 [pii]
Pucci F, Venneri MA, Biziato D, Nonis A, Moi D, Sica A, Di Serio C, Naldini L, De Palma M (2009) A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood 114(4):901–914. doi:10.1182/blood-2009-01-200931blood-2009-01-200931 [pii]
Ojalvo LS, Whittaker CA, Condeelis JS, Pollard JW (2010) Gene expression analysis of macrophages that facilitate tumor invasion supports a role for Wnt-signaling in mediating their activity in primary mammary tumors. J Immunol 184(2):702–712. doi:10.4049/jimmunol.0902360
Hagemann T, Wilson J, Burke F, Kulbe H, Li NF, Pluddemann A, Charles K, Gordon S, Balkwill FR (2006) Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol 176(8):5023–5032. doi:176/8/5023 [pii]
Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS (2007) CD4 + CD25 + Foxp3 + regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A 104(49):19446–19451. doi:0706832104 [pii]10.1073/pnas.0706832104
DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM (2009) CD4( + ) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 16 (2):91–102. doi:10.1016/j.ccr.2009.06.018S1535-6108(09)00216-5 [pii]
Wong SC, Puaux AL, Chittezhath M, Shalova I, Kajiji TS, Wang X, Abastado JP, Lam KP, Biswas SK (2010) Macrophage polarization to a unique phenotype driven by B cells. Eur J Immunol 40(8):2296–2307. doi:10.1002/eji.200940288
Zhang J, Chen L, Xiao M, Wang C, Qin Z (2011) FSP1 + fibroblasts promote skin carcinogenesis by maintaining MCP-1-mediated macrophage infiltration and chronic inflammation. Am J Pathol 178(1):382–390. doi:10.1016/j.ajpath.2010.11.017S0002-9440(10)00063-5 [pii]
Sinha P, Bunt S, Clements VK, Albelda S, Ostrand-Rosenberg S (2007) Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity towards a type 2 response. J Immunol in press
Bunt SK, Clements VK, Hanson EM, Sinha P, Ostrand-Rosenberg S (2009) Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. J Leukoc Biol 85(6):996–1004. doi:10.1189/jlb.0708446jlb.0708446 [pii]
Spence S, Fitzsimons A, Boyd CR, Kessler J, Fitzgerald D, Elliott J, Gabhann JN, Smith S, Sica A, Hams E, Saunders SP, Jefferies CA, Fallon PG, McAuley DF, Kissenpfennig A, Johnston JA (2012) Suppressors of Cytokine Signaling 2 and 3 Diametrically Control Macrophage Polarization. Immunity. doi:S1074-7613(12)00476-1 [pii]10.1016/j.immuni.2012.09.013
Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S (2005) Macrophage receptors and immune recognition. Annu Rev Immunol 23:901–944
Squadrito ML, Pucci F, Magri L, Moi D, Gilfillan GD, Ranghetti A, Casazza A, Mazzone M, Lyle R, Naldini L, De Palma M (2012) miR-511-3p modulates genetic programs of tumor-associated macrophages. Cell Rep 1(2):141–154. doi:10.1016/j.celrep.2011.12.005S2211-1247(11)00015-5 [pii]
Acquati F, Bertilaccio S, Grimaldi A, Monti L, Cinquetti R, Bonetti P, Lualdi M, Vidalino L, Fabbri M, Sacco MG, van Rooijen N, Campomenosi P, Vigetti D, Passi A, Riva C, Capella C, Sanvito F, Doglioni C, Gribaldo L, Macchi P, Sica A, Noonan DM, Ghia P, Taramelli R (2011) Microenvironmental control of malignancy exerted by RNASET2, a widely conserved extracellular RNase. Proc Natl Acad Sci U S A 108(3):1104–1109. doi:10.1073/pnas.10137461081013746108 [pii]
Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, Costa S, Vinckier S, Dresselaer T, Akerud P, De Mol M, Salomaki H, Phillipson M, Wyns S, Larsson E, Buysschaert I, Botling J, Himmelreich U, Van Ginderachter JA, De Palma M, Dewerchin M, Claesson-Welsh L, Carmeliet P (2011) HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell 19(1):31–44. doi:10.1016/j.ccr.2010.11.009S1535-6108(10)00474-5 [pii]
De Santa F, Narang V, Yap ZH, Tusi BK, Burgold T, Austenaa L, Bucci G, Caganova M, Notarbartolo S, Casola S, Testa G, Sung WK, Wei CL, Natoli G (2009) Jmjd3 contributes to the control of gene expression in LPS-activated macrophages. EMBO J 28(21):3341–3352. doi:10.1038/emboj.2009.271emboj2009271 [pii]
Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, Miyake T, Matsushita K, Okazaki T, Saitoh T, Honma K, Matsuyama T, Yui K, Tsujimura T, Standley DM, Nakanishi K, Nakai K, Akira S (2010) The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol 11(10):936–944. doi:10.1038/ni.1920ni.1920 [pii]
Ishii M, Wen H, Corsa CA, Liu T, Coelho AL, Allen RM, Carson WFt, Cavassani KA, Li X, Lukacs NW, Hogaboam CM, Dou Y, Kunkel SL (2009) Epigenetic regulation of the alternatively activated macrophage phenotype. Blood 114(15):3244–3254. doi:10.1182/blood-2009-04-217620blood-2009-04-217620 [pii]
Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, Bast MA, Rosenwald A, Muller-Hermelink HK, Rimsza LM, Campo E, Delabie J, Braziel RM, Cook JR, Tubbs RR, Jaffe ES, Lenz G, Connors JM, Staudt LM, Chan WC, Gascoyne RD (2010) Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med 362(10):875–885. doi:10.1056/NEJMoa0905680362/10/875 [pii]
Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R (2007) High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res 13(5):1472–1479. doi:10.1158/1078-0432.CCR-06-2073
Zhou Q, Peng RQ, Wu XJ, Xia Q, Hou JH, Ding Y, Zhou QM, Zhang X, Pang ZZ, Wan DS, Zeng YX, Zhang XS (2010) The density of macrophages in the invasive front is inversely correlated to liver metastasis in colon cancer. J Transl Med 8:13. doi:10.1186/1479-5876-8-13
Algars A, Irjala H, Vaittinen S, Huhtinen H, Sundstrom J, Salmi M, Ristamaki R, Jalkanen S (2012) Type and location of tumor-infiltrating macrophages and lymphatic vessels predict survival of colorectal cancer patients. Int J Cancer 131(4):864–873. doi:10.1002/ijc.26457
Kinouchi M, Miura K, Mizoi T, Ishida K, Fujibuchi W, Sasaki H, Ohnuma S, Saito K, Katayose Y, Naitoh T, Motoi F, Shiiba KI, Egawa S, Shibata C, Unno M (2012) Infiltration of CD40-positive tumor-associated macrophages indicates a favorable prognosis in colorectal cancer patients. Hepato-gastroenterology 60(121). doi:10.5754/hge12372
Weiss JM, Ridnour LA, Back T, Hussain SP, He P, Maciag AE, Keefer LK, Murphy WJ, Harris CC, Wink DA, Wiltrout RH (2010) Macrophage-dependent nitric oxide expression regulates tumor cell detachment and metastasis after IL-2/anti-CD40 immunotherapy. J Exp Med 207(11):2455–2467. doi:10.1084/jem.20100670
Buddingh EP, Kuijjer ML, Duim RA, Burger H, Agelopoulos K, Myklebost O, Serra M, Mertens F, Hogendoorn PC, Lankester AC, Cleton-Jansen AM (2011) Tumor-infiltrating macrophages are associated with metastasis suppression in high-grade osteosarcoma: a rationale for treatment with macrophage activating agents. Clin Cancer Res 17(8):2110–2119. doi:10.1158/1078-0432.CCR-10-2047
Watkins SK, Egilmez NK, Suttles J, Stout RD (2007) IL-12 rapidly alters the functional profile of tumor-associated and tumor-infiltrating macrophages in vitro and in vivo. J Immunol 178(3):1357–1362. doi:178/3/1357 [pii]
Chmielewski M, Kopecky C, Hombach AA, Abken H (2011) IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res 71(17):5697–5706. doi:10.1158/0008-5472.CAN-11-01030008-5472.CAN-11-0103 [pii]
Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, Leonardi AJ, Morgan RA, Wang E, Marincola FM, Trinchieri G, Rosenberg SA, Restifo NP (2011) IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest 121(12):4746–4757. doi:10.1172/JCI5881458814 [pii]
Duluc D, Corvaisier M, Blanchard S, Catala L, Descamps P, Gamelin E, Ponsoda S, Delneste Y, Hebbar M, Jeannin P (2009) Interferon-gamma reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor-associated macrophages. Int J Cancer 125(2):367–373. doi:10.1002/ijc.24401
Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR (2008) “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med 205(6):1261–1268. doi:10.1084/jem.20080108jem.20080108 [pii]
Wang YC, He F, Feng F, Liu XW, Dong GY, Qin HY, Hu XB, Zheng MH, Liang L, Feng L, Liang YM, Han H (2010) Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res 70(12):4840–4849. doi:10.1158/0008-5472.CAN-10-02690008-5472.CAN-10-0269 [pii]
Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP (2005) Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res 65(8):3437–3446
De Palma M (2012) Partners in crime: VEGF and IL-4 conscript tumour-promoting macrophages. J Pathol 227(1):4–7. doi:10.1002/path.4008
Linde N, Lederle W, Depner S, van Rooijen N, Gutschalk CM, Mueller MM (2012) Vascular endothelial growth factor-induced skin carcinogenesis depends on recruitment and alternative activation of macrophages. J Pathol 227(1):17–28. doi:10.1002/path.3989
Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, Letterio JJ, Paul WE, Berzofsky JA (2003) Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med 198(11):1741–1752
Gabrilovich DI, Ostrand-Rosenberg S, Bronte V (2012) Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 12(4):253–268. doi:10.1038/nri3175
Rogers TL, Holen I (2011) Tumour macrophages as potential targets of bisphosphonates. J Transl Med 9:177. doi:10.1186/1479-5876-9-177
Weiss JM, Back TC, Scarzello AJ, Subleski JJ, Hall VL, Stauffer JK, Chen X, Micic D, Alderson K, Murphy WJ, Wiltrout RH (2009) Successful immunotherapy with IL-2/anti-CD40 induces the chemokine-mediated mitigation of an immunosuppressive tumor microenvironment. Proc Natl Acad Sci U S A 106(46):19455–19460. doi:10.1073/pnas.0909474106
Rauh MJ, Ho V, Pereira C, Sham A, Sly LM, Lam V, Huxham L, Minchinton AI, Mui A, Krystal G (2005) SHIP represses the generation of alternatively activated macrophages. Immunity 23(4):361–374
Coscia M, Quaglino E, Iezzi M, Curcio C, Pantaleoni F, Riganti C, Holen I, Monkkonen H, Boccadoro M, Forni G, Musiani P, Bosia A, Cavallo F, Massaia M (2010) Zoledronic acid repolarizes tumour-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. J Cell Mol Med 14(12):2803–2815. doi:10.1111/j.1582-4934.2009.00926.x
Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL (1999) Inhibitiono f T cell proliferation by macrophage tryptophan catabolism. J Exp Med 189:1363–1372
Munn DH, Mellor AL (2004) IDO and tolerance to tumors. Trands Mol Med 10:15–18
Munn DH, Mellor AL (2006) The tumor-draining lymph nodes as an immune-privileged site. Immunol Rev 213:146–158
Acknowledgments
This work was supported by grants from the NIH (RO1CA84232 and RO1CA115880).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Ostrand-Rosenberg, S. (2014). Macrophages and Tumor Development. In: Gabrilovich, D., Hurwitz, A. (eds) Tumor-Induced Immune Suppression. Springer, New York, NY. https://doi.org/10.1007/978-1-4899-8056-4_6
Download citation
DOI: https://doi.org/10.1007/978-1-4899-8056-4_6
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4899-8055-7
Online ISBN: 978-1-4899-8056-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)