Abstract
For many years, the nuclear envelope was viewed as a passive barrier that separates the genetic material in the nucleus from the cytoplasm of the cell and permits regulated trafficking of various molecules through the nuclear pores. Research in the past two decades has shown that the nuclear envelope is a complex cellular compartment, which harbors tissue-specific resident proteins, extensively interacts with chromatin and contributes to spatial genome organization and regulation of gene expression. Chromatin at the nuclear periphery is organized into active and silenced domains punctuated by insulator elements. The nuclear envelope transmembrane proteins and the nuclear lamina serve as anchoring sites for heterochromatin. They recruit chromatin that has been modified with specific epigenetic marks, provide silencing factors that add new epigenetic modifications to genes located at the nuclear periphery, and sequester transcription factors away from the nuclear interior. On the other hand, proteins of the nuclear pores anchor as well as help generate active chromatin, promote transcription, and coordinate gene expression with mRNA export. The importance of these functions is underscored by aberrant distribution of peripheral chromatin and changes in gene expression that occur in cancer and heritable human diseases linked to mutations in nuclear envelope proteins. Although many mechanistic questions addressing the role of the nuclear envelope in genome organization and function have been answered in recent years, a great deal remains to be discovered in this exciting and rapidly moving field.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The nuclear envelope (NE) forms extensive connections with the cytoplasm of the cell and chromatin in the nuclear space. Thus, the NE is uniquely positioned to integrate extracellular, cytoplasmic and nuclear signaling networks. There are multiple ways in which the NE contributes to the regulation of gene expression ranging from transmitting signals from the cytoplasm to the nucleus to spatial genome organization and fine-tuning of gene expression at the nuclear periphery. A wide range of cellular functions that are highly relevant to tissue homeostasis, human disease, and cancer biology are dependent on the composition and the organization of the NE. Therefore, there is an ever-growing interest in exploring the mechanisms of nuclear architecture and gene expression that are regulated by spatial cues at the nuclear periphery in conjunction with the NE and its resident proteins.
Structurally, the NE is a complex structure consisting of two separate concentric lipid bilayers, the outer (ONM) and the inner (INM) nuclear membranes, respectively (Fig. 1). The ONM is studded with ribosomes and continuous with the endoplasmic reticulum [1], but it has many unique proteins that connect to the cytoskeleton as well as to the proteins residing in the INM [2]. The connectivity of the NE to the endoplasmic reticulum also means that the NE lumen is continuous with the endoplasmic reticulum lumen. The ONM provides an impenetrable barrier for proteins and most small molecules except where the nuclear pore complexes (NPCs) are inserted. The NPCs regulate directional transport of soluble macromolecules in and out of the nucleus and are extremely large (>40 MDa) structures made up of at least 30 distinct polypeptides present in multiple copies [3]. Where NPCs are inserted, the ONM curves around the outer face of the NPCs into the INM. The INM has its own unique set of transmembrane proteins, many of which interact with the intermediate filament lamins (in higher eukaryotes) that form a polymeric meshwork under the INM [4, 5]. The integral membrane proteins of both membranes are generally referred to as NETs for nuclear envelope transmembrane proteins [6].
Chromatin organization at the nuclear envelope. The nuclear envelope is a double membrane system perforated by nuclear pore complexes (NPCs) that regulate transport of molecules in and out of the nucleus. Between NPCs the inner nuclear membrane is lined by a filamentous polymer of the intermediate filament lamin proteins (lamina). At a simplistic level, peripheral heterochromatin tends to be associated with the lamina while peripheral euchromatin tends to be associated with the NPCs. Insulators on the DNA separate the peripheral euchromatin from peripheral heterochromatin
Lamins, many NETs, and some NPC proteins bind directly to chromatin and/or transcriptional regulators and some have been reported to directly bind DNA (Fig. 1; reviewed in [7]). Moreover, some of the chromatin-binding partners of NETs specifically reflect transcriptionally silenced chromatin [8–10]. These interactions, accordingly, have been shown to direct the distribution of heterochromatin at the nuclear periphery [11, 12]. The affinity between lamins/NETs and chromatin provides a mechanism whereby binding interactions with lamina proteins can both sequester certain parts of the genome to the periphery and provide regulatory proteins to these genome regions.
Most of the NE proteins initially found to bind chromatin were widely expressed. However, subsequent proteomics studies found a much larger set of proteins at the NE than previously expected [13–16] and more recent studies further revealed that many of these NETs possess a high degree of tissue specificity [17–19]. Recent work indicates that tissue-specific NETs can influence spatial genome organization and gene expression [17, 20]. These findings suggest that the NE is a complex cellular compartment, which integrates both cytoplasmic and extracellular signals to regulate gene expression.
Spatial Genome Organization Directed from the Nuclear Periphery
The three-dimensional architecture of the genome is not random. For example, the heterochromatin, historically defined as denser chromatin observed by electron microscopy, tends to be concentrated at the nuclear periphery and around nucleoli and centromeres in most cell types [21]. Of these structures, the NE provides a large two-dimensional surface with a specific set of proteins to organize the genome. In general, if one were to consider the area spanning 50 nm inwards from the NE against the total volume of a typical mammalian nucleus (~5–10 μm in diameter), the NE could be considered to control roughly 1/30th of the nucleus. As each chromosome is one long folded strand of DNA, this NE tethering could physically propagate effects along the DNA polymer deep inside the nucleus. The idea that there appears to be a direct physical contact between chromatin and the NE has been supported by microscopy studies [22, 23] and biochemical experiments detecting retention of chromatin components after NE purification and extraction with high ionic strength buffers [24].
The notion that higher order chromatin structure might play regulatory roles was implied by observations that the distribution of heterochromatin is relatively uniform within a given cell type, yet it can vary widely between different cell types. For example, neurons tend to have very little peripheral heterochromatin while hepatocytes display a uniform and patchy heterochromatin distribution, epithelial cells have a less uniform patchy distribution, fibroblasts have a uniform distribution, and lymphoblasts tend to have an enormous amount of heterochromatin that extends several microns into the nucleus from the periphery [21]. In the case of lymphoblast cells, this dense chromatin largely dissipates upon immune activation, consistent with the idea that the strongly negatively stained material represents transcriptionally inert, but plastic facultative chromatin [25, 26].
Heterochromatin is now defined by specific chromatin epigenetic modifications such as histone H3 lysine 9 dimethylation and trimethylation (H3K9me2 and H3K9me3) and lack of histone acetylation and H3 K4 dimethylation and trimethylation (H3K4me2 and H3K4me3), which are normally present at genes that are actively transcribed or poised for transcription. Recent studies clearly demonstrate that antibodies against H3K9me2, a mark characteristic of facultative heterochromatin, detect chromatin positioned close to the NE, while antibodies against H3K9me3, a modification found at constitutive heterochromatin associated with telomeres and centromeres in higher eukaryotes, stains internal heterochromatic domains [27]. These data support earlier biochemical and microscopic observations indicating that the heterochromatin protein 1 alpha (HP1α) seems to have a distinct subpopulation at the NE [28]. Recently, many specific interactions have been reported between the NETs, lamins and silent chromatin as well as cross talk between NPCs and transcriptional regulatory mechanisms. However, the precise relationship between gene activity and higher order chromatin organization at the nuclear periphery is not yet entirely clear.
Centromere and Telomere Patterns of Spatial Genome Organization
While early observations made by clinicians suggested that changes in nuclear morphology in tumor cells might be functionally important, it was not until the late 1800s that cell biologists began to notice that genome organization is not completely random [29]. The first basic description of such nonrandom nuclear organization came from Carl Rabl who found that the centromeres in nuclei from salamander larvae were located at the nuclear periphery, concentrating at the side of the nucleus where the centrosome was located (though on the outside) [29]. This “Rabl configuration” has since been observed in many plants and higher mammals, though it is more often transiently occurring just before or during mitosis and particularly meiosis [30, 31]. It also has been observed for telomeres, with associations at one pole of the nucleus occurring in both mitotic cells and in some interphase cells. One possible role for the Rabl configuration in meiosis is to orient the synaptonemal complex and align chromosomes for homologous recombination [32].
In interphase mammalian cells centromeres are not typically located at the periphery, but they do accumulate at the NE in certain cell types such as human neutrophils [33]. Though NE tethering of telomeres is also usually transient, this connection appears to be permanent in budding and fission yeast [34, 35] and is maintained throughout sperm development in mammals [36]. The NET SUN proteins have been linked to both telomere and centromere tethering to the NE in certain systems [36–40]. Most recently, the interaction between the fission yeast Sad1, a SUN family member, and a novel kinetochore protein Csi has been found to direct the tethering of centromeres to the NE [40]. Older studies suggested that lamins are also able to bind to specific chromatin structures such as centromeres and telomeres [41, 42]; however, as lamins also bind SUN proteins, it is possible that these reports reflect the SUN interactions.
Centromere function can be very important for cancer biology both by being essential for proper chromosome segregation in mitosis and, through these NE connections, for the formation of the synaptonemal complex. Improper formation of synaptonemal complex could contribute to chromosome translocations and aneuploidy. Telomeres also have been shown to play important roles in aging, cancer, and cell immortalization as maintenance of telomere length is essential for the immortalization process. Although it is not known what effect telomere length has on spatial positioning, the NE tethering of telomeres and centromeres provides potential links between the NE, genomic instability and cancer, which merit further studies.
Large-Scale Patterns of Tissue-Specific Chromosome and Chromatin Organization
While the positioning of structurally important chromosome regions, such as centromeres and telomeres that are rich in repetitive sequences and present on all chromosomes, tends to be transient in higher organisms, the nonrandom distribution of individual chromosomes tends to be tissue-specific. Theodor Boveri first suggested roughly 100 years ago that chromosomes tend to occupy particular domains in the interphase nucleus while studying eggs of the worm Ascaris [43]. However, only recently has work indicated that specific chromosomes have higher than random probability to occupy characteristic positions within the three-dimensional framework of the nucleus with respect to the NE. Whole chromosome fluorescence in situ hybridization (FISH) revealed that in human fibroblasts chromosome 18 tends to be located at the nuclear periphery while chromosome 19 tends to be positioned internally [44]. Much of this positioning appears to correlate with gene density of individual chromosomes [45, 46]; however, gene density cannot fully account for differences in chromosome positioning. For example in hybrid nuclei containing mouse and human chromosomes, the human chromosomes adopt a spatial position in the mouse cell nucleus based on the synteny with the mouse chromosomes rather than their gene density [47]. Moreover, the positioning of some chromosomes can vary between cell types in a tissue-specific manner although the molecular mechanisms that determine this phenomenon are as yet very poorly understood. For example mouse chromosome 5 tends to be peripheral in lung cells while being internal in blood and liver cells; also chromosome 6 is peripheral in CD8+ T-cells but internal in CD4+ T-cells [48, 49].
The spatial organization of chromosomes is often altered in tumors. Determining the position of chromosomes 18 and 19 in both normal and tumor cell lines revealed that their respective radial positioning with respect to the NE was highly conserved in normal fibroblasts, but in seven of eight tumor cell lines this particular positioning was much less pronounced [46]. It is not clear whether these observations reflect tissue-specific differences in nuclear organization between fibroblasts and epithelial tumors or altered chromosome numbers that are characteristic of tumor cells: further studies using matched cancer and normal tissues should elucidate this question. Importantly, the maintenance of tissue-specific chromosome positioning patterns may explain why some chromosomal translocations occur with higher frequency in certain tumor types. Recently, a study aiming to test whether chromosomes most commonly involved in tissue-specific tumor translocations were positioned adjacent to one another during interphase has found a statistically significant correlation between adjacent positioning of chromosomes in normal tissues and translocation frequency observed in tumors [50].
Large-scale differences in chromosome positioning may also reflect the above- mentioned tissue-specific patterns of heterochromatin distribution in different cell types or, as an extreme example, the evolutionary adaptation of similar cell types to function under diverse conditions. For example, a large comparative survey of heterochromatin organization in a variety of tissues and species detected profound tissue-specific differences in heterochromatin patterning as well as differences between similar cell types, such as the eye rod cell nuclei, in diurnal, nocturnal and aquatic mammals [11, 51]. Further experiments have suggested that heterochromatin positioning in some cases could be explained by tissue- and cell type-specific expression of lamin B receptor (LBR) and lamins A/C, which contribute to tethering of heterochromatin to the nuclear periphery [11]. Whether this is the most prominent mechanism to achieve tissue-specific genome architecture is yet to be determined. Notably, many tissue-specific NETs could also fulfill this function.
Specific Gene Positioning with Respect to the NE
The finding that centromeres, telomeres, and gene-poor chromosomes associate with the NE together with the knowledge that most of the genome is noncoding might suggest that this spatial positioning is directed by general repetitive elements in the genome. The first indication that specific coding gene loci could be preferentially positioned at the NE came from Drosophila where several individual gene loci were observed to be reproducibly proximal to the NE [52]. This conserved positioning would be expected to have functional consequences.
We now know that many individual genes have nonrandom positions in the nucleus and many genes change position under certain conditions, particularly those under strong regulation during development. For example, the immunoglobulin H (IgH) locus moves from the nuclear periphery to the nuclear interior during B lymphocyte development at a critical time when the locus undergoes V(D)J recombination. Specifically, the IgH locus is at the NE in early lymphocyte lineages such as Pro-B cells and T-cells but is in the nuclear interior in later stages such as Pre-B cells [53]. Also during neurogenesis the Mash1 (Ascl1) locus moves away from the periphery [54] and during adipogenesis several genes involved in lipid biogenesis that are upregulated during differentiation have been observed to move from the nuclear periphery to the interior [55]. Finally, the cystic fibrosis transmembrane conductance receptor (CFTR) gene is positioned at the NE in some cell types and in the interior in other cell types in a reproducible fashion [56]. Notably, in most cases described genes associate with the nuclear periphery in their inactive state and reposition towards the nuclear interior when active. Some genes, however, have been observed to exhibit a spatial preference for the nuclear periphery that is maintained rather than changing with differentiation and activation. These include the proteolipid protein (PLP) gene locus [57], the interferon-γ locus [58], the breast cancer ERBB2 locus [59], and the osteogenesis collagen type 1 alpha 1 (COL1A1) locus [60].
In most studies, when the repositioning of individual genes between the nuclear periphery and interior was observed the rest of the chromosome was not tested. In cases when this was tested, many genes were found to move between the periphery and interior without a corresponding change in the position of the whole chromosome territory [55, 61]. This is consistent with numerous observations that chromosome territories often exhibit “looping out” of small regions [62–64]. However, the FABP4 gene and its host chromosome both strongly shift from the nuclear periphery to the nuclear interior during adipocyte differentiation [55]. Thus genes and chromosomes may not always exhibit synergistic behavior and it remains unclear if the spatial gene positioning regulates or simply reflects gene activity.
Tethering of Chromosomes and Loci to the NE
In attempts to investigate the consequences of gene and/or chromosome positioning within the nuclear space several laboratories developed artificial tethering systems allowing recruitment of a single locus to the NE [65–67]. Three independent studies used mammalian cell lines with bacterial lac operator repeats (lacO) inserted into a genomic region that tended to be in the nuclear interior. Separately, the bacterial lac repressor (lacI) that specifically binds these repeats was fused to either GFP or to a NE protein (lamin B1 or the NETs LAP2β and emerin) and expressed in the cells carrying the array. Expression of the lacI-reporter fusion to GFP had no effect on the position of the lacO locus within the three-dimensional organization of the nucleus, but when the lacI fused to a NE protein was expressed the lacO array repositioned from the nuclear interior to the NE [65–67]. Once at the NE, the tethering could be reversed because the binding of lacI to lacO sequences can be disrupted by addition of isopropyl β-d-1-thiogalactopyranoside (IPTG). Addition of IPTG to disrupt this interaction caused the locus to be released from the periphery. Several important conclusions stemmed from these studies. In all cases, tethering of the lacO array to the nuclear envelope led to repositioning of the entire chromosome carrying the lacO array from the nuclear interior to the nuclear periphery. However, the effect of this repositioning on gene expression was not consistent between individual studies (see below). It was also observed that when chromosome 11 containing the lacO repeats moved to the periphery, another chromosome, chromosome 4, moved away from the periphery suggesting that genes/chromosomes may compete for space based on the strength of affinity interactions. As the NE represents only ~1/30th of the nuclear volume it would not be likely to be able to accommodate all chromosomes in a typical nucleus. Lastly, the positioning of lacO repeats and their recipient chromosome was not heritable - the loss of lacO-lacI affinity interaction upon treatment of the cells with IPTG resulted in loss of peripheral localization of the lacO array in daughter cells. This argues that in the case of endogenous loci/chromosomes a particular pattern of affinity interactions must be restored at the end of each mitosis for a specific cell type to achieve a particular chromosome configuration. Such reestablishment would not be expected to be completely accurate. Thus, not surprisingly, a particular organizational pattern using the directed lac array system was never achieved in more than 89 % of cells and endogenous patterns tend to be achieved at frequencies of 60–80 %. A recent study using an elegant experimental set up to follow specifically the localization of peripheral chromatin through several rounds of mitosis also clearly demonstrates that sequences labeled as peripheral in mother cells may (with some degree of probability) end up localizing internally in daughter cells [27]. This is likely to reflect the randomness in chromosome movements when aligning at the metaphase plate that may result in some chromosomes not being accessible when NETs bind to reform the NE in telophase. Such lack of accurate heritable propagation places the spatial genome organization outside the classical definition of epigenetics.
The lacO-lacI system provides an extremely strong tether not just because of the high binding affinities between lacO and lacI, but also because the lacO sequence usually is amplified 128–256 times in the array. Though this is certain to be stronger than any individual chromatin–NE interactions in mammalian cells, interaction sites on human chromosomes responsible for spatial genome organization would likely be both abundant and widely distributed, thus providing many tether points that would in the end have the same effect as the amplified lacO array. Alternatively, large gene clusters such as at the IgH locus, Hox loci, and olfactory receptor gene clusters might provide unique binding sites that would create distinct microenvironments at the NE. Indeed, a recent study found that olfactory receptor gene clustering is associated with the NE [68].
So how are loci and/or chromosomes tethered to the NE? The NE tethers for chromatin should be strongly embedded core components of the NE, e.g., lamins and NETs. Lamins form an intermediate filament polymer resistant to most standard cell extraction conditions such as 1 M NaCl and 2 % detergent and some lamin-lamin interactions can withstand 6 M urea extraction [69]. Thus, the nuclear lamina provides a strong scaffold to tether chromosomes. Lamins have been shown to bind core histones, particularly the H2A/H2B subtypes [70–72]. These interactions would not be expected to discriminate any particular areas of the genome, however, these studies were performed before the identification of many histone modifications and it is possible that if revisited some specificity might be observed. Some specificity was also observed in lamin binding to DNA, in particular to repetitive AT-rich sequences and the minor groove of single-stranded DNA in matrix- and scaffold-attachment regions (MARs and SARs; [41, 73–75]).
Just as the NET SUN proteins are responsible for the tethering of centromeres and telomeres to the NE, other NETs are likely to contribute to specific chromosome attachments. As the NETs are embedded in the membrane, they provide a strong anchor to chromatin, but this strength is further increased when considering that most NETs tested thus far have been shown to bind the lamin polymer [6]. That the NETs could contribute specificity to genome organization was first supported by observations that LBR and emerin bind to distinct positions on chromosomes at the earliest stages in NE assembly in telophase [76]. LBR has, like lamins, been found to bind histones, though in this case histones H3/H4 [77], and also to heterochromatin protein 1 (HP1) [10], which binds with high affinity to methylated histone H3 tails (H3K9me2 and H3K9me3) [78, 79]. These interactions provide a mechanistic explanation for the enrichment of H3K9me2 modified heterochromatin at the nuclear periphery. Notably, either pharmacological inhibition or knockdown by RNA interference of the enzymes responsible for depositing H3K9me2 (G9a and GLP, also known as EHMT2 and EHMT1, respectively) led to dissociation of peripheral chromatin from its proximity to the nuclear lamina [27].
The NET LAP2β has also been shown to bind to core histones [80], but also binds directly to DNA and to the barrier-to-autointegration factor (BAF) [81, 82]. BAF is a soluble protein that binds both to histones and DNA and so can contribute to higher order chromatin structure [83]. BAF binding could bring some specificity to NE–chromatin interactions because it has particular affinity for selected linker histones including H1.1 [84]. The NET MAN1 can also bind BAF, but separately binds directly to DNA through a winged helix fold domain in its carboxyl-terminal domain [85].
The NETs mentioned above are all widely expressed and so it is relatively easy to imagine how they could contribute to general spatial genome organization patterns such as those related to gene density—and indeed LAP2 and LBR have been linked to such general organization [11, 12, 68]. However, it is not easy to explain how these ubiquitously expressed proteins, except perhaps LBR, could contribute to the tissue-specific patterns of genome organization observed for certain chromosomes and gene loci. It would seem more likely that tissue-specific NETs recently discovered in proteomic analyses of NEs isolated from several different tissues [17–19] might perform this function. Indeed, screening of novel blood-specific NETs identified one that promoted chromatin condensation and two others that repositioned a gene locus [17]. Moreover, a recent study indicates that several NETs expressed preferentially in either liver, fat, or muscle cells can reposition chromosomes to the NE when exogenously expressed in fibroblasts and, in the case of the liver-specific NETs 45 and 47, their knockdown resulted in release of certain chromosomes from the NE in liver cells [20]. The same study showed that different NETs are able to affect the positioning of different subsets of chromosomes, indicating that these are indeed the likely endogenous players that provide tissue specificity to spatial genome organization [20].
Relationships Between Nuclear Positioning and Gene Expression State
The idea that changing the position of a gene with respect to the nuclear periphery could lead to a change in gene expression received its first strong support in Drosophila when an insulator sequence called gypsy was found to be preferentially located at the nuclear periphery. When gypsy and a reporter gene were inserted into a more internal area of the genome, the locus was translocated to the periphery and the expression from the reporter was correspondingly reduced [86]. This came to be known as one of the many examples of position effect variegation in gene expression that have been observed in Drosophila, yeast and mammalian cells. The example above is somewhat anecdotal and may reflect caveats with experimental design as insulator sequences, such as gypsy, serve as boundary elements protecting active genes from spreading of nearby heterochromatin [87, 88]. Insulator elements bind specific proteins and are involved in long-range chromatin interactions (chromatin looping), blocking of enhancer activity, and notably, delineation of subnuclear localization of chromosomes [87, 89].
In mammalian cells, insulator sequences are bound by zinc-finger proteins CTCF (CCCTC-binding factor) and its relative BORIS (Brother Of the Regulator of Imprinted Sites) [90, 91]. In recent years, the mapping of CTCF binding sites in the genome [92] and the lamina-associated chromatin domains [93, 94] in conjunction with maps of chromatin modifications and gene expression profiles have provided important insights into the relationship between nuclear positioning, chromatin architecture, chromatin modifications and gene expression state.
Gene Activity at the Nuclear Periphery
Apart from the gypsy insulator experiment mentioned above, multiple examples can be found in literature suggesting that genes located at the nuclear periphery tend to be transcriptionally inactive, late replicating and marked by chromatin modifications indicative of silenced chromatin [95]. When genes were found to migrate to the nuclear interior upon differentiation or under the influence of external stimuli, this in some cases was accompanied by upregulation of transcriptional activity, increase of histone acetylation and the presence of RNA polymerase II at gene promoters [53, 54]. However, as alluded to earlier, examples exist demonstrating that not all genes located at the nuclear periphery are transcriptionally silenced. Moreover, the cause and consequence relationship between gene location and gene activity has always been difficult to infer from studies on individual gene loci.
The experiments using lacO arrays to tether genomic loci to the NE [65–67] were partly designed to test the effect of nuclear positioning on gene expression in a more controlled fashion. Two of these studies had a selectable marker inserted in the array and both found that transcription of this particular marker was reduced when the locus was at the periphery [65, 67]. Correspondingly they found that release of the locus from the periphery with IPTG restored the lost activity to the marker genes [65, 67]. One of the studies also tested endogenous genes close to the area where the lacO array was inserted, finding that repression was not general with only some genes being repressed when the locus was at the periphery [65]. In the third study no repressive effects or deficiencies in the induction dynamics of a reporter gene inserted by the array were observed between its internal and peripheral positioning [66]. However, in this study the reporter was strongly and actively induced from a promoter that could potentially overcome any repressive effects of the periphery. Therefore, the question of whether and how tethering a locus to the periphery directly results in its repression remained unresolved by these studies, but it is clear that changes in gene regulation can occur concomitantly with changes in gene positioning.
The general features of gene activity at loci in close proximity to the NE became apparent when the nuclear lamina associated chromatin domains were mapped on a genome-wide scale in Drosophila and mammalian cells by a technique known as DamID [93, 94]. The DamID method used in these studies employed a fusion of lamin B with a GATC sequence-specific bacterial DNA adenine methylase (Dam). As adenine methylation in GATC context does not exist in higher eukaryotes, any DNA carrying this mark in cells expressing the Dam-laminB fusion signifies proximity to the nuclear lamina. This approach defined the lamina-associated domains (LADs) as stretches of DNA 0.1–1 mega bases (Mb) in length. In human fibroblasts, there are more than 1,300 of such genomic regions in close contact with the nuclear lamina. LADs are present on all chromosomes, associated with repressive histone modifications (H3K9me2 and H3K27me3) and are relatively gene-poor (Fig. 2). Genes embedded in LADs have low levels of expression with very few active genes that escape silencing. Most LADs have sharp boundaries that are marked by insulator elements bound by CTCF [94] (Fig. 2). This organization of chromatin into LADs and inter-LAD domains is also conserved in Drosophila [93] with a different set of insulator elements marking the LAD boundaries [87].
Anchoring and silencing of chromatin at the nuclear envelope. Silenced chromatin is both recruited to and maintained at the NE through interactions of lamins and NETs with chromatin and chromatin modifying enzymes. Lamina-associated domains (LADs) are bounded by CTCF on chromatin. Silent marks such as histone H3 lysine 9 di and tri methylation (H3K9Me2 and Me3) recruit the additional silencing factor heterochromatin protein 1 (HP1), which in turn can bind to the NET lamin B receptor (LBR, so named because it in turn binds the lamin polymer). One part of the barrier-to-autointegration factor (BAF) can bind histones while another part binds to the NETs emerin, LAP2β and MAN1. LAP2β and emerin have also been found to bind histone deacetylases, which further promote and maintain silencing marks at the periphery
Given the vast changes in gene expression that occur during differentiation and the observations that the radial positioning of chromosomes varies in a tissue-specific manner, one would expect LADs and CTCF binding sites also to change substantially during differentiation. Surprisingly, the LADs and H3K9me2-rich domains, which largely overlap with LADs, remain largely invariable during differentiation of embryonic stem cells into neuronal progenitors and further into mature neurons [96–98]. Instead of a global rearrangement of genome-nuclear lamina interactions, small local changes affecting individual genes were observed in these studies. Thus, LAD-embedded genes, which were upregulated during neuronal differentiation, were seen to dissociate from the lamina and lose silencing histone modifications [96, 97]. This may seem surprising, however, such global maps represent population average and cell-to-cell variations are lost in such studies. Thus, LADs can be viewed as a probabilistic map of genome-nuclear lamina interactions. Techniques that permit global genomic studies on a single-cell level are starting to emerge and promise to be instrumental in determining cell-to-cell variation in cultured cells and, excitingly, cells derived from specific tissues. As mentioned earlier, tracking individual cells through mitosis detected substantial rearrangement and dissociation of LADs from the nuclear lamina in daughter cells [27]. This, perhaps, allows rearrangement of LADs and activation of LAD-embedded genes. It also suggests that rearrangement of chromatin–lamina interactions may be proportional to the number of cell divisions undertaken by cells before they acquire the differentiated state. In the case of in vitro differentiation models using ES cells, the differentiated state is achieved within very few mitotic divisions, which may not allow sufficient time for significant LAD rearrangements to take place.
Is the dissociation of genes from the nuclear periphery sufficient to induce gene expression? In the lacO tethering experiments to the NE, it was found that the release of the locus from the periphery either by IPTG (disrupting lacO-lacI interactions) or treatment with histone deacetylase (HDAC) inhibitors could restore the lost activity to marker genes and few, but not all, endogenous genes in close proximity to the lacO array [65–67]. However, recruitment to peripheral chromatin of the strong transcriptional activator VP16, which interacts with histone acetyltransferases (HATs) and disrupts nuclear lamina–chromatin contacts, induces relatively few changes in gene expression on a global scale [27]. Taken together, these experiments suggest that movement of loci away from the nuclear lamina is perhaps permissive, but not sufficient for gene activation.
Recruitment and Silencing of Chromatin at the NE
The question of whether or not genes are silenced before they are anchored to the NE is not yet comprehensibly answered. Notably, many interactions between chromatin, the nuclear lamina and the NETs may require preexisting histone modifications. Thus, chromatin anchoring to the INM embedded lamin B receptor LBR via HP1α and HP1γ [10] requires H3K9me2 to allow HP1 binding (Fig. 2). Although there is some evidence that BAF (barrier-to-autointegration factor), which binds the NETs LAP2β, emerin, and MAN1 through a shared sequence motif called the LEM domain [81, 99, 100] interacts with histone H3 methylase G9a (EHMT2) [101], in most mammalian cell types G9a is distributed throughout the nucleoplasm and not exclusively anchored to the NE. However, additional evidence suggests that NETs emerin and LAP2ß interact with histone deacetylases, HDAC3 being one of them [102, 103] (Fig. 2). Therefore, it is possible that localized HDAC activity at the NE provides G9a with a suitable substrate for subsequent methylation of H3 tails generating H3K9me2 required for anchoring to LBR-interacting HP1 proteins.
The interactions of BAF with NETs raise another interesting issue regarding anchoring to the NE of silenced chromatin. The NET LAP2β has several soluble splice variants that also bind BAF [104–106]. One of these, LAP2ζ, principally resides in the cytoplasm and its upregulation compared to LAP2β causes BAF to be captured in the cytoplasm before nuclear import, thus reducing intranuclear pools and their corresponding functions in cross-linking chromatin and recruiting it to the NE [106]. As many NETs have multiple splice variants, this type of competitive inhibition will likely prove to be used commonly as a regulatory mechanism for chromatin recruitment to the NE.
In addition to chromatin-mediated interactions, several DNA-binding factors have been found to interact with NETs and the nuclear lamina. One of these is the transcription factor Oct1, which interacts with lamin B and localizes to the nuclear periphery in a lamin B-dependent manner [107]. As Oct1 motifs are enriched within LADs, this suggests that Oct1 may contribute to tethering to the NE of genomic loci in a sequence-specific manner.
Activation of Chromatin at the Nuclear Periphery
Among the proteins identified in a proteomic study of rat liver NEs were several that can modify histones for not only repression, but also activation. One of these, NET43/hALP, is a histone acetyltransferase [16]. Interestingly, the membrane prediction for NET43/hALP only occurs in some organisms and was absent in the human homologue. Nonetheless, the human version localizes to mitotic chromosomes in mammalian cells by binding to another NET, SUN1 [108]. Depletion of SUN1 in human tissue culture cells resulted in delayed chromosome decondensation and a reduction in histone H2B and H4 acetylation in a manner dependent on hALP [108]. Interestingly NET43/hALP appears to be upregulated during lymphocyte activation when the large amount of dense peripheral chromatin of resting lymphocytes becomes decondensed [17].
Functions of the NPC in Spatial Genome Organization and Gene Regulation
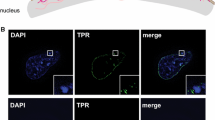
Each NPC is a large protein assembly of >40 MDa in yeast and >60 MDa in mammalian cells made up of at least 30 core proteins [13, 15]. The assembly has an eightfold symmetry so that each protein is represented in a minimum of eight copies and in some cases in as many as 64 copies in a single NPC. Among these proteins is Tpr, a 270 kDa coiled-coil protein that extends roughly 100 nm into the nucleoplasm in a structure generally referred to as the nuclear basket [109], and Nup50, which also associates with the nuclear basket extending roughly 50 nm from the central plane of the NPC into the nucleoplasm [110]. These extensions allow interactions with chromatin of a different character to those at the level of the membrane. Electron micrographs and recent high-resolution imaging of mammalian nuclei [111] show that while heterochromatin contacts the areas of the NE which are lined with nuclear lamina, the NPCs are surrounded by less dense chromatin. Studies in yeast and higher eukaryotes demonstrate that NPCs contribute in several different ways to the spatial genome organization and regulation of gene expression.
The NPC in Spatial Genome Organization
Although in mammalian cells telomeres are tethered to the NE through interactions with SUN proteins, in yeast they are tethered by the NPC. In fact, the first specific interactions between telomeres and the NPC were determined in yeast with the yeast Tpr homologue, Mlp, as the anchoring site [34, 35]. The difference in telomere tethering between yeast and mammals together with the lack of yeast homologues for many NETs, particularly the more tissue-specific ones [18], suggests that many regulatory functions in yeast carried out by the NPC have been subsequently taken over by other NE proteins during evolution.
The recruitment of telomeres to the periphery in budding yeast is essential for silencing of subtelomeric genes. In addition to Mlp (Tpr), peripheral localization of telomeres also involves the soluble non-NPC protein Ku [112]. Mutation in Mlp and Ku proteins results in derepression of subtelomeric silenced genes [34, 35, 113, 114]. As a potential epigenetic mechanism, the heritability of this silencing was also addressed. Derepression of reporter genes integrated close to telomeres upon deletion of Mlp and Ku implied that silencing requires NE/NPC association [34, 114]. However, deletion of the NPC proteins also results in a redistribution of Sir3p fused to GFP [114]. The latter result argues that the observed derepression of genes could be a secondary consequence of NPC disruption, rather than due to relocation of the telomeres away from the nuclear periphery. Another study, using an elegant experimental setup to break the connection between the NPC and a silenced reporter after the silencing was established, found that the release from the periphery did not derepress the silent reporter [115]. This suggests that once established at the nuclear periphery the silenced chromatin state can be stably maintained without NPC association.
Active Chromatin at the NPC
In contrast to chromatin at the nuclear lamina, the NPCs in many species including yeast, flies and mammals associate with active chromatin [116–119]. Adaptor proteins that mediate these connections have been identified in a variety of systems. In yeast, the nuclear basket protein Mlp1 interacts with the chromatin-bound co-activator complex SAGA (Spt-Ada-Gcn5-Acetyltransferase), which is known to promote active transcription [120]. SAGA is a large protein assembly, containing two chromatin-modifying enzymes, the Gcn5 histone acetyltransferase and Ubp8 histone deubiquitinase [121, 122]. SAGA, via its Sus1 component, was also shown to interact with the NPC-bound TERX-2 complex, which plays important roles in transcript elongation and mRNA transport [123–125] (Fig. 3). Budding yeast TREX-2 consists of four proteins Sac3-Thp1, Cdc31, and Sus1) [126]. Thus, tethering of active acetylated chromatin to the NPC via Sus1, a shared component of SAGA and TREX-2 complexes, may help to coordinate transcription-coupled mRNA export [127]. This arrangement seems to be conserved in other species. In Drosophila, the Sus1 ortholog E(y)2 via the TREX-2 complex protein Xmas-2, the equivalent of yeast Sac3, promotes transcription, mRNA export and positioning close to the NE of the hsp70 gene cluster [128, 129]. Whether tethering of active chromatin follows a similar arrangement in mammalian cells is yet to be determined. Notably, the SAGA complex is highly conserved [122] and a Sac3-related protein GANP involved in mRNA export is present in human cells [130]. Therefore, it is likely that the positioning of active SAGA-bound chromatin at the NPC may operate in a similar manner across most eukaryotes.
Active chromatin at the nuclear pore. NPC proteins Nup98, Nup153, and Tpr form the nuclear basket, which tethers active chromatin through binding a complex of TREX-2 and SAGA. The SAGA complex promotes active transcription while the TREX complex is important for RNA export. Thus, recruitment of active genes to the periphery by this mechanism also facilitates rapid translocation of the mRNAs out of the nucleus so they can be translated on ribosomes in the cytoplasm. It is noteworthy that the average mammalian nucleus is estimated to have 2,000–3,000 NPCs and as they are 125 MDa complexes of greater than 100 nm diameter this translates to up to 40 % of the nuclear surface
Studies in yeast and Drosophila have also shown that several nucleoporins located at the nuclear side of the NPC are required for tethering of active chromatin to the nuclear periphery. Nup1, Nup2, Nup60, and Mlp1 (Tpr in metazoan species) have been implicated in mediating connections between active genes and the NPC [120, 131, 132]. One of the most interesting examples signifying the functional importance of such tethering is dosage compensation in Drosophila. Male flies, having one X chromosome, achieve balanced expression of X-linked genes relative to female flies with two X chromosomes by upregulating gene expression on the single male X. This upregulation requires Drosophila dosage compensation complex, which includes noncoding RNAs, histone acetyltransferase MOF and additional proteins, as well as the nuclear basket proteins Nup153 and Tpr [117, 133]. Taken together, these examples implicate nucleoporins located at the nuclear side of the NPC in spatial genome organization and regulation of gene activity.
It has been suggested that, topologically, positioning of active genes at the NPC may occur via formation of chromatin loops mediated by promoter–terminator interactions as well as the boundary elements in yeast and their equivalent, the insulator elements, in other metazoan species [92, 134, 135] (Fig. 1). Such elements are often found at the boundaries between active and repressed chromatin and serve to antagonize heterochromatin spreading. Consistent with such a function, studies in yeast and human cells have shown that chromatin in close proximity to the NPC carries modifications indicative of both, active and repressed, chromatin states [92, 136]. A screen for proteins involved in boundary activity in budding yeast identified Mlp, Nup2 (the yeast homologue of Nup153 in humans), the NPC-associated proteins Nup60p, and the Ran-GTP exchange factor Prp20p [134, 137]. The typically mobile Prp20 is bound to the core structure of the NPC through Nup2 on one side and on the other it binds H2A.Z (also called Htz1), a variant of histone H2A that is loaded by the SWR-C chromatin-remodeling complex [138]. H2A.Z marks relatively immobile nucleosomes in the yeast genome and can be found at most gene promoters and some intergenic regions of the budding yeast genome [139]. Interestingly, the SAGA complex may also contribute to boundary/insulator element function. It has been reported that the Ada2 component of SAGA is recruited to yeast telomeres and required to suppress the spreading of telomeric heterochromatin into subtelomeric regions [140]. Moreover, Sus1, a shared component of SAGA and TREX-2 complexes, functions together with Su(Hw) at insulator elements in Drosophila [129]. The overall similarity between the function of boundary elements in yeast and the insulators in Drosophila and mammalian cells indicates that the compartmentalization of chromatin into active and inactive domains at the NE is widely used in evolution.
Transcription Factors at the Nuclear Envelope
The nuclear surface provides a large scaffold on which the genome can be spatially organized and regulated; however, in relative terms it represents only ~1/30th of the total nuclear volume. If a spherical nucleus has a radius of ~5 μm, then the volume of the nucleus would be 523 μm3 and the surface area would be 314 μm2. However, the thickness of the NE from ONM to INM is only ~50 nm, and the penetration of NETs and lamins from the inner surface into the nucleoplasm is likely much less than this, while the NPC nuclear baskets have been measured to project roughly 100 nm into the nucleoplasm. Thus, if one considered an average penetration of 50 nm for the “volume” of the NE, the volume of the nuclear surface would be less than 16 μm3 or roughly 30-fold smaller than the volume of the nucleoplasm. Thus, if, in addition to the genes tethered by the NE (discussed above), the NE also tethered transcriptional regulators for those genes, it would have the equivalent effect of increasing the local concentration of the transcriptional regulator by 30-fold.
The Function of NE in Direct and Indirect Repression of Transcription
Both LAP2β and emerin bind the transcriptional repressor germ cell-less (gcl) that is known to affect E2F/DP transcription factor heterodimers [141, 142]. Moreover, overexpression of LAP2β in tissue culture cells inhibited E2F-dependent transcription from a reporter construct [142]. Emerin also binds Btf, another transcriptional repressor with a different target specificity [143]. Of the lacO-lacI studies that used NE affinity tethering to recruit a lacO array to the periphery one fused the lacI to LAP2β and the other fused lacI to emerin [65, 67]. The overlap and discrepancies in results between these studies might be in part because of the partly shared and partly different specific transcriptional repressors binding to these two NETs. In the emerin study the amino-terminus was deleted to minimize this potential criticism [67]; however, the deleted region only partly overlaps with the binding site on emerin for germ cell-less [141], so this may not have been sufficient.
Whereas it has been demonstrated that multiple different NPC proteins can recruit transcription factors and their target genes to the same location to effectively increase the relative local concentration of the transcription factor, NETs and lamins that have been found to bind to transcription factors appear to function in the opposite fashion. They sequester the transcription factor at the periphery away from the gene target in the nuclear interior. Thus, NE binding of transcription factors appears to function to prevent gene activation.
The first demonstration of transcription factor binding to NE proteins was binding of lamin A to the retinoblastoma protein (Rb) [144]. However, the effect of lamins on Rb may be due mostly to the nucleoplasmic pool of lamins. This is supported by findings that a soluble splice variant of the NET LAP2β, LAP2α, forms a complex with lamin A and Rb in the nuclear interior [145]. Interestingly, this can have both positive and negative effects on gene regulation depending on cell type. Although this complex on the one hand sequesters Rb away from gene targets, on the other hand the complex stabilizes Rb, which, without this binding, turns over rapidly. As a consequence of this, in cells stimulated by phosphorylation of Rb to initiate progression into S-phase, the higher levels of stabilized available Rb enable much stronger activation. Thus, cells with more or less LAP2α and lamin would have different propensities to engage cell cycle progression upon the same activation stimulus. Consistent with this, LAP2α knockout mice exhibit hyperproliferation of both erythroid and epidermal lineage cells [146]. Lamins are ideal for sequestering transcriptional regulators as their abundance (~3,000,000 copies per mammalian nucleus; [147]) could easily saturate any transcription factor. Lamin A has also been shown to bind cFos [148].
In addition to lamins, NETs also repress gene function by sequestering transcription factors. Emerin interacts with the transcription factor Lmo7 [149]. Intriguingly, this interaction has been found to function in a tightly regulated feedback loop to regulate the emerin gene itself. Lmo7 activates the emerin gene (EMD) so that emerin protein binding of Lmo7 sequesters Lmo7 at the periphery away from the emerin gene; thus, the more emerin is produced, the more it can sequester Lmo7 to repress its own expression. Similarly, the NET MAN1 interacts with Smads and sequesters them away from target genes located in the nuclear interior [150, 151]. Very few of the many tissue-specific NETs recently identified have been characterized in detail, but it seems likely that some of these may bind to transcription factors for sequestration. The tissue specificity of these NETs would add a much greater complexity to NE regulation of gene expression.
Transcriptional Activation at the NE
In addition to binding Smad transcription factors, MAN1 directly binds DNA through a winged helix fold in its carboxyl-terminal domain [85]. Thus, though this has not been specifically demonstrated yet, it could potentially also tether both Smads and Smad-regulated genes to the NE to additionally activate transcription as shown for the NPC proteins.
While older studies showing the dissipation of dense chromatin at the nuclear periphery concomitant with activation of lymphocytes suggested a role for the NE in gene activation [26], several more recent studies show with certainty that transcriptional activation occurs from the NE. The PLP gene, involved in myelin production, becomes activated when already at the periphery [57]. It is interesting that this gene is active in glial cells, as it was noted earlier that brain cells tend to have minimal peripheral heterochromatin. It is thus reasonable to postulate that the ability to activate a gene at the NE may depend on cell type and the amount of peripheral heterochromatin. Other differentiation/cell state-associated genes observed to be active at the NE are the breast cancer ERBB2 gene, the osteogenesis COL1A1 gene and the interferon gamma IFN-γ locus [58–60].
Stabilization of the Genome by the Nuclear Envelope
Though only theoretical at this stage, the physical tethering of chromatin to the NE could play a major role in stabilizing the genome to protect against translocations and other factors that can lead to cancer. Proteins in the nuclear interior, even those associated with nucleoplasmic structures, tend to be relatively dynamic in FRAP studies [152]. In contrast, the movements of proteins at the NE are much less dynamic and indicate local constraints [153, 154]. One potential consequence of NE tethering could be to physically stabilize the genome, minimizing movement. Indeed, transgenes located near the nuclear periphery in mammalian cells have been shown to be less mobile than those residing in more internal positions [155]. In theory, tethering of chromatin to the NE could help maintain chromosome territories and prevent entanglement of chromosomes and potentially associated chromosome translocations that could lead to tumors. This could be particularly important during replication and even the reason why late-replicating DNA tends to be at the periphery [93, 156], i.e., because a peripheral tether combined with silent chromatin helps to stabilize chromosome territories so that chromosomes do not get entangled during replication.
The NE is a good tethering point for chromatin because it is a relatively stable structure due to the intermediate filament lamin polymer lining the inner surface of the NE [4]. It is striking that while the cytoplasm has actin filaments, microtubules and intermediate filaments, the NE has just the intermediate filament lamins. Unlike other cytoskeletal systems, intermediate filaments are highly elastic. Under compression or tension forces that would break actin filaments and microtubules the intermediate filaments are unaffected [157]. It is not surprising thus that spider’s webs are made of intermediate filaments, tough yet elastic.
These properties are important because live cell microscopy indicates that nuclei move and exhibit frequent morphological aberrations while chromatin also moves dynamically. Thus, the NE needs to have a structural support that can bend, but not break connections. In this light it is not surprising that both lamins and INM NETs bind chromatin and each other, thus providing multiple contacts in an overlapping network embedded with a wide range of proteins into the membrane to create a very strong tether. Other INM NETs make connections across the lumen of the NE to outer membrane NETs [158] and these in turn connect the NE to the cytoskeleton providing an anchor for the nucleus in the cell [159]. Together these properties enable the NE to keep chromatin tethered while still being able to stretch in response to forces placed on the polymer by genome movements or the cytoskeleton. If the peripheral lamina nucleoskeleton were rigid like microtubules, it would likely break in response to such forces and genes and chromosomes would lose their tethering. Similarly, if tethered merely by transmembrane proteins, strong forces from chromosome movements might rip the tethering NETs out of the lipid bilayer. Thus, the use of both lamins and NETs is a sensible strategy to support the many dynamic movements of chromatin within the interphase nucleus.
Although much of the above is merely a hypothesis, it is clear that lamins contribute to the mechanical stability of the nucleus. Lamin depletion or mutant expression resulted in nuclear lobulation and increased deformation under mechanical stress [69, 160–163]. In theory the importance of this is manifold. Increased lobulation would also increase the ratio of NE to nucleoplasmic volume, thus enabling greater silencing and gene regulation from the NE. Due to the DNA of chromosomes being single rope-like molecules, connections to the NE could influence the ability of internal sections to engage in transcription factories.
Peripheral Chromatin Organization and Nuclear Envelope Disease
Though lamins, NETs, and NPC proteins have all been linked to cancer in a variety of ways, most of these are indirect, e.g., having functions in processes that are critical to cancer progression as opposed to mutations in a particular NE protein causing cancer. In contrast, mutations in NE proteins have been found to be causative of a wide variety of inherited disorders ranging from muscular dystrophies to the premature aging progeroid syndromes [164–166]. In fact lamin A is now the most mutated gene in the human genome. Lamin diseases include muscular dystrophies [167–169], lipodystrophy [170–172], neuropathy [173, 174], cardiomyopathy [175], dermopathy [176], and the aging disease progeria [177, 178]. Several NETs and associated proteins also cause diseases or syndromes affecting muscle [179–182], bone [183, 184], brain [185–189], skin [176] and immune cells [190]. Because most NETs tested bind to lamins, lamin mutations could also affect NET distribution and function. Thus, the pathology of lamin-based diseases could be as much due to secondary effects on NET function as to the loss of lamin function.
Chromatin Organization in Heritable Diseases Linked to the NE
The overall distribution of heterochromatin as defined by electron dense material in electron microscopy is altered in several NE-linked diseases. Normal fibroblasts typically have a reasonable amount of this dense peripheral chromatin generally distributed throughout the periphery. In fibroblasts from patients with Emery–Dreifuss muscular dystrophy (EDMD), the dense chromatin often seems to have broken away from the NE and resides about 500 nm in from the NE. This altered pattern is observed for two different NE-linked muscular dystrophies, EDMD and Limb-girdle muscular dystrophy, and for both NET-linked and lamin-linked disease [191–193]. It is also observed in cardiomyopathy linked to the NE [194]. In contrast, fibroblasts from patients with NE-linked progeroid diseases tended to lose all peripheral dense chromatin [195], and fibroblasts from NE-linked lipodystrophy patients exhibited an intermediate phenotype with partial loss of peripheral dense chromatin in some areas at the periphery and partial clumping in other areas [193]. The fact that the patterns are not just disrupted, but disrupted in reproducible ways for each disorder suggests that these spatial genome organizational patterns are functionally relevant.
These NE protein mutations had specific and reproducible effects on spatial genome organization also in controlled experimental systems. For example, a mutation in lamin A that causes Hutchinson–Gilford progeria syndrome (HGPS) yielded an abnormal distribution of telomeres and clustering of centromeres [196] while other mutations that cause variously a neuropathy, lipodystrophy and muscular dystrophy reposition chromosomes 13 and 18 away from the nuclear periphery [197]. However, the relevance of these changes to specific disease pathology is uncertain as different mutations that cause the same disease can yield different effects on chromosome positioning. For example, both E161K and D596N lamin A mutations cause cardiomyopathy, but only E161K causes chromosome 13 to lose its normal peripheral localization [198].
Epigenetic Changes in Heritable NE Diseases and Cancer
Some of the reorganization of chromosomes, observed by FISH, and altered distribution of peripheral dense chromatin, observed by electron microscopy, could be explained by changes to epigenetic heterochromatin marks. This appears to be the case for HGPS where primary fibroblasts from patients have a significant loss of silenced chromatin marks associated with facultative heterochromatin, such as H3K9me2 and H3K27me3, while marks of constitutive heterochromatin such as H4K20me3 were increased [199]. Similar changes could be induced in normal cells upon overexpression of lamin A carrying a progeria-causing mutation [199]. Fibroblasts from a female patient also lost silencing marks on the inactive X chromosome and this chromosome lost its tight association with the periphery. Similar changes in silent chromatin marks have also been reported in normal aging cells [200]. Most cases of HGPS are due to a splice site mutation that causes loss of an exon that contains a cleavage site close to the C-terminus of lamin A. similar to the small G proteins, Lamin A acquires a farnesyl group at its C-terminal CaaX box. This modification is transient as the last 18 amino acids are cleaved at this cleavage site in mature lamin A. Blocking lamin A farnesylation had a positive effect on HP1α accumulation [201] and some of the heterochromatin defects observed in progeroid cells in culture could be reversed by treatment of the cells with farnesyltransferase inhibitors [202].
A number of studies have detected altered expression of NE components in cancer cells. For example, reduced expression of Emerin in ovarian cancer [203] and Lamin A/C overexpression in colorectal, ovarian, and prostate cancer correlates with poor prognosis and advanced tumor stage [204, 205]. Although such examples are numerous [206], a direct causal relationship between either mutations or altered expression of NE proteins has been established in very few cases. Nevertheless, indications that NE–chromatin interactions play an important role in genome organization and gene expression in cancer have started to emerge. It is well known that tumors exhibit altered patterns of DNA methylation in comparison to normal tissues [207]. These alterations include localized gain of DNA methylation at normally methylation-free CpG-rich gene promoters (CpG islands), leading to stable silencing of tumor suppressor genes, and widespread loss of DNA methylation from extensive chromosomal regions potentially promoting genomic instability. Interestingly, recent reports have shown that loss of DNA methylation in colorectal and other types of cancer occurs in regions of the genome that coincide with LADs and the silencing H3K9me2 [98, 208]. Moreover, gain of DNA methylation at promoters also occurs at genes located in these domains [208]. Taken together, these findings suggest that genome organization mediated by chromatin–NE interactions is not only profoundly different in cancer cells, but that these rearrangements may have direct functional implications in cancer development.
Another striking connection between NE components and cancer has emerged when translocations involving the nucleoporin Nup98 were found in patients with acute myeloid leukemia (AML) [209]. The most commonly detected translocations generate a fusion between Nup98 and homeobox transcription factor HoxA9 genes [207, 210]. The resulting Nup98-HoxA9 chimeric protein is nucleoplasmic and contains the GLFG repeats of Nup98, normally involved in shuttling of nuclear transport receptors through the NPC, fused to the DNA binding domain of HoxA9. The mechanism by which the fusion protein induces AML is as yet unclear. It has been suggested that GLFG repeats bind transcriptional co-activator CBP/p300 histone acetyltransferase [211], thus maintaining expression of HoxA9 target genes, which are normally downregulated during differentiation of hematopoietic stem cells following downregulation of HoxA9 itself. Fusions of Nup98, all containing the Nup98 GLGF repeats, with a variety of other partners have also been identified in AML. These fusion partners include a variety of co-activator proteins, histone methyltransferases such as NSD1, DNA topoisomerases, and RNA helicases [209]. Similar to Nup98-HoxA9, the Nup98-NSD1 fusion protein was found to bind regulatory elements of polycomb target genes, normally silenced during myeloid differentiation, and induce high levels of H3K36me3 (a mark associated with active transcription elongation) and histone acetylation, thus maintaining the expression of several homeotic genes, which contribute to malignancy [212]. Mutations abolishing H3K36me3 activity of NSD1 as well as deletion of GLGF repeats of Nup98 within the Nup98-NSD1 fusion protein context abolish activation of homeotic genes and inhibit leukemogenesis [212]. By a broadly similar mechanism, fusion proteins between Nup98 GLGF repeat region and PHD (plant homeobox domain) zinc fingers, which bind to histone H3 tails methylated at K4, function as potent oncogenes and induce AML in mouse models [213]. These observations suggest that most chimeric Nup98 proteins function to promote AML by generating aberrant active chromatin, which counteracts polycomb-mediated gene repression and allows self-renewal and maintenance of the undifferentiated state of myeloid progenitors [214]. Thus, epigenetic changes caused by a mutant NE protein are an essential component of AML and, perhaps, other malignancies.
Conclusions
Research in the past few decades has led us to conclude that the NE is a complex compartment of the cell, with functions that extend beyond the separation of the genetic material from the cytoplasm of the cell and the control of nuclear trafficking. The NE contributes to tissue-specific genome organization and regulation of gene expression through many different mechanisms, the details of which remain to be fully elucidated. Lamins provide a structural framework for the nuclear periphery and interact with silenced chromatin. NETs bind both the lamin polymer and the membrane in addition to both general and specific chromatin proteins. Moreover, NETs and NPC proteins recruit transcriptional regulators, epigenetically modified chromatin, and separately recruit enzymes that add epigenetic modifications to chromatin. As only a fraction of the many novel NETs identified by proteomics have been characterized in detail, there are a great many possibilities for diverse mechanisms in the regulation of spatial genome architecture and chromatin modifications from the NE. The finding that many NETs are tissue specific further adds to this complexity.
There are many questions that remain to be elucidated. To what degree does the NE silence genes by bringing them into an environment that is rich with silencing factors that directly modify the chromatin versus bringing already silenced chromatin to the periphery by affinity interactions? Does NE tethering also contribute a steric effect to this regulation? To what degree are the NE influences on chromatin organization heritable? To what degree does NE tethering stabilize the genome to protect it from inappropriate recombination events? And how much do these various functions contribute to the initiation of tumorigenesis? The NE provides many layers for regulating genome function, and many important discoveries are certainly yet to be made in this exciting and rapidly moving field.
Abbreviations
- BAF:
-
Barrier-to-autointegration factor
- EDMD:
-
Emery–Dreifuss muscular dystrophy
- HP1:
-
Heterochromatin protein 1
- HDAC3:
-
Histone deacetylase 3
- HGPS:
-
Hutchinson–Gilford progeria syndrome
- INM:
-
Inner nuclear membrane
- IPTG:
-
Isopropyl β-d-1-thiogalactopyranoside
- lacO:
-
Lac operator repeats
- lacI:
-
Bacterial lac repressor
- NE:
-
Nuclear envelope
- NET:
-
Nuclear envelope transmembrane protein
- NPC:
-
Nuclear pore complex
References
Callan HG, Tomlin SG (1950) Experimental studies on amphibian oocyte nuclei. I. Investigation of the structure of the nuclear membrane by means of the electron microscope. Proc R Soc Lond B Biol Sci 137(888):367–378
Tapley EC, Starr DA (2013) Connecting the nucleus to the cytoskeleton by SUN-KASH bridges across the nuclear envelope. Curr Opin Cell Biol 25(1):57–62, doi:S0955-0674(12)00177-9 [pii] 10.1016/j.ceb.2012.10.014
Suntharalingam M, Wente SR (2003) Peering through the pore: nuclear pore complex structure, assembly, and function. Dev Cell 4(6):775–789
Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, Goldman RD (2008) Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev 22(7):832–853
Stuurman N, Heins S, Aebi U (1998) Nuclear lamins: their structure, assembly, and interactions. J Struct Biol 122(1–2):42–66
Schirmer EC, Foisner R (2007) Proteins that associate with lamins: many faces, many functions. Exp Cell Res 313(10):2167–2179
Mattout-Drubezki A, Gruenbaum Y (2003) Dynamic interactions of nuclear lamina proteins with chromatin and transcriptional machinery. Cell Mol Life Sci 60(10):2053–2063
Brown CR, Kennedy CJ, Delmar VA, Forbes DJ, Silver PA (2008) Global histone acetylation induces functional genomic reorganization at mammalian nuclear pore complexes. Genes Dev 22:627–639
Makatsori D, Kourmouli N, Polioudaki H, Shultz LD, McLean K, Theodoropoulos PA, Singh PB, Georgatos SD (2004) The inner nuclear membrane protein lamin B receptor forms distinct microdomains and links epigenetically marked chromatin to the nuclear envelope. J Biol Chem 279(24):25567–25573
Ye Q, Worman HJ (1996) Interaction between an integral protein of the nuclear envelope inner membrane and human chromodomain proteins homologous to Drosophila HP1. J Biol Chem 271(25):14653–14656
Solovei I, Wang AS, Thanisch K, Schmidt CS, Krebs S, Zwerger M, Cohen TV, Devys D, Foisner R, Peichl L, Herrmann H, Blum H, Engelkamp D, Stewart CL, Leonhardt H, Joffe B (2013) LBR and Lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell 152(3):584–598, doi:S0092-8674(13)00012-3 [pii]10.1016/j.cell.2013.01.009
Zullo JM, Demarco IA, Pique-Regi R, Gaffney DJ, Epstein CB, Spooner CJ, Luperchio TR, Bernstein BE, Pritchard JK, Reddy KL, Singh H (2012) DNA sequence-dependent compartmentalization and silencing of chromatin at the nuclear lamina. Cell 149(7):1474–1487, doi:S0092-8674(12)00591-0 [pii]10.1016/j.cell.2012.04.035
Cronshaw J, Krutchinsky A, Zhang W, Chait B, Matunis M (2002) Proteomic analysis of the mammalian nuclear pore complex. J Cell Biol 158(5):915–927
Dreger M, Bengtsson L, Schoneberg T, Otto H, Hucho F (2001) Nuclear envelope proteomics: novel integral membrane proteins of the inner nuclear membrane. Proc Natl Acad Sci U S A 98(21):11943–11948
Rout MP, Aitchison JD, Suprapto A, Hjertaas K, Zhao Y, Chait BT (2000) The yeast nuclear pore complex: composition, architecture, and transport mechanism. J Cell Biol 148(4):635–651
Schirmer EC, Florens L, Guan T, Yates JR 3rd, Gerace L (2003) Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science 301(5638):1380–1382
Korfali N, Wilkie GS, Swanson SK, Srsen V, Batrakou DG, Fairley EA, Malik P, Zuleger N, Goncharevich A, de Las Heras J, Kelly DA, Kerr AR, Florens L, Schirmer EC (2010) The leukocyte nuclear envelope proteome varies with cell activation and contains novel transmembrane proteins that affect genome architecture. Mol Cell Proteomics 9(12):2571–2585, doi:M110.002915 [pii]10.1074/mcp.M110.002915
Korfali N, Wilkie GS, Swanson SK, Srsen V, de Las Heras J, Batrakou DG, Malik P, Zuleger N, Kerr AR, Florens L, Schirmer EC (2012) The nuclear envelope proteome differs notably between tissues. Nucleus 3(6):552–564, doi:22257 [pii]10.4161/nucl.22257
Wilkie GS, Korfali N, Swanson SK, Malik P, Srsen V, Batrakou DG, de Las Heras J, Zuleger N, Kerr AR, Florens L, Schirmer EC (2011) Several novel nuclear envelope transmembrane proteins identified in skeletal muscle have cytoskeletal associations. Mol Cell Proteomics 10(1):M110.003129, doi:M110.003129 [pii]10.1074/mcp.M110.003129
Zuleger N, Boyle S, Kelly DA, de Las Heras JI, Lazou V, Korfali N, Batrakou DG, Randles KN, Morris GE, Harrison DJ, Bickmore WA, Schirmer EC (2013) Specific nuclear envelope transmembrane proteins can promote the location of chromosomes to and from the nuclear periphery. Genome Biol 14(2):R14, doi:gb-2013-14-2-r14 [pii]10.1186/gb-2013-14-2-r14
Fawcett DW (1981) The cell. Saunders, Philadelphia
Paddy MR, Belmont AS, Saumweber H, Agard DA, Sedat JW (1990) Interphase nuclear envelope lamins form a discontinuous network that interacts with only a fraction of the chromatin in the nuclear periphery. Cell 62(1):89–106
Belmont AS, Zhai Y, Thilenius A (1993) Lamin B distribution and association with peripheral chromatin revealed by optical sectioning and electron microscopy tomography. J Cell Biol 123(6 Pt 2):1671–1685
Bouvier D, Hubert J, Seve AP, Bouteille M (1985) Characterization of lamina-bound chromatin in the nuclear shell isolated from HeLa cells. Exp Cell Res 156(2):500–512
Mirsky AE, Allfrey V (1960) Biochemical activities of the cell nucleus. Dis Nerv Syst 21(2)Suppl:23–28
Hirschhorn R, Decsy MI, Troll W (1971) The effect of PHA stimulation of human peripheral blood lymphocytes upon cellular content of euchromatin and heterochromatin. Cell Immunol 2(6):696–701
Kind J, Pagie L, Ortabozkoyun H, Boyle S, de Vries SS, Janssen H, Amendola M, Nolen LD, Bickmore WA, van Steensel B (2013) Single-cell dynamics of genome-nuclear lamina interactions. Cell 153(1):178–192, doi:S0092-8674(13)00217-1 [pii]10.1016/j.cell.2013.02.028
Minc E, Allory Y, Worman HJ, Courvalin JC, Buendia B (1999) Localization and phosphorylation of HP1 proteins during the cell cycle in mammalian cells. Chromosoma 108(4):220–234, doi:91080220.412 [pii]
Rabl C (1885) Über Zelltheilung. Morphol Jahrb 10:214–330
Cowan CR, Carlton PM, Cande WZ (2001) The polar arrangement of telomeres in interphase and meiosis. Rabl organization and the bouquet. Plant Physiol 125(2):532–538
Bass HW, Marshall WF, Sedat JW, Agard DA, Cande WZ (1997) Telomeres cluster de novo before the initiation of synapsis: a three-dimensional spatial analysis of telomere positions before and during meiotic prophase. J Cell Biol 137(1):5–18
Scherthan H, Weich S, Schwegler H, Heyting C, Harle M, Cremer T (1996) Centromere and telomere movements during early meiotic prophase of mouse and man are associated with the onset of chromosome pairing. J Cell Biol 134(5):1109–1125
Aquiles Sanchez J, Karni RJ, Wangh LJ (1997) Fluorescent in situ hybridization (FISH) analysis of the relationship between chromosome location and nuclear morphology in human neutrophils. Chromosoma 106(3):168–177
Galy V, Olivo-Marin JC, Scherthan H, Doye V, Rascalou N, Nehrbass U (2000) Nuclear pore complexes in the organization of silent telomeric chromatin. Nature 403(6765):108–112
Scherthan H, Jerratsch M, Li B, Smith S, Hulten M, Lock T, de Lange T (2000) Mammalian meiotic telomeres: protein composition and redistribution in relation to nuclear pores. Mol Biol Cell 11(12):4189–4203
Schmitt J, Benavente R, Hodzic D, Hoog C, Stewart CL, Alsheimer M (2007) Transmembrane protein Sun2 is involved in tethering mammalian meiotic telomeres to the nuclear envelope. Proc Natl Acad Sci U S A 104(18):7426–7431
Bupp JM, Martin AE, Stensrud ES, Jaspersen SL (2007) Telomere anchoring at the nuclear periphery requires the budding yeast Sad1-UNC-84 domain protein Mps3. J Cell Biol 179(5):845–854, doi:jcb.200706040 [pii]10.1083/jcb.200706040
Chikashige Y, Tsutsumi C, Yamane M, Okamasa K, Haraguchi T, Hiraoka Y (2006) Meiotic proteins bqt1 and bqt2 tether telomeres to form the bouquet arrangement of chromosomes. Cell 125(1):59–69, doi:S0092-8674(06)00318-7 [pii]10.1016/j.cell.2006.01.048
Conrad MN, Lee CY, Wilkerson JL, Dresser ME (2007) MPS3 mediates meiotic bouquet formation in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 104(21):8863–8868
Hou H, Zhou Z, Wang Y, Wang J, Kallgren SP, Kurchuk T, Miller EA, Chang F, Jia S (2012) Csi1 links centromeres to the nuclear envelope for centromere clustering. J Cell Biol 199(5):735–744, doi:jcb.201208001 [pii]10.1083/jcb.201208001
Baricheva EA, Berrios M, Bogachev SS, Borisevich IV, Lapik ER, Sharakhov IV, Stuurman N, Fisher PA (1996) DNA from Drosophila melanogaster beta-heterochromatin binds specifically to nuclear lamins in vitro and the nuclear envelope in situ. Gene 171(2):171–176
Shoeman RL, Traub P (1990) The in vitro DNA-binding properties of purified nuclear lamin proteins and vimentin. J Biol Chem 265(16):9055–9061
Boveri T (1909) Die blastomerenkerne von Ascaris megalocephala und die Theorie der Chromosomen-indiviüalitat. Arch Zellforsch 3:181–268
Croft JA, Bridger JM, Boyle S, Perry P, Teague P, Bickmore WA (1999) Differences in the localization and morphology of chromosomes in the human nucleus. J Cell Biol 145(6):1119–1131
Bickmore WA, Teague P (2002) Influences of chromosome size, gene density and nuclear position on the frequency of constitutional translocations in the human population. Chromosome Res 10(8):707–715
Cremer M, Kupper K, Wagler B, Wizelman L, von Hase J, Weiland Y, Kreja L, Diebold J, Speicher MR, Cremer T (2003) Inheritance of gene density-related higher order chromatin arrangements in normal and tumor cell nuclei. J Cell Biol 162(5):809–820, doi:10.1083/jcb.200304096jcb.200304096 [pii]
Meaburn KJ, Newbold RF, Bridger JM (2008) Positioning of human chromosomes in murine cell hybrids according to synteny. Chromosoma 117(6):579–591
Kim SH, McQueen PG, Lichtman MK, Shevach EM, Parada LA, Misteli T (2004) Spatial genome organization during T-cell differentiation. Cytogenet Genome Res 105(2–4):292–301
Parada LA, McQueen PG, Misteli T (2004) Tissue-specific spatial organization of genomes. Genome Biol 5(7):R44
Parada LA, McQueen PG, Munson PJ, Misteli T (2002) Conservation of relative chromosome positioning in normal and cancer cells. Curr Biol 12(19):1692–1697
Solovei I, Kreysing M, Lanctot C, Kosem S, Peichl L, Cremer T, Guck J, Joffe B (2009) Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell 137(2):356–368, doi:S0092-8674(09)00137-8 [pii]10.1016/j.cell.2009.01.052
Marshall WF, Dernburg AF, Harmon B, Agard DA, Sedat JW (1996) Specific interactions of chromatin with the nuclear envelope: positional determination within the nucleus in Drosophila melanogaster. Mol Biol Cell 7(5):825–842
Kosak ST, Skok JA, Medina KL, Riblet R, Le Beau MM, Fisher AG, Singh H (2002) Subnuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science 296(5565):158–162
Williams RR, Azuara V, Perry P, Sauer S, Dvorkina M, Jorgensen H, Roix J, McQueen P, Misteli T, Merkenschlager M, Fisher AG (2006) Neural induction promotes large-scale chromatin reorganisation of the Mash1 locus. J Cell Sci 119(Pt 1):132–140
Szczerbal I, Foster HA, Bridger JM (2009) The spatial repositioning of adipogenesis genes is correlated with their expression status in a porcine mesenchymal stem cell adipogenesis model system. Chromosoma 118(5):647–663. doi:10.1007/s00412-009-0225-5
Zink D, Amaral MD, Englmann A, Lang S, Clarke LA, Rudolph C, Alt F, Luther K, Braz C, Sadoni N, Rosenecker J, Schindelhauer D (2004) Transcription-dependent spatial arrangements of CFTR and adjacent genes in human cell nuclei. J Cell Biol 166(6):815–825
Nielsen JA, Hudson LD, Armstrong RC (2002) Nuclear organization in differentiating oligodendrocytes. J Cell Sci 115(Pt 21):4071–4079
Hewitt SL, High FA, Reiner SL, Fisher AG, Merkenschlager M (2004) Nuclear repositioning marks the selective exclusion of lineage-inappropriate transcription factor loci during T helper cell differentiation. Eur J Immunol 34(12):3604–3613
Park PC, De Boni U (1998) A specific conformation of the territory of chromosome 17 locates ERBB-2 sequences to a DNase-hypersensitive domain at the nuclear periphery. Chromosoma 107(2):87–95
Johnson C, Primorac D, McKinstry M, McNeil J, Rowe D, Lawrence JB (2000) Tracking COL1A1 RNA in osteogenesis imperfecta. splice-defective transcripts initiate transport from the gene but are retained within the SC35 domain. J Cell Biol 150(3):417–432
Morey C, Da Silva NR, Kmita M, Duboule D, Bickmore WA (2008) Ectopic nuclear reorganisation driven by a Hoxb1 transgene transposed into Hoxd. J Cell Sci 121(Pt 5):571–577, doi:jcs.023234 [pii]10.1242/jcs.023234
Albiez H, Cremer M, Tiberi C, Vecchio L, Schermelleh L, Dittrich S, Kupper K, Joffe B, Thormeyer T, von Hase J, Yang S, Rohr K, Leonhardt H, Solovei I, Cremer C, Fakan S, Cremer T (2006) Chromatin domains and the interchromatin compartment form structurally defined and functionally interacting nuclear networks. Chromosome Res 14(7):707–733
Morey C, Da Silva NR, Perry P, Bickmore WA (2007) Nuclear reorganisation and chromatin decondensation are conserved, but distinct, mechanisms linked to Hox gene activation. Development 134(5):909–919. doi:10.1242/dev.02779
Misteli T (2007) Beyond the sequence: cellular organization of genome function. Cell 128(4):787–800. doi:10.1016/j.cell.2007.01.028
Finlan LE, Sproul D, Thomson I, Boyle S, Kerr E, Perry P, Ylstra B, Chubb JR, Bickmore WA (2008) Recruitment to the nuclear periphery can alter expression of genes in human cells. PLoS Genet 4(3):e1000039
Kumaran RI, Spector DL (2008) A genetic locus targeted to the nuclear periphery in living cells maintains its transcriptional competence. J Cell Biol 180(1):51–65
Reddy KL, Zullo JM, Bertolino E, Singh H (2008) Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature 452(7184):243–247
Clowney EJ, LeGros MA, Mosley CP, Clowney FG, Markenskoff-Papadimitriou EC, Myllys M, Barnea G, Larabell CA, Lomvardas S (2012) Nuclear aggregation of olfactory receptor genes governs their monogenic expression. Cell 151(4):724–737, doi:S0092-8674(12)01286-X [pii]10.1016/j.cell.2012.09.043
Schirmer EC, Gerace L (2004) The stability of the nuclear lamina polymer changes with the composition of lamin subtypes according to their individual binding strengths. J Biol Chem 279(41):42811–42817
Hoger TH, Krohne G, Kleinschmidt JA (1991) Interaction of Xenopus lamins A and LII with chromatin in vitro mediated by a sequence element in the carboxyterminal domain. Exp Cell Res 197(2):280–289
Taniura H, Glass C, Gerace L (1995) A chromatin binding site in the tail domain of nuclear lamins that interacts with core histones. J Cell Biol 131(1):33–44
Goldberg M, Harel A, Brandeis M, Rechsteiner T, Richmond TJ, Weiss AM, Gruenbaum Y (1999) The tail domain of lamin Dm0 binds histones H2A and H2B. Proc Natl Acad Sci U S A 96(6):2852–2857
Luderus ME, den Blaauwen JL, de Smit OJ, Compton DA, van Driel R (1994) Binding of matrix attachment regions to lamin polymers involves single-stranded regions and the minor groove. Mol Cell Biol 14(9):6297–6305
Luderus ME, de Graaf A, Mattia E, den Blaauwen JL, Grande MA, de Jong L, van Driel R (1992) Binding of matrix attachment regions to lamin B1. Cell 70(6):949–959
Rzepecki R, Bogachev SS, Kokoza E, Stuurman N, Fisher PA (1998) In vivo association of lamins with nucleic acids in Drosophila melanogaster. J Cell Sci 111(Pt 1):121–129
Haraguchi T, Koujin T, Hayakawa T, Kaneda T, Tsutsumi C, Imamoto N, Akazawa C, Sukegawa J, Yoneda Y, Hiraoka Y (2000) Live fluorescence imaging reveals early recruitment of emerin, LBR, RanBP2, and Nup153 to reforming functional nuclear envelopes. J Cell Sci 113(Pt 5):779–794
Polioudaki H, Kourmouli N, Drosou V, Bakou A, Theodoropoulos PA, Singh PB, Giannakouros T, Georgatos SD (2001) Histones H3/H4 form a tight complex with the inner nuclear membrane protein LBR and heterochromatin protein 1. EMBO Rep 2(10):920–925
Lachner M, O’Carroll D, Rea S, Mechtler K, Jenuwein T (2001) Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410(6824):116–120. doi:10.1038/35065132
Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, Kouzarides T (2001) Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410(6824):120–124. doi:10.1038/35065138
Foisner R, Gerace L (1993) Integral membrane proteins of the nuclear envelope interact with lamins and chromosomes, and binding is modulated by mitotic phosphorylation. Cell 73(7):1267–1279
Furukawa K (1999) LAP2 binding protein 1 (L2BP1/BAF) is a candidate mediator of LAP2-chromatin interaction. J Cell Sci 112(Pt 15):2485–2492
Cai M, Huang Y, Ghirlando R, Wilson KL, Craigie R, Clore GM (2001) Solution structure of the constant region of nuclear envelope protein LAP2 reveals two LEM-domain structures: one binds BAF and the other binds DNA. EMBO J 20(16):4399–4407
Zheng R, Ghirlando R, Lee MS, Mizuuchi K, Krause M, Craigie R (2000) Barrier-to-autointegration factor (BAF) bridges DNA in a discrete, higher-order nucleoprotein complex. Proc Natl Acad Sci U S A 97(16):8997–9002
Montes de Oca R, Lee KK, Wilson KL (2005) Binding of barrier to autointegration factor (BAF) to histone H3 and selected linker histones including H1.1. J Biol Chem 280(51):42252–42262
Caputo S, Couprie J, Duband-Goulet I, Konde E, Lin F, Braud S, Gondry M, Gilquin B, Worman HJ, Zinn-Justin S (2006) The carboxyl-terminal nucleoplasmic region of MAN1 exhibits a DNA binding winged helix domain. J Biol Chem 281(26):18208–18215
Gerasimova TI, Byrd K, Corces VG (2000) A chromatin insulator determines the nuclear localization of DNA. Mol Cell 6(5):1025–1035
Sun FL, Elgin SC (1999) Putting boundaries on silence. Cell 99(5):459–462
Ramos E, Ghosh D, Baxter E, Corces VG (2006) Genomic organization of gypsy chromatin insulators in Drosophila melanogaster. Genetics 172(4):2337–2349. doi:10.1534/genetics.105.054742
Yang J, Corces VG (2012) Insulators, long-range interactions, and genome function. Curr Opin Genet Dev 22(2):86–92. doi:10.1016/j.gde.2011.12.007
Bell AC, West AG, Felsenfeld G (1999) The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell 98(3):387–396
Loukinov DI, Pugacheva E, Vatolin S, Pack SD, Moon H, Chernukhin I, Mannan P, Larsson E, Kanduri C, Vostrov AA, Cui H, Niemitz EL, Rasko JE, Docquier FM, Kistler M, Breen JJ, Zhuang Z, Quitschke WW, Renkawitz R, Klenova EM, Feinberg AP, Ohlsson R, Morse HC 3rd, Lobanenkov VV (2002) BORIS, a novel male germ-line-specific protein associated with epigenetic reprogramming events, shares the same 11-zinc-finger domain with CTCF, the insulator protein involved in reading imprinting marks in the soma. Proc Natl Acad Sci U S A 99(10):6806–6811. doi:10.1073/pnas.092123699
Handoko L, Xu H, Li G, Ngan CY, Chew E, Schnapp M, Lee CW, Ye C, Ping JL, Mulawadi F, Wong E, Sheng J, Zhang Y, Poh T, Chan CS, Kunarso G, Shahab A, Bourque G, Cacheux-Rataboul V, Sung WK, Ruan Y, Wei CL (2011) CTCF-mediated functional chromatin interactome in pluripotent cells. Nat Genet 43(7):630–638. doi:10.1038/ng.857
Pickersgill H, Kalverda B, de Wit E, Talhout W, Fornerod M, van Steensel B (2006) Characterization of the Drosophila melanogaster genome at the nuclear lamina. Nat Genet 38(9):1005–1014
Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, Eussen BH, de Klein A, Wessels L, de Laat W, van Steensel B (2008) Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 453(7197):948–951, doi:nature06947 [pii]10.1038/nature06947
Kind J, van Steensel B (2010) Genome-nuclear lamina interactions and gene regulation. Curr Opin Cell Biol 22(3):320–325, doi:S0955-0674(10)00054-2 [pii]10.1016/j.ceb.2010.04.002
Peric-Hupkes D, Meuleman W, Pagie L, Bruggeman SW, Solovei I, Brugman W, Graf S, Flicek P, Kerkhoven RM, van Lohuizen M, Reinders M, Wessels L, van Steensel B (2010) Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol Cell 38(4):603–613, doi:S1097-2765(10)00321-7 [pii]10.1016/j.molcel.2010.03.016
Lienert F, Mohn F, Tiwari VK, Baubec T, Roloff TC, Gaidatzis D, Stadler MB, Schubeler D (2011) Genomic prevalence of heterochromatic H3K9me2 and transcription do not discriminate pluripotent from terminally differentiated cells. PLoS Genet 7(6):e1002090. doi:10.1371/journal.pgen.1002090
Wen B, Wu H, Shinkai Y, Irizarry RA, Feinberg AP (2009) Large histone H3 lysine 9 dimethylated chromatin blocks distinguish differentiated from embryonic stem cells. Nat Genet 41(2):246–250. doi:10.1038/ng.297
Lee KK, Haraguchi T, Lee RS, Koujin T, Hiraoka Y, Wilson KL (2001) Distinct functional domains in emerin bind lamin A and DNA-bridging protein BAF. J Cell Sci 114(Pt 24):4567–4573
Mansharamani M, Wilson KL (2005) Direct binding of nuclear membrane protein MAN1 to emerin in vitro and two modes of binding to barrier-to-autointegration factor. J Biol Chem 280(14):13863–13870
Montes de Oca R, Shoemaker CJ, Gucek M, Cole RN, Wilson KL (2009) Barrier-to-autointegration factor proteome reveals chromatin-regulatory partners. PLoS One 4(9):e7050. doi:10.1371/journal.pone.0007050
Holaska JM, Wilson KL (2007) An emerin “proteome”: purification of distinct emerin-containing complexes from HeLa cells suggests molecular basis for diverse roles including gene regulation, mRNA splicing, signaling, mechanosensing, and nuclear architecture. Biochemistry 46(30):8897–8908. doi:10.1021/bi602636m
Somech R, Shaklai S, Geller O, Amariglio N, Simon AJ, Rechavi G, Gal-Yam EN (2005) The nuclear-envelope protein and transcriptional repressor LAP2beta interacts with HDAC3 at the nuclear periphery, and induces histone H4 deacetylation. J Cell Sci 118(Pt 17):4017–4025. doi:10.1242/jcs.02521
Dechat T, Gajewski A, Korbei B, Gerlich D, Daigle N, Haraguchi T, Furukawa K, Ellenberg J, Foisner R (2004) LAP2alpha and BAF transiently localize to telomeres and specific regions on chromatin during nuclear assembly. J Cell Sci 117(Pt 25):6117–6128
Suzuki Y, Yang H, Craigie R (2004) LAP2alpha and BAF collaborate to organize the Moloney murine leukemia virus preintegration complex. EMBO J 23(23):4670–4678
Shaklai S, Somech R, Gal-Yam EN, Deshet-Unger N, Moshitch-Moshkovitz S, Hirschberg K, Amariglio N, Simon AJ, Rechavi G (2008) LAP2zeta binds BAF and suppresses LAP2beta-mediated transcriptional repression. Eur J Cell Biol 87(5):267–278
Malhas AN, Lee CF, Vaux DJ (2009) Lamin B1 controls oxidative stress responses via Oct-1. J Cell Biol 184(1):45–55. doi:10.1083/jcb.200804155
Chi YH, Haller K, Peloponese JM Jr, Jeang KT (2007) Histone acetyltransferase hALP and nuclear membrane protein hsSUN1 function in de-condensation of mitotic chromosomes. J Biol Chem 282(37):27447–27458
Jarnik M, Aebi U (1991) Toward a more complete 3-D structure of the nuclear pore complex. J Struct Biol 107(3):291–308
Guan T, Kehlenbach RH, Schirmer EC, Kehlenbach A, Fan F, Clurman BE, Arnheim N, Gerace L (2000) Nup50, a nucleoplasmically oriented nucleoporin with a role in nuclear protein export. Mol Cell Biol 20(15):5619–5630
Schermelleh L, Carlton PM, Haase S, Shao L, Winoto L, Kner P, Burke B, Cardoso MC, Agard DA, Gustafsson MG, Leonhardt H, Sedat JW (2008) Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science 320(5881):1332–1336. doi:10.1126/science.1156947
Laroche T, Martin SG, Gotta M, Gorham HC, Pryde FE, Louis EJ, Gasser SM (1998) Mutation of yeast Ku genes disrupts the subnuclear organization of telomeres. Curr Biol 8(11):653–656
Maillet L, Gaden F, Brevet V, Fourel G, Martin SG, Dubrana K, Gasser SM, Gilson E (2001) Ku-deficient yeast strains exhibit alternative states of silencing competence. EMBO Rep 2(3):203–210
Feuerbach F, Galy V, Trelles-Sticken E, Fromont-Racine M, Jacquier A, Gilson E, Olivo-Marin JC, Scherthan H, Nehrbass U (2002) Nuclear architecture and spatial positioning help establish transcriptional states of telomeres in yeast. Nat Cell Biol 4(3):214–221
Gartenberg MR, Neumann FR, Laroche T, Blaszczyk M, Gasser SM (2004) Sir-mediated repression can occur independently of chromosomal and subnuclear contexts. Cell 119(7):955–967
Akhtar A, Gasser SM (2007) The nuclear envelope and transcriptional control. Nat Rev Genet 8(7):507–517
Vaquerizas JM, Suyama R, Kind J, Miura K, Luscombe NM, Akhtar A (2010) Nuclear pore proteins nup153 and megator define transcriptionally active regions in the Drosophila genome. PLoS Genet 6(2):e1000846. doi:10.1371/journal.pgen.1000846
Capelson M, Liang Y, Schulte R, Mair W, Wagner U, Hetzer MW (2010) Chromatin-bound nuclear pore components regulate gene expression in higher eukaryotes. Cell 140(3):372–383, doi:S0092-8674(09)01681-X [pii]10.1016/j.cell.2009.12.054
Kalverda B, Pickersgill H, Shloma VV, Fornerod M (2010) Nucleoporins directly stimulate expression of developmental and cell-cycle genes inside the nucleoplasm. Cell 140(3):360–371, doi:S0092-8674(10)00012-7 [pii]10.1016/j.cell.2010.01.011
Luthra R, Kerr SC, Harreman MT, Apponi LH, Fasken MB, Ramineni S, Chaurasia S, Valentini SR, Corbett AH (2007) Actively transcribed GAL genes can be physically linked to the nuclear pore by the SAGA chromatin modifying complex. J Biol Chem 282(5):3042–3049. doi:10.1074/jbc.M608741200
Baker SP, Grant PA (2007) The SAGA continues: expanding the cellular role of a transcriptional co-activator complex. Oncogene 26(37):5329–5340. doi:10.1038/sj.onc.1210603
Nagy Z, Tora L (2007) Distinct GCN5/PCAF-containing complexes function as co-activators and are involved in transcription factor and global histone acetylation. Oncogene 26(37):5341–5357. doi:10.1038/sj.onc.1210604
Rodriguez-Navarro S, Fischer T, Luo MJ, Antunez O, Brettschneider S, Lechner J, Perez-Ortin JE, Reed R, Hurt E (2004) Sus1, a functional component of the SAGA histone acetylase complex and the nuclear pore-associated mRNA export machinery. Cell 116(1):75–86
Cabal GG, Genovesio A, Rodriguez-Navarro S, Zimmer C, Gadal O, Lesne A, Buc H, Feuerbach-Fournier F, Olivo-Marin JC, Hurt EC, Nehrbass U (2006) SAGA interacting factors confine sub-diffusion of transcribed genes to the nuclear envelope. Nature 441(7094):770–773. doi:10.1038/nature04752
Pascual-Garcia P, Govind CK, Queralt E, Cuenca-Bono B, Llopis A, Chavez S, Hinnebusch AG, Rodriguez-Navarro S (2008) Sus1 is recruited to coding regions and functions during transcription elongation in association with SAGA and TREX2. Genes Dev 22(20):2811–2822. doi:10.1101/gad.483308
Klockner C, Schneider M, Lutz S, Jani D, Kressler D, Stewart M, Hurt E, Kohler A (2009) Mutational uncoupling of the role of Sus1 in nuclear pore complex targeting of an mRNA export complex and histone H2B deubiquitination. J Biol Chem 284(18):12049–12056. doi:10.1074/jbc.M900502200
Rodriguez-Navarro S (2009) Insights into SAGA function during gene expression. EMBO Rep 10(8):843–850. doi:10.1038/embor.2009.168
Kurshakova MM, Krasnov AN, Kopytova DV, Shidlovskii YV, Nikolenko JV, Nabirochkina EN, Spehner D, Schultz P, Tora L, Georgieva SG (2007) SAGA and a novel Drosophila export complex anchor efficient transcription and mRNA export to NPC. EMBO J 26(24):4956–4965. doi:10.1038/sj.emboj.7601901
Kurshakova M, Maksimenko O, Golovnin A, Pulina M, Georgieva S, Georgiev P, Krasnov A (2007) Evolutionarily conserved E(y)2/Sus1 protein is essential for the barrier activity of Su(Hw)-dependent insulators in Drosophila. Mol Cell 27(2):332–338. doi:10.1016/j.molcel.2007.05.035
Wickramasinghe VO, McMurtrie PI, Mills AD, Takei Y, Penrhyn-Lowe S, Amagase Y, Main S, Marr J, Stewart M, Laskey RA (2010) mRNA export from mammalian cell nuclei is dependent on GANP. Curr Biol 20(1):25–31. doi:10.1016/j.cub.2009.10.078
Jani D, Lutz S, Marshall NJ, Fischer T, Kohler A, Ellisdon AM, Hurt E, Stewart M (2009) Sus1, Cdc31, and the Sac3 CID region form a conserved interaction platform that promotes nuclear pore association and mRNA export. Mol Cell 33(6):727–737. doi:10.1016/j.molcel.2009.01.033
Casolari JM, Brown CR, Komili S, West J, Hieronymus H, Silver PA (2004) Genome-wide localization of the nuclear transport machinery couples transcriptional status and nuclear organization. Cell 117(4):427–439
Mendjan S, Taipale M, Kind J, Holz H, Gebhardt P, Schelder M, Vermeulen M, Buscaino A, Duncan K, Mueller J, Wilm M, Stunnenberg HG, Saumweber H, Akhtar A (2006) Nuclear pore components are involved in the transcriptional regulation of dosage compensation in Drosophila. Mol Cell 21(6):811–823. doi:10.1016/j.molcel.2006.02.007
Ishii K, Arib G, Lin C, Van Houwe G, Laemmli UK (2002) Chromatin boundaries in budding yeast: the nuclear pore connection. Cell 109(5):551–562
Gerasimova TI, Corces VG (2001) Chromatin insulators and boundaries: effects on transcription and nuclear organization. Annu Rev Genet 35:193–208. doi:10.1146/annurev.genet.35.102401.090349
Oki M, Valenzuela L, Chiba T, Ito T, Kamakaka RT (2004) Barrier proteins remodel and modify chromatin to restrict silenced domains. Mol Cell Biol 24(5):1956–1967
Dilworth DJ, Tackett AJ, Rogers RS, Yi EC, Christmas RH, Smith JJ, Siegel AF, Chait BT, Wozniak RW, Aitchison JD (2005) The mobile nucleoporin Nup2p and chromatin-bound Prp20p function in endogenous NPC-mediated transcriptional control. J Cell Biol 171(6):955–965
Luk E, Ranjan A, Fitzgerald PC, Mizuguchi G, Huang Y, Wei D, Wu C (2010) Stepwise histone replacement by SWR1 requires dual activation with histone H2A.Z and canonical nucleosome. Cell 143(5):725–736. doi:10.1016/j.cell.2010.10.019
Li B, Pattenden SG, Lee D, Gutierrez J, Chen J, Seidel C, Gerton J, Workman JL (2005) Preferential occupancy of histone variant H2AZ at inactive promoters influences local histone modifications and chromatin remodeling. Proc Natl Acad Sci U S A 102(51):18385–18390. doi:10.1073/pnas.0507975102
Jacobson S, Pillus L (2009) The SAGA subunit Ada2 functions in transcriptional silencing. Mol Cell Biol 29(22):6033–6045. doi:10.1128/MCB.00542-09
Holaska JM, Lee K, Kowalski AK, Wilson KL (2003) Transcriptional repressor germ cell-less (GCL) and barrier to autointegration factor (BAF) compete for binding to emerin in vitro. J Biol Chem 278(9):6969–6975
Nili E, Cojocaru GS, Kalma Y, Ginsberg D, Copeland NG, Gilbert DJ, Jenkins NA, Berger R, Shaklai S, Amariglio N, Brok-Simoni F, Simon AJ, Rechavi G (2001) Nuclear membrane protein LAP2beta mediates transcriptional repression alone and together with its binding partner GCL (germ-cell-less). J Cell Sci 114(18):3297–3307
Haraguchi T, Holaska JM, Yamane M, Koujin T, Hashiguchi N, Mori C, Wilson KL, Hiraoka Y (2004) Emerin binding to Btf, a death-promoting transcriptional repressor, is disrupted by a missense mutation that causes Emery-Dreifuss muscular dystrophy. Eur J Biochem 271(5):1035–1045
Ozaki T, Saijo M, Murakami K, Enomoto H, Taya Y, Sakiyama S (1994) Complex formation between lamin A and the retinoblastoma gene product: identification of the domain on lamin A required for its interaction. Oncogene 9(9):2649–2653
Markiewicz E, Dechat T, Foisner R, Quinlan R, Hutchison C (2002) Lamin A/C binding protein LAP2alpha is required for nuclear anchorage of retinoblastoma protein. Mol Biol Cell 13(12):4401–4413
Naetar N, Korbei B, Kozlov S, Kerenyi MA, Dorner D, Kral R, Gotic I, Fuchs P, Cohen TV, Bittner R, Stewart CL, Foisner R (2008) Loss of nucleoplasmic LAP2alpha-lamin A complexes causes erythroid and epidermal progenitor hyperproliferation. Nat Cell Biol 10(11):1341–1348, doi:ncb1793 [pii]10.1038/ncb1793
Gerace L, Burke B (1988) Functional organization of the nuclear envelope. Annu Rev Cell Biol 4:335–374
Ivorra C, Kubicek M, Gonzalez JM, Sanz-Gonzalez SM, Alvarez-Barrientos A, O’Connor JE, Burke B, Andres V (2006) A mechanism of AP-1 suppression through interaction of c-Fos with lamin A/C. Genes Dev 20(3):307–320, doi:20/3/307 [pii]10.1101/gad.349506
Holaska JM, Rais-Bahrami S, Wilson KL (2006) Lmo7 is an emerin-binding protein that regulates the transcription of emerin and many other muscle-relevant genes. Hum Mol Genet 15(23):3459–3472
Osada S, Ohmori SY, Taira M (2003) XMAN1, an inner nuclear membrane protein, antagonizes BMP signaling by interacting with Smad1 in Xenopus embryos. Development 130(9):1783–1794
Pan D, Estevez-Salmeron LD, Stroschein SL, Zhu X, He J, Zhou S, Luo K (2005) The integral inner nuclear membrane protein MAN1 physically interacts with the R-Smad proteins to repress signaling by the transforming growth factor-{beta} superfamily of cytokines. J Biol Chem 280(16):15992–16001
Misteli T (2001) Protein dynamics: implications for nuclear architecture and gene expression. Science 291(5505):843–847
Marshall WF, Straight A, Marko JF, Swedlow J, Dernburg A, Belmont A, Murray AW, Agard DA, Sedat JW (1997) Interphase chromosomes undergo constrained diffusional motion in living cells. Curr Biol 7(12):930–939, doi:S0960-9822(06)00412-X [pii]
Strickfaden H, Zunhammer A, van Koningsbruggen S, Kohler D, Cremer T (2010) 4D chromatin dynamics in cycling cells: Theodor Boveri’s hypotheses revisited. Nucleus 1(3):284–297. doi:10.4161/nucl.1.3.11969
Chubb JR, Boyle S, Perry P, Bickmore WA (2002) Chromatin motion is constrained by association with nuclear compartments in human cells. Curr Biol 12(6):439–445
Zink D, Bornfleth H, Visser A, Cremer C, Cremer T (1999) Organization of early and late replicating DNA in human chromosome territories. Exp Cell Res 247(1):176–188
Janmey PA, Euteneuer U, Traub P, Schliwa M (1991) Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J Cell Biol 113(1):155–160
Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D (2006) Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol 172(1):41–53
Starr DA, Fischer JA (2005) KASH ‘n Karry: the KASH domain family of cargo-specific cytoskeletal adaptor proteins. Bioessays 27(11):1136–1146
Lammerding J, Schulze P, Takahashi T, Kozlov S, Sullivan T, Kamm R, Stewart C, Lee R (2004) Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest 113(3):370–378
Liu J, Ben-Shahar T, Riemer D, Treinin M, Spann P, Weber K, Fire A, Gruenbaum Y (2000) Essential roles for Caenorhabditis elegans lamin gene in nuclear organization, cell cycle progression, and spatial organization of nuclear pore complexes. Mol Biol Cell 11(11):3937–3947
Schirmer EC, Guan T, Gerace L (2001) Involvement of the lamin rod domain in heterotypic lamin interactions important for nuclear organization. J Cell Biol 153(3):479–489
Lenz-Bohme B, Wismar J, Fuchs S, Reifegerste R, Buchner E, Betz H, Schmitt B (1997) Insertional mutation of the Drosophila nuclear lamin Dm0 gene results in defective nuclear envelopes, clustering of nuclear pore complexes, and accumulation of annulate lamellae. J Cell Biol 137(5):1001–1016
Capell BC, Collins FS (2006) Human laminopathies: nuclei gone genetically awry. Nat Rev Genet 7(12):940–952
Foisner R, Aebi U, Bonne G, Gruenbaum Y, Novelli G (2007) 141st ENMC International Workshop inaugural meeting of the EURO-Laminopathies project “Nuclear Envelope-linked Rare Human Diseases: From Molecular Pathophysiology towards Clinical Applications”, 10-12 March 2006, Naarden, The Netherlands. Neuromuscul Disord 17(8):655–660
Worman HJ, Bonne G (2007) “Laminopathies”: a wide spectrum of human diseases. Exp Cell Res 313(10):2121–2133
Bonne G, Di Barletta MR, Varnous S, Becane HM, Hammouda EH, Merlini L, Muntoni F, Greenberg CR, Gary F, Urtizberea JA, Duboc D, Fardeau M, Toniolo D, Schwartz K (1999) Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet 21(3):285–288
Raffaele Di Barletta M, Ricci E, Galluzzi G, Tonali P, Mora M, Morandi L, Romorini A, Voit T, Orstavik KH, Merlini L, Trevisan C, Biancalana V, Housmanowa-Petrusewicz I, Bione S, Ricotti R, Schwartz K, Bonne G, Toniolo D (2000) Different mutations in the LMNA gene cause autosomal dominant and autosomal recessive Emery-Dreifuss muscular dystrophy. Am J Hum Genet 66(4):1407–1412
Muchir A, Bonne G, van der Kooi AJ, van Meegen M, Baas F, Bolhuis PA, de Visser M, Schwartz K (2000) Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B). Hum Mol Genet 9(9):1453–1459
Cao H, Hegele RA (2000) Nuclear lamin A/C R482Q mutation in Canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum Mol Genet 9(1):109–112
Hegele RA, Cao H, Liu DM, Costain GA, Charlton-Menys V, Rodger NW, Durrington PN (2006) Sequencing of the reannotated LMNB2 gene reveals novel mutations in patients with acquired partial lipodystrophy. Am J Hum Genet 79(2):383–389
Shackleton S, Lloyd DJ, Jackson SN, Evans R, Niermeijer MF, Singh BM, Schmidt H, Brabant G, Kumar S, Durrington PN, Gregory S, O’Rahilly S, Trembath RC (2000) LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet 24(2):153–156
De Sandre-Giovannoli A, Chaouch M, Kozlov S, Vallat J, Tazir M, Kassouri N, Szepetowski P, Hammadouche T, Vandenberghe A, Stewart C, Grid D, Levy N (2002) Homozygous defects in LMNA, encoding lamin A/C nuclear-envelope proteins, cause autosomal recessive axonal neuropathy in human (Charcot-Marie-Tooth disorder type 2) and mouse. Am J Hum Genet 70(3):726–736
Padiath QS, Saigoh K, Schiffmann R, Asahara H, Yamada T, Koeppen A, Hogan K, Ptacek LJ, Fu YH (2006) Lamin B1 duplications cause autosomal dominant leukodystrophy. Nat Genet 38(10):1114–1123
Fatkin D, MacRae C, Sasaki T, Wolff MR, Porcu M, Frenneaux M, Atherton J, Vidaillet HJ Jr, Spudich S, De Girolami U, Seidman JG, Seidman C, Muntoni F, Muehle G, Johnson W, McDonough B (1999) Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease [see comments]. N Engl J Med 341(23):1715–1724
Navarro CL, De Sandre-Giovannoli A, Bernard R, Boccaccio I, Boyer A, Genevieve D, Hadj-Rabia S, Gaudy-Marqueste C, Smitt HS, Vabres P, Faivre L, Verloes A, Van Essen T, Flori E, Hennekam R, Beemer FA, Laurent N, Le Merrer M, Cau P, Levy N (2004) Lamin A and ZMPSTE24 (FACE-1) defects cause nuclear disorganization and identify restrictive dermopathy as a lethal neonatal laminopathy. Hum Mol Genet 13(20):2493–2503
Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, Dutra A, Pak E, Durkin S, Csoka AB, Boehnke M, Glover TW, Collins FS (2003) Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423(6937):293–298
De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, Lyonnet S, Stewart C, Munnich A, Le Merrer M, Levy N (2003) Lamin A truncation in Hutchinson-Gilford progeria. Science 300(5628):2055
Bione S, Maestrini E, Rivella S, Mancini M, Regis S, Romeo G, Toniolo D (1994) Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat Genet 8(4):323–327
Taylor MR, Slavov D, Gajewski A, Vlcek S, Ku L, Fain PR, Carniel E, Di Lenarda A, Sinagra G, Boucek MM, Cavanaugh J, Graw SL, Ruegg P, Feiger J, Zhu X, Ferguson DA, Bristow MR, Gotzmann J, Foisner R, Mestroni L (2005) Thymopoietin (lamina-associated polypeptide 2) gene mutation associated with dilated cardiomyopathy. Hum Mutat 26(6):566–574
Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A, Ragnauth CD, Yi Q, Mellad JA, Warren DT, Wheeler MA, Ellis JA, Skepper JN, Vorgerd M, Schlotter-Weigel B, Weissberg PL, Roberts RG, Wehnert M, Shanahan CM (2007) Nesprin-1 and -2 are involved in the pathogenesis of Emery-Dreifuss Muscular Dystrophy and are critical for nuclear envelope integrity. Hum Mol Genet 16(23):2816–2833
Cronshaw JM, Matunis MJ (2003) The nuclear pore complex protein ALADIN is mislocalized in triple A syndrome. Proc Natl Acad Sci U S A 100(10):5823–5827
Hellemans J, Preobrazhenska O, Willaert A, Debeer P, Verdonk PC, Costa T, Janssens K, Menten B, Van Roy N, Vermeulen SJ, Savarirayan R, Van Hul W, Vanhoenacker F, Huylebroeck D, De Paepe A, Naeyaert JM, Vandesompele J, Speleman F, Verschueren K, Coucke PJ, Mortier GR (2004) Loss-of-function mutations in LEMD3 result in osteopoikilosis, Buschke-Ollendorff syndrome and melorheostosis. Nat Genet 36(11):1213–1218
Waterham H, Koster J, Mooyer P, Noort GG, Kelley R, Wilcox W, Wanders R, Hennekam R, Oosterwijk J (2003) Autosomal recessive HEM/Greenberg skeletal dysplasia is caused by 3beta-hydroxysterol delta14-reductase deficiency due to mutations in the lamin B receptor gene. Am J Hum Genet 72(4):1013–1017
Gros-Louis F, Dupre N, Dion P, Fox MA, Laurent S, Verreault S, Sanes JR, Bouchard JP, Rouleau GA (2007) Mutations in SYNE1 lead to a newly discovered form of autosomal recessive cerebellar ataxia. Nat Genet 39(1):80–85
Basel-Vanagaite L, Muncher L, Straussberg R, Pasmanik-Chor M, Yahav M, Rainshtein L, Walsh CA, Magal N, Taub E, Drasinover V, Shalev H, Attia R, Rechavi G, Simon AJ, Shohat M (2006) Mutated nup62 causes autosomal recessive infantile bilateral striatal necrosis. Ann Neurol 60(2):214–222
Gonzalez-Alegre P, Paulson HL (2004) Aberrant cellular behavior of mutant torsinA implicates nuclear envelope dysfunction in DYT1 dystonia. J Neurosci 24(11):2593–2601
Naismith TV, Heuser JE, Breakefield XO, Hanson PI (2004) TorsinA in the nuclear envelope. Proc Natl Acad Sci U S A 101(20):7612–7617
Goodchild RE, Dauer WT (2004) Mislocalization to the nuclear envelope: an effect of the dystonia-causing torsinA mutation. Proc Natl Acad Sci U S A 101(3):847–852
Hoffmann K, Dreger C, Olins A, Olins D, Shultz L, Lucke B, Karl H, Kaps R, Muller D, Vaya A, Aznar J, Ware R, Sotelo Cruz N, Lindner T, Herrmann H, Reis A, Sperling K (2002) Mutations in the gene encoding the lamin B receptor produce an altered nuclear morphology in granulocytes (Pelger-Huet anomaly). Nat Genet 31(4):410–414
Fidzianska A, Toniolo D, Hausmanowa-Petrusewicz I (1998) Ultrastructural abnormality of sarcolemmal nuclei in Emery-Dreifuss muscular dystrophy (EDMD). J Neurol Sci 159(1):88–93
Sewry CA, Brown SC, Mercuri E, Bonne G, Feng L, Camici G, Morris GE, Muntoni F (2001) Skeletal muscle pathology in autosomal dominant Emery-Dreifuss muscular dystrophy with lamin A/C mutations. Neuropathol Appl Neurobiol 27(4):281–290
Maraldi NM, Lattanzi G, Capanni C, Columbaro M, Mattioli E, Sabatelli P, Squarzoni S, Manzoli FA (2006) Laminopathies: a chromatin affair. Adv Enzyme Regul 46:33–49, doi:S0065-2571(06)00002-1 [pii]10.1016/j.advenzreg.2006.01.001
Verga L, Concardi M, Pilotto A, Bellini O, Pasotti M, Repetto A, Tavazzi L, Arbustini E (2003) Loss of lamin A/C expression revealed by immuno-electron microscopy in dilated cardiomyopathy with atrioventricular block caused by LMNA gene defects. Virchows Arch 443(5):664–671
Goldman RD, Shumaker DK, Erdos MR, Eriksson M, Goldman AE, Gordon LB, Gruenbaum Y, Khuon S, Mendez M, Varga R, Collins FS (2004) Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A 101(24):8963–8968
Taimen P, Pfleghaar K, Shimi T, Moller D, Ben-Harush K, Erdos MR, Adam SA, Herrmann H, Medalia O, Collins FS, Goldman AE, Goldman RD (2009) A progeria mutation reveals functions for lamin A in nuclear assembly, architecture, and chromosome organization. Proc Natl Acad Sci U S A 106(49):20788–20793, doi:0911895106 [pii]10.1073/pnas.0911895106
Meaburn KJ, Cabuy E, Bonne G, Levy N, Morris GE, Novelli G, Kill IR, Bridger JM (2007) Primary laminopathy fibroblasts display altered genome organization and apoptosis. Aging Cell 6(2):139–153
Mewborn SK, Puckelwartz MJ, Abuisneineh F, Fahrenbach JP, Zhang Y, MacLeod H, Dellefave L, Pytel P, Selig S, Labno CM, Reddy K, Singh H, McNally E (2010) Altered chromosomal positioning, compaction, and gene expression with a lamin A/C gene mutation. PLoS One 5(12):e14342. doi:10.1371/journal.pone.0014342
Shumaker DK, Dechat T, Kohlmaier A, Adam SA, Bozovsky MR, Erdos MR, Eriksson M, Goldman AE, Khuon S, Collins FS, Jenuwein T, Goldman RD (2006) Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc Natl Acad Sci U S A 103(23):8703–8708
Scaffidi P, Misteli T (2006) Lamin A-dependent nuclear defects in human aging. Science 312(5776):1059–1063
Lattanzi G, Columbaro M, Mattioli E, Cenni V, Camozzi D, Wehnert M, Santi S, Riccio M, Del Coco R, Maraldi NM, Squarzoni S, Foisner R, Capanni C (2007) Pre-Lamin A processing is linked to heterochromatin organization. J Cell Biochem 102(5):1149–1159
Columbaro M, Capanni C, Mattioli E, Novelli G, Parnaik VK, Squarzoni S, Maraldi NM, Lattanzi G (2005) Rescue of heterochromatin organization in Hutchinson-Gilford progeria by drug treatment. Cell Mol Life Sci 62(22):2669–2678
Capo-chichi CD, Cai KQ, Smedberg J, Ganjei-Azar P, Godwin AK, Xu XX (2011) Loss of A-type lamin expression compromises nuclear envelope integrity in breast cancer. Chin J Cancer 30(6):415–425
Willis ND, Cox TR, Rahman-Casans SF, Smits K, Przyborski SA, van den Brandt P, van Engeland M, Weijenberg M, Wilson RG, de Bruine A, Hutchison CJ (2008) Lamin A/C is a risk biomarker in colorectal cancer. PLoS One 3(8):e2988. doi:10.1371/journal.pone.0002988
Skvortsov S, Schafer G, Stasyk T, Fuchsberger C, Bonn GK, Bartsch G, Klocker H, Huber LA (2011) Proteomics profiling of microdissected low- and high-grade prostate tumors identifies Lamin A as a discriminatory biomarker. J Proteome Res 10(1):259–268. doi:10.1021/pr100921j
Chow KH, Factor RE, Ullman KS (2012) The nuclear envelope environment and its cancer connections. Nat Rev Cancer 12(3):196–209. doi:10.1038/nrc3219
Baylin SB, Jones PA (2011) A decade of exploring the cancer epigenome—biological and translational implications. Nat Rev Cancer 11(10):726–734. doi:10.1038/nrc3130
Berman BP, Weisenberger DJ, Aman JF, Hinoue T, Ramjan Z, Liu Y, Noushmehr H, Lange CP, van Dijk CM, Tollenaar RA, Van Den Berg D, Laird PW (2012) Regions of focal DNA hypermethylation and long-range hypomethylation in colorectal cancer coincide with nuclear lamina-associated domains. Nat Genet 44(1):40–46. doi:10.1038/ng.969
Romana SP, Radford-Weiss I, Ben Abdelali R, Schluth C, Petit A, Dastugue N, Talmant P, Bilhou-Nabera C, Mugneret F, Lafage-Pochitaloff M, Mozziconacci MJ, Andrieu J, Lai JL, Terre C, Rack K, Cornillet-Lefebvre P, Luquet I, Nadal N, Nguyen-Khac F, Perot C, Van den Akker J, Fert-Ferrer S, Cabrol C, Charrin C, Tigaud I, Poirel H, Vekemans M, Bernard OA, Berger R (2006) NUP98 rearrangements in hematopoietic malignancies: a study of the Groupe Francophone de Cytogenetique Hematologique. Leukemia 20(4):696–706. doi:10.1038/sj.leu.2404130
Nakamura T, Largaespada DA, Lee MP, Johnson LA, Ohyashiki K, Toyama K, Chen SJ, Willman CL, Chen IM, Feinberg AP, Jenkins NA, Copeland NG, Shaughnessy JD Jr (1996) Fusion of the nucleoporin gene NUP98 to HOXA9 by the chromosome translocation t(7;11)(p15;p15) in human myeloid leukaemia. Nat Genet 12(2):154–158. doi:10.1038/ng0296-154
Kasper LH, Brindle PK, Schnabel CA, Pritchard CE, Cleary ML, van Deursen JM (1999) CREB binding protein interacts with nucleoporin-specific FG repeats that activate transcription and mediate NUP98-HOXA9 oncogenicity. Mol Cell Biol 19(1):764–776
Wang GG, Cai L, Pasillas MP, Kamps MP (2007) NUP98-NSD1 links H3K36 methylation to Hox-A gene activation and leukaemogenesis. Nat Cell Biol 9(7):804–812. doi:10.1038/ncb1608
Wang GG, Song J, Wang Z, Dormann HL, Casadio F, Li H, Luo JL, Patel DJ, Allis CD (2009) Haematopoietic malignancies caused by dysregulation of a chromatin-binding PHD finger. Nature 459(7248):847–851. doi:10.1038/nature08036
Wang GG, Allis CD (2009) “Misinterpretation” of a histone mark is linked to aberrant stem cells and cancer development. Cell Cycle 8(13):1982–1983
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Stancheva, I., Schirmer, E.C. (2014). Nuclear Envelope: Connecting Structural Genome Organization to Regulation of Gene Expression. In: Schirmer, E., de las Heras, J. (eds) Cancer Biology and the Nuclear Envelope. Advances in Experimental Medicine and Biology, vol 773. Springer, New York, NY. https://doi.org/10.1007/978-1-4899-8032-8_10
Download citation
DOI: https://doi.org/10.1007/978-1-4899-8032-8_10
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4899-8031-1
Online ISBN: 978-1-4899-8032-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)