Abstract
Pharmacotherapy in children often consists of oral medication. Effectiveness of oral prescriptions may be influenced by extrinsic (formulation, nutrition, and co-medication) and intrinsic factors (physiological and disease-related variation).
During development the GI characteristics change: swallowing reflexes, excretion of digestive enzymes, intestinal motility, transit time and intestinal transporters and drug metabolizing enzymes. For example, changes in drug efflux transporters result in a decrease or increase in expelling drugs back into the intestinal lumen and thereby in variation in oral bioavailability.
Closing the main information gaps on the ontogeny of GI processes governing oral drug absorption would allow for more accurate prediction of the oral disposition of drugs in children of all ages. Different ex- and in vitro study designs, as drug dissolution/solubility tests, in vitro drug metabolism and transporter studies and in vivo drug-microdosing can be used to elucidate the age-related changes in GI processes to better understand oral drug disposition in children. Using these data in PB-PK models may further guide individualized pediatric drug therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
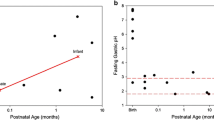
Pharmacotherapy in children will usually consist of oral medication [1]. Absorption in the gastro-intestinal (GI) tract—and thereby effectiveness of oral medication—may be influenced by drug formulation, food intake, co-medication, and physiological factors. The oral route is characterized by changing environments from the oral cavity with saliva to the GI tract with interplay of digestive enzymes, intestinal motility, transit time and, moreover, intestinal transporters and drug metabolizing enzymes on the cellular level [2]. In children, the interaction between the oral drug and the developmental continuum will influence the systemic exposure to and effectiveness of the medication. Few studies are available on changes in bioavailability and other oral absorption parameters in the pediatric age range, a selection of which is presented in Fig. 3.1.
1.1 Swallowing Reflex
Swallowing is a multifactorial mechanism transporting food or liquid from the oral cavity to the esophagus. Swallowing involves coordination of neurologic and aerodigestive systems from the oral cavity to the esophagus.
Quantitative measures such as time at which a (feeding) bolus reaches a specific location and time for a bolus to move from one place to another during a swallow may serve to determine if the process is successful [3]. Besides the voluntary component of swallowing, three different reflexes are also involved in the process; the pharyngeal swallowing reflex, the pharyngo-upper esophageal sphincter contractile reflex, and the pharyngoglottal closure reflex. Reflexive swallowing is crucial to airway protection, as it prevents food or fluid from directly entering the trachea [4], or returning from the esophagus to the trachea. Moreover, all reflexes have a distinct reaction on air and water stimuli [4, 5].
The first swallowing activity appears at around 11 weeks gestation, and further matures over time. Non-nutritive swallowing is well patterned by 34 weeks postmenstrual age. The suction and expression/compression component of sucking in preterm infants can be assessed on a five-point scale [6]. Recently an objective indicator of infants’ feeding ability has been developed assessing feeding skills and endurance [7] as an expression of proficiency (number of ml taken during the first 5 min/total ml prescribed × 100) and milk transfer (ml/min).
Overall transfer (percentage of total volume transferred/volume to be taken) and rate of transfer (ml/min) of the feeding bolus is positively correlated with increased gestational age. So, although preterm babies could have developed similar oral motor skills as term babies, term babies can drink faster and more [8].
An important part of oral feeding is pharyngeal and upper esophageal sphincter function but this is not easy to assess. Compared with older neonates who are adapted to feeding, most preterm infants demonstrate poor pharyngeal pressures at the laryngeal inlet coupled with poor coordination of the pharyngeal propulsion with upper esophageal sphincter relaxation [9].
Research does not indicate that growth of the oropharyngeal cavity during childhood may influence swallowing [9], although the position of the feeding bolus in relationship to the laryngeal closure changes from the age of 2 to 48 months [10]. Furthermore, with increasing age or initiation of cup feeding, laryngeal vestibule closure occurs later in the swallowing process.
To our knowledge, salivary content has never been studied in relation to development. Two studies have addressed salivary flow rates in young fasting children and found that the mean salivary flow went up from 28.2 to 39.6 ml/min between 18 and 42 months of age, these changes are not statistically significant [11, 12].
On a more molecular level, recent studies on the salivary transcriptome revealed up-regulation of specific genes involved in the different aspects of oral feeding in newborns. The genes in question are involved in the sensory development, neurodevelopment, cartilage and bone development, cranial nerve development, feeding behavior, and muscular development [13].
Oral medication intake (tablets, capsules, liquids) is also influenced by the maturation state of normal feeding, unless liquid formulations are given via a naso-gastric tube. Although liquid formulations overcome the problem of difficulty of swallowing solids, in children who are not fully accustomed to oral feeds, oral suspensions may also present a problem.
Moreover, children’s taste sensation can negatively affect liquid ingestion. Sweet tastes are innately preferred by most or all herbivores and omnivores, presumably since sweetness reflects the presence of caloric sugars in plants. Bitter taste signals the presence of potentially toxic compounds and hence substances that are bitter are generally disliked and avoided. Neonates and infants therefore react adversely to bitter taste. A sensitivity to and preference for salty substances also appears to have an innate component, which develops at around 4 months of age. By 2 years of age, children’s preferences for salty foods are even greater than those of adults [14]. These preferences in children should be accounted for by masking the taste of drugs, either by formulation or by dissolving the drug in a suitable vehicle. The latter is often challenged by the increased volume of the bottle feeding. However, this problem is not restricted to the (preterm) infant. Also older children and even adolescents (age between 11 and 20 years) often have difficulty swallowing tablets and capsules [15]. In a Danish study 8.5 % of the documented oral medication had been interrupted [16]. This problem might be explained by the smaller dimensions of the puerile pharynx and the developing oropharyngeal musculature [17] and by lack of experience in swallowing drugs. Children can be trained in swallowing oral medication, it was shown that training was successful from 3 years of age [18, 19].
Moreover, adapting formulations from a tablet or capsule to a liquid formulation may negatively influence drug efficacy [1]. Tablets and capsules cannot be divided multiple times without losing dosing accuracy. Regrettably, pediatric formulations for many drugs are lacking, and children often receive unlicensed and off-label drugs of which safety and efficacy are unknown. It is important, therefore, to speed up the licensing of drugs for children. Recently, much research effort was spent on pediatric medicines formulation with a view to facilitating easier oral drug administration. Especially the development of micro-particulate solid dose formulations and mini-tablets seems promising [20, 21] as it may overcome some of the inherent stability issues of oral liquid formulations.
1.2 pH
Gastric acid has a main role in food digestion and is an important barrier in gastro-intestinal defense. Gastric pH is usually measured by intermittent gastric fluids aspirates or by continuous intragastric pH monitoring (24 h).
In all age ranges gastric pH is strongly acidic. Only in newborn children just seconds after delivery the mean is around 7, but dropping to 2–3 a few hours after birth [22, 23]. This phenomenon is explained by the swallowing of amniotic fluid during birth [22]. Other studies have subsequently shown that the gastric pH remains low at values between 2 and 3 in children of all ages [24–40].
In continuous measurement of gastric pH we usually consider the proportion of time in 24 h that values above 4 occur. In preterm infants this proportion ranged from 46 to 70 % [41, 42]. The age of 2 years seems to be a turning point as this proportion decreased from 51 in children under the age of 2 years to 34 % in older children [43]. This effect is most likely caused by the buffering capacity of the formula or breast milk with which the younger children are fed, seeing that the older children receive more solids [37, 38, 43]. The influence of the buffering capacity of feeding was also seen in preterm neonates, as the postprandial pH of 7 dropped to 2 within 3 h after feeding [37, 38]. The buffering capacity of milk feeding is often believed to produce a non-acidic gastric environment in neonates. This is true to some extent; however, gastric pH reaches low adult values in between the feeds.
Little is known about the maturation of intestinal pH. One small study using a radio-transmitting pH-sensitive capsule showed an increase from 6.4 to 7.4 from the duodenum to the distal part of the small intestine in children aged between 8 and 14 years. The pH profile was almost identical to that found in healthy adults [44].
Different pH values can induce different absorption profiles to orally given drugs. For example, the antifungal drug ketoconazole is less effectively absorbed in preterm infants, most likely because the gastric fluid is not acidic during drug absorption [45].
1.3 pH Changes in GI tract
A drug’s chemical nature determines the effect of prolonged periods of elevated gastric pH on its absorption. Weakly basic drugs such as ketoconazole [45] may be absorbed less well, but weakly acidic drugs somewhat better as they are more soluble at higher pH values. In addition, drugs that are unstable at acidic pH may be absorbed better at higher gastric pH values [46].
The effect of changes in gastric pH is probably most evident with drugs from Biopharmaceutical Classification System (BCS) classes II (low solubility, extensive metabolism) and IV (low solubility, low metabolism) [47]. It may be difficult to predict the effect of an age-related change in gastric pH (due to buffering effect of frequent oral feeds in neonates) on oral drug absorption as it is the result of interplay between changing feeding regimens and other developmental changes in oral drug absorption.
1.4 Gastric Emptying
The duration of a drug’s exposure to a highly acidic environment is determined by the gastric emptying time.
Various techniques are available to establish gastric emptying time, including the gastric emptying breath test, scintigraphic procedure by Technetium-99M liquid gastric emptying scan, and the paracetamol absorption test. The results are expressed in various ways: gastric emptying time, gastric half-emptying time, or residual gastric activity at 1 h.
Gastric emptying time is influenced by stomach content. The l-glycine-1-13C breath test performed in four healthy children (age range 12.1–16.0 years) showed different gastric half-emptying times after fructose or glucose intake (45.5 and 64.3 min, respectively). After ingestion of both sugars the gastric half-emptying time increased significantly to 85.3 min [48].
A meal of solids can increase the gastric half-emptying time even more as shown by a 13C-octanoic acid breath test in nine healthy control patients (mean age 9 years; age range 4–16 years)after eating a meal of bread, ham, juice, and eggs [49]. The mean gastric half-emptying time was 121 min.
The influence of GI reflux on gastric emptying time was evaluated with the 13C-octanoic acid breath test in 22 patients, mean age 13.2 year, with symptoms of gastroesophageal- or duodenogastroesophageal reflux. Surprisingly, gastric emptying time did not significantly differ from that in healthy controls [50]. Celiac disease, which affects the small bowel mucosa villi, was associated with a longer gastric emptying time, but the effect disappeared after initiating a gluten free diet [49]. The authors speculate that mucosal inflammation of the duodenum, with impaired smooth muscle contraction or neurotransmitter release, leads to motor abnormalities in the stomach-duodenal passage and thus longer emptying time. There are no data on gastric emptying in very young children established with breath tests.
Scintigraphic imaging can help establish gastric emptying time, gastric half-emptying time, and residual gastric activity.
This method showed a gastric half-emptying time of 60 min in preterm infants (median gestational age 28.9 weeks; range 26–33) at a median postnatal age of 9 days [51]. They were fed hourly although not using a standard meal size. The residual gastric activity at 1 h was 37.5 % (range 19–100 %) [51].
The influence of GERD (based on pH and/or scintigraphic imaging) on gastric emptying was studied in 477 patients aged 0–18 years [52]. In children without bolus or acidic gastroesophageal reflux, gastric residual activity at 1 h declined with increasing age: 65, 51, and 45 % in the age group up to 3 years, 4–6 years, and over 6 years, respectively. Gastric emptying was significantly delayed only in those children over 6 years suffering from reflux, these results were in line with findings from another study in children suffering from GERD; residual gastric activity was 52 % in 44 infants up to 23 months of age, and 49 % in eight children up to 14 years of age [53]. In yet another study, residual gastric activity in a healthy control group of 11 children with a mean age of 5.6 years was almost similar at 43.3 % [54].
Yahav et al. [55] reported a mean gastric emptying time of 87.8 min in a control group with a mean age 10.4 months. Gastric emptying times in healthy adult controls ranged between 56 (32–85) and 104 (49–126) min, for liquid and solid markers, respectively [56, 57].
In the paracetamol absorption test a pharmacological tracer is applied for measuring the rate of gastric emptying. This technique has been rarely applied in children, therefore age-related changes in outcomes are not known. A small study in 15 critically ill but food-tolerant children (median age 5.3 years) showed a median 1.5 (interquartile range 0.7–2.2) ratio of time to reach paracetamol peak to the maximum paracetamol concentration (T max/C max) [58]. The influence of diet was shown in adolescent participants, as the paracetamol absorption ratio was 1.4 for high-fat meals and 0.5 for low-fat meals [59].
Viewed from a different angle, a population pharmacokinetic study in newly born children showed a low oral paracetamol absorption rate in the first days of life, which then increased and stabilized after 1 week [60]. Gastric emptying time seems to influence paracetamol absorption as a lag time, which is the time to reach and permeate the absorbing surface of the intestine [2], only occurs after oral paracetamol administration and not after rectal administration. This suggests that gastric emptying time may be the primary determinant of a lag time for oral absorption of paracetamol.
A recent meta-analysis of gastric emptying data from 49 studies covering 1991 healthy subjects ranging from preterm birth to adulthood showed that postnatal and gestational age were not significant covariates for changes in gastric emptying. The only significant influence was meal type; gastric emptying was faster in the order: breast milk > formula milk > semi-solid meal > solid meal [61]. A separate analysis of the data did not reveal a significant relationship between volume of feeds and gastric emptying time.
1.5 Antroduodenal Contractions
The rate of gastric emptying is determined by an orchestrated combination of antroduodenal motor activity, fundic contraction, pyloric sphincter relaxation, and intestinal motor activity. Contraction and relaxation of the distal stomach and proximal small intestine can be measured by antroduodenal manometry.
Both in the fasting and intraduodenally fed state, antral motor activity does not differ between preterm and term neonates [62]. Yet, in preterm neonates the proportion of antral clusters upon duodenal activity is much lower than that in term neonates. With increasing gestational age not only the degree of association of antral and duodenal activity [63] rises but also the effectiveness of the contractions on motility [64, 65].
Duodenal cluster activity during fasting lasted shorter in preterm neonates than in term infants but duodenal motor activity in response to feeding increased similarly in both preterm and term infants [62]. Maturation of the duodenal activity is dependent on the timing of the introduction of oral food, as maturation was more pronounced after early (days 3–4) rather than after late (days 10–14) introduction [66]. Moreover, duodenal motor activity response to bolus feeding shows a more immature pattern in preterm infants compared to term infants [67].
In contrast to antral motor activity, proximal intestinal (duodenal) motor activity matures throughout the first weeks of life, with increasing frequency, amplitude, and duration of propagating contractions. No data are available on children beyond the neonatal period.
1.6 Intestinal Transit Time
The effectiveness of gastro-intestinal motility is reflected by the orocecal transit time (OCTT), which can be measured with the hydrogen breath test, 13C-ureide breath test, radio-transmitting capsule, red carmine marker test, or scintigraphy. However, each of these techniques has its limitations.
The hydrogen breath test with lactulose as non-absorbable carbohydrate substrate has limited use in the general population, which may include hydrogen-non-responders. Moreover, lactulose may accelerate transit time by its osmotic laxative effect, this was clearly shown in healthy subjects: OCTT after a meal was significantly longer than after lactulose [68]. Studies in the pediatric population using the hydrogen breath test to measure OCTT did not reveal an association with age [68–72]. The transit time differs from 60 to 110 min in children from 1 to 17 years of age, which is the same as in adults [72].
OCTT measured with the lactose-13C-ureide breath test had a mean of 255 min in children from 3 to 17 years of age [69]. In adults the lactose-13C-ureide test was validated against scintigraphy [73]. This test is unsuitable in infants below 6 months because they lack the required enzymatic activity to convert lactose ureide. The significantly longer OCTT with the labeled ureide test than the lactulose-H2 breath test is thought to be caused by the laxative effect of lactulose [74].
In healthy children aged 4–14 years, intestinal transit times have been established using a radio-transmitting capsule, the mean values were 7.5 and 17.2 h in the small bowel and colon, respectively [44]. From the number of observations it was estimated that the capsule resided in the duodenum for 8 %, in the proximal part for 5 %, the mid part for 12 %, and the distal part for 75 % of the small intestinal passage time. The small intestinal transit time of 7.5 h established with this method is considerably longer than that established by the breath tests. The fact that the capsule, which was larger than 2 mm, moved through the distal part of the terminal ileum for three quarters of the time suggests a longer ileo-cecal transit for large particles.
Lastly, scintigraphy performed in premature neonates (gestational age 29 weeks) showed a mean OCTT of 3.1 h [51].
All techniques show a wide range of intestinal transit times with no clear age-association. Differences in reported transit times seem more or less related to the specific test properties.
Gastric emptying and intestinal transit time are the primary determinants of the rate at which drugs are presented to and dispersed along the mucosal surface of the small intestine. This rate is further influenced by intestinal disease. The time to reach maximal plasma levels of an orally absorbed drug could therefore be prolonged in the very young sick child.
1.7 Bile Salts and Pancreatic Enzymes
Bile, secreted from the liver, aids the digestion and absorption of lipids by the intestine.
A study in preterm neonates established intestinal bile concentration in the first few weeks postnatally at 4.55 mmol/l, for small- and appropriate-for-gestational-age neonates alike [75]. Another study showed that type of feeding influenced bile acid concentration, as it was higher in breast-fed infants than in formula-fed infants, but this difference is not statistically significant [76]. Measurements at 2, 7, and 10 days to 7 months postnatally made clear that the total bile acid concentration increases during the first few days to months, reaching comparable adult levels between 10 days and 7 months of age [77]. Digestive enzymes secreted by the exocrine pancreas aid in the digestion of nutrients, his digestive function is measured by the fecal Elastase-1 concentration, which is highly specific for the pancreas and is not degraded during the intestinal passage.
The fecal Elastase-1 concentration was abnormal in all of a group of preterm infants for the first 2 days after birth; while concentrations were normal in 43 % of a group of term infants. This discrepancy may be due to immaturity or insufficiency of the exocrine pancreatic function in premature neonates. Other than this there are no age-related changes in fecal Elastase-1 concentrations [78]. In both preterm and term neonates adult levels of fecal Elastase had been reached after 2 weeks [79].
The body’s ability to solubilize and absorb lipophilic drugs is influenced by the effectiveness of the biliary function. Immature conjugation, decreased intestinal–hepatic-loop, and transport defects of bile salts into the intestinal lumen may reduce uptake of fat-soluble vitamins and lipophilic drugs.
1.7.1 Drug “First Pass” Metabolism in the Intestine
High levels of drug transporters such as multidrug resistance protein 1 (MDR1/MRP1/P-glycoprotein) in the villus tips on the apical side of small bowel enterocytes, along with CYP3A4 within the cells, form a concerted “first pass” defense mechanism limiting the oral bioavailability of drugs, dietary mycotoxins and other xenobiotics [80].
1.8 Development of Intestinal Transporters
Intestinal transporters are quite important to oral drug availability. Drug efflux transporters expelling drugs back into the intestinal lumen may reduce their availability. MDR1 is one of the most important efflux transporters [81]. Found in the brush border of the small intestine, this glycoprotein is genetically controlled by the ABCB1 gene [82].
MDR1 ontogeny can be described by mRNA expression and protein content (total and glycosylated) and localization in the gut wall can be determined by immunohistochemistry. In duodenal biopsies from children aged from 1 month to 17 years, MDR1 mRNA expression was highly variable and not related to age [83, 84]. This observation was backed up Konieczna et al. [85] who investigated the differential expression of ABC transporters MDR1, MRP1, and BCRP in the intestinal epithelium of developing human embryos. Expression of all three transporters had reached adult levels after 12 weeks of intrauterine development. In contrast, Miki et al. showed an age relationship: mRNA expression was low in fetuses and neonates (14–20 weeks, 1–24 days post delivery) but generally higher in the adult group (15–38 years) [86]. Van Kalken et al. failed to detect MDR1 expression in the intestine until 28 weeks gestational age [87]. Immunohistochemistry found the MDR1 protein on the apical surface of all enterocytes. In children younger than 3 years, MDR1 was also found on a small upper part of the lateral surface [88].
Variant alleles will often lower the activity of transporters. However, the effect of various alleles for MDR1 on activity of transporters on specific substrates is not always clear cut.
Pediatric post-renal transplant patients (age 0.36–16.3 years) carrying the ABCB1 c.1236C > T or c.2677G > T variant allele showed higher oral bioavailability and lower pre-hepatic extraction ratios of the MDR1 and CYP3A4 substrate cyclosporine than did over 8-year-old non-carriers [89]. There is some evidence linking genotype of MDR1 with CYP3A4 mRNA expression, suggesting it is a compounded result of altered MDR1 and CYP3A4 activity [90].
Moreover, local or systemic inflammation may influence intestinal transporter activity. MDR1 mRNA expression in non-inflamed duodenal biopsies of children with Crohn’s disease was significantly higher than that in normal biopsies [91]. The authors speculate that the discrepancy is due to the systemic inflammation present in Crohn’s disease. Other studies, however, have shown down-regulation of drug transporter expression in inflammatory states [92].
Little is known about the postnatal development of the other members of the ATP binding cassette transporters found in the small bowel, such as multidrug resistance protein 2 (MRP2/ABCC2) or breast cancer resistance protein (BCRP/ABCG2) [81, 93–95].
1.9 Development of Intestinal Metabolism
Our understanding of the ontogeny of intestinal metabolism is far from complete. The 3A (CYP3A) subfamily of cytochrome P450 is probably most studied. This enzyme subfamily is abundantly expressed in the gut and is involved in the first pass metabolism of numerous orally administered drugs in adults [96].
The ontogeny of CYP3A can be described as changes in mRNA expression, protein expression, or activity level. A striking discrepancy is seen between intestinal CYP3A mRNA and protein expression, which may reflect the influence of a post-transcriptional regulatory mechanism. CYP3A protein expression increases with age [97], whereas CYP3A4 and CYP3A5 mRNA expressions are high in the first year of life and then drop to adult levels [88].
Immunohistochemistry in intestinal biopsies showed that CYP3A protein was present in only half of the enterocytes in children younger than 6 months. In the older children (up to 17 years of age) CYP3A protein was expressed in all enterocytes [88]. This suggests that CYP3A ontogeny is determined by the proportion of enterocytes expressing the enzyme rather than by a gradual turning on of enzyme expression in individual enterocytes. Further study should confirm this assumption as the manner of specimen collection, storage and pre-treatment to immunohistochemistry may have been of influence. Dissociation between protein and mRNA levels during maturation has also been reported for hepatic CYP2D6 [98].
The age-related increase in CYP3A protein levels is mirrored with increasing CYP3A4 activity, which can be measured by the degree of formation of 6beta-hydroxytestosterone from testosterone. This method did not detect CYP3A activity in fetal samples [97] butneonates showed much lower CYP3A activity compared to children older than 5 years [97].
Both mean intestinal CYP3A4 and CYP3A5 mRNA expression did not differ between young (age 0.1–15 years) and adult liver transplant recipients [81]. This finding suggests that intestinal CYP3A mRNA expression does not change beyond childhood. The authors did not study the effect of age within the pediatric cohort. However, these data suggest no age-related changes in CYP3A mRNA expression, although this cannot be excluded [83].
The influence of the CYP3A5 gene polymorphism has been studied in the transplant population. For children and adults alike, CYP3A5*1 gene carriers express higher levels of intestinal CYP3A5 mRNA levels than do CYP3A5*3 homozygous patients. In CYP3A5*1 gene carriers, CYP3A5 mRNA accounted for 20–30 % of all CYP3A mRNA detected [83, 84].
Pediatric clinical trials on the oral bioavailability of CYP3A substrates are rare. Midazolam is a validated probe drug for CYP3A4/5 activity. In agreement with its age-related intestinal expression, the oral bioavailability of Midazolam in preterm infants (28–32 weeks, <10 days of age) is significantly higher than in adults (50 versus 30 %) [99, 100].
Of great clinical interest is evidence that type of feeding (breast milk or formula) seems to impact the developmental pattern of combined intestinal and hepatic CYP3A in neonates. CYP3A4 activity, expressed as the urinary metabolite/dextromethorphan ratio, increased in between two weeks and 6 months of age, but the repeated measurements showed that this increase was faster for formula- versus breast milk-fed children [101].
It is important to reiterate that intestinal MDR1 and CYP3A appear to work in concert to potentially limit oral drug bioavailability [90]. Hence, age-associated variation in intestinal MDR1/CYP3A4 activity may differentially impact substrates depending on their affinity for MDR1 and/or CYP3A4.
Increases in MDR1/P-glycoprotein causes increased efflux and therefore a decrease in substrate uptake. Consequently a lesser amount of intraepithelial drug is presented to the metabolizing enzyme. Decreased efflux can consequently cause greater risk on drug toxicity. However, as the MDR-1 substrate subsequently is presented to the liver the same efflux mechanism can have different consequences.
Increased CYP3A4 expression and activity with age, consequently causes higher oral bioavailability of, e.g. midazolam in premature children compared to adults.
1.10 Challenges in Research
To gain more insight in the ontogeny of oral drug absorption we will need to use a study design in which specific factors that are subject to change can be elucidated. After all, oral drug absorption is influenced by the interplay of age, genetic, and disease-related changes and co-medication, in addition to ethnicity and gender.
Nevertheless, the limited number of patients and reluctant willingness of parents and patients to cooperate is a challenge for pediatric studies. Moreover, breath tests are not feasible in all age groups and radioactivity of probe medication in the developing child raises ethical concerns, as does the invasive nature of tissue harvesting.
1.11 Research Options
1.11.1 GI Tract Model
To gain more insight in physiological influences on oral substrates, different parameters could be tested in an in vitro drug dissolution and solubility model. The Dutch Institute of Innovative Research has developed the TNO Gastro-Intestinal Tract model (TIM), a computer-controlled dynamic system which mimics the physiological human conditions in stomach and intestines [102, 103]. This system allows researchers to measure possible changes in the effective dose of the drug presented to the intestinal mucosa.
1.11.2 Modeling and Simulation: PB-PK Models and Population PK
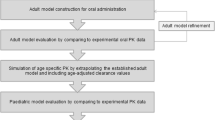
The available data on age-related changes in relevant GI processes can be incorporated into population-based pharmacokinetics (PB-PK) software programs such as Simcyp® or PKsim®. These programs can then simulate the fate of drugs given to children of different ages and provide guidance for age-appropriate dosing. Modeling pediatric drug absorption by this approach still has a long way to go, however, it becomes more feasible as more research data becomes available and will eventually enable the prediction of oral absorption rate and bioavailability in children. However, in the meantime such a modeling approach can be used in terms of “what if” scenarios to investigate the effects of changing parameters on the prediction of absorption parameters.
The usefulness of these programs is limited by the lack of data on changing physiological and biochemical parameters across the pediatric age range. The current data availability is shown in Fig. 3.2, which also indicates areas that require further research including intestinal transporter ontogeny, intestinal fluid dynamics, and characteristics of the intestinal unstirred boundary layer. Moreover, validation of the models is still challenging as pharmacokinetic data on neonates and infants are scarce [104]. Opportunistic sampling and PK analysis in leftover blood drawn for clinical purposes from all patients receiving medication could provide more data. And then, more pediatric population pharmacokinetics (POPPK) studies involving oral drugs are needed, aimed at quantifying drug absorption parameters across the age spectrum rather than using fixed values for oral bioavailability (F) and absorption rate constant (ka).
1.11.3 In Vitro Drug Metabolism and Transporter Studies
The ontogeny of drug metabolizing enzymes and transporters can be studied in intestinal samples from children of all ages. Methods like RT-PCR for drug transporter expression (mRNA) and sensitive LC–MS–MS to measure protein content are used more widespread. The disconnect between drug transporter mRNA and activity should be considered by researchers especially where the solute carriers are involved, e.g. OATP1B1. Leftover tissue from surgical procedures should be collected consistently over a long time to provide enough samples for research purposes.
1.11.4 Microdosing
Ontogeny of drug absorption can also be addressed by a mechanism-based approach [105], e.g. studying one specific drug which represents a specific (intestinal) drug metabolizing enzyme. Pharmacokinetic studies in children of all ages may provide valuable information on the ontogeny of that specific pathway. For example, the plasma clearance of midazolam is a validated and widely used method to study variation in CYP3A4/5 activity in both adults and children [106].
Full PK studies to determine oral bioavailability for a probe drug using a multi-day cross-over design are hardly feasible in children for ethical and practical reasons. Children will not benefit from the drug but rather experience the drug effect and run the risk of adverse events.
Alternatively a stable-labeled isotope or a (very weak) radioactive-labeled microdose can be used [107, 108]. Both stable and radioactive approaches make it possible to administer a labeled probe drug in addition to an intravenous therapeutic dose. Parent compound and metabolites can therefore be traced in serum and urine. This enables simultaneous determination of the pharmacokinetics of therapeutic IV and the labeled oral dose. It eliminates the risk of therapeutic effect and toxicity. A prerequisite for the use of microdosing in this context, however, is dose-linearity across the dosing range.
1.12 Concluding Remarks
Closing the main information gaps on the ontogeny of GI processes governing oral drug absorption would allow for more accurate prediction of the oral disposition of drugs in children of all ages. Suggested approaches, both in vitro and in vivo, could provide more understanding of oral drug absorption in children. Clinical trials on the influence of age on drug absorption and thereby effectiveness are indispensable to formulate age-dependent drug dosing protocols.
References
Schirm E et al (2003) Lack of appropriate formulations of medicines for children in the community. Acta Paediatr 92(12):1486–1489
Atkinson AJ et al (2007) Drug absorption and bioavailability, in principles of clinical pharmacology. Academic, Burlington, pp 37–58
Kim Y, McCullough GH, Asp CW (2005) Temporal measurements of pharyngeal swallowing in normal populations. Dysphagia 20(4):290–296
Dua K et al (2011) Pharyngeal airway protective reflexes are triggered before the maximum volume of fluid that the hypopharynx can safely hold is exceeded. Am J Physiol Gastrointest Liver Physiol 301(2):G197–G202
Jadcherla SR et al (2007) Pharyngeal swallowing: defining pharyngeal and upper esophageal sphincter relationships in human neonates. J Pediatr 151(6):597–603
Amaizu N et al (2008) Maturation of oral feeding skills in preterm infants. Acta Paediatr 97(1):61–67
Lau C, Smith EO (2011) A novel approach to assess oral feeding skills of preterm infants. Neonatology 100(1):64–70
Lau C et al (2000) Characterization of the developmental stages of sucking in preterm infants during bottle feeding. Acta Paediatr 89(7):846–852
Rommel N et al (2003) Development of the orohypopharyngeal cavity in normal infants and young children. Cleft Palate Craniofac J 40(6):606–611
Weckmueller J, Easterling C, Arvedson J (2011) Preliminary temporal measurement analysis of normal oropharyngeal swallowing in infants and young children. Dysphagia 26(2):135–143
Dezan CC et al (2002) Flow rate, amylase activity, and protein and sialic acid concentrations of saliva from children aged 18, 30 and 42 months attending a baby clinic. Arch Oral Biol 47(6):423–427
Siqueira WL et al (2007) Salivary parameters in infants aged 12 to 60 months with Down syndrome. Spec Care Dentist 27(5):202–205
Maron JL (2012) Insights into neonatal oral feeding through the salivary transcriptome. Int J Pediatr 2012:195153
Beauchamp GK, Mennella JA (2011) Flavor perception in human infants: development and functional significance. Digestion 83(Suppl 1):1–6
Hansen DL, Tulinius D, Hansen EH (2008) Adolescents’ struggles with swallowing tablets: barriers, strategies and learning. Pharm World Sci 30(1):65–69
Steffensen GK, Pachai A, Pedersen SE (1998) Peroral medicinsk behandling af born—er der problemer? [Peroral drug administration to children—are there any problems?]. Ugeskr Laeger 160(15):2249–2252
Derkay CS, Schechter GL (1998) Anatomy and physiology of pediatric swallowing disorders. Otolaryngol Clin North Am 31(3):397–404
Garvie PA, Lensing S, Rai SN (2007) Efficacy of a pill-swallowing training intervention to improve antiretroviral medication adherence in pediatric patients with HIV/AIDS. Pediatrics 119(4):e893–e899
Czyzewski I et al (2000) Teaching and maintaining pill swallowing in HIV-infected children. AIDS Read 10(2):88–94
Spomer N et al (2012) Acceptance of uncoated mini-tablets in young children: results from a prospective exploratory cross-over study. Arch Dis Child 97(3):283–286
Zajicek A et al (2013) A report from the pediatric formulations task force: perspectives on the state of child-friendly oral dosage forms. AAPS J 15(4):1072–1081
Datta S, Houle GL, Fox GS (1975) Concentration of lidocaine hydrochloride in newborn gastric fluid after elective caesarean section and vaginal delivery with epidural analgesia. Can Anaesth Soc J 22(1):79–83
Whetstine LJ et al (1995) Supplemental oxygen and gastric pH in unfed preterm infants. South Med J 88(4):458–461
Splinter WM, Schaefer JD (1990) Unlimited clear fluid ingestion two hours before surgery in children does not affect volume or pH of stomach contents. Anaesth Intensive Care 18(4):522–526
Splinter WM, Stewart JA, Muir JG (1990) Large volumes of apple juice preoperatively do not affect gastric pH and volume in children. Can J Anaesth 37(1):36–39
Nishina K et al (1994) Omeprazole reduces preoperative gastric fluid acidity and volume in children. Can J Anaesth 41(10):925–929
Cote CJ et al (1982) Assessment of risk factors related to the acid aspiration syndrome in pediatric patients-gastric ph and residual volume. Anesthesiology 56(1):70–72
Oderda G et al (2002) Measurement of postprandial changes in urine acid output to detect changes of gastric acid secretion after proton pump inhibitors in children. Dig Dis Sci 47(8):1843–1849
Kraus G et al (1990) Famotidine. Pharmacokinetic properties and suppression of acid secretion in paediatric patients following cardiac surgery. Clin Pharmacokinet 18(1):77–81
Jahr JS et al (1991) Effects of famotidine on gastric pH and residual volume in pediatric surgery. Acta Anaesthesiol Scand 35(5):457–460
Maekawa N et al (1993) Effects of 2-, 4- and 12-hour fasting intervals on preoperative gastric fluid pH and volume, and plasma glucose and lipid homeostasis in children. Acta Anaesthesiol Scand 37(8):783–787
Goresky GV et al (1992) Efficacy, duration, and absorption of a paediatric oral liquid preparation of ranitidine hydrochloride. Can J Anaesth 39(8):791–798
Yildiz F et al (1984) Reduction of gastric acid secretion. The efficacy of pre-anaesthetic oral cimetidine in children. Anaesthesia 39(4):314–318
Gupta L et al (2011) Long-term follow-up of patients with esophageal replacement by reversed gastric tube. Eur J Pediatr Surg 21(2):88–93
Miller BR, Tharp JA, Issacs WB (1990) Gastric residual volume in infants and children following a 3-hour fast. J Clin Anesth 2(5):301–305
Rogers IM et al (1975) Plasma gastrin in congenitial hypertrophic pyloric stenosis. A hypothesis disproved. Arch Dis Child 50(6):467–471
Smith LJ, Kaminsky S, D'Souza SW (1986) Neonatal fat digestion and lingual lipase. Acta Paediatr Scand 75(6):913–918
Omari TI, Davidson GP (2003) Multipoint measurement of intragastric pH in healthy preterm infants. Arch Dis Child Fetal Neonatal Ed 88(6):F517–F520
Kelly EJ et al (1993) Gastric acid secretion in preterm infants. Early Hum Dev 35(3):215–220
Kelly EJ et al (1993) The effect of intravenous ranitidine on the intragastric pH of preterm infants receiving dexamethasone. Arch Dis Child 69(1 Spec No):37–39
Lopez-Alonso M et al (2006) Twenty-four-hour esophageal impedance-pH monitoring in healthy preterm neonates: rate and characteristics of acid, weakly acidic, and weakly alkaline gastroesophageal reflux. Pediatrics 118(2):e299–e308
Omari TI et al (2007) Effect of omeprazole on acid gastroesophageal reflux and gastric acidity in preterm infants with pathological acid reflux. J Pediatr Gastroenterol Nutr 44(1):41–44
Demir H et al (2005) Does simultaneous gastric and esophageal pH monitoring increase the diagnosis of gastroesophageal reflux disease? Turk J Pediatr 47(1):14–16
Fallingborg J et al (1990) Measurement of gastrointestinal pH and regional transit times in normal children. J Pediatr Gastroenterol Nutr 11(2):211–214
van den Anker JN et al (1993) Insufficient ketoconazole concentrations in preterm infants with fungal infections. Eur J Pediatr 152(6):538
Budha NR et al (2012) Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH-dependent solubility the Achilles heel of targeted therapy? Clin Pharmacol Ther 92(2):203–213
Chen ML et al (2011) The BCS, BDDCS, and regulatory guidances. Pharm Res 28(7):1774–1778
Hoekstra JH et al (1996) Evaluation of 13CO2 breath tests for the detection of fructose malabsorption. J Lab Clin Med 127(3):303–309
Perri F et al (2000) Gastric emptying of solids is delayed in celiac disease and normalizes after gluten withdrawal. Acta Paediatr 89(8):921–925
Hoffman I et al (2007) Duodenogastro-esophageal reflux in children with refractory gastro-esophageal reflux disease. J Pediatr 151(3):307–311
Bode S, Dreyer M, Greisen G (2004) Gastric emptying and small intestinal transit time in preterm infants: a scintigraphic method. J Pediatr Gastroenterol Nutr 39(4):378–382
Di Lorenzo C et al (1987) Gastric emptying with gastro-oesophageal reflux. Arch Dis Child 62(5):449–453
Seibert JJ, Byrne WJ, Euler AR (1983) Gastric emptying in children: unusual patterns detected by scintigraphy. AJR Am J Roentgenol 141(1):49–51
Miele E et al (2000) Persistence of abnormal gastrointestinal motility after operation for Hirschsprung's disease. Am J Gastroenterol 95(5):1226–1230
Yahav J et al (1985) Assessment of intestinal and cardiorespiratory function in children with congenital heart disease on high-caloric formulas. J Pediatr Gastroenterol Nutr 4(5):778–785
Graff J, Brinch K, Madsen JL (2000) Simplified scintigraphic methods for measuring gastrointestinal transit times. Clin Physiol 20(4):262–266
Bennink R et al (1999) Evaluation of small-bowel transit for solid and liquid test meal in healthy men and women. Eur J Nucl Med 26(12):1560–1566
Mayer AP et al (2002) Amylin is associated with delayed gastric emptying in critically ill children. Intensive Care Med 28(3):336–340
Lodefalk M, Aman J, Bang P (2008) Effects of fat supplementation on glycaemic response and gastric emptying in adolescents with Type 1 diabetes. Diabet Med 25(9):1030–1035
Anderson BJ et al (2002) Acetaminophen developmental pharmacokinetics in premature neonates and infants: a pooled population analysis. Anesthesiology 96(6):1336–1345
Vijjah P, Bonner J et al (2013) A meta-analysis of the effects of age and meal type on gastric emptying in premature neonates through adults. Abstract presented at the ESDPPP meeting in Sapzburg
Berseth CL, Ittmann PI (1992) Antral and duodenal motor responses to duodenal feeding in preterm and term infants. J Pediatr Gastroenterol Nutr 14(2):182–186
Ittmann PI, Amarnath R, Berseth CL (1992) Maturation of antroduodenal motor activity in preterm and term infants. Dig Dis Sci 37(1):14–19
Morriss FH Jr et al (1986) Ontogenic development of gastrointestinal motility: IV. Duodenal contractions in preterm infants. Pediatrics 78(6):1106–1113
Bisset WM et al (1988) Measurement of small-intestinal motor activity in the preterm infant. J Biomed Eng 10(2):155–158
Berseth CL et al (1992) Responses of gastrointestinal peptides and motor activity to milk and water feedings in preterm and term infants. Pediatr Res 31(6):587–590
al Tawil Y, Berseth CL (1996) Gestational and postnatal maturation of duodenal motor responses to intragastric feeding. J Pediatr 129(3):374–381
Vajro P et al (1988) Orocoecal transit time in healthy and constipated children. Acta Paediatr Scand 77(4):583–586
Van Den Driessche M et al (2000) Lactose-[13C]ureide breath test: a new, noninvasive technique to determine orocecal transit time in children. J Pediatr Gastroenterol Nutr 31(4):433–438
Khin M et al (1999) Investigation of small-intestinal transit time in normal and malnourished children. J Gastroenterol 34(6):675–679
Murphy MS, Nelson R, Eastham EJ (1988) Measurement of small intestinal transit time in children. Acta Paediatr Scand 77(6):802–806
Vreugdenhil G, Sinaasappel M, Bouquet J (1986) A comparative study of the mouth to caecum transit time in children and adults using a weight adapted lactulose dose. Acta Paediatr Scand 75(3):483–488
Geypens B et al (1999) Validation of the lactose-[13C]ureide breath test for determination of orocecal transit time by scintigraphy. J Nucl Med 40(9):1451–1455
Wutzke KD et al (1997) Evaluation of oro-coecal transit time: a comparison of the lactose-[13C, 15N]ureide 13CO2- and the lactulose H2-breath test in humans. Eur J Clin Nutr 51(1):11–19
Boehm G et al (1991) Activities of lipase and trypsin in duodenal juice of infants small for gestational age. J Pediatr Gastroenterol Nutr 12(3):324–327
Jarvenpaa AL et al (1983) Feeding the low-birth-weight infant. III. Diet influences bile acid metabolism. Pediatrics 72(5):677–683
Challacombe DN, Edkins S, Brown GA (1975) Duodenal bile acids in infancy. Arch Dis Child 50(11):837–843
Walkowiak J et al (2002) Fecal elastase-1 is superior to fecal chymotrypsin in the assessment of pancreatic involvement in cystic fibrosis. Pediatrics 110(1 Pt 1):e7
Nissler K et al (2001) Pancreatic elastase 1 in feces of preterm and term infants. J Pediatr Gastroenterol Nutr 33(1):28–31
Zhang Y, Benet LZ (2001) The gut as a barrier to drug absorption: combined role of cytochrome P450 3A and P-glycoprotein. Clin Pharmacokinet 40(3):159–168
Dietrich CG, Geier A, Oude Elferink RP (2003) ABC of oral bioavailability: transporters as gatekeepers in the gut. Gut 52(12):1788–1795
Cascorbi I (2011) P-glycoprotein: tissue distribution, substrates, and functional consequences of genetic variations. Handb Exp Pharmacol 201:261–283
Fukudo M et al (2008) Impact of MDR1 and CYP3A5 on the oral clearance of tacrolimus and tacrolimus-related renal dysfunction in adult living-donor liver transplant patients. Pharmacogenet Genomics 18(5):413–423
Fukudo M et al (2006) Population pharmacokinetic and pharmacogenomic analysis of tacrolimus in pediatric living-donor liver transplant recipients. Clin Pharmacol Ther 80(4): 331–345
Konieczna A et al (2011) Differential expression of ABC transporters (MDR1, MRP1, BCRP) in developing human embryos. J Mol Histol 42(6):567–574
Miki Y et al (2005) Steroid and xenobiotic receptor (SXR), cytochrome P450 3A4 and multidrug resistance gene 1 in human adult and fetal tissues. Mol Cell Endocrinol 231(1–2):75–85
van Kalken CK et al (1992) Multidrug resistance gene (P-glycoprotein) expression in the human fetus. Am J Pathol 141(5):1063–1072
Fakhoury M et al (2005) Localization and mRNA expression of CYP3A and P-glycoprotein in human duodenum as a function of age. Drug Metab Dispos 33(11):1603–1607
Fanta S et al (2008) Pharmacogenetics of cyclosporine in children suggests an age-dependent influence of ABCB1 polymorphisms. Pharmacogenet Genomics 18(2):77–90
Lamba J et al (2006) MDR1 genotype is associated with hepatic cytochrome P450 3A4 basal and induction phenotype. Clin Pharmacol Ther 79(4):325–338
Fakhoury M et al (2006) Impact of inflammation on the duodenal mRNA expression of CYP3A and P-glycoprotein in children with Crohn's disease. Inflamm Bowel Dis 12(8):745–749
Vet NJ et al (2011) The effect of inflammation on drug metabolism: a focus on pediatrics. Drug Discov Today 16(9–10):435–442
Zhao W et al (2010) Population pharmacokinetics and pharmacogenetics of mycophenolic acid following administration of mycophenolate mofetil in de novo pediatric renal-transplant patients. J Clin Pharmacol 50(11):1280–1291
Ohmann EL et al (2010) Genetic polymorphisms influence mycophenolate mofetil-related adverse events in pediatric heart transplant patients. J Heart Lung Transplant 29(5):509–516
Zhao W et al (2009) Population pharmacokinetics and pharmacogenetics of tacrolimus in de novo pediatric kidney transplant recipients. Clin Pharmacol Ther 86(6):609–618
Hines RN (2008) The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol Ther 118(2):250–267
Johnson TN et al (2001) Enterocytic CYP3A4 in a paediatric population: developmental changes and the effect of coeliac disease and cystic fibrosis. Br J Clin Pharmacol 51(5):451–460
Treluyer JM et al (1991) Expression of CYP2D6 in developing human liver. Eur J Biochem 202(2):583–588
Paine MF et al (1996) First-pass metabolism of midazolam by the human intestine. Clin Pharmacol Ther 60(1):14–24
Mandema JW et al (1992) Pharmacokinetic-pharmacodynamic modeling of the central nervous system effects of midazolam and its main metabolite alpha-hydroxymidazolam in healthy volunteers. Clin Pharmacol Ther 51(6):715–728
Blake MJ et al (2006) Effect of diet on the development of drug metabolism by cytochrome P-450 enzymes in healthy infants. Pediatr Res 60(6):717–723
Blanquet S et al (2004) A dynamic artificial gastrointestinal system for studying the behavior of orally administered drug dosage forms under various physiological conditions. Pharm Res 21(4):585–591
Brouwers J et al (2011) Food-dependent disintegration of immediate release fosamprenavir tablets: in vitro evaluation using magnetic resonance imaging and a dynamic gastrointestinal system. Eur J Pharm Biopharm 77(2):313–319
Johnson TN, Rostami-Hodjegan A, Tucker GT (2006) Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin Pharmacokinet 45(9): 931–956
Ince I et al (2009) Tailor-made drug treatment for children: creation of an infrastructure for data-sharing and population PK-PD modeling. Drug Discov Today 14(5–6):316–320
de Wildt SN, Ito S, Koren G (2009) Challenges for drug studies in children: CYP3A phenotyping as example. Drug Discov Today 14(1–2):6–15
Gorski JC et al (1998) The contribution of intestinal and hepatic CYP3A to the interaction between midazolam and clarithromycin. Clin Pharmacol Ther 64(2):133–143
Lappin G et al (2011) Comparative pharmacokinetics between a microdose and therapeutic dose for clarithromycin, sumatriptan, propafenone, paracetamol (acetaminophen), and phenobarbital in human volunteers. Eur J Pharm Sci 43(3):141–150
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 American Association of Pharmaceutical Scientists
About this chapter
Cite this chapter
de Koning, B.A.E., Mooij, M., Johnson, T.N., de Wildt, S.N. (2014). Developmental Changes in the Processes Governing Oral Drug Absorption. In: Bar-Shalom, D., Rose, K. (eds) Pediatric Formulations. AAPS Advances in the Pharmaceutical Sciences Series, vol 11. Springer, New York, NY. https://doi.org/10.1007/978-1-4899-8011-3_3
Download citation
DOI: https://doi.org/10.1007/978-1-4899-8011-3_3
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4899-8010-6
Online ISBN: 978-1-4899-8011-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)