Abstract
Prediction of the pharmacokinetics of orally administered drugs in children is of importance to optimize the efficacy and safety of pediatric medicines. Physiologically based pharmacokinetic (PBPK) models can be helpful for this purpose. However, application of these tools is limited by significant knowledge gaps regarding the physiological and anatomical changes which occur with age. This study aimed at investigating the age-dependent differences in oral absorption of a poorly soluble model compound, carbamazepine (CBZ) in children, infants, and neonates. We developed an oral absorption model in GastroPlus® and, after evaluation of the PBPK model for adults, extrapolation to younger ages was verified with clinical data and sensitivity analyses were applied for uncertain model parameters. We found that age-based scaling of physiological parameters, with clearance in particular, was important to obtain adequate simulation results. The sensitivity analysis revealed that CBZ absorption was influenced by solubility, particle radius, and small intestinal transit time depending on the pediatric age group and CBZ dose. However, in vitro dissolution testing using proposed pediatric biorelevant media suggested no major age dependency of dissolution kinetics. Such better understanding of oral absorption in pediatric patients is required to improve the prediction of exposure in children and the confidence in oral biopharmaceutical tools.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The Best Pharmaceuticals for Children Act (BPCA) and the Pediatric Research Equity Act (PREA) encourage the pharmaceutical industry to increase drug safety and reduce off-label use in children by conducting clinical studies in pediatric populations (1,2). Drug development in pediatric patients requires careful attention due to ethical and logistical constraints associated with the conduct of clinical studies in these patient groups. Pediatric subjects are not small adults. They are a heterogeneous population that is undergoing significant physiological and anatomical alterations during developmental growth which can affect the rate and extent of drug absorption, as well as drug distribution, metabolism, and excretion. As all of these processes can influence safety and efficacy of drug compounds, it is extremely important to provide accurate predictions of pharmacokinetics in all age groups.
From an oral delivery perspective, the maturation of the gastrointestinal (GI) tract is of particular interest. The GI tract grows rapidly during the first years of life with an associated increase in luminal volumes and absorptive surface area. In addition, maturation of the GI tract affects its physical and biochemical environment, which can directly influence drug solubility, dissolution, and permeation across the intestinal membrane. For example, the stomach pH is known to be close to neutral at birth and decreases to more acidic conditions within hours after birth, before increasing again during the first month of life. Similarly, bile secretion is poor at birth and results in comparatively low concentrations of bile acids in the intestinal fluids during the first weeks of life (3,4).
Conventional oral dosage forms used for adult subjects are often not suitable for administration to children due to the impaired swallowing capabilities, sensory preferences, and physiological functions in the very young. For this reason, the development of age-appropriate formulations is strongly recommended and drives the need for bridging studies if more than one formulation is available for different age groups. Such studies are often conducted in adults to minimize the conduct of studies in children or may be entirely omitted if the compound is highly soluble and well-permeable, or if an in vitro-in vivo correlation has been established. Both cases imply that the biopharmaceutical performance of the compound and the drug product is comparable in adults and in children. However, such comparison is generally difficult to establish. While it would be important to apply age-specific biopharmaceutical prediction methods during pediatric drug development, such tools are currently limited by significant knowledge gaps regarding the physiological and anatomical changes which occur with age (3–8).
Biopharmaceutical tools such as in silico physiologically based pharmacokinetic (PBPK) models and in vitro biorelevant dissolution tests are routinely applied to predict the rate and extent of oral absorption in adults. Similarly, the biopharmaceutics classification system (BCS) is extensively used to guide formulation development. However, while they have been extensively validated in adults, validation in pediatric populations is still lagging behind due to limited knowledge of pediatric GI tract physiology and poor availability of pediatric clinical data. The BCS is a scientific tool that classifies the absorption behavior of oral compounds based on their solubility and permeability properties in adults. However, drug dose, intestinal membrane permeability, and GI fluid volumes and composition can be different between adults and children and, therefore, extrapolation of the BCS to pediatric populations is not straightforward (3,4,9,10). PBPK models are commonly used to study drug absorption processes in adults and some animal species by integrating drug material properties (e.g., ionization, particle size, solubility, dose) with physiological and biochemical processes occurring in the GI tract (e.g., GI transit, bile secretion, pH of GI fluids, active transport across the intestinal membrane, enterohepatic circulation). Pediatric physiologies are being implemented in PBPK software tools; however, verification of their predictive capability is still limited (3).
Although the prediction of drug absorption using pediatric PBPK models is still related to uncertainties, these models provide the possibility to study the complex interplay of processes involved in drug absorption and evaluate potential differences between adult and pediatric subjects (11,12). In a previous work, Villiger et al. studied the physiological factors affecting oral drug absorption in children using pediatric PBPK modeling and in vitro dissolution testing (11). These tools were applied for two highly soluble and well permeable model compounds, paracetamol and sotalol, and suggested that gastric transit was significantly slower in newborns and infants (< 2 years of age) compared to older children and adults. Recently, Batchelor et al. recommended that further research on pediatric biopharmaceutics should be undertaken with BCS class 2 and 4 compounds (9). Cristofoletti et al. highlighted the ability of advanced in silico absorption models to separate pre-absorptive from post-absorptive processes and to determine the rate-limiting steps in oral absorption of two weak bases in children (13). In line with these reports and while recognizing the need for further verification of drug absorption models in children, we conducted this study of pediatric PBPK absorption modeling for the BCS class 2 model compound carbamazepine (CBZ) to improve the understanding of oral absorption of a poorly soluble model compound in children. Clinical data in children are very limited and the selection of an ideal model compound is challenging. CBZ exhibits a complex pharmacokinetic behavior but has been extensively used to prevent epileptic seizures in pediatric patients and, therefore, the availability of clinical data is comparatively good. It has also been recommended as model compound by the Pediatric Biopharmaceutics Classification System Working Group of the National Institute of Child Health and Human Development (10). Moreover, Maharaj et al. recently found that the solubility of CBZ in media simulating the pediatric GI fluids fell outside an 80–125% range compared to solubility values in adult media, thereby indicating a potential age-related alteration in absorption (14).
To the best of our knowledge, no pediatric CBZ PBPK model has yet been published. CBZ pharmacokinetics is complex as it includes auto-induction of metabolism through the CYP3A4 pathway (15,16,17,18) and influence of frequent co-medication with other enzyme-inducing antiepileptic drugs (AEDs) such as phenobarbital (PB) and phenytoin (PT). The present study describes how we overcame these challenges and used modeling and in vitro tests to simulate CBZ absorption in children and investigate the sensitivity to dosing factors and age-dependent changes in GI physiology. While carbamazepine-epoxide is an active major metabolite and should also be considered in children, the modeling of this metabolite was beyond the scope of our work as the main aim was to explore oral absorption.
MATERIALS AND METHODS
Materials
The marketed tablet formulation Tegretol® 200 mg was obtained from Novartis Pharma GmbH (Nuremberg, Germany). CBZ was obtained from Chemie Brunschwig AG (Basel, Switzerland). FaSSIF/FeSSIF/FaSSGF Instant powder® was from biorelevant.com (Croyden, UK), Bimbosan® Classic from Bimbosan AG (Welschenrohr, Switzerland), and standardized heat-treated and homogenized milk (UHT-milk) containing 3.5% fat was from Mopro Luzern AG (Luzern, Switzerland). Acetonitrile was purchased from Biosolve Chimie (Dieuze, France). Acetic acid, dimethyl sulfoxide, sodium hydroxide pellets, hydrochloric acid, sodium taurocholate, sodium oleate, maleic acid, and pepsin were from Sigma-Aldrich Chemie GmbH (Steinheim, Germany). Sodium dihydrogen phosphate monohydrate, methanol, sodium acetate, and water for chromatography were from Merck KGaA (Darmstadt, Germany). Lecithin was purchased from Lipoid AG (Ludwigshafen, Germany), sodium chloride from Fluka Chemie AG (Buchs, Switzerland), and glycerol monooleate from Gattefossé AG (Saint Priest, France).
Pharmacokinetic Data Collection
Pharmacokinetic (PK) data in adults and pediatric populations were collected from the literature. The PK data in adults were used for model building and were included if the data were from healthy subjects, receiving a single CBZ dose formulated as immediate release (IR) suspension, solution, or tablet. In addition to the plasma concentration-time profiles, demographic and dosing information were extracted from the published reports. This resulted in a database of adult PK data from 34 different studies published between 1974 and 2004 (19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52), which included either single subject data or average data from studies in up to 24 subjects. The collected plasma concentration-time profiles were digitized using the software DigitizeIt® version 2.0.5 (I. Bormann, Braunschweig, Germany).
Weighted average plasma concentration-time profiles were calculated across the studies obtained after administration of IR Tegretol to healthy, fasted adults at either 200 mg (time points 0.5, 1, 2, 4, 6, 8, 12, 24, 48, 72, and 96 h) or at 400 mg (time points 1, 2, 4, 6, 8, 12, 24, 48, and 72 h). These two profiles were based on data from 7 (19,20,21,22,23,24,25) and 10 (26,27,28,29,30,31,32,33,34,35) different studies involving a total of 76 and 94 patients, respectively.
One study in healthy children (53) and four different pediatric patient studies (54,55,56) were found, which reported plasma concentration profiles as well as details of the medication history and other clinically relevant information. After further examination, one of the patient studies was considered as not representative and therefore excluded because it was performed in critically ill children suffering from additional complications such as ileus and impaired renal and hepatic function (56). After classifying the remaining datasets into age groups according to the International Conference on Harmonisation (ICH) E11 guideline (57), data were available for 11 newborns, (0–28 days of age), 3 infants (1–24 months of age), 7 children (2–11 years of age), and 3 adolescents (12–18 years of age). However, as the literature reports did not group their studies according to the ICH age classification and did not provide individual data, we followed a categorization based on mean age and age range as reported in Table I. Table I also provides information on patient weight, CBZ dose, and co-medications. More detailed information of individual patient characteristics and co-medication can be found in the Supplementary Material (Table S1).
PBPK Modeling Software
PBPK modeling was performed using GastroPlus version 9.0 (SimulationsPlus Inc., Lancaster, USA). GastroPlus incorporates the Advanced Compartmental and Transit (ACAT) model for predicting oral drug absorption, separating the GI tract into nine compartments. A general description of GastroPlus is available in the user’s manual (58) and in several publications (59,60,61,62).
Physiologically Based Scaling with Age
Adult and child physiologies, including age- and body weight-matched organ weights, volumes, and blood perfusion rates, were generated using the GastroPlus PEAR® module (Population Estimates for Age-Related Physiology). In brief, this module scales the physiological model framework based on published data for age-related changes in children (63,64). Thus, hepatic clearance was scaled to children accounting for changes in liver size and perfusion (65,66), protein binding and hematocrit (67,68,69), and enzyme expression maturation (e.g., for CYP3A (70)). Gut size in children was scaled according to data from Brown (71), and the GI transit times for children are based on reported data from Zwart et al. (72), Van Den Driessche et al. (73), Zhang et al. (74), and Bautista et al. (75). Due to lack of data, age dependencies in regional luminal solubility or in gut wall permeability were not considered in the model calculations. A list of anatomical and physiological parameters important for drug absorption and the basis for default values included in the GastroPlus pediatric absorption model is provided in the Supplementary Materials.
PBPK Modeling Strategy
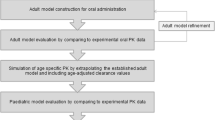
The workflow of the modeling strategy is shown in Fig. 1. The general approach to developing a pediatric CBZ model was to first establish a robust adult model based on single-dose clinical data from healthy adults prior to extrapolation to pediatric age groups. CBZ clearance is complex because CBZ induces its own CYP450 3A metabolism and it is also commonly administered with other antiepileptic drugs (AEDs) which are also CYP450 3A inducers. Clearance scaling to children had to consider these effects because most of the pediatric plasma concentration profiles were obtained in patients receiving co-medications. The oral plasma concentration profiles in children were simulated using the default physiological age-based scaling of the GI tract after having adjusted the systemic clearance to children using empirical relationships taken from statistical analysis of clinical data (described in “Models for Clearance Estimation in Children” section). Simulated profiles were then compared to observed profiles and model parameter sensitivity analyses were employed to scrutinize discrepancies between simulated and observed data.
Carbamazepine PBPK Model
The baseline model for CBZ in adults was constructed using input data listed in Table II. CBZ disposition was modeled using physiologies generated with PEAR with demographic parameters (gender, race, age) set to match the study specifications. If body weight, gender, and age were not specified in the source publication, a 70-kg and 30-year-old American male was assumed. CBZ is a neutral molecule and distributes well into tissues with a volume of distribution of approximately 1 L/kg. Drug distribution was predicted using perfusion-limited tissue models with tissue partition coefficients predicted using the Lukacova (Rodgers single) method (76). A good match to the observed volume was achieved using a logP of 2 which is within the range of reported measurements (Table II). CBZ metabolism by CYP450 3A4 was defined using metabolism rate data extracted from Kerr et al. (15) and, for single-dose studies, the clearance was scaled to different body weights and ages based on the physiological changes in liver size and enzyme expression.
For the simulations in children, except for one study in healthy subjects (53), the studies were for single doses in patients co-medicated with AEDs (54,55) and an adjustment to clearance was needed to account for enzyme induction (see below). Demographic data and dosing information for studies in children were set appropriately and when details were not reported, the following assumptions were made. Dose volume was set to 5 mL for children under 5 years of age and to 10 mL for children of 5 years and older (77). For a study in which the generic tablet formulation Mazetol® was administered (53) and for two studies in which a suspension was prepared from crushed Tegretol tablets (54,55), the same values of particle density, particle radius, particle radius standard deviation, and number of bins as for the Tegretol tablet formulation were used (Table II).
Models for Clearance Estimation in Children
The pediatric clinical data collected were all for a first dose of CBZ but the patients in four studies had been previously treated with CYP3A4 inducing AEDs and so induction of clearance needed to be accounted for. We conducted a literature search on published CBZ clearance models and four empirical models were retrieved based on patient characteristics and co-administered medications (78,79,80,81). The equations for these statistical models are provided below and the patient population characteristics for the studies used to derive the models are detailed in Table S2 (Supplementary Materials).
-
$$ \mathrm{Reith}\; et\ al.(78):\mathrm{CL}\ \left(\mathrm{L}/\mathrm{h}\right)=2.24\times \mathrm{Body}\ \mathrm{surface}\ \mathrm{area}\ \left({\mathrm{m}}^2\right)+0.047\times \mathrm{Dose}\ \left(\mathrm{mg}/\mathrm{kg}\right) $$(1)
-
$$ \mathrm{Gray}\; et\ al.(81):\mathrm{CL}\ \left(\mathrm{L}/\mathrm{h}\right)=\left(0.7\times {\mathrm{BW}}^{0.4}\right)\times 1.{4}^{\mathrm{M}} $$(2)
-
$$ \mathrm{Yukawa}\; et\ al.(80):\mathrm{CL}\ \left(\mathrm{mL}/\mathrm{h}/\mathrm{kg}\right)=64.9\times {\mathrm{BW}}^{-0.336}\times {\mathrm{Dose}}^{0.465}\ \left(\mathrm{mg}/\mathrm{kg}/\mathrm{d}\right)\times 1.0{7}^{\mathrm{VPA}}\times 1.{16}^{\mathrm{PB}}\times 1.{27}^{\mathrm{POLY}} $$(3)
-
$$ \mathrm{Delgado}\ \mathrm{Iribarnegaray}\kern0.28em et\ al.(79):\mathrm{CL}\ \left(\mathrm{L}/\mathrm{h}\right)=\left(0.0122\times \mathrm{BW}+0.0467\times Dose\left(\mathrm{mg}/\mathrm{kg}/\mathrm{d}\right)\right)\times {\mathrm{Age}}^{0.331}\times \left(1+0.289\times 1.{4}^{\mathrm{PB}}\right) $$(4)
where BW is body weight in kilograms, M (= 1) is a general scaling factor for concomitant CYP3A-inducing medication (M = 0 in the absence of co-medication), VPA (= 1) and PB (= 1) are scaling factors for concomitant medication with valproic acid and phenobarbital, respectively (VPA and PB = 0 in the absence of co-medication), and POLY (= 1) is a scaling factor for concomitant mediation more than two AEDs (POLY = 0 for co-medication with two or less AEDs). The conversion of body weight to body surface area for the calculation of CBZ clearance of the Reith model used tables from the National Institute for Health and Care Excellence Great Britain (82).
These empirical models are all based on clinical concentration measurements after repeated CBZ treatment and thus estimate the steady-state oral CBZ clearance, which includes both the bioavailable fraction and the systemic clearance. Since no data were available after intravenous dosing in children, a definitive separation of systemic clearance from bioavailability was not possible. However, since oral bioavailability is high in adults (most literature references report values > 0.9 (15,16,18)) and CBZ oral clearance, even after induction, remains very low compared to hepatic blood flow, we made the assumption that first-pass metabolism in gut and liver was negligible in children and the systemic clearance was derived from the predicted oral clearance according to CL sys = CL oral × F a, where the fraction of the dose absorbed (F a) was estimated from simulations using the PEAR module in GastroPlus. The sensitivity of simulated profiles to the assumed systemic clearance was explored subsequently.
Parameter Sensitivity Analysis
We conducted parameter sensitivity analysis (PSA) for critical input parameters to explore their impact on oral CBZ exposure. These parameters included physiological properties (stomach volume and transit time, fraction of small intestine fluid volume, small intestine length, radius, and transit time), compound-related properties (bile salt solubilization ratio, reference solubility, permeability, particle radius), and dosing variables (drug dose and dose volume). The baseline values as well as upper and lower bounds are detailed in Table S3. PSA was conducted for each of the four pediatric populations and assuming fasted conditions.
Preparation of Biorelevant Media
Adult fasted-state simulated gastric and fasted- and fed-state simulated intestinal fluids (FaSSGF, FaSSIF, and FeSSIF, respectively) were prepared according to Galia et al. (83) and the instructions provided by biorelevant.com (84). Pediatric fasted- and fed-state simulated gastric fluid representative of neonates (Pn-FaSSGF and Pn-FeSSGF, respectively), as well as pediatric fasted- and fed-state simulated intestinal fluids representative of neonates (P-FaSSIF and Pnc-FeSSIF, respectively), were prepared as proposed by Maharaj et al. (14). The composition of the simulated gastric and intestinal media is detailed in Tables S4 and S5.
Solubility Measurement
Solubility in Non-Milk-Based Media
For the assessment of CBZ solubility in non-milk-based media (FaSSGF, Pn-FaSSGF FaSSIF, P-FaSSIF 50%, P-FaSSIF 150%, FeSSIF, and Pnc-FeSSIF), 5 mL of each medium was filled into screw cap glass vials and an excess amount of crystalline CBZ was added. The experiments were conducted in triplicate. The samples were equilibrated at 37°C on a GFL 3025 rotation mixer at 20 rpm and aliquots of 200 μL were taken after 1, 2, 4, 6, and 21 h and centrifuged at 13,000 rpm for 90 s. The supernatant was removed and diluted 1:10 in a methanol-water mixture (60:40 v/v) prior to high-performance liquid chromatography (HPLC) analysis. Undissolved material was collected via centrifugation (13,000 rpm, 90 s) and this residual solid was analyzed via X-ray powder diffraction (XRPD). As a reference for XRPD, an excess amount of solid drug was suspended in fresh medium and immediately separated via centrifugation prior to analysis. The patterns showed no polymorphic conversion during solubility testing.
Solubility in Milk-Based Media
Solubility measurements in milk-based media (FeSSGF and Pnc-FeSSGF, respectively) were conducted according to the procedure described by Maharaj et al. (14) after 24 and 48 h of equilibration. Briefly, milk-based media were filled into the donor and acceptor compartments of a Vivacon® 500 tube (molecular weight cutoff 500 kDa, Sartorius Stedim Biotech GmbH, Goettingen, Germany). An excess amount of solid drug was added to the donor compartment and the tubes were equilibrated on a rotation mixer at 20 rpm and 37°C. Samples were removed after 24 h from the acceptor compartment and diluted with methanol (1:2) to remove milk proteins and centrifuged (8000 rpm, 15 min). The supernatant was finally removed for HPLC analysis. Undissolved material was collected and prepared for XRPD analysis as described for non-milk-based media and XRPD patterns showed no polymorphic conversion. The experiment was conducted in four replicates. Drug recovery in milk-based media was tested as described in (14).
Dissolution Testing
Dissolution experiments were carried out using a μDISS Profiler® (Pion Inc., Billerica, MA). The total CBZ concentration in the dissolution test was kept constant in relation to the equilibrium solubility and corresponded to about 80% of the experimentally determined solubility value. Establishing real sink conditions in biorelevant media is generally challenging for poorly soluble compounds, and therefore, a constant dose-to-volume ratio was selected for direct comparison of potential influence of media composition on the dissolution rate. A fraction of Tegretol tablet was accurately weighted and added to 20 mL of the pre-heated dissolution medium (37°C). Samples were stirred at 100 rpm and the amount of dissolved drug was determined over 2 h. For Pn-FaSSGF, P-FaSSIF 50%, and P-FaSSIF 150%, the concentration of dissolved drug was measured using in situ UV analytics (path length 2 mm, wavelength 300 to 310 nm). In situ UV spectra were analyzed using AuPro® (Pion Inc., MA). For Pn-FaSSGF, FeSSGF, Pnc-FeSSGF, FaSSIF, FeSSIF, and Pnc-FeSSIF, drug quantification was done off-line via sample removal and HPLC analysis. Samples were removed at 2.5, 5, 10, 20, 30, 45, 60, 75, 90, 105, and 120 min. The milk-based media (FeSSGF and Pnc-FeSSGF) were centrifuged (5000 rpm, 2 min). A range of centrifugation conditions was evaluated to ensure complete separation of undissolved drug material. The supernatant was diluted with methanol (1:2 v/v) and centrifuged (5000 rpm, 45 s) to remove milk proteins. The sample was finally diluted 1:10 in methanol-water (60:40 v/v) prior to HPLC analysis. The non-milk-based media samples (Pn-FaSSGF, FaSSIF, FeSSIF, and Pnc-FeSSIF) were centrifuged (90 s, 13,000 rpm) and the supernatant was diluted 1:10 in methanol-water (60:40 v/v) prior to HPLC analysis.
HPLC Method
Chromatographic analysis was performed on a Waters HPLC system (2795 Seperations Module, Waters Corp., Milford, USA) with a reversed-phase C18 column (XTerra®, 280 × 4.6 mm, 5 μm) in connection with a photodiode array detector (Waters 2996, Waters Corp., Milford, USA). An injection volume of 10 μL at a flow rate of 1 mL/min was eluted with mobile phase (methanol:water, 60:40 v/v) and the compound was detected at a wavelength of 285 nm. HPLC samples were analyzed using Empower® 3 (Waters Corp., Milford, USA).
RESULTS
Adult PBPK Model
Simulations with the adult PBPK model were in good agreement with plasma concentrations measured after a wide range of CBZ doses (50 to 800 mg) in both the fasted and fed states, and for different oral IR formulations (solution, suspension, and tablet). A selection of simulated profiles is presented in Fig. 2. Goodness-of-fit plots and additional simulated profiles are provided in the Supplementary Material (Figures S1 and S6–12). A total of 86% of simulated plasma concentrations were within twofold of observed values and 85% of predicted area under the plasma concentration-time curve (AUC0-t ) values were within 1.25-fold of observed values.
A selection of simulated plasma concentration-time profiles (dashed line) in adult subjects receiving different doses and formulations in the fasted (a, c, d) and the fed states (b). Observed data from the selected studies are shown as circles with error bars (mean ± standard deviation). Source: a (37), b (36), c mean weighted profile (19,20,21,22,23,24,25), d mean weighted profile (26,27,28,29,30,31,32,33,34,35)
Scaling of PBPK Model to Pediatric Population
The systemic clearances estimated using the four statistical models for the pediatric patients and by considering the default PEAR scaling for the healthy children are shown in Table III. The highest and lowest systemic clearance estimations from Table III were added to the GastroPlus model and the simulations were compared to the observed profiles. Figure 3a–d shows the simulated and observed plasma concentration profiles in pediatric subjects.
Observed (black circle, mean ± standard deviation) and simulated CBZ plasma concentration-time profiles for four different studies in pediatric subjects. Mean ages and doses are specified on the figure. The formulations used were as follows: a Bano (Mazetol tablet) (53); b Rey (suspension) (54); c Rey (suspension) (54); d MacKintosh (suspension) (55). The dashed line shows the simulated high clearance scenario while the continuous line is the low clearance scenario. Clearances were estimated as described in “MATERIALS AND METHODS” section and values used are given in Table III. In a, the lower clearance (0.43 L/h) was based on default physiological-based scaling in GastroPlus (Table III) and the higher clearance value of 0.75 L/h was increased to match the terminal half-life of the observed data
Simulated profiles in healthy children (Fig. 3a) and in child patients (Fig. 3b) were in quite close agreement with the observed profiles irrespective of the clearance used. For the healthy children, the clearance of 0.43 L/h based on default physiologically based scaling led to a slight underestimate of the terminal phase slope and a higher clearance of 0.75 L/h (shown as a dotted line) better matched the observed data. The clearances predicted for a 5.1-year-old child patient taking enzyme-inducing AEDs were higher (ranging from 1.5 to 2.2 L/h) and both values gave simulations close to the observed profile (Fig. 3b).
Figure 3c, d represents data in neonatal patients taking enzyme-inducing co-medications. In both cases, the simulations with the lower clearance value were found to be in better agreement with the observed data. However, while the profile was well predicted in newborns receiving 5 mg/kg CBZ (Fig. 3d), C max was underpredicted in the newborns dosed 17 mg/kg CBZ (Fig. 3c), even when assuming the lower clearance.
The data in Fig. 3 were all simulated using the fasted-state physiology. However, with the exception of the Bano study (53), which reported drug administration in the fasted state, the published studies did not report any details on the food status. Due to this uncertainty, the simulations were also run assuming fed-state conditions and are shown in Fig. 4. The resulting oral fractions absorbed are provided in Table IV. When assuming fed-state conditions, a slightly higher systemic exposure was predicted in neonates and infants receiving comparatively high doses (> 17 mg/kg). In contrast, the difference was small in children and neonates administered low doses of CBZ (< 10 mg/kg). The fed-state model resulted in a better match between predicted and observed PK profiles in the neonates receiving high CBZ doses, whereas oral exposures was overpredicted in infants dosed 19 mg/kg CBZ.
Observed (black circle, mean ± standard deviation) and simulated plasma concentration profiles assuming either fed (dashed line) or fasted (continuous line) state in pediatric age groups. Source: a Bano study (children/adolescents; Mazetol tablet) (53); b Rey study (children; suspension) (54); c Rey study (neonates; suspension) (54); d MacKintosh study (neonates; suspension) (55). All simulations were run using the lower clearance values
Analysis of Oral CBZ Absorption in Pediatric Age Groups
The pediatric PBPK models were used to study the extent and rate-limiting steps of CBZ absorption in the different age groups. Figure 5a illustrates the simulated fractions of drug dissolved and absorbed from the intestine following fasted-state administration for the four pediatric studies. There is very little separation between the dissolution and absorption profiles, suggesting that permeation is not a rate-limiting step for CBZ absorption as expected for the given BCS class 2 compound. No significant differences were observed in this regard between different age groups. Almost complete drug absorption within 4–6 h was predicted in the older children and adolescents (mean age 11.3 years) receiving a mean dose of 9 mg/kg. In newborns receiving low CBZ dose (5 mg/kg), drug absorption was complete but comparatively slow. In contrast, absorption was incomplete in children and newborns receiving high CBZ doses (17 and 19 mg/kg).
Figure 5b shows the simulations for the fed-state conditions. The most significant difference in absorption with food was observed at the higher doses both in the newborns and children. At these doses, CBZ fasted-state absorption was incomplete at only 50–60% and food resulted in a 10–20% increase in the fraction absorbed in both age groups. While the predicted fraction absorbed was > 80% with low-dose CBZ (5–9 mg/kg) in both younger and older age groups, the extent of drug absorption was notably reduced in the high dose range (17–19 mg/kg) irrespective of age.
Parameter Sensitivity Analysis
Parameter sensitivity analysis was performed on critical parameters for oral drug absorption for the four pediatric models. The results depicted in Fig. 6a, b show that the fraction absorbed was most sensitive to variations in CBZ solubility and dose. The influence of small intestine transit time (Fig. 6c), particle size (Fig. 6d), fraction of small intestine fluid volume, small intestine radius and length, permeability, and bile salt solubilization ratio was more pronounced for high-dose CBZ, but was minor for low-dose CBZ, independent from the age group. In general, stomach volume, stomach transit time, and dose volume were less influential on drug absorption. The sensitivity analysis plots for all parameters are given in the Supplementary Materials (Figure S3).
Parameter sensitivity analysis results for a reference solubility, b dose, c small intestine transit time, and d drug particle radius. Gray-colored profiles represent data in neonates; black profiles are data in older children; low doses are illustrated as continuous lines and high doses as dotted lines. The baseline values used in the models are shown as a red circle
In Vitro Dissolution Testing
In vitro dissolution of CBZ was generally fast with almost 80% dissolved within 20 min. No difference in dissolution kinetics was observed between pediatric and adult simulated gastric and intestinal fluids, suggesting similar dissolution behavior of CBZ in adults and pediatric subjects. The results of the in vitro dissolution tests of CBZ in biorelevant media are displayed in Figure S4.
DISCUSSION
In this study, PBPK modeling and in vitro dissolution testing were employed to investigate the age dependency in oral absorption of the poorly water-soluble compound CBZ. A good match of simulations to observed data in healthy adults was seen for solution, suspension, and tablet formulations in both fasted and fed states which is consistent with previous GastroPlus modeling of immediate and extended release CBZ (36).
The verified adult model was extrapolated to simulate the single oral dose concentration versus time profile obtained in a study in healthy children/adolescents of average age 11 years (53) by using the default GastroPlus physiologically based age-dependent scaling. The simulated profile was in excellent agreement to observations for the absorption phase, but a slight underestimate of the terminal half-life was seen and an increase in clearance by a factor of 1.7 was necessary to match the observed profile. The current CYP3A ontogeny profile in GastroPlus is based on work of Johnson (70) which relies on in vitro measurements of enzyme levels in children. More recently, Upreti et al. (85) reported a method to derive in vivo ontogenies and showed higher in vivo CYP3A activities than has been estimated from in vitro data. Indeed in Upreti’s study with the probe substrate sufentanil, the CYP3A activity in the infant and child exceeded adult values. Our example of CBZ seems to support this finding although other reasons for the apparent under prediction of CBZ clearance in these children are possible and further analysis with different CYP3A substrates is needed to fully understand the ontogeny of this enzyme. The estimated single-dose clearance of 0.75 L/h is 2.4 to 3.8 times lower than the estimated steady-state clearance for an 11-year-old child taking AEDs as predicted by the four models used in this study (Table III). This is consistent with the increase which is expected due to a combination of auto-induction of CBZ (estimated as approximately twofold in adults (86) and in children (87)), and the additional induction due to co-medications (predicted to be less than ~2-fold based on the four models used in this study).
All other pediatric studies in our analysis were from children being co-medicated with enzyme-inducing AEDs and so it was necessary to estimate the induced CBZ clearances before simulating single-dose oral profiles. To obtain full plasma concentration profiles showing the effect of enzyme-inducing co-medications on CBZ proved to be difficult. However, we did find four different statistical models for prediction of steady-state oral CBZ clearance in patients taking AEDs. These models gave consistent clearance estimates in a 5-year-old child with a range of only 1.5-fold between the model predictions (Table III). However, a much wider range of prediction was obtained in very young children with a fourfold range in a 21-day-old and 16-fold range in a 6-day-old (Table III). This uncertainty is not unexpected since none of the data sets used to build these clearance models included data in neonates (Table S2). Indeed, the ages of the children on which these models are based are biased towards older ages and only the Iribarnegaray model includes age as an independent predictor of clearance while the other models are based on body size (either body weight or body surface area). As CYP3A activity is low at birth and develops rapidly, an over prediction of clearance in neonates is to be expected with these models. This was indeed seen when using the range of predicted clearances in the PBPK absorption models. Simulated oral profiles were close to the observed clinical data (54) in the 5-year-old child independent of the systemic clearance used. However, simulated oral profiles in neonates were in much better agreement to observed data when using the lowest clearance estimates. Overall, it seems likely that the development in enzyme expression is not captured in these models due to the low number of data in very young children. However, as simulations with the lowest clearance were close to observations and the simulated and observed half-life for the neonates were in good agreement where concentrations were measured up to 48 h (54), we were reassured that the predicted clearance was appropriate. Interestingly, these estimated CBZ clearances in neonates are significantly higher (15- to 20-fold) than predicted with the default scaling in GastroPlus (Table III). This default scaling has assumed that CYP3A4 metabolism is the sole clearance route whereas other enzymes including CYP3A5 and CYP2C8 are involved (88,89). Furthermore, the assumed ontogeny of CYP3A starting from zero activity at birth and reaching only 4% of adult levels by the age of 6 days is also questionable based on recent in vivo analyses (85).
Simulation of fraction of the dose absorbed was almost complete in adults (96% of a 200-mg dose and 91% of a 400-mg dose) while in children it was reduced and showed a clear dose dependency. Neonates receiving a dose of 5 mg/kg showed almost complete simulated absorption (> 90%) but this was reduced in the same age group after administration of 17 mg/kg. A similar behavior was predicted in older children, with complete absorption at 9 mg/kg, while 19 mg/kg resulted in a fraction absorbed of only 66%. These observations are comparable with the reduced fraction of dose absorbed in children compared to that in adults reported for another BCS class 2 molecule, montelukast, using the SimCYP pediatric module (12). Further work on additional molecules from different BCS classes is needed before conclusions on behavior of different BCS classes in pediatrics can be drawn.
It must be considered that the currently available pediatric PBPK models are based on certain assumptions (Table S7, Supplementary Materials) and their predictive capability needs more thorough verification. Although good predictions of oral CBZ exposure were obtained with the current datasets, sensitivity analyses were conducted to scrutinize how age-dependent changes in the GI tract may affect oral exposure. At high dose levels, the oral fraction absorbed was sensitive to the intestine length and transit time, suggesting that, due to the smaller GI tract, the residence time in the gut is critical for complete absorption of CBZ. In contrast, this effect was less pronounced for lower doses (e.g., 5 mg/kg in neonates), in which case almost complete absorption was predicted for a broad range of small intestine transit times and lengths. Similarly, use of fed-state conditions had almost no impact on the extent of absorption simulated at low dose, but showed a significant increase for high CBZ doses.
Aqueous solubility was another sensitive parameter for oral exposure. This parameter showed the most pronounced influence on the oral fraction absorbed in high-dose patients (also illustrated in Figure S5), along with the physiological and anatomical parameters determining the residence time in the intestine (length, radius, transit times). This observation highlights the importance of accurate solubility prediction for reliable simulations with poorly water-soluble compounds. As newborns and infants are fed in regular, short intervals, there is likely to be a prolonged presence of milk in the intestine which could facilitate drug dissolution and increase the fraction absorbed. These considerations might explain the more accurate simulations seen when assuming fed-state physiology in neonates. A similar observation was made for two BCS 1 compounds where a prolonged gastric emptying improved simulations (11). The differences in gastric and intestinal fluids between adults and children (e.g., the presence of milk due to frequent infant feeding) may not only affect the overall solubility of the compound but as well its dissolution rate. The PBPK model assumed no age-dependent difference in dissolution rate and, to test this model assumption, we conducted dissolution studies using the proposed pediatric (14) and adult biorelevant media. The in vitro studies suggested no influence of media composition on the dissolution rate of CBZ from immediate release tablets. It must be noted that the simulated pediatric gastric and intestinal media used for dissolution testing were based on age-specific changes in GI fluid parameters which were obtained from the literature (e.g., pH, osmolarity, concentration of bile salts) (14). However, there still exist significant knowledge gaps in this regard, and therefore, the media represent a rough estimate of GI fluid composition. Prospective studies are still required to confirm their biorelevance.
Some uncertainty was related to the drug particle size parameter, which was found to be a sensitive parameter at high doses. The uncertainty in this parameter value could not be fully ruled out due to the lack of product-specific particle size data. However, dissolution data showed that the dissolution rate from Tegretol tablets was generally fast, with almost 80% dissolved within 20 min, and suggested no influence of medium (with or without milk) on the kinetics of drug release.
The impact of age-specific differences in GI tract physiology on oral exposure have been discussed by several authors recently who have provided theoretical considerations supporting the need for a pediatric BCS for drug development and regulatory purposes (3,4,9,10,13,90,91,92). However, clinical data in children are very limited and the challenge of isolating pre-absorptive from post-absorptive processes has restricted a clear assessment of the in vivo relevance of the proposals. Mechanistic absorption modeling, as presented in the current study, provides valuable insights into the oral absorption process in children and shows that age can influence both the rate and extent of oral absorption. Furthermore, the fraction absorbed of CBZ was strongly dose-dependent which also needs to be carefully considered as children may sometimes require higher doses per unit body weight than adults. Differences in dose and physiology should also be considered when conducting relative bioavailability studies to compare different formulations since the rate and extent of oral absorption might be limited by different factors depending on the dose and age of the patient population. The relevance of studies conducted in adults for data extrapolation to children needs careful consideration, and oral absorption modeling should be an important component in the toolkit for pediatric drug development and regulatory questions.
CONCLUSIONS
This work has shown that if a poorly soluble drug like CBZ is given at high doses, the reduced GI size and transit times may lead to an age-dependent reduced fraction absorbed in pediatric patients. Furthermore, assuming fed-state conditions showed an improvement in simulation results in neonates, believed to be due to slower gastric emptying rates and higher solubility caused by presence of milk. Overall, the simulation results in this study supported the GastroPlus age-based scaling of absorption in pediatric populations and work on additional molecules from different BCS classes is needed to further verify the predictive capability of pediatric PBPK models for compounds of different BCS classes. Care is needed when extrapolating the biopharmaceutical performance of orally administered compounds from adults to pediatric patients. There still exist significant knowledge gaps with regard to the characteristics of the pediatric GI tract which requires further research. Such knowledge will also help in the design of more biorelevant in vitro and in silico tools to predict the biopharmaceutical performance of drug products.
This study further demonstrates the importance of research to develop a pediatric relevant BCS and improve pediatric absorption modeling tools which can aid the design of formulations and support appropriate clinical studies in children.
References
Best Pharmaceuticals for Children Act, January 4, 2002 (Public Law No. 107–109).
Pediatric Research Equity Act, December 3, 2003 (Public Law No. 108–155).
Batchelor HK, Fotaki N, Klein S. Paediatric oral biopharmaceutics: key considerations and current challenges. Adv Drug Deliv Rev. 2014;73:102–26.
Batchelor HK, Kendall R, Desset-Brethes S, Alex R, Ernest TB. Application of in vitro biopharmaceutical methods in development of immediate release oral dosage forms intended for paediatric patients. Eur J Pharm Biopharm. 2013;85:833–42.
Frattarelli DA, Galinkin JL, Green TP, Johnson TD, Neville KA, Paul IM, et al. American Academy of Pediatrics Committee on Drugs. Off-label use of drugs in children. Pediatrics. 2014;133(3):563–7.
Jamei M. Recent advances in development and application of physiologically-based pharmacokinetic (PBPK) models: a transition from academic curiosity to regulatory acceptance. Curr Pharmacol Rep. 2016;2:161–9.
Leong R, Vieira ML, Zhao P, Mulugeta Y, Lee CS, Huang SM, et al. Regulatory experience with physiologically based pharmacokinetic modeling for pediatric drug trials. Clin Pharmacol Ther. 2012;91(5):926–31.
Grillo JA. Pediatric applications of PBPK modeling and simulation in drug regulatory science: where are we now? AAPS Annual Meeting and Exposition. San Diego, USA; 2014.
Batchelor H, Ernest T, Flanagan T, Klein S, Turner R, Fotaki N, et al. Towards the development of a paediatric biopharmaceutics classification system: results of a survey of experts. Int J Pharm. 2016;511(2):1151–7.
Abdel-Rahman SM, Amidon GL, Kaul A, Lukacova V, Vinks AA, Knipp GT. Summary of the National Institute of Child Health and Human Development-best pharmaceuticals for Children Act Pediatric Formulation Initiatives Workshop–Pediatric Biopharmaceutics Classification System Working Group. Clin Ther. 2012;34:S11–23.
Villiger A, Stillhart C, Parrott N, Kuentz M. Using physiologically based pharmacokinetic (PBPK) modelling to gain insights into the effect of physiological factors on oral absorption in paediatric populations. AAPS J. 2016;18(4):933–47.
Jones HM, Chen Y, Gibson C, Heimbach T, Parrott N, Peters SA, et al. Physiologically based pharmacokinetic modeling in drug discovery and development: a pharmaceutical industry perspective. Clin Pharmacol Ther. 2015;97(3):247–62.
Cristofoletti R, Charoo NA, Dressman JB. Exploratory investigation of the limiting steps of oral absorption of fluconazole and ketoconazole in children using an in silico pediatric absorption model. J Pharm Sci. 2016;105(9):2794–803.
Maharaj AR, Edginton AN, Fotaki N. Assessment of age-related changes in pediatric gastrointestinal solubility. Pharm Res. 2016;33(1):52–71.
Kerr BM, Thummel KE, Wurden CJ, Klein SM, Kroetz DL, Gonzalez FJ, et al. Human liver carbamazepine metabolism. Role of CYP3A4 and CYP2C8 in 10,11-epoxide formation. Biochem Pharmacol. 1994;47(11):1969–79.
Bertilsson L, Tomson T. Clinical pharmacokinetics and pharmacological effects of carbamazepine and carbamazepine-10,11-epoxide. An update. Clin Pharmacokinet. 1986;11(3):177–98.
Tulloch JK, Carr RR, Ensom MH. A systematic review of the pharmacokinetics of antiepileptic drugs in neonates with refractory seizures. J Pediatr Pharmacol Ther. 2012;17(1):31–44.
Patsalos PN. Carbamazepine. In: Patsalos PN, editor. Antiepileptic drug interactions. A clinical guide. 2nd ed. London: Springer; 2013. p. 11–21.
Rawlins MD, Collste P, Bertilsson L, Palmér L. Distribution and elimination kinetics of carbamazepine in man. Eur J Clin Pharmacol. 1975;8(2):91–6.
Gérardin AP, Abadie FV, Campestrini JA, Theobald W. Pharmacokinetics of carbamazepine in normal humans after single and repeated oral doses. J Pharmacol Biopharm. 1976;4(6):521–35.
Anttila M, Kahela P, Panelius M, Yrjänö T, Tikkanen R, Aaltonen R. Comparative bioavailability of two commercial preparations of carbamazepine tablets. Eur J Clin Pharmacol. 1979;15:421–5.
De Aurajo Ferreira AA, Coelho Guerra GB, da Silva SG, Dibildox E, Perez-Urizar J, et al. Comparative bioavailability of two extemporaneous solid formulations of carbamazepine against the innovator in Mexican healthy subjects. J Bioequiv Availab. 2014;6:2.
Meyer MC, Straughn AB, Jarvi EJ, Wood GC, Pelsor FR, Shah VP. The bioinequivalence of carbamazepine tablets with a history of clinical failures. Pharm Res. 1992;9(12):1612–6.
Revankar SN, Desai ND, Bhatt AD, Bolar HV, Sane SP, Gupta C, et al. Comparison of absorption rate and bioavailability of two brands of carbamazepine. J Assoc Physicians India. 1999;47(7):699–702.
Kayali A, Tuğlular I, Ertaş M. Pharmacokinetics of carbamazepine. Part I: a new bioequivalency parameter based on a relative bioavailability trial. Eur J Drug Metab Pharmacokinet. 1994;19(4):319–25.
Strandjord RE, Johannessen SI. A preliminary study of serum carbamazepine levels in healthy subjects and in patients with epilepsy. Clin Pharmacol Ther. 1975:181–8.
Morselli PL, Gerna M, de Maio D, Zanda G, Viani F, Garattini S. Pharmacokinetic studies on carbamazepine in volunteers and epileptic patients. Clin Pharmacol Anti-Epileptic Drugs. 1975:166–80.
Chan KK, Sawchuk RJ, Thompson TA, Redalieu E, Wagner WE Jr, LeSher AR, et al. Bioequivalence of carbamazepine chewable and conventional tablets: single-dose and steady-state studies. J Pharm Sci. 1985;74(8):866–70.
Kovacević I, Parojcić J, Homsek I, Tubić-Grozdanis M, Langguth P. Justification of biowaiver for carbamazepine, a low soluble high permeable compound, in solid dosage forms based on IVIVC and gastrointestinal simulation. Mol Pharm. 2009;6(1):40–7.
Cotter LM, Eadie MJ, Hooper WD, Lander CM, Smith GA, Tyrer JH. The pharmacokinetics of carbamazepine. Eur J Clin Pharmacol. 1977;12(6):451–6.
Pynnönen S, Mäntylä R, Iisalo E. Bioavailability of four different pharmaceutical preparations of carbamazepine. Acta Pharmacol Toxicol (Copenh). 1978;43(4):306–10.
Olling M, Mensinga TT, Barends DM, Groen C, Lake OA, Meulenbelt J. Bioavailability of carbamazepine from four different products and the occurrence of side effects. Biopharm Drug Dispos. 1999;20(1):19–28.
Bhatia SC, Bhatt AD, Bakshi RJ, Revankar SN, Bharucha ED, Doshi KJ, et al. Comparative bioavailability with two brands of carbamazepine-Tegretol and Mazetol in healthy volunteers. J Assoc Physicians India. 1988;36(10):611–2.
Neuvonen PJ. Bioavailability and central side effects of different carbamazepine tablets. Int J Clin Pharmacol Ther Toxicol. 1985;23(4):226–32.
Wong YY, Ludden TM, Bell RD. Effect of erythromycin on carbamazepine kinetics. Clin Pharmacol Ther. 1983;33(4):460–4.
Zhang X, Lionberger RA, Davit BM, Yu LX. Utility of physiologically based absorption modeling in implementing quality by design in drug development. AAPS J. 2011;13(1):59–71.
Levy RH, Pitlick WH, Troupin AS, Green JR, Neal JM. Pharmacokinetics of carbamazepine in normal man. Clin Pharmacol Ther. 1975;17(6):657–68.
Johannessen SI, Henriksen O, Munthe-Kaas AW, Salvesen B. Serum concentration profile studies of tablets and suppositories of valproate and carbamazepine in healthy subjects and patients with epilepsy. In: Levy RH, Pitlick WH, Eichelbaum M, Meijer J, editors. Metabolism of antiepileptic drugs. New York: Raven Press; 1984. p. 61–71.
Dam M, Christiansen J, Kristensen CB, Helles A, Jaegerskou A, Schmiegelow M. Carbamazepine: a clinical biopharmaceutical study. Eur J Clin Pharmacol. 1981;20(1):59–64.
Cotter LM, Smith G, Hooper WD, Tyrer JH, Eadie MJ. The bioavailability of carbamazepine. Proc Aust Assoc Neurol. 1975;12:123–8.
Maas B, Garnett WR, Pellock JM, Comstock TJ. A comparative bioavailability study of carbamazepine tablets and a chewable tablet formulation. Ther Drug Monit. 1987;9(1):28–33.
Sumi M, Watari N, Umezawa O, Kaneniwa N. Pharmacokinetic study of carbamazepine and its epoxide metabolite in humans. Aust J Pharm. 1987;10(11):652–61.
Kaneniwa N, Umezawa O, Watari N, Kawakami K, Asami H, Sumi M. Bioavailability and dissolution test of commercial carbamazepine tablets. Yakugaku Zasshi 1984;104(1):83–90. Japanese.
Paxton JW, Donald RA. Concentrations and kinetics of carbamazepine in whole saliva, parotid saliva, serum ultrafiltrate, and serum. Clin Pharmacol Ther. 1980;28(5):695–702.
Riad LE, Chan KK, Wagner WE Jr, Sawchuk RJ. Simultaneous first- and zero-order absorption of carbamazepine tablets in humans. J Pharm Sci. 1986;75(9):897–900.
Jung H, Milán RC, Girard ME, Montoya MA. Bioequivalence study of carbamazepine tablets: in vitro/in vivo correlation. Int J Pharm. 1997;152(1):37–44.
Neuvonen PJ, Tokola O. Bioavailability of rectally administered carbamazepine mixture. Br J Clin Pharmacol. 1987;24(6):839–41.
Hooper WD, King AR, Patterson M, Dickinson RG, Eadie MJ. Simultaneous plasma carbamazepine and carbamazepine-epoxide concentrations in pharmacokinetic and bioavailability studies. Ther Drug Monit. 1985;7(1):36–40.
Wada JA, Troupin AS, Friel P, Remick R, Leal K, Pearmain J. Pharmacokinetic comparison of tablet and suspension dosage forms of carbamazepine. Epilepsia. 1978;19(3):251–5.
Graves NM, Kriel RL, Jones-Saete C, Cloyd JC. Relative bioavailability of rectally administered carbamazepine suspension in humans. Epilepsia. 1985;26(5):429–33.
Popović J, Mikov M, Jakovljevic V. Pharmacokinetics of carbamazepine derived from a new tablet formulation. Eur J Drug Metab Pharmacokinet. 1995;20(4):297–300.
Kauko K, Tammisto P. Comparison of two generically equivalent carbamazepine preparations. Ann Clin Res. 1974;6(Suppl 11):21–5.
Bano G, Raina RK, Sharma DB. Pharmacokinetics of carbamazepine in protein energy malnutrition. Pharmacology. 1986;32(4):232–6.
Rey E, d'Athis P, de Lauture D, Dulac O, Aicardi J, Olive G. Pharmacokinetics of carbamazepine in the neonate and in the child. Int J Clin Pharmacol Biopharm. 1979;17(2):90–6.
MacKintosh DA, Baird-Lampert J, Buchanan N. Is carbamazepine an alternative maintenance therapy for neonatal seizures? Dev Pharmacol Ther. 1987;10(2):100–6.
Miles MV, Lawless ST, Tennison MB, Zaritsky AL, Greenwood RS. Rapid loading of critically ill patients with carbamazepine suspension. Pediatrics. 1990;86(2):263–6.
European Medicines Agency. ICH Topic E 11: Clinical investigation of medicinal products in the paediatric population. CPMP/ICH/2711/99. London; 2001.
Simulations Plus, Inc. GastroPlus version 9.0: simulation software for drug discovery and development (user manual). 2015; Lancaster, CA.
Parrott N, Lave T. Applications of physiologically based absorption models in drug discovery and development. Mol Pharm. 2008;5(5):760–75.
Heikkinen AT, Baneyx G, Caruso A, Parrott N. Application of PBPK modeling to predict human intestinal metabolism of CYP3A substrates—an evaluation and case study using GastroPlus. Eur J Pharm Sci. 2012;47(2):375–86.
Jones H, Parrott N, Ohlenbusch G, Lavé T. Predicting pharmacokinetic food effects using biorelevant solubility media and physiologically based modelling. Clin Pharm. 2006;45(12):1213–26.
Parrott N, Lave T. Computer models for predicting drug absorption. In: Dressman J, Reppas C, editors. Oral drug absorption—prediction and assessment. Boca Raton: CRC Press; 2010. p. 338–55.
Jorga K, Chavanne C, Frey N, Lave T, Lukacova V, Parrott N, et al. Bottom-up meets top-down: complementary physiologically based pharmacokinetic and population pharmacokinetic modeling for regulatory approval of a dosing algorithm of valganciclovir in very young children. Clin Pharmacol Ther. 2016;100(6):761–9.
Lukacova V, Goelzer P, Reddy M, Greig G, Reigner B, Parrott N. A physiologically based pharmacokinetic model for ganciclovir and its prodrug valganciclovir in adults and children. AAPS J. 2016;18(6):1453–63.
Haddad S, Restieri C, Krishnan K. Characterization of age-related changes in body weight and organ weights from birth to adolescence in humans. J Toxicol Environ Health A. 2001;64(6):453–64.
Johnson TN, Tucker GT, Tanner MS, Rostami-Hodjegan A. Changes in liver volume from birth to adulthood: a meta-analysis. Liver Transpl. 2005;11(12):1481–93.
McNamara PJ, Alcorn J. Protein binding predictions in infants. AAPS PharmSciTech. 2002;4(1):E4.
Rubin MI, Bruck E, Rapport M. Maturation of renal function in childhood: clearance studies. J Clin Invest. 1949;28(5 Pt 2):1144–62.
Yanowitz TD, Yao AC, Pettigrew KD, Werner JC, Oh W, Stonestreet BS. Postnatal hemodynamic changes in very-low-birth weight infants. J Appl Physiol. 1999;87(1):370–80.
Johnson TN, Rostami-Hodjegan A, Tucker GT. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin Pharmacokinet. 2006;45:931–56.
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health. 1997;13(4):407–84.
LL de Zwart, CJM Rompelberg, AJAM Sips, Welink J, JGM van Engelen. Anatomical and physiological differences between various species used in studies on the pharmacokinetics and toxicology of xenobiotics. A review of literature. Bilthoven (NL): National Institute of Public Health and the Environment; 1999 Report No.: 623860 010.
Van Den Driessche M, Van Malderen N, Geypens B, Ghoos Y, Veereman-Wauters G. Lactose-[13C]ureide breath test: a new, noninvasive technique to determine orocecal transit time in children. J Pediatr Gastroenterol Nutr. 2000;31(4):433–8.
Zhang SC, Wang WL, Bai YZ, Yuan ZW, Wang W. Determination of total and segmental colonic transit time in constipated children. Zhonghua Er Ke Za Zhi 2003;41(3):176–179. Chinese.
Bautista Casasnovas A, Varela Cives R, Villanueva Jeremias A, Castro-Gago M, Cadranel S, Tojo SR. Measurement of colonic transit time in children. J Pediatr Gastroenterol Nutr. 1999;13(1):42–5.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95(6):1238–57.
European Medicines Agency; Committee for Medicinal Products for Human Use (CHMP). Reflection paper: formulations of choice for the paediatric population. EMEA/CHMP/PEG194810/2005. London; 2006.
Reith DM, Hooper WD, Parke J, Charles B. Population pharmacokinetic modeling of steady state carbamazepine clearance in children, adolescents, and adults. J Pharmacokinet Pharmacodyn. 2001;28(1):79–92.
Delgado Iribarnegaray MF, Santo Bueldga D, García Sánchez MJ, Otero MJ, Falcão AC, Domínguez-Gil A. Carbamazepine population pharmacokinetics in children: mixed-effect models. Ther Drug Monit. 1997;19(2):132–9.
Yukawa E, Aoyama T. Detection of carbamazepine drug interaction by multiple peak approach screening using routine clinical pharmacokinetic data. J Clin Pharmacol. 1996;36(8):752–9.
Gray AL, Botha JH, Miller R. A model for the determination of carbamazepine clearance in children on mono- and polytherapy. Eur J Clin Pharmacol. 1998;54(4):359–62.
Table for body surface area—body weight correlation in children. [Internet] National Institute for Health and Care Excellence Great Britain [cited 21 Sep 2016]. Available from: https://www.evidence.nhs.uk/formulary/bnfc/current/body-surface-area-in-children.
Galia E, Nicolaides E, Hörter D, Löbenberg R, Reppas C, Dressman JB. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharm Res. 1998;15(5):698–705.
biorelevant.com [Internet]. London: Instructions for preparation of FaSSIF, FeSSIF, and FaSSGF [cited 3 June 2017]. Available from: https://biorelevant.com/fassif-fessif-fassgf/how-to-make/.
Upreti VV, Wahlstrom JL. Meta-analysis of hepatic cytochrome P450 ontogeny to underwrite the prediction of pediatric pharmacokinetics using physiologically based pharmacokinetic modeling. J Clin Pharmacol. 2015;2(10):585.
Magnusson M, et al. Pharmacodynamics of carbamazepine-mediated induction of CYP3A4, CYP1A2, and Pgp as assessed by probe substrates midazolam, caffeine, and digoxin. Clin Pharmacol Ther. 2008;84(1)
Bertilsson L, et al. Autoinduction of carbamazepine metabolism in children examined by a stable isotope technique. Clin Pharmacol Ther. 1980;27(1):83–8.
Biochemical pharmacology Volume 47, Issue 11, 1 June 1994, Pages 1969–1979.
Wang P, et al. Effects of CYP3A4/5 and ABCB1 genetic polymorphisms on carbamazepine metabolism and transport in Chinese patients with epilepsy treated with carbamazepine in monotherapy and bitherapy. Epilepsy Res. 2015;117:52–7.
Gandhi SV, Rodriguez W, Khan M, Polli JE. Considerations for a Pediatric Biopharmaceutics Classification System (BCS): application to five drugs. AAPS PharmSciTech. 2014;15(3):601–11.
Shawahna R. Pediatric biopharmaceutical classification system: using age-appropriate initial gastric volume. AAPS J. 2016;18(3):728–36.
Nicolas JM, Bouzom F, Hugues C, Ungell AL. Oral drug absorption in pediatrics: the intestinal wall, its developmental changes and current tools for predictions. Biopharm Drug Dispos. 2017;38(3):209–30.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(DOCX 1580 kb)
Rights and permissions
About this article
Cite this article
Kohlmann, P., Stillhart, C., Kuentz, M. et al. Investigating Oral Absorption of Carbamazepine in Pediatric Populations. AAPS J 19, 1864–1877 (2017). https://doi.org/10.1208/s12248-017-0149-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-017-0149-6