Abstract
The mammary gland is an apocrine organ that undergoes multiple periods of robust change marked with proliferation, differentiation and apoptosis. The profound regenerative potential observed in the mammary gland implies the presence of a population of mammary stem cells (MaSCs) with the capacity to both self-renew and give rise to all mammary lineages. Furthermore, a single mammary epithelial cell enriched for specific cell surface markers has been shown to reconstitute an entire, functional mammary gland in vivo, thereby demonstrating multipotent stem cell potential. The purpose of this chapter is to briefly outline the current state of knowledge on the identity and location of the MaSC, as well as provide a critical overview of the assays utilized to examine MaSC potential.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The mammary gland is composed of an organized bi-layered epithelial ductal network, embedded within mesenchymal components, and serves to effectively deliver milk containing vital nutrients and immune factors to offspring. In humans, the epithelial ductal network arises as a bundle of 5–10 lactiferous ducts extending from the nipples into the mammary fat pads. Bifurcating radially, each lactiferous duct branches off into segmental ducts that end in discrete pyramidal lobules. These lobular structures, called terminal ductal lobulo-alveolar units (TDLUs), are the main functional secretory units of the gland and include an intralobular duct that diverges into terminal ducts (Fig. 1a) [1–3]. These terminal ducts contain clusters of smaller blind-ended ductules that differentiate into milk-secreting acini during lactation. At the cellular level, both the TDLUs and the subtending mammary ducts are bi-layered in nature, with an inner layer of luminal epithelial cells and an outer layer of myoepithelial cells. These latter cells, also referred to as basal cells, are in direct contact with the basement membrane and contract to aid in milk ejection during lactation while luminal cells differentiate into milk-secreting cells during pregnancy and lactation [4, 5]. Mesenchymal components of the mammary fat pad consist of fibroblasts and adipocytes that are interspersed with a variety of immune cells and blood vessels [6]. Connective tissue proteins such as collagen, laminin, fibronectin, tenascin, and others lend structural support to the intricate epithelial ductal tree to build the breast tissue [7, 8].
The mammary gland structure. (a) Schematic of the human ductal system. Arising as 5–10 lactiferous ducts from the nipple, the mammary ductal tree bifurcates in a radial manner with terminal ductal lobular units (TDLUs) as the functional unit. TDLUs form at the end of terminal ducts and consist of an intralobular terminal duct and smaller blind-ended tubules lined with secretory cells. (b) Schematic of the murine mammary gland. The murine mammary gland consists of a single lactiferous duct that bifurcates into 5–10 secondary ducts linearly. The functional units of the murine gland are lobuloalveoli. During puberty, growth primarily occurs at the club-like structures at the distal tip of ducts called terminal end buds. Terminal end buds contain of an inner layer of body cells that align with luminal cells and an outer layer of cap cells that align with basal cells of the subtending duct
The murine mammary gland often serves as an instructive model and has proven to be an insightful tool for investigating mammary stem cell dynamics. There are five pairs of mammary glands in mice, with each gland bearing a single lactiferous duct that bifurcates linearly into 5–10 secondary ducts, with multiple side branches [1]. Analogous in function to TDLUs, lobuloalveoli are the main secretory unit in the murine gland [2, 3]. However, unlike TDLUs, lobuloalveoli have the propensity to develop along both a duct and at the end of a terminal duct (Fig. 1b) [1]. The mesenchyme surrounding the mouse mammary epithelial network is less fibrous and has higher adipocyte content compared to the human breast. Unlike the human breast that has loose intralobular connective tissue and dense interlobular connective tissue forming a slightly exclusive collagenous compartment around the epithelial network, murine epithelial cells are encased in a periductal stroma which is in turn imbedded in fat tissue [9, 10].
Despite these structural differences, similarities in the development and function of the mouse mammary gland inform human mammary biology. The developmental progression of the murine mammary gland observed over a female’s reproductive lifetime, from embryonic development to pregnancy and lactation, recapitulates critical aspects of the human breast. Similarly, the cellular organization of the murine ductal system mirrors the human bi-layered epithelial network consisting of luminal and basal cells (Fig. 2). Comparative transcriptome analyses of normal mouse mammary epithelial populations and human counterparts revealed many conserved gene signatures and pathways with the MaSC-enriched subpopulation showing the highest rate of conservation [11]. Thus, similarities between the two species allow emerging knowledge on murine MaSCs to guide the study of human mammary stem cells.
Mammary cell compartments. (a) The mammary ducts are bi-layered in structure that consist of an inner layer of luminal cells lining the lumen of the duct and an outer layer of basal cells which is in contact with the basement membrane. (b) Cross section of the bi-layered mammary ducts stained with antibodies for cytokeratin 5 (green) and cytokeratin 18 (red), labeling basal and luminal cell layers, respectively
2 Mammary Gland Development and MaSCs
The mammary ductal network primarily develops postnatally and undergoes episodes of distinct but highly regulated morphological changes before maturing into a functional organ. The striking growth and structural remodeling which occur repeatedly over the reproductive life span of a female have been well characterized in both the human breast and murine mammary tissue. Epidemiological studies link these developmental phases to an altered predisposition for breast cancer and experimental evidence from murine models supports a role for MaSCs in driving these morphological developments.
2.1 Prepubertal Mammary Gland and MaSCs
Mammary tissue formation first begins embryonically around day E10–11 as a mammary streak from the anterior to the posterior limb bud, forming a bulbous mammary rudiment by day E12.5 with ducts arising by day E16 in the mouse [12]. Comparably, human breast development begins as the mammary epithelium forms between week 7 and 8 of gestation (typically when the embryo is 5.5 mm in size) and subsequently invades the stroma whilst continuing through various stages of development [13, 14]. Chimera studies using fused blastomeres initially indicate the presence of at least two stem cells embryonically, but the frequency of fetal mammary stem cells has since been characterized [15, 16].
At birth, the gland in both species consists of a primitive rudimentary ductal tree. The gland undergoes isometric growth postnatally until the onset of puberty, when hormones from the hypothalamic–pituitary–ovarian axis trigger the development of the intricate ductal network. Specifically, the ovarian hormone estrogen elicits ductal elongation and expansion while the ovarian hormone progesterone stimulates tertiary branching and lobuloalveologenesis. In mice, growth of the mammary duct during puberty primarily occurs at the distal tips to form enlarged bulbous structures called terminal end buds (TEBs) [17]. Consisting of inner body cells that align with luminal cells of the subtending duct and a single outer layer of cap cells that is continuous with the basal layer, TEBs are the site of active proliferation as the ductal system is generated. During this process, some of the cap cells from the TEBs have been shown to reposition themselves along the extended duct as basal cells. It has been postulated that these cells are a stem cell population in rodents. In humans, although TEB-like structures are found and are the sites of active epithelial proliferation, the corresponding cap cell population is somewhat indiscernible. As a result, the precise nature of the population driving human pubertal mammary changes remains unknown. The epithelial ductal network continues to invade the surrounding stroma until the boundaries of the mammary fat pad are reached, giving rise to the virgin mammary gland by the end of puberty.
The occurrence of label-retaining epithelial cells in the mammary pubertal gland has been examined through the long-term maintenance of bromodeoxyuridine during DNA replication [18–20]. Label retention is thought to be a characteristic of stem cells through asymmetric cell division and label-retaining cells (LRCs) have been specifically detected in the basal fraction during puberty [21]. Identified as a stem cell marker in the hematopoietic system, src homology 2 domain-containing 5′-inositol phosphatase (s-SHIP) is another proposed marker for activated MaSCs. Green fluorescent protein (GFP) expression driven by s-SHIP promoter was found in a subpopulation of cap cells at puberty. This supports the presence of an activated stem cell pool within the cap cell population [22].
2.2 MaSCs and the Adult Mammary Gland
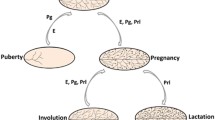
In the adult female, an expansion and regression of the mammary epithelium is observed during each reproductive cycle. The human reproductive cycle, known as the menstrual cycle, is 28 days long on average whereas the rodent estrous cycle generally lasts 4–6 days. Cyclical hormonal changes in the hypothalamic–pituitary–ovarian axis lead to potent fluctuations in ovarian estrogen and progesterone, evoking transient but repeated morphological alterations in the mammary gland. These cellular changes in the gland are often overlooked due to the fact that they are less extensive than the growth observed at puberty or pregnancy. However, the peak of progesterone during the murine diestrus stage, corresponding to the human luteal phase, results in significant side branching and lobuloalveologenesis [23, 24]. Recent studies have demonstrated that mammary epithelium and MaSC frequency undergo notable alterations during each reproductive cycle with MaSCs being defined as cells that have the ability to reconstitute all lineages of a mammary gland in vivo [23]. Specifically, increased progesterone during diestrus drives an up to sixfold expansion in the basal populations and a threefold expansion in the luminal population relative to the estrous stage [23]. These alterations are accompanied by a 7.6-fold increase in functional MaSC activity when comparing diestrus- and estrous-staged mammary cell transplants [23]. This expansion accompanies increased cell proliferation that is tightly regulated and counteracted by increased cell death, thereby preventing an accumulation of mammary epithelium [23]. Overall, the dynamic nature of the gland can be appreciated even in the adult premenopausal female regardless of parity.
2.3 MaSCs During Pregnancy, Lactation, and Involution
The gland undergoes a period of copious proliferation and differentiation during pregnancy and lactation. A prominent formation of alveolar buds takes place under the influence of placental progesterone and prolactin during murine gestation, with these buds differentiating into the milk-secreting alveoli by the end of pregnancy [25]. During human gestation, TDLUs transition from lobule type (Lob)-1 that resemble TEBs to Lob-3 which is the most differentiated lobule type [13, 26, 27]. By the end of pregnancy, not only does the number of cells per TDLU increase dramatically due to proliferation, but the size of each cell also increases due to cytoplasmic enlargement [13, 26, 27].
Post-lactation involution depends on extensive apoptosis peaking at 3–4 days after weaning and results in mammary gland remodeling back to a non-parous-like state by 8 days in the mouse. Although these events have been more intensely studied in rodents, the process occurs in a similar manner in humans. It is characterized by cellular autolysis leading to the collapse of acinar lobules and narrowing tubules, infiltration of phagocytes and round cells, and connective tissue regeneration surrounding the ducts and lobules. The post-involutional breast tissue does not completely return to the virgin state. Instead, the human parous gland contains slightly more glandular tissue and Lob-3 type lobules, reflecting a more differentiated state [13, 26, 27]. These changes in the mammary gland occur again upon subsequent pregnancies.
A unique population of cells, termed parity-induced mammary epithelial cells (PI-MECs), were found to expand within alveolar units during pregnancy, survive involution to persist in the nonpregnant parous female, and serve as progenitors in subsequent pregnancies [28]. Transplantation into cleared mammary fat pads demonstrated that PI-MECs could contribute to both ductal and alveolar development, further implicating self-renewing and multipotent capabilities. In the mouse, s-SHIP expressing cells are also found restricted to the distal tips of alveolar buds during early-mid gestation, before the formation of differentiated alveoli suggesting the alveolar unit to be the putative niche for activated MaSCs during pregnancy [22].
Overall, the mammary gland endures many cycles of remodeling throughout the female life span where it undergoes significant changes in size and function. Pubertal development of the mammary gland alludes to the existence of cells that have the ability to give rise to the full spectrum of mammary epithelial cell types. The successive cycles of epithelial cell turnover that occur as a function of the reproductive cycle or pregnancy further indicate the presence of activated stem or progenitor cell pool(s) in the mature gland which have an inherent ability to self-renew. What is not clear is whether a subpopulation of MaSCs drives the morphological changes observed over the female reproductive life span in vivo or if concerted progenitor activity also contributes to these changes.
3 Mammary Epithelial Stem and Progenitor cells
3.1 Murine MaSCs
Early transplantation studies first introduced the concept of a self-renewing multipotent mammary cell in murine models. In these experiments, mammary epithelial fragments as small as 0.5 mm could regenerate a functional mammary gland when transplanted into the mammary fat pad of a syngeneic host cleared of all endogenous mammary epithelium [29, 30]. The regenerated glands retained hormone responses and successfully lactated, demonstrating the regenerative capacity of select mammary cells to repopulate a mammary fat pad with the appropriate mammary epithelial differentiation program [29–31]. Furthermore, the regenerated gland possessed a finite ability to serially transplant for five to eight generations, unlike neoplastic tissues that have unlimited outgrowth potential [32]. Concurrently, early attempts to identify a putative stem cell population were also based on electron microscopy analyses. Specifically, based on in vitro differentiation potential, small light cells were the candidate MaSC population, characterized with unique ultrastructural features, mitotic figures, and situated between the luminal and basal layers of the gland [33, 34].
Building on the above pioneering work by the Deome laboratory subsequent studies demonstrated that regenerative potential was scattered throughout the epithelial network and persists throughout the life span of a mouse, irrespective of parity [34]. For instance, the mammary epithelial cells from both 26-month-old virgin mice and 3-week-old prepubertal mice were successfully propagated and transplanted for up to five generations [34, 35]. In the same manner, the reconstitution potential of cells from virgin glands, nulliparous, uniparous, and multiparous mice were also found to have little variation [34, 35]. Thus, the consistent presence of select multipotent cells within the mammary gland was proposed since outgrowth potential was affected neither by the age of transplanted mammary tissue nor the developmental stage.
However, the existence of a multipotent MaSC in the gland was most convincingly supported by the formation of a functional mammary gland from a single cell. This phenomenon was established using a retroviral-tagged cell that clonally expanded to produce an extensive ductal tree in an epithelium-divested mammary fat pad [36]. The resulting gland contained both luminal and basal epithelial components and retained the ability to serially transplant [36].
With the foundation built by these studies, the primary goal within the mammary stem cell field subsequently transitioned towards purifying a highly select MaSC population. Initially, based on approaches used to identify stem cells in other systems, a number of candidate populations were examined for putative MaSC activity. Drawing from the hematopoietic system, the dye hoechst33342 was used to isolate a subpopulation of putative stem cells, referred to as the side population (SP) [37]. Stem cells generally have the ability to efflux dye more effectively due to the presence of ABC transporters. The ability to effectively efflux Hoechst dye more rapidly in MaSCs than differentiated cells may arise from the presence of ABCG2, a breast cancer resistance protein belonging to the ABC transporter super family [38, 39]. Originally, the SP population in the mammary gland was thought to enrich for MaSCs since this population was able to generate ductal and alveolar structures in epithelium-divested fat pads [20, 40]. However, current evidence suggests this population enriches for a luminal progenitor. Similarly, cells expressing the marker stem cell antigen-1 (Sca-1) were found to regenerate limited structures upon transplantation, but it too is now proposed to enrich for a luminal hormone receptor positive population [21, 22]. At present, dissociated mammary epithelial cells continue to be separated into distinct subpopulations on the basis of various cell surface markers and assayed for repopulating potential. Notably, studies by Shackleton et al. [21] and Stingl et al. [41] have provided a cell surface marker profile that distinctly isolates the luminal, basal, and stromal cellular subpopulations. Furthermore, a single basal cell has been shown to be capable of reconstituting an entire, functional mammary gland when transplanted into an epithelium-divested fat pad in vivo [21, 41]. Despite the significant progress in characterizing various mammary epithelial subpopulations and in the enrichment of MaSCs through the use of combinations of various cell surface markers, a cell surface signature exclusive to MaSCs remains to be defined.
In addition to evidence from transplantation assays, other approaches utilizing unique transgenic reporter mice have been insightful in understanding MaSC dynamics. A lineage-tracing study has been done to examine stem cell dynamics from embryogenesis to just after birth, through puberty, and following multiple pregnancies [42]. Using yellow fluorescent protein (YFP) expression under tamoxifen or doxycycline inducible lineage-specific cytokeratin promoters, stem cell and progenitor activity was monitored. In this manner, multipotent MaSC were identified embryonically, giving rise to both luminal and basal cell. When YFP expression was induced during puberty, candidate unipotent progenitors were instead identified that solely gave rise to either luminal cell or basal cell, but not both. Moreover, the study proposed that a unipotent basal progenitor reverts to a multipotent progenitor in order to reconstitute a cleared mammary fat pad following transplantation and that this cell does not assume such a function physiologically [42]. Contrastingly, however, more recent lineage-tracing experiments have suggested that Axin-2-positive cells, which are largely restricted to the basal population in adult virgin females, are able to contribute to both the luminal alveolar and basal lineages during pregnancy [43]. To date, the field remains divided as to whether or not MaSCs and/or bipotent progenitors do in fact contribute to mammary gland remodeling, or if physiologically these events are mediated solely by a combined action of basal and luminal unipotent progenitor cells.
3.2 Human MsSC
Studies of microdissected human breast tissues showed conserved X inactivation patterns in contiguous regions of breast epithelium implying that the cells originated from the same progenitor [44]. Similarly, entire ducts or lobules with identical patterns of loss of heterozygosity were also observed, again implicating a common progenitor [45]. Even luminal and basal cells in the same region were found to possess identical chromosomal alterations insinuating a shared ancestor [46]. However, until recently, evidence for human MaSCs was mainly observational due to limitations in our technical ability to test human stem cell potential in vivo. Lack of appropriate in vitro and in vivo assays initially delayed the characterization of human mammary epithelial cells for stem cell potential. In vivo transplantation assays of human mammary cells were compromised by differences in the mouse host stroma in comparison to the human stroma resulting in unsuccessful transplants. Primary mammary epithelial cells also have restricted colony-forming ability in vitro due to limited replication and differentiation capacity in solid matrix culturing systems. Thus advances in establishing in vivo and in vitro measures for human stem cell potential have been paramount in strengthening evidence for human MaSCs.
Progressive improvements in the dissociation of mammary tissue, the use of feeder layers, and the development of special culturing media have enabled human epithelial cells to be successfully cultured in vitro [47–50]. The technique of culturing mammospheres has even provided the first evidence of human mammary epithelial differentiation ex vivo through the formation of mixed and basal staining colonies from a single clonal monolayer under differentiating stimuli [49]. Additionally, improvements have also been made towards measuring stem cell potential in vivo by “humanizing” the murine mammary fat pad. Cleared murine fat pads colonized with immortalized human fibroblasts have helped render the murine fat pad a suitable environment for supporting human mammary outgrowths [51, 52]. Transplanting mammary tissue under the renal capsule of CD-1 nude mice maintained viable mammary epithelium that expressed appropriate luminal and basal markers and hormone receptors and even produced beta-casein and milk fat globule membrane proteins when the hosts became pregnant [53]. A method for quantifying human MaSC frequency has been established by combining human breast epithelium with immortalized human breast fibroblasts and co-inoculating this mix into either the mammary fat pad or under the renal kidney capsule of an immunocompromised mouse which is followed by in vitro assays [54]. This technique has been successful in generating outgrowths from select subpopulations of mammary epithelial cells, paralleling what is often observed in murine mammary epithelial transplant studies [51, 54, 55]. Using these in vivo and in vitro techniques in conjunction has also proved fruitful as staining mammospheres with PKH26, a lipophilic dye that is retained in slowly dividing cells, has shown to further enrich for cells with MaSC activity. This activity was tested by transplantation into a humanized mouse mammary gland [56]. In this manner, many new avenues of characterizing human MaSCs are now possible and will undoubtedly broaden the field of knowledge.
3.3 Progenitors
In the attempts to uncover the multipotent mammary stem cell, distinct progenitors with a parent–progeny relationship to the multipotent stem cell have also been identified. For instance, early limiting dilution transplantations lead to the identification of three distinct progenitor populations: a progenitor that forms both ducts and alveoli, a progenitor that gives rise to ducts alone, and another that solely forms alveoli [57]. As a result, the notion of a mammary epithelial hierarchy was established. However, over the years, due to the use of different experimental approaches, the above hierarchy has been questioned. Other studies have suggested the presence of progenitors that form colonies with only luminal epithelial cells, only myoepithelial cells, or colonies with a mixture of both luminal and myoepthelial cells, indicating the presence of a bipotent progenitor [47, 48, 58]. As a result, it remains to be determined whether the lineage-restricting step for ductal vs. alveolar progenitor commitment or basal vs. luminal progenitor commitment occurs first.
Overall, there are two proposed mammary epithelial hierarchies supported by different experimental approaches, but it still remains unclear as to which is the more biologically relevant hierarchy. The first model proposes that a bipotent progenitor downstream of the mammary stem cell gives rise to a luminal progenitor and a myoepithelial progenitor. The luminal progenitor is postulated to then give rise to ductal progenitors and alveolar progenitors, which in turn form ductal cells and alveolar cells respectively. The myoepithelial progenitor is thought to form basal cells. In the second model, the multipotent stem cell gives rise to a ductal progenitor and an alveolar progenitor. The ductal progenitor then goes on to form ductal cells or basal cells while the alveolar progenitor goes on to form alveolar cells or basal cells (Fig. 3).
Mammary epithelial cell hierarchy models. Based on different experimental approaches, there are currently two main models for the mammary epithelial hierarchy. The first model (left) contains a ductal progenitor and an alveolar progenitor with the latter giving rise to both basal cells and alveolar cells. The second model (right) contains a bipotent progenitor which gives rise to basal, alveolar, and luminal progenitors
4 Characterizing Mammary Stem Cells
Considerable effort has been focused on isolating a pure MaSC population. Using purification strategies from other systems, such as the hematopoietic and digestive system where the stem cell hierarchy is better established, various putative markers have been tested. Although current methods have enabled for the enrichment of this small cell fraction, a signature strictly unique to MaSC remains to be elucidated.
4.1 Murine and Human MaSC Markers
Based on the expression of heat stable antigen (CD24) in conjunction with either α6 integrin (CD49f) or β1 integrin (CD29), the mammary gland can be resolved into three distinct isolated subpopulations [21, 41, 59]. Luminal epithelial cells are characterized as Lin− CD24med/+ CD49flo or Lin− CD24med/+ CD29lo, basal cells as Lin− CD24med/+ CD49fhi or Lin− CD24med/+ CD29hi, and stromal cells as Lin− CD24lo/− CD49flo or Lin− CD24lo/− CD29lo.
MaSCs are enriched specifically within the basal compartment, with transplantation of FACS-purified basal cells, but not luminal or basal cells, yielding functional mammary outgrowths. Containing both ductal and alveolar structures, glands generated from basal cells possessed the full spectrum of epithelial cells and exhibited the ability to serially transplant. The generation of an entire mammary gland from a single cell from the basal population further solidified the location of the MaSC within the basal compartment [21, 41]. It has also been suggested that the tip of the basal population highest in expression for CD49 and CD24 may further enrich for MaSCs, also referred to as the mammary repopulating unit (MRU) population [41]. The marker CD29 has even been suggested to play a functional role in mammary stem cell biology as deletion of the β1 integrin from the basal compartment resulted in a lower reconstitution frequency in secondary transplants [60]. LRG5 and Axin2 are two other markers shown to further enrich for MaSCs within the basal compartment [61, 62]. The multipotent MaSCs enriched in the basal population have also been further characterized as hormone receptor negative and were not observed to express the estrogen receptor (ERα), the progesterone receptor, or the ErbB2 receptor [63]. Transplantations of sorted murine basal cells at limiting dilution have led to the estimate that a mammary stem cell is situated at a frequency of about 1 in a few hundred cells within the basal population, although estimates range from 1 in 100 to 1 in 2,500 basal cells [23, 41].
Using hormone-treated immunodeficient mice, human breast epithelial cells incorporated with human fibroblasts and collagen injected at a non-orthotopic site under the kidney capsule have resulted in the regeneration of a mammary gland [54]. The gland was only regenerated from the CD49fhi EpCAM−/lo basal cell population but not the luminal fraction and the regenerated glands were also able to form clonogenic progenitors in vitro [54]. Implantation of the CD49fhi EpCAM−/lo basal cell population was again shown to regenerate a functional mammary gland when combined with immortalized human breast fibroblasts in an immunodeficient mouse mammary fat pad [55]. At this orthotopic site, the resulting regenerated gland contained lobular regions similar to TDLUs that were capable of fully differentiating into terminal alveolar structures. Although a suboptimal regenerative ability was observed in serial transplants, likely resulting from nonoptimal growth conditions, the CD49fhi EpCAM−/lo basal human breast epithelial cell population is thought to contain human MaSCs. These developments in quantifying MaSC frequency in humans have led to estimates of between 1/1,000 and 1/10,000 MaSc in the human breast [54].
4.2 Murine Progenitor Markers
Various candidate luminal progenitors pools have been identified in the mouse mammary gland within the Lin− CD24med/+ CD49flo/CD29lo population. Based on colony forming assays, the luminal fraction seems to contain luminal progenitor cells that form discrete colonies in vitro when placed in low cell-density adherent cultures. Cells derived from MRU outgrowths also result in these types of colonies and are therefore believed to be the parent population to the luminal progenitors. Different populations of luminal progenitors have been further resolved from the Lin− CD24med/+ CD49flo/CD29lo population using additional markers. Notably, enrichment for a luminal progenitor from a more differentiated luminal cell has been shown using the β3 integrin marker (CD61+) or the lack of CD133 prominin1 or Sca-1 [64, 65]. The exact degree to which these two populations overlap is still unclear; however, the CD61+ luminal progenitor is the first cell in the mammary epithelial hierarchy believed to express estrogen receptor (ER α), while the Sca-1− luminal progenitor population is generally thought to be a hormone receptor negative progenitor population based on high colony-forming ability [66]. Sca-1− cells are also candidate alveolar progenitors since they have been shown to express more milk protein genes while Sca-1+ populations are thought to be hormone receptor positive. Moreover, the expression of c-Kit in conjunction with Sca-1 expression was shown to enrich for estrogen receptor positive luminal progenitors (c-Kit+ Sca-1+) and estrogen receptor negative luminal progenitors (c-Kit+ Sca-1−) that are also believed to be the alveolar progenitors [67]. Recently the expression of the α2 integrin (CD49b) has been found to better resolve these luminal progenitors into the estrogen receptor positive (CD49b+ Sca-1+) and estrogen receptor negative luminal progenitor populations (CD49b+ Sca-1−) [68].
4.3 Human Progenitor Markers
Although the ability to test stem potential in vivo has been limited until recently, bipotent mammary epithelial progenitors that form either luminal or basal colonies as well as mixed luminal plus basal colonies have been detected previously based on in vitro cultures. These bipotent progenitors have been enriched using flow cytometry and immunomagnetic sorting strategies based on the expression of a cohort of markers including MUC1 [69], CD10/CALLA [70], ESA/EpCAM [58, 71], CD49f [72], CD24, CD133, and Thy1 [47, 54, 55, 73]. EpCAM+ MUC1+ cells have been shown to enrich for progenitors that form luminal colonies while CD10+ progenitors form basal colonies [47, 54, 55, 73, 74]. Cells that are EpCAM−MUC−/weakCD10+/weak have been shown to make mixed colonies and were also found to express high levels of α6 integrin (CD49f), indicating a basal position in vivo. This data insinuates that the bipotent progenitor in humans is EpCAM−MUC−/weakCD10+/weak and immortalized EpCAM− MUC1− further support this idea since they are able to self-renew and generate luminal as well as basal cells while immortalized EpCAM+ MUC1+ cells are restricted to the luminal lineage [47]. EpCAM+ MUC1− also expressed high level of keratin 19, a feature of TDLUs in vivo and EpCAM+ MUC1− cells formed branching structures similar to uncultured TDLUs in 3D cultures as well as in vivo transplantations, suggesting that they may be a TDLU precursor in the breast. The markers EpCAM and CD49f in conjunction with the marker ALDH are also proposed to separate nonclonogenic luminal cells from relatively differentiated luminal progenitors (EpCAM+ CD49f+ ALDH−) and undifferentiated luminal progenitors (EpCAM+ CD49f+ ALDH+). Possessing a gene signature related to alveolar differentiation, ALDH luminal progenitors are proposed to be analogous to CD49b+ Sca-1− luminal progenitors in the mouse [68]. Recent evidence also suggests GATA3 as a luminal marker and ErbB2 as an estrogen receptor positive luminal progenitor marker [52, 64]. Other basal cell markers in humans include p63 and SMA [75].
5 Assessing Stem Cell Potential
Until quite recently, a select number of assays have been utilized as standard techniques to measure the stem cell potential of cells in the mammary gland. However, recent advances in the way stem cell potential is examined have led to a number of unique insights regarding MaSC dynamics. Although all of these tools have been informative, there is still an array of underlying limitations and caveats that must not be overlooked.
5.1 FACS-Based Analyses
The application of fluorescence-activated cell sorting (FACS) has greatly advanced mammary epithelial characterization. Using total dissociated mammary cells, a cocktail of antibodies, and a series of gates that deplete for doublets, immune cells, and dead cells while marking cells of interest, the mammary epithelial subpopulations can specifically be isolated and purified from the total gland (Fig. 4).
Cell surface characterization of mammary cell subpopulations by flow cytometry. FACS plots showing the gating strategy used to isolate mammary epithelial subpopulations. Excluding for debris, dead cells (PI+), and lineage-positive cells (CD45+, Ter 119+, CD31+), mammary cells can be separated into the luminal, basal, and stromal compartment using the markers CD24 and CD49f. Further gating on just the luminal subpopulation, the markers Sca-1 and CD49b can further segregate the population into distinct progenitor and differentiated luminal cell fractions
Although FACS-based transplantation assays have yielded an inscrutable amount of useful data, there are a number of technical issues that should be taken into consideration. To begin with, using freshly dissociated mammary cells is imperative for gaining biologically relevant data. Although this is often difficult for human breast epithelium, it is important to avoid culturing cells before analysis as the inherent biology of the cells becomes altered. For instance, Sca-1, a progenitor cell surface marker, becomes induced in mammary epithelial cells after culturing, which can confound results. The dissociation protocol itself can significantly affect results and has the potential to alter the types of cells that are successfully dissociated. A number of different techniques have been developed to circumvent some of the concerns associated with dissociating the mammary gland into single cells, although each still has its own caveats [76]. Other factors that should be taken into account include the antibodies themselves since some antibodies, such as those for CD24, have been shown to bind with variable efficacy when doing flow cytometry and alter the cellular profiles obtained [76].
When using mouse models, the stage of the mouse during the reproductive cycle is often overlooked which can have profound effects, particularly when conducting transplantations assays or FACS-based analyses. The profound influence of hormones on the mammary gland has always been accepted, but the specific mitogenic effects of progesterone during the reproductive cycle on the mammary gland and MaSCs in particular have been clearly reported [23]. As a result, it is important to take into account the reproductive stage of a mouse when conducting these analyses since MaSC numbers can be greatly confounded by the hormone status of the animal.
5.2 In Vitro Colony-Forming Assays
Since transplantation studies were not possible with human breast epithelium until recently, the colony-forming assays became an imperative tool for exploring multipotent ability of human mammary cells. There are two major methods of conducting colony-forming assays, the first is a culture that utilizes a feeder layer of NIH3T3 cells and the second is a 3D culture in Matrigel [21, 41, 64, 65] (Fig. 5).
An outline of colony-forming assays. Mammary tissue is dissociated using collagenase treatment to generate a single cell preparation. CFC assays can be performed on total mammary cells or on FACS-purified cell populations. The cells are plated onto a layer of irradiated feeder cells and incubated in 20 % O2 if assessing luminal colony-forming potential and in 5 % O2 if assessing basal stem cell potential, followed by scoring for number of colonies generated. Cells can alternatively be resuspended in matrigel and grown for 16 days to develop 3D colonies that are either acinar or solid in nature. These 3D colonies can be subsequently fixed, sectioned, and stained for luminal and basal markers
The colony-forming assay using the feeder layer requires the initial irradiation of NIH3T3 cells. The irradiated cells are then mixed with mammary epithelial cells and plated on a dish. To generate luminal type colonies, the plates are cultured for 7 days at 37 °C at 20 % oxygen levels while basal colonies form when cultured for 7 days at 5 % oxygen levels at 37 °C. This method is primarily used to quantify progenitor frequencies and colony-forming capacity within a population.
Matrigel cultures are done by resuspending mammary epithelial cells in 50 μl of Matrigel covered with 4 ml of Epicult B medium containing 5 % FCS. After 16 days of culture, the 50 μl Matrigel culture is fixed in 4 % paraformaldehyde, embedded in 1 % agarose, and then fixed again in 4 % paraformaldehyde. Finally the colonies can be sectioned and stained using hematoxylin and eosin. Mainly used to stain colonies, this method is not typically used for calculating progenitor frequencies due to concerns about obtaining accurate colony counts across the various planes of the plate.
A caveat with culturing cells in vitro is that it likely alters the inherent biological nature of the cells being examined. In a dynamic system such as the mammary gland where there is a complex interplay of signaling factors from not only the microenvironment but also systemically in the case of hormones, it is difficult to recapitulate the biologically relevant signaling milieu. As a result, capturing a response in vitro may not always be possible. Furthermore, mammary epithelial cells have already been shown to alter their expression of certain cell surface markers upon culture. Therefore, in vitro studies provide a readout for cellular potency, although they are not the ideal assays.
5.3 In Vivo Transplantation Assays
Transplantation assays have become the gold standard for assessing stem cell potential and have been widely used in the field for some time. Using prepubertal mice between the ages of day 19 and 21, mice are cleared of endogenous mammary epithelium in their fourth inguinal glands and cells for transplantation are injected into the mammary fat pad. Serial transplantations done in this manner thus assess stem cell potential since both the ability to self-renew and the multipotent ability to generate the full epithelial hierarchy can be examined. Transplanting cells at limiting numbers also allows for the quantification of MaSC frequency. In conjunction with recent advances in flow cytometry, the ability to sort specific subpopulations and transplant cells to evaluate stem cell function has been invaluable.
The ability to do transplantation assays in humans was originally limited due to differences in the mouse stroma in comparison to the human breast leading to unsuccessful transplants. However, advances in “humanizing” the murine mammary fat pad with immortalized human breast fibroblasts in immunocompromised mice by simulating human stroma have resulted in the successful transplantation of human breast epithelium. Outgrowths in the orthotopic site of a mouse mammary gland as well as the non-orthotopic site under the kidney capsule have been successfully generated when a combination of immortalized human breast fibroblasts and human breast epithelium were injected in a collagen gel [51, 54, 55].
Although transplantations allow for the assessment of multipotency through the regeneration of a complete functional mammary gland and self-renewal through serial transplantations, lineage-tracing studies suggest that this does not accurately reflect the way basal cells behave physiologically. Instead it is proposed that basal unipotent progenitors revert to a bipotent state during transplantation assays responding to meet the homeostatic maintenance demands of a system which perhaps simulates injury [42]. Thus although single cells have been shown to generate an entire mammary gland, it is important not to overlook the potential impact of placing cells in a environment that may extraneously stimulate MaSC activity which would not occur in the intact physiological state. It is important to consider the critical role the stem cell niche plays in directing MaSC activity and how transplantation assays may not necessarily reflect this crucial component [77].
6 Conclusions
There have been significant advances in providing evidence for a mammary stem cell but much remains to be uncovered about the true identity as well as the precise location of this stem cell population. The mammary epithelial hierarchy is constantly beginning redefined. Specifically, the identification of a highly defined cell surface marker signature for MaSCs, apart from mature basal cells, awaits identification. Various mammary progenitor pools are also being identified, refined, and characterized. Moreover, lineage-tracing studies are beginning to raise new questions and suggest that the gold standard transplantation assay may not accurately reflect the physiological behavior of mammary epithelial cells. Further murine studies coupled with advances in the ability to assess human MaSC/progenitor activity will undoubtedly lead to a better understanding of the human breast.
Abbreviations
- FACS:
-
Fluorescence-activated cell sorting
- FCS:
-
Fetal calf serum
- GFP:
-
Green fluorescent protein
- LRC:
-
Label-retaining cells
- MaSC:
-
Mammary stem cell
- MRU:
-
Mammary repopulating unit
- PI-MEC:
-
Parity-induced mammary epithelial cells
- SP:
-
Side population
- TDLU:
-
Terminal ductal lobular units
- TEB:
-
Terminal end buds
- YFP:
-
Yellow fluorescent protein
References
Cardiff RD, Wellings SR (1999) The comparative pathology of human and mouse mammary glands. J Mammary Gland Biol Neoplasia 4(1):105–122
Cardiff RD (1998) Are the TDLU of the human the same as the LA of mice? J Mammary Gland Biol Neoplasia 3(1):3–5
Russo J et al (1990) Comparative study of human and rat mammary tumorigenesis. Lab Invest 62(3):244–278
Murrell TG (1995) The potential for oxytocin (OT) to prevent breast cancer: a hypothesis. Breast Cancer Res Treat 35(2):225–229
Ronnov-Jessen L, Petersen OW, Bissell MJ (1996) Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev 76(1):69–125
Wiseman BS, Werb Z (2002) Stromal effects on mammary gland development and breast cancer. Science 296(5570):1046–1049
Schedin P et al (2004) Mammary ECM composition and function are altered by reproductive state. Mol Carcinog 41(4):207–220
Muschler J, Streuli CH (2010) Cell-matrix interactions in mammary gland development and breast cancer. Cold Spring Harb Perspect Biol 2(10):a003202
Hovey RC, McFadden TB, Akers RM (1999) Regulation of mammary gland growth and morphogenesis by the mammary fat pad: a species comparison. J Mammary Gland Biol Neoplasia 4(1):53–68
Weaver VM et al (1996) The importance of the microenvironment in breast cancer progression: recapitulation of mammary tumorigenesis using a unique human mammary epithelial cell model and a three-dimensional culture assay. Biochem Cell Biol 74(6):833–851
Lim E et al (2010) Transcriptome analyses of mouse and human mammary cell subpopulations reveal multiple conserved genes and pathways. Breast Cancer Res 12(2):R21
Veltmaat JM et al (2003) Mouse embryonic mammogenesis as a model for the molecular regulation of pattern formation. Differentiation 71(1):1–17
Russo J, Russo IH (2004) Development of the human breast. Maturitas 49(1):2–15
Hovey RC, Trott JF, Vonderhaar BK (2002) Establishing a framework for the functional mammary gland: from endocrinology to morphology. J Mammary Gland Biol Neoplasia 7(1):17–38
Mintz B, Slemmer G (1969) Gene control of neoplasia. I. Genotypic mosaicism in normal and preneoplastic mammary glands of allophenic mice. J Natl Cancer Inst 43(1):87–109
Spike BT et al (2012) A mammary stem cell population identified and characterized in late embryogenesis reveals similarities to human breast cancer. Cell Stem Cell 10(2):183–197
Williams JM, Daniel CW (1983) Mammary ductal elongation: differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev Biol 97(2):274–290
Smith GH (2005) Label-retaining epithelial cells in mouse mammary gland divide asymmetrically and retain their template DNA strands. Development 132(4):681–687
Zeps N et al (1998) Estrogen receptor-negative epithelial cells in mouse mammary gland development and growth. Differentiation 62(5):221–226
Welm BE et al (2002) Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev Biol 245(1):42–56
Shackleton M et al (2006) Generation of a functional mammary gland from a single stem cell. Nature 439(7072):84–88
Bai L, Rohrschneider LR (2010) s-SHIP promoter expression marks activated stem cells in developing mouse mammary tissue. Genes Dev 24(17):1882–1892
Joshi PA et al (2010) Progesterone induces adult mammary stem cell expansion. Nature 465(7299):803–807
Andres AC, Strange R (1999) Apoptosis in the estrous and menstrual cycles. J Mammary Gland Biol Neoplasia 4(2):221–228
Richert MM et al (2000) An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia 5(2):227–241
Russo J et al (2005) The protective role of pregnancy in breast cancer. Breast Cancer Res 7(3):131–142
Russo J, Rivera R, Russo IH (1992) Influence of age and parity on the development of the human breast. Breast Cancer Res Treat 23(3):211–218
Matulka LA, Triplett AA, Wagner KU (2007) Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol 303(1):29–44
Deome KB et al (1959) Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res 19(5):515–520
Faulkin LJ Jr, Deome KB (1960) Regulation of growth and spacing of gland elements in the mammary fat pad of the C3H mouse. J Natl Cancer Inst 24:953–969
Daniel CW, Deome KB (1965) Growth of mouse mammary glands in vivo after monolayer culture. Science 149(3684):634–636
Daniel CW et al (1975) Unlimited division potential of precancerous mouse mammary cells after spontaneous or carcinogen-induced transformation. Fed Proc 34(1):64–67
Chepko G, Smith GH (1997) Three division-competent, structurally-distinct cell populations contribute to murine mammary epithelial renewal. Tissue Cell 29(2):239–253
Smith GH, Medina D (1988) A morphologically distinct candidate for an epithelial stem cell in mouse mammary gland. J Cell Sci 90(Pt 1):173–183
Young LJ et al (1971) The influence of host and tissue age on life span and growth rate of serially transplanted mouse mammary gland. Exp Gerontol 6(1):49–56
Kordon EC, Smith GH (1998) An entire functional mammary gland may comprise the progeny from a single cell. Development 125(10):1921–1930
Goodell MA et al (1996) Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 183(4):1797–1806
Zhou S et al (2002) Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc Natl Acad Sci U S A 99(19):12339–12344
Jonker JW et al (2005) Contribution of the ABC transporters Bcrp1 and Mdr1a/1b to the side population phenotype in mammary gland and bone marrow of mice. Stem Cells 23(8):1059–1065
Alvi AJ et al (2003) Functional and molecular characterisation of mammary side population cells. Breast Cancer Res 5(1):R1–R8
Stingl J et al (2006) Purification and unique properties of mammary epithelial stem cells. Nature 439(7079):993–997
Van Keymeulen A et al (2011) Distinct stem cells contribute to mammary gland development and maintenance. Nature 479(7372):189–193
van Amerongen R, Bowman AN, Nusse R (2012) Developmental stage and time dictate the fate of Wnt/beta-catenin-responsive stem cells in the mammary gland. Cell Stem Cell 11(3):387–400
Tsai YC et al (1996) Contiguous patches of normal human mammary epithelium derived from a single stem cell: implications for breast carcinogenesis. Cancer Res 56(2):402–404
Lakhani SR et al (1999) Genetic alterations in ‘normal’ luminal and myoepithelial cells of the breast. J Pathol 189(4):496–503
Deng G et al (1996) Loss of heterozygosity in normal tissue adjacent to breast carcinomas. Science 274(5295):2057–2059
Stingl J et al (2001) Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res Treat 67(2):93–109
Gudjonsson T et al (2002) Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev 16(6):693–706
Dontu G et al (2003) In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 17(10):1253–1270
Villadsen R et al (2007) Evidence for a stem cell hierarchy in the adult human breast. J Cell Biol 177(1):87–101
Kuperwasser C et al (2004) Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci U S A 101(14):4966–4971
Ginestier C et al (2007) ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1(5):555–567
Parmar H et al (2002) A novel method for growing human breast epithelium in vivo using mouse and human mammary fibroblasts. Endocrinology 143(12):4886–4896
Eirew P et al (2008) A method for quantifying normal human mammary epithelial stem cells with in vivo regenerative ability. Nat Med 14(12):1384–1389
Lim E et al (2009) Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med 15(8):907–913
Pece S et al (2010) Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell 140(1):62–73
Smith GH (1996) Experimental mammary epithelial morphogenesis in an in vivo model: evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Res Treat 39(1):21–31
Stingl J et al (1998) Phenotypic and functional characterization in vitro of a multipotent epithelial cell present in the normal adult human breast. Differentiation 63(4):201–213
Sleeman KE et al (2006) CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Res 8(1):R7
Taddei I et al (2008) Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat Cell Biol 10(6):716–722
Zeng YA, Nusse R (2010) Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell 6(6):568–577
Plaks V et al (2013) Lgr5-expressing cells are sufficient and necessary for postnatal mammary gland organogenesis. Cell Rep 3(1):70–78
Asselin-Labat ML et al (2006) Steroid hormone receptor status of mouse mammary stem cells. J Natl Cancer Inst 98(14):1011–1014
Asselin-Labat ML et al (2007) Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol 9(2):201–209
Sleeman KE et al (2007) Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. J Cell Biol 176(1):19–26
Kendrick H et al (2008) Transcriptome analysis of mammary epithelial subpopulations identifies novel determinants of lineage commitment and cell fate. BMC Genomics 9:591
Regan JL et al (2012) c-Kit is required for growth and survival of the cells of origin of Brca1-mutation-associated breast cancer. Oncogene 31(7):869–883
Shehata M et al (2012) Phenotypic and functional characterization of the luminal cell hierarchy of the mammary gland. Breast Cancer Res 14(5):R134
Taylor-Papadimitriou J et al (2002) MUC1 and the immunobiology of cancer. J Mammary Gland Biol Neoplasia 7(2):209–221
Gusterson BA et al (1986) Identification of myoepithelial cells in human and rat breasts by anti-common acute lymphoblastic leukemia antigen antibody A12. J Natl Cancer Inst 77(2):343–349
Latza U et al (1990) Ber-EP4: new monoclonal antibody which distinguishes epithelia from mesothelial. J Clin Pathol 43(3):213–219
Koukoulis GK et al (1991) Immunohistochemical localization of integrins in the normal, hyperplastic, and neoplastic breast. Correlations with their functions as receptors and cell adhesion molecules. Am J Pathol 139(4):787–799
Raouf A et al (2008) Transcriptome analysis of the normal human mammary cell commitment and differentiation process. Cell Stem Cell 3(1):109–118
Clayton H, Titley I, Vivanco M (2004) Growth and differentiation of progenitor/stem cells derived from the human mammary gland. Exp Cell Res 297(2):444–460
Skalli O et al (1986) A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol 103(6 Pt 2):2787–2796
Smalley MJ et al (2012) Isolation of mouse mammary epithelial subpopulations: a comparison of leading methods. J Mammary Gland Biol Neoplasia 17(2):91–97
Joshi PA, Di Grappa MA, Khokha R (2012) Active allies: hormones, stem cells and the niche in adult mammopoiesis. Trends Endocrinol Metab 23(6):299–309
Acknowledgments
The authors would like to thank Mr. Hartland Jackson for providing the images of colonies from various colony-forming assays and for sample flow cytometry plots. The authors would also like to thank Dr. Alison Casey, Mr. Hartland Jackson, and Dr. Purna Joshi for their critical reading of the manuscript. This work was supported by funding to the Khokha lab from the Canadian Institutes of Health Research (CIHR), the Canadian Cancer Society Research Institute (CCSRI), and the Canadian Breast Cancer Foundation (CBCF). Pirashaanthy Tharmapalan is supported by a CBCF studentship.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Tharmapalan, P., Khokha, R. (2014). Adult Mammary Stem Cells: Identity, Location, and Functional Assays. In: Turksen, K. (eds) Adult Stem Cells. Stem Cell Biology and Regenerative Medicine. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4614-9569-7_9
Download citation
DOI: https://doi.org/10.1007/978-1-4614-9569-7_9
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4614-9568-0
Online ISBN: 978-1-4614-9569-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)