Abstract
An entire mammary epithelial outgrowth, capable of full secretory differentiation, may comprise the progeny of a single stem cell. Experimental evidence has indicated three distinct mammary progenitor subtypes: unlimited progenitor cells, duct-limited progenitors, and lobule-limited progenitor cells. These different progenitor cell types drive the individual stages of mammary development taking direction from the surrounding microenvironment and systemic hormones. Mammary epithelial stem cells reside in basal positions, in contact with the basement membrane, but not in luminal positions throughout the mammary ductal tree. Stem cell morphology is characterized by the absence of cellular organelles resulting in their light or pale appearance in thin and ultrathin tissue sections. In recent years mammary stem and progenitor cells have been isolated based on the expression of cell surface markers, thus allowing increased study of these cells. The purpose of this chapter is to summarize the experimental history of mammary stem cells and to address future areas and challenges for the investigation of these cells.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Experimental Evidence Supporting the Existence of Mammary Stem/Progenitor Cells
The experiments that originally demonstrated the existence of stem cells in the mammary gland were based on the pioneering studies of DeOme and his students, Les Faulkin and Charles Daniel. They developed and optimized serial transplantation of normal mammary gland into the cleared mammary fat pad of syngeneic mice [1, 2]. They demonstrated that the normal mammary gland contains cells that will grow and fill the fat pad with a normal ductal mammary tree and respond to hormones with a normal differentiation program [3]. The progeny of the transplanted cells could be serially transplanted into the appropriate recipients for multiple times; however, unlike preneoplastic or neoplastic cells, the normal cells always senesced after multiple serial transplants, generally 5–8 transplant generations [3]. This was interpreted as indicating mammary stem cells possessed a finite proliferative activity (i.e., life span). This finite life span is a fundamental difference between normal and preneoplastic/neoplastic mammary cells. Cells with an indefinite in vivo life span (i.e., immortalized) have been identified in numerous mammary model systems, including MMTV-induced alveolar hyperplasias [4], chemical carcinogen-induced ductal and alveolar hyperplasias [5, 6], hormonally induced alveolar hyperplasia, spontaneously immortalized ductal hyperplasias [7, 8], and cells containing specific genetic alterations (i.e., p53 deletion, polyoma mT antigen) [9, 10]. These immortalized populations can be non-tumorigenic, weakly tumorigenic, or highly tumorigenic [10–12]. One might speculate that the ability to proliferate over 8–12 serial transplant generations before exhibiting a decrease and loss of proliferation activity would indicate an increase of stem cell number or activity as a consequence of some treatment. As of the end of 2011, this assay has not yet been applied in any stem cell study.
Subsequent studies demonstrated that stem cells were located along the entire mammary ductal tree and represented in all the different developmental states of the mammary gland. Host age and reproductive history had little influence on the frequency of stem cells as measured by percent successful takes and life span assay [13, 14]. Mammary cells taken from 26-month-old virgin mice had the same transplant potential as cells taken from 3-week-old mice. Both cell populations senesced after five transplant generations. Similarly, mammary cells in 12-month-old multiparous mice had the same serial transplant potential as cells from 3-week-old virgin mice [13]. Finally, continuous hormone stimulation did not induce additional loss of ductal growth potential. These results have important implications for understanding the role of mammary stem cells in normal mammary development because they emphasize that the mammary stem cell is a relatively quiescent cell that is only activated under conditions of gland repopulation (i.e., fetal growth stage, pubertal growth phase). Under other conditions, such as pregnancy, it is likely that ductal and alveolar progenitor cells form the bulk of the increased mammary epithelial cell population [15] (see discussion in next section).
These early studies emphasized that stem cell life span is intricately linked to proliferation activity. For example, life span was correlated with the interval of serial transplantation. Thus, transplanting at 12-month intervals instead of 3-month intervals prolonged the ultimate life span of normal cells [13, 16]. Similarly, transplanting from the periphery of the ductal outgrowth (i.e., such cells would have undergone more cell divisions) resulted in earlier senescence than transplanting cells from the center (i.e., the original transplant site) of the outgrowth. In summary, these early studies suggested the presence of a mammary cell that could repopulate the mammary gland and undergo a normal and complete morphogenetic program (i.e., a stem cell). Such cells are spaced throughout the mammary tree, are quiescent, and have a finite life span. A commonly stated assumption that normal mammary stem cells are an ideal target for oncogenic transformation because they, like cancer cells, share a long life span (i.e., replicative potential) is not supported by the transplantation results. At least for the mammary gland, the evidence to date suggests that mammary stem cells have a finite life span.
Morphologic Evidence of Stem Cells Among Mammary Epithelium
Distinguishing mammary cells was first based on their ultrastructural appearance [14]. Undifferentiated (pale) cells were found which exhibited the expected behavior of stem cells in mammary explants induced in vitro to differentiate toward secretory cell fates. It was discovered that mouse mammary explants, like mammary epithelium in situ, contained pale- or light-staining cells and that it was only these cells that entered mitosis when mammary explants were cultured.
Light cells were analyzed in mouse and rat mammary glands in the electron microscope utilizing their ultrastructural features to distinguish them from other mammary epithelial cells (Fig. 1) [17]. Both small light cells (SLC) and undifferentiated large light cells (ULLC) (Fig. 1) were observed with condensed mitotic chromosomes indicative of their replicative competence in mouse mammary explants, pregnant and lactating mouse mammary glands, and rat mammary gland from 17 stages of development beginning with nulliparity through pregnancy, lactation, and involution [17–20]. Partially differentiated ULLC or differentiating large light cells (DLLC) were observed in rapidly proliferating mammary epithelium during pregnancy and probably represent transient-amplifying epithelial cells committed to a secretory fate. Using all of the above features, a more detailed description of the epithelial subtypes that comprise the mammary epithelium was established.
Electron micrographs taken of a secretory acinus in a fully lactating female mouse showing (a) large (ULLC) light cell juxtaposed (but undifferentiated) to differentiated secretory dark epithelial cells and (b) a small light (SLC) cell depicted in a secretory acinus. SLC are found exclusively located near the basement membrane and never are found in contact with the lumen (shown here at the bottom of the figure characterized by the presence of microvilli on the surface of the secretory cells and the presence of dark casein micelles)
Evaluation of the 17 stages of mammary gland development showed that the population density (number of cells/mm2) of SLC among mammary epithelium did not change from puberty through post-lactation involution. The proportion of SLC in the epithelial population remained unchanged. This means that although the number of mammary epithelial cells increased by 27-fold during pregnancy in the mouse, the percent of SLC in the population did not change [21, 22]. Therefore SLC increase and decrease in absolute number at the same relative rate as the expanding epithelial cell population, suggesting that they have a capacity for self-renewal. In contrast, ULLC numbers were much more variable, perhaps indicative of their transitional nature.
Absence of SLC and ULLC in Growth Senescent Mammary Tissues
Mammary epithelial cells bearing the morphological characteristics of undifferentiated stem cells (i.e., SLC and ULLC) likewise disappear from senescent populations simultaneous with growth cessation [23]. In premalignant mammary epithelial populations, which exhibit indefinitely prolonged growth potential, both of these cell types (SLC and ULLC) are maintained.
A study of human breast epithelium demonstrated the presence of mammary epithelial cells possessing the ability to regenerate elaborate branching structures resembling mammary terminal ductal lobular units both by morphology and marker expression, in vivo and in vitro [24]. The experimental approach was based upon ultrastructural studies in the mouse mammary gland, which described SLC and ULLC as putative epithelial stem cells. SLC and ULLC do not commonly contact the duct or lobule lumen [25]. Indeed suprabasal breast epithelial cells were found with these properties and demonstrated that these cells possessed stem cell properties. This discovery lends strong experimental support for the conclusion that the undifferentiated SLC and ULLC represent a multipotent epithelial cell population in the mouse and that a similar epithelial subset exists in the human breast.
Mammary Stem/Progenitor Cell Hierarchy
Evidence for lobule-limited and duct-limited pluripotent mammary epithelial cell activities has been established for both rats and mice by transplantation of limiting dilutions of dispersed mammary epithelial cells into hosts that were subsequently impregnated and/or treated with hormone combinations to produce alveologenesis [15, 22, 26, 27]. Studies with retroviral-marked clonal mammary populations demonstrated that both of these lineage-limited activities were present within clonal populations through repeated transplant generations indicating their derivation from a single pluripotent antecedent [22, 28]. In addition, serial passage of the retroviral-marked mammary epithelial clones in pregnant hosts showed that the capacity of individual outgrowths to produce lobulogenesis or ductal elongation was independently lost during the acquisition of growth senescence among individual transplants [28]. With the development of the WAP-Cre model used in combination with the Rosa26LacZ reporter mice (R26R), evidence for a LacZ-marked lobule-limited progenitor observable in parous mouse mammary epithelium surfaced [29]. These LacZ-positive, parity-identified mammary cells (PI-MEC) were found to be pluripotent, self-renewing, and capable of maintaining their lobule-limited progenitor activities following serial transplantation in epithelium-free mammary fat pads when the hosts were subsequently impregnated (Fig. 2) [30]. During pregnancy in these hosts, the PI-MEC proliferated and gave rise to luminal progeny that were PR- or ERα-positive and luminal progeny that were bereft of these steroid receptors. Further, in the developing secretory acini, they contributed not only secretory progeny but also myoepithelial cells. Further study indicated that these cells were present in the mammary tissue of nulliparous females and that they could be detected in explant cultures after treatment of the fragments with growth factors that do not induce lactogenic differentiation [31]. Additional evidence demonstrates that PI-MEC are found to be virtually 100 % present in the CD49fhi population [32]. This population was shown earlier to possess essentially all of the mammary repopulating activity [33]. Subsequent transplantation of CD49fhi-positive PI-MEC and the CD49flo epithelial cells into epithelium-divested mammary fat pads indicated that all the repopulating activity was associated with the PI-MEC fraction [32].
(a–d) Parity-identified multipotent mammary epithelial cells (PI-MEC) marked by lacZ expression (blue) are capable of producing both myoepithelial (a) progeny characterized by the simultaneous expression of lacZ and smooth muscle actin (brown) and luminal epithelial progeny during lobulogenesis (b–d). Luminal epithelial progeny may be positive for progesterone receptor (brown nuclear stain in b and c) or estrogen receptor (brown nuclear stain in d) or luminal progeny negative for either progesterone or estrogen receptor. This evidence indicates that PI-MEC represent the lobule-limited multipotent epithelial progenitor cells in the mouse. Scale bars = 15 mm
Functional Assays for Monitoring Mammary Stem and Progenitor Cells (Limitations)
The accepted standard of functional mammary stem cell assays remains the repopulation of a cleared mammary fat pad and subsequent secondary transplantation of any ensuing mammary outgrowth first reported in 1959 [1]. The main deterrent to these experiments is that they are expensive, time-consuming, and not amenable to high-throughput assays. While these assays work well for the detection of murine stem cells and other rodent sources, no equivalent model has yet been established for the study of human mammary stem cells. Human tissue nonresponsiveness and the murine host mammary stroma are the main causes for experimental failure [34–37].
One alternative method for testing engraftment capacity of human mammary stem/progenitor cells involves the injection of human mammary fibroblasts into the cleared murine fat pad prior to the transplantation of the human mammary epithelial cells [38, 39]. This assay allows for the establishment of human mammary stroma or “humanization” creating a basement structure allowing for the engraftment and expansion of the human mammary epithelial cells. In this system the epithelial cells are able to expand and differentiate into histologically normal-appearing human mammary structures comprised of luminal and myoepithelial cells.
A second human into mouse implantation model has been investigated. In this model human mammary epithelial cells are mixed with irradiated fibroblast, embedded within collagen gels, and implanted into highly vascularized areas such as underneath the kidney capsule [40]. After 4 weeks the histologically sectioned tissue resembles normal human breast tissue with both luminal and myoepithelial cells. These outgrowths have fully differentiated luminal cells that express ER and PR and form functional secretory epithelial cells that synthesize milk proteins if the host becomes pregnant.
Phenotypic Analysis of Mammary Stem and Progenitor Cells
The most common technique used to identify and isolate mammary stem and progenitor cells is based on cell surface markers using magnetic and/or fluorescent sorting methods. By sorting for combinations of cell surface markers, researchers have been able to establish a rough idea of what markers are expressed by different classes of mammary progenitor cells.
Mouse
The cell surface markers used currently to establish mammary progenitor populations include CD14, CD24 (a pan-epithelial marker used to discriminate against stromal cells), β1-integrin (CD29), α6-integrin (CD49f), β3-integrin (CD61), and Sca-1. Cells bearing these markers can be sorted based on the intensity of the fluorescent activity that correlates to the expression levels of each of the markers. The populations are sorted into high, med, and low populations (e.g., CD24medSca-1lowCD29highCD49fhigh). A CD24medSca-1lowCD29highCD49fhigh cell is referred to as a mammary repopulating unit (MRU). It is estimated that 1 MRU can be isolated from every 60–90 mammary epithelial cells [33, 41]. The MRU designation is based on its ability to form a mammary colony in vitro; its in vivo regenerative capacity is yet to be determined. Based on the expression levels of CD24, CD29, and CD49f, it is believed that MRUs occupy basal positions in the mammary epithelium. These cells express basal keratin 5 further evidence of the basal position in situ [42].
These markers have been useful but only to a limited extent. Recently the Cre-Lox recombination system was used in the mouse, and the results indicate that many mammary cell types, as characterized by keratin expression, contribute progeny to outgrowths generated by injection of dispersed cells [43]. In addition, it has been shown in human breast cancer cell lines that the markers for tumor-initiating cells and for luminal non-tumor-initiating cells do not indicate the exclusivity of these markers to tumorigenesis per se [44].
There are conflicting reports regarding the importance of these surface markers and their relevance to the prospective isolation of populations of epithelial cells enriched for their ability to produce competent mammary epithelial reconstitution in transplanted mammary fat pads. Two groups have claimed that CD49fhi/CD24pos or CD29hi/CD24pos cells constitute populations highly enriched for mammary stem cell activities competent for regeneration of a complete and functional mammary gland and capable of self-renewal [33, 41]. Reports from another group indicate that the bulk of in vivo reconstituting activity resides in the CD24lo population, and practically none is associated with CD24hi in cells isolated from mammary tissue using this single-cell surface marker [45]. Removal of CD24 from the genome has little to no effect on mammary gland development or function in the mouse [46].
Human
In vitro and in situ studies indicate that the mammary stem cells reside in the intralobular ducts of the human mammary gland and not the terminal ductal lobule units (TDLUs) [47]. The markers used to isolate human mammary stem cells include epithelial cell adhesion molecule (EpCAM; also known as epithelial cell antigen (ESA) and CD326), CD49f, and luminal-specific glyco-mucin protein MUC1 [40, 47–50]. CD49f is expressed at higher levels on basal epithelial cells and lower levels on luminal cells, while EpCAM is expressed at higher levels on luminal cells and lower levels on basal cells. Human MRUs have an EpCAMloCD49fhighMUC1 phenotype indicating a basal position similar to those of the mouse [48, 51].
The lack of a species specific in vivo model has hampered the characterization of the human mammary stem cells as all of these results are based on in vitro experiment or transplantation studies utilizing immune-deficient mice.
Functional Assays for Monitoring Mammary Stem and Progenitor Cells (Limitations)
The early serial transplantation studies did not provide precise data on stem cell frequency as the experiments utilized fragments of mammary cells instead of cell suspensions. One study provided an upper estimate of stem cell frequency in different portions of the mammary fat pad [14]. This study calculated the total number of mammary epithelial cells in a mammary fat pad and then divided the fat pad into 80–100 fragments for transplantation. Using this approach, the authors calculated the upper frequency of stem cells in virgin duct and end buds from 6-week-old virgin mice as 1/7,200 and 1/2,200, respectively. Studies done with semi-purified cell suspensions prepared by enzymatic digestion and using a limiting dilution approach provided more definite results, although the frequency was very dependent upon the procedure used for preparation of the cells. For example, using cells prepared from 10- to 12-week-old virgin BALB/c female mice a 3-h digestion with collagenase-hyaluronidase yielded a repopulating frequency of 1/2,200 when cells were injected in PBS solution. However, when cells were digested overnight and followed by a short exposure to trypsin and implanted with Matrigel (in a 1:1 volume ratio), the repopulating frequency was 1/250. These results were evaluated using Poisson distribution statistics, which required five dilutions, thus imposing very stringent criteria. Using other less demanding approaches can only provide estimates, which are less reliable. The improved protocol was developed by Moraes et al. [52]. In their study, they provided estimates of repopulating frequency of 1:100 for cells taken from FVB strain normal adult virgin mammary gland. These studies have implications for any study on mammary stem cells [53]. Recent approaches using sorted cell populations estimate stem cell frequency at least an order of magnitude greater than the above studies. One has to consider the factors that might contribute to the underestimate of stem cell frequency in studies using flow-cytometry-guided cell sorting. What is the impact of cell damage and cell loss on the interpretation of the results?
Is there a fundamental difference between implanting a cell suspension and a fragment of mammary cells? Surprisingly, this question has not been addressed in any recent study that focuses on the identification of mammary stem cells. In the older published literature, there is limited data and discussion of the events occurring within 72 h after implantation of a mammary fragment. An early study demonstrated that transplanted fragments of normal ductal tissue dissociate into small aggregates within 24 h after transplantation [54]. By 72 h, ductal tubular organization is established with an intact basement membrane and significant mitotic activity. A similar pattern of histogenesis is observed upon the transplantation of hyperplastic alveolar nodules [55]. It is unknown (although highly likely) if this initial cell dispersion and reaggregation represents the interaction of different subsets of mammary cells. If this early histogenic activity is critical for subsequent cell proliferation, how does one interpret the results where cell suspensions of sorted populations representing one subset of mammary cells are cited as evidence for the existence of the “stem” cell? The current assays do not distinguish between engraftment capability and stemness.
Dispersed Cell Implantation Compared to Fragment: Clonal or Combinatorial
It has been shown, both directly by retroviral-tagging in serially transplanted MMTV-infected mammary outgrowths and more recently by implantation of “visually confirmed” single cells, that an entire functional mammary gland may be developed from the progeny of a single cell [22]. On the other hand, considerable evidence exists that transplantation of dispersed mammary epithelial cells comprised of unsorted heterogeneously marked epithelial cells produces complete outgrowths that are frequently (in some cases invariably) mixtures of the progeny derived from the variously marked donor cells [28, 30, 32, 56, 57]. In the absence of ERα expression, duct elongation and development fails both in pubertal and in parous females [56]. The amphiregulin null (ARnull) mouse mammary gland phenocopies this deficiency indicating that AR is a major duct-specific growth signal mediated through ERα-positive mammary epithelial cells, Despite this, both ERαnull and ARnull mammary epithelial cells are capable of contributing progeny to all mammary epithelial subtypes when dispersed and mixed with wild-type mammary epithelium before injection into cleared mammary fat pads [56, 57]. The evidence from progesterone receptor (PR) null models reveals that alveologenesis cannot proceed in the absence of paracrine signals from PR+ epithelial cells [58]; nevertheless dispersed PRnull cells marked by LacZ expression contribute alveolar progeny when mixed together with wild-type epithelial cells in pregnant hosts. This clearly demonstrates that a complete mammary epithelial outgrowth cannot be formed without ERα+ and PR+ epithelial cell subtypes. These findings argue that a single mammary cell injected into an empty mammary fat pad must at a minimum divide asymmetrically (and remain a stem/progenitor cell) to produce an ERα+ daughter and later again to produce cap cell progeny in order to begin ductal growth and still later to produce a PR+ cell to support side branching and, subsequently, alveologenesis. The clear existence of lineage-limited, pluripotent duct and lobule progenitors within the nulliparous mouse’s mammary epithelium raises the strong probability that these cells might combine to produce mammary outgrowths comprising both ductal and lobular development when inoculated in dispersed cell populations. PI-MEC (i.e., lobule-limited stem/progenitor cells) produce PR+ and ERα+ as well as progeny negative for these receptors when contributing to mammary outgrowths in pregnant host [30]. Similar findings were obtained when duct-limited outgrowths were tested for the presence of these steroid nuclear receptors. These results indicate that each of these lineage-limited stem/progenitors is capable of producing cell progeny shown above to be indispensable for complete mammary development. Thus the lines between the primary antecedent and the downstream stem/progenitors become blurred regarding their relative importance in producing complete mammary outgrowths in transplanted fat pads. Serial transplantation of clonal populations by fragment implantation into subsequently impregnated hosts showed that the capacity of any given fragment to produce alveologenesis and/or duct elongation was lost independently during the onset of growth senescence [28]. Earlier, serially transplanted growth senescent duct fragments were shown to be able to generate lobulo-alveolar growth upon impregnation of the transplant host [59]. More recently, it has been shown that fragment versus dispersed cell implantation demonstrates that no change in the ability to produce regenerated glandular structures (hence no change in stem cell function) results from either age or reproductive longevity [60]. The conclusion drawn from these observations postulates that either each lineage-limited stem/progenitor activity decays independently from the other during outgrowth development or that the primary mammary stem cell loses the capacity to produce one or the other lineage-limited downstream stem/progenitor during its own self-renewal during its expansion in the previous generation.
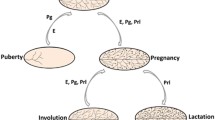
To summarize, both dispersed cell and fragment implantation led to mammary epithelial outgrowths comprised of progeny produced by independently self-renewing stem/progenitor populations. These facts do not in any way dispute the existence of a primary mammary stem cell antecedent. However, they do indicate the persistence of multiple pluripotent stem/progenitor cell activities within the mammary epithelial population that is capable of independently contributing diverse epithelial progeny during mammary gland growth and regeneration. The current understanding of the mouse mammary stem/progenitor cell hierarchy is summarized in Fig. 3.
Schematic illustration depicting classical hierarchy of mammary stem and progenitor cells where one stem cell results in two lineage-restricted progenitors (upper panel). The current understanding of the mouse mammary stem cell hierarchy where bi-potent progenitors participate in embryonic development or during regeneration following transplantation of basal progenitors while unipotent progenitors maintain luminal and basal lineages in adult mice
Influence of the Mammary Microenvironment over Stem Cells
To highlight the influence of diverse mammary epithelial cell types in bringing about the successful regeneration, near-limiting dilutions of dispersed mammary epithelial cells were comingled with testicular cells isolated from adult WAP-Cre/Rosa26R mice [61]. The resulting mixtures were inoculated into cleared fat pads, and mammary ductal morphogenesis was allowed to proceed. Subsequently, a fraction of the transplant hosts were maintained as virgins, and the rest were mated and permitted to complete a full pregnancy, lactation, and involution cycle. Only male cells possess the WAP-Cre and Rosa26 LacZ reporter gene. Thus, LacZ-positive cells among the regenerated mammary epithelium indicate the presence of testicular cell progeny. The mammary nature of these LacZ-positive cells was confirmed by staining for mammary-specific markers for milk protein synthesis, cytokeratins K5/K14, and smooth muscle actin. FISH analysis confirmed that these cells were male and indicated the absence of fusion between male and female cells. LacZ-positive cells were found in all second-generation transplants from the male/female chimeric outgrowths, indicating their capacity for self-renewal. These experiments demonstrate the overarching importance of the signals provided by mammary epithelial cells for the development of microenvironment(s) capable of sustaining stem cell activity and differentiation. Experiments have also demonstrated that neural stem cells and lineage-negative bone marrow cells isolated from WAP-Cre/Rosa26 LacZ reporter mice responded in the same manner as the testicular cells in this mammary niche assay (Fig. 4) [62, 63]. Not only is the normal mammary microenvironment able to direct stem cells derived from non-mammary tissues but also directs tumor-derived cells, mouse mammary tumor, or human testicular carcinoma to adopt a normal mammary phenotype [64, 65]. In both cases differentiation of the tumor-derived cells required the presence of ERα+ and PR+ cells in the surrounding environment. Without the cues provided by these cells, tumors formed.
A neural stem cell/mammary epithelial cell chimeric outgrowth from a lactating host is shown. Casein protein expression is indicated by the red fluorescence and beta-galactosidase by the green fluorescence, Yellow indicates the overlapping of the two stains. In the inset, an electron micrograph of a lactating acinus is depicted. The arrows indicate the presence of casein micelles in the secretory cells at their luminal surface. The black arrows in the fluorograph show that casein and beta-galactosidase staining is present (yellow) at the luminal surface of the beta-galactosidase-positive (neural-derived) cells
In the human breast, little transplantation biology is available due to technical difficulties in establishing mammary outgrowths in vivo. Recently, progress has been made in this area through humanization of the mouse mammary fat pad with human-derived stromal cells [38]. The results of successful implantations of normal human organoids indicate that independent ductal, lobular and acinar structures may be generated within humanized mouse mammary fat pads by human mammary epithelial cells. This result and those demonstrating the association of bi-potency with individual mammary epithelial cells (of the mouse mammary fat pad with human-derived stromal cells suggests that a similar stem/progenitor cell hierarchy exists in human breast epithelium) [38, 39].
Future Directions and Challenges for Mammary Stem Cell Biology
The foregoing discussion supports the concept that the tissue microenvironment can affect the cellular repertoire of an adult stem cell. This influence in the murine mammary gland appears to be manifest in signals emanating from the epithelial cells as well as the stromal elements of the mammary fat pad. Several questions remain to be answered. For example, what is the role if any of mammary stem/progenitor cells in this process? Does the mammary fat pad selectively support the reprogramming in conjunction with the mammary epithelial cells or can any fat pad in the female mouse demonstrate this activity? Both testes and neural tissues develop from ectodermal precursors, will cells developing from mesoderm or endoderm precursors respond similarly when mixed with mammary epithelial cells in the context of the mammary fat pad? In fact cells derived from mesoderm tissue demonstrate this capacity [63]. Finally, what are the cellular, genetic and molecular components that define the mammary epithelial-specific stem cell niche and how can these factors be utilized for developing new paradigms for stem cell control and cancer therapy?
Preliminary experiments have shed a small amount of light on the questions mentioned above. First, enriching or depleting the mammary epithelial cells for cells expressing the currently accepted cell surface markers for mammary stem/progenitor cells (CD49f, CD29, or CD24) did not affect the efficiency of reprogramming non-mammary cells [33, 41, 45]. Testing mammary epithelial cell populations from various gene knockout models has thus far not revealed any particular gene product that is essential for reprogramming. However recent findings have delineated at least one essential epithelial cell characteristic necessary for the process of reprogramming.
Serial transplantation of the mammary epithelium inevitably leads to growth senescence, which has clearly been linked to the number of mitotic events required for stem cell activity to reach the outermost periphery of the regenerated gland. Studies designed to determine whether growth senescent mammary epithelial cell populations that are unable to support in vivo mammary epithelial regeneration by themselves may be able to reprogram non-mammary stem/progenitor cells have begun. Thus far, those growth-deficient mammary populations that have been tested were able to reprogram non-mammary stem cells and in the process were able to generate full mammary outgrowths in cleared mammary fat pads. These findings have strong implications for recruitment of transformed cells to growth-deficient niches and neoplasia. In addition, these studies have led to the examination of the response of cancer cells in this experimental model, as cancer cells show considerable plasticity when placed in developing tissue environments [66, 67]. Present work demonstrates that signals from the mammary microenvironment in the context of the regenerating gland are capable of redirecting the repertoire of adult somatic stem cells from at least three non-mammary tissues. Further efforts to extend these initial findings will elucidate at least some of the mechanisms involved.
Although untested, another possibility for the appearance of growth senescence might be due to failure of the microenvironment (niche) to provide the signals appropriate for stem cell self-renewal. This deficiency would by necessity involve the epithelial cell population surrounding the stem cell proper since transplantation always occurs into young mammary fat pad stroma. This possibility is easily tested in current model systems where mammary cells carry the β-gal marker. A corollary to this possibility would be that signals emanating from the transformed progeny surrounding the self-renewing premalignant/tumorigenic cell rather than a property intrinsic to the premalignant/tumorigenic cell are responsible for the infinite replicative lifetime of an immortalized mammary population. This latter situation would require that asymmetric divisions from the self-renewing tumorigenic cell generate these supporting “niche” cells.
Our challenge is not to sort out from this mixture the primal mammary stem cell but instead to comprehend the interaction among these components that allows the long-term maintenance of mammary stem cell activity. We want to emphasize that focusing our primary deliberations upon the primordial mammary stem cell deflects our attention from the important issue of extending our understanding of how stem/progenitor cells and their progeny interact to maintain mammary homeostasis and how this may be disturbed during neoplastic transformation.
Abbreviations
- AR:
-
Amphiregulin
- DLLC:
-
Differentiating large light cells
- ER:
-
Estrogen receptor
- MMTV:
-
Mouse mammary tumor virus promoter
- MRU:
-
Mammary repopulating unit
- PI-MEC:
-
Parity-identified mammary epithelial cells
- PR:
-
Progesterone receptor
- SLC:
-
Small light cells
- TDLU:
-
Terminal ductal lobule unit
- ULLC:
-
Undifferentiated large light cells
- WAP:
-
Whey acidic protein promoter
References
DeOme KB, Faulkin Jr LJ, Bern HA, Blair PB. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959;19:515–20.
Faulkin Jr LJ, Deome KB. Regulation of growth and spacing of gland elements in the mammary fat pad of the C3H mouse. J Natl Cancer Inst. 1960;24:953–69.
Daniel CW, Aidells BD, Medina D, Faulkin Jr LJ. Unlimited division potential of precancerous mouse mammary cells after spontaneous or carcinogen-induced transformation. Fed Proc. 1975;34:64–7.
Callahan R, Smith GH. MMTV-induced mammary tumorigenesis: gene discovery, progression to malignancy and cellular pathways. Oncogene. 2000;19:992–1001.
Smith GH, Pauley RJ, Socher SH, Medina D. Chemical carcinogenesis in C3H/StWi mice, a worthwhile experimental model for breast cancer. Cancer Res. 1978;38:4504–9.
Smith GH, Arthur LA, Medina D. Evidence of separate pathways for viral and chemical carcinogenesis in C3H/StWi mouse mammary glands. Int J Cancer. 1980;26:373–9.
Medina D. The preneoplastic phenotype in murine mammary tumorigenesis. J Mammary Gland Biol Neoplasia. 2000;5: 393–407.
Medina D. Biological and molecular characteristics of the premalignant mouse mammary gland. Biochim Biophys Acta. 2002;1603:1–9.
Maglione JE, Moghanaki D, Young LJ, et al. Transgenic Polyoma middle-T mice model premalignant mammary disease. Cancer Res. 2001;61:8298–305.
Medina D, Kittrell FS, Shepard A, et al. Biological and genetic properties of the p53 null preneoplastic mammary epithelium. FASEB J. 2002;16:881–3.
Medina D, Kittrell FS. Immortalization phenotype dissociated from the preneoplastic phenotype in mouse mammary epithelial outgrowths in vivo. Carcinogenesis. 1993;14:25–8.
Medina D, Kittrell FS, Liu YJ, Schwartz M. Morphological and functional properties of TM preneoplastic mammary outgrowths. Cancer Res. 1993;53:663–7.
Young LJ, Medina D, DeOme KB, Daniel CW. The influence of host and tissue age on life span and growth rate of serially transplanted mouse mammary gland. Exp Gerontol. 1971;6:49–56.
Smith GH, Medina D. A morphologically distinct candidate for an epithelial stem cell in mouse mammary gland. J Cell Sci. 1988;90(Pt 1):173–83.
Smith GH. Experimental mammary epithelial morphogenesis in an in vivo model: evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Res Treat. 1996;39: 21–31.
Daniel CW, Young LJ. Influence of cell division on an aging process. Life span of mouse mammary epithelium during serial propagation in vivo. Exp Cell Res. 1971;65:27–32.
Chepko G, Smith GH. Three division-competent, structurally-distinct cell populations contribute to murine mammary epithelial renewal. Tissue Cell. 1997;29:239–53.
Smith GH, Vonderhaar BK. Functional differentiation in mouse mammary gland epithelium is attained through DNA synthesis, inconsequent of mitosis. Dev Biol. 1981;88:167–79.
Vonderhaar BK, Smith GH. Dissociation of cytological and functional differential in virgin mouse mammary gland during inhibition of DNA synthesis. J Cell Sci. 1982;53:97–114.
Graham DE, Medina D, Smith GH. Increased concentration of an indigenous proviral mouse mammary tumor virus long terminal repeat-containing transcript is associated with neoplastic transformation of mammary epithelium in C3H/Sm mice. J Virol. 1984;49: 819–27.
Nicoll CS, Tucker HA. Estimates of parenchymal, stromal, and lymph node deoxyribonucleic acid in mammary glands of C3H/Crgl-2 mice. Life Sci. 1965;4:993–1001.
Kordon EC, Smith GH. An entire functional mammary gland may comprise the progeny from a single cell. Development. 1998;125:1921–30.
Smith GH, Strickland P, Daniel CW. Putative epithelial stem cell loss corresponds with mammary growth senescence. Cell Tissue Res. 2002;310:313–20.
Gudjonsson T, Villadsen R, Nielsen HL, Ronnov-Jessen L, Bissell MJ, Petersen OW. Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev. 2002;16:693–706.
Smith GH, Chepko G. Mammary epithelial stem cells. Microsc Res Tech. 2001;52:190–203.
Kim ND, Clifton KH. Characterization of rat mammary epithelial cell subpopulations by peanut lectin and anti-Thy-1.1 antibody and study of flow-sorted cells in vivo. Exp Cell Res. 1993;207:74–85.
Kamiya K, Gould MN, Clifton KH. Quantitative studies of ductal versus alveolar differentiation from rat mammary clonogens. Proc Soc Exp Biol Med. 1998;219:217–25.
Smith GH, Boulanger CA. Mammary stem cell repertoire: new insights in aging epithelial populations. Mech Ageing Dev. 2002;123:1505–19.
Wagner KU, Boulanger CA, Henry MD, Sgagias M, Hennighausen L, Smith GH. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development. 2002;129:1377–86.
Boulanger CA, Wagner KU, Smith GH. Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-beta1 expression. Oncogene. 2005;24:552–60.
Booth BW, Boulanger CA, Smith GH. Alveolar progenitor cells develop in mouse mammary glands independent of pregnancy and lactation. J Cell Physiol. 2007;212:729–36.
Matulka LA, Triplett AA, Wagner KU. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol. 2007;303:29–44.
Stingl J, Eirew P, Ricketson I, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–7.
Gusterson BA, Williams J, Bunnage H, O’Hare MJ, Dubois JD. Human breast epithelium transplanted into nude mice. Proliferation and milk protein production in response to pregnancy. Virchows Arch A Pathol Anat Histopathol. 1984;404:325–33.
Knight J, Gusterson BA, Cowley G, Monaghan P. Differentiation of normal and malignant human squamous epithelium in vivo and in vitro: a morphologic study. Ultrastruct Pathol. 1984;7:133–41.
Sheffield LG, Welsch CW. Transplantation of human breast epithelia to mammary-gland-free fat-pads of athymic nude mice: influence of mammotrophic hormones on growth of breast epithelia. Int J Cancer. 1988;41:713–9.
Hovey RC, McFadden TB, Akers RM. Regulation of mammary gland growth and morphogenesis by the mammary fat pad: a species comparison. J Mammary Gland Biol Neoplasia. 1999;4: 53–68.
Kuperwasser C, Chavarria T, Wu M, et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci U S A. 2004;101:4966–71.
Proia DA, Kuperwasser C. Reconstruction of human mammary tissues in a mouse model. Nat Protoc. 2006;1:206–14.
Eirew P, Stingl J, Raouf A, et al. A method for quantifying normal human mammary epithelial stem cells with in vivo regenerative ability. Nat Med. 2008;14:1384–9.
Shackleton M, Vaillant F, Simpson KJ, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–8.
Taddei I, Deugnier MA, Faraldo MM, et al. Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat Cell Biol. 2008;10:716–22.
Van Keymeulen A, Rocha AS, Ousset M, et al. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479:189–93.
Gupta PB, Fillmore CM, Jiang G, et al. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146:633–44.
Sleeman KE, Kendrick H, Ashworth A, Isacke CM, Smalley MJ. CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Res. 2006;8:R7.
Cremers N, Deugnier MA, Sleeman J. Loss of CD24 expression promotes ductal branching in the murine mammary gland. Cell Mol Life Sci. 2010;67:2311–22.
Villadsen R, Fridriksdottir AJ, Ronnov-Jessen L, et al. Evidence for a stem cell hierarchy in the adult human breast. J Cell Biol. 2007;177:87–101.
Stingl J, Eaves CJ, Kuusk U, Emerman JT. Phenotypic and functional characterization in vitro of a multipotent epithelial cell present in the normal adult human breast. Differentiation. 1998;63:201–13.
Stingl J, Eaves CJ, Zandieh I, Emerman JT. Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res Treat. 2001;67:93–109.
Raouf A, Zhao Y, To K, et al. Transcriptome analysis of the normal human mammary cell commitment and differentiation process. Cell Stem Cell. 2008;3:109–18.
Carter WG, Kaur P, Gil SG, Gahr PJ, Wayner EA. Distinct functions for integrins alpha 3 beta 1 in focal adhesions and alpha 6 beta 4/bullous pemphigoid antigen in a new stable anchoring contact (SAC) of keratinocytes: relation to hemidesmosomes. J Cell Biol. 1990;111:3141–54.
Moraes RC, Zhang X, Harrington N, et al. Constitutive activation of smoothened (SMO) in mammary glands of transgenic mice leads to increased proliferation, altered differentiation and ductal dysplasia. Development. 2007;134:1231–42.
Vaillant F, Lindeman GJ, Visvader JE. Jekyll or Hyde: does Matrigel provide a more or less physiological environment in mammary repopulating assays? Breast Cancer Res. 2011;13:108.
Chew EC, Hoshino K. Early histogenesis of transplanted mouse mammary glands. II. Within 96 hours following isografting. Z Anat Entwicklungsgesch. 1970;132:318–24.
Medina D, Vaage J, Sedlacek R. Mammary noduligenesis and tumorigenesis in pathogen-free C3Hf mice. J Natl Cancer Inst. 1973;51:961–5.
Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci U S A. 2006;103:2196–201.
Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc Natl Acad Sci U S A. 2007;104:5455–60.
Brisken C, Park S, Vass T, Lydon JP, O’Malley BW, Weinberg RA. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci U S A. 1998;95: 5076–81.
Raafat A, Strizzi L, Lashin K, et al. Effects of age and parity on mammary gland lesions and progenitor cells in the FVB/N-RC mice. PLoS One. 2012;7:e43624.
Daniel CW, Young LJ, Medina D, DeOme KB. The influence of mammogenic hormones on serially transplanted mouse mammary gland. Exp Gerontol. 1971;6:95–101.
Boulanger CA, Mack DL, Booth BW, Smith GH. Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proc Natl Acad Sci U S A. 2007;104:3871–6.
Booth BW, Mack DL, Androutsellis-Theotokis A, McKay RD, Boulanger CA, Smith GH. The mammary microenvironment alters the differentiation repertoire of neural stem cells. Proc Natl Acad Sci U S A. 2008;105:14891–6.
Boulanger CA, Bruno RD, Rosu-Myles M, Smith GH. The mouse mammary microenvironment redirects mesoderm-derived bone marrow cells to a mammary epithelial progenitor cell fate. Stem Cells Dev. 2011;21:948–54.
Bussard KM, Boulanger CA, Booth BW, Bruno RD, Smith GH. Reprogramming human cancer cells in the mouse mammary gland. Cancer Res. 2010;70:6336–43.
Booth BW, Boulanger CA, Anderson LH, Smith GH. The normal mammary microenvironment suppresses the tumorigenic phenotype of mouse mammary tumor virus-neu-transformed mammary tumor cells. Oncogene. 2011;30:679–89.
Kasemeier-Kulesa JC, Teddy JM, Postovit LM, et al. Reprogramming multipotent tumor cells with the embryonic neural crest microenvironment. Dev Dyn. 2008;237:2657–66.
Postovit LM, Margaryan NV, Seftor EA, et al. Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proc Natl Acad Sci U S A. 2008;105: 4329–34.
Acknowledgment
Figure 3 was illustrated by Eve E. Kingsley Booth.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science + Business Media New York
About this chapter
Cite this chapter
Booth, B.W., Medina, D., Smith, G.H. (2013). Mammary Epithelial Stem Cells. In: Sell, S. (eds) Stem Cells Handbook. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4614-7696-2_18
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7696-2_18
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4614-7695-5
Online ISBN: 978-1-4614-7696-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)