Abstract
The introduction of PET, and especially 18F-FDG PET, has transformed pediatric nuclear medicine. In particular, hybrid imaging with 18F-FDG PET/CT has transformed pediatric nuclear oncology. However, PET and PET/CT have a wide variety of pediatric indications including neurology, sports medicine and orthopedics, pediatric cardiology, and infection imaging, as well as pediatric oncology. Most PET and PET/CT studies utilize the glucose analogue, 18F-fluorodeoxyglucose (18F-FDG). Infants and children provide special challenges to acquiring a technically adequate and diagnostically satisfactory PET or PET/CT scan. Adequate pre-study preparation of the patient and family is critical. Imaging protocols must pay particular attention to the pediatric spectrum of disease, the developmental needs of pediatric patients, and the goal of minimizing radiation exposure. Interpretation of pediatric 18F-FDG PET and PET/CT requires knowledge of pediatric diseases and an appreciation for the patterns of tracer biodistribution that can be seen in infants and children. Issues such as sedation or anesthesia, rarely a concern in adult nuclear medicine, are a normal part of routine pediatric PET and PET/CT. Protocols and departmental procedures must balance the developmental needs of children with the goal of acquiring a diagnostic imaging study that answers the clinical question.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Brown Adipose Tissue
- Uptake Period
- Attenuation Artifact
- Nuclear Medicine Technologist
- Pediatric Nuclear Medicine
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
The introduction of PET, and especially 18F-FDG PET, has transformed pediatric nuclear medicine. In particular, hybrid imaging with 18F-FDG PET/CT has transformed pediatric nuclear oncology [1–4]. However, PET and PET/CT have a wide variety of pediatric indications [1] including neurology [5, 6], sports medicine and orthopedics [7], pediatric cardiology [8], and infection imaging [9], as well as pediatric oncology. Most PET and PET/CT studies utilize the glucose analogue, 18F-fluorodeoxyglucose (18F-FDG) (Figs. 3.1 and 3.2). Few other PET radiopharmaceuticals have been approved for routine clinical use, and few of those have extensive pediatric experience [6, 10]. One with some pediatric experience is the bone imaging agent [18F] sodium fluoride, which can be used for skeletal imaging of both benign and malignant conditions (Fig. 3.3) [7].
Whole-body 18F-FDG PET in a 14-year-old female. A MIP of a whole-body 18F-FDG PET demonstrates a typical pattern of normal physiological uptake of 18F-FDG. Physiological uptake of 18F-FDG is most intense in the brain. Other sites of normal physiological uptake include the liver, tonsils, and gastrointestinal system. Variable uptake can be seen in muscles, including myocardium, skeletal muscle, and small muscles in the larynx and orbits. Due to renal excretion, tracer accumulation usually is seen in the renal collecting system, ureters, and bladder. Physiological uptake in the thymus is more common in pediatric patients than adults
Brain 18F-FDG PET in a 16-year-old male. A brain 18F-FDG PET displayed in axial (a), coronal (b), and sagittal (c) planes demonstrates the normal pattern of 18F-FDG uptake in the brain. Within the cerebral cortex, physiological uptake of 18F-FDG is greater in the gray matter than in the white matter. Uptake in the cerebellum typically is less than in the cerebral cortex. Intense uptake of 18F-FDG also is seen in the striatum. Increased uptake in the visual cortex reflects visual activity during the uptake period
Fluorine-18-sodium fluoride PET bone scan in a 14-year-old female. A MIP of an 18F-sodium fluoride PET bone scan demonstrates that 18F-sodium fluoride uptake reflects bone turnover in a pattern similar to 99mTc-labeled diphosphonates, but image quality can be higher with PET acquisition. In pediatric patients, physeal uptake indicates skeletal immaturity
Radiopharmaceuticals
[18F] Sodium Fluoride
Fluorine-18 (physical half-life 110 min) is a positron emitter and is the predominant radiolabel for PET radiopharmaceuticals in routine clinical use. It is a cyclotron product typically produced by the nuclear reaction 18O (p,n) 18F and isolated as an aqueous solution of [18F] sodium fluoride. Fluorine-18 may be the ideal PET tracer for medical imaging due to its high specific activity, efficient labeling of a wide variety of PET radiopharmaceuticals, and nearly 2-h half-life. The emitted positron has the lowest kinetic energy (630 keV) of any common PET radionuclide. This limits the positron range before annihilation and may contribute to improved spatial resolution with 18F compared to other PET radionuclides [11].
Fluorine-18 sodium fluoride also may be used as a radiopharmaceutical for skeletal imaging [7]. When used as a bone imaging agent, no further chemical processing is required. The North American consensus guidelines [12] recommend a pediatric dose of 2.22 MBq/kg (0.06 mCi/kg) with a minimum dose of 18.5 mBq (0.5 mCi). After intravenous administration, there is rapid bone uptake of [18F] fluoride as it exchanges with hydroxyl ions on the surface of the hydroxyapatite matrix of bone [13]. Fluorine-18 fluoride is rapidly cleared from plasma, so that within 1 h of administration, only about 10 % of the dose remains in the circulation [14].
18F-Fluoro-2-Deoxyglucose
Fluorine-18-fluoro-2-deoxyglucose (18F-FDG) is a glucose analogue labeled with 18F. Fluorine-18-FDG is taken into cells through one of the transmembrane glucose transporters, but does not enter the energy producing metabolic pathways [15]. Insulin-mediated stimulation of expression of some glucose transporters (GLUT-4) will increase cellular uptake of 18F-FDG in insulin-sensitive tissues such as skeletal muscle, liver, and myocardium [16]. Fluorine-18-FDG is phosphorylated by cellular hexokinases, but after monophosphorylation, FDG-6 phosphate cannot be further phosphorylated and is trapped in the cell [17], which provides a mechanism for amplification of the 18F signal. Only a few tissues, such as liver, express sufficient phosphorylase activity to dephosphorylate large amounts FDG-6 phosphate, which permits secretion out of the cell. The North American consensus guidelines [12] recommend a pediatric dose of 3.7–5.2 MBq/kg (0.10–0.14 mCi/kg) for torso/whole-body imaging and 3.7 mBq/kg (0.10 mCi/kg) for brain imaging, with a minimum dose of 37 MBq (1.0 mCi) for all studies.
Other PET Radiopharmaceuticals
Other 18F-labeled PET agents, including 18F-fluoro-L-thymidine, 18F-deoxy-phenylalanine (18F-DOPA), and 18F-dopamine, remain investigational, but may acquire a role for imaging specific oncological or other indications [18]. Other PET radiopharmaceuticals [19], including those labeled with 11C or 15N, have limited pediatric experience, in part due to their short half-lives and the need for ready access to a cyclotron. PET radiopharmaceuticals labeled with 68Ga may have a role in identification and localization of neuroendocrine tumors (see Chap. 20). PET with 124I has been used to image thyroid cancer in adults, but has not been used routinely in children or young adults (see Chap. 5).
Patient Preparation for 18F-FDG PET and 18F-FDG PET/CT
Patient preparation is important for successful completion of a technically adequate and diagnostically satisfactory PET or PET/CT [20, 21]. Patient and family preparation is of particular importance for 18F-FDG studies [22], but may be less important with PET of the skeleton with [18F] sodium fluoride [23]. Although much information can be provided in a mailing or on a departmental website, individual telephone contact made by a knowledgeable healthcare professional, such as a registered nurse or nuclear medicine technologist, provides an opportunity for individualized education and encouragement. One-to-one contact provides a way for the healthcare professional to answer questions, dispel misunderstandings, identify potential problems that may affect the study, and assess the developmental needs of the patient. For nearly all pediatric patients, family contact with a healthcare professional prior to the study increases the likelihood of a technically adequate and diagnostically successful study.
Pre-study fasting is important before 18F-FDG PET or 18F-FDG PET/CT of the body for an oncological indication [20] or for imaging infection or inflammation [24]. Pre-study fasting may be less important for brain imaging, but most guidelines recommend that it be done [21]. Although rarely performed in children, 18F-FDG PET for myocardial imaging requires enhancement of 18F-FDG uptake in the myocardium [22]. Caloric intake will stimulate insulin secretion, which can increase uptake of 18F-FDG in skeletal and myocardial muscle. For most 18F-FDG PET studies, patients should be fasting for at least 4 h before administration of 18F-FDG and must remain fasting during the 1-h uptake period after tracer administration. This restriction includes caloric intake by nasogastric tube or percutaneous feeding tube. Patients with delayed gastric emptying may need to fast for longer periods to ensure a technically adequate study. Unless the study will be performed with sedation or general anesthesia, patients can continue to drink water before the study. However, they must be instructed to avoid sweetened or caffeine-containing beverages [20, 21]. Even nonnutritive sweeteners may have a mild stimulatory effect on insulin secretion [25]. In patients receiving parenteral nutrition or hydration, all intravenous glucose sources must be discontinued for at least 4 h before 18F-FDG administration. If intravenous hydration must be continued, this can be done with saline-containing solutions, but it is important to confirm that all glucose-containing solutions, including solutions such as D5 Ringer’s lactate, have been discontinued.
In infants and young children, adequate fasting can be a challenge and may require coordination of the imaging schedule with the child’s eating and sleeping routine. Infants may be breastfed or given a bottle just before the start of the scan. However, this approach is rarely used, as patients usually must have nothing by mouth before undergoing sedation or general anesthesia, which is commonly used in this age group. In older children, allowing a small snack after the uptake period and just before the start of imaging may improve patient cooperation and permit successful completion of a scan. Special attention should be given patients at increased risk of hypoglycemia during prolonged fasting, including infants. Caloric intake still must be restricted before tracer administration and during the 18F-FDG uptake period, but caloric intake after the 1-h uptake period may have little effect on 18F-FDG PET quality. If hypoglycemia is a clinical concern, then a dextrose-containing intravenous solution to maintain adequate blood glucose levels can be started after the 1-h 18F-FDG uptake period.
Elevated blood glucose levels can compete with 18F-FDG for cellular uptake and diminish 18F-FDG uptake in tissues of interest. This effect may be greater when the FDG PET is performed for oncological indications and less when performed to evaluate infection or inflammation [24]. Before 18F-FDG is administered, blood glucose should be checked, typically using a glucometer to test a finger-stick sample of capillary blood. Most guidelines recommend delaying an 18F-FDG PET study if the blood glucose level is greater than 200 mg/dl [20, 21].
Patients with diabetes mellitus can provide special challenges when planning an 18F-FDG PET study. Excess insulin administration may produce hypoglycemia as well as lead to increased nonspecific muscle uptake of 18F-FDG. With inadequate therapy, the resulting hyperglycemia can competitively inhibit tissue uptake of 18F-FDG. Both of these effects can degrade the quality and decrease the diagnostic accuracy of an 18F-FDG PET study. The patient’s endocrinologist or primary care physician should be involved in any decision to alter the patient’s medical or nutritional regimen. However, if these clinicians are not familiar with the techniques of 18F-FDG PET, they may depend on the nuclear medicine physician to help develop an appropriate plan.
The management approach will depend on whether the patient has type 1 (insulin-dependent) or type 2 (insulin-resistant) diabetes. For most patients with diabetes, scheduling an 18F-FDG PET for early morning will facilitate satisfactory management of blood glucose levels. Patients with type 1 diabetes must continue to receive sufficient insulin to maintain appropriate basal blood insulin levels while fasting. This can be accomplished with either subcutaneous injection of a long-acting insulin (typically at bedtime) or subcutaneous infusion of insulin at a basal rate from an insulin pump. Patients should not take additional pre-meal or sliding scale insulin before the 18F-FDG PET study, as this may result in fasting hypoglycemia and can increase nonspecific muscle uptake of 18F-FDG. Patients demonstrating fasting hyperglycemia may require adjustment of basal insulin doses.
Although type 2 diabetes is more common in adults than in children, increasing numbers of children are developing this disease. Patients with type 2 diabetes have insulin resistance, which can be managed by diet, oral medications, or insulin. With an overnight fast, many of these patients will not develop significant hyperglycemia if they delay taking morning diabetes medications or insulin until midmorning, after completion of an early morning 18F-FDG PET or PET/CT. Treatment with the diabetic drug metformin can been associated with intense 18F-FDG uptake in the colon, and sometimes in the small intestine [26]. Metformin also increases 18F-FDG uptake in skeletal muscle and liver [27]. Although it is used rarely in children, metformin should be discontinued, if possible, two to three days before the 18F-FDG PET study [28]. Some patients will arrive to their scheduled appointment with marked hyperglycemia due to either poorly controlled diabetes or unrecognized nocturnal hyperglycemia. Occasionally, a patient may develop marked hyperglycemia after initiation of glucocorticoid therapy. Ideally, 18F-FDG PET or PET/CT can be postponed while awaiting improved glycemic control, but this may not be possible in all clinical situations.
Depending on the season and climate, brown adipose tissue may be seen in as many as a third of all children having an 18F-FDG PET or PET/CT study (Fig. 3.4). A variety of maneuvers have been used to minimize 18F-FDG uptake in brown adipose tissue. Heating in a warm room for at least 30 min before 18F-FDG administration and for the duration of the uptake period can decrease the incidence of significant uptake in brown adipose tissue from 33 to 9 % [29]. Other interventions have been used in an attempt to decrease 18F-FDG uptake in brown adipose tissue, including oral administration of a low dose of a beta-blocker such as propanolol [30] or a low dose of a benzodiazepine such as diazepam [31], intravenous administration of fentanyl [32], or dietary restriction to a high-fat diet [33] for at least 12 h before the study. Compared to these medical and dietary interventions, pre-study warming avoids the need to assess potential drug interactions or side effects and does not require patient compliance with a special diet. In some institutions, administration of diazepam or fentanyl may be considered sedation and thus require the documentation and consultation needed before any sedation procedure. Regardless of the method used to decrease uptake in brown adipose tissue, it is helpful to instruct patients to avoid cold exposure and to dress warmly while travelling from home for the 18F-FDG PET or PET/CT study.
Fluorine-18-FDG uptake in brown adipose tissue. Increased 18F-FDG uptake in brown adipose tissue can be a common finding on 18F-FDG PET of the body. Many pediatric patients may demonstrate a mild degree of brown adipose uptake (a), but some may have moderate (b) or marked (c) uptake. Extensive uptake in brown adipose tissue can obscure sites of pathological 18F-FDG uptake and decrease confidence in study interpretation. A variety of strategies have been used to decrease brown adipose uptake. Note variable myocardial uptake of 18F-FDG, despite similar pretest preparation in all three patients
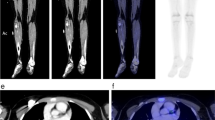
Intense muscle use prior to 18F-FDG PET will increase uptake of 18F-FDG in the involved muscles (Fig. 3.5). Depending on the indication for the 18F-FDG PET/CT, this may decrease the sensitivity of the study. Therefore, patients should be advised to refrain from heavy physical exertion for at least 24 h before the 18F-FDG PET or PET/CT. Similarly, exercise, repetitive muscle activity, maintaining a static posture, talking, or chewing should be avoiding during the uptake period. In young children, increased uptake may be seen in the muscles of respiration after prolonged crying during the uptake period [34].
Fluorine-18-FDG uptake in skeletal muscle. Non-pathological muscle uptake can occur with muscle use or exertion before or during the uptake period. (a) In a 14-year-old patient with treated synovial cell sarcoma of the left upper leg, increased muscle uptake in the right leg reflects a change in gait and altered weight-bearing. In muscles of the right arm and hand, increased uptake could be related to weight-bearing on a cane, but also reflect use of an electronic entertainment device during the uptake period. (b) In a 17-year-old male athlete, diffusely increased uptake throughout the skeletal muscles, but most intense in the lower legs and forearms, was attributed to extreme physical exertion 1 day before the 18F-FDG PET was performed. The patient denied dietary indiscretion, but a similar pattern of uptake can be seen if 18F-FDG PET is performed in a non-fasting patient
Patients should be provided with a quiet environment in which to stay during the uptake period. Although patients may be tempted to use electronic devices, including cell phones, electronic games, or laptop computers, the repetitive hand motion required to operate these devices may result in increased muscle uptake of 18F-FDG. Quiet reading or movie watching usually is not a problem prior to imaging the torso, but patients still need to be encouraged to avoid staying in a single posture requiring prolonged static contraction of neck or back muscles, as this also can result in increased 18F-FDG uptake in affected muscles (Fig. 3.5). In preparation for 18F-FDG PET of the brain [21], the patient should stay in a quiet, dimly lit room for a half an hour before tracer administration and during the uptake period to limit visual stimulation (Fig. 3.2). Ideally, the patients should have limited interaction with others and not talk, speak, or read, but this may not be feasible with every pediatric patient. Depending on the indication for the study, some interaction with caregivers or quiet reading may be allowed or even necessary to gain patient cooperation. Prior to all studies, emotional stimulation should be limited. The indications for 18F-FDG PET and the region of interest will guide the appropriate environment during the uptake period. However, dealing successfully with young children and teenagers requires flexibility to achieve a balance between acquiring an ideal imaging study and provoking an adverse behavioral response to an environment that is sterile or unappealing.
PET Acquisition
The acquisition procedures for pediatric PET and PET/CT depend on the radiopharmaceutical, camera geometry, the clinical indication, and the anatomic region to be imaged. Imaging protocols must be optimized for the equipment available in each department. For example, if a camera detector has septa, images can be acquired in “2-D” mode, while if the septa are retracted or not available on a camera model, then imaging is performed in “3-D” mode [21]. Other camera features, such as time-of-flight circuitry, also can influence acquisition parameters. FDG PET image acquisition in 2-D may require 4–6 min per bed position, while 3-D mode may require 1.5–3 min per bed position. The number of bed positions required to complete a study will depend on the size of the child and camera. Brain imaging typically takes no more than two bed positions, and in small children may require only one bed position.
The uptake period, the time between radiopharmaceutical administration and the start of imaging, depends on the radiopharmaceutical and the clinical indication. Fluorine-18-FDG PET of the torso (base of skull to thighs) or whole body (top of skull to feet) typically is started after a 60-min uptake period. If standard uptake values (SUVs) are to be compared from one study to the next, then it is critical that the uptake period be very consistent (less than 5 min of variation) from one study to the next [34]. Some authors have advocated a longer uptake period or acquiring a second delayed 18F-FDG PET to better characterize tumors [35], but this has not become routine practice. Other technical factors that may affect the standard uptake value include improper calibration of the dose calibrator and PET camera, subcutaneous infiltration during intravenous administration of tracer, and inappropriate image reconstruction parameters [34].
Fluorine-18-FDG PET of the brain usually is started after a 30-min uptake period [21]. For the occasional patient that needs both brain and body imaging, two approaches are possible. One approach is to start the brain PET after a 30-min uptake. Then, with only a small delay, acquisition of the torso scan can be started 60 min after 18F-FDG administration. However, this approach limits the time a patient may be kept in a heated room as a method to decrease uptake of 18F-FDG in brown adipose tissue. An alternative approach is to acquire the torso or whole-body scan starting at 60 min and then to acquire the brain PET. Imaging for 18F-NaF bone PET begins typically 30–45 min after tracer administration for whole-body or torso imaging, but some departments may wait 90–120 min before acquiring images of the extremities [23].
Special Issues
Some management issues must be considered for all pediatric PET and PET/CT studies. Sedation or anesthesia may be needed in younger patients or patients with intellectual disabilities that may limit adequate cooperation. The preferred approaches and policies for sedation or general anesthesia vary among institutions (see Chap. 2). For other patients, distraction techniques may be sufficient to accomplish a successful study [22]. If sedation or anesthesia will be needed for brain 18F-FDG PET, it should be administered as late as possible after 18F-FDG administration and before imaging [21]. The requirements for sedation or anesthesia may mandate a longer period of fasting than needed for the 18F-FDG PET or PET/CT alone.
The approach to bladder catheterization also varies among institutions. Placement of a bladder catheter will decrease accumulation of excreted tracer in the bladder, which can improve visualization of pelvic pathology on torso or whole-body studies. This is less important in a study limited to the brain. A bladder catheter also prevents the contamination of the scanner bed and patient that can occur if a young patient inadvertently voids during the study. However, if the patient can void before the start of imaging and if torso imaging is performed from the pelvis to head, then only small amount of tracer should be in the bladder when the pelvis is imaged. Placement of a bladder catheter will be unpleasant for the patient and may make an otherwise cooperative child become uncooperative. Some facilities perform bladder catheterization after induction of sedation or anesthesia [22, 36]. However, in patients undergoing conscious sedation, catheterization may arouse the patient and necessitate using deeper sedation than originally planned. In our facility, we routinely do not use bladder catheterization and use it only for patients with known voiding disorders. Rarely, catheterization will be used if a sedated patient is discovered to have a large, filled bladder after imaging has started.
Although pregnancy is not an issue for most pediatric patients, the possibility must be considered in postpubertal girls and young women. Most institutions have standard policies guiding the determination of pregnancy status. At a minimum, postpubertal females should be asked if they might be pregnant. Any patient expressing concern about pregnancy should be able to have a pregnancy test before proceeding with a PET or PET/CT scan. Depending on institutional policies and local statutes and regulations, this may raise issues about patient confidentiality, parental or partner involvement, and the risks and benefits of the scheduled study, which may necessitate involvement of the ordering clinician or primary care provider. Similarly, if a pediatric patient’s mother or other family caregiver is pregnant, then special arrangements may be needed for other family members to accompany the patient during the PET or PET/CT study [37].
Image Co-registration
Co-registration with anatomic imaging studies (CT, MR) or with prior PET studies can improve diagnostic accuracy. Nearly all commercially available PET scanners are integrated devices that include both PET and CT capabilities. Thus, PET/CT fusion images can be obtained with a minimum of image manipulation. However, fusion software can be used for co-registration of PET images with a separately acquired CT or MR studies or with a prior PET study. This can be particularly useful with a patient who has had a recent diagnostic CT that was not acquired as a PET/CT. Software co-registration also is becoming more important for planning radiation therapy [38]. For brain PET, such as for evaluation of a seizure disorder or brain tumor, correlative MR studies almost always are available and can be used to create PET/MR fusion images [6]. Elsewhere in the body, PET/MR software co-registration can be helpful for evaluating musculoskeletal tumors or intra-abdominal tumors that may be conspicuous on MR rather than CT. With increased concern about radiation dose, MR is being used more for follow-up and surveillance of pediatric oncology patients. Due to the lower radiation dose, recently introduced hybrid PET/MR scanners may be well suited to pediatric applications [39], but until these reach wider availability (see Chaps. 26 and 27), software co-registration of PET and MR can be used to improve diagnostic certainty while decreasing radiation dose.
PET/CT
The availability of integrated PET/CT scanners provides the possibility that an 18F-FDG PET/CT and diagnostic CT can be acquired as part of the same study and that the diagnostic CT can be used for attenuation correction the 18F-FDG PET. However, implementing this approach in a pediatric department requires careful planning and imaging protocols that can be customized to the medical and developmental needs of each patient. Development of all protocols should be guided by the principle of ALARA [40]. For example, the need for a diagnostic CT may be influenced by other available imaging, such as a recently acquired diagnostic CT or MR.
Depending on the energy settings, the CT acquired as part of a PET/CT study can serve as a stand-alone diagnostic study (with or without intravenous contrast, as indicated), as a “nondiagnostic” study for anatomic correlation, or for attenuation correction only [20]. If appropriately acquired, any of these studies can be used for attenuation correction of the PET, although metallic implants such as pacemakers or prosthetics may create an attenuation artifact.
CT energy settings should be determined based on patient size. An attenuation-only CT can be acquired with an energy of only 16–30 mA, depending on patient size [41]. Any CT scan that is to be used for attenuation correction of an 18F-FDG PET should be acquired with the same patient positioning and with the same breathing pattern as the PET [20]. Thus, a chest CT should be acquired during tidal breathing or with a mid-breath breathhold. Otherwise, misregistration of the attenuation map and 18F-FDG PET may create attenuation artifacts that can decrease the diagnostic accuracy of the PET. However, a chest CT acquired without a full inspiration may have decreased diagnostic accuracy compared to a standard chest CT. Therefore, it may be necessary to acquire multiple chest CT scans, for example, a diagnostic chest CT acquired with a full inspiration and a low-dose attenuation correction CT scan acquired with a mid-breath breathhold. How these scans link to other CT scans of the body will depend on the clinical indication, the need for diagnostic CT imaging of other body regions, and the availability of recent prior imaging. For example, in our institution, we have found that in most patients with lymphoma, it is helpful to perform a diagnostic CT of the “extended neck” that includes the region from the base of the skull to the aorta and is acquired with the arms down and without full lung inspiration. This improves the diagnostic accuracy of both PET and CT, by providing a high-quality diagnostic scan and by facilitating high-fidelity co-registration in a region of the body most likely to have pathological findings.
The appropriate use of intravenous and oral contrast for PET/CT in pediatric patients remains unclear [20, 34]. The use of either contrast agent implies the acquisition of a diagnostic quality CT scan. However, if a high-quality CT scan is not indicated, then performing one will result in a higher radiation dose than necessary (see Chap. 30). On the other hand, if a diagnostic CT can be acquired as part of the PET/CT study, then this may avoid another separate diagnostic CT and lessen the overall radiation dose to the patient. Some authors have suggested that the use of barium-containing oral contrast may risk attenuation artifacts that can result from minor misregistration between the CT and PET acquisitions. The risk of this may be lower with iodine-containing oral contrast agents, probably as these agents typically are used in diluted form and have a lower density than barium contrast agents, or with a negative contrast agent such as water. If oral contrast agents will be administered before the 18F-FDG PET, then they should be given only with noncaloric, non-sweetened liquids. Similarly, if a CT acquired early during intravenous contrast injection is used for attenuation correction of the PET, then attenuation artifacts may occur around a vessel (e.g., the subclavian vein) containing a transiently high concentration of contrast.
Developing the CT and PET imaging protocols for 18F-FDG PET/CT must be customized for the medical and developmental needs of each patient. For example, in the absence of a specific clinical indication, the routine acquisition of high-energy diagnostic CT throughout the PET field of view is particularly inappropriate in the pediatric population. For each patient, the PET and CT imaging protocol must take into account the clinical indication, the sites of known disease, the information that can be provided by each study, and the goal of limiting radiation exposure.
Normal Patterns of FDG Uptake
Accurate interpretation of 18F-FDG PET and PET/CT requires familiarity with the normal patterns of FDG distribution in children. Normal patterns of physiological uptake and excretion of 18F-FDG should be distinguished from pathological uptake [42–44].
The normal brain demonstrates high 18F-FDG uptake, with the most intense uptake in gray matter and basal ganglia (Figs. 3.1 and 3.2). Glucose provides approximately 95 % of the energy required by the brain [21], and up to 6 % of the administered dose of 18F-FDG may be taken up in the brain [45]. Increased 18F-FDG uptake can be seen with neuronal activation, such as in the visual cortex. This pattern of uptake limits the use of 18F-FDG PET for evaluation of brain tumors as it may be difficult to discriminate uptake in tumor from uptake in nearby normal brain. Similarly, intense uptake in the brain can obscure pathological uptake in the adjacent scalp or skull base.
Normal physiological 18F-FDG uptake is seen in lymphatic tissue in Waldeyer’s ring, including the palatine tonsils and adenoids (Fig. 3.1). In children, prominent physiological uptake can obscure disease, while increased uptake in response to an upper respiratory infection can be difficult to distinguish from disease. Symmetrical 18F-FDG uptake is more likely to represent physiological uptake [46]. Mild, and usually symmetrical, 18F-FDG uptake also can be seen in salivary glands and pharyngeal muscles. Laryngeal uptake can range from mild to intense, depending, at least in part on recent talking. As in adults, asymmetrical laryngeal uptake is concerning for pathology, suggesting either FDG-avid local disease or unilateral vocal cord paralysis.
In children, cardiac uptake of 18F-FDG can be variable, and intense myocardial uptake can be seen even after well-documented fasting (Figs. 3.4 and 3.6). Additional dietary interventions, with high-fat, low-carbohydrate diets, to decrease myocardial uptake have been tried in adults [33], especially those with disease located near the heart, but there has been little reported experience using these approaches in children.
Variable physiological uptake of 18F-FDG. Variable patterns of physiological 18F-FDG uptake can be seen in many organs. (a) In postpubertal females, physiological uptake of 18F-FDG is most prominent in the ovaries and fallopian tubes around the time of ovulation and in the uterus at the end of menstrual cycle. (b) Mild-to-intense testicular uptake of 18F-FDG can be seen in peripubertal and postpubertal males. (c) Thymic uptake of 18F-FDG is as common in pediatric PET. Intense thymic uptake of 18F-FDG also can be seen due to “thymic rebound” after completion of chemotherapy. (d) Diffusely increased 18F-FDG uptake in the expected locations of bone marrow typically reflects physiological or pharmacological stimulation of marrow and rarely represents disease involvement of bone marrow
Diffuse thymic uptake of 18F-FDG rarely indicates disease in children (Fig. 3.5). This pattern of uptake usually represents age-related physiological activation or post-chemotherapy stimulation (thymic rebound) [47]. Although correlation or co-registration with CT can be used to confirm thymic uptake, the conformation of uptake is typical of the thymus. Diffuse bone marrow uptake occasionally represents widespread disease, but marrow uptake also is a well-described response to cancer therapy, including physiological rebound after chemotherapy and pharmacological stimulation with colony-stimulating factors used hasten marrow recovery (Fig. 3.6) [42, 48]. On the other hand, focal uptake rarely reflects treatment-related stimulation and raises concern for marrow involvement by disease. Occasionally, diffuse marrow uptake of 18F-FDG can represent incidental nontreatment-related marrow stimulation, such as in response to anemia or a systemic inflammatory process [42].
Normal physiological distribution of 18F-FDG can be seen throughout the gastrointestinal tract [43]. Focal uptake at the gastroesophageal junction is a normal finding, while substantial diffuse or regional uptake along the esophagus more likely represents an inflammatory process, such as posttreatment mucositis. Fluorine-18-FDG uptake by gastric mucosa can be physiological, but also may indicate a pathological neoplastic or inflammatory process. In the small and large intestines, the pattern of 18F-FDG uptake helps distinguish physiological from pathological uptake. Typical physiological 18F-FDG uptake can be multifocal and widespread. A single intense focus of 18F-FDG uptake is worrisome for disease, while regional, segmental, or widespread diffuse intestinal uptake more likely represents an inflammatory process [49]. For example, terminal ileitis may come to attention due to incidental 18F-FDG uptake identified on an 18F-FDG PET/CT.
The urinary system is the usual path of 18F-FDG excretion, so tracer can be seen in the kidneys, renal collecting systems, ureters, and bladder (Fig. 3.1) [43, 50]. Tracer accumulation in ureters usually can be identified by the distinctive contours of the ureters and by correlation with CT. This may be particularly helpful if there is focal urinary tracer accumulation within the urinary collecting system. Tracer accumulation in the collecting system can be minimized by encouraging patient hydration. Although routine use of pharmacological diuresis has been advocated in the past, diuretics are rarely, if ever, used for imaging children [44]. Bladder catheterization may be helpful in selected circumstances, such as in a sedated patient in whom tracer accumulation within the bladder may obscure 18F-FDG uptake in nearby disease. Rarely, collecting system obstruction or hydronephrosis will be identified on 18F-FDG PET or PET/CT. Tracer accumulation in congenital variants such as a dilated renal calyx, an ectopic or horseshoe kidney, or a bladder diverticulum must be recognized to avoid misinterpretation as a site of FDG-avid disease [51]. Persistent uptake in the renal cortex is abnormal and has a broad differential, including infection, infiltrative disorder, and lymphoma.
Physiological uptake of 18F-FDG can be seen in the testes [52], especially in peripubertal and postpubertal young men (Fig. 3.6). In premenarchal females, 18F-FDG uptake should not be seen in the ovaries or uterus. After menarche (Fig. 3.6), 18F-FDG uptake in the ovaries is most intense during follicular genesis and ovulation, while endometrial uptake appears most intense at the end of the menstrual cycle, but also may be increased in the ovulatory phase of the menstrual cycle [53]. Therefore, it can be important to know the menstrual phase to adequately interpret these findings in a female patient.
Increased muscle 18F-FDG uptake can be seen for up to 2 days after heavy exertion or if there is repetitive muscle use during the uptake (Fig. 3.5). Diffusely increased muscle uptake also can be seen in patients who did not fast before 18F-FDG administration. Rarely, widespread muscle uptake represents an inflammatory myositis or rhabdomyolysis. Increased uptake can be seen with compensatory muscle activity, such as increased muscle uptake in a leg after disuse or amputation of the contralateral limb. Increased 18F-FDG uptake is seen with increased use of the accessory muscles of respiration related to respiratory distress or even crying.
Summary
Infants and children provide special challenges to acquiring a technically adequate and diagnostically satisfactory PET or PET/CT scan. Adequate pre-study preparation of the patient and family is critical. Imaging protocols must pay particular attention to the pediatric spectrum of disease, the developmental needs of pediatric patients, and the goal of minimizing radiation exposure. Interpretation of pediatric 18F-FDG PET and PET/CT requires knowledge of pediatric diseases and an appreciation for the patterns of tracer biodistribution that can be seen in infants and children. Issues such as sedation or anesthesia, rarely a concern in adult nuclear medicine, are a normal part of routine pediatric PET and PET/CT. Protocols and departmental procedures must balance the developmental needs of children with the goal of acquiring a diagnostic imaging study that answers the clinical question. Achieving this goal can be facilitated by trained professional staff, including nuclear medicine physicians, nuclear medicine technologists, registered nurses, and child-life specialists, who have experience in pediatric imaging and a desire to work with infants and children.
References
Jadvar H, Connolly LP, Fahey FH, Shulkin BL. PET and PET/CT in pediatric oncology. Semin Nucl Med. 2007;37:316–31.
Grant FD, Dubach L, Treves ST. 18F-fluorodeoxyglucose PET and PET/CT in pediatric musculoskeletal malignancies. PET Clin. 2010;5:349–61.
Portwine C, Marriot C, Barr RD. PET imaging for pediatric oncology: an assessment of the evidence. Pediatr Blood Cancer. 2010;55:1048–61.
Grant FD, Treves ST. Nuclear medicine and molecular imaging of the pediatric chest: current practical imaging assessment. Radiol Clin North Am. 2011;49:1025–51.
Patil S, Biassoni L, Borgwardt L. Nuclear medicine in pediatric neurology and neurosurgery: epilepsy and brain tumors. Semin Nucl Med. 2007;37:357–81.
Kim S, Salamon N, Jackson HA, Blüml S, Panigraphy A. PET imaging in pediatric neuroradiology: current and future applications. Pediatr Radiol. 2010;40:82–96.
Grant FD, Fahey FH, Packard AB, DAvis RT, Alavi A, Treves ST. Skeletal PET with 18F-fluoride: applying new technology to an old tracer. J Nucl Med. 2008;49:68–78.
Jadvar H, Alavi A, Mavi A, Shulkin BL. PET in pediatric diseases. Radiol Clin North Am. 2005;43:135–52.
Kaste SC. PET-CT in children: where is it appropriate? Pediatr Radiol. 2011;41:S509–13.
Weckesser M. Molecular imaging with positron emission tomography in paediatric oncology – FDG and beyond. Pediatr Radiol. 2009;39:S450–5.
Levin CS, Hoffman EJ. Calculation of positron range and its effect on the fundamental limit of positron emission tomography. Phys Med Biol. 1999;44:781–99.
Treves ST, Parisi MT, Gelfand MJ. Pediatric radiopharmaceutical doses: new guidelines. Radiology. 2011;261:347–9.
Wootton R, Doré C. The single-passage extraction of 18F in rabbit bone. Clin Phys Physiol Meas. 1986;7:333–43.
Costeas A, Woodward HQ, Laughlin JS. Depletion of 18F from blood flowing though bone. J Nucl Med. 1970;11:43–5.
Gatley SJ. Labeled glucose analogs in the genomic era. J Nucl Med. 2003;44:1082–6.
The MICAD Research Team. [18F]fluoro-2-deoxy-2-D-glucose. In: Molecular imaging and contrast agent database (MICAD) [internet]. Bethesda: National Center for Biotechnology Information (US); 2004–2012. Bookshelf ID: NBK23335PMID: 20641537.
Fowler JS, Ido T. Initial and subsequent approach for the synthesis of 18FDG. Semin Nucl Med. 2002;32:6–12.
Vallabhajosula S. 18F-labeled positron emission tomographic radiopharmaceuticals in oncology: an overview of radiochemistry and mechanisms of tumor localization. Semin Nucl Med. 2007;37:400–19.
Rice SL, Roney CA, Daumar P, Lewis JS. The next generation of positron emission tomography radiopharmaceuticals in oncology. Semin Nucl Med. 2011;41:265–82.
Dulbeke D, Coleman RE, Guiberteau MJ, et al. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med. 2006;47:885–95.
Waxman AD, Herholz K, Lewis DH, et al. Society of Nuclear Medicine procedure guideline for FDG PET brain imaging. http://interactive.snm.org/docs/SocietyofNuclearMedicineGuidelineforFDGPETBrainImaging.pdf.
McQuattie S. Pediatric PET/CT imaging: tips and techniques. J Nucl Med Technol. 2008;36:171–8.
Segall G, Delbeke D, Stabin MG, Even-Sapir E, Fair J, Sajdak R. SNM practice guideline for sodium 18F-fluoride PET/CT bone scans 1.0. J Nucl Med. 2010;51:1813–20.
Rabkin Z, Israel O, Keidar Z. Do hyperglycemia and diabetes affect the incidence of false-negative 18F-FDG PET/CT studies in patients evaluated for infection or inflammation and cancer? A comparative analysis. J Nucl Med. 2010;51:1015–20.
Liang Y, Steinbach G, Maier V, Pfeiffer EF. The effect of artificial sweetener on insulin secretion. 1. The effect of acesulfame K on insulin secretion in the rat (studies in vivo). Horm Metab Res. 1987;19:233–8.
Gontier E, Fourme E, Wartski M, Blondet C, Donardel G, Le Stanc E, Marzarides M, Foehrenbach H, Peckig AP, Alerini JL. High and typical 18F-FDG bowel uptake in patients treated with metformin. Eur J Nucl Med Mol Imaging. 2008;35:95–9.
Bybel B, Greenberg ID, Paterson J, Ducharme J, Leslie WD. Increased F-18 FDG intestinal uptake in diabetic patients on metformin: a matched case–control analysis. Clin Nucl Med. 2011;36:452–6.
Ozülker T, Ozülker F, Mert M, Ozpaçaci T. Clearance of the high intestinal 18F-FDG uptake associated with metformin after stopping the drug. Eur J Nucl Med Mol Imaging. 2010;37:1011–7.
Zukotynski KA, Fahey FA, Laffin S, Davis R, Treves ST, Grant FD, Drubach LA. Constant ambient temperature of 24 °C significantly reduces FDG uptake by brown adipose tissue in children. Eur J Nucl Med Mol Imaging. 2009;36:602–6.
Söderlund V, Larsson SA, Jacobsson H. Reduction of FDG uptake in brown adipose tissue in clinical patients by a single dose of propanolol. Eur J Nucl Med Mol Imaging. 2007;34:1018–22.
Barrington SF, Maisey MN. Skeletal muscle uptake of fluorine-18-FDG: effect of oral diazepam. J Nucl Med. 1996;37:1127–9.
Gelfand MJ, O’Hara SM, Curtwright LA, MacLean JR. Pre-medication to block [18F]FDG uptake in the brown adipose tissue of pediatric and adolescent patients. Pediatr Radiol. 2005;35:984–90.
Williams G, Kolodny GM. Method for decreasing uptake of 18F-FDG by hypermetabolic brown adipose tissue on PET. Am J Roentgenol. 2008;190:1406–9.
Boellaard R. Standards for PET image acquisition and quantitative data analysis. J Nucl Med. 2009;50:11S–20.
Hustinx R, Smith RJ, Benard F, et al. Dual time point fluorine-18 fluorodeoxyglucose positron emission tomography: a potential method to differentiate malignancy from inflammation and normal tissue in the head and neck. Eur J Nucl Med. 1999;26:1345–8.
Roberts EG, Shulkin BA. Technical issues in performed PET studies in pediatric patients. J Nucl Med Technol. 2004;32:5–9.
McCarville MB. PET-CT imaging in pediatric oncology. Cancer Imaging. 2009;9:35–43.
Scripes PG, Yaparpalvi R. Technical aspects of positron emission tomography/computed tomography in radiotherapy treatment planning. Semin Nucl Med. 2012;42:283–8.
Antoch G, Bockisch A. Combined PET/MRI: a new dimension in whole-body oncology imaging? Eur J Nucl Med Mol Imaging. 2009;36:S113–20.
Chawla SC, Federman N, Zhang D, et al. Estimated cumulative radiation dose from PET/CT in children with malignancies: a 5-year retrospective review. Pediatr Radiol. 2010;40:681–6.
Fahey FH. Dosimetry of pediatric PET/CT. J Nucl Med. 2009;50:1483–91.
Gordon BA, Flanagan FL, Dehdashti F. Whole-body positron emission tomography: normal variations, pitfalls, and technical considerations. Am J Roentgenol. 1997;169:1675–80.
Shreve PD, Anzai Y, Wahl RL. Pitfalls in oncologic diagnosis with FDG PET imaging: physiologic and benign variants. Radiographics. 1999;19:61–77.
Shammas A, Lim R, Charron M. Pediatric FDG PET/CT: physiological uptake, normal variants, and benign conditions. Radiographics. 2009;29:1467–86.
Ak I, Stokkel MP, Pauwels EK. Positron emission tomography with 2-[18F]fluoro-2-deoxy-D-glucose in oncology. II: the clinical value in detecting and staging primary tumours. J Cancer Res Clin Oncol. 2000;126:560–74.
Sarji SA. Physiological uptake in FDG PET simulating disease. Biomed Imaging Interv J. 2006;2:e59.
Brink I, Reinhardt MJ, Hoegerle S, Altehoefer C, Moser E, Nitzsche EU. Increased metabolic activity in the thymus studied with FDG PET: age dependency and frequency after chemotherapy. J Nucl Med. 2001;42:591–5.
Yao WJ, Hoh CK, Hawkins RA, et al. Quantitative PET imaging of bone marrow glucose metabolic response to hematopoietic cytokines. J Nucl Med. 1995;36:794–9.
Tatlidil R, Jadvar H, Bading JR, Conti PS. Incidental colonic fluorodeoxyglucose uptake: correlation with colonoscopic and histopathological findings. Radiology. 2002;224:783–7.
Vesselle HJ, Miraldi FD. FDG PET of the retroperitoneum: normal anatomy, variants, pathologic conditions, and strategies to avoid diagnostic pitfalls. Radiographics. 1998;18:805–23.
Subhas N, Patel PV, Pannu HK, Jacene HA, Fishman EK, Wahl RL. Imaging of pelvic malignancies with in-line FDG PET-CT: case examples and common pitfalls of FDG PET. Radiographics. 2005;25:1031–43.
Kitajima K, Nakamoto Y, Senda M, Onishi Y, Okizuka H, Sugimara K. Normal uptake of 18F-FDG in the testis: an assessment by PET/CT. Ann Nucl Med. 2007;21:405–10.
Lerman H, Metser U, Grisaru D, Fishman A, Lievshitz G, Even-Sapir E. Normal and abnormal 18F-FDG endometrial and ovarian uptake in pre- and postmenopausal patients: assessment by PET/CT. J Nucl Med. 2004;45:266–71.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Grant, F.D. (2014). PET and PET/CT in Children and Young Adults. In: Treves, S. (eds) Pediatric Nuclear Medicine and Molecular Imaging. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-9551-2_3
Download citation
DOI: https://doi.org/10.1007/978-1-4614-9551-2_3
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-9550-5
Online ISBN: 978-1-4614-9551-2
eBook Packages: MedicineMedicine (R0)