Abstract
Diabetes mellitus increases the risk of cardiomyopathy independently of underlying comorbidities, and heart failure is a major cause of death in diabetic patients. The development of this distinct cardiomyopathy in both type 1 and type 2 diabetes is associated with complex and multifactorial cellular and molecular perturbations. It is widely recognized that cardiac dysfunction in chronic diabetes involves hormonal imbalance, oxidative stress, proteases activation, defects in Ca2+ cycling, and varying degrees of subcellular remodeling of organelles.
Ca2+ -handling abnormalities in diabetic cardiomyocytes have primarily been attributed to changes in the sarcolemmal Na+–Ca2+ exchanger, L-type Ca2+ channel, Na+–K+ ATPase, and Na+–H+ exchanger proteins as well as Ca2+-release channels and Ca2+-pump proteins embedded in the sarcoplasmic reticulum. Intracellular Ca2+ overload has been implicated in the impairment of excitation–contraction coupling as a result of alterations in Ca2+-entry, Ca2+-removal, Ca2+-uptake, and Ca2+-release processes in the diabetic heart. These observations are consistent with the view that defects in Ca2+-handling proteins play a critical role in the pathogenesis of cardiac dysfunction during the development of diabetic cardiomyopathy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Diabetic heart
- Diabetic cardiomyopathy
- Calcium cycling proteins
- Sarcolemma remodeling
- Sarcoplasmic reticulum remodeling
- Cardiac dysfunction
- Na+–Ca2+ exchange
- Na+–K+ ATPase
- Ca2+-pump ATPase
- Ca2+-release channels
1 Introduction
Cardiovascular disease is the leading cause of death in the diabetic population. Although diabetic cardiomyopathy is associated with several comorbidities including atherosclerosis, hypertension, coronary artery disease, and valvular malfunction, it has been demonstrated that chronic diabetes impairs ventricular function independently of other risk factors [1, 2]. This distinct diabetic cardiomyopathy is characterized by reduced diastolic compliance and rate of myocardial relaxation as well as a decrease in absolute force development [3, 4]. The exact underlying pathological mechanisms are not clear; however, several studies have suggested that cardiac dysfunction in chronic diabetes is intimately associated with varying degrees of defects in subcellular organelles such as sarcolemma (SL), sarcoplasmic reticulum (SR), mitochondria (MT), myofibrils (MF), and extracellular matrix (ECM) [3, 5, 6]. Remodeling of these components in the diabetic heart primarily occurs in response to hormonal imbalance, oxidative stress, activation of different proteases, changes in gene expression, and metabolic shift caused by increased levels of cholesterol and fatty acids. It is worthwhile to note that remodeling of SL and SR along with altered calcium metabolism has been shown to be an early sign in the process for the development of diabetic cardiomyopathy [7–9].
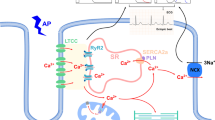
It is well known that intracellular Ca2+ is a major regulator of excitation–contraction coupling, and multiple aspects of calcium handling are considered to underlie the subcellular mechanisms responsible for the impaired cardiac contraction and relaxation in diabetic cardiomyopathy [6]. Indeed, several studies have reported the occurrence of intracellular Ca2+ overload in diabetic cardiomyocytes [3, 7, 10]. This alteration have been mostly attributed to the SL and SR remodeling, leading to depressed SL Na+–Ca2+ exchanger activity, decreased SR Ca2+-pump ATPase (SERCA2a) activity, reduced SR Ca2+ load, and Ca2+-release channel (ryanodine receptor) dysfunction [11–13]. It is pointed out that the inward Ca2+ current is the critical initiator of the contractile and relaxation cycle in the heart. Cardiac depolarization opens L-type Ca2+ channels in the SL membrane and allows the entry of Ca2+ into cardiomyocytes. This transient increase in cytoplasmic Ca2+ concentration triggers Ca2+ release from SR, mainly through the Ca2+-release channel or ryanodine receptor2 (RyR2) and by inositol triphosphate receptors (InsP3R) to a lesser extent. This event, described as calcium-induced calcium release (CICR), is crucial for excitation–contraction coupling in cardiac muscle [14, 15]. Following the opening of a RyR2 cluster on the SR, Ca2+ sparks are generated; this local, rapid, and brief elevation in [Ca2+] i elevates cytosolic-free Ca2+ by tenfold or more and initiates contraction. The relaxation of cardiac muscle occurs upon lowering the concentration of free Ca2+ by intracellular SR uptake via SERCA2a as well as SL efflux via the Na+–Ca2+ exchanger and the SL Ca2+ pump in the SL membrane [6, 14]. Although the MT and nucleus are also known to accumulate a significant amount of Ca2+ in cardiomyocytes, their role in the regulation of cytoplasmic concentration of free Ca2+ during the contraction and relaxation processes is not well established [6, 14]. This chapter is therefore focused on discussion regarding the status Ca2+-handling proteins in SL and SR during the development of diabetic cardiomyopathy.
2 SL Defects in Diabetic Heart
Alterations in SL L-type Ca2+ channels, Na+–Ca2+ exchanger, Na+–K+ ATPase, and Na+–H+ exchanger proteins, which are involved in Ca2+ handling directly or indirectly, have been shown to occur in diabetic cardiomyopathy [2, 6]. L-type Ca2+ channels are voltage-gated channels mostly located in the transverse tubules in proximity with RyR in SR, thereby suggesting the existence of a physical coupling between both Ca2+-entry and Ca2+-release channels [16]. SL Ca2+ channels in cardiomyocytes are modulated by several pathways including calmodulin (CaM), β-adrenergic receptors, phosphatidylinositol-3-kinase (PI3K), protein kinase A (PKA), and protein kinase C (PKC) [17, 18]. Although most of the calcium for cardiac contraction is provided by the SR, the activity of L-type Ca2+ channels is of critical importance for heart function. For instance, genetic mutation of SL Ca2+ channels leading to their impaired function has been linked with short QT syndrome, arrhythmia, and sudden death [19]. One of the early alterations detected in diabetic hearts was the prolongation of the ventricular action potential, which was attributed mainly to depressed transient outward K+ current and to L-type Ca2+ current [3, 20]. Experimental investigations in diabetic animals have revealed an unaltered [21, 22] or decreased [23–26] SL Ca2+-channel density. These disparities in results seems to reflect differences in models used, especially regarding the progression of disease, because alteration of the Ca2+ current has been shown to occur only in later stages of diabetes. The reduced Ca2+-channel density has been attributed to decreased levels of protein content [23, 25], depressed cell-surface expression [24–26], and changes in the phosphorylation status [23]. Lu et al. [24], after a series of investigations using type 1 (Ins2 Akita rats) and type 2 (db/db rats) [23] diabetes models, have reported decreased Ca2+-current density in both groups of diabetic animals, although the reduction was more intense in db/db than in Ins2 Akita myocytes as compared to nondiabetic cells. Because reduced phosphorylation status of the L-type Ca2+ channel was observed, it was hypothesized that Ca2+-current alteration could be related to a lack of insulin in type 1 diabetes and downregulation of the Akt pathway.
Following intracellular infusion of phosphatidylinositol-3,4,5-trisphosphate (PIP3), a second messenger produced by PI3K, and consequently because of stimulation of the Akt pathway, depression in Ca2+-current density was fully restored in Ins2 Akita myocytes in contrast with the partial restoration seen in db/db myocytes. The reduced levels of SL Ca2+-channel protein in the db/db cardiomyocytes were not seen in Ins2 Akita cardiomyocytes, thereby leading to the hypothesis that hyperglycemia in combination with obesity and insulin resistance in type 2 diabetes could cause more damage to SL Ca2+-channel function than hyperglycemia and lack of insulin in type 1 diabetes [23, 24]. It is important to highlight that another study has revealed that the activation of the PI3K-dependent Akt signaling pathway by insulin-like growth factor 1 restored L-type Ca2+ channels function in type 1 diabetic animals [27]. Taken together, these data suggest that insulin may have a positive inotropic effect and could explain how insulin resistance can affect heart function in several pathological states [3]. Despite the fact that most of the investigations support the idea that L-type Ca2+-channel activity is not impaired in cardiac hypertrophy, Ca2+ transients trigged by Ca2+-channel current have shown to be desynchronized, presenting a decreased amplitude and slow kinetics. These findings support the view that the intermolecular failure state would also apply to SL Ca2+ channels, the SR Ca2+-release channels, considering that the Ca2+ current becomes less effective in triggering SR Ca2+ release in the diabetic heart [16, 28–30].
It has become clear that Na+–Ca2+ exchanger 1 (NCX1) is the major SL protein for extruding Ca2+ that enters the cardiac cell via SL Ca2+ channels [31, 32]. This exchanger promotes the influx of 3 Na+ for the extrusion of each Ca2+, and its activity is controlled by both internal and external Na+ and Ca2+ levels as well as by the membrane potential. Under certain pathological conditions, NCX1 also works in the reverse mode, contributing to the development of intracellular Ca2+ overload in cardiomyocytes. It is noteworthy that the direction and amplitude of NCX1 current relies on the activity of SL Na+–K+-ATPase, which is responsible for maintaining the intracellular Na+ concentration at a low level [31, 32]. In type 1 diabetes, both depressed NCX activity [10, 33] and expression [10, 34, 35] were observed in the heart. It has been suggested that NCX1 dysfunction is related to alterations in the phospholipid composition of SL and reduced stimulation of the transporter by protein kinase C [36]. Furthermore, marked depression in SL Na+–K+ ATPase activity in insulin-dependent diabetes animals is considered to stimulate the NCX activity in a reverse mode to normalize the cytosolic Na+ concentration [37–39]. Depressed SL activity of Ca2+-pump ATPase was also reported in the diabetic heart [40, 41]. Consequently, a net gain of Ca2+ would occur as a result of the impaired efflux and increased Ca2+ entry, leading to intracellular Ca2+ overload as well as mechanical and electrical dysfunction in diabetic cardiomyocytes. On the other hand, in some studies involving type 2 diabetes, the NCX1 activity was either increased [25] or unchanged [42, 43], and no difference in mRNA level or protein content was detected [36, 42, 43]. Thus, the role of NCX in the etiology of cardiomyocyte dysfunction is complex, and changes in its expression or activity are viewed as compensatory or causal, depending upon the stage and severity of diabetes.
SL Na+–K+ ATPase plays a key role in maintenance of the resting membrane potential in cardiac cells by removing intracellular Na+ in exchange for extracellular K+. It has been demonstrated that Na+–K+ ATPase dysfunction in diabetic cardiomyopathy is related to downregulation of its subunit expression as well as alteration in the enzyme kinetics [37, 39]. The activity of this enzyme may also be influenced by alterations in composition of SL membrane observed in diabetes [44]. The abnormality in Na+–K+ ATPase activity in the diabetic heart results in cytosolic Ca2+ overload involving the NCX exchanger. It is important to emphasize that treatment of diabetic animals with insulin upregulates the expression of Na+–K+ ATPase and improves cardiac function [45]. Moreover, antioxidant agents, including vitamin E [46] and fish oil containing n-3 fatty acids [47], were able to attenuate and even prevent the diabetic-induced changes in SL Na+–K+ ATPase and cardiac dysfunction. These observations suggest the role of the observed depression in Na+–K+ in Ca2+-handling abnormalities in cardiomyocytes during the development of diabetic cardiomyopathy.
Another integral SL protein, Na+–H+ exchanger (NHE), is involved in intracellular Ca2+ modulation. NHE-1, which isoform is mostly expressed in cardiac cells, regulates intracellular pH by exchanging one intracellular H+ ion for an extracellular Na+ ion. In addition, NHE-1 participates in the regulation of Na+ fluxes and cell volume. Although emerging evidence supports NHE-1 involvement in diabetic cardiomyopathy, the results are controversial, and its potential role has not been established [48]. The NHE-1 activity has been shown to be decreased in isolated cardiomyocytes as well as the SL membranes of the diabetic heart [49, 50]. In another study, the reduced activity of NHE-1 in diabetes has been considered responsible for resistance of diabetic hearts to ischemia–reperfusion injury [51]. An increase in NHE-1 activity in cardiomyocytes of the Goto-Kakizaki rat model of type 2 diabetes has also been detected [52]. It has been suggested that intracellular acidification in cardiac cells stimulates the Akt signaling pathway, which could represent a likely mechanism that mediates the myocardial hypertrophy observed in the diabetic animals. In addition, chronic treatment with cariporide, a NHE-1-selective inhibitor, has been shown to prevent the phenotype of hypertrophy [52]. It is worth noting that some studies have also indicated that chronic administration of NHE-1-selective inhibitors may prevent vascular hypertrophy in diabetic rats [53] and also attenuate or even reverse the development of cardiac hypertrophy and its progression to heart failure in different animal models [54–56]. Thus, the observed alterations in SL Na+–H+ exchanger in diabetes can be seen to indirectly affect the Ca2+ handling by cardiomyocytes and participate in the development of diabetic cardiomyopathy.
3 SR Changes in Diabetic Heart
Several studies have revealed that different Ca2+-handling proteins embedded in the SR membrane become abnormal during the development of diabetic cardiomyopathy [2, 6, 46]. SR channel or RyR is a key component in Ca2+ handling and excitation–contraction coupling in the heart. Cardiac cells express mostly the RyR2 isoform, which is regulated by proteins such as calmodulin (CaM), Ca2+-CaM-dependent kinase (CaMKII), and PKA [57]. Following the opening of a RyR2 cluster on the SR, Ca2+ sparks are generated and result in local, rapid, and brief elevation in cytosolic-free Ca2+ by tenfold or more and trigger cardiac contraction. It has been demonstrated that RyR2 function in diabetic cardiomyocytes is compromised, becoming leaky to Ca2+ during diastole and accounting for a reduced SR Ca2+ load. In addition, a leaky RyR would promote Ca2+ accumulation in the cytosol, resulting in increased SL NCX activity to remove the intracellular excess Ca2+ in exchange for Na+. Consequently, the increased Na+ influx would induce cell membrane depolarization, thereby leading to extrasystolic depolarizations and development of premature beats [58–60]. It has been suggested that these abnormalities may be linked to reduced levels of FKBP12.6 and increased activity of PKA [25, 61]. It should be mentioned that FKBP 12.6 is an accessory protein that plays a role in coordinating the opening and closing of individual RyRs in an array. The hyperphosphorylation of RyR2 by PKA leads to the dissociation of FKBP 12.6 and increasing the open probability of the RyR2 receptor [43]. This increased phosphorylation at Ser2809 and Ser2814 of RyR2 is also observed in stress/exercise-induced cardiac arrhythmias, sudden death, and catecholaminergic ventricular tachycardia [62, 63].
In a model of diabetic cardiomyopathy, Bidasee et al. [58] have reported that RyR2 proteins of 6-week streptozotocin (STZ)-induced diabetes rats bound less [3H]ryanodine in comparison to control, although the affinity of this specific ligand and protein expression of the receptor remained unchanged in comparison to control. In a later study using 6- and 8-week STZ-induced diabetes rats [64], they also observed impaired binding ability of RyR2 to [3H]ryanodine, which was even more pronounced in 8-week STZ-induced diabetes cardiomyocytes. In addition, 8-week STZ-induced diabetes rats showed a decrease in RyR2 expression (mRNA and protein). In both studies [58, 64], 2 weeks of insulin treatment initiated after 4 and 6 weeks of untreated diabetes was able to minimize the loss in function and expression of RyR2. Taken together, the findings indicate that the loss of functional integrity of the receptor precedes reduction in its expression and that the severity depends on the duration of untreated disease. The underlying mechanisms for RyR2 dysfunction remain unclear, but it has been shown that it could be caused by oxidative stress, nonenzymatic glycation reactions, and increased formation of disulfide bonds between adjacent sulfhydryl groups of the receptor [65–67].
The InsP3R plays a minor role in excitation–contraction coupling compared to the RyR in ventricular cardiomyocytes, but in atrial myocytes InsP3Rs are much more numerous and coexist with RyR on the SR, suggesting a prominent role in atrial contraction [68]. Several studies have shown that the InsP3R pathway is involved in progression of heart failure and delayed after depolarizations arrhythmias [69, 70]. In an experiment involving animals with obesity and type 2 diabetes, InsP3R expression was unaltered in ventricles from ob/ob mice [71], but in other diabetes studies it was shown to be decreased in diabetic rats [72] and in the atrium from diabetic patients [73]. The existing data indicate that altered InsP3R signaling may account for impaired Ca2+ handling and arrhythmogenesis in diabetic cardiomyopathy. However, the precise role of InsP3R in such pathological conditions requires further study.
Most of the intracellular Ca2+ is stored in SR via SERCA, which transfers two Ca2+ ions from the cytosol to the lumen at the expense of the hydrolysis of one ATP molecule. SERCA2a, the isoform predominately expressed by cardiomyocytes, is regulated by phosphorylation of a SR protein, phospholamban (PLB) [32, 74]. In its dephosphorylated form, PLB interacts with the pump, reducing its affinity for Ca2+. However, when phosphorylated by PKC or CAMK, PLB is not able to inhibit SERCA2a activity [75, 76]. SR function in diabetic cardiomyocytes has been shown to be compromised, presenting a reduced Ca2+ uptake that could explain the prolonged cardiac relaxation observed. As a consequence, SR calcium storage declines, resulting in reduced systolic calcium release and therefore a weaker cardiac contraction [74]. In this regard, some investigations with STZ-induced type 1 diabetes rats have reported decreased protein content and SERCA2a pumping dysfunction, which might be partly associated with an upregulation of activity and inhibitory PLB expression [35, 77]. In addition, it was proposed that products from advanced glycosylation reactions would form irreversible crosslinks within many proteins, leading to impairment of SERCA2a activity in diabetes [78, 79]. Thus far, it has been difficult to establish a general conclusion regarding myocardial SERCA2a and PLB changes in type 2 diabetes, most likely because of data limitation and ambiguity, especially taking in consideration the differences in animals models used.
Several studies using different type 2 diabetes animal models observed compromised SERCA2a function [25, 42, 43, 80]. SERCA2a expression was shown to be downregulated in Otsuka Long-Evans Tokushima fatty rats [80] and db/db mice [43] but unaltered in sucrose (SU)-fed rats [42]. Increased protein level of inhibitory PLB was only detected by one study [43]. Furthermore, Fredersdorf et al. [81] evaluated cardiac function and protein expression of Zucker diabetic fatty (ZDF) rats in the early stages of type 2 diabetes. They were able to demonstrate that animals in transition from insulin resistance to type 2 diabetes developed significant myocardial hypertrophy initially characterized by an increased systolic function and an intense SR Ca2+ uptake. In addition, myocardial expression of SERCA2a was markedly elevated and PLB expression was depressed. These changes were attributed to Akt signaling pathway activation induced by high levels of insulin, thereby supporting the view that upregulation of myocardial SERCA2a expression may be seen as a feedback mechanism in handling volume overload in the early phase of diabetes type 2. Taken together, the conflicting results regarding gene and protein expressions for SERCA2a and PLB can be explained by differences in the duration and severity of diabetes in various studies. Nonetheless, these observations are consistent with the view that alterations in SR function and SR remodeling occur in the diabetic heart [74]. Moreover, the critical role of SERCA2a in excitation–relaxation coupling is reinforced with the evidence that upregulation of its expression is able to reverse contractile dysfunction and abnormal calcium flux in established diabetic cardiomyopathy [82–84].
4 Mechanisms of SL and SR Alterations in the Diabetic Heart
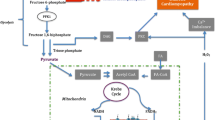
It has been suggested that hyperglycemia along with metabolic shift, as a result of the hormonal imbalance caused by elevated plasma levels of catecholamines and angiotensin II, leads to oxidative stress and contributes to diabetic injury to multiple organs, especially the cardiac muscle [2, 4, 46]. The shift in myocardial metabolism, marked by decreased use of glucose and excessive utilization of long-chain fatty acids as an energy substrate, intensify the production of reactive oxygen species (ROS) that damage the respiratory and oxidative phosphorylation activities of mitochondria, contributing to decreased myocardial efficiency [14, 85]. In addition, there is experimental evidence to suggest that mitochondria under several pathological conditions can act as a Ca2+ sink [86, 87]. Although this mechanism initially seems to play an important compensatory role in Ca2+ regulation by preventing or delaying intracellular Ca2+ overload in cardiomyocytes, it also accounts for the development of oxidative stress at late stages of diabetes. The generation of ROS can lead to leakage of toxic proteins through opening of mitochondrial pores and further damage of cardiomyocytes [65, 88]. Another mechanism of oxidative stress is mediated by advanced glycation end products (AGE), which are able to activate signaling pathways that induce ROS production, and its accumulation is related to structural and functional alterations of proteins in chronic diabetic tissues. It is worthwhile to note that hyperglycemia can impair and decrease the antioxidant system capacity in the heart and other organs in diabetes [85, 89, 90]. Thus, both the intense generation of ROS and reduced antioxidant capacity contribute significantly to oxidative stress and therefore myocardial damage in chronic diabetes.
It is now well established that genomic alterations lead to myocardial dysfunction in diabetic cardiomyopathy. Numerous studies have also been relating the diabetic state with activation of proteases and changes in signal transduction pathways, including PKC, PKA, CaM kinase, and mitogen-activated protein kinase, contributing to subcellular remodeling [91]. With respect to Ca2+ cycling, downregulation of SERCA2a expression, as well as its promoter activity were reported. Some investigations also detected reduced protein levels of SL Ca2+ channels, NCX1, Na+–K+ ATPase, and RyR2 [74, 92]. These alterations have been attributed to an increased nuclear O-GlcN acylation, as a result of oxidative stress induced by hyperglycemia and enhanced activity of the PKC signaling pathway [93]. Moreover, genomic alterations also seem to underlie myosin dysfunction [94, 95]. In models of diabetic cardiomyopathy, abnormal myosin isozyme distribution, shift in myosin content from V1 to V3, and increased troponin I phosphorylation via the PKC pathway have been detected. Taken together, this could contribute to the decrease in Ca2+ sensitivity of myofilaments [96–101].
5 Conclusions
From the foregoing discussion it can be appreciated that diabetes is a complex pathology and that a wide variety of mechanisms contributes to cardiac dysfunction. The hormonal imbalance along with metabolic shift enhances oxidative stress, which leads to several abnormalities including activation of proteases, increased intracellular concentration of free Ca2+, and alterations in cardiac gene expression (Fig. 1). Intracellular Ca2+ overload has been implicated not only in the process of excitation–contraction impairment but also in subcellular remodeling of organelles in cardiac cells. This event has been attributed to decreased SR Ca2+ load, depressed SERCA2a activity, and RyR2 dysfunction as well as changes in L-type Ca2+ channels. Abnormalities of SL proteins such as SL NCX, Na+–K+ ATPase, NHE-1, and Ca2+-pump ATPase have also been shown to be involved in diabetic cardiomyopathy. Molecular targeting approaches to revert or even attenuate alterations in proteins associated with Ca2+ handling hold promise as a new therapeutic modality. In addition, recent data have suggested that the insulin signaling pathway and Ca2+ regulatory processes are clearly interrelated, although many of these relationships are yet to be defined. Thus, further in-depth studies regarding the interactions between these pathways should lay the foundations for the design of new therapeutic approaches for diabetic heart disease.
References
Go AS, Mozaffarian D, Roger VL et al (2013) Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 127:e6–e245
Schaffer SW (1991) Cardiomyopathy associated with noninsulin-dependent diabetes. Mol Cell Biochem 107:1–20
Lebeche D, Davidoff AJ, Hajjar RJ (2008) Interplay between impaired calcium regulation and insulin signaling abnormalities in diabetic cardiomyopathy. Nat Clin Pract Cardiovasc Med 5:715–724
Hayat SA, Patel B, Khattar RS, Malik RA (2004) Diabetic cardiomyopathy: mechanisms, diagnosis and treatment. Clin Sci (Lond) 107:539–557
Boudina S, Abel ED (2007) Diabetic cardiomyopathy revisited. Circulation 115:3213–3223
Dhalla NS, Takeda N, Rodriguez-Leyva D, Elimban V (2013) Mechanisms of subcellular remodeling in heart failure due to diabetes. Heart Fail Rev. doi:10.1007/s10741-013-9385-8
Ligeti L, Szenczi O, Prestia CM et al (2006) Altered calcium handling is an early sign of streptozotocin-induced diabetic cardiomyopathy. Int J Mol Med 17:1035–1043
Fein FS, Sonnenblick EH (1994) Diabetic cardiomyopathy. Cardiovasc Drugs Ther 8:65–73
Op den Buijs J, Miklós Z, Van Riel NAW et al (2005) β-Adrenergic activation reveals impaired cardiac calcium handling at early stage of diabetes. Life Sci 76:1083–1098
Hattori Y, Matsuda N, Kimura J et al (2000) Diminished function and expression of the cardiac Na+- Ca2+ exchanger in diabetic rats: implication in Ca2+ overload. J Physiol 527(Pt 1):85–94
Ren J, Davidoff AJ (1997) Diabetes rapidly induces contractile dysfunctions in isolated ventricular myocytes. Am J Physiol 272:H148–H158
Bai S, Sun J, Wu H et al (2012) Decrease in calcium-sensing receptor in the progress of diabetic cardiomyopathy. Diabetes Res Clin Pract 95:378–385
Kralik P, Ye G, Metreveli N et al (2005) Cardiomyocyte dysfunction in models of type 1 and type 2 diabetes. Cardiovasc Toxicol 5:285–292
Dhalla NS, Saini HK, Tappia PS et al (2007) Potential role and mechanisms of subcellular remodeling in cardiac dysfunction due to ischemic heart disease. J Cardiovasc Med (Hagerstown) 8:238–250
Halling DB, Aracena-Parks P, Hamilton SL (2005) Regulation of voltage-gated Ca2+ channels by calmodulin. Sci STKE 2005:re15
Xu M, Zhou P, Xu S-M et al (2007) Intermolecular failure of L-type Ca2+ channel and ryanodine receptor signaling in hypertrophy. PLoS Biol 5:e21
Petrovic MM, Vales K, Putnikovic B et al (2008) Ryanodine receptors, voltage-gated calcium channels and their relationship with protein kinase A in the myocardium. Physiol Res 57:141–149
Shaw RM, Colecraft HM (2013) L-type calcium channel targeting and local signalling in cardiac myocytes. Cardiovasc Res 98:177–186
Splawski I, Timothy KW, Sharpe LM et al (2004) Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell 119:19–31
Shimoni Y, Firek L, Severson D, Giles W (1994) Short-term diabetes alters K+ currents in rat ventricular myocytes. Circ Res 74:620–628
Lacombe VA, Viatchenko-Karpinski S, Terentyev D et al (2007) Mechanisms of impaired calcium handling underlying subclinical diastolic dysfunction in diabetes. Am J Physiol 293:R1787–R1797
Lengyel C, Virág L, Bíró T et al (2007) Diabetes mellitus attenuates the repolarization reserve in mammalian heart. Cardiovasc Res 73:512–520
Lu Z, Ballou LM, Jiang Y-P et al (2011) Restoration of defective L-type Ca2+ current in cardiac myocytes of type 2 diabetic db/db mice by Akt and PKC-ι. J Cardiovasc Pharmacol 58:439–445
Lu Z, Jiang Y-P, Xu X-H et al (2007) Decreased L-type Ca2+ current in cardiac myocytes of type 1 diabetic akita mice due to reduced phosphatidylinositol 3-kinase signaling. Diabetes 56:2780–2789
Pereira L, Matthes J, Schuster I et al (2006) Mechanisms of [Ca2+]i transient decrease in cardiomyopathy of db/db type 2 diabetic mice. Diabetes 55:608–615
Malhotra A, Sanghi V (1997) Regulation of contractile proteins in diabetic heart. Cardiovasc Res 34:34–40
Sun H, Kerfant B-G, Zhao D et al (2006) Insulin-like growth factor-1 and PTEN deletion enhance cardiac L-type Ca2+ currents via increased PI3Kα/PKB signaling. Circ Res 98:1390–1397
Gómez AM, Valdivia HH, Cheng H et al (1997) Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science 276:800–806
Bénitah J-P, Kerfant BG, Vassort G et al (2002) Altered communication between L-type calcium channels and ryanodine receptors in heart failure. Front Biosci 7:e263–e275
Shao C-H, Rozanski GJ, Patel KP, Bidasee KR (2007) Dyssynchronous (non-uniform) Ca2+ release in myocytes from streptozotocin-induced diabetic rats. J Mol Cell Cardiol 42:234–246
Eisner DA, Choi HS, Diaz ME et al (2000) Integrative analysis of calcium cycling in cardiac muscle. Circ Res 87:1087–1094
Bers DM (2008) Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70:23–49
Chattou S, Diacono J, Feuvray D (1999) Decrease in sodium-calcium exchange and calcium currents in diabetic rat ventricular myocytes. Acta Physiol Scand 166:137–144
Wold LE, Ceylan-Isik AF, Fang CX et al (2006) Metallothionein alleviates cardiac dysfunction in streptozotocin-induced diabetes: role of Ca2+ cycling proteins, NADPH oxidase, poly(ADP-Ribose) polymerase and myosin heavy chain isozyme. Free Radic Biol Med 40:1419–1429
Choi KM, Zhong Y, Hoit BD et al (2002) Defective intracellular Ca2+ signaling contributes to cardiomyopathy in type 1 diabetic rats. Am J Physiol Heart Circ Physiol 283:H1398–H1408
Schaffer SW, Ballard-Croft C, Boerth S, Allo SN (1997) Mechanisms underlying depressed Na+-Ca2+ exchanger activity in the diabetic heart. Cardiovasc Res 34:129–136
Pierce GN, Dhalla NS (1983) Sarcolemmal Na+–K+-ATPase activity in diabetic rat heart. Am J Physiol 245:C241–C247
Kjeldsen K, Braendgaard H, Sidenius P et al (1987) Diabetes decreases Na+–K+ pump concentration in skeletal muscles, heart ventricular muscle, and peripheral nerves of rat. Diabetes 36:842–848
Golfman L, Dixon IM, Takeda N et al (1998) Cardiac sarcolemmal Na+–Ca2+ exchange and Na+–K+ATPase activities and gene expression in alloxan-induced diabetes in rats. Mol Cell Biochem 188:91–101
Schaffer SW, Allo S, Punna S, White T (1991) Defective response to cAMP-dependent protein kinase in non-insulin-dependent diabetic heart. Am J Physiol 261:E369–E376
Allo SN, Lincoln TM, Wilson GL et al (1991) Non-insulin-dependent diabetes-induced defects in cardiac cellular calcium regulation. Am J Physiol 260:C1165–C1171
Wold LE, Dutta K, Mason MM et al (2005) Impaired SERCA function contributes to cardiomyocyte dysfunction in insulin resistant rats. J Mol Cell Cardiol 39:297–307
Belke DD, Swanson EA, Dillmann WH (2004) Decreased sarcoplasmic reticulum activity and contractility in diabetic db/db mouse heart. Diabetes 53:3201–3208
Stubbs CD, Smith AD (1984) The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta 779:89–137
Vér Á, Szántó I, Bányász T et al (1997) Changes in the expression of Na+–K+-ATPase isoenzymes in the left ventricle of diabetic rat hearts: effect of insulin treatment. Diabetologia 40:1255–1262
Dhalla NS, Liu X, Panagia V, Takeda N (1998) Subcellular remodeling and heart dysfunction in chronic diabetes. Cardiovasc Res 40:239–247
Gerbi A, Barbey O, Raccah D et al (1997) Alteration of Na, K-ATPase isoenzymes in diabetic cardiomyopathy: effect of dietary supplementation with fish oil (n-3 fatty acids) in rats. Diabetologia 40:496–505
Chen S, Khan ZA, Karmazyn M, Chakrabarti S (2007) Role of endothelin-1, sodium hydrogen exchanger-1 and mitogen activated protein kinase (MAPK) activation in glucose-induced cardiomyocyte hypertrophy. Diabetes Metab Res Rev 23:356–367
Pierce GN, Ramjiawan B, Dhalla NS, Ferrari R (1990) Na+/H+exchange in cardiac sarcolemmal vesicles isolated from diabetic rats. Am J Physiol 258:H255–H261
Le Prigent K, Lagadic-Gossmann D, Feuvray D (1997) Modulation by pH0 and intracellular Ca2+ of Na+–H+ exchange in diabetic rat isolated ventricular myocytes. Circ Res 80:253–260
Khandoudi N, Bernard M, Cozzone P, Feuvray D (1990) Intracellular pH and role of Na+–H+ exchange during ischaemia and reperfusion of normal and diabetic rat hearts. Cardiovasc Res 24:873–878
Darmellah A, Baetz D, Prunier F et al (2007) Enhanced activity of the myocardial Na+–H+ exchanger contributes to left ventricular hypertrophy in the Goto–Kakizaki rat model of type 2 diabetes: critical role of Akt. Diabetologia 50:1335–1344
Jandeleit-Dahm K, Hannan KM, Farrelly CA et al (2000) Diabetes-induced vascular hypertrophy is accompanied by activation of Na+–H+ exchange and prevented by Na+–H+ exchange inhibition. Circ Res 87:1133–1140
Kusumoto K, Haist JV, Karmazyn M (2001) Na+/H+ exchange inhibition reduces hypertrophy and heart failure after myocardial infarction in rats. Am J Physiol Heart Circ Physiol 280:H738–H745
Baartscheer A, Hardziyenka M, Schumacher CA et al (2008) Chronic inhibition of the Na+–H+-exchanger causes regression of hypertrophy, heart failure, and ionic and electrophysiological remodelling. Br J Pharmacol 154:1266–1275
Vial G, Dubouchaud H, Couturier K et al (2008) Na+–H+ exchange inhibition with cariporide prevents alterations of coronary endothelial function in streptozotocin-induced diabetes. Mol Cell Biochem 310:93–102
Meissner G (2004) Molecular regulation of cardiac ryanodine receptor ion channel. Cell Calcium 35:621–628
Bidasee KR, Dinçer ÜD, Besch HR (2001) Ryanodine receptor dysfunction in hearts of streptozotocin-induced diabetic rats. Mol Pharmacol 60:1356–1364
Lanner JT (2012) Ryanodine receptor physiology and its role in disease. Adv Exp Med Biol 740:217–234
Dincer UD, Araiza A, Knudson JD et al (2006) Dysfunction of cardiac ryanodine receptors in the metabolic syndrome. J Mol Cell Cardiol 41:108–114
Netticadan T, Temsah RM, Kent A et al (2001) Depressed levels of Ca2+-cycling proteins may underlie sarcoplasmic reticulum dysfunction in the diabetic heart. Diabetes 50:2133–2138
Lehnart SE, Mongillo M, Bellinger A et al (2008) Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest 118:2230–2245
Turan B, Vassort G (2011) Ryanodine receptor: a new therapeutic target to control diabetic cardiomyopathy. Antioxid Redox Signal 15:1847–1861
Bidasee K, Nallani K, Henry B et al (2003) Chronic diabetes alters function and expression of ryanodine receptor calcium-release channels in rat hearts. Mol Cell Biochem 249:113–123
Dhalla NS, Temsah RM, Netticadan T (2000) Role of oxidative stress in cardiovascular diseases. J Hypertens 18:655–673
Bidasee KR, Nallani K, Besch HR, Dincer UD (2003) Streptozotocin-induced diabetes increases disulfide bond formation on cardiac ryanodine receptor (RyR2). J Pharmacol Exp Ther 305:989–998
Wolff SP, Jiang ZY, Hunt JV (1991) Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med 10:339–352
Hund TJ, Ziman AP, Lederer WJ, Mohler PJ (2008) The cardiac IP3 receptor: uncovering the role of “the other” calcium-release channel. J Mol Cell Cardiol 45:159–161
Mackenzie L, Bootman MD, Laine M et al (2002) The role of inositol 1,4,5-trisphosphate receptors in Ca2+ signalling and the generation of arrhythmias in rat atrial myocytes. J Physiol 541:395–409
Zima AV, Blatter LA (2004) Inositol-1,4,5-trisphosphate-dependent Ca2+ signalling in cat atrial excitation-contraction coupling and arrhythmias. J Physiol 555:607–615
Fauconnier J, Lanner JT, Zhang S-J et al (2005) Insulin and inositol 1,4,5-trisphosphate trigger abnormal cytosolic Ca2+ transients and reveal mitochondrial Ca2+ handling defects in cardiomyocytes of ob/ob mice. Diabetes 54:2375–2381
Zhou B-Q, Hu S-J, Wang G-B (2006) The analysis of ultrastructure and gene expression of sarco/endoplasmic reticulum calcium handling proteins in alloxan-induced diabetic rat myocardium. Acta Cardiol 61:21–27
Guner S, Arioglu E, Tay A et al (2004) Diabetes decreases mRNA levels of calcium-release channels in human atrial appendage. Mol Cell Biochem 263:143–150
Dhalla NS, Rangi S, Zieroth S, Xu Y-J (2012) Alterations in sarcoplasmic reticulum and mitochondrial functions in diabetic cardiomyopathy. Exp Clin Cardiol 17:115–120
Dutta K, Carmody MW, Cala SE, Davidoff AJ (2002) Depressed PKA activity contributes to impaired SERCA function and is linked to the pathogenesis of glucose-induced cardiomyopathy. J Mol Cell Cardiol 34:985–996
Davidoff AJ, Davidson MB, Carmody MW et al (2004) Diabetic cardiomyocyte dysfunction and myocyte insulin resistance: role of glucose-induced PKC activity. Mol Cell Biochem 262:155–163
Zhong Y, Ahmed S, Grupp IL, Matlib MA (2001) Altered SR protein expression associated with contractile dysfunction in diabetic rat hearts. Am J Physiol Heart Circ Physiol 281:H1137–H1147
Bidasee KR, Nallani K, Yu Y et al (2003) Chronic diabetes increases advanced glycation end products on cardiac ryanodine receptors/calcium-release channels. Diabetes 52:1825–1836
Bidasee KR, Zhang Y, Shao CH et al (2004) Diabetes increases formation of advanced glycation end products on sarco(endo)plasmic reticulum Ca2+-ATPase. Diabetes 53:463–473
Abe T, Ohga Y, Tabayashi N et al (2002) Left ventricular diastolic dysfunction in type 2 diabetes mellitus model rats. Am J Physiol Heart Circ Physiol 282:H138–H148
Fredersdorf S, Thumann C, Zimmermann WH et al (2012) Increased myocardial SERCA expression in early type 2 diabetes mellitus is insulin dependent: in vivo and in vitro data. Cardiovasc Diabetol 11:57
Trost SU, Belke DD, Bluhm WF et al (2002) Overexpression of the sarcoplasmic reticulum Ca2+-ATPase improves myocardial contractility in diabetic cardiomyopathy. Diabetes 51:1166–1171
Vetter R, Rehfeld U, Reissfelder C et al (2002) Transgenic overexpression of the sarcoplasmic reticulum Ca2+ ATPase improves reticular Ca2+ handling in normal and diabetic rat hearts. FASEB J 16:1657–1659
Golfman L, Dixon IC, Takeda N et al (1999) Differential changes in cardiac myofibrillar and sarcoplasmic reticular gene expression in alloxan-induced diabetes. Mol Cell Biochem 200:15–25
Cai L, Kang YJ (2001) Oxidative stress and diabetic cardiomyopathy: a brief review. Cardiovasc Toxicol 1:181–193
Boudina S, Abel ED (2006) Mitochondrial uncoupling: a key contributor to reduced cardiac efficiency in diabetes. Physiology (Bethesda) 21:250–258
An D, Rodrigues B (2006) Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol 291:H1489–H1506
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552: 335–344
Chen Y, Saari JT, Kang YJ (1994) Weak antioxidant defenses make the heart a target for damage in copper-deficient rats. Free Radic Biol Med 17:529–536
Yan SD, Schmidt AM, Anderson GM et al (1994) Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem 269:9889–9897
Müller AL, Dhalla NS (2012) Role of various proteases in cardiac remodeling and progression of heart failure. Heart Fail Rev 17:395–409
Dhalla NS, Rangi S, Babick AP et al (2012) Cardiac remodeling and subcellular defects in heart failure due to myocardial infarction and aging. Heart Fail Rev 17:671–681
Clark RJ, McDonough PM, Swanson E et al (2003) Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem 278:44230–44237
Belin RJ, Sumandea MP, Allen EJ et al (2007) Augmented protein kinase C-induced myofilament protein phosphorylation contributes to myofilament dysfunction in experimental congestive heart failure. Circ Res 101:195–204
Shimoni Y, Liu X-F (2003) Role of PKC in autocrine regulation of rat ventricular K+ currents by angiotensin and endothelin. Am J Physiol Heart Circ Physiol 284:H1168–H1181
Rupp H, Elimban V, Dhalla NS (1989) Diabetes-like action of intermittent fasting on sarcoplasmic reticulum Ca2+ pump ATPase and myosin isoenzymes can be prevented by sucrose. Biochem Biophys Res Commun 164:319–325
Dillmann WH (1982) Influence of thyroid hormone administration on myosin ATPase activity and myosin isoenzyme distribution in the heart of diabetic rats. Metabolism 31:199–204
Afzal N, Pierce GN, Elimban V et al (1989) Influence of verapamil on some subcellular defects in diabetic cardiomyopathy. Am J Physiol 256:E453–E458
Dillmann WH (1980) Diabetes mellitus induces changes in cardiac myosin of the rat. Diabetes 29:579–582
Yu JZ, Rodrigues B, McNeill JH (1997) Intracellular calcium levels are unchanged in the diabetic heart. Cardiovasc Res 34:91–98
Pierce GN, Russell JC (1997) Regulation of intracellular Ca2+ in the heart during diabetes. Cardiovasc Res 34:41–47
Acknowledgments
This work was supported by the St. Boniface Hospital Research Foundation, and A.F.P. Pinto was supported by the National Council for Scientific and Technological Development (CNPq), Brazil.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Elimban, V., Pinto, A.F.P., Dhalla, N.S. (2014). Calcium-Handling Proteins in Diabetic Cardiomyopathy. In: Turan, B., Dhalla, N. (eds) Diabetic Cardiomyopathy. Advances in Biochemistry in Health and Disease, vol 9. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-9317-4_17
Download citation
DOI: https://doi.org/10.1007/978-1-4614-9317-4_17
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-9316-7
Online ISBN: 978-1-4614-9317-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)