Abstract
This study is the first to assess the impact of habitat degradation and fragmentation on sleeping site choice and activity budgets of the Sahamalaza sportive lemur (Lepilemur sahamalazensis), first described in February 2006 and exclusively confirmed for the remaining forest fragments on the Sahamalaza Peninsula. Seventeen individual Lepilemurs in forests of different degradation levels that used two classes of sleeping sites (tree holes vs. tree tangles) were observed for 606 h during the day and 324 h at night. 24-h activity budgets were quantified. Preliminary analyses show differences in the ratio of active to inactive behaviour: (a) between different types of sleeping sites, and (b) between differently degraded forest fragments. Individuals resting in tree tangles were active during 7.4 % of daylight hours, while those resting in tree holes were active for 25.4 % of the time. During the day, Lepilemurs never left their chosen resting site. Individuals in a young secondary forest fragment were active for 14.3 min/h during daylight hours, in mixed and mature secondary forest fragments 12.6 and 9.1 min/h, respectively, and in a degraded primary forest fragment 2.7 min/h. However, the latter group had a higher percentage of time out of sight. These differences are most likely predator avoidance strategies and highlight the importance of intact mature forests for this species. Further research into the diurnal habits of this nocturnal primate, investigating their anti-predator responses, and detailed habitat requirements is ongoing.
An erratum to this chapter can be found at http://dx.doi.org/10.1007/978-1-4614-8839-2_35
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The deforestation rate of Madagascar continues to be enormous with a reduction of 33 % of primary forest types since the 1970s (Moat and Smith 2007) endangering all forest-dwelling organisms. The current rate of forest loss is roughly 1,500 km2/year, which means that if this rate continues, all primary vegetation of Madagascar will be gone by 2067 (Moat and Smith 2007). The lemur superfamily Lemuroidea counts approximately 100 endemic species that are exclusively found in the forests of Madagascar. Members of the genus Lepilemur (family Lepilemuridae; Gray 1870), known as sportive lemurs, are small folivores (446–1,000 g) that have received notably little scientific attention (Groves 2001; Harcourt and Thornback 1990; Mittermeier et al. 2006; Tattersall 1982). Based on differences in mtDNA sequences, the number of Lepilemur species recently increased from a previous total of 7 species (Harcourt and Thornback 1990; Tattersall 1982) to 26 species (Andriaholinirina et al. 2006; Craul et al. 2007; Lei et al. 2008; Louis et al. 2006; Rabarivola et al. 2006; Ramaromilanto et al. 2009). Many of these Lepilemur species are only confirmed for single locations (Andriaholinirina et al. 2006; Craul et al. 2007; Louis et al. 2006; Rabarivola et al. 2006), and with the limited reproductive rates typical for similar-sized primates (female maximum reproductive output is one offspring/year; Randrianambinina et al. 2007) they are particularly vulnerable to human-caused disturbances.
The Sahamalaza sportive lemur (Lepilemur sahamalazensis; Fig. 18.1) is one of the numerous lemurs recently described (Andriaholinirina et al. 2006). Since it received species status, the Sahamalaza sportive lemur has been included on the World’s Top 25 Most Endangered Primates 2006–2008 (Mittermeier et al. 2007). L. sahamalazensis is the first Lepilemur, and the first nocturnal lemur, to be included on this list. It is currently red-listed as Data Deficient by the IUCN, but likely to be reassessed as Critically Endangered (Olivieri et al. 2008).
Taking into account their limited distribution, the small extent of remaining forest cover within their distribution range, as well as an average of 2.8 individuals found per hectare by Ruperti (2007), there are probably around 3,000 individual L. sahamalazensis remaining in their natural habitat. Although the range of this cat-sized, nocturnal primate is not precisely known, it is probably limited to the Sahamalaza Peninsula in northwestern Madagascar (Olivieri et al. 2007). This area has experienced rapid deforestation and habitat destruction over recent history.
Strictly arboreal species such as Lepilemur, who are unable to cross the non-forest matrix within their distribution range, will eventually become extinct when their habitat patches get too small and disconnected to support a viable gene pool/flow (Fahrig 2003; Frankham et al. 2002). The smallest forest fragment with confirmed Lepilemur persistence has been found to be 6 ha for L. ruficaudatus in western dry deciduous forest (Ganzhorn et al. 2000; Gibbons and Harcourt 2009). As an effect of a combination of all these and other factors, several Lepilemur species are now confirmed or thought to be at risk of imminent extinction (Bachmann et al. 2000; Mittermeier et al. 2007; Ravaoarimanana et al. 2001).
Besides deforestation and habitat fragmentation, general forest degradation (e.g. through selective logging, disturbance of sapling growth by zebu cattle, bush pigs, goats, other livestock) is an aggravating factor putting lemur populations under further stress. Habitat degradation might also mean easier access for predators and hence increased predation pressure (Andren and Angelstam 1988; Estrada and Coates-Estrada 1996; Wilcove 1985; Wilcove et al. 1986; but see Onderdonk and Chapman 2000). Better visibility and accessibility resulting from logging and fragmentation has been discussed as one reason for increases in some raptor populations, leading to the conclusion that fragmentation may be more disruptive to lemur populations than to predatory raptors (Colquhoun 2006; Karpanty 2003). Very little is currently known regarding how nocturnal primates respond to predation threats, especially when they are vulnerable to predators that have different daily activity patterns to them (Fichtel 2007). During the day, hawks and eagles may hunt sleeping nocturnal lemurs, like Lepilemur, as well as active diurnal lemurs, and fossas and boas hunt during day and night (Karpanty 2006; Wright 1998). In forests exposed to human activity, hunters are an additional predation threat for many lemur species. For sportive lemurs, the threat from humans is thought to greatly outweigh that from natural predators, such as the Madagascar harrier hawk (Polyboroides radiatus), the Fossa (Cryptoprocta ferox) and constricting snakes such as Acrantophis madagascariensis, Acrantophis dumereli, and Sanzinia madagascariensis (Colquhoun 2006).
The ability to develop foraging and resting strategies for risk avoidance is theorized to be an important factor in primate sociality (Janson and Goldsmith 1995; Janson and van Schaik 1993; Stanford 1995). Thus, the selection of an appropriate sleeping site may be essential for survival in primates (Rasoloharijaona et al. 2006). Sleeping sites should not only provide shelter from aerial and terrestrial predators but also protect from difficult climatic conditions like rain, wind, or temperature fluctuations in order to limit energy expenses for maintaining basal body metabolism (e.g. Anderson 1998). This might be particularly important for sportive lemurs, as they are known to exhibit the lowest known metabolic rates among mammals (Schmid and Ganzhorn 1996), and may profit particularly from sleeping site selection guided by metabolic constraints. Since the number of suitable sleeping sites is potentially limited, they are also considered as one factor shaping sociality in these primates (Rasoloharijaona et al. 2008).
Preliminary data from a study conducted by Ruperti in 2007 at Sahamalaza National Park suggested that factors associated with primary forest areas were vital for the continued existence of the Sahamalaza sportive lemur. In particular, a high density of large trees, extensive canopy cover, as well as the availability of vegetation tangles, tree holes, and food plants were correlated with higher densities of L. sahamalazensis (F. Ruperti, pers. comm.). Sahamalaza sportive lemurs were observed to react differently to aerial predators (Polyboroides radiatus and Buteo brachypterus) than to humans and other ground-dwelling animals (F. Ruperti, pers. comm.). The author of that same study commented that L. sahamalazensis was also easy and defenceless prey to humans because of its choice of exposed sleeping sites and therefore heavily hunted by natives. The combination of a very limited range, rapidly decreasing suitable habitats, and high hunting pressure thus renders this species particularly vulnerable to extinction (Olivieri et al. 2007).
Based on the preliminary data collected by Ruperti (2007), we hypothesised that the level of diurnal activity shown by Sahamalaza sportive lemurs differed in differently degraded forest fragments. Our prediction was that the animals were more active in more degraded fragments, as the latter would have less dense canopies and would thus demand a higher level of vigilance concerning aerial predators. Additionally, we hypothesised that the level of diurnal activity differed between different types of sleeping sites, and predicted that individuals were more active when they occupied tree holes than when resting in vegetation tangles.
Methods
Study Site
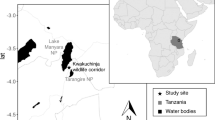
The Ankarafa Forest is situated within the UNESCO Biosphere Reserve and National Park on the Sahamalaza Peninsula, and is part of the Province Autonome de Mahajanga, NW Madagascar. It extends between 13°52′S and 14°27′S and 45°38′E and 47°46′E (WCS/DEC 2002; Fig. 18.2). The climate is strongly seasonal, with a cool, dry season from May to October and a hot, rainy season from November to April. The Ankarafa Forest lies within a transition zone between the Sambirano region in the North and the western dry deciduous forest region in the South, harbouring semi-humid forests with tree heights of up to 30 m (Schwitzer et al. 2006). The forests in this area include a mixture of plant species typical of the western dry deciduous forest as well as some typical of the Sambirano domain (Birkinshaw 2004) and comprise primary and secondary forest fragments.
There are no large connected areas of intact primary forest left on the Sahamalaza Peninsula, and the remaining fragments all show some degree of anthropogenic disturbance and/or edge effects (Schwitzer et al. 2007a, b). The forests and forest fragments are separated through grassland with shrubs. The Sahamalaza sportive lemur has so far been confirmed exclusively for this area. Other lemur species in Sahamalaza include the blue-eyed black lemur (Eulemur flavifrons), the aye–aye (Daubentonia madagascariensis), the western bamboo lemur (Hapalemur occidentalis), the giant mouse lemur (Mirza zaza), and an as yet unidentified species of dwarf lemur (Cheirogaleus sp.). The lemur species living in Sahamalaza are threatened by hunting and forest destruction (Schwitzer et al. 2006). The Ankarafa Forest is home to the Ankarafa Research Station, where previous research efforts in the region have taken place and which was also the research base for this study.
Selection of Forest Fragments
Within a 2 km radius from the field station there are several secondary forest fragments of various degrees of degradation, interspersed with small remainders of primary vegetation and separated by grass savannah and mosaics of low- to medium-height shrubs. Ruperti (2007) showed differences in forest characteristics of 1 ha plots between fragments as well as within different sections of larger fragments. She referred these differences to past and ongoing anthropogenic disturbance. For our study we selected three forest fragments based on Ruperti (2007) work and one additional fragment where L. sahamalazensis was also present (M. Craul, pers. comm.). After visual inspection on arrival at the field site in mid-2009 we ranked the four fragments along a degradation gradient A–D (with A being least and D being most degraded) based mainly on perceived tree density (see Gerwing 2002), which concurred with the results of Ruperti (2007) habitat description, where A = mature secondary forest, B = degraded primary forest, C = secondary forest, and D = vegetation mosaic (Fig. 18.1). The two fragments A and C as well as parts of the vegetation mosaic D were in the process of regeneration after significant human disturbance of the original forest vegetation over an extended period in the past. We considered them to be at least 35 years old, based on aerial and satellite images and GIS data (Harper et al. 2007), and to exhibit the key characteristics of post-abandonment secondary forest according to the definition by Chokkalingam and de Jong (2001). In order to confirm our visual assessment and to verify that there had not been any major changes to the forest structure since Ruperti (2007) study, a quantitative structural description and quantification of differences in forest characteristics between the fragments was one aim of this study.
Forest Characterisation
The four forest fragments were described using the Point-Centered-Quarter method (Ganzhorn 2003). For this, 252 points (63 points per fragment), distributed along line transects through the forest fragments with a distance of 25 m between them, were selected. Distance to the nearest small tree (5–10 cm DBH) and the nearest large tree (>10 cm DBH) was measured; trees were described in terms of species, DBH, height, and crown diameter. We calculated the density of trees per hectare as 10,000/d2, where d is the mean distance between the centre point and the nearest tree. Trees were identified to at least genus level by our local field assistants, referring to Schatz (2001) and to tree species identifications carried out by the Département de Flore at Parc Botanique et Zoologique de Tsimbazaza in Antananarivo as part of an earlier study in the same forest fragments by Schwitzer et al. (2007b). We identified 94.6 % of large and 93.7 % of small trees. During comprehensive but nonsystematic searches in the four forest fragments, each Lepilemur sleeping hole (N = 25) was marked and recorded using a handheld GPS device.
Behavioural Monitoring
During preliminary observations of four L. sahamalazensis during 2 days and 2 nights each in early July 2009, a basic ethogram of both diurnal and nocturnal behaviours was obtained using continuous focal animal sampling (Altmann 1974; Martin and Bateson 1993). Whenever new behaviours were encountered during subsequent data collection, they were added to the ethogram. From July to October 2009, 606 h of diurnal behavioural observations (06:00–18:00 h) and 324 h of nocturnal observations (18:00–06:00 h) were conducted on 18 sportive lemurs, 17 of which (3 males, 7 females, 7 individuals of unknown sex) were observed during the day and 4 (2 males, 2 females) during the night. The latter four individuals were fitted with radio tags (TW3 SM, Biotrack, Dorset, UK) and were followed using a portable TR-4 receiver (Telonics Inc., Arizona, USA) and a three-element yagi antenna (Biotrack, Dorset, UK). Diurnal and nocturnal behaviours and additional information related to spatial and ecological factors (e.g., location within the home range, climatic conditions) were recorded continuously during 6- or 12-h observation sessions using focal animal sampling. The exact time (hour, minute, second) of each activity was noted. Each individual was observed for a minimum of 3 days and 3 nights to quantify home range size, habitat use, food preferences, activity budget, social behaviour, anti-predator behaviour, vocal spectrum, and context of vocalisations. We compared the behaviour of individuals in differently degraded forest fragments and between different sleeping sites to assess the effects of habitat destruction and the influence of different day roosts. Here, we only refer to diurnal behaviour and resting site usage of the species.
Statistics
To test for differences in structural habitat characteristics and in diurnal activity levels between fragments of different degradation levels we used non-parametric Kruskal–Wallis ANOVA. When differences between fragments were statistically significant, we applied multiple Mann–Whitney U tests with Holm’s Sequential Bonferroni corrections as post hoc tests. Units of statistical analysis were centre points in case of the habitat description (n = 63 points/forest fragment) and individual sportive lemurs observed in case of the comparison of diurnal activity levels (n = 3, 4, 4, and 7 for fragments A, B, C, and D, respectively). To compare diurnal activity levels between two different types of sleeping site we used non-parametric Mann–Whitney U tests. The significance level α was chosen as 5 % (p ≤ 0.05). All statistical tests were carried out using SPSS 16.0 (SPSS Inc., Chicago, USA).
Results
Habitat Description
The four described forest fragments differed significantly in density of large and small trees, height of large trees, and crown diameter of small trees (Mann–Whitney U tests with Holm’s Sequential Bonferroni corrections after Kruskal–Wallis ANOVA; p ≤ 0.05; Table 1). Whereas the secondary forest fragment (C) had the highest tree density and largest crown diameter, the degraded primary forest fragment (B) contained the overall largest and tallest trees and had the highest species diversity (Table 1).
Diurnal Activity Budget in Differently Degraded Forest Fragments
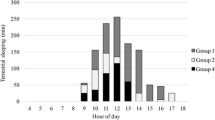
During diurnal observations, 4.5–23.9 % of behaviours were considered active (i.e. autogrooming, resting vigilant, monitoring other species, biting/licking tree, and changing position), although the animals never left their sleeping sites. We found a trend towards higher levels of activity in secondary forest and vegetation mosaic than in mature secondary or degraded primary forest with the respective Kruskal–Wallis ANOVA approaching significance (p = 0.082; Table 2).
Diurnal Activity Budget at Different Types of Sleeping Sites
We found a significant difference in diurnal activity between two different types of sleeping site, with a higher level of activity in tree holes as compared to vegetation tangles (p = 0.0008, Mann–Whitney U test, two-tailed; Table 3).
Discussion
Our results showed significant differences in forest structure between the four study fragments, which were not consistent for all measured variables. Contrary to our expectations and our initial ranking, the secondary forest fragment (C) had the highest tree density and largest crown diameter, whilst the degraded primary forest fragment (B) contained the overall largest and tallest trees and had the highest plant species diversity. Thus, based on our results we would have to rank the secondary forest fragment (C) as the least degraded, followed by the degraded primary forest fragment (B), the vegetation mosaic (D), and finally the mature secondary forest fragment (A) as the most degraded. This sequence does not concur with the results of Ruperti (2007), who described the mature secondary forest fragment (A) as the least degraded one, whilst, as in our study, the degraded primary forest came out second. The differences between our results and those of Ruperti (2007) may be due to different methods of data collection. Whereas Ruperti and coworkers measured habitat structure variables inside 1-ha plots (one for each fragment), we worked along line transects that covered a considerably larger area and possibly greater detail of the respective forest fragments. After several centuries of anthropogenic and subsequent natural degradation, the forests of the Sahamalaza Peninsula seem to be heterogeneous on an increasingly small scale, which makes it difficult to assign entire fragments to existing categories such as primary or secondary forest. In order to measure habitat quality for a forest-dwelling species in a biologically meaningful way in forests such as those in our study area, it may make more sense to concentrate on areas known to be within the home ranges of the species in question, which in case of Lepilemur are usually below 1 ha of size (Albignac 1981; Hladik and Charles-Dominique 1974; Russell 1978; Warren and Crompton 1997; Zinner et al. 2003). Another factor that may have contributed to the differences between Ruperti et al. (2007) and our habitat description is ongoing habitat alteration, which has increased in magnitude since the political crisis in Madagascar in early 2009. Forest and bush fires have been occurring on the Sahamalaza Peninsula on an almost daily basis during recent years, and illegal logging of hardwoods is also a common occurrence. Due to the methodological differences between the two studies it is impossible to assess how much of a role these activities have played, however.
Based on the preliminary data collected by Ruperti (2007), we hypothesised that the level of diurnal activity shown by Sahamalaza sportive lemurs differed in differently degraded forest fragments. Our prediction was that the animals were more active during the day in more degraded fragments, as the latter would have less dense canopies and would thus demand a higher level of vigilance concerning aerial predators. We found a trend towards higher levels of activity in secondary forest and vegetation mosaic than in mature secondary or degraded primary forest. However, the results from our habitat description, which showed the mature secondary forest fragment to be the most degraded, disproved our prediction. Aligning structural habitat differences with differences in diurnal activity levels between the four study fragments, there was a trend for L. sahamalazensis to be less active in habitat with larger and taller trees and higher plant species diversity.
Additionally, we hypothesised that levels of diurnal activity differed between different types of sleeping sites, and predicted that individuals were more active when they occupied tree holes than when resting in vegetation tangles. We found a statistically highly significant difference in diurnal activity levels between lemurs resting in tree holes and such resting in tree tangles, with activity more than three times higher in individuals resting in tree holes. As almost the entire diurnal activity of L. sahamalazensis consisted of vigilantly scanning their surroundings, our results indicated a higher level of vigilance in those animals having chosen tree holes as resting sites. The observed Lepilemurs seemed to be more sensitive to birds of prey and moving leaves than animals or noise on the ground, suggesting that especially the individuals resting in tree holes, possibly due to the less dense cover of foliage in the immediate vicinity (tree holes were often found in dead trees of Bridelia pervilleana), are more vulnerable to aerial predators (M. Seiler, personal observation). The threat to Lepilemur from predation is however not restricted to birds of prey. Faecal analyses of fossa (Cryptoprocta ferox), Madagascar’s largest mammalian terrestrial predator, revealed that Lepilemur are this species’ most common mammalian prey item in the dry forests of Western Madagascar (Dollar et al. 2007). Ruperti (2007) speculated that the multitude of escape routes available from vegetation tangles rather than tree holes explained why many L. sahamalazensis were found in tangles during her study, even when tree holes previously used by Lepilemur were available nearby—similar to woolly lemurs (Avahi spp.), nocturnal lemurs similar in body size to Lepilemur, that prefer to sleep in tangled vegetation. Overall, the results of our study indicate that resting site type seems to be a more relevant constraint on diurnal activity levels of L. sahamalazensis than the degree of habitat degradation. The availability of suitable shelters for daytime use is also suggested to be associated with the distribution of the Milne-Edwards’ sportive lemur L. edwardsi (Rasoloharijaona et al. 2003). There seem to be regional differences regarding the use of tree holes or vegetation tangles as resting sites by Lepilemur (Ruperti 2007). Both sexes of L. edwardsi use dense vegetation and holes in hollow trees high above the ground as shelters for sleeping during the day (Rasoloharijaona et al. 2008) with the majority of sleeping sites (92 %) being tree holes (Rasoloharijaona et al. 2003). The apparent preference of L. edwardsi for tree holes led Rasoloharijaona et al. (2008) to conclude that survival of this species will strongly depend on the availability of mature rain forests with suitable hollow trees. On the other hand, Charles-Dominque and Hladik (1971) found L. leucopus to sleep in tree holes only rarely. During strategic diurnal searches in Ankarafa by Ruperti (2007), only 7 % of L. sahamalazensis were found in tree holes and the great majority rested in vegetation tangles; the number of tree holes available inside the 1-ha study plots, which differed between differently degraded forest types, did not seem to have an influence on the percentage of individuals resting in this type of sleeping site in Ruperti’s study. We did not count the number of available tree holes in any of the fragments in this study, since a comparative count of the number of available tree tangles was impossible due to insufficient knowledge on the species’ criteria for the choice of tree tangles as resting sites.
The differences in Lepilemur diurnal activity levels between different types of resting sites and between differently degraded forest fragments are most likely predator avoidance strategies and highlight the importance of intact mature forests for this species. Further research into the diurnal habits of this nocturnal primate, investigating their anti-predator responses and detailed habitat requirements, is ongoing.
References
Albignac R (1981) Lemurine social and territorial organization in a north-western Malagasy forest (restricted area of Ampijoroa). In: Chiarelli AB, Corruccini RS (eds) Primate behaviour and sociobiology. Springer, New York, pp 25–29
Altmann J (1974) Observational study of behaviour: sampling methods. Behaviour 49:227–265
Anderson JR (1998) Sleep, sleeping sites, and sleep-related activities: awakening to their significance. Am J Primatol 46:63–75
Andren H, Angelstam P (1988) Elevated predation rates as an edge effect in habitat islands: experimental evidence. Ecology 69:544–547
Andriaholinirina N, Fausser J-L, Roos C, Zinner D, Thalmann U, Rabarivola C, Ravoarimanana I, Ganzhorn JU, Meier B, Hilgartner R, Walter L, Zaramody A, Langer C, Hahn T, Zimmermann E, Radespiel U, Craul M, Tomiuk J, Tattersall I, Rumpler Y (2006) Molecular phylogeny and taxonomic revision of the sportive lemurs (Lepilemur, primates). BMC Evol Biol 6:17
Bachmann L, Rumpler Y, Ganzhorn JU, Tomiuk J (2000) Genetic differentiation among natural populations of Lepilemur ruficaudatus. Int J Primatol 21:853–864
Birkinshaw CR (2004) Priority areas for plant conservation. Ravintsara 2:14–15
Charles-Dominque P, Hladik CM (1971) Le Lepilemur du sud de Madagascar: écologie, alimentation et vie sociale. Terre et Vie 1:3–66
Chokkalingam U, de Jong W (2001) Secondary forest: a working definition and typology. Int For Rev 3:19–26
Colquhoun IC (2006) Predation and cathemerality: comparing the impact of predators on the activity patterns of Lemurids and Ceboids. Folia Primatol 77:143–165
Craul M, Zimmermann E, Rasoloharijaona S, Randrianambinina B, Radespiel U (2007) Unexpected species diversity of Malagasy primates (Lepilemur spp.) in the same biogeographical zone: a morphological and molecular approach with the description of two new species. BMC Evol Biol 7:83
Dollar L, Ganzhorn J, Goodman SM (2007) Primates and other prey in the seasonally variable diet of Cryptoprocta ferox in the dry deciduous forest of Western Madagascar. In: Gursky SL, Nekaris KAI (eds) Primate anti-predator strategies. Springer, New York, pp 63–99
Estrada A, Coates-Estrada R (1996) Tropical rain forest fragmentation and wild populations of primates at Los Tuxtlas, Mexico. Int J Primatol 17:759–783
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Fichtel C (2007) Avoiding predators at night: antipredator strategies in red-tailed sportive lemurs (Lepilemur ruficaudatus). Am J Primatol 69:611–624
Frankham R, Ballou JD, Briscoe DA (2002) Introduction to conservation genetics. Cambridge University Press, New York
Ganzhorn JU (2003) Habitat description and phenology. In: Setchell JM, Curtis DJ (eds) Field and laboratory methods in primatology. A practical guide. Cambridge University Press, Cambridge, pp 40–56
Ganzhorn JU, Goodman SM, Ramanamanjato JB, Ralison J, Rakotondravony D, Rakotosamimanana B (2000) Effects of fragmentation and assessing minimum viable populations of lemurs in Madagascar. In: Rheinwald G (ed) Isolated vertebrate communities in the tropics. Bonner zoologische monographien 46. Museum Alexander Koenig, Bonn, pp 265–272
Gerwing JJ (2002) Degradation of forests through logging and fire in the eastern Brazilian Amazon. Forest Ecol Manag 157:131–141
Gibbons MA, Harcourt AH (2009) Biological correlates of extinction and persistence of primates in small forest fragments: a global analysis. Trop Conserv Sci 2:388–403
Groves C (2001) Why taxonomic stability is a bad idea, or why are there so few species of primates (or are there?). Evol Anthropol 10:192–198
Harcourt C, Thornback J (1990) Lemurs of Madagascar and the Comoros. The IUCN red data book. IUCN, Gland, Switzerland
Harper G, Steininger M, Tucker C, Juhn D, Hawkins F (2007) Fifty years of deforestation and forest fragmentation in Madagascar. Environ Conserv 34:325–333
Hladik CM, Charles-Dominique P (1974) The behaviour and ecology of the sportive lemur (Lepilemur mustelinus) in relation to its dietary peculiarities. In: Martin RD, Doyle GA, Walker AC (eds) Prosimian biology. Duckworth, London, pp 23–37
Janson CH, Goldsmith ML (1995) Predicting group size in primates: foraging costs and predation risks. Behav Ecol 6:326–336
Janson CH, van Schaik CP (1993) Ecological risk aversion in juvenile primates: slow and steady wins the race. In: Pereira ME, Fairbanks LA (eds) Juvenile primates: life history, development, and behavior. Oxford University Press, New York, pp 57–74
Karpanty SM (2003) Rates of predation by diurnal raptors on the lemur community of Ranomafana National Park, Madagascar. Am J Phys Anthropol 36:126–127
Karpanty SM (2006) Direct and indirect impacts of raptor predation on lemurs in southeastern Madagascar. Int J Primatol 27:239–261
Lei RH, Engberg SE, Andriantompohavana R, McGuire SM, Mittermeier RA, Zaonarivelo JR, Brenneman RA, Louis EE Jr (2008) Nocturnal lemur diversity at Masoala National Park. Spec Publ Mus Tex Tech Univ 53:1–41
Louis EE Jr, Engberg SE, Lei RH, Geng HM, Sommer JA, Randriamampionona R, Randriamanana JC, Zaonarivelo JR, Andriantomphohavana R, Randria G, Prosper RB, Rakotoarisoa G, Rooney A, Brenneman RA (2006) Molecular and morphological analyses of the sportive lemurs (family Megaladapidae: genus Lepilemur) reveals 11 previously unrecognized species. Spec Publ Mus Tex Tech Univ 49:1–47
Martin P, Bateson P (1993) Measuring behaviour. An introductory guide, 2nd edn. Cambridge University Press, Cambridge
Mittermeier RA, Konstant WR, Hawkins F, Louis EE, Langrand O, Ratsimbazafy J, Rasoloarison R, Ganzhorn JU, Rajaobelina S, Tattersall I, Meyers DM (2006) Lemurs of Madagascar, 2nd edn. Conservation International, Washington, DC
Mittermeier RA, Ratsimbazafy J, Rylands AB, Williamson L, Oates JF, Mbora D, Ganzhorn JU, Rodriguez-Luna E, Palacios E, Heymann EW, Kierulff MCM, Long YC, Supriatna J, Roos C, Walker S, Aguiar JM (2007) Primates in Peril: The World’s 25 most endangered primates 2006–2008. Primate Conserv 22:1–40
Moat J, Smith P (2007) Atlas of the vegetation of Madagascar. Royal Botanic Gardens, Kew, Richmond
Olivieri G, Zimmermann E, Randrianambinina B, Rasoloharijaona S, Rakotondravony D, Guschanski K, Radespiel U (2007) The ever-increasing diversity in mouse lemurs: three new species in north and northwestern Madagascar. Mol Phylogenet Evol 1:309–327
Olivieri G, Schwitzer C, Schwitzer N, Craul M (2008) Lepilemur sahamalazensis. In: IUCN 2010. IUCN red list of threatened species. Version 2010.2. www.iucnredlist.org. Accessed 29 June 2010
Onderdonk DA, Chapman CA (2000) Coping with forest fragmentation: the primates of Kibale National Park, Uganda. Int J Primatol 21:587–611
Rabarivola C, Zaramody A, Fausser JL, Andriaholinirina N, Roos C, Zinner D, Marcel H, Rumpler Y (2006) Cytogenetic and molecular characteristics of a new species of sportive lemur from northern Madagascar. Lemur News 11:45–49
Ramaromilanto B, Lei RH, Engberg SE, Johnson SE, Sitzmann BD, Jouis EE Jr (2009) Sportive lemur diversity at Mananara-Nord Biosphere Reserve, Madagascar. Occas Pap Mus Tex Tech Univ 286:1–22
Randrianambinina B, Mbotizafy S, Rasoloharijaona S, Ravoahangimalala RO, Zimmermann E (2007) Seasonality in reproduction of Lepilemur edwardsi. Int J Primatol 28:783–790
Rasoloharijaona S, Rakotosamimanana B, Randrianambinina B, Zimmermann E (2003) Pair-specific usage of sleeping sites and their implications for social organization in a nocturnal malagasy primate, the Milne Edwards’ sportive Lemur (Lepilemur edwardsi). Am J Phys Anthropol 122:251–258
Rasoloharijaona S, Randrianambinina B, Braune P, Zimmermann E (2006) Loud calling, spacing, and cohesiveness in a nocturnal primate, the Milne Edwards’ sportive lemur (Lepilemur edwardsi). Am J Phys Anthropol 129:591–600
Rasoloharijaona S, Randrianambinina B, Zimmermann E (2008) Sleeping site ecology in a rain-forest dwelling nocturnal lemur (Lepilemur mustelinus): implications for sociality and conservation. Am J Primatol 70:247–253
Ravaoarimanana B, Fausser JL, Rumpler Y (2001) Genetic comparison of wild populations of Lepilemur septentrionalis and Lepilemur dorsalis using RAPD markers. Primates 42:221–231
Ruperti FS (2007) Population density and habitat preferences of the Sahamalaza sportive lemur (Lepilemur sahamalazensis) at the Ankarafa Research Site, NW Madagascar. MSc thesis, Oxford Brookes University
Russell RJ (1978) The behavior, ecology and environmental physiology of a nocturnal primate, Lepilemur mustelinus (Strepsirhini, Lemuriformes, Lepilemuridae). Diss Abstr Int B38:5793–5794
Schatz GE (2001) Generic tree flora of Madagascar. [flore generique des arbres de Madagascar]. Missouri Botanical Garden, Saint Louis, USA and Royal Botanic Gardens, Kew
Schmid J, Ganzhorn J (1996) Resting metabolic rates of Lepilemur mustelinus ruficaudatus. Am J Primatol 38:169–174
Schwitzer C, Schwitzer N, Randriatahina GH, Rabarivola C, Kaumanns W (2006) “Programme Sahamalaza”: new perspectives for the in situ and ex situ study and conservation of the blue-eyed black lemur (Eulemur macaco flavifrons) in a fragmented habitat. In: Schwitzer C et al (eds) Proceedings of the German–Malagasy research cooperation in life and Earth sciences. Concept Verlag, Berlin, pp 135–149
Schwitzer N, Kaumanns W, Seitz PC, Schwitzer C (2007a) Cathemeral activity patterns of the blue- eyed black lemur Eulemur macaco flavifrons in intact and degraded forest fragments. Endang Species Res 3:239–247
Schwitzer N, Randriatahina GH, Kaumanns W, Hoffmeister D, Schwitzer C (2007b) Habitat utilization of blue-eyed black lemurs, Eulemur macaco flavifrons (Gray, 1867), in primary and altered forest fragments. Primate Conserv 22:79–87
Stanford C (1995) The influence of chimpanzee predation on group size and anti-predator behaviour in red colobus monkeys. Anim Behav 49:577–587
Tattersall I (1982) The primates of Madagascar. Columbia University Press, New York
Warren RD, Crompton RH (1997) A comparative study of the ranging behaviour, activity rhythms and sociality of Lepilemur edwardsi (Primates, Lepilemuridae) and Avahi occidentalis (Primates, I ndriidae) at Ampijoroa, Madagascar. J Zool 243:397–415
WCS/DEC (2002) Etude de faisabilité de la création d’APMC et plan de développement pour le site de la RBM Sahamalaza – Nosy Radama. Wildlife Conservation Society/Development Environment Consult, Antananarivo
Wilcove DS (1985) Nest predation in forest tracts and the decline of migratory songbirds. Ecology 66:1212–1214
Wilcove DS, McLellan CH, Dobson AP (1986) Habitat fragmentation in the temperate zone. In: Soulé ME (ed) Conservation biology. The science of scarcity and diversity. Sinauer Associates, Sunderland, MA, pp 237–256
Wright P (1998) Impact of predation risk on the behaviour of Propithecus diadema edwardsi in the rainforest of Madagascar. Behaviour 135:483–512
Zinner D, Hilgartner RD, Kappeler PM, Pietsch T, Ganzhorn JU (2003) Social organization of Lepilemur ruficaudatus. Int J Primatol 24:869–888
Acknowledgments
We would like to thank Madagascar National Parks (MNP), especially the director of Sahamalaza-Iles Radama National Park, M. ISAIA Raymond, for their continuing collaboration. Thank you also to the DGEF and CAFF/CORE for granting us research permits for our work in Sahamalaza, and to Prof. Rabarivola Clément for his ongoing help. Tantely Ralantoharijaona and Bronwen Daniel, along with all Ankarafa field guides, contributed substantially to the data collection. MS was funded by Bristol Conservation and Science Foundation, Association Européenne pour l’Etude et la Conservation des Lémuriens (AEECL), Conservation International Primate Action Fund, Margot Marsh Biodiversity Foundation, Mohamed bin Zayed Species Conservation Fund, International Primatological Society, and Christian-Vogel-Fonds.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Seiler, M., Holderied, M., Schwitzer, C. (2013). Effects of Habitat Alteration on Resting Behaviour of the Sahamalaza Sportive Lemur in North West Madagascar. In: Marsh, L., Chapman, C. (eds) Primates in Fragments. Developments in Primatology: Progress and Prospects. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-8839-2_18
Download citation
DOI: https://doi.org/10.1007/978-1-4614-8839-2_18
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-8838-5
Online ISBN: 978-1-4614-8839-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)