Abstract
Behaviour, diet and population demography were sampled and compared between two forest fragment-living populations of wild ringtailed lemurs (Lemur catta) in south-central Madagascar. Both sites—a fragment in the Tsaranoro Valley near Andringitra National Park, and a more densely populated fragment at Anja, much closer to human habitation—are sacred forests (sites of human burial) surrounded by anthropogenically produced savannah, and are subject to traditional protective prohibitions (fady). Both sites attract tourists, but are operated differently, with Anja receiving considerably more tourists; the resources available to the L. catta also differ at each site, affecting their behaviour. L. catta at Tsaranoro spent more time feeding, and less time resting and engaging in social behaviour than those at Anja, where abundant fruit from introduced trees, as well as plentiful drinking water, are available and resource abundance is relatively higher. Although the fragments are of similar size and were expected to differ little, many significant behavioural and population differences were observed, suggesting the importance of the refinement of rapid assessment techniques for judging the habitat suitability and conservation value of small forest fragments.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Madagascar is regarded as a conservation hotspot due to its intense biodiversity—nearly 80 % of Madagascar’s flora and fauna are endemic to the island (Lourenco 1996; Goodman and Benstead 2003). Humans arrived on Madagascar roughly 2000 years ago, bringing slash and burn agriculture, and the process of forest clearing has been ongoing since then, fragmenting the island’s forests and driving many species extinct (Burney et al. 2004). The fragmentation of forest threatens the island’s remaining nonhuman primates, leaving them vulnerable in terms of both the immediate survival of populations and in terms of the genetic diversity of remaining populations (Ganzhorn et al. 2001; Mittermeier et al. 2008). In early 2009, a political coup resulted in increased economic instability and relaxed enforcement of logging restrictions in Madagascar, in many areas resulting in hardwood poaching and increased deforestation (Innes 2010). The area capable of supporting wild lemur populations is currently less than a tenth of the island (Mittermeier et al. 2008) and isolated forest fragments remain underrepresented among study sites due to the focus on continuous tracts in protected private reserves and national parks.
This is particularly true regarding the body of research on Madagascar’s flagship nonhuman primate, the ring-tailed lemur (Lemur catta). In brief: L. catta is a medium-sized strepsirrhine averaging 2.2 kg (Sussman 1991; Gould et al. 2003). The species is found throughout parts of southern, south-western, and south-central Madagascar: L. catta range from Parc National de Kirindy-Mitea in the west; to the southwest and the southernmost point of the island (Cap Sainte Marie); east to Petriky and Parc National de Andohahela; and to the south-central region where our study occurred, with Anja Reserve likely the furthest northeast of their distribution (Goodman et al. 2006; Gould 2006). They are remarkably ecologically flexible, occurring in areas of gallery, xerophytic, brush and scrub, spiny, and dry deciduous forest as well as high-altitude ericoid bush (in the Andringitra mountain range) and rocky outcrop mixed vegetation forest surrounded by anthropogenic savannah (Goodman et al. 2006; Gould 2006; Jolly 2003; Sussman et al. 2003; Gabriel 2013). L. catta is the most terrestrial lemur, spending on average one-third of the time on the ground (Jolly 1966; Sussman 1974).
L. catta have been described as opportunistic omnivores (Sauther et al. 1999), feeding on fruit and other plant parts, insects, soil, and termitaria (Jolly 1966; Sauther 1992; Sauther et al. 1999; Sussman 1977). Seasonality of food resources is extreme in southern Madagascar, and droughts are common throughout L. catta’s range (Jolly 1966; Gould et al. 1999; 2003; Sauther 1992; Sussman 1977). As a result, L. catta embodies the traits expected in primate species that persist in fragments, as outlined by Marsh (2003): a low or flexible degree of frugivory, small or variable home range sizes, broad behavioural and dietary plasticity, and the capacity to move through or utilize matrix habitat surrounding the fragment (Gould et al. 1999; Gould 2006; Jolly et al. 1993; Jolly 2003; Sauther 1992, 1993 2002; Soma 2006; Gabriel 2013).
Although the species has been extensively studied for over 50 years, it is only rarely reported on at sites other than Beza Mahafaly Special Reserve (BMSR) in the southwest and Berenty Private Reserve in the far south, both largely gallery forest habitats (histories in Jolly 2012; Jolly et al. 2006; Sussman and Ratsirarson 2006; Sussman et al. 2012). This work has focused heavily on L. catta’s dry habitat adaptations in continuous tracts of gallery and scrub forests: correlations have been suggested between seasonality and behaviour, e.g., female dominance, ranging patterns (e.g., Jolly 1984; Jolly et al. 1993; Mertl-Millhollen et al. 2003; Sauther 1993), and seasonality and demography, e.g., rapid population recovery after extreme droughts, group size fluctuations (e.g., Gould et al. 1999; Jolly et al. 2002; Pride 2005; Sauther and Cuozzo 2008). These studies commonly lack comparative data from other habitats, because little has been generated (but see recent work on L. catta in spiny forest/spiny bush by Gould et al. 2011; Kelley 2011; LaFleur 2012).
This is particularly pertinent from the perspective of maintaining wild populations, as southern gallery forest, the predominant habitat type at both BMSR and Berenty, is considered among the most endangered types of forest on the island (Sussman et al. 2003). Livestock grazing and overcutting for agriculture have created narrow forest fragments running along river and stream edges, and this habitat has been considered optimal for L. catta (based on population densities, health profiles, population recovery in this habitat, and resource availability (Goodman et al. 2006; Gould et al. 1999, 2003; Jolly et al. 2002; Koyama et al. 2002). However, evidence of river shifts may hold implications regarding the efficacy of current efforts to protect these gallery forests (Blumenfeld-Jones et al. 2006; Koyama et al. 2006), which suggests the utility of studies aimed at determining the conservation potential of other sites and habitats. Particularly, many small fragments of other forest types exist throughout L. catta’s range (Sussman et al. 2003; Gould and Gabriel under review), and the work presented here was conducted at two such fragments. Few studies have been conducted to investigate how populations of L. catta in rocky outcrop fragments differed from conspecifics in terms of diet, demography, or behaviour (but see Gabriel 2013a), limiting our understanding of range of L. catta’s flexibility along these vectors, and leaving the conservation value of such sites unknown.
In this chapter, we present population densities, behavioural surveys and dietary data on two populations of L. catta living in south-central Madagascar, at a fragment in the Tsaranoro Valley and at Anja Special Reserve. Both small fragments remain intact due to past use by local Betsileo as a burial area, which renders the sites sacred, and subject to various fadys or taboos/restrictions on their use. The adherence to these traditional restrictions is a feature of tribal groups across the island, but the fadys themselves can vary intensely from area to area and village to village, as do the penalties, which may include divine retribution (as when the ancestors have forbidden the harvesting of a wild species, a very strict fady) or compensating the head of the village in which the violation occurred (in the areas where we worked this would generally be in the form of cattle or tree plantation). It has been suggested that fady, while not necessarily promoting sustainable resource management, are adhered to more than imposed, external conservation-oriented rules, and that the introduction of restrictions rooted in governmental and foreign authority may instead weaken the traditional safeguards against poaching and sacred site use (Jones et al. 2008).
Approximately 60 L. catta live in the Tsaranoro fragment in approximately 6–8 groups, ranging in size from 4 to ~20 individuals. During the study period, large groups of individuals appeared to fission and fusion, aggregating alternately throughout the day via contact calls between much smaller subgroups. There are approximately 225 L. catta living at Anja (Gould and Gabriel under review). Groups at this site average 16 individuals, although groups size ranges from 4 to 21 animals—no fusing of small groups was observed here, although large groups appear to time-share feeding sites peacefully and sometimes spread into slightly smaller groups while feeding.
Behavioural and dietary variations between these populations and L. catta studied elsewhere highlight this species’ intense plasticity, suggesting the potential of small forest fragments for L. catta’s persistence in the wild. However, while comparisons with data from other habitats indicate that the two fragment sites are generally similar to one another, significant differences between the two populations in terms of behaviour and diet suggest that generalizing on the basis of habitat type and forest size is less useful for conservation planning than rapid assessment on a site-by-site basis.
Study Sites
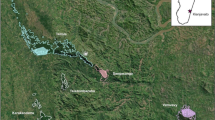
Our study was conducted during the austral winter in June and July 2009 and July 2010, at a fragment in the Tsaranoro Valley (22°05′11 S, 46°46′ 14 E), and at Anja Community Reserve (21°51′12 S, 46°50′40 E), 55 km NE of Tsaranoro, and 13 km south of the town of Ambalavao, in south-central Madagascar (Fig. 16.1). Both fragments remain due to past use by local Betsileo as a burial area, which renders the sites sacred, and subject to various fady; hunting or harming lemurs is also considered fady by locals and thus L. catta are protected by traditional beliefs twice over. Malagasy guides are required for entrance into either fragment to ensure that these prohibitions are followed. At both sites the L. catta have not been previously studied but are habituated to human presence, although the Anja lemurs are far more habituated due to constant tourism since the designation of the fragment as a protected reserve in 1999 (http://anjacommunityreserve.netai.net/).
The major difference between sites is the presence at Anja of a human-made fish-farm lake surrounded by vegetable and tobacco gardens bordering the southwest corner of the forest. The lake also provides irrigation to rice terraces on the north side of the fragment. The broader area differs little from the Tsaranoro Valley in terms of water availability, but, fragment-to-fragment, water is much more accessible at Anja, as lemurs residing in the lower area of the reserve come down daily to drink at the lake, small ponds, or near the rice terraces, and there are small streams further up the mountain.
The Tsaranoro Fragment
The Tsaranoro Valley is 12 km east of Parc National Andringitra, and peak elevation in the fragment is 1,104 m (Google Earth 2012). Average cold season temperatures are similar (23–32 °C) to other L. catta sites in the south and south-west (Jury 2003; Sauther 1992; Sussman 1991). The Tsaranoro Massif makes up the western edge of the Tsaranoro fragment (Fig. 16.2a). The forest is 53.7 ha of continuous and semi-continuous rocky-outcrop vegetation, with a mix of temperate, rainforest (e.g., bamboo, some palms), and xerophytic scrub species similar to that found in southern Madagascar’s spiny bush habitat.
The Anja Fragment
The Anja fragment is 13 km south of Ambalavao, located immediately adjacent to Route Nationale 7, Madagascar’s main highway (Fig. 16.2b). The fragment area is 34 ha including continuous forest running up the mountain, with 3–5 L. catta groups living at this higher altitude. The reserve is bordered by large rock formations (sandwiching the fragment with the highway, which runs parallel). Maximum elevation of the mountain is 1,206 m (Google Earth 2012); but lemurs do not range above 1,020 m. The forest is more strictly rocky-outcrop vegetation than the Tsaranoro fragment (Fig. 16.3), and fruiting trees, especially Melia azedarach and P. cattleianum have become well established at Anja since the removal of large Adansonia and Ficus trees before the fragment was designated as a protected area (Razafimandimby, pers. comm.) (Fig. 16.4).
The majority of the forest grows around large boulders at the centre of the fragment, which has increased in size substantially from 8 ha in 1999 to 34 ha today (Rahaovalahy pers. comm.).
Tourists with guides are much more common at Anja; the reserve is maintained by the Anja-Miray Association as a community conservation and development effort. Tourism entrance fees go directly towards local projects such as the building of a school, horticultural projects, reserve maintenance and guide wages (http://anjacommunityreserve.netai.net/, Rahaovalahy pers. comm.). Because Anja is located directly off a national highway, it attracts tourists interested in seeing easily accessible wildlife and purchasing crafts. Anja saw a much greater number of tourists, approximately 10 times the Tsaranoro fragment’s daily average in 2009, and as of 2010, Anja received approximately 12,000 visitors/year (Raharovalahy 2012). The L. catta groups ranging in the lower portion of the reserve are highly habituated to human presence, groups further up the mountain, off the tourist circuit, are not.
Data Collection and Sampling Techniques
Due to the time available at each site during 2009, data collection was designed as a rapid assessment process (RAP), with methodological development, data collection, and site interpretation coordinated between three researchers (A. Cameron, L. Gould, D. Gabriel) assessing related variables at the same sites; data collection by each team member was assisted by the guides at each site. Often such studies focus on modelling the expansion of protected forest, i.e., assessing land use by wild species in terms of population density over large areas, for purposes of forming new governmentally recognized parks that optimize the park population’s numbers. Our methodology was oriented toward generating predictive information about population status in preexisting (although more circumstantially established) reserves, in addition to obtaining general information on the ecological impact of different habitats and microhabitat features on these fragment populations.
Population counts were averaged after collection, with each researcher tallying the individuals in recognized groups at Tsaranoro and at Anja, via a coordinated walking survey of the fragment from group to group, counting individuals.
Behavioural data were collected by A. Cameron at the group level, noting behaviour (including food type when feeding) for each visible group member at intervals of 5 min. Data were recorded throughout the fragment and surrounding area, including the anthropogenic savannah as well as the gardened grounds of Camp Catta at the Tsaranoro site, which is visited regularly by at least one group of L. catta—data from this group are not included in these results as these individuals return nightly to remote sleeping trees to the north of the camp and likely never interact with fragment groups (Gabriel pers. comm.). Five minute group scan sampling (Martin and Bateson 1993) was chosen because it obtains data that are representative across all individuals, and allows for the rapid collection of data. In total, 3,598 observations of individual behaviour were recorded at Anja and 1,640 were recorded at Tsaranoro. Differences between the sites were measured in these data via two-tailed Pearson's chi-square test with significance level set at 0.05.
L. Gould collected phenological specimens of every type of food eaten during behavioural observations, and these were identified, when possible, by the guides at each site. Many were identified by vernacular name, and some by scientific names . Because we were unable to obtain identification for all plants consumed by the L. catta at the sites, food items were grouped as fruit, vegetation (leaves, vine, stems—at all stages of maturity) insects, or soil. Simplified plant-part categories allow for quick recording during behavioural sampling, and were used to observe broad trends in food resource use between sites and habitats.
Results
Population Demographics
These findings are summarized in Table 16.1.
Behaviour
Activity
The overall similarity and relatively close geographic proximity of the two fragments suggested that the populations would proportion their time similarly. Relative to other sites the two populations are generally similar in behaviour (Table 16.2). Following Rasamimanana et al. (2006), we grouped behaviours as active or inactive, with resting, sunning, and resting in contact as inactive behaviours and the remainder as active behaviours. Tsaranoro L. catta were inactive during 46.9 % of scan records, and active for 53.1 % and Anja L. catta were inactive during 46.8 % of scan records, and active for 53.2 %, which suggests overall similarity between the populations in terms of balancing the conservation and expenditure of energy.
Statistical comparison between the sites’ activity budget figures indicates that although the fragments are similar in size and habitat type, local-level differences cause divergences in the populations’ allocation of time. Table 16.3 illustrates the percentage of feeding observations at each site according to food item class.
Diet
The difference in proportion of diet is significant in the case of the insect category (χ2 = 68.374, df = 1, p = <0.0001). The fruit and vegetation categories are not markedly different, and neither reached statistical significance (χ2 = 2.816, df = 1, p = 0.0933 and χ2 = 0.108, df = 1, p = 0.7426, respectively).
Discussion
Population Demographics
Population density has been seen as a vector for inferring habitat quality—for instance, the large populations supported by Berenty and Beza Mahafaly Reserves’ gallery forests have been cited as indicating both that gallery forests have a high overall carrying capacity for L. catta and that this habitat is particularly suited to L. catta (Goodman et al. 2006; Gould et al. 1999, 2003; Jolly et al. 2002; Koyama et al. 2002). Table 16.4 compares the fragment populations’ demographics to those from other sites.
The low population density of Tsaranoro suggests that rocky outcrop forest may be approximate in carrying capacity to dry and spiny forests, and that L. catta in regions increasingly dominated by cleared land and anthropogenic savannah are attracted to the remaining forest where they may become entrenched, with populations expanding extensively when resources allow, as they do at Anja. We suggest, however, that the extremely high density of the Anja population is an anomaly, and has grown rapidly (since the designation of the reserve as a protected area) as a function of excellent seed dispersal by the lemurs, particularly of the fruiting trees M. azedarach and P. cattleianum, the protected status of the reserve, year-round water availability, and access for some groups to village gardens which are planted at the edge of the lower reserve paths.
Gould et al. (1999) suggested that L. catta is an r-selected species, a strategy that aids in populations rebounding after decimation by a drought or other disaster . In small forests like our study fragments, this trait may inflate a population rapidly, possibly beyond the fragment’s ability to sustain it. In the case of fragments isolated from other forests, such a trend may result in limited emigration, leading to stress due to population density and possibly to inbreeding depression. The availability of water year-round at Anja is unusual in L. catta’s geographic range, and this site-specific feature may decrease resource strain for this population, even as the crowding allowed may increase stress. A comparison of stress hormone levels in both populations is forthcoming (Gabriel 2013b), and a study of the population genetics at each site was conducted in 2012 (Clarke in prep).
Activity Budget
The patterning in activity budgets previously reported for L. catta across sites indicates that in habitats or microhabitats with low resource availability, L. catta have a higher resting-to-feeding-time ratio than conspecifics with greater resource access in the same season, as seen in studies at both Berenty, during the early hot season (Ellwanger 2007), and BMSR, during the cooler austral winter (Sauther et al. 2006). There is also a broad tendency to decrease the proportion of time devoted to affiliative social behaviour when resources are less available (Ellwanger 2007; Ellwanger and Gould 2011; Gemmill 2007; Sauther et al. 2006). As a species with a moderately low basal metabolic rate, L. catta spends a substantial portion of the day at rest or inactive (Rasamimanana et al. 2006). Comparison of activity budget, phenological, and dietary data across sites and habitats indicates activity decreases as habitat suitability increases, even as higher resource availability correlates with increased time spent feeding (Ellwanger 2007; Gemmill 2007; Gemmill and Gould 2008; Rasamimanana et al. 2006; Sauther et al. 2006). The two sites, while exhibiting significant intersite differences in the proportion of time allocated to particular behaviours, were very similar to one another relative to other habitats in the cold season (e.g., Opuntia forest (Kelley 2011), gallery forest at BMSR (Gemmill 2007)). This may be due to the overall greater continuity of forest type and size between the two fragments relative to the habitats in which previous studies have been conducted, despite local differences between the fragments such as tourist flow, isolation, and in particular the availability of water—although these factors likely contribute to the statistically significant intersite variation observed in some behaviours.
L. catta at Tsaranoro were observed feeding and resting less than L. catta at Anja (46.29–51.9 % respectively) in our cold season study, and both populations engaged in social behaviours (allogrooming, resting in contact, agonism) or locomoting in roughly one third of our scan record (33.6 % at Tsaranoro and 32.1 % at Anja). These patterns of time allocation suggests that L. catta at both sites were under low pressure to minimize energy expenditure. The Tsaranoro L. catta were inactive in 53 % of observations and active in 47 % and the Anja L. catta inactive in 46.8 % and active in 53.2 %, both echoing the figures from larger reserve sites considered to be near-optimal for L. catta (Rasamimanana et al. 2006).
Given the dense concentration of L. catta at Anja, agonism and scent marking were rare, and territoriality may be mitigated by the social flexibility of the species; low agonism and the relaxation of home range defence, including daily multigroup aggregations and fission, seem reasonable mechanisms for alleviating stress from crowding in an environment with ample resources. This flexibility has been observed sporadically in L. catta: groups at BMSR with overlapping home ranges meet in both benign and agonistic capacities (Gould and Overdorff 2002; Sauther 2002).
Diet
The results are in line with the similarity observed in activity budgets between sites. The majority of insects consumed were aphids and aphid secretions licked off of fig leaves (Ficus baroni), an extremely seasonal resource—during the austral winter. L. catta in the spiny forest at Berenty also include a relatively higher proportion of insects in their diet during the cold season (Gould et al. 2011), and L. catta in Berenty gallery forest begin to allocate increased feeding time to insects in July, peaking at 15.7 and 14.1 % of feeding time in November and December respectively, when preferred resources are low (Soma 2006). The greater number of regularly fruiting trees at Anja may mitigate the need to shift the diet toward insects at this site, where licking of aphid secretions was rare. Fruit consumption rates are expected to reflect total fruit availability, in which case the Tsaranoro and Anja populations appear to have much greater access to fruit compared with gallery and spiny forest L. catta sampled in the same season (Gould et al. 2011; Soma 2006). Concomitantly, naturally occurring vegetation makes up less of the Tsaranoro and Anja diets than those in the gallery and spiny forest at Berenty (Gould et al 2011; Soma 2006). Research conducted in 2010 (Gould and Gabriel under review) revealed that the groups residing in the lower part of Anja reserve regularly consume tomatoes from gardens planted along the lower edges of the reserve. One notable diet difference between the sites is that the Anja lemurs’ diet is overwhelmingly made up of the fruit and leaves of Melia azedarach, an introduced tree to Madagascar, which is ubiquitous throughout the Anja reserve, even at higher altitudes (Gabriel 2013a). The abundance of fruit produced by this tree year-round is likely an important variable in the unusually high density of L. catta in this 34 ha site (Gould and Gabriel under review).
Summary
The variation in social structure, time allocation, and diet observed between sites during this study suggest that these fragment-dwelling populations of L. catta echo observations made of cercopithecoid primates that persist in fragments and exhibit high degrees of behavioural and dietary plasticity (Tutin 1999). The flexible home ranges, variable degree of frugivory (and variable diet in general), and willingness to utilize matrix habitat that were observed in the Tsaranoro and Anja populations fall in line with the other major traits of fragment living species (Bicca-Marques 2003; Estrada and Coates-Estrada 1996; Laurance 1991; Lovejoy et al. 1986; Marsh 2003; Onderdonk and Chapman 2000; Silver and Marsh 2003; Tutin and White 1999). The L. catta at Tsaranoro and Anja follow patterns of behaviour similar to conspecifics in high quality habitat, suggesting the attractiveness of small fragments of rocky outcrop forest as habitat under conditions of adequate food resources. Our population, diet, and activity budget data emphasize the importance of generating basic knowledge about population health in, and particularly gene flow between, these isolated populations.
The significant variations observed between the two fragments, despite their overall similarity to one another relative to other habitats in southern Madagascar’s cool season (austral winter), indicate that site-specific assessments accounting for local differences in fragment management, and site-specific features (e.g., sources of water) are of greater practical use for conservation purposes than generalization on the basis of forest size or habitat type. Our research in these small fragments suggests the utility of a roughly standardized, broadly applicable rapid assessment methodology for fragment-living primate populations
A more detailed examination of diet, activity patterns and parasite loads of the L. catta populations at these two sites over an annual cycle is forthcoming (Gabriel 2013a; Gould and Gabriel under review). Future work at these sites should include phenological surveys, which will more clearly indicate resource availability in and the carrying capacities of the fragments by season. Endocrinological research conducted in 2010–2011 (Gabriel and Gould under review) further illuminates the interaction between resource bases, population density, stress, and population health and behaviour. The data collection protocols established in our study may be applicable in local level management efforts at other fragmented sites containing primate populations.
References
Bicca-Marques JC (2003) How do howler monkeys cope with habitat fragmentation? In: Marsh LK (ed) Primates in fragments: ecology and conservation. Springer, New York, pp 283–304
Blumenfeld-Jones K, Randriamboavonjy TM, Williams G, Mertl-Millhollen AS, Pinkus S, Rasamimanana H (2006) Tamarind recruitment and long-term stability in the Gallery Forest at Berenty, Madagascar. In: Jolly A, Sussman RW, Koyama N, Rasamimanana H (eds) Ringtailed lemur biology: Lemur catta in Madagascar. Springer, New York, pp 69–85
Burney DA, Burney LP, Godfrey LR, Jungers WL, Goodman SM, Wright HT, Jull AJT (2004) A chronology for late prehistoric Madagascar. J Human Evol 47(1–2):25–63
Cameron A (2010) Behavioural surveys and edge-sensitivity estimates of two populations of free-ranging ringtailed lemurs (Lemur catta) in rocky outcrop/savannah mosaic habitat at Anja Special Reserve and the Tsaranoro Valley, Southcentral Madagascar. [MA Thesis] University of Victoria, Victoria, BC
Ellwanger NW (2007) Behavioural strategies of the ring-tailed lemur (Lemur catta) in a sub-desert spiny forest habitat at Berenty Reserve, Madagascar. [MA Thesis] University of Victoria, Victoria, BC
Ellwanger N, Gould L (2011) Variations in behavioural patterns between Lemur catta groups living in different forest types: implications for conservation. Endangered Species Res 14:259–270
Estrada A, Coates-Estrada R (1996) Tropical rain forest fragmentation and wild populations of primates at Los Tuxtlas, Mexico. Int J Prim 17:759–778
Gabriel DN (2013a) Habitat use and activity patterns as an indication of fragment quality in a strepsirrhine primate. Int J Primatol 34:388–406
Gabriel DN (2013b) Ecological flexibility in a disturbed landscape: an assessment of the behavioural and health ecology of ring-tailed lemurs (Lemur catta) in relation to forest fragmentation. Ph.D. dissertation, University of Victoria
Gemmill AL (2007). Adult female feeding competition within two groups of free-ranging ringtailed lemurs (Lemur catta) in different habitats at the Beza Mahafaly Special Reserve, Southwestern Madagascar. [MA Thesis] University of Victoria, Victoria, BC
Gemmill A, Gould L (2008) Microhabitat variation and its effects on dietary composition and intragroup feeding interactions between adult female Lemur catta during the dry season at Beza Mahafaly Special Reserve, southwestern Madagascar. Int J Primatol 29:1511–1533
Ganzhorn JU, Lowry PP, Schatz GE, Sommer S (2001) The biodiversity of Madagascar: one of the world’s hottest hotspots on its way out. Oryx 35(4):346–348
Goodman S, Benstead J (eds) (2003). The natural history of Madagascar. University of Chicago Press, Chicago
Goodman SM, Rakotoarisoa SV, Wilme L (2006) The distribution and biogeography of the ringtailed lemur (Lemur catta) in Madagascar. In: Jolly A, Sussman RW, Koyama N, Rasamimanana H (eds) Ringtailed lemur biology: Lemur catta in Madagascar. Springer, New York, pp 3–15
Google Inc. (2012) Google earth version 6.0.2.2074 Available at: http://www.google.com/earth/index.html
Gould L, Sussman RW, Sauther ML (1999) Natural disasters and primate populations: the effects of a two-year drought on a naturally occurring population of ringtailed lemurs in southwestern Madagascar. Int J Primatol 20:69–84
Gould L, Overdorff DJ (2002) Adult male scent-marking in Lemur catta and Eulemur fulvus rufus. Int J Primatol 23(3):575–586
Gould L, Sussman RW, Sauther ML (2003) Demographic and life-history patterns in a population of ring-tailed lemurs (Lemur catta) at Beza Mahafaly Reserve, Madagascar: A 15-year perspective. Am J Phys Anthropol 120(2):182–194
Gould L (2006) Lemur catta ecology: what we know and what we need to know. In: Gould L, Sauther ML (eds) Lemurs: ecology and adaptation. Springer, New York, pp 255–274
Gould L, Power ML, Ellwanger N, Rambeloarivony H (2011) Feeding behavior and nutrient intake in spiny forest-dwelling ring-tailed lemurs (Lemur catta) during early gestation and early to mid-lacttation periods: compensating in a harsh environment. Am J Phys Anth 145:469–479
Innes JL (2010) Madagascar rosewood, illegal logging and the tropical timber trade. Madagascar Conservat Dev 5(1):6–10
Jolly A (1966) Lemur behavior. University of Chicago Press, Chicago
Jolly A (1984) The puzzle of female feeding priority. In: Small MF (ed) Female primates: studies by women primatologists. Alan R. Liss, Inc., New York, pp 197–215
Jolly A (2003) Lemur catta, ring-tailed lemur, maky. In: Goodman SM, Benstead JP (eds) The natural history of Madagascar. University of Chicago Press, Chicago, pp 1329–1331
Jolly A (2012) Berenty reserve, Madagascar: a long time in a small space. In: Kappeler PM, Watts DP (eds) Long-term field studies of primates. Springer, New York, pp 21–44
Jolly A, Rasamimanana HR, Kinnaird MF, O’Brien TG, Crowley HM, Harcourt CS, Gardner S, Davidson J (1993) Territoriality in Lemur catta groups during the birth season at Berenty, Madagascar. In: Kappeler PM, Ganzhorn JU (eds) Lemur social systems and their ecological basis. Plenum Press, New York, pp 85–109
Jolly A, Dobson A, Rasamimanana HM, Walker J, O’Connor S, Solberg M, Perel V (2002) Demography of Lemur catta at Berenty Reserve, Madagascar: effects of troop size, habitat and rainfall. Int J Primatol 23(2):327–353
Jolly A, Koyama N, Rasamimanana HM, Crowley H, Williams G (2006) Berenty Reserve: a research site in southern Madagascar. In: Jolly A, Sussman RW, Koyama N, Rasamimanana H (eds) Ringtailed lemur biology: Lemur catta in Madagascar. Springer, New York, pp 32–42
Jones JPG, Andriamarovololona MM, Hockley N (2008) The importance of taboos and social norms to conservation in Madagascar. Cons Bio 22(4):976–986
Jury M (2003) The climate of Madagascar. In: Goodman S, Benstead J (eds) The natural history of Madagascar. University of Chicago Press, Chicago, pp 75–87
Kelley EA (2011) Lemur catta in the Region of Cap Sainte-Marie, Madagascar: introduced cacti, xerophytic Didiereaceae-Euphorbia bush, and tombs. PhD dissertation, Washington University, St. Louis
Koyama N, Nakamichi M, Ichino S, Takahata Y (2002) Population and social dynamics changes in ring-tailed lemur troops at Berenty, Madagascar between 1989 and 1999. Primates 43(4):291–314
Laurance WF (1991) Edge effects in tropical forest fragments: application of a model for the design of nature reserves. Biolog Cons 57(2):205–219
LaFleur M (2012) Ecology of ring-tailed lemur (Lemur catta) at the Tsimanampetsotsa National Park, Madagascar: implications for female dominance and the evolution of lemur traits. Ph.D. Dissertation, University of Colorado
Lourenço WR (1996) Biogéographie de Madagascar. Paris:ORSTOM
Lovejoy TE, Bierregaard RO Jr, Rylands AB, Malcolm JR, Quintela CE, Harper LH, Brown K Jr, Powell AH, Powell GVN, Powell, Schubart HO, Hays MB (1986) Edge and other effects of isolation on Amazon forest fragments. In: Soulé ME (ed) Conservation biology: the science of scarcity and diversity. Sinauer, Sunderland, MA, pp 257–285
Martin P, Bateson P (1993) Measuring behaviour: an introductory guide. Cambridge University Press, Cambridge
Marsh LK (2003) The nature of fragmentation. In: Marsh LK (ed) Primates in fragments: ecology and conservation. Springer, New York, pp 1–10
Mertl-Millhollen AS, Moret ES, Felantsoa D, Rasamimanana H, Blumenfeld-Jones KC, Jolly A (2003) Ring-tailed lemur home ranges correlate with food abundance and nutritional content at a time of environmental stress. Int J Prim 24(5):969–985
Mittermeier R, Ganzhorn J, Konstant W, Glander K, Tattersall I, Groves C, Rylands A, Hapke A, Ratsimbazafy J, Mayor M, Louis E, Rumpler Y, Schwitzer C, Rasoloarison R (2008) Lemur diversity in Madagascar. Int J Primatol 29:1607–1656
Onderdonk DA, Chapman CA (2000) Coping with forest fragmentation: the primates of Kibale National Park, Uganda. Int J Prim 21:587–611
Pride ER (2005) Optimal group size and seasonal stress in ring-tailed lemurs (Lemur catta). Behav Ecol 16:550–560
Raharovalahy VS (2012) Anja-Miray, Madagascar. Presentation given to the UN Equator Initiative ICCA Panel Discussion, June, 2012. http://www.equatorinitiative.org/
Rasamimanana H, Andrianome V, Ramibeloarivony H, Pasquet P (2006) Male and female ringtailed lemur’s energetic strategy does not explain female dominance. In: Jolly A, Sussman R, Koyama N, Rasimimanana H (eds) Ringtailed lemur biology: Lemur catta in Madagascar. Stringer, New York, pp 271–295
Sauther ML (1992) The effect of reproductive state, social rank and group size on resource use among free-ranging ringtailed lemurs (Lemur catta) of Madagascar. Ph.D. dissertation. Washington University, St. Louis
Sauther ML (1993) Resource competition in wild populations of ringtailed lemurs (Lemur catta): implications for female dominance. In: Kappeler PM, Ganzhorn JU (eds) Lemur social systems and their ecological basis. Plenum Press, New York, pp 135–152
Sauther ML, Sussman RW, Gould L (1999) The socioecology of the ringtailed lemur: thirty-five years of research. Evol Anthropol 8:120–132
Sauther M (2002) Group size effects on predation sensitive foraging in wild ring-tailed lemurs (Lemur catta). In: Miller L (ed) Eat or be Eaten: predator sensitive foraging among primates. Cambridge University Press, Cambridge, pp 107–125
Sauther ML, Cuozzo FP (2008) Somatic and dental variation in living, wild ring-tailed lemurs. Folia Primatologica 79:58–78
Sauther ML, Fish KD, Cuozzo FP, Miller DS, Hunter-Ishikawa M, Culbertson H (2006) Patterns of health, disease, and behaviour among wild ringtailed lemurs, Lemur catta: effects of habitat and sex. In: Jolly A, Sussman RW, Koyama N, Rasamimanana H (eds) Ringtailed lemur biology: Lemur catta in Madagascar. Springer, New York, pp 313–331
Silver SC, Marsh LK (2003) Dietary flexibility, behavioural plasticity, and survival in fragments: lessons from translocated howlers. In: Marsh LK (ed) Primates in fragments: ecology and conservation. Springer, New York, pp 251–266
Soma T (2006) Tradition and novelty: Lemur catta feeding strategy on introduced tree species at Berenty Reserve. In: Jolly A, Sussman RW, Koyama N, Rasamimanana H (eds) Ringtailed lemur biology: Lemur catta in Madagascar. Springer, New York, pp 141–159
Sussman RW (1974) Ecological distinctions in sympatric species of Lemur. In: Martin RD, Doyle GA, Walker AC (eds) Prosimian biology. Duckworth, London, pp 75–108
Sussman RW (1977) Socialization, social structure and ecology of two sympatric species of lemur. In: Chevalier-Skolnikoff S, Poirier FE (eds) Primate bio-social development: biological, social, and ecological determinants. Garland, New York, pp 515–528
Sussman RW (1991) Demography and social organization of free-ranging Lemur catta in the Beza Mahafaly Reserve, Madgascar. Am J Phys Anthropol 84:43–58
Sussman RW, Green GM, Porton I, Andrianasolondaibe OL, Ratsirarson JA (2003) Survey of the habitat of Lemur catta in southwestern and southern Madagascar. Primate Conserv 19:32–57
Sussman RW, Ratsirarson JA (2006) Beza Mahafaly special reserve: a research site in southwestern Madagascar. In: Jolly A, Sussman RW, Koyama N, Rasamimanana H (eds) Ringtailed lemur biology: Lemur catta in Madagascar. Springer, New York, pp 43–51
Sussman RW, Richard AF, Ratsirarson J, Sauther ML, Brockman DK, Gould L, Lawler R, Cuozzo FP (2012) Beza Mahafaly Special Reserve: long-term research on lemurs in Southwestern Madagascar. In: Kappeler PM, Watts DP (eds) Long-term field studies of primates. Springer, New York, pp 45–66
Tutin CG (1999) Fragmented living: behavioural ecology of primates in a forest fragment in the Lopé Reserve, Gabon. Primates 40(1):249–265
Tutin CG, White L (1999) The recent evolutionary past of primate communitiesL likely environmental impacts during the past three millennia. In: Fleagle JG, Janson CH, Reed KE (eds) Primate communities. Cambridge University Press, Cambridge, pp 220–236
Acknowledgments
We thank the Département des Eaux et Forêts, Madagascar for granting us research permission, and to MICET (2009) and GERP (2010) for logistical assistance. We also thank the guides at both sites: Jean-Paul (Tsaranoro) and Clovis (Anja) in 2009, Patrick Andrianomejanahary (Tsaranoro) and Alexandre Bruno (Anja) in 2010, and the Anja-Miray Village Association and Association Tantely which manage the Anja and Tsaranoro Valley forests, respectively. We are grateful to Tara Clarke for her excellent research assistance in 2010. Funding was provided by a grant to LG from the Natural Sciences and Engineering Research Council of Canada (NSERC).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Cameron, A., Gould, L. (2013). Fragment-Adaptive Behavioural Strategies and Intersite Variation in the Ring-Tailed Lemur (Lemur catta) in South-Central Madagascar. In: Marsh, L., Chapman, C. (eds) Primates in Fragments. Developments in Primatology: Progress and Prospects. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-8839-2_16
Download citation
DOI: https://doi.org/10.1007/978-1-4614-8839-2_16
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-8838-5
Online ISBN: 978-1-4614-8839-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)