Abstract
Squamous cell carcinomas of the upper aerodigestive tract exhibit complex interactions with the host immune system that may simultaneously explain resistance to various therapeutic modalities and that may also provide opportunities for therapeutic intervention. The interplay between developing or established malignancy and the host immune system is best understood through a careful analysis of the key components and effector arms of the immune system. These include the complex cellular network of immune modulation as well as tumor-derived factors such as chemokines and cytokines. While the host response to the developing tumor may successfully curtail tumor growth in some cases (immunosurveillance), squamous cell carcinomas of the head and neck are characterized by their ability to create an immunosuppressive environment powerful enough to evade the immune response. It is increasingly apparent that efforts to stimulate a therapeutically effective immune response against established tumors must be coupled with strategies to abrogate this immune-suppressive environment. Preclinical studies and clinical trials have yielded promising results and provide the foundation for further refinements in a broad variety of immunotherapeutic strategies targeting all components of the immune system. Combining such approaches with the established treatment options of surgical resection, radiotherapy, and chemotherapy may ultimately yield substantive improvements in overall survival that to date have been lacking and simultaneously reduce disease-related and treatment-related morbidities for this debilitating and deadly disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Squamous cell carcinoma
- Immunosurveillance
- Immunosuppression
- Immunotherapy
- Cytokines
- T regulatory cells
- Myeloid derived suppressor cells
- Tumor vaccines
- Antibody therapies
- Adoptive cell transfer
1 Introduction
The vast majority of tumors (95 %) that arise in the head and neck region are squamous cell carcinomas arising from the epithelium of the upper aerodigestive tract. Head and neck squamous cell carcinomas (HNSCCs) infringe on the highly critical functions of speech, swallowing, and respiration. Current therapies, surgery alone, radio- or chemo-radiotherapy, or combinations of these modalities, leave many of these patients with significant functional deficits that exact a unique physical and social toll. Although significant advances in the areas of reconstructive surgery, minimally invasive surgery, precisely targeted radiotherapy, chemotherapy, and monoclonal antibody therapy have been achieved in the last three decades, the overall survival rates for patients with these cancers have been modestly affected. HNSCC accounts for approximately 2.5 % of all newly diagnosed cancer cases in the United States, with 40,250 new cases estimated in 2012 [1]. Globally, HNSCC (including the oral cavity, oro/hypo/nasopharynx, and larynx) represents the sixth most common malignancy encountered [2] with a high case fatality (ratio of mortality to incidence of 0.53) and with more than 644,000 new cases reported in 2002 worldwide [3]. Although the cause of HNSCC is multifactorial, its risk has been historically associated with tobacco and alcohol use, especially those who use both. Processed tobacco, in fact, contains more than 3,000 chemical compounds, including at least 30 known carcinogens, while cigarette smoke contains approximately 50 known carcinogens and pro-carcinogens [4]. The epidemiology of HNSCC has dramatically changed over the past two decades, however, particularly as this relates to oropharyngeal SCC. As tobacco use, traditionally the most important risk factor for HNSCC, has decreased in the USA, the incidence of tobacco-associated human papillomavirus (HPV)-negative HNSCC has also decreased [5, 6]. Instead, the incidence of HPV-associated oropharyngeal cancers overall is increasing worldwide [7, 8]. The incidence of tonsillar cancer in the USA, especially among men under age 60, increased by 2–3 % each year between 1973 and 1995; however this incidence has increased more rapidly in the last decade [9]. Indeed, while only 16 % of the US oropharyngeal cases were HPV positive in 1984–1989, 73 % of tumors were positive for this virus in 2000–2004 [10]. Interestingly, survival of HPV-positive HNSCC patients is notably better than survival of HPV-negative HNSCC patients (3-year survival of 84 % vs. 57 %, respectively) [11]. Recent analysis of oropharyngeal cancer patient survival among cases from 1984 to 2004 in SEER suggested that median survival was fourfold higher among HPV-positive than HPV-negative oropharyngeal cases (131 vs. 20 months) in the USA during the past two decades. In addition, while survival increased significantly for HPV-positive oropharyngeal cases between 1984 and 2004 (p = 0.003) survival did not improve for HPV-negative cases (p = 0.18) [12]. The effect of tobacco remains powerful, however, as patients with HPV-positive tumors who smoke have a prognosis intermediate between smokers whose tumors are HPV negative and nonsmokers with HPV-positive tumors [11].
The better survival and lower rate of recurrence observed in HPV-positive HNSCC highlight the importance that the immune system plays in this malignancy. Indeed, despite the best efforts of the virus to evade host defenses, most HPV infections resolve with time as a result of a successful cell-mediated immune response [13] directed against the early HPV proteins (i.e., E2 and E6) [14, 15]. Furthermore, even in the absence of viral induced cytolysis and cell death, the HPV-infected cells can activate the production of type 1 interferons and evoke a powerful, generic, antiviral, and innate immune system response. The type 1 interferons (IFN-α and IFN-β) have antiviral, antiproliferative, anti-angiogenic, and immunostimulatory properties that act as a bridge between innate and adaptive immunity, activating immature dendritic cells and thus facilitating antigen processing and generation of antiviral immunity [16]. The possible role of the immune system is further suggested in an HPV-positive and HPV-negative preclinical model of tonsil squamous cell carcinoma [17]. While in immune-deficient mice no differences in tumor growth were observed between HPV+ and HPV− tumors, in immune-competent mice a significant delay in tumor progression was observed in the group bearing the HPV+ carcinoma with 20–30 % of animals able to completely clear the tumor [17]. Tumor rejection was dependent on both CD4+ and CD8+ T cells that are spontaneously primed and expanded in the mice bearing the HPV+ tumor [17]. However, it is important to remember that significant differences exist between the murine transplantable model and spontaneously arising tumors. While transplantable tumors derived from immortalized cell lines grew and developed rapidly when injected in the mice, spontaneous tumors developed slowly through a long interaction with the host. Indeed, while strong evidence exists that specific immune surveillance operates at early stages of tumorigenesis, causing inflammation and neoplastic stabilization, established tumors appear to be able to induce immune tolerance [18] and T cell anergy that allow tumor growth. In the presence of this tumor-driven tolerogenic environment, immune surveillance is restrained and immune interventions, such as vaccination or adoptive cell transfer, are likely to be much less effective. The presence of these suppressive mechanisms generated by growing tumor can explain the low clinical success rates obtained by immunotherapy in the last decades [19].
2 Interaction Between HNSCC and the Immune System
As in many other cancers, the interaction between the immune system and the transformed epithelial cells plays a critical role in the genesis and in the progression of HNSCC. In this malignancy the concept of immune surveillance [20] and tumor–host immune system interaction is sustained by both clinical and experimental observations. For example, one clear indication of the contribution of the immune system in controlling HNSCC is the relative increase in its incidence in the context of pharmaceutical immunosuppression or acquired immunodeficiency. Premalignant leukoplakia is identified in 13 % of renal transplant patients as compared to 0.6 % of control age- and sex-matched individuals [21, 22]. In the majority of these patients leukoplakia evolves into dysplasia, and 10 % develop frank SCC [21, 22]. Similar results are observed in patients who have undergone bone marrow transplantation [23–25] and/or are receiving chronic treatment for GVHD [26]. In these latter cases, the major risk factors for the development of SCC were long duration of chronic GVHD therapy and the use of azathioprine, particularly when combined with cyclosporine and steroids [26]. Although HNSCC is not an AIDS-defining illness, the appearance of this malignancy is seen in excess among HIV-infected individuals [27]. HNSCC patients infected with HIV are significantly younger than non-infected patients, and while there are no differences in tumor location, HIV-infected patients generally present with larger and more advanced tumors and significantly poorer prognosis [28]. Interestingly, despite the fact that HPV is a causative agent of HNSCC and opportunistic infection in HIV patients, a large study in AIDS patients with laryngeal squamous cell carcinoma proved the lack of association with HPV infection. However, it is important to remember that this subsite is not typically associated with HPV-associated malignancy in immunocompetent individuals, suggesting that the increased tumor frequency in this cohort of patients could be primarily due to a defective immune surveillance even in the absence of tumor-promoting HPV infection [29].
Although acquired or iatrogenic immune suppression increases the risk of HNSCC and seems to worsen the prognosis, this malignancy most commonly arises in individuals with a normal and healthy immune system. Indeed, immune surveillance is suggested to clear most preclinical lesions, while immunoediting [30] is the process that characterizes all clinically relevant lesions. This process is thought to play a key role during malignant progression, promoting a selective pressure in the tumor microenvironment that leads to the growth of extremely aggressive neoplastic clones capable of escaping tumor immunity. Indeed, it has long been thought that the immune system functions during tumor formation to select for tumor variants that are better suited to survive in an immunologically intact environment, very much like it does with viruses, bacteria, and parasites [30]. Many studies demonstrate that the repassage of transplantable tumors through immunocompetent hosts generates tumor variants with reduced immunogenicity. Cancer immunoediting is composed of three processes: elimination, equilibrium, and escape. Immunosurveillance occurs during the elimination process, whereas the Darwinian selection of tumor variants occurs during the equilibrium process. This, in turn, can ultimately lead to escape and the appearance of clinically apparent tumors [30]. Indeed, these three processes are not necessarily temporally separated but rather they can coexist.
Although initially immune editing was thought to allow the growth of only those neoplastic clones able to escape immune recognition by losing particular immune-dominant epitopes, by down-regulating the major histocompatibility complex (MHC), or by affecting the antigen processing machinery, it is now clear that the selection of malignant cells with intrinsic immunosuppressive activity is particularly common. Indeed, like many other solid malignancies, almost all HNSCC tumors express or secrete factors that are able to prevent immunological recognition or that can promote apoptosis of tumor-specific T cells. These factors can be expressed on the membrane of neoplastic cells such as in the case of B7-H1 (PDL-1) that is found in the tonsillar crypts, the site of initial HPV infection. In HPV+ HNSCCs, PD-L1 expression on both tumor cells and CD68+ tumor-associated macrophages (TAMs) is geographically localized to sites of lymphocyte fronts [31]. Despite the strong immunogenicity driven by HPV protein, the majority of CD8+ tumor-infiltrating lymphocytes (TILs) express high levels of PD-1 that upon binding to PDL-1 promote T cell anergy, exhaustion, or apoptosis [32]. These findings support a role for the PD-1:PD-L1 interaction in creating an “immune-privileged” site for initial viral infection and subsequent adaptive immune resistance once tumors are established. In addition to PDL-1, HNSCC tumors can also express other molecules that promote tumoricidal T cell apoptosis. For example, these tumors can express FAS-L [33, 34] or TRAIL [35] that, upon engagement with the cognate receptors on T cells, induces the apoptosis of tumor-specific lymphocytes [35].
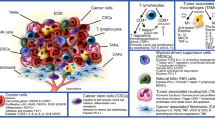
Membrane expression of molecules able to promote T cell apoptosis is not the only immunosuppressive mechanism that is exploited by HNSCC as a result of the immunoediting pressure. Indeed, it is now evident that tumors can secrete different factors able to alter normal hematopoiesis and to induce the appearance and the recruitment of cells from the innate and adaptive immune system with an intrinsic immunosuppressive and pro-tumoral phenotype (Fig. 1). For example, the vast majority of HNSCC tumors secrete interleukin (IL)-4, IL-6, IL-8, IL-10, granulocytes macrophage-colony stimulating factor (GM-CSF), granulocytes-colony stimulating factor (G-CSF), vascular endothelial growth factor (VEGF), prostaglandin E2 (PGE2), basic fibroblast growth factor (bFGF), and chemokines that are able to shape not only the tumor microenvironment but also distal sites creating de facto a tumor macro-environment that predisposes the host to the neoplastic growth and to metastatic dissemination of tumor cells.
HNSSC–immune system interactions and current immune therapeutic interventions. (A) Tumor antigens released by apoptotic cells are uptaken by immature DC that, after migration to the lymph nodes, (B) mature and cross-present the antigens and expand the tumor-specific effector T cells. Driven by the release of inflammatory molecules (i.e., CCL2), effector T cells migrate to the tumor site where they exert their tumoricidal action. (C) Immunoediting promotes the selection of neoplastic clones able to secrete (D) tumor-derived factors (TDF, i.e., GM-CSF, VEGF) that alter normal myelopoiesis (E) arresting DC differentiation while promoting MDSC and tolerogenic DC accumulation. (F) MDSCs and tolerogenic DCs can inhibit effector T cells directly or indirectly. The direct mechanisms of immunosuppression include the secretion of ROS, nitric oxide, and TGF-β; the depletion of semi-essential amino acids (i.e., l-Arg, Trp); or the inhibition of T cell trafficking by, for example, chemokine nitration. The indirect mechanisms of immunosuppression mediate the expansion of Tregs that further block effector cell function and DC maturation (G) by the secretion of TGF-β and/or IL-10. Additionally, MDSCs promote tumor progression by immune-independent mechanisms such as the promotion of tumor angiogenesis and metastasis by the secretion of metalloproteinases that regulate VEGF bioavailability and tissue modifications. Different therapeutic strategies (in blue) have been developed and are currently being tested in ongoing clinical trials to either restrain tumor immunosuppression or promote tumor immunity
3 Tumor-Derived Factors in HNSCC
Numerous findings indicate that tumor-derived factors (TDFs) greatly influence the interaction between tumor and the host and can orchestrate important changes in the hematopoietic differentiation generating a tumor macro-environment that facilitates the malignant progression and metastasis (Fig. 1). For example, conditioned media from tumor cell lines can inhibit the in vitro differentiation of dendritic cells from their precursors [36]. Normal bone marrow cells could give rise to immunosuppressive elements simply by culturing them for a few days with supernatants from a highly metastatic Lewis lung carcinoma variant [37]. For more than 25 years efforts have been made to identify and understand the role of these TDFs in tumor progression [38–44]. Tumors secrete a large panel of cytokines, chemokines, or other diffusible molecules that, alone or in combination, can induce myeloid derived suppressor cells (MDSC) recruitment and increase their maturation into fully suppressive cells. To date, a number of candidate proteins (discussed below) have been identified in HNSCC.
3.1 Granulocyte Macrophage-Colony Stimulating Factor
Even though GM-CSF has long been considered an immune adjuvant, different evidence has uncovered its dual role in stimulating as well as suppressing the immune system: First, almost 31 % of tested human tumor cell lines (including HNSCC [45]) secreted this cytokine [46]. GM-CSF is also secreted by many mouse cell lines such as squamous cell carcinoma [47], colon and mammary adenocarcinoma [46], and plasmacytoma [48]. Second, its secretion by HNSCC is associated with a negative prognosis [45]. Third, tumor-transduced GM-CSF, administration of recombinant GM-CSF protein, or use of high doses of GM-CSF vaccines are sufficient to recruit MDSCs into the secondary lymphoid organs to suppress antigen-specific CD8+ T cells and promote tolerance [46, 49, 50]. Fourth, the ability of different tumor-conditioned media to promote MDSC differentiation is inhibited by the use of a GM-CSF-neutralizing antibody, and, conversely, MDSCs can be generated in vitro from BM precursors by the use of either GM-CSF and G-CSF or GM-CSF and IL-6 [51, 52]. Fifth, GM-CSF promotes HNSCC cell invasiveness and malignant phenotype in nude mice [53].
GM-CSF has also been shown to elicit powerful immune responses when combined with γ-irradiated tumor cell vaccines, in various mouse models and in the clinical setting [54, 55], which has led to its widespread use as an immune adjuvant to augment antitumor immunity. In the therapy of HNSCC, oropharyngeal mucositis is a painful, often dose-limiting side effect of radiotherapy and chemotherapy [56, 57]. G-CSF and GM-CSF decrease the incidence of mucositis, and GM-CSF directly promotes wound healing of the mucosa [58]. In addition, G-CSF and GM-CSF are used to prevent potentially life-threatening febrile neutropenia. Nevertheless, the survival benefit for patients under adjuvant therapy with G-CSF and GM-CSF is a matter of controversial discussion. While the beneficial effect on neutropenia and mucositis is shown in several clinical trials [59], a large randomized clinical trial in advanced HNSCC even identified adjuvant G-CSF treatment as a poor prognostic factor with reduced locoregional control [60], others have not shown any significant effect of G-CSF and GM-CSF on overall survival or disease-free survival [61] or a beneficial action when GM-CSF was used in conjunction with radiotherapy and an oncolytic virus [62].
To better understand this dual role of GM-CSF, we used a bystander vaccine strategy in which the antigen dose and steric hindrance could be maintained constant while altering the GM-CSF dose to assess the impact of high vs. low concentrations of GM-CSF. While we confirmed the efficacy of low doses of GM-CSF-secreting vaccine, we also defined a threshold above which the vaccine not only lost its efficacy but also resulted in significant in vivo immunosuppression mediated by MDSC recruitment [50]. A systematic analysis of different clinical trials performed with this cytokine suggests that the same phenomenon can take place in humans. Although in some of these studies GM-CSF appeared to help the generation of an immune response, in others no effect or even a suppressive effect was reported. GM-CSF may increase the vaccine-induced immune response when administered repeatedly at relatively low doses (range 40–80 μg for 1–5 days), whereas an opposite effect was often reported at dosages between 100 and 500 μg [63]. These findings support the dual role of GM-CSF on the immune response and highlight several critical parameters such as dose, systemic concentration, and duration of exposure as key factors for GM-CSF effect on the immune system, all of which need to be considered when utilizing GM-CSF as a vaccine adjuvant.
3.2 Prostaglandins (PGEs)
The overexpression of cyclooxygenase (COX)-2 is a frequent event in squamous cell carcinomas of the head and neck [64, 65], and nonsteroidal anti-inflammatory drugs, which are potent inhibitors of COX-1 and COX-2, exert chemopreventive effects on HNSCC cancer development [66]. COX-2 promotes the release of the pro-inflammatory mediator prostaglandin E2 (PGE2), which acts on its cell surface G protein-coupled receptors EP1, EP2, EP3, and EP4. The products of COX2 enzyme activity, prostaglandins and mainly PGE2, have been implicated in tumor-associated subversion of immune functions, since inhibitors of prostaglandin synthesis typically enhanced antitumor immunity. PGE2 is one of the best-characterized and -studied isoform of eicosanoids that possesses both pro-inflammatory and immunosuppressive properties and that is produced during the course of inflammation following cellular stresses, and in response to growth factors, hormones, endotoxin, and inflammatory cytokines, or by growing tumors. Freshly excised solid human tumor cells produce substantially more PGE than established tumor cell lines [67]: interestingly, while primary tumor cell-conditioned media profoundly hampered the in vitro DC differentiation from CD14+ monocytes or CD34+ myeloid precursors, the effects of supernatants derived from established tumor cell lines were minor [67]. Both tumors and MDSCs can actively produce and secrete PGE2. This production and secretion correlate with arginase overexpression, STAT3 and STAT1 phosphorylation, and IL-10 and MIP-2 production, a phenotype typically associated with MDSC suppressive activity [68].
3.3 Interleukin-4 and -13
IL-13 and IL-4 are central T helper 2 (Th2) anti-inflammatory and immunomodulatory cytokines with close structural and biological homology. Both are produced mainly by T and B cells, mast cells, and basophils. In HNSCC these cytokines are produced in the tumor microenvironment by the infiltrating leukocytes [69] and by the tumor itself [70, 71]. The promiscuous receptor for IL-4 and IL-13 (alias IL4R type II) is composed of the IL4Rα chain and IL13Rα1 chain [72], while IL4Rα and the gamma chain (γc), common to the receptors for different members of the cytokine family comprising IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21, associate to compose the IL-4 receptor (alias IL4R type I). Since the IL4Rα chain is the only component that possesses kinase-sensitive tyrosine residues in the cytoplasmic domain, signals from both type I and type II IL4R are transduced by the IL4Rα chain [73]. IL4Rα phosphorylation, upon engagement and dimerization, recruits and phosphorylates STAT6 that dimerizes and migrates to the nucleus to activate the transcription of several proteins including arginase 1 [74]. Interestingly, IL4Rα and IL13Rα are constitutively over-expressed in several HNSCC cell lines and, upon engagement with their cognate ligand, were shown to promote neoplastic cell proliferation [75] suggesting a pleiotropic function of these cytokines in this disease.
IL4Rα expression on MDSCs and monocytes is required for their suppressive phenotype [76] and survival [77], and genetic ablation of this receptor on monocytes and granulocytes is sufficient to revert MDSC-mediated immune suppression in vivo whereas its aptamer-mediated blockade is sufficient to promote MDSCs and TAM apoptosis [77]. MDSC and TAM produce IL-13 and IFN-γ and integrate the downstream signals of these cytokines to trigger the molecular pathways suppressing antigen-activated CD8+ T lymphocytes [76].
3.4 Interleukin-6
High levels of IL-6 have been detected in leukemia, lymphoma, multiple myeloma, melanoma, as well as breast, lung, ovarian, renal cell, and pancreatic cancers [78] and are associated with a poor prognosis. Elevated IL-6 serum levels are found in the majority of HNSCC cancer patients, and its concentration correlates with tumor stage and lymph node status [79]. Because of the role of IL-6 in the acute-phase response in the liver and in the regulation of the systemic immune response, it is believed that high serum levels of the cytokine contribute to weight loss, night sweats, fever, and other systemic symptoms [80].
The physiological activity of IL-6 is complex, producing both pro-inflammatory and anti-inflammatory effects. In addition, IL-6 affects the differentiation of myeloid lineages, including macrophages and DCs, both in vitro and in vivo [81] through the activation of the transcription factor STAT3, which exerts a negative regulatory function on the adaptive and innate immune system during tumor development. Indeed, treatment in vitro with combinations of GM-CSF, G-CSF, IL-6, and IL-13 induces the rapid differentiation of human and mouse bone marrow precursor cells into cells that resemble suppressive MDSC [82, 83].
Beside its role on MDSC differentiation, IL-6 has an important function on dendritic cell differentiation. Indeed, tumor-derived factors can inhibit the generation of DC [84]. Dendritic cell differentiation can be restored by the use of VEGF- and/or IL-6-specific antibodies that neutralize this inhibitory effect [85].
3.5 Vascular Endothelial Growth Factor
Increased expression of VEGF and its receptors in HNSCCs underscores the importance of the VEGF pathway in angiogenesis and survival of tumor cells under hypoxic conditions [86]. VEGF expression is regulated by hypoxia-inducible factor-1α (HIF-1α)-dependent and -independent processes, both of which involve PI3-K and AKT [87]. VEGF plays an important role in the formation of blood vessels during embryogenesis, hematopoiesis, and tumor neovascularization [88]. It is secreted by most tumors, and high levels correlate with a poor prognosis [89]. Neutralizing antibodies against VEGF restored DC differentiation from hematopoietic precursors blocked by tumor-conditioned media [90]. VEGF has been directly linked with the systemic MDSC expansion. The administration of recombinant VEGF to tumor-free mice, in fact, resulted in inhibition of DC development and was associated with an increase in the number of MDSCs in the spleen [90]. Besides playing a direct role in tumor angiogenesis, this factor promotes cross talk between tumor and tumor-associated MDSCs [91]. By expressing high levels of matrix metalloproteinase 9, tumor-associated MDSCs regulate the bioavailability of VEGF by releasing it from the extracellular matrix [92], suggesting the presence of a positive feedback loop by which MDSCs increase VEGF release that in turn promotes MDSC differentiation and expansion. MMP9 inhibition by amino-biphosphonates significantly decreased MMP-9 expression and the number of macrophages in tumor stroma and reduced MDSC expansion both in bone marrow and peripheral blood [93]. In HNSCC, VEGF-A expression correlated with microvessel density, disease progression, a reduced number of mature DCs, and an increased number of immature DCs and MDSC, confirming the importance of this factor in the progression of this malignancy [94]. These findings underlie the importance of the VEGF–MDSC connection in HNSCC and suggest that treatments aimed to block MDSC or VEGF should have an effect on both tumor immunosuppression and angiogenesis.
3.6 Chemokines
Chemokines are leukocyte chemoattractants that are usually classified into two main subgroups: the inflammatory and the homeostatic. While the first group promotes leukocyte infiltration at the inflammation site and is inducible by proinflammatory cytokines, the homeostatic chemokines are constitutively expressed and regulate hematopoiesis and lymphoid organ development [95]. Both classes of chemokines can play a role in various aspects of malignancies. Because of the importance of leukocytes in HNSCC outcome, it is not surprising that CCL-3, CCL-4, and CCL-5 [96] are expressed by HNSCC tumors and that CCL-2 [97] and its receptor have been proposed as genetic markers for oral squamous cell carcinoma [98]. Similarly, the expression of CXC chemokine receptor 2 (CXCR2) is increased in the laryngeal squamous cell carcinoma, and its expression correlates with the lymph node metastases, with the histological grade, and with 5-year survival [99]. Additionally, CXCR4 was shown to be important in HNSCC tumor progression and organ-specific metastasis [100, 101] and could be used as a prognostic marker [102].
Approximately 50 chemokines and 20 receptors have been identified to date, and they can interact in a complex network in which the signal can be differentially integrated in each target cell (depending on the particular chemokine receptor profile). Each chemokine can bind multiple receptors, and a receptor can be activated by different chemokines, allowing chemokine redundancy [103], robustness [103], integration [104], and synergy [105]. While the initially attributed importance of redundancy was challenged by subsequent studies [106], the significance of signal integration, robustness, and synergy are being confirmed and explored by numerous studies. Chemokines can form homo- and hetero-dimers, integrating or modulating their own signal and the one from the dimeric partner [107, 108]. For example, CXCL4 can form a heterodimer with CCL5 that promotes monocyte arrest [109], while each chemokine alone is chemotactic. Chemokine integration and synergy can also involve the binding of different chemokines in the same cell that can result not only in the activation of the different individual pathways mediated by each chemokine but also in the integration of both transduced signals [110–113]. In human monocytes, CCL21 engagement of chemokine receptor (CCR)-7 dramatically amplifies the effect of CCL2 binding to CCR2 [114]. Because of the signal complexity and the different effects on the target cell population, it is not surprising that individual chemokines are reported to have opposite effects on tumor outcome. For example CCL5 in Ewing sarcoma [115] can promote tumor immunity by recruiting T cells at the tumor site, whereas in other cancers it is thought to inhibit tumor immunity and promote tumor angiogenesis and metastasis through MDSC and macrophage (tumor-educated myeloid cells (TEMCs)) recruitment [116, 117]. Interestingly, the antitumor effect of adoptively transferred T cells is increased by CCL5 but only when intratumoral CD11c+ cells are depleted [118]. Similarly, CCL21 was shown to induce Th1 polarization [119], boost the efficacy of DNA vaccine [120], and promote the antitumor immunity [121]. Nevertheless the very same chemokine is secreted by many human tumors [122], and the expression of its receptor (CCR7) correlates with the metastatic activity in HNSCC [123] and is necessary for the formation of a tolerogenic lymphoid-like organ within the tumor [124]. Although the examples of contradictory roles of the same chemokines are too numerous to be listed here, it is noteworthy to report the opposite roles of CCL2. CCL2 secreted by the majority of solid tumors [125, 126] is able to attract both TEMCs and T cells in the tumor microenvironment and plays an important role in the proper homing into the tumor of adoptively transferred tumor-specific T cells [127]. Nevertheless, CCL2 mediates the recruitment of TEMC to the primary and secondary tumor sites, promoting tumor progression and metastasis [128–131]. Several studies have demonstrated that, contrary to TEMCs, T cells do not freely travel within the tumor but rather they remain trapped in the stroma surrounding the cancer cells [132]. The explanation of this phenomenon was recently clarified: reactive nitrogen species produced by TEMCs and neoplastic cells induce the nitration/nitrosilation of CCL2 that, once nitrosilated, can no longer attract cytotoxic T cells but can still recruit myeloid cells to the tumor [133]. These findings implicate the existence of a protumoral positive feedback mechanism by which TAM and MDSC promote the recruitment of new protumoral myeloid cells while hindering T cell infiltration.
4 Cellular Network of Immune Modulation in HNSCC
It is increasingly clear that tumors enforce strict connections with the surrounding environment creating a “microenvironment” that supports tumor progression. Moreover, by releasing soluble factors and exosomes, neoplastic cells can also condition distant sites, such as bone marrow, to sustain the demand of myeloid cells and precursors necessary for tumor neovascularization and spreading to local and distant anatomical sites. This creates de facto a complex interplay that can be viewed as a tumor-driven “macro-environment.” The recognition that tumor macro- and microenvironments play pivotal roles in tumor progression suggests that innovative attempts should be made to block tumor/environment interactions that facilitate tumor progression and to enhance those counteracting malignancy.
Cancers are not only a mass of neoplastic cells; instead, they contain several noncancerous stromal cells. In many cases tumor stromal cells, which include TAMs, MDSCs, granulocytes, endothelial cells, fibroblasts, and T cells, may outnumber the malignant cells (Fig. 1) [134]. These accessory cells, most of which are leukocytes, are not innocent bystanders, but, rather, they interact with the malignant cells and play a key role in the disease outcome [135]. Depending on the composition and activation status of the immune infiltrate, the net effect can be either favorable or detrimental to tumor progression and metastasis. While effector T cells (quality and quantity) infiltrating the tumor correlate with a better survival in HNSCC patients [136–138] by either destroying or inducing dormancy in neoplastic cells, the infiltration of tumor-educated myeloid cells is associated with a higher mortality [45, 139].
4.1 Effector T Cells
Effector CD4+ and CD8+ T cells are considered the most important immune cells acting against cancer promotion and progression, as discussed elsewhere in this book. Although patients with HNSCC have reduced number of lymphocytes in their blood compared with healthy individuals [140], a significant shift from naive to effector memory T cells is observed in patients with oropharynx or larynx squamous cell carcinomas with an increased number of effector memory T cells in HPV+ oropharyngeal squamous cell carcinomas [141], suggesting that a strong immune response against the tumor can be generated. Indeed, in recent years extraordinary progress has been made in the identification of tumor-associated antigens (TAA) in HNSCC. For example an analysis of the TILs in HNSCC revealed the prevalence of effector lymphocytes against the TAA cyclin B1 and NY ESO-1 that can be expanded in vitro and potentially used as a treatment modality [138]. Additionally, HNSCCs were found to express melanoma-associated antigens (i.e., MAGE 1 and MAGE 3) [142, 143] and, because of the changes in the epidemiology of this malignancy, viral antigens such as HPV E6 and E7 [144, 145]. Despite the presence of effector/memory tumor-specific T cells, these cells are unable to either reach the tumor or fully perform their tumoricidal action most likely because of the presence of a suppressive network orchestrated by the tumor. This hypothesis is supported by the finding that the functional impairment of T cells from HNSCC conferred by intrinsic molecular defects that have been demonstrated in this cell population can be reversed by removal of immune suppression by a pharmacologic treatment (Serafini, Weed unpublished data) or by radio- or chemotherapy [146]. This reversal is sufficient to reestablish T cell functionality in these tumors.
4.2 Dendritic Cells
Dendritic cells (DCs) are a family of specialized APCs and are essential mediators of immunity and tolerance [147]. DC may derive from the lymphoid (i.e., plasmacytoid DC) or myeloid precursors. While plasmacytoid DCs are mainly found in the blood and in the secondary lymphoid organs, myeloid DCs can infiltrate the dermis (dermal/interstitial myeloid dendritic cells) or the epidermis (Langerhans cells) of the mucosa of the upper aerodigestive tract [148] where they show an immature phenotype and a great capacity to uptake antigens. Upon encountering inflammatory signals (i.e., IL-1, TNF-α) or microbial products (i.e., TLR ligands) they migrate to the secondary lymphoid organs, assuming a mature phenotype and the capacity to cross-present the captured antigens, promoting the priming and the expansion of effector T cells. Because of their immunological role and their localization, myeloid DCs and Langerhans are particularly important in orchestrating the interaction between the immune system and HNSCC. Interestingly, tobacco and alcohol consumption, two main risk factors in HNSCC, are associated with an increased number of oral mucosal Langerhans cells that could suggest an active role of these cells in the initial phase of immunosurveillance [149, 150]. Indeed, their number seems to be higher in benign lesions than in normal mucosa or in neoplastic lesions [151]. Furthermore, their number seems to decrease with tumor grade [152], and, in laryngeal and nasopharyngeal carcinomas, a strong infiltration of Langerhans cells has been associated with longer disease-free survival, less locoregional recurrence [153], and a better prognosis [154]. Similarly, a larger number of DCs was found to be present in nonmetastatic lymph nodes than in metastatic lymph nodes in a series of hypopharyngeal and laryngeal carcinomas [155]. However, molecular defects, an inability to mature, and a reduced number of circulating myeloid DCs are found in patients with HNSCC (discussed in more detail below). The fact that surgical removal of the tumor is sufficient to restore the number and the function of DC [156] highlights that this reduction is due to the presence of tumor, is reversible, and is most likely one of the mechanisms of immune escape in HNSCC patients.
4.3 Myeloid Derived Suppressor Cells and Tumor-Associated Macrophages
MDSCs have been described in patients affected by different tumors. In HNSCC, for example, the release of GM-CSF and the tumor infiltration with CD34+ MDSCs were determined to be negative prognostic factors and were associated with an increased rate of tumor metastasis and recurrence [157]. The increased frequency of CD34+ cells in the PBMCs was also correlated with the suppression of the anamnestic responses to recall antigens, a frequent finding in HNSCC patients [158]. A more extensive study of the peripheral blood of patients with HNSSC, breast cancer, and non-small-cell lung cancer better characterized the phenotype of MDSCs that were described as immature cells positive for the markers CD34, CD33, and CD13 but negative for the myelomonocytic marker CD15. We recently confirmed these markers as associated with MDSCs in HNSCC and determined also that IL4Rα+ CD33+ cells are the most immunosuppressive myeloid cell subsets in these patients (Serafini, Weed, unpublished data). Although the murine counterpart of MDSC is characterized by the expression of the markers CD11b and Gr1, murine MDSCs share many functional features with human MDSC (such as the expression of the functional marker IL4Rα, the immature phenotype, and the molecular mechanisms of immunosuppression) and have allowed the dissection of the molecular mechanisms employed to restrain the immune response. These mechanisms were shown to utilize either the metabolism of l-arginine (l-Arg) or TGF-β production to render lymphocytes unresponsive to antigen stimulation. l-Arg is metabolized in myeloid cells (macrophages, granulocytes, and DCs) by two enzymes: (1) nitric oxide synthase (NOS), which oxidizes l-Arg in two steps that generate NO and citrulline, and (2) arginase (ARG), which converts l-Arg into urea and l-ornithine [159, 160]. By up-regulating Arg1 and consuming l-Arg in the surrounding microenvironment MDSC inhibited re-expression of the ζ-chain of CD3 complex in T lymphocytes, thereby impairing their function [161]. Alternatively, by NOS2 up-regulation, MDSC can S-nitrosilate, on T cells, crucial cysteine residues of important signaling proteins in the IL-2-receptor pathway including JAK1, JAK3, STAT5, ERK, and AKT [162]. S-nitrosilation makes T cells unresponsive to IL-2 inhibiting their proliferation and effector function [162]. Furthermore MDSC can express both Arg1 and NOS2. In these conditions MDSCs produce high quantities of peroxynitrite [163] that can induce either apoptosis [163] or anergy [164] in activated T cells and promote the nitration of particular chemokines affecting T cell infiltration into the tumor [133]. This hypothesis is further confirmed by the presence of NOS [165] and peroxynitrate metabolites [166] in the tumor bed of HNSSC patients. These observations are of clinical importance, because a reduced T cell proliferative capability to mitogenic stimulation has been associated with a poorer outcome for patients with HNSCC [167]. Moreover, the maturation of dendritic cells in patients with HNSCC is impaired [168] and associated with an increase of immature CD34+ MDSC in the blood and in the tumor bed [169]. These observations are supported by murine data in which inhibition of DC maturation correlates with MDSC accumulation in the blood [170]. Attempts to overcome the immune dysfunction of patients with HNSCC have included combining in vivo immunization with autologous, irradiated HNSCC plus GM-CSF [171, 172]. Such treatments of patients with recurrent and metastatic HNSCC disease have shown the capacity to stimulate in vitro antitumor immune reactivity. However, in most cases the in vitro antitumoral activities of these T cells do not translate with a tumoricidal activity in vivo. These paradoxical results can be explained by the immunosuppressive network generated by the tumor that prevents CTL activities in vivo. These considerations are consistent with the preclinical data demonstrating that tumor-associated MDSC can induce anergy or apoptosis in tumor-specific T cells [173–175]. Similar inhibitory CD34+ cells are also present within the cancer mass of patients with HNSCC where they can inhibit the activity of intratumoral T cells [157, 176–178].
The pro-tumoral activity of MDSCs and TAMs is not limited to their immunosuppressive role. Upon activation, these leukocytes secrete matrix-remodeling proteases and serine proteases that are associated with more advanced tumor grade and metastasis [179–181]. Additionally, following IL4Rα engagement, TAMs and MDSCs express elevated levels of the cysteine protease cathepsin B and expression of this protease is found within macrophages at the invasive edge of pancreatic cancers [179–181]. Metalloproteinase (MMP) and cathepsin B secretion by TAMs and MDSCs are partially regulated by IL-6 [182]. It is important to note that this cytokine, in concert with GM-CSF, is one of the key elements that regulate MDSC differentiation [52] and levels of both are particularly elevated in the sera of HNSCC [183]. In particular, GM-CSF, G-CSF, and IL-6 allowed a rapid generation of MDSCs from precursors present in mouse and human bone marrow (BM). These cytokines induce the activation of C/EBPβ in the myeloid lineage, a transcription factor necessary for MDSC differentiation. Genetic inactivation of this factor in the myeloid lineage blocked MDSC differentiation and reestablished the efficacy of antitumor immune interventions [52].
4.4 Regulatory T Cells
Tregs share the capacity to induce antigen-specific T cell tolerance and play an important role in preventing the development of autoimmune responses [184]. The very same cells are recruited by growing tumors to protect themselves from the immunological assault. In fact, the in vivo depletion of CD4+CD25+ T cells by anti-CD25 antibody (PC61), prior to tumor challenge, enhances tumor immunosurveillance and induces the rejection of multiple immunogenic tumors in different mouse strains [185]. Phenotypically, Treg cells express CD4 and CD25 and the functional marker forkhead/winged helix transcription factor (FoxP3) [186, 187]. Functionally, Treg cells inhibit T cell activity through their production of soluble inhibitory mediators such as TGF-β and IL-10 [187, 188]. Increased levels of CD4+CD25+FoxP3+ Treg have been shown in the peripheral blood of patients with HNSCC [186] and have been associated with poor prognosis [189–191]. Tregs localizing to tumor tissue in HNSCC comprise a unique subset of CD4+CD25high Foxp3+ T cells, which secrete IL-10 and TGF-β1 and mediate a strong suppressor function [192]. Studies with patients with hepatocellular carcinoma showed increased levels of Treg within tumor tissue compared with normal tissue and increased levels of TGF-β expression in the peripheral blood of these patients compared with controls [193, 194]. The presence at the tumor site of these CD4+CD25highFOXP3+ T cells seems to be a characteristic feature of T3/T4-stage HNSCC tumors and can be associated with a poor prognosis [192]. Nevertheless, the extent to which Treg cells contribute to the immune depression of patients with HNSCC is still unclear. Indeed, contradictory reports exist on the role of Treg in patients with oral cavity carcinoma: while initial studies associate the tumor infiltration of FOXP3+ T cell with a worse prognosis [195, 196], other reports associate the infiltration of FOXP3+ T cells with a better survival [197] or with better locoregional control of the tumor [198]. No significant associations were found in other studies [199]. Although technical differences in the Treg quantification (i.e., the antibody used, scoring system, number of markers) may explain these contradictory reports, the role of biological components also needs to be considered. Indeed, it is known that, contrary to murine Treg, human T cells may transiently express FOXP3 upon activation [200]. In this case, FOXP3 expression is not indicative of a regulatory function but, instead, of either incompletely activated effector cells [201, 202] or activated memory effector T cells [202]. Thus, although the effect of FOXP3 on activated T cells may be to down-regulate some of their effector functions, its expression could identify two distinct subsets of TILs with opposite effects on tumor outcome. An important breakthrough can derive from the work of Magg et al. [203] demonstrating that activated human effector T cells express FOXP3 mainly in the cytoplasm whereas Tregs are characterized mostly by a nuclear localization of this important transcription factor [203]. In accordance with this observation we recently found in patients with oral tongue SCC that the presence of CD4+ cells expressing FOXP3 in the cytoplasm is associated with a favorable prognosis whereas its nuclear localization correlates with the risk of recurrence [204].
5 Immunologic Defects in HNSCC Patients
The impact of HNSCC on immune function is underscored by numerous molecular defects and alteration induced by the tumor in the immune system of the patients. The impact of HNSCC on immune function is underscored by the reduced number of CD3+, CD4+, and CD8+ T cells (with CD3+CD4+ cell levels being more prominently reduced in patients with active disease) in the peripheral blood [205]. Even after curative surgery these levels remain low for several years further highlighting the profound consequences of this tumor on the immune system. The mechanisms by which HNSCC reduce T cell number seem to be multiple and complex. The induction of apoptosis can explain a reduction in the number of tumor-specific T cells. Indeed, neoplastic and stromal cells can trigger different pro-apoptotic signaling on T cells by the engagement of FAS by FAS-L or of DR4 and 5 by TRAIL [2]. Alternatively MDSCs and TAMs were shown to promote T cell apoptosis by different mechanisms that include nitration, deprivation of semi-essential amino acids, or production of reactive oxygen species [83]. Nevertheless additional mechanisms mediated by the tumor macro-environment also seem to affect the T cells with a specificity different from the tumor. Indeed, an increase in the ratio between the pro-apoptotic protein BAX and the anti-apoptotic factor Bcl-2 is found in most of the CD8 T cells of HNSCC patients [34], suggesting that apoptosis is induced in T cells regardless of the specificity. The defects associated with T cells in HNSCC regardless of their intrinsic specificity can be also partially explained by the changes in the cytokine macro-environment induced by the tumor. Indeed, compared with plasma cytokine levels of age-matched controls, cytokine levels in HNSCC patients demonstrated a shift to a Th2 bias with an increase of IL-4, IL-6, and IL-10 and a reduction of IFN-γ [69]. IFN-γ, besides being extremely important for many immunological processes (i.e., increasing antigen presentation, promotion of Th1 and CTL activity, induction of MHC class I), can inhibit the expression of BAX in T cells upon engagement of the beta chain of its receptor [206]. Thus, a significant and prolonged reduction of this cytokine could increase BAX expression, promoting T cell apoptosis. Interestingly, although the number of IL-2+CD4+ and CD8+ T cells seems to be reduced in HNSCC patients [207], the serological level of IL-2 seems to be higher in this cohort of patients [69], partially excluding a role for IL-2 deprivation in T cell apoptosis.
Besides the reduced number of T cells, intrinsic molecular defects are detectable in the T cells of HNSCC patients. For example, lymphocytes of HNSCC patients show a profound down-regulation of the ζ-chain of the CD3 complex, a low responsiveness to IL-2 [208], and a reduced proliferative capability to mitogenic stimulation. The degree of reduction to the mitogenic stimula correlates with a poorer outcome for patients with HNSCC [167]. Considering that both CD3 ζ-chain down-regulation (by l-Arg deprivation) and IL-2 unresponsiveness (by STAT5 nitration) are two of the mechanisms by which MDSCs control the immune response [209], these observations can highlight the role of MDSC accumulation in the immunological defects observed in HNSCC.
HNSCC not only alters T cells but, as mentioned above, also has important consequences on the DC and myelopoiesis. Indeed, defects in DC function are considered a hallmark of immune system dysfunction in HNSCC [210]. For example, the accumulation of histiocytes/DCs in the distended sinuses of lymph nodes is a reflection of DC defects and is present in the lymph nodes of HNSCC patients. The buildup of these cells in the nodal sinuses prevents their entry into the node parenchyma, and maturation is, therefore, impaired, preventing optimal T cell stimulation [211]. A drastic reduction of circulating DC is also observed in HNSCC: while patients with early disease show a twofold decrease in circulating DC, patients with advanced disease show a fourfold reduction [168]. The decreased number of circulating DC seems to be confined to the myeloid subset, whereas the number of lymphoid DC is not affected [156]. The reduction of DC seems to be dependent on the release of GM-CSF and VEGF by the tumor that hijacks the physiological hematopoietic differentiation, promoting the accumulation of MDSCs and immature DCs in the lymphoid organs and at the tumor site [91]. The accumulation at the tumor site can be explained by the impaired migratory function of DC in hypoxic conditions [212] and by the presence of extracellular adenosine that characterize the tumor microenvironment. Under hypoxic conditions, DC not only failed to migrate in the lymph node but also acquired the chemokine receptor profile necessary for homing to the peripheral tissue. Moreover, hypoxia reduced DC maturation [212] and antigen uptake capability [213]. Finally, the up-regulation of the hypoxia-inducible factor 1α induced the expression of the adenosine receptor A2B that, once engaged, caused the DC to drive CD4+ T cells toward a Th2 phenotype [213], a characteristic of HNSCC.
6 Immunotherapy for HNSCC
Immunotherapeutic strategies for HNSCC can be broadly categorized as antigen specific or antigen nonspecific (Fig. 1). Antigen-nonspecific therapies are designed to broadly enhance the immune response either by the selective addition of various immune stimulatory cytokines or by strategies to reverse or abrogate the immunosuppression mediated by the tumor. Intuitively it would seem that antigen-specific therapies would be the most powerful, and perhaps the least likely to generate systemic toxicity, due to their tumor cell-specific targeting. Yet it is precisely the host’s own failure to generate a sufficient immune response against the developing tumor that suggests the importance of therapeutic strategies to nonspecifically enhance the immune response against already established tumor antigens. Clinically evident tumors have already survived the processes of immunosurveillance and immunoediting to be by their nature resistant to the immune response. Overcoming this resistance is essential to the success of immunotherapeutic strategies.
6.1 Antigen-Nonspecific Therapies
Antigen-nonspecific therapies may be used alone or in combination with antigen-specific immunotherapies. For example, IL-2 is often used in conjunction with adoptive cell transfer therapies to sustain the vitality and efficacy of transferred CTLs. Moreover, removal of immunosuppression is thought to be extremely important to potentiate the efficacy of antigen-specific immunotherapeutic intervention.
6.1.1 Cytokine Treatment Approaches
Cytokines have been used therapeutically either alone or in combination with chemotherapy, with varying degrees of success. These represent some of the earliest immunotherapeutic strategies employed against HNSCC. Administration of IL-2 to HNSCC patients has resulted in measurable increases in IL-2 levels, an increase in the number of NK cells within the tumor, and an increase in the overall activity of TIL [215, 216]. In both of these studies the cytokine was delivered locally to the tumor by peritumoral [215] or intranodal injection or intraarterial infusion [216]. Injection of recombinant IL-2 around the cervical lymph node chain for 10 days preoperatively in patients with T3-4, N0-3, M0 SCC of the oral cavity or oropharynx who subsequently underwent surgery for definitive tumor resection and radiotherapy resulted in significant increases in disease-free and overall survival with limited toxicity [217]. Systemic infusion of recombinant IL-2 combined with intramuscular or subcutaneous administration of interferon alpha resulted in an 18 % partial response rate (2/11) with substantial associated toxicity [218]. High doses of IL-2 may result in severe systemic toxicities (hypotension, capillary leak syndrome, and oliguria) [215], while at low doses the therapeutic efficacy might not be reached. Specific delivery of IL-2 at the tumor site is being explored in order to reach the therapeutic concentration locally while maintaining tolerable concentration systemically. For example, ALT-801 [216], a fusion protein composed of IL-2 and a TCR specific for the p53 (aa264-272)/HLA-A*0201 complex, is a new drug that moves toward this direction that targets IL-2 to the tumor cells overexpressing p53. Its use, in patients with different malignancies including head and neck cancer, seems to suggest a higher efficacy and a lower toxicity than high doses of IL-2 [216]. Interferon alpha has been used in combination with cisplatin and 5-fluorouracil in the treatment of advanced esophageal carcinoma (SCC and adenocarcinoma), with an overall response rate of 55 and a response rate of 61 % with esophageal SCC. Significant toxicities were reported [219]. Interferon gamma infused intravenously over a 24-h period once weekly for four infusions resulted in measurable response in three of eight patients with advanced resectable HNSCC with minimal toxicity noted and with histopathologic changes attributed to the therapy noted at the time of resection [220]. Intratumoral administration of recombinant IL-12 in patients with HNSCC resulted in a significant activation of B cells and the B cell compartment, with the presence of tumor-infiltrating B cells correlating with overall survival in 30 patients studied (irrespective of IL-12 treatment) [221]. IL-12 intratumoral administration resulted in an increase in the number of B cells and B cell proliferation in regional lymph nodes, a measurable increase in B cell interferon gamma mRNA expression, and a highly significant IgG subclass switch measured in plasma, indicating a switch to a T helper 1 phenotype [222]. Intratumoral administration of IL-12 resulted in increased number of CD56+ NK cells in the primary tumor with no differences seen in primary tumor infiltration by CD8+ and CD4+ lymphocytes, with increased production of interferon gamma measured in CD56+ NK cells and CD8+ and CD4+ lymphocytes in regional lymph nodes [222]. A more recent strategy undergoing ongoing clinical trials is to employ a cocktail of cytokines administered subcutaneously in preoperative fashion in patients with HNSCC [223–226]. IRX-2 is a primary cell-derived biologic containing physiologic quantities of T helper type 1 cytokines and monokines. Its primary active components are IL-2, IL-1β, IFNγ, and tumor necrosis factor alpha (TNF-alpha), which are combined with zinc (important in the development and function of cellular immunity), indomethacin (activates immune response and reduces immunosuppressive effects of prostaglandins), and low-dose cyclophosphamide (enhances the cell-mediated immune response by depleting and inhibiting immunosuppressive Tregs) as complementary methods to enhance immune responsiveness [225]. Preliminary studies have shown increases in CD3+, CD20+, and CD68+ cells in surgical tumor specimens compared with pretreatment biopsies, with CD3+ T cells localized to intratumoral regions. CD4+ cells were localized to peritumoral areas, while CD8+ T cells were mainly intratumoral. CD20+ B cells were primarily peritumoral, with CD68+ macrophages localized to intratumoral regions [226]. The treatment was well tolerated, with CD3+ lymphocyte infiltration in the surgical specimen having the strongest association with overall survival (all patients were treated without comparison control group) [225, 226].
6.1.2 Reversal of Immunosuppression
While the above-described nonspecific strategies for immune stimulation have the potential to generate increased quantity and quality of antitumor effector cells, their efficacy can be severely limited by tumor-mediated immunosuppression. Indeed, a tumor-specific immune tolerance and a generalized immunosuppression are the main immunological characteristics of HNSCC. Thus, it is not surprising that different anti-immunosuppressive strategies are being developed now that the multiple mechanisms of immunosuppression in HNSCC are being delineated. These strategies are designed to (1) reestablish a micro- and macro-environment favorable for immune surveillance, (2) deplete the suppressive populations that are recruited by the tumor, or (3) block the molecular mechanisms by which the negative regulators of the immune response shield the tumor from immunological recognition.
In the first class of immune therapeutic intervention, the signaling by which the HNSCC promotes the expansion of immune-modulatory cells (i.e., MDSC, Treg) is targeted. For example, it is known that VEGF, PGE2, GM-CSF, IL-6, and other tumor-derived factors activate aberrant intracellular pathways (i.e., IL6st, STAT3) in the myeloid lineage, expand the pool of MDSCs, and prevent DC maturation. Thus inhibition of these intracellular pathways can be a strategy to revert the suppressive tumor macro-environment and restore effective immune surveillance. Sunitinib for example is a small molecule that inhibits multiple tyrosine kinases (VEGFR-1, VEGFR-2, VEGFR-3, PDGFR, c-kit, ret, and STAT3) and that has shown potent effects against MDSC in both animal models and human studies [227]. Clinical studies in advanced renal cell carcinomas have found reversal of MDSC accumulation in addition to tumor cell apoptosis in sunitinib-treated patients [228]. However, a clinical trial using sunitinib in HNSCC demonstrated important hematological toxicities (i.e., lymphopenia, neutropenia, and thrombocytopenia) or bleeding complications in many patients [229] and poor therapeutic efficacy [230, 231]. Considering the capacity of this molecule to inhibit multiple tyrosine kinases and the fact that some of these pathways need to be transiently activated during normal myelopoiesis and lymphopoiesis, these results are not completely surprising. Nevertheless positive antitumor results were obtained when sunitinib was administered in conjunction with image-guided radiotherapy for the treatment of patients with oligometastases [232]. Thus, despite the intrinsic toxicity (that could be significantly reduced by a nanotargeted delivery in the future) sunitinib may yet maintain its therapeutic promise as a component of a possible multimodal therapy or in situations of minimal residual disease.
1α,25-dihydroxyvitamin D3 is another compound that may have important therapeutic potential in the treatment of HNSCC. This well-tolerated vitamin has previously been shown to induce, in vitro, the maturation of immune-suppressive CD34+ MDSC into immune-stimulatory dendritic cells [233, 234]. Further studies demonstrate that patients treated with 1α,25-dihydroxyvitamin D3 for 21 days before surgery had reduced intratumoral levels of MDSCs, an increased level of mature dendritic cells, and a higher number of effector CD4+ and CD8+ T cells infiltrating the tumor and expressing the early activator marker CD69. More importantly, this short presurgical treatment was sufficient to double the time of HNSCC recurrence in the treated patients [235]. Interestingly, these antitumor effects of 1α,25-dihydroxyvitamin D3 were characterized by a profound modulation of the cytokine concentration in the plasma and in the tumor specimen [236]. Although induction of MDSC differentiation into immune-stimulatory DC may be one of the mechanisms that promotes the immunomodulatory activity of 1α,25-dihydroxyvitamin D3, other actions might be involved. Indeed, 1α,25-dihydroxyvitamin D3 has been shown to inhibit tumor angiogenesis in vivo and the production of VEGF and hypoxia factor 1α in many human tumor cell lines [237]. Since both VEGF and HIF1α are implicated in the induction of MDSCs by the tumor, it is possible that by modulating the transcriptome profile of neoplastic cells, 1α,25-dihydroxyvitamin D3 deprives tumors of those elements that allow for their escape from immune surveillance.
Another agent that has been used to reverse immune suppression in HNSCC belongs to the class of cyclooxygenase-2 (COX2) inhibitors. As we mentioned above, COX2 is overexpressed in the tumor microenvironment and its expression correlates with a poor prognosis in HNSCC [65]. COX2 expression is localized both in the neoplastic cells and in the surrounding stroma [65], and by producing PGE2 it is able not only to activate the suppressive phenotype in the MDSCs but also to facilitate the differentiation of MDSCs from their hematopoietic precursors [68]. Thus, COX2 inhibition can be seen as an important opportunity to reverse HNSCC-induced immune suppression by blocking both MDSC differentiation and activation (i.e., by inducing arginase 1 and iNOS [238]) at the tumor site. Interestingly, celecoxib (a specific COX2 inhibitor) has demonstrated antitumor activity in advanced HNSCC when used in combination with erlotinib (a specific inhibitor of EGFR) and in combination with radiotherapy. In combination with erlotinib, 25 % of the treated patients with unresectable recurrent locoregional and/or distant metastatic HNSCC show a partial response and a low toxicity profile [239]. Instead, when the same treatment was used in combination with local irradiation on patients with previously irradiated HNSCC, 60 % of the patients showed locoregional control and 37 % progression-free survival at 1 year [240]. Celecoxib unfortunately was also found to be associated with a dose-dependent cardiovascular morbidity, which limited its dosage and prevented its long-term use in reversing tumor-induced immunosuppression [241–243].
In addition to the use of drug to restrain MDSC differentiation, other strategies have been developed targeting specifically the suppressive mechanisms by which these cells inhibit antitumor immunity. We previously demonstrate in preclinical models that PDE5 is a key protein that mediates MDSC suppression [175]. This enzyme, by controlling the intracellular concentration of cGMP, directly controls the expression of iNOS, IL4Rα, and arginase 1 on MDSCs [175], thereby controlling their suppressive action. Indeed, pharmacologic inhibition of PDE5 using sildenafil or tadalafil (FDA-approved drugs for the treatment of erectile dysfunction) was sufficient to restrain tumor-induced immune suppression, prime a spontaneous antitumor immune response, and drastically reduce tumor progression in murine models of breast and colon cancer [175]. Furthermore, in a lymphoma model of tumor-induced tolerance, PDE5 was sufficient to restrain the MDSC-mediated expansion of tumor-specific Treg [244]. Finally, and more importantly, when Sildenafil was added to anti-CD3/anti-CD28-stimulated PBMCs from patients with HNSCC and multiple myeloma, PDE5 blockade was sufficient to restore the otherwise repressed T cell proliferation [244]. Based on these results two independent clinical trials (at Johns Hopkins University and at the University of Miami) were started in HNSCC patients to test the immune modulatory capacity of tadalafil daily administration before surgical resection of the primary tumor. Interim analyses in both clinical trials seem to suggest that PDE5 blockade lowers MDSC and Treg concentrations in the blood and in the tumor tissue, promotes the tumor infiltration of activated (CD69+) CD4+ and CD8+ T cells, and expands the systemic pool of tumor-specific T cells. Only reversible negative side effects (back pain) have been found in a small percentage of treated patients suggesting the possible use of PDE5 inhibitors to down-modulate immune suppression in HNSCC (Weed and Serafini unpublished observation).
It is important to remember that MDSCs are not the only mediator of immunosuppression in HNSCC. As described above Tregs are significantly expanded in HNSCC patients, and this cell population is thought to play an important role in tumor-induced T cell anergy, inhibition of DC maturation, and malignant progression [192]. Despite therapies specifically aimed at depleting or inactivating Tregs in HNSCCs that have not yet been clinically tested (or results are still unavailable) there is the indication that low dosage of cyclophosphamide or ifosfamide can selectively deplete Treg in cancer patients [245]. These alkylating agents have been shown to be effective in the treatment of HNSCC even when used as a single agent [246]. Interestingly ifosfamide’s therapeutic efficacy was increased when it was administered in association with cisplatin and 13-Cis retinoic acid, with a response rate of 72 % in distant metastatic HNSCC [247]. In this study a restoration of tumor immunity was hypothesized because of the prolonged response observed once the chemotherapeutic regimen was terminated. In light of the new understanding that 13-Cis retinoic acid can trans-isomerize in vivo to produce all-trans-retinoic acid (ATRA) [248] and that ATRA can force MDSC to differentiate into mature DC [249], it is highly possible that at least part of the beneficial effect of this chemotherapeutic combination was due to the restoration of a correct immunological milieu by MDSC conversion and Treg depletion. Because of the possible beneficial role that cyclophosphamide-dependent Treg depletion can act in synergy with other therapies in head and neck cancer, different trials are currently ongoing, but results are still unavailable.
In summary, the better understanding of the cellular and molecular mechanisms that regulate tumor immunosuppression in HNSCC and the technical advances in drug isolation and development are offering many strategies for the immunomodulation of tumor immunity. Nevertheless, many of the identified pathways are implicated in normal physiological activity, and thus their inhibition may lead to extremely serious side effects. A specific targeting using nanoparticle or other similar strategies needs to be developed in order to minimize the negative side effect and maximize antitumor efficacy. It is important to note that despite the coexistence of multiple suppressive pathways, the inhibition of only one is often sufficient to tilt the balance and generate a micro- and macro-environment favorable to an effective immune surveillance.
6.2 Antigen-Specific Approaches
Immune-specific therapeutic strategies seek to harness the natural immune response to tumor by virtue of amplifying one or more of the effector arms of the immune system targeting specific tumor antigens. These strategies include antibody therapies, adoptive cell transfer, and various tumor vaccine approaches. The efficacy of these approaches is likely to be substantially improved when combined with nonspecific immunotherapeutic approaches and particularly with strategies designed to reverse tumor-induced immunosuppression. Additionally, the efficacy of combinations of both antigen-specific and -nonspecific immunotherapeutic strategies with conventional chemotherapy, radiation therapy, and surgical protocols is only beginning to be elucidated.
6.2.1 Antibody Therapies
The most widely studied antibody therapy for HNSCC involves the use of antibodies targeting the epidermal growth factor receptor (EGFR). These strategies are summarized in useful reviews by De Costa and Young [250], Ferris et al. [251], and Russell and Colevas [252]. The EGFR is overexpressed in oral premalignant and malignant lesions [253] and is the most common tumor antigen against which antibody therapies are directed in HNSCC. Other antibody targets include HER2/neu and VEGF. Corresponding therapeutic antibodies include cetuximab (EGFR/HER1), panitumimab (EGFR/HER/1), trastuzumab (HER2/neu), and bevacizumab (VEGF).
The tumor antigens mentioned above are cell surface molecules that have functional capabilities as mediators of signaling pathways responsible for cell growth and endothelial cell proliferation as examples. These receptors are found on normal cells as well as tumor, indicating that toxicity to normal tissues can occur with these therapies. Monoclonal antibody-based therapies can therefore have a dual effect on tumor cells bearing increased expression of these receptor antigens. The signaling pathway initiated by the receptor may be inhibited by antibody blockade, typically a preferred outcome for these tumors. Additionally, cell-mediated cytotoxicity may be initiated by the monoclonal antibody bound to the tumor antigen resulting in tumor cell death. Fortunately treatment with these antibodies does not typically result in severe allergic reactions or systemic toxicity [251]. Treatment with these antibodies alone can result in limited responses, even in advanced pretreated disease [254]. Their efficacy is typically increased in combination with chemotherapy and radiation therapy.
The clinical success of cetuximab has been demonstrated in single agent and combinational phase I studies, showing both good patient tolerance and clinical efficacy [255–257]. The most common side effect of treatment is an acneiform rash. A phase III trial of 424 HNSCC patients randomized to radiation alone or cetuximab and radiation resulted in an increase in locoregional control in the cetuximab group (24.4 months vs. 14.9 months, p = 0.005) [258]. This study, and its relatively limited toxicity, resulted in FDA approval of cetuximab for use in combination with radiation therapy for locally advanced HNSCC in 2006. The longer term follow-up of this trial still showed efficacy of the cetuximab-treated patients, with 45.6 % vs. 36.4 % overall 5-year survival and the notable observation that improved overall survival was seen in patients experiencing at least a grade 2 acneiform rash during treatment [259]. Studies combining cetuximab with both chemotherapy and radiation therapy have noted considerably greater toxicities that become therapy limiting, making demonstration of efficacy difficult over standard platinum-based chemo-radiation strategies [252, 260, 261]. Studies comparing the efficacy of cetuximab combined with radiation therapy vs. cisplatin-based chemoradiation therapy are ongoing [252].
The mechanism of action of monoclonal antibody blockade leading to tumor response and death is likely twofold, as mentioned above. Blockade of signal transduction alone is not likely to yield the clinical benefits described above, with an immunologic mediated cell death being an important component of the success of monoclonal antibody-based therapies as well as a key contributor to the variable responses seen with such therapies [251, 262–264]. In cell culture tumor cell apoptosis does not occur with treatment by monoclonal antibody alone but only when lymphocytes are added to the culture system [251, 265]. Clinical response can be correlated in patients with polymorphisms of monoclonal antibody-binding receptors on NK cells, monocytes, and granulocytes that are known to have lytic activity [251]. Interestingly, the level of EGFR expression, activation, or gene amplification does not correlate with clinical response to monoclonal antibody therapy. This finding suggests that factors other than blockade of signal transduction contribute to the observed clinical effect [251, 266]. Possible explanations for the variability of response are many. The monoclonal antibody isotype subclass plays an important role in the extent of cell-dependent lysis of the target cell. IgG1 and IgG3 subclasses are more efficient than IgG2 and IgG4 subclasses at mediating lysis of target cells [251, 267, 268]. This is clinically relevant as cetuximab is an IgG1 isotype, whereas panitumimab, another monoclonal antibody targeting the EGFR, is an IgG2 isotype and is predicted to have a lower ability to induce cellular immune reactions [251, 265, 269]. Complement-dependent cytotoxicity can be seen with IgG1 isotype monoclonal antibodies, but this mechanism of cell death occurs rapidly and is not consistent with the observed clinical responses seen in cetuximab therapy in HNSCC where tumor shrinkage is noted over weeks. This is also inconsistent with an NK cell-mediated cell death, which should occur over a matter of hours, and is more indicative of a T-lymphocyte-mediated lytic effect [251]. Another factor which may influence clinical response to monoclonal antibody-based therapies includes Fc gamma R polymorphisms, particularly as this relates to the role of NK cell-mediated cytotoxicity, although clinical relevance of such polymorphisms is not well elucidated [251, 267]. It is also possible that an initial antibody-mediated neoplastic cell death and macrophage infiltration result in the release of tumor antigens and increase cross-priming that allows the generation of an adaptive immune response that promotes tumor regression.
Taken together, data regarding antibody-based immunotherapies for HNSCC have clearly demonstrated the greatest clinical efficacy and certainly the most widespread use in clinical practice today. The complexity of interactions that occur at the cellular level with such strategies likely explains the variable responses seen and the lack of clear correlation with antigen (receptor) levels and treatment response. This complexity also argues for further investigation of treatment strategies designed to exploit the immunologic effects of monoclonal antibody-based therapies, in the form of either combined strategies to reverse immunosuppression or perhaps adoptive T cell therapies that may ultimately further strengthen the already established clinical efficacy of these treatments [270].
6.2.2 Adoptive Cell Transfer
Adoptive cell transfer is a therapeutic strategy designed to provide a primed antigen-specific population of effector cells that can result in cell-mediated tumor cytotoxicity. Studies utilizing this method are few, in part due to the technically challenging and cumbersome nature of the therapies. One such trial [171] involved a study of 17 patients with recurrent and metastatic HNSCC who were vaccinated in the thigh with irradiated autologous tumor cells admixed with GM-CSF followed by three additional daily injections of GM-CSF at the vaccination site. Eight to ten days later inguinal lymph nodes draining the vaccine site were resected, and lymphocytes harvested from these nodes were activated with staphylococcal enterotoxin A and expanded in IL-2 in vitro. The cultured cells were then infused back into the patients peripherally as outpatients. 15 patients were successfully infused (2 showed insufficient vaccine response), with the toxic effects of infusion limited to grade 2 reactions in 3 of 16 total treatments. The infused cells were predominately CD3+, a mixture of CD4+ and CD8+ cells. Three patients showed disease stabilization where progression had been evident pre-treatment, with two patients described as having a favorable clinical course [171].
Another adoptive cell strategy [271] was employed utilizing the antibody catumaxomab that binds epithelial cell adhesion molecule (EpCAM) with one arm and CD3+ T cells with its other arm. Peripheral blood mononuclear cells (PBMC) of four patients were collected by leukopheresis, then incubated ex vivo with catumaxomab for 24 h, and cleared and released from cytokines. Each patient received an escalated dose of the opsonized PBMC intravenously at bi-weekly intervals. The opsonized PBMC released significant amounts of IFN-γ and TNF-α in vitro, which was removed prior to administration. Catumaxomab up-regulated CD25, CD69, and CD83 on PBMC, and catumaxomab-loaded PBMC released IFN-γ and granzyme B when coincubated with EpCAM(+)BHY cells. This suggested cell activation and target-directed biological activity. Adverse events were significant at higher doses, but lower doses were well tolerated and one patient showed stable disease at 6 months and one in complete remission at 27 months [271].
A recent study evaluated a bimodal ex vivo expansion method to harvest tumor-specific T cells [272]. TIL bulk cultures were established from primary and recurrent HNSCC in high-dose IL-2. Next selected bulk cultures were rapidly expanded using anti-CD3 antibody, feeder cells, and high-dose IL-2. T cell subsets were phenotypically characterized using flow cytometry. Interferon gamma detection by Elispot and 51Cr release assay was used to determine the specificity and functional capacity of selected TIL pre- and post-rapid expansion. Bulk TIL cultures were expanded in 80 % of the patients included with tumor specificity demonstrated in 60 %. Rapid expansions generated up to 3,500-fold expansion of selected TIL cultures within 17 days. The cultures consisted primarily of T-effector memory cells, with varying distributions of CD8+ and CD4+ subtypes. TCR clonotype mapping demonstrated oligoclonal expanded cultures with 10–30 T cell clonotypes. The TIL from large-scale rapid expansions maintained both functional capacity and contained tumor-specific T cells. This study provides the basis for future clinical trials utilizing this method of ex vivo T cell expansion in adoptive cell transfers in HNSCC [272].
Nevertheless adoptive cell transfer strategies are limited by the need to isolate and expand antitumor reactive lymphocytes that preexist in the patient and often are anergic to the in vitro restimulation [273]. Gene modification of T lymphocytes [217, 225] may overcome the requirement for preexisting tumor-specific immunity. With this strategy, PBMCs from patients are retrovirally transduced with TCR specific for the tumor, thus conferring them with additional specificity for the neoplastic cells before reinfusion. Additional genes that protect the lymphocytes from the tumor-suppressive mechanisms can also be added, making this strategy extremely interesting [220]. As a proof of concept for the therapy, PBMCs from melanoma patients were retrovirally transfected with high-affinity TCR specific for p53–HLA-A2 complex and, as anticipated, were shown to be able to recognize different p53-expressing human tumor cell lines [274]. Considering the importance that p53 antigen plays in HNSCC and that this strategy is already being tested in other malignancies, the use of TCR-transduced PBMCs could be rapidly tested in P53+ head and neck cancer.
Besides the transfer of CTL, the antitumor efficacy of adoptively transferred effector Vα24 NKT cells has been tested in HNSCC [275]. Since NKT cells have an antitumor effect, Yamasaki et al. evaluated the safety and therapeutic efficacy of ex vivo-expanded NKT cells adoptively transferred to ten locally recurrent and operable HNSCC patients. One course of nasal submucosal administration of αGalCer-pulsed APCs and intra-arterial infusion of activated NKT cells via tumor-feeding arteries was given before salvage surgery. Five patients achieved objective tumor regression. The number of NKT cells increased in cancer tissues in seven cases and was associated with tumor regression [275].
Adoptive cell transfer of HNSCC-reactive cells seems to be a promising therapeutic option that could be used in association with anti-immunosuppressive strategies and/or with other standard therapeutic options.
6.2.3 Antitumor Vaccines
The intrinsic genetic instability and the particular etiology of HNSCC result in the expression of both unique and shared TAA by the malignant cells. These differences provide an important therapeutic opportunity to educate the immune system to recognize and destroy the neoplastic cells while preserving the normal tissues. TAA are presented as epitope by the MHC of the cancerous and precancerous cells, allowing their identification by the cytotoxic T cells. Many HNSCC-associated antigens have been identified and characterized, and vaccine strategies aimed to mount an immune response against these antigens are being developed. For example, 71 % of HNSCC express antigens from at least one of the six melanoma antigen genes (MAGE) [142, 276–278]. Additionally, NY-ESO-1, a testis-specific antigen, is highly expressed in HNSCC [279]. Moreover, mutations of normal protein (i.e., p53) are extremely common in HNSCC because they contribute to the malignant phenotype, and these give rise to tumor-specific antigens [62]. Finally, because of the changes in HNSCC epidemiology, the HPV-associated antigens E6 and E7 can be used as epitopes to target the immune response against the tumor [280].
Different strategies (described elsewhere in this book) can be used to promote an immune response against the tumor; however, the clinical evaluation of these strategies in HNSCC remains in its infancy, with few clinical studies available. Nevertheless several lines of evidence make the development of HNSCC-specific vaccines extremely appealing. For example, p53-specific CTL can be expanded in patients with HNSCC [281]. By using autologous DC pulsed with the HLA-A2.1-restricted wt p53264–272 CTL-specific clones could be expanded in vitro and detected in vivo from the PBMCs of many HNSCC patients. Interestingly, while p53-specific T cells could be expanded and detected in vivo from the group of patients whose tumors express low levels of p53, the group of patients whose tumor expresses high levels of p53 showed fewer circulating P53-specific CTLs that only rarely could be expanded in vitro [281]. These data are in line with the hypothesis that immune selection and immune editing might have intervened in the first group selecting those neoplastic clones with a lower p53 expression but also indicate that it might be difficult to raise an immune response in patients in whom immune surveillance has already failed. Nevertheless, in these cases different strategies might be adopted: more immunogenic modified epitope might be used for the immunization or the immunogen can be given with an adequate adjuvant such as on DC co-pulsed with adjuvant helper peptides. Indeed, more immunogenic variants of the p53264–272 are able to rescue the expansion even from the PBMCs of patients with high p53-expressing tumor [282]. Furthermore, an appreciable immune response was observed when autologous DC pulsed with the p53 peptide and a tetanus-derived helper peptide were given as a vaccine in stage I–IVa patients (pts) with HNSCC with no active disease [266]. Vaccination was well tolerated by all HNSCC patients. Increased p53-specific T cells were seen in 11/16 patients (69 %) with positive IFN-γ secretion in 4/16 patients (25 %). Frequencies and absolute number of Treg were significantly decreased after vaccination (p = 0.006). Disease-free survival (85 %) at 24 months of follow-up appeared to be favorable as compared to historical unvaccinated HNSCC patients [283].
6.3 HPV as a Potential Immunological Target
With the recognition of the important role of HPV infection in HNSCC tumorigenesis, particularly in nonsmokers, this expanding subset of patients is a particularly attractive patient population in which to study novel vaccine strategies. One reason for this is that the HPV is an immunoreactive target for which a vaccine has already been established and currently approved for prophylactic use [272]. One strategy for vaccine therapy in HNSCC, therefore, is a preventive one. Vaccination of not just girls but also boys should result in more than an additive effect in the long-term prevention of HPV-related malignancies given that HPV-related malignancies are sexually transmitted diseases [284]. Vaccination programs targeting both boys and girls will likely result in a significant reduction in the overall incidence of HPV-related HNSCC in the future. Nevertheless therapeutic HPV vaccines are also being developed. These vaccines are being designed to target not the HPV capside antigens L1 and L2, that are not expressed once the virus is integrated, but rather E6 and E7 that are constitutively expressed in all levels of epithelium and that are critical for the maintenance of malignant transformation in HPV-infected cells by inactivating the tumor-suppressor protein p53 and retinoblastoma (RB) [280]. Different methods of immunization are currently being tested in cervical and head and neck cancer. These vaccines include the use of HPV-E6 peptides, live attenuated listeria monocytogenes bacteria carrying E7 fusion protein, vaccinia-based vaccines, naked DNA vaccines, and DC-based vaccines. Live attenuated listeria monocytogenes bacteria encoding E6 and E7 vaccines (ADXS11-001) are a relatively new and interesting method of immunization that takes advantage of capacity of listeria monocytogenes to both stimulate the innate immunity and, by naturally infecting the antigen-presenting cells, to promote DC antigen cross-presentation activating both CD4+ and CD8+ T cells [285]. Preclinical data demonstrate that ADXS11-001 vaccine induces the regression of established tumors, by reducing the suppressive activity of Treg and MDSCs at the tumor site, by promoting the chemotaxis and maturation of dendritic cells, and by generating memory effector T cells [286]. An initial phase I clinical trial was performed in a population of refractory cervical cancer patients in which no therapy had been shown to extend survival [287]. Despite the presence of a transient adverse flu-like effect in 100 % of the patients, 73 % of the patients had a performance status ECOG 2–4, 1-year survival increased to 53 % from the historical 5 %, and a significant reduction of tumor size was appreciated in 33 % of the patients [287]. Considering that listeria monocytogenes can be killed by the use of antibiotics, this strategy is extremely promising for the treatment of HPV+ HNSCC. Based on these and other results, a clinical trial started in the UK in which patients with HPV16+ oropharyngeal SCC are being treated with three different doses of ADXS11-001 in addition to the current chemo-, radio-, and/or surgical treatment (T.M. Jones, Liverpool CR-UK Centre, personal communication). Results of this ongoing trial are still unavailable.
Another strategy being explored for inducing a strong immune response against HPV epitope is the use of “Trojan” peptides. Trojan peptide-based vaccines contain a penetrin peptide sequence derived from HIV-TAT which allows the entire peptide to translocate through the cell membrane and penetrate directly into the endoplasmatic reticulum and the Golgi apparatus. There, they can form peptide–HLA complexes without the need of proteosomal processing and TAP transportation. This strategy has been used to vaccinate patients with advanced HNSCC against the TAA MAGE 3 and HPV-16 [288]. In particular the penetrin peptide was fused via furin cleavable linker to MAGE and HPV-16-derived HLA-I- and HLA-II-restricted peptides. Following four immunizations with GM-CSF and Montanide ISA 51 as adjuvant, systemic immune response against the Trojan and the HLA-II-restricted peptides were measurable in most patients whereas the CD8-mediated responses were less pronounced [288]. Interestingly, analysis of the tumor specimen of one patient that underwent surgical resection of the malignancy after treatment revealed great infiltration of MAGE-specific CD4+ and CD8+ T cells (absent in the pretreatment biopsies) and large areas of apoptotic tumor cells [288]. Although this was only a proof-of-concept pilot study, the obtained data strengthen the enthusiasm for the development of tumor vaccines in HNSCC.
6.4 Whole Tumor Vaccines in HNSCC
Since the use of a small number of epitopes in a vaccine formulation can result in further immune editing and relevant antigen lost from tumor, other vaccination methods include the use of the whole tumor with the rationale to target the whole repertoire of tumor antigens. As mentioned above, patients have been vaccinated with autologous irradiated tumor cells and then received adoptive transfer of in vitro-expanded CD4+ and CD8+ lymphocytes harvested from lymph nodes draining the vaccination site [171]. This strategy achieved a limited clinical response while being well tolerated and is an example of one way in which two separate but related types of cellular immunotherapies can be combined in an effort to achieve greater efficacy with appropriate toxicity profile [171]. Another example of a vaccine strategy that utilized autologous tumor cells involved modification of autologous tumor cells harvested at the time of tumor resection with Newcastle disease virus [271]. The treated patients were divided into groups receiving IL-2 alone or IL-2 combined with vaccination with virus-modified autologous tumor. The vaccinated group showed increased levels of tumor-reactive T cells, enhanced antitumor delayed-type hypersensitivity responses, and a prolonged long-term survival that was associated with an increase in immune reactivity [271]. Finally, another strategy exploited to prime an HNSCC-specific immune response is based on the use of oncolytic virus encoding for immune-stimulatory cytokines. Indeed, oncolytic virus therapy is a promising approach to cancer treatment, particularly for the locoregional control of solid tumors. The rationale for the use of these viruses is that they selectively replicate in tumor in a way that tumor cells are killed by lytic virus replication while normal cells are spared. Thus, having the virus encoding immune-stimulatory cytokines such as GM-CSF, the tumor mass is reduced not only by the direct viral effect but also by the release of tumor antigens in an immune-stimulatory environment, inducing a tumor-specific immune response that protects the host from local and distant recurrence.
One of the first examples reported in HNSCC was a phase I clinical trial using an oncolytic herpes simplex virus expressing GM-CSF [289]. In this clinical trial, the virus was injected intratumorally in patients with head and neck cancer, breast cancer, and malignant melanoma that had failed previous therapies. The virus was generally well tolerated with local inflammation, erythema, and febrile responses being the main side effects. Nineteen of 26 patient posttreatment biopsies contained residual tumor, of which 14 showed tumor necrosis, which in some cases was extensive, or apoptosis. The overall responses to treatment were that three patients had stable disease, six patients had tumors flattened, and four patients showed inflammation of un-injected as well as the injected tumor, which, in nearly all cases, became inflamed [289]. Interestingly, in some patients both injected and un-injected lesions became inflamed and flattened over time suggesting that either the virus spread to distant metastases or an important immune response was generated. Additional studies were performed in patients with stage III/IVA/IVB HNSCC in conjunction with chemoradiotherapy (cisplatin plus 70 Gy/35 fractions) and underwent neck dissection 6–10 weeks later. 82.3 % of the patients showed tumor response by Response Evaluation Criteria in Solid Tumors, and pathologic complete remission was confirmed in 93 % of patients at neck dissection. HSV was detected in injected and adjacent un-injected tumors at levels higher than the input dose, indicating viral replication. All patients were seropositive at the end of the treatment. No patient developed locoregional recurrence, and disease-specific survival was 82.4 % at a median follow-up of 29 months [290]. Although an extensive analysis of tumor immunity in head and neck cancer patients treated with oncoviral vector has not yet been performed, similar studies performed in melanoma as well as preclinical studies indicate that the therapeutic potential of oncolytic virus is linked to both innate and adaptive immunity [291]. Indeed, in melanoma patients, regression of untreated lesions has been reported after treatment with oncolytic GM-CSF-encoding virus [291, 292]. In a murine model, tumor-bearing mice treated and cured are resistant to subsequent challenge with the same but not an unrelated tumor [292]. In summary, significant progress is being made in promoting an HNSCC-specific antitumor immunity using a variety of strategies in currently ongoing clinical trials.
7 Conclusion
The promise of immunotherapy in HNSCC remains elusive, yet its realization is closer now than ever. Advances in our understanding of the complex interactions of head and neck squamous cell carcinomas and the immune system have led to innovative immunotherapeutic approaches tested in both preclinical and clinical settings. It is increasingly apparent that efforts to stimulate an effective immune response must be coupled with strategies to abrogate the immune-suppressive environment characteristic of these tumors. Preclinical studies and clinical trials have yielded very promising results and provide the foundation for further refinements in a broad variety of immunotherapeutic strategies targeting all components of the immune system. Combining such approaches with the established treatment options of surgical resection, radiotherapy, and chemotherapy may ultimately yield substantive improvements in overall survival that to date have been lacking. Novel combinations of immunotherapies with traditional therapies may further reduce both disease-related and treatment-related morbidities for this debilitating and deadly disease.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi:10.3322/caac.20138.
Duray A, Demoulin S, Hubert P, Delvenne P, Saussez S. Immune suppression in head and neck cancers: a review. Clin Dev Immunol. 2010;2010:701657. doi:10.1155/2010/701657.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108.
Ho T, Wei Q, Sturgis EM. Epidemiology of carcinogen metabolism genes and risk of squamous cell carcinoma of the head and neck. Head Neck. 2007;29(7):682–99.
Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–9. doi:10.1200/JCO.2007.14.1713.
Ryerson AB, Peters ES, Coughlin SS, Chen VW, Gillison ML, Reichman ME, et al. Burden of potentially human papillomavirus-associated cancers of the oropharynx and oral cavity in the US, 1998–2003. Cancer. 2008;113(10 Suppl):2901–9. doi:10.1002/cncr.23745.
Auluck A, Hislop G, Bajdik C, Poh C, Zhang L, Rosin M. Trends in oropharyngeal and oral cavity cancer incidence of human papillomavirus (HPV)-related and HPV-unrelated sites in a multicultural population: the British Columbia experience. Cancer. 2010;116(11):2635–44. doi:10.1002/cncr.25087.
Hocking JS, Stein A, Conway EL, Regan D, Grulich A, Law M, et al. Head and neck cancer in Australia between 1982 and 2005 show increasing incidence of potentially HPV-associated oropharyngeal cancers. Br J Cancer. 2011;104(5):886–91. doi:10.1038/sj.bjc.6606091.
Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11(8):781–9. doi:10.1016/S1470-2045(10)70017-6.
Chaturvedi AK. Epidemiology and clinical aspects of HPV in head and neck cancers. Head Neck Pathol. 2012;6 Suppl 1:S16–24. doi:10.1007/s12105-012-0377-0.
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi:10.1056/NEJMoa0912217.
Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–301. doi:10.1200/JCO.2011.36.4596.
Coleman N, Birley HD, Renton AM, Hanna NF, Ryait BK, Byrne M, et al. Immunological events in regressing genital warts. Am J Clin Pathol. 1994;102(6):768–74.
de Jong A, van der Burg SH, Kwappenberg KM, van der Hulst JM, Franken KL, Geluk A, et al. Frequent detection of human papillomavirus 16 E2-specific T-helper immunity in healthy subjects. Cancer Res. 2002;62(2):472–9.
Welters MJ, de Jong A, van den Eeden SJ, van der Hulst JM, Kwappenberg KM, Hassane S, et al. Frequent display of human papillomavirus type 16 E6-specific memory t-Helper cells in the healthy population as witness of previous viral encounter. Cancer Res. 2003;63(3):636–41.
Le Bon A, Tough DF. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol. 2002;14(4):432–6.
Williams R, Lee DW, Elzey BD, Anderson ME, Hostager BS, Lee JH. Preclinical models of HPV+ and HPV− HNSCC in mice: an immune clearance of HPV+ HNSCC. Head Neck. 2009;31(7):911–8. doi:10.1002/hed.21040.
Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–39.
Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10(9):909–15.
Burnet M. Cancer; a biological approach. I. The processes of control. Br Med J. 1957;1(5022):779–86.
King GN, Healy CM, Glover MT, Kwan JT, Williams DM, Leigh IM, et al. Increased prevalence of dysplastic and malignant lip lesions in renal-transplant recipients. N Engl J Med. 1995;332(16):1052–7. doi:10.1056/NEJM199504203321602.
Harris JP, Penn I. Immunosuppression and the development of malignancies of the upper airway and related structures. Laryngoscope. 1981;91(4):520–8.
Bhatia S, Louie AD, Bhatia R, O'Donnell MR, Fung H, Kashyap A, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19(2):464–71.
Avital I, Moreira AL, Klimstra DS, Leversha M, Papadopoulos EB, Brennan M, et al. Donor-derived human bone marrow cells contribute to solid organ cancers developing after bone marrow transplantation. Stem Cells. 2007;25(11):2903–9. doi:10.1634/stemcells.2007-0409.
Baker KS, DeFor TE, Burns LJ, Ramsay NK, Neglia JP, Robison LL. New malignancies after blood or marrow stem-cell transplantation in children and adults: incidence and risk factors. J Clin Oncol. 2003;21(7):1352–8.
Curtis RE, Metayer C, Rizzo JD, Socie G, Sobocinski KA, Flowers ME, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case–control study. Blood. 2005;105(10):3802–11. doi:10.1182/blood-2004-09-3411.
Haigentz Jr M. Aerodigestive cancers in HIV infection. Curr Opin Oncol. 2005;17(5):474–8.
Singh B, Balwally AN, Shaha AR, Rosenfeld RM, Har-El G, Lucente FE. Upper aerodigestive tract squamous cell carcinoma. The human immunodeficiency virus connection. Arch Otolaryngol Head Neck Surg. 1996;122(6):639–43.
Moyano S, Ordi J, Caballero M, Garcia F, Diaz A, de Sanjose S, et al. Laryngeal squamous cell carcinoma in HIV-positive patients: lack of association with human papillomavirus infection. HIV Med. 2009;10(10):634–9. doi:10.1111/j.1468-1293.2009.00737.x.
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–8. doi:10.1038/ni1102-991.
Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733–41. doi:10.1158/0008-5472.CAN-12-2384.
Zha Y, Blank C, Gajewski TF. Negative regulation of T-cell function by PD-1. Crit Rev Immunol. 2004;24(4):229–37.
Gastman BR, Atarshi Y, Reichert TE, Saito T, Balkir L, Rabinowich H, et al. Fas ligand is expressed on human squamous cell carcinomas of the head and neck, and it promotes apoptosis of T lymphocytes. Cancer Res. 1999;59(20):5356–64.
Gastman BR, Yin XM, Johnson DE, Wieckowski E, Wang GQ, Watkins SC, et al. Tumor-induced apoptosis of T cells: amplification by a mitochondrial cascade. Cancer Res. 2000;60(24):6811–7.
Kassouf N, Thornhill MH. Oral cancer cell lines can use multiple ligands, including Fas-L, TRAIL and TNF-alpha, to induce apoptosis in Jurkat T cells: possible mechanisms for immune escape by head and neck cancers. Oral Oncol. 2008;44(7):672–82. doi:10.1016/j.oraloncology.2007.08.013.
Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2(10):1096–103.
Young MR, Young ME, Wright MA. Myelopoiesis-associated suppressor-cell activity in mice with Lewis lung carcinoma tumors: interferon-gamma plus tumor necrosis factor-alpha synergistically reduce suppressor cell activity. Int J Cancer. 1990;46(2):245–50.
Pegoraro L, Fierro MT, Lusso P, Giovinazzo B, Lanino E, Giovarelli M, et al. A novel leukemia T-cell line (PF-382) with phenotypic and functional features of suppressor lymphocytes. J Natl Cancer Inst. 1985;75(2):285–90.
Cirillo C, Montaldo P, Lanciotti M, Parodi MT, Castagnola E, Ponzoni M. Immunosuppressive factors produced by a T cell line derived from acute lymphoblastic leukemia. Boll Ist Sieroter Milan. 1988;67(4):295–308.
Lim SH, Worman CP, Jewell A, Goldstone AH. Production of tumour-derived suppressor factor in patients with acute myeloid leukaemia. Leuk Res. 1991;15(4):263–8.
Kunicka JE, Fox FE, Seki H, Oleszak EL, Platsoucas CD. Hybridoma-derived human suppressor factors: inhibition of growth of tumor cell lines and effect on cytotoxic cells. Hum Antibodies Hybridomas. 1991;2(3):160–9.
Young MR, Wright MA, Coogan M, Young ME, Bagash J. Tumor-derived cytokines induce bone marrow suppressor cells that mediate immunosuppression through transforming growth factor beta. Cancer Immunol Immunother. 1992;35(1):14–8.
Moore SC, Shaw MA, Soderberg LS. Transforming growth factor-beta is the major mediator of natural suppressor cells derived from normal bone marrow. J Leukoc Biol. 1992;52:596–601.
Heldin CH. Development and possible clinical use of antagonists for PDGF and TGF-beta. Ups J Med Sci. 2004;109(3):165–78.
Young MR, Wright MA, Lozano Y, Prechel MM, Benefield J, Leonetti JP, et al. Increased recurrence and metastasis in patients whose primary head and neck squamous cell carcinomas secreted granulocyte-macrophage colony-stimulating factor and contained CD34+ natural suppressor cells. Int J Cancer. 1997;74(1):69–74.
Bronte V, Chappell DB, Apolloni E, Cabrelle A, Wang M, Hwu P, et al. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J Immunol. 1999;162(10):5728–37.
Smith CW, Chen Z, Dong G, Loukinova E, Pegram MY, Nicholas-Figueroa L, et al. The host environment promotes the development of primary and metastatic squamous cell carcinomas that constitutively express proinflammatory cytokines IL-1alpha, IL-6, GM-CSF, and KC. Clin Exp Metastasis. 1998;16(7):655–64.
Merchav S, Apte RN, Tatarsky I, Ber R. Effect of plasmacytoma cells on the production of granulocyte-macrophage colony-stimulating activity (GM-CSA) in the spleen of tumor-bearing mice. Exp Hematol. 1987;15(9):995–1000.
Adeegbe D, Serafini P, Bronte V, Zoso A, Ricordi C, Inverardi L. In vivo induction of myeloid suppressor cells and CD4Foxp3 T regulatory cells prolongs skin allograft survival in mice. Cell Transplant. 2011;20:941–54. doi:ct0121adeegbe [pii]10.3727/096368910X540621.
Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64(17):6337–43.
Young MR, Wright MA, Young ME. Antibodies to colony-stimulating factors block Lewis lung carcinoma cell stimulation of immune-suppressive bone marrow cells. Cancer Immunol Immunother. 1991;33:146–52.
Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32(6):790–802. doi:S1074-7613(10)00202-5 [pii]10.1016/j.immuni.2010.05.010.
Gutschalk CM, Herold-Mende CC, Fusenig NE, Mueller MM. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor promote malignant growth of cells from head and neck squamous cell carcinomas in vivo. Cancer Res. 2006;66(16):8026–36. doi:10.1158/0008-5472.CAN-06-0158.
Dranoff G. GM-CSF-based cancer vaccines. Immunol Rev. 2002;188:147–54.
Dranoff G. GM-CSF-secreting melanoma vaccines. Oncogene. 2003;22(20):3188–92.
American Society of Clinical Oncology. Recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. J Clin Oncol. 1994;12(11):2471–508.
Carl W, Havens J. The cancer patient with severe mucositis. Curr Rev Pain. 2000;4(3):197–202.
Biron P, Sebban C, Gourmet R, Chvetzoff G, Philip I, Blay JY. Research controversies in management of oral mucositis. Support Care Cancer. 2000;8(1):68–71.
Palmeri S, Leonardi V, Danova M, Porta C, Ferrari S, Fincato G, et al. Prospective, randomized trial of sequential interleukin-3 and granulocyte- or granulocyte-macrophage colony-stimulating factor after standard-dose chemotherapy in cancer patients. Haematologica. 1999;84(11):1016–23.
Staar S, Rudat V, Stuetzer H, Dietz A, Volling P, Schroeder M, et al. Intensified hyperfractionated accelerated radiotherapy limits the additional benefit of simultaneous chemotherapy—results of a multicentric randomized German trial in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50(5):1161–71.
Papaldo P, Lopez M, Cortesi E, Cammilluzzi E, Antimi M, Terzoli E, et al. Addition of either lonidamine or granulocyte colony-stimulating factor does not improve survival in early breast cancer patients treated with high-dose epirubicin and cyclophosphamide. J Clin Oncol. 2003;21(18):3462–8. doi:10.1200/JCO.2003.03.034.
Rapidis AD, Wolf GT. Immunotherapy of head and neck cancer: current and future considerations. J Oncol. 2009;2009:346345. doi:10.1155/2009/346345.
Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol. 2006;18:226–32.
Nathan CO, Leskov IL, Lin M, Abreo FW, Shi R, Hartman GH, et al. COX-2 expression in dysplasia of the head and neck: correlation with elF4E. Cancer. 2001;92(7):1888–95.
Itoh S, Matsui K, Furuta I, Takano Y. Immunohistochemical study on overexpression of cyclooxygenase-2 in squamous cell carcinoma of the oral cavity: its importance as a prognostic predictor. Oral Oncol. 2003;39(8):829–35.
Hoshikawa H, Goto R, Mori T, Mitani T, Mori N. Expression of prostaglandin E2 receptors in oral squamous cell carcinomas and growth inhibitory effects of an EP3 selective antagonist, ONO-AE3-240. Int J Oncol. 2009;34(3):847–52.
Sombroek CC, Stam AG, Masterson AJ, Lougheed SM, Schakel MJ, Meijer CJ, et al. Prostanoids play a major role in the primary tumor-induced inhibition of dendritic cell differentiation. J Immunol. 2002;168(9):4333–43.
Serafini P. Editorial: PGE2-producing MDSC: a role in tumor progression? J Leukoc Biol. 2010;88(5):827–9. doi:10.1189/jlb.0510303.
Lathers DM, Achille NJ, Young MR. Incomplete Th2 skewing of cytokines in plasma of patients with squamous cell carcinoma of the head and neck. Hum Immunol. 2003;64(12):1160–6.
Pries R, Thiel A, Brocks C, Wollenberg B. Secretion of tumor-promoting and immune suppressive cytokines by cell lines of head and neck squamous cell carcinoma. In Vivo. 2006;20(1):45–8.
Squarize CH, Castilho RM, Sriuranpong V, Pinto Jr DS, Gutkind JS. Molecular cross-talk between the NFkappaB and STAT3 signaling pathways in head and neck squamous cell carcinoma. Neoplasia. 2006;8(9):733–46. doi:10.1593/neo.06274.
Terabe M, Park JM, Berzofsky JA. Role of IL-13 in regulation of anti-tumor immunity and tumor growth. Cancer Immunol Immunother. 2004;53(2):79–85.
Keegan AD, Nelms K, White M, Wang LM, Pierce JH, Paul WE. An IL-4 receptor region containing an insulin receptor motif is important for IL-4-mediated IRS-1 phosphorylation and cell growth. Cell. 1994;76(5):811–20.
Gray MJ, Poljakovic M, Kepka-Lenhart D, Morris Jr SM. Induction of arginase I transcription by IL-4 requires a composite DNA response element for STAT6 and C/EBPbeta. Gene. 2005;353(1):98–106.
Kawakami K, Leland P, Puri RK. Structure, function, and targeting of interleukin 4 receptors on human head and neck cancer cells. Cancer Res. 2000;60(11):2981–7.
Gallina G, Dolcetti L, Serafini P, Santo CD, Marigo I, Colombo MP, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8 T cells. J Clin Invest. 2006;116(10):2777–90.
Roth F, De La Fuente AC, Vella JL, Zoso A, Inverardi L, Serafini P. Aptamer-mediated blockade of IL4Ralpha triggers apoptosis of MDSCs and limits tumor progression. Cancer Res. 2012;72(6):1373–83. doi:10.1158/0008-5472.CAN-11-2772.
Trikha M, Corringham R, Klein B, Rossi JF. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res. 2003;9(13):4653–65.
Riedel F, Zaiss I, Herzog D, Gotte K, Naim R, Hormann K. Serum levels of interleukin-6 in patients with primary head and neck squamous cell carcinoma. Anticancer Res. 2005;25(4):2761–5.
Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for translational therapeutics. Cancer. 2007;110(9):1911–28. doi:10.1002/cncr.22999.
Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol. 2004;173(6):3844–54.
Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32(6):790–802. doi:S1074-7613(10)00202-5 [pii]10.1016/j.immuni.2010.05.010.
Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–68. doi:10.1038/nri3175.
Ratta M, Fagnoni F, Curti A, Vescovini R, Sansoni P, Oliviero B, et al. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood. 2002;100(1):230–7.
Hayashi T, Hideshima T, Akiyama M, Raje N, Richardson P, Chauhan D, et al. Ex vivo induction of multiple myeloma-specific cytotoxic T lymphocytes. Blood. 2003;102(4):1435–42.
Shang ZJ, Li ZB, Li JR. VEGF is up-regulated by hypoxic stimulation and related to tumour angiogenesis and severity of disease in oral squamous cell carcinoma: in vitro and in vivo studies. Int J Oral Maxillofac Surg. 2006;35(6):533–8. doi:10.1016/j.ijom.2005.09.006.
Liang X, Yang D, Hu J, Hao X, Gao J, Mao Z. Hypoxia inducible factor-alpha expression correlates with vascular endothelial growth factor-C expression and lymphangiogenesis/angiogenesis in oral squamous cell carcinoma. Anticancer Res. 2008;28(3A):1659–66.
Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62.
Toi M, Kondo S, Suzuki H, Yamamoto Y, Inada K, Imazawa T, et al. Quantitative analysis of vascular endothelial growth factor in primary breast cancer. Cancer. 1996;77(6):1101–6.
Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92(11):4150–66.
Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4(12):941–52.
Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6(4):409–21.
Melani C, Sangaletti S, Barazzetta FM, Werb Z, Colombo MP. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 2007;67(23):11438–46. doi:10.1158/0008-5472.CAN-07-1882.
Strauss L, Volland D, Kunkel M, Reichert TE. Dual role of VEGF family members in the pathogenesis of head and neck cancer (HNSCC): possible link between angiogenesis and immune tolerance. Med Sci Monit. 2005;11(8):BR280–92.
Murooka TT, Rahbar R, Fish EN. CCL5 promotes proliferation of MCF-7 cells through mTOR-dependent mRNA translation. Biochem Biophys Res Commun. 2009;387(2):381–6. doi:10.1016/j.bbrc.2009.07.035.
Chakraborty K, Bose A, Chakraborty T, Sarkar K, Goswami S, Pal S, et al. Restoration of dysregulated CC chemokine signaling for monocyte/macrophage chemotaxis in head and neck squamous cell carcinoma patients by neem leaf glycoprotein maximizes tumor cell cytotoxicity. Cell Mol Immunol. 2010;7(5):396–408. doi:10.1038/cmi.2010.29.
Kross KW, Heimdal JH, Olsnes C, Olofson J, Aarstad HJ. Tumour-associated macrophages secrete IL-6 and MCP-1 in head and neck squamous cell carcinoma tissue. Acta Otolaryngol. 2007;127(5):532–9. doi:10.1080/00016480600951384.
Bektas-Kayhan K, Unur M, Boy-Metin Z, Cakmakoglu B. MCP-1 and CCR2 gene variants in oral squamous cell carcinoma. Oral Dis. 2012;18(1):55–9. doi:10.1111/j.1601-0825.2011.01843.x.
Han L, Jiang B, Wu H, Wang X, Tang X, Huang J, et al. High expression of CXCR2 is associated with tumorigenesis, progression, and prognosis of laryngeal squamous cell carcinoma. Med Oncol. 2012;29(4):2466–72. doi:10.1007/s12032-011-0152-1.
Tan CT, Chu CY, Lu YC, Chang CC, Lin BR, Wu HH, et al. CXCL12/CXCR4 promotes laryngeal and hypopharyngeal squamous cell carcinoma metastasis through MMP-13-dependent invasion via the ERK1/2/AP-1 pathway. Carcinogenesis. 2008;29(8):1519–27. doi:10.1093/carcin/bgn108.
Rehman AO, Wang CY. CXCL12/SDF-1 alpha activates NF-kappaB and promotes oral cancer invasion through the Carma3/Bcl10/Malt1 complex. Int J Oral Sci. 2009;1(3):105–18. doi:10.4248/IJOS.09059.
Wang N, Wu QL, Fang Y, Mai HQ, Zeng MS, Shen GP, et al. Expression of chemokine receptor CXCR4 in nasopharyngeal carcinoma: pattern of expression and correlation with clinical outcome. J Transl Med. 2005;3:26. doi:10.1186/1479-5876-3-26.
Zlotnik A. Involvement of chemokine receptors in organ-specific metastasis. Contrib Microbiol. 2006;13:191–9. doi:10.1159/000092973.
Nath A, Chattopadhya S, Chattopadhyay U, Sharma NK. Macrophage inflammatory protein (MIP)1alpha and MIP1beta differentially regulate release of inflammatory cytokines and generation of tumoricidal monocytes in malignancy. Cancer Immunol Immunother. 2006;55(12):1534–41. doi:10.1007/s00262-006-0149-3.
Vaday GG, Peehl DM, Kadam PA, Lawrence DM. Expression of CCL5 (RANTES) and CCR5 in prostate cancer. Prostate. 2006;66(2):124–34. doi:10.1002/pros.20306.
Cozar JM, Canton J, Tallada M, Concha A, Cabrera T, Garrido F, et al. Analysis of NK cells and chemokine receptors in tumor infiltrating CD4 T lymphocytes in human renal carcinomas. Cancer Immunol Immunother. 2005;54(9):858–66. doi:10.1007/s00262-004-0646-1.
Sun Y, Cheng Z, Ma L, Pei G. Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem. 2002;277(51):49212–9. doi:10.1074/jbc.M207294200.
Azenshtein E, Luboshits G, Shina S, Neumark E, Shahbazian D, Weil M, et al. The CC chemokine RANTES in breast carcinoma progression: regulation of expression and potential mechanisms of promalignant activity. Cancer Res. 2002;62(4):1093–102.
Rainwater SM, Wu X, Nduati R, Nedellec R, Mosier D, John-Stewart G, et al. Cloning and characterization of functional subtype A HIV-1 envelope variants transmitted through breastfeeding. Curr HIV Res. 2007;5(2):189–97.
Aoki MN, Da Silva do Amaral Herrera AC, Amarante MK, do Carneiro JL, Fungaro MH, Watanabe MA. CCR5 and p53 codon 72 gene polymorphisms: implications in breast cancer development. Int J Mol Med. 2009;23(3):429–35.
Davidson B, Dong HP, Holth A, Berner A, Risberg B. The chemokine receptor CXCR4 is more frequently expressed in breast compared to other metastatic adenocarcinomas in effusions. Breast J. 2008;14(5):476–82. doi:10.1111/j.1524-4741.2008.00625.x.
Darbon JM. A new model of metastatic dissemination of breast cancer bringing into play mesenchymal stem cells. Bull Cancer. 2007;94(12):1035–6. doi:10.1684/bdc.2007.0523.
Walker UA. Antiretroviral therapy-induced liver alterations. Curr Opin HIV AIDS. 2007;2(4):293–8. doi:10.1097/COH.0b013e328122dbaa.
Levina V, Nolen BM, Marrangoni AM, Cheng P, Marks JR, Szczepanski MJ, et al. Role of eotaxin-1 signaling in ovarian cancer. Clin Cancer Res. 2009;15(8):2647–56. doi:10.1158/1078-0432.CCR-08-2024.
Berghuis D, Santos SJ, Baelde HJ, Taminiau AH, Egeler RM, Schilham MW, et al. Pro-inflammatory chemokine-chemokine receptor interactions within the Ewing sarcoma microenvironment determine CD8(+) T-lymphocyte infiltration and affect tumour progression. J Pathol. 2011;223(3):347–57. doi:10.1002/path.2819.
Mi Z, Bhattacharya SD, Kim VM, Guo H, Talbot LJ, Kuo PC. Osteopontin promotes CCL5-mesenchymal stromal cell-mediated breast cancer metastasis. Carcinogenesis. 2011;32(4):477–87. doi:10.1093/carcin/bgr009.
Yaal-Hahoshen N, Shina S, Leider-Trejo L, Barnea I, Shabtai EL, Azenshtein E, et al. The chemokine CCL5 as a potential prognostic factor predicting disease progression in stage II breast cancer patients. Clin Cancer Res. 2006;12(15):4474–80. doi:10.1158/1078-0432.CCR-06-0074.
Nesbeth Y, Scarlett U, Cubillos-Ruiz J, Martinez D, Engle X, Turk MJ, et al. CCL5-mediated endogenous antitumor immunity elicited by adoptively transferred lymphocytes and dendritic cell depletion. Cancer Res. 2009;69(15):6331–8. doi:10.1158/0008-5472.CAN-08-4329.
Flanagan K, Moroziewicz D, Kwak H, Horig H, Kaufman HL. The lymphoid chemokine CCL21 costimulates naive T cell expansion and Th1 polarization of non-regulatory CD4+ T cells. Cell Immunol. 2004;231(1–2):75–84. doi:10.1016/j.cellimm.2004.12.006.
Yamano T, Kaneda Y, Hiramatsu SH, Huang S, Tran AN, Giuliano AE, et al. Immunity against breast cancer by TERT DNA vaccine primed with chemokine CCL21. Cancer Gene Ther. 2007;14(5):451–9. doi:10.1038/sj.cgt.7701035.
Wu S, Xing W, Peng J, Yuan X, Zhao X, Lei P, et al. Tumor transfected with CCL21 enhanced reactivity and apoptosis resistance of human monocyte-derived dendritic cells. Immunobiology. 2008;213(5):417–26. doi:10.1016/j.imbio.2007.10.003.
Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007;11(6):526–38. doi:10.1016/j.ccr.2007.04.020.
Wang J, Xi L, Hunt JL, Gooding W, Whiteside TL, Chen Z, et al. Expression pattern of chemokine receptor 6 (CCR6) and CCR7 in squamous cell carcinoma of the head and neck identifies a novel metastatic phenotype. Cancer Res. 2004;64(5):1861–6.
Shields JD, Kourtis IC, Tomei AA, Roberts JM, Swartz MA. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science. 2010;328(5979):749–52. doi:10.1126/science.1185837.
Viola A, Sarukhan A, Bronte V, Molon B. The pros and cons of chemokines in tumor immunology. Trends Immunol. 2012;33:496–504. doi:10.1016/j.it.2012.05.007.
Heimdal JH, Olsnes C, Olofsson J, Aarstad HJ. Monocyte and monocyte-derived macrophage secretion of MCP-1 in co-culture with autologous malignant and benign control fragment spheroids. Cancer Immunol Immunother. 2001;50(6):300–6.
Brown CE, Vishwanath RP, Aguilar B, Starr R, Najbauer J, Aboody KS, et al. Tumor-derived chemokine MCP-1/CCL2 is sufficient for mediating tumor tropism of adoptively transferred T cells. J Immunol. 2007;179(5):3332–41.
Loberg RD, Day LL, Harwood J, Ying C, St John LN, Giles R, et al. CCL2 is a potent regulator of prostate cancer cell migration and proliferation. Neoplasia. 2006;8(7):578–86. doi:10.1593/neo.06280.
Bailey C, Negus R, Morris A, Ziprin P, Goldin R, Allavena P, et al. Chemokine expression is associated with the accumulation of tumour associated macrophages (TAMs) and progression in human colorectal cancer. Clin Exp Metastasis. 2007;24(2):121–30. doi:10.1007/s10585-007-9060-3.
Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6(8):3282–9.
Huang B, Lei Z, Zhao J, Gong W, Liu J, Chen Z, et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007;252(1):86–92. doi:10.1016/j.canlet.2006.12.012.
Molon B, Viola A, Bronte V. Smoothing T cell roads to the tumor: chemokine post-translational regulation. Oncoimmunology. 2012;1(3):390–2. doi:10.4161/onci.19069.
Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208(10):1949–62. doi:10.1084/jem.20101956.
Olkhanud PB, Baatar D, Bodogai M, Hakim F, Gress R, Anderson RL, et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69(14):5996–6004. doi:10.1158/0008-5472.CAN-08-4619.
Velasco-Velazquez M, Jiao X, De La Fuente M, Pestell TG, Ertel A, Lisanti MP, et al. CCR5 antagonist blocks metastasis of basal breast cancer cells. Cancer Res. 2012;72(15):3839–50. doi:10.1158/0008-5472.CAN-11-3917.
Ogino T, Shigyo H, Ishii H, Katayama A, Miyokawa N, Harabuchi Y, et al. HLA class I antigen down-regulation in primary laryngeal squamous cell carcinoma lesions as a poor prognostic marker. Cancer Res. 2006;66(18):9281–9. doi:10.1158/0008-5472.CAN-06-0488.
Wolf GT, Hudson JL, Peterson KA, Miller HL, McClatchey KD. Lymphocyte subpopulations infiltrating squamous carcinomas of the head and neck: correlations with extent of tumor and prognosis. Otolaryngol Head Neck Surg. 1986;95(2):142–52.
Junker N, Kvistborg P, Kollgaard T, Straten P, Andersen MH, Svane IM. Tumor associated antigen specific T-cell populations identified in ex vivo expanded TIL cultures. Cell Immunol. 2012;273(1):1–9. doi:10.1016/j.cellimm.2011.12.004.
Marcus B, Arenberg D, Lee J, Kleer C, Chepeha DB, Schmalbach CE, et al. Prognostic factors in oral cavity and oropharyngeal squamous cell carcinoma. Cancer. 2004;101(12):2779–87. doi:10.1002/cncr.20701.
Kuss I, Hathaway B, Ferris RL, Gooding W, Whiteside TL. Decreased absolute counts of T lymphocyte subsets and their relation to disease in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2004;10(11):3755–62. doi:10.1158/1078-0432.CCR-04-0054.
Turksma A, Bontkes H, van den Heuvel H, de Gruijl T, von Blomberg B, Braakhuis B, et al. Effector memory T-cell frequencies in relation to tumour stage, location and HPV status in HNSCC patients. Oral Dis. 2012. doi:10.1111/odi.12037.
Eura M, Ogi K, Chikamatsu K, Lee KD, Nakano K, Masuyama K, et al. Expression of the MAGE gene family in human head-and-neck squamous-cell carcinomas. Int J Cancer. 1995;64(5):304–8.
Kienstra MA, Neel HB, Strome SE, Roche P. Identification of NY-ESO-1, MAGE-1, and MAGE-3 in head and neck squamous cell carcinoma. Head Neck. 2003;25(6):457–63. doi:10.1002/hed.10223.
Albers A, Abe K, Hunt J, Wang J, Lopez-Albaitero A, Schaefer C, et al. Antitumor activity of human papillomavirus type 16 E7-specific T cells against virally infected squamous cell carcinoma of the head and neck. Cancer Res. 2005;65(23):11146–55. doi:10.1158/0008-5472.CAN-05-0772.
Devaraj K, Gillison ML, Wu TC. Development of HPV vaccines for HPV-associated head and neck squamous cell carcinoma. Crit Rev Oral Biol Med. 2003;14(5):345–62.
Gelbard A, Garnett CT, Abrams SI, Patel V, Gutkind JS, Palena C, et al. Combination chemotherapy and radiation of human squamous cell carcinoma of the head and neck augments CTL-mediated lysis. Clin Cancer Res. 2006;12(6):1897–905. doi:10.1158/1078-0432.CCR-05-1761.
Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev. 2010;234(1):45–54. doi:10.1111/j.0105-2896.2009.00879.x.
Valladeau J, Saeland S. Cutaneous dendritic cells. Semin Immunol. 2005;17(4):273–83. doi:10.1016/j.smim.2005.05.009.
Cruchley AT, Williams DM, Farthing PM, Speight PM, Lesch CA, Squier CA. Langerhans cell density in normal human oral mucosa and skin: relationship to age, smoking and alcohol consumption. J Oral Pathol Med. 1994;23(2):55–9.
Boyle JO, Gumus ZH, Kacker A, Choksi VL, Bocker JM, Zhou XK, et al. Effects of cigarette smoke on the human oral mucosal transcriptome. Cancer Prev Res (Phila). 2010;3(3):266–78. doi:10.1158/1940-6207.CAPR-09-0192.
Girod SC, Kuhnast T, Ulrich S, Krueger GR. Langerhans cells in epithelial tumors and benign lesions of the oropharynx. In Vivo. 1994;8(4):543–7.
Albuquerque Jr RL, Miguel MC, Costa AL, Souza LB. Correlation of c-erbB-2 and S-100 expression with the malignancy grading and anatomical site in oral squamous cell carcinoma. Int J Exp Pathol. 2003;84(6):259–65.
Yilmaz T, Gedikoglu G, Celik A, Onerci M, Turan E. Prognostic significance of Langerhans cell infiltration in cancer of the larynx. Otolaryngol Head Neck Surg. 2005;132(2):309–16. doi:10.1016/j.otohns.2004.04.018.
Gallo O, Libonati GA, Gallina E, Fini-Storchi O, Giannini A, Urso C, et al. Langerhans cells related to prognosis in patients with laryngeal carcinoma. Arch Otolaryngol Head Neck Surg. 1991;117(9):1007–10.
Li X, Takahashi Y, Sakamoto K, Nakashima T. Expression of dendritic cell phenotypic antigens in cervical lymph nodes of patients with hypopharyngeal and laryngeal carcinoma. J Laryngol Otol Suppl. 2009;31:5–10.
Hoffmann TK, Muller-Berghaus J, Ferris RL, Johnson JT, Storkus WJ, Whiteside TL. Alterations in the frequency of dendritic cell subsets in the peripheral circulation of patients with squamous cell carcinomas of the head and neck. Clin Cancer Res. 2002;8(6):1787–93.
Young MR, Wright MA, Lozano Y, Prechel MM, Benefield J, Leonetti JP, et al. Increased recurrence and metastasis in patients whose primary head and neck squamous cell carcinomas secreted granulocyte-macrophage colony- stimulating factor and contained CD34+ natural suppressor cells. Int J Cancer. 1997;74(1):69–74.
Pak AS, Wright MA, Matthews JP, Collins SL, Petruzzelli GJ, Young MRI. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin Cancer Res. 1995;1(1):95–103.
Wu G, Morris Jr SM. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336(Pt 1):1–17.
Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2(10):907–16.
Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64(16):5839–49.
Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168(2):689–95.
Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor- bearing mice. J Immunol. 2003;170(1):270–8.
Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, et al. Altered recognition of antigen is a mechanism of CD8(+) T cell tolerance in cancer. Nat Med. 2007;13(7):828–35.
Rosbe KW, Prazma J, Petrusz P, Mims W, Ball SS, Weissler MC. Immunohistochemical characterization of nitric oxide synthase activity in squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg. 1995;113(5):541–9.
Bentz BG, Haines 3rd GK, Radosevich JA. Increased protein nitrosylation in head and neck squamous cell carcinogenesis. Head Neck. 2000;22(1):64–70.
Heimdal JH, Aarstad HJ, Olofsson J. Peripheral blood T-lymphocyte and monocyte function and survival in patients with head and neck carcinoma. Laryngoscope. 2000;110(3 Pt 1):402–7.
Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, et al. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6(5):1755–66.
Young MR, Cigal M. Tumor skewing of CD34+ cell differentiation from a dendritic cell pathway into endothelial cells. Cancer Immunol Immunother. 2006;55(5):558–68.
Serafini P, De Santo C, Marigo I, Cingarlini S, Dolcetti L, Gallina G, et al. Derangement of immune responses by myeloid suppressor cells. Cancer Immunol Immunother. 2004;53(2):64–72.
To WC, Wood BG, Krauss JC, Strome M, Esclamado RM, Lavertu P, et al. Systemic adoptive T-cell immunotherapy in recurrent and metastatic carcinoma of the head and neck: a phase 1 study. Arch Otolaryngol Head Neck Surg. 2000;126(10):1225–31.
Chang AE, Li Q, Jiang G, Teknos TN, Chepeha DB, Bradford CR. Generation of vaccine-primed lymphocytes for the treatment of head and neck cancer. Head Neck. 2003;25(3):198–209.
Kusmartsev S, Nagaraj S, Gabrilovich DI. Tumor-associated CD8+ T cell tolerance induced by bone marrow-derived immature myeloid cells. J Immunol. 2005;175(7):4583–92.
Kusmartsev S, Gabrilovich DI. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J Immunol. 2005;174(8):4880–91.
Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203(12):2691–702.
Pandit R, Lathers DM, Beal NM, Garrity T, Young MR. CD34+ immune suppressive cells in the peripheral blood of patients with head and neck cancer. Ann Otol Rhinol Laryngol. 2000;109(8 Pt 1):749–54.
Pak AS, Wright MA, Matthews JP, Collins SL, Petruzzelli GJ, Young MR. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin Cancer Res. 1995;1(1):95–103.
Wiers K, Wright MA, Vellody K, Young MR. Failure of tumor-reactive lymph node cells to kill tumor in the presence of immune-suppressive CD34+ cells can be overcome with vitamin D3 treatment to diminish CD34+ cell levels. Clin Exp Metastasis. 1998;16(3):275–82.
Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103(3):481–90.
Pahler JC, Tazzyman S, Erez N, Chen YY, Murdoch C, Nozawa H, et al. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia. 2008;10(4):329–40.
Xiang M, Gu Y, Zhao F, Lu H, Chen S, Yin L. Mast cell tryptase promotes breast cancer migration and invasion. Oncol Rep. 2010;23(3):615–9.
Mohamed MM, Cavallo-Medved D, Rudy D, Anbalagan A, Moin K, Sloane BF. Interleukin-6 increases expression and secretion of cathepsin B by breast tumor-associated monocytes. Cell Physiol Biochem. 2010;25(2–3):315–24. doi:000276564 [pii]10.1159/000276564.
Lathers DM, Young MR. Increased aberrance of cytokine expression in plasma of patients with more advanced squamous cell carcinoma of the head and neck. Cytokine. 2004;25(5):220–8. doi:10.1016/j.cyto.2003.11.005.
Jonuleit H, Schmitt E. The regulatory T cell family: distinct subsets and their interrelations. J Immunol. 2003;171(12):6323–7.
Terabe M, Berzofsky JA. Immunoregulatory T cells in tumor immunity. Curr Opin Immunol. 2004;16(2):157–62.
Schaefer C, Kim GG, Albers A, Hoermann K, Myers EN, Whiteside TL. Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer. 2005;92(5):913–20.
Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172(9):5213–21.
Huber S, Schramm C, Lehr HA, Mann A, Schmitt S, Becker C, et al. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol. 2004;173(11):6526–31.
Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169(5):2756–61.
Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9(2):606–12.
Okita R, Saeki T, Takashima S, Yamaguchi Y, Toge T. CD4+CD25+ regulatory T cells in the peripheral blood of patients with breast cancer and non-small cell lung cancer. Oncol Rep. 2005;14(5):1269–73.
Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-{beta}1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007;13(15):4345–54.
Unitt E, Rushbrook SM, Marshall A, Davies S, Gibbs P, Morris LS, et al. Compromised lymphocytes infiltrate hepatocellular carcinoma: the role of T-regulatory cells. Hepatology. 2005;41(4):722–30.
Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65(6):2457–64.
Zhang YL, Li J, Mo HY, Qiu F, Zheng LM, Qian CN, et al. Different subsets of tumor infiltrating lymphocytes correlate with NPC progression in different ways. Mol Cancer. 2010;9:4. doi:10.1186/1476-4598-9-4.
Distel LV, Fickenscher R, Dietel K, Hung A, Iro H, Zenk J, et al. Tumour infiltrating lymphocytes in squamous cell carcinoma of the oro- and hypopharynx: prognostic impact may depend on type of treatment and stage of disease. Oral Oncol. 2009;45(10):e167–74. doi:10.1016/j.oraloncology.2009.05.640.
Pretscher D, Distel LV, Grabenbauer GG, Wittlinger M, Buettner M, Niedobitek G. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+ B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer. 2009;9:292. doi:10.1186/1471-2407-9-292.
Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay Nel H, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12(2):465–72. doi:10.1158/1078-0432.CCR-05-1886.
Zingg U, Montani M, Frey DM, Dirnhofer S, Esterman AJ, Went P, et al. Tumour-infiltrating lymphocytes and survival in patients with adenocarcinoma of the oesophagus. Eur J Surg Oncol. 2010;36(7):670–7. doi:10.1016/j.ejso.2010.05.012.
Morgan ME, van Bilsen JH, Bakker AM, Heemskerk B, Schilham MW, Hartgers FC, et al. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol. 2005;66(1):13–20. doi:10.1016/j.humimm.2004.05.016.
Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37(1):129–38. doi:10.1002/eji.200636435.
Kmieciak M, Gowda M, Graham L, Godder K, Bear HD, Marincola FM, et al. Human T cells express CD25 and Foxp3 upon activation and exhibit effector/memory phenotypes without any regulatory/suppressor function. J Transl Med. 2009;7:89. doi:10.1186/1479-5876-7-89.
Magg T, Mannert J, Ellwart JW, Schmid I, Albert MH. Subcellular localization of FOXP3 in human regulatory and nonregulatory T cells. Eur J Immunol. 2012;42(6):1627–38. doi:10.1002/eji.201141838.
Weed DT, Walker G, De La Fuente AC, Nazarian R, Vella JL, et al. FOXP3 Subcellular localization predicts recurrence in oral squamous cell carcinoma. PLoS ONE. 2013;8(8):e71908. doi:10.1371/journal.pone.0071908.
Kuss I, Hathaway B, Ferris RL, Gooding W, Whiteside TL. Imbalance in absolute counts of T lymphocyte subsets in patients with head and neck cancer and its relation to disease. Adv Otorhinolaryngol. 2005;62:161–72.
Gomez JA, Sun W, Gama V, Hajkova D, Yoshida T, Wu Z, et al. The C-terminus of interferon gamma receptor beta chain (IFNgammaR2) has antiapoptotic activity as a Bax inhibitor. Cancer Biol Ther. 2009;8(18):1771–86.
Manchanda P, Sharma SC, Das SN. Differential regulation of IL-2 and IL-4 in patients with tobacco-related oral squamous cell carcinoma. Oral Dis. 2006;12(5):455–62. doi:10.1111/j.1601-0825.2005.01220.x.
Farace F, Angevin E, Vanderplancke J, Escudier B, Triebel F. The decreased expression of CD3 zeta chains in cancer patients is not reversed by IL-2 administration. Int J Cancer. 1994;59(6):752–5.
Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16(1):53–65.
Dunn G, Oliver KM, Loke D, Stafford ND, Greenman J. Dendritic cells and HNSCC: a potential treatment option? (Review). Oncol Rep. 2005;13(1):3–10.
Meneses A, Verastegui E, Barrera JL, de la Garza J, Hadden JW. Lymph node histology in head and neck cancer: impact of immunotherapy with IRX-2. Int Immunopharmacol. 2003;3(8):1083–91. doi:10.1016/S1567-5769(03)00017-1.
Mancino A, Schioppa T, Larghi P, Pasqualini F, Nebuloni M, Chen IH, et al. Divergent effects of hypoxia on dendritic cell functions. Blood. 2008;112(9):3723–34. doi:10.1182/blood-2008-02-142091.
Elia AR, Cappello P, Puppo M, Fraone T, Vanni C, Eva A, et al. Human dendritic cells differentiated in hypoxia down-modulate antigen uptake and change their chemokine expression profile. J Leukoc Biol. 2008;84(6):1472–82. doi:10.1189/jlb.0208082.
Yang M, Ma C, Liu S, Shao Q, Gao W, Song B, et al. HIF-dependent induction of adenosine receptor A2b skews human dendritic cells to a Th2-stimulating phenotype under hypoxia. Immunol Cell Biol. 2010;88(2):165–71. doi:10.1038/icb.2009.77.
Rosenberg SA, Lotze MT, Yang JC, Aebersold PM, Linehan WM, Seipp CA, et al. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210(4):474–84. discussion 484–75.
Fishman MN, Thompson JA, Pennock GK, Gonzalez R, Diez LM, Daud AI, et al. Phase I trial of ALT-801, an interleukin-2/T-cell receptor fusion protein targeting p53 (aa264-272)/HLA-A*0201 complex, in patients with advanced malignancies. Clin Cancer Res. 2011;17(24):7765–75. doi:10.1158/1078-0432.CCR-11-1817.
Rossig C, Brenner MK. Genetic modification of T lymphocytes for adoptive immunotherapy. Mol Ther. 2004;10(1):5–18. doi:10.1016/j.ymthe.2004.04.014.
Shirakura Y, Mizuno Y, Wang L, Imai N, Amaike C, Sato E, et al. T-cell receptor gene therapy targeting melanoma-associated antigen-A4 inhibits human tumor growth in non-obese diabetic/SCID/gammacnull mice. Cancer Sci. 2012;103(1):17–25. doi:10.1111/j.1349-7006.2011.02111.x.
Linnemann C, Schumacher TN, Bendle GM. T-cell receptor gene therapy: critical parameters for clinical success. J Invest Dermatol. 2011;131(9):1806–16. doi:10.1038/jid.2011.160.
Jorritsma A, Schotte R, Coccoris M, de Witte MA, Schumacher TN. Prospects and limitations of T cell receptor gene therapy. Curr Gene Ther. 2011;11(4):276–87.
Davis JL, Theoret MR, Zheng Z, Lamers CH, Rosenberg SA, Morgan RA. Development of human anti-murine T-cell receptor antibodies in both responding and nonresponding patients enrolled in TCR gene therapy trials. Clin Cancer Res. 2010;16(23):5852–61. doi:10.1158/1078-0432.CCR-10-1280.
Ochi T, Fujiwara H, Yasukawa M. Application of adoptive T-cell therapy using tumor antigen-specific T-cell receptor gene transfer for the treatment of human leukemia. J Biomed Biotechnol. 2010;2010:521248. doi:10.1155/2010/521248.
Bendle GM, Linnemann C, Hooijkaas AI, Bies L, de Witte MA, Jorritsma A, et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med. 2010;16(5):565–70. doi:10.1038/nm.2128. 561–70.
Leisegang M, Turqueti-Neves A, Engels B, Blankenstein T, Schendel DJ, Uckert W, et al. T-cell receptor gene-modified T cells with shared renal cell carcinoma specificity for adoptive T-cell therapy. Clin Cancer Res. 2010;16(8):2333–43. doi:10.1158/1078-0432.CCR-09-2897.
Govers C, Sebestyen Z, Coccoris M, Willemsen RA, Debets R. T cell receptor gene therapy: strategies for optimizing transgenic TCR pairing. Trends Mol Med. 2010;16(2):77–87. doi:10.1016/j.molmed.2009.12.004.
Bendle GM, Haanen JB, Schumacher TN. Preclinical development of T cell receptor gene therapy. Curr Opin Immunol. 2009;21(2):209–14. doi:10.1016/j.coi.2009.02.007.
Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69(6):2514–22. doi:10.1158/0008-5472.CAN-08-4709.
Porta C, Paglino C, Imarisio I, Ganini C, Pedrazzoli P. Immunological effects of multikinase inhibitors for kidney cancer: a clue for integration with cellular therapies? J Cancer. 2011;2:333–8.
Choong NW, Kozloff M, Taber D, Hu HS, Wade 3rd J, Ivy P, et al. Phase II study of sunitinib malate in head and neck squamous cell carcinoma. Invest New Drugs. 2010;28(5):677–83. doi:10.1007/s10637-009-9296-7.
Fountzilas G, Fragkoulidi A, Kalogera-Fountzila A, Nikolaidou M, Bobos M, Calderaro J, et al. A phase II study of sunitinib in patients with recurrent and/or metastatic non-nasopharyngeal head and neck cancer. Cancer Chemother Pharmacol. 2010;65(4):649–60. doi:10.1007/s00280-009-1070-1.
Machiels JP, Henry S, Zanetta S, Kaminsky MC, Michoux N, Rommel D, et al. Phase II study of sunitinib in recurrent or metastatic squamous cell carcinoma of the head and neck: GORTEC 2006–01. J Clin Oncol. 2010;28(1):21–8. doi:10.1200/JCO.2009.23.8584.
Kao J, Packer S, Vu HL, Schwartz ME, Sung MW, Stock RG, et al. Phase 1 study of concurrent sunitinib and image-guided radiotherapy followed by maintenance sunitinib for patients with oligometastases: acute toxicity and preliminary response. Cancer. 2009;115(15):3571–80. doi:10.1002/cncr.24412.
Garrity T, Pandit R, Wright MA, Benefield J, Keni S, Young MR. Increased presence of CD34+ cells in the peripheral blood of head and neck cancer patients and their differentiation into dendritic cells. Int J Cancer. 1997;73(5):663–9.
Young MR, Wright MA, Vellody K, Lathers DM. Skewed differentiation of bone marrow CD34+ cells of tumor bearers from dendritic toward monocytic cells, and the redirection of differentiation toward dendritic cells by 1alpha,25-dihydroxyvitamin D3. Int J Immunopharmacol. 1999;21(10):675–88.
Walsh JE, Clark AM, Day TA, Gillespie MB, Young MR. Use of alpha,25-dihydroxyvitamin D3 treatment to stimulate immune infiltration into head and neck squamous cell carcinoma. Hum Immunol. 2010;71(7):659–65. doi:10.1016/j.humimm.2010.04.008.
Walker DD, Reeves TD, de Costa AM, Schuyler C, Young MR. Immunological modulation by 1alpha,25-dihydroxyvitamin D3 in patients with squamous cell carcinoma of the head and neck. Cytokine. 2012;58(3):448–54. doi:10.1016/j.cyto.2012.03.002.
Ben-Shoshan M, Amir S, Dang DT, Dang LH, Weisman Y, Mabjeesh NJ. 1alpha,25-dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol Cancer Ther. 2007;6(4):1433–9. doi:10.1158/1535-7163.MCT-06-0677.
Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13(2 Pt 2):721s–6. doi:13/2/721s [pii]10.1158/1078-0432.CCR-06-2197.
Wirth LJ, Haddad RI, Lindeman NI, Zhao X, Lee JC, Joshi VA, et al. Phase I study of gefitinib plus celecoxib in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2005;23(28):6976–81. doi:10.1200/JCO.2005.02.4182.
Kao J, Genden EM, Chen CT, Rivera M, Tong CC, Misiukiewicz K, et al. Phase 1 trial of concurrent erlotinib, celecoxib, and reirradiation for recurrent head and neck cancer. Cancer. 2011;117(14):3173–81. doi:10.1002/cncr.25786.
Dajani EZ, Islam K. Cardiovascular and gastrointestinal toxicity of selective cyclo-oxygenase-2 inhibitors in man. J Physiol Pharmacol. 2008;59 Suppl 2:117–33.
Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352(11):1071–80. doi:10.1056/NEJMoa050405.
Huang WF, Hsiao FY, Wen YW, Tsai YW. Cardiovascular events associated with the use of four nonselective NSAIDs (etodolac, nabumetone, ibuprofen, or naproxen) versus a cyclooxygenase-2 inhibitor (celecoxib): a population-based analysis in Taiwanese adults. Clin Ther. 2006;28(11):1827–36. doi:10.1016/j.clinthera.2006.11.009.
Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68(13):5439–49.
Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56(5):641–8. doi:10.1007/s00262-006-0225-8.
Airoldi M, Cortesina G, Giordano C, Pedani F, Bumma C. Ifosfamide in the treatment of head and neck cancer. Oncology. 2003;65 Suppl 2:37–43.
Recchia F, Lalli A, Lombardo M, De Filippis S, Saggio G, Fabbri F, et al. Ifosfamide, cisplatin, and 13-Cis retinoic acid for patients with advanced or recurrent squamous cell carcinoma of the head and neck: a phase I–II study. Cancer. 2001;92(4):814–21.
Schroeder M, Zouboulis CC. All-trans-retinoic acid and 13-cis-retinoic acid: pharmacokinetics and biological activity in different cell culture models of human keratinocytes. Horm Metab Res. 2007;39(2):136–40. doi:10.1055/s-2007-961813.
Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74. doi:10.1038/nri2506.
De Costa AM, Young MR. Immunotherapy for head and neck cancer: advances and deficiencies. Anticancer Drugs. 2011;22(7):674–81. doi:10.1097/CAD.0b013e328340fd18.
Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J Clin Oncol. 2010;28(28):4390–9. doi:10.1200/JCO.2009.27.6360.
Russell JS, Colevas AD. The use of epidermal growth factor receptor monoclonal antibodies in squamous cell carcinoma of the head and neck. Chemother Res Pract. 2012;2012:761518. doi:10.1155/2012/761518.
Young MR, Neville BW, Chi AC, Lathers DM, Boyd Gillespie M, Day TA. Oral premalignant lesions induce immune reactivity to both premalignant oral lesions and head and neck squamous cell carcinoma. Cancer Immunol Immunother. 2007;56(7):1077–86. doi:10.1007/s00262-006-0242-7.
Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23(9):1147–57. doi:nbt1137 [pii]10.1038/nbt1137.
Baselga J, Pfister D, Cooper MR, Cohen R, Burtness B, Bos M, et al. Phase I studies of anti-epidermal growth factor receptor chimeric antibody C225 alone and in combination with cisplatin. J Clin Oncol. 2000;18(4):904–14.
Robert F, Ezekiel MP, Spencer SA, Meredith RF, Bonner JA, Khazaeli MB, et al. Phase I study of anti–epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J Clin Oncol. 2001;19(13):3234–43.
Shin DM, Donato NJ, Perez-Soler R, Shin HJ, Wu JY, Zhang P, et al. Epidermal growth factor receptor-targeted therapy with C225 and cisplatin in patients with head and neck cancer. Clin Cancer Res. 2001;7(5):1204–13.
Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–78. doi:10.1056/NEJMoa053422.
Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11(1):21–8. doi:10.1016/S1470-2045(09)70311-0.
Pfister DG, Su YB, Kraus DH, Wolden SL, Lis E, Aliff TB, et al. Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: a pilot phase II study of a new combined-modality paradigm. J Clin Oncol. 2006;24(7):1072–8. doi:10.1200/JCO.2004.00.1792.
Merlano M, Russi E, Benasso M, Corvo R, Colantonio I, Vigna-Taglianti R, et al. Cisplatin-based chemoradiation plus cetuximab in locally advanced head and neck cancer: a phase II clinical study. Ann Oncol. 2011;22(3):712–7. doi:10.1093/annonc/mdq412.
Harari PM, Allen GW, Bonner JA. Biology of interactions: antiepidermal growth factor receptor agents. J Clin Oncol. 2007;25(26):4057–65. doi:10.1200/JCO.2007.11.8984.
Harari PM, Huang S. Radiation combined with EGFR signal inhibitors: head and neck cancer focus. Semin Radiat Oncol. 2006;16(1):38–44. doi:10.1016/j.semradonc.2005.08.005.
Kim S, Grandis JR, Rinaldo A, Takes RP, Ferlito A. Emerging perspectives in epidermal growth factor receptor targeting in head and neck cancer. Head Neck. 2008;30(5):667–74. doi:10.1002/hed.20859.
Lopez-Albaitero A, Ferris RL. Immune activation by epidermal growth factor receptor specific monoclonal antibody therapy for head and neck cancer. Arch Otolaryngol Head Neck Surg. 2007;133(12):1277–81. doi:10.1001/archotol.133.12.1277.
Andrade P, Deleo A, Visus C, Butterfield L, Argiris A, Ferris RL. Phase I adjuvant trial of multi-epitope p53 vaccine for patients with squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:15s.
Lopez-Albaitero A, Lee SC, Morgan S, Grandis JR, Gooding WE, Ferrone S, et al. Role of polymorphic Fc gamma receptor IIIa and EGFR expression level in cetuximab mediated, NK cell dependent in vitro cytotoxicity of head and neck squamous cell carcinoma cells. Cancer Immunol Immunother. 2009;58(11):1853–64. doi:10.1007/s00262-009-0697-4.
Kurai J, Chikumi H, Hashimoto K, Yamaguchi K, Yamasaki A, Sako T, et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin Cancer Res. 2007;13(5):1552–61. doi:10.1158/1078-0432.CCR-06-1726.
Andrade Filho PA, Lopez-Albaitero A, Gooding W, Ferris RL. Novel immunogenic HLA-A*0201-restricted epidermal growth factor receptor-specific T-cell epitope in head and neck cancer patients. J Immunother. 2010;33(1):83–91. doi:10.1097/CJI.0b013e3181b8f421.
Dechant M, Weisner W, Berger S, Peipp M, Beyer T, Schneider-Merck T, et al. Complement-dependent tumor cell lysis triggered by combinations of epidermal growth factor receptor antibodies. Cancer Res. 2008;68(13):4998–5003. doi:10.1158/0008-5472.CAN-07-6226.
Riechelmann H, Wiesneth M, Schauwecker P, Reinhardt P, Gronau S, Schmitt A, et al. Adoptive therapy of head and neck squamous cell carcinoma with antibody coated immune cells: a pilot clinical trial. Cancer Immunol Immunother. 2007;56(9):1397–406. doi:10.1007/s00262-007-0283-6.
Junker N, Andersen MH, Wenandy L, Dombernowsky SL, Kiss K, Sorensen CH, et al. Bimodal ex vivo expansion of T cells from patients with head and neck squamous cell carcinoma: a prerequisite for adoptive cell transfer. Cytotherapy. 2011;13(7):822–34. doi:10.3109/14653249.2011.563291.
Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3(9):666–75. doi:10.1038/nrc1167.
Cohen CJ, Zheng Z, Bray R, Zhao Y, Sherman LA, Rosenberg SA, et al. Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR. J Immunol. 2005;175(9):5799–808.
Yamasaki K, Horiguchi S, Kurosaki M, Kunii N, Nagato K, Hanaoka H, et al. Induction of NKT cell-specific immune responses in cancer tissues after NKT cell-targeted adoptive immunotherapy. Clin Immunol. 2011;138(3):255–65. doi:10.1016/j.clim.2010.11.014.
Lee KD, Lee HH, Joo HB, Lee HS, Yu TH, Chang HK, et al. Expression of MAGE A 1–6 mRNA in sputa of head and neck cancer patients–a preliminary report. Anticancer Res. 2006;26(2B):1513–8.
Lee KD, Chang HK, Jo YK, Kim BS, Lee BH, Lee YW, et al. Expression of the MAGE 3 gene product in squamous cell carcinomas of the head and neck. Anticancer Res. 1999;19(6B):5037–42.
Lee KD, Eura M, Ogi K, Nakano K, Chikamatsu K, Masuyama K, et al. Expression of the MAGE-1, -2, -3, -4, and −6 genes in non-squamous cell carcinoma lesions of the head and neck. Acta Otolaryngol. 1996;116(4):633–9.
Clark CE, Vonderheide RH. Cancer-testis antigens in tumor biology and immunotherapy. Cancer Biol Ther. 2006;5(9):1226–7.
Ma B, Yijie X, Hung C-F, Wu T-C (2010) HPV and therapeutic vaccines: where we stand in 2010. Curr Cancer Ther Rev 6:81–103
Hoffmann TK, Bier H, Donnenberg AD, Whiteside TL, De Leo AB. p53 as an immunotherapeutic target in head and neck cancer. Adv Otorhinolaryngol. 2005;62:151–60. doi:10.1159/000082505.
Hoffmann TK, Loftus DJ, Nakano K, Maeurer MJ, Chikamatsu K, Appella E, et al. The ability of variant peptides to reverse the nonresponsiveness of T lymphocytes to the wild-type sequence p53(264–272) epitope. J Immunol. 2002;168(3):1338–47.
Schuler PJH, Harasymczuk M, Visus C, Hoffmann TK, Lang S, Argiris A, DeLeo AB, Whiteside TL, Ferris RL (2012) Adjuvant P53 peptide loaded dendritic cell vaccination in patients with head and neck cancer – a phase I/II clinical trial 8th international conference on head and neck cancer. Metro Toronto Convention Centre, 21–25 July 2012. http://ahns.jnabstracts.com/Detail.aspx?ID=0252
Marty R, Roze S, Bresse X, Largeron N, Smith-Palmer J. Estimating the clinical benefits of vaccinating boys and girls against HPV-related diseases in Europe. BMC Cancer. 2013;13(1):10. doi:10.1186/1471-2407-13-10.
Wallecha A, French C, Petit R, Singh R, Amin A, Rothman J. Lm-LLO-based immunotherapies and HPV-associated disease. J Oncol. 2012;2012:542851. doi:10.1155/2012/542851.
Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol. 2001;167(11):6471–9.
Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a Phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009;27(30):3975–83. doi:10.1016/j.vaccine.2009.04.041.
Voskens CJ, Sewell D, Hertzano R, DeSanto J, Rollins S, Lee M, et al. InducTION of mage-A3 and HPV-16 immunity by Trojan vaccines in patients with head and neck carcinoma. Head Neck. 2012;34(12):1734–46. doi:10.1002/hed.22004.
Hu JC, Coffin RS, Davis CJ, Graham NJ, Groves N, Guest PJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12(22):6737–47. doi:10.1158/1078-0432.CCR-06-0759.
Harrington KJ, Hingorani M, Tanay MA, Hickey J, Bhide SA, Clarke PM, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16(15):4005–15. doi:10.1158/1078-0432.CCR-10-0196.
Prestwich RJ, Errington F, Diaz RM, Pandha HS, Harrington KJ, Melcher AA, et al. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum Gene Ther. 2009;20(10):1119–32. doi:10.1089/hum.2009.135.
Naik JD, Twelves CJ, Selby PJ, Vile RG, Chester JD. Immune recruitment and therapeutic synergy: keys to optimizing oncolytic viral therapy? Clin Cancer Res. 2011;17(13):4214–24. doi:10.1158/1078-0432.CCR-10-2848.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Serafini, P., Weed, D.T. (2014). The Immune System in Head and Neck Squamous Cell Carcinoma: Interactions and Therapeutic Opportunities. In: Rosenblatt, J., Podack, E., Barber, G., Ochoa, A. (eds) Advances in Tumor Immunology and Immunotherapy. Current Cancer Research. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-8809-5_13
Download citation
DOI: https://doi.org/10.1007/978-1-4614-8809-5_13
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-8808-8
Online ISBN: 978-1-4614-8809-5
eBook Packages: MedicineMedicine (R0)