Abstract
Papaya (Carica papaya L.) is the first fleshy fruit with a climacteric ripening pattern to be sequenced. Many of the predicted papaya genes potentially involved in fruit growth, development, and ripening have homology to those involved in tomato fruit size and shape. Fewer genes that may impact sugar accumulation in papaya, ethylene synthesis and response, respiration, chlorophyll degradation, and carotenoid synthesis are predicted than in tomato. Similar or fewer genes were found in papaya for the enzymes leading to volatile production than so far determined for tomato. Similar numbers or fewer genes are found in papaya for the enzymes leading to volatile production than so far determined for tomato. The presence of fewer papaya genes in most fruit development and ripening categories suggests less subfunctionalization of gene action. The lack of whole genome duplication and reductions in most gene families and biosynthetic pathways make papaya a valuable and unique tool to study the convergent evolution of fruit ripening. The available data suggest that few phylogenetic constraints exist in the evolution of fruit type with fleshy fruits appearing independently in different plant families. The evolutionary origin and fundamental molecular mechanisms that lead to the development of the fruit ripening syndrome are unknown. Abscission-like cell separation processes that occur in leaves, petals, flowers, and pollen show parallel to fruit ripening in terms of physiology and biochemistry and potentially in the network and clusters of genes expressed. A global understanding of the evolution of the complex regulatory networks controlling the modules active during fruit ripening could lead to new regulatory controls that could limit postharvest losses in fruits.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The papaya fruit is a fleshy berry and fruit growth follows a single sigmoid growth curve (Zhou and Paull 2001). During development, all tissues in the gynoecium less than 1 mm are meristematic (Roth and Clausnitzer 1972). Later the outer layer of the epidermis increases in size, while the subepidermal layer continues to divide both anticlinally and periclinally. The central parenchyma of the pericarp increases in size and divides with the placenta forming opposite the marginal vascular bundles. This meristematic activity lasts 28–42 days and determines final fruit size. Fruit growth shows two major phases. The first lasts about 80 days post-anthesis, with a large increase in dry weight occurring just before fruit maturity. Fruit development takes 150–164 days that is extended another 14–21 days in Hawaii in the colder months (Paull and Chen 1983; Qiu et al. 1995). Mesocarp growth parallels seed and total fruit growth.
Papaya fruit shape is a sex-linked character and ranges from spherical to ovoid in female flowers to long, cylindrical, or pyriform (pear shaped) in hermaphrodite flowers (Table 14.1). The fruit is normally composed of five carpels united to form a central ovarian cavity that is lined with the placenta carrying numerous black seeds. Placentation is parietal with the seeds attached by 0.5–1 mm stalks. The ovarian cavity is larger in female fruit than hermaphrodite. The shape of the cavity at the transverse cut ranges from star shape with five to seven furrows to smooth and circular (Chan and Paull 2007).
Papaya ripening is climacteric with the rise in ethylene production occurring at the same time as the respiratory rise (Paull and Chen 1983). Respiration is a critical factor in fruit growth and development, especially as it relates to fruit ripening. Fruits are divided into two broad groups based on the role of ethylene in the ripening process and its relationship to the respiratory pattern. Climacteric fruits (e.g., banana, papaya, peach, tomato) demonstrate a peak in respiration and ethylene production during ripening, including autostimulatory (System 2) ethylene production (Biale 1964). The timing of the autostimulatory ethylene peak (Lelievre et al. 1997; Barry et al. 2000) and of other ripening events (skin color changes, carotenoid synthesis, flavor development, softening) varies widely between species and cultivars (Biale 1964; Burg and Burg 1965; Bruinsma and Paull 1984; Gussman et al. 1993). Non-climacteric fruit shows a gradual decline in the respiration rate and shows no marked peak in ethylene production and gradual change in other ripening parameters.
Fruit Development
Fruit Growth
The plant growth regulator abscisic acid (ABA) plays a crucial role in the plant’s adaptation to stress and in seed maturation and dormancy, fruit ripening, and senescence (Zeevart and Creelman 1988). ABA and strigolactone are products from the cleavage of carotenoids at one of its double bonds. Carotenoid cleavage is carried out by two groups of related enzymes. One group uses multiple carotenoid substrates and is referred to as carotenoid cleavage dioxygenase (CCD). The other group has C40-9-cis-epoxycarotenoid as the preferred substrate and referred to as 9-cis-epoxycarotenoid dioxygenase (NCED). The NCEDs are thought to be involved in ABA synthesis while the role of CCDs is less clear. Some evidences suggest that CCDs have a role in lateral shoot growth (Auldridge et al. 2006b; Azarkan et al. 2006), possibly by cleavage of carotenoids to strigolactone or a related compound (Gomez-Roldan et al. 2008).
Two Arabidopsis genes CCD7/MAX3 and CCD8/MAX4 control lateral shoot growth (Booker et al. 2004; Auldridge et al. 2006a; Gomez-Roldan et al. 2008). The citrus homolog gene (CsNCED1) likely plays a role in ABA synthesis in leaves and fruits (Rodrigo et al. 2006). ABA has also been given a role in the regulation of citrus fruit skin coloration (Rodrigo et al. 2003). Citrus are non-climacteric fruit and normally respond to ethylene by induced fruit coloration (Alferez and Zacarias 1999). Papaya is a climacteric fruit though fruit mesocarp carotenoid development and fruit degreening are ethylene independent (Manenoi et al. 2007). ABA via CCD and/or NCED may therefore play a role in fruit mesocarp carotenoid development and skin degreening. One CCD (CpCCD1) and two NCEDs (CpNCED1, CpNCED2) are predicted in papaya (Paull et al. 2008). A partial open reading frame (ORF) was found for another possible CpCCD that had 67 % identity to Arabidopsis CCD1. A papaya expressed sequence tag (EST) is not found for CpCCD1 or CpNCED1, and a chloroplast transit peptide was not found for CpNCED1. The Arabidopsis carotenoid cleavage family has five putative NCEDs and four putative CCDs (Auldridge et al. 2006b), and tomato has two: LeCCD1A and LeCCD1B (Schwab et al. 2008). CpCCD1 had 69 % identity with ArabidopsisAtNCED4; CpNCED1 has 73 % identity with ArabidopsisAtNCED5. CpNCED2 had 77 % identity with AtNECED3. The CpCCD and the CpNCEDs were all predicted to have a carotenoid oxygenase motif.
Fruit Growth
The plasticity in papaya size from 0.1 to 12 kg (Nakasone and Paull 1998) provides a unique opportunity to study fruit development control (Table 14.1). Tomato fruit can similarly vary from small berries (~2 g) to large fruit (1,000 g) (Lippman and Tanksley 2001) with a single gene ORFX (QTL loci fw 2.2) being responsible for 30 % of the difference (Frary et al. 2000; Bartley and Ishida 2003; Cong and Tanksley 2006). ORFX appears to act at or near the plasma membrane β subunit of CKII kinase (Cong and Tanksley 2006) and exerts its control through early fruit cell division (Lippman and Tanksley 2001). One homolog to ORFX was predicted in papaya (CpORFX) with 63 % identity with ORFX from Solanum pennellii and 62 % with Solanum lycopersicum. CpORFX shows homology to the cysteine-rich PLAC8 domain of unknown function that is found in animals and plants (Marchler-Bauer et al. 2005). No papaya ESTs were detected for CpORFX. The function of CpORFX in papaya is unknown though the wide range of papaya fruit sizes found presents the possibility it may have a role similar to that in tomato.
Fruit Shape
The shape of papaya fruit varies with fruit from female flowers being spherical to ovoid (Table 14.1), while fruit from hermaphrodite flowers being cylindrical or pear shaped (Nakasone and Paull 1998). A mutation in the quantitative trait locus (QTL) OVATE changes the shape of tomato from round to pear shaped (Liu et al. 2002). This regulatory gene apparently has its impact early in flower development (Ku et al. 2000a, b). A similar QTL in eggplant has a similar effect on shape (Ku et al. 1999). Ovate is a negative regulatory hydrophilic protein with a putative bipartite nuclear localization signal (Liu et al. 2002). Three ovate proteins are predicted in the papaya genome with homology to ovate sequences in Arabidopsis and S. lycopersicum (Blas et al. 2012). Papaya ESTs are only found for one of the gene (CpOVATE1) with 38 % identity to S. lycopersicum OVATE and 39 % identity with Arabidopsis. All three predicted papaya genes had the ~70 aa plant-specific motif (DUF623) of unknown function, also reported for the tomato OVATE gene (Liu et al. 2002). CpOVATE 2 has 31 % identity with Arabidopsis Ovate family (AtOFP8) and 58 % identity with tomato. CpOVATE3 which was on the same linkage group as the papaya sex-related gene has 67 % identity with tomato ovate protein and an ovate-like protein from tobacco with 65 % identity.
Another major gene controlling the elongated fruit shape of tomato is SUN that acts in a dosage-dependent manner (Xiao et al. 2008). The gene belongs to the IQD family with AtIQD1 being the only member with a known function. A homolog to SUN is found in papaya (Blas et al. 2012). This papaya homolog has four introns, the same as SUN and is 446 aa long versus 405 aa for SUN (Xiao et al. 2008). The homology of the predicted papaya gene is moderate (37 %) for SUN and 50 % for Arabidopsis IQD11 and 35 % for IQD12. Another predicted papaya homolog is two-thirds the length and has only two introns possibly due to a gap between the two sequenced contigs. This second predicted gene is 45 % homologous to SUN and 53 % to Arabidopsis IQD12. The variation in papaya shape from round to elongated, between female and hermaphrodite fruit, presents a unique model to ascertain the role of SUN homologs in determining fruit shape.
Expansins
The cell wall proteins termed expansin are involved in cell wall relaxation and growth (McQueen-Mason et al. 1992). A number of expansin genes are recognized (Cosgrove 2007). Expansins have been shown to be expressed during tomato fruit growth (Rose et al. 2000), and different isoforms are expressed during fruit ripening (Rose et al. 1997; Brummell et al. 1999, 2004). Papaya fruit has been shown to express an expansin (CpEXPA1), and four have been reported in banana fruit (Asha et al. 2007). Immunoblots have also detected expansins in ripening pear, persimmon, kiwi fruit, strawberry, and pineapple but not detected in pepper (Rose et al. 2000). The papaya genome contains at least 15 CpEXPA (Expansin A), three CpEXPB (Expansin B), and one CpEXPLA (Expansin Like A) (Table 14.2). Secretory sequences are not predicted on four of the CpEXPAs. All CpEXPs had similar intron positions and lengths to those described for Arabidopsis (Choi et al. 2006). Sampedro et al. (2005, 2006) proposed that the number in the last common ancestor for expansin EXLB is four, though none are predicted for papaya (Paull et al. 2008). The monocot/dicot ancestor has 15–17 expansin genes (Sampedro et al. 2005). Papaya is classified as a basal clade in the order Brassicales (Ronse de Craene and Haston 2006) with at least 19 expansin genes close to the number predicted for the monocot/dicot ancestor (15) (Sampedro et al. 2005).
The expansins are believed to have arisen and diversified early if not before colonization of plants on land (Li et al. 2002; Choi et al. 2006). Expansins are found in monocots, pines, ferns, and mosses. The closely related expansin-like sequences are similarly widely found, and the absence of any EXLB predicted in papaya is unexpected. One EXLB is found in Arabidopsis and rice, and four in poplar, and one predicted in pine (Sampedro et al. 2005, 2006). This suggests that EXLBs were present before the angiosperms/gymnosperms separation (Table 14.2). The Arabidopsis (At4g17030) and Pinus EXLB are distantly related (Sampedro et al. 2006), but no match was found to any predicted papaya gene sequences or in the papaya EST database. When predicted papaya protein models are queried using the Arabidopsis expansin-like B sequence, homology is found to EXPLA and EXPB sequences predicted in papaya. A number of other papaya sequences are predicted with an EG45 motif though these peptides had less than 100 amino acids and had no CBD motif.
Cell Wall Synthesis Genes
The major components of plant cell walls are structural proteins, cellulose, matrix polysaccharides, pectins, and in secondary cell walls, lignin (Carpita and Gibeaut 1993; Fry 2004). Matrix polysaccharides and pectins are two of the most important components of the cell wall, but little is known about their biosynthesis, assembly, and degradation (Cosgrove 1999). Matrix polysaccharides comprise the cross-linking glycan molecules that are bound to the cell wall through covalent and non-covalent bonds (Carpita and Gibeaut 1993; Fry 2004). Pectins are a family of complex polysaccharides that all contain the acidic 1,4-linked α-d-galacturonic acid and surround the cellulose microfibrils and cross-linked matrix polysaccharides (Carpita and Gibeaut 1993; Cosgrove 2001).
Cell wall synthesis genes potentially associated with growth and development involve numerous glycosyltransferase (GT) genes in different families (Henrissat et al. 2001). Papaya has at least 55 putative β-glucosyl, xylosyl, and rhamnosyltransferases (GT1) genes compared to 121 in Arabidopsis and 162 in tomato and higher numbers in other species. The higher number in tomato probably represents the triplicate replication that occurred on two separate occasions in this genome (The Tomato Genome Consortium 2012). These GT1 members are involved in hemicellulose synthesis. Twenty-one glycosyltransferases that are cellulose synthase-related genes (GT2) are identified in papaya versus the 48 Populus and 42 Arabidopsis and other sequenced species with the exception of poplar where only 6 are predicted (Table 14.3). These results suggest that an increase in the number of cellulose-related structural genes (Ces, Csl) might be due to genome duplication in Arabidopsis and specifically required for the biosynthesis of wood in poplar.
Galacturonosyltransferases are in family GT8 and are associated with pectin synthesis; 41 genes have been reported in Arabidopsis though only 25 have been found in papaya based upon sequence comparison with all other species so far having higher numbers than papaya (Table 14.3). There is more similarity for GT47 the glucuronosyltransferases, with papaya having 28 predicted genes and Arabidopsis having 39 genes. Papaya has at least 3 genes and Arabidopsis 10 genes for xyloglucan α-1,2-fucosyltransferase (GT37). Three genes for β-1,3-glucan (callose) synthase (GT48) are found in papaya with 13 reported for Arabidopsis and 9 genes in tomato. For most GT families, papaya has fewer genes for cell wall synthesis and metabolism than Arabidopsis and often similar number or less than that reported for other species. The exceptions are that papaya has the same number of genes for sialyltransferase (GT29) and β-glucuronosyltransferase (GT43) as other species listed, suggesting selected gene loss. The fewer genes for cell wall synthesis possibly reflect, in part, the absence of genome duplication in papaya.
Cell Wall Expansion and Degradation
Plant cell walls are capable of both plastic and elastic extension and control the rate and direction of cell expansion (Fry 2004). Modification of cell wall components during plant growth and development can significantly alter the cellular mechanics and the control of growth and morphogenesis. Enzymes that modify the cell wall structure are thought to play a central role in turnover and modification. The enzymes have been classified into a number of groups based upon catalytic activity [hydrolases (GH), esterases (CE), lyases (PL)] and then placed into families based upon their amino acid sequence and structure. This classification is used to analyze the Arabidopsis genome (Coutinho and Henrissat 1999; Henrissat et al. 2001). In fruit ripening, endo- and exo-polygalacturonase (PG), pectin methyl esterase, glucanase, galactosidase, and xylanase have been detected in papaya fruit (Paull and Chen 1983; Chen and Paull 2003; Thumdee et al. 2010). In papaya fruit, α-galactosidase protein and activity are detected and may be involved in pectin-related textural change during ripening (Soh et al. 2006). As tomato fruit ripens, the PG and β-galactosidase II mRNA increases in parallel with an increase in enzyme activities (Smith and Gross 2000). There are three beta-galactosidase genes expressed during papaya fruit ripening (Othman et al. 2011). The authors suggested that pPBGII and pBG(a) cDNA clones characterized in this work may be involved in fruit softening during papaya ripening while the fruit-specific pBG(b) may be related to early ripening stage.
As with the GT families, papaya has fewer members in most catalytic families than Arabidopsis or other species (Table 14.4). The exceptions are the chitinases (GH18), α-mannosidase (GH47), and carboxylesterase (CE1). In the case of the chitinases (GH18, GH19), this may reflect increased need for fungal resistance (Zhu et al. 2003). Pectin methyl esterases that remove methyl groups from the carbonyl group are present in multiple copies with 58 potential genes (CE8) in papaya (Table 14.4), 67 genes in Arabidopsis, and at least 79 in tomato and a higher number in other species. Forty-five genes for polygalacturonase (GH28) are found in papaya with 68 in Arabidopsis and at least 56 in tomato, some of which are exclusively expressed in fruit ripening (The Tomato Genome Consortium 2012) with similar numbers in other species.
Hemicellulose degradative enzymes include endo-1,4-β-glucanases (GH5, GH9), endo-1,4-β-xylanases (GH10), and GH16, xyloglucan endotransglycosylase/hydrolase (Table 14.4). Fourteen genes for β-1,4-endoglucanase (GH9) are found in papaya, 26 in Arabidopsis, and 23 in tomato. Similarly, Arabidopsis has 12 predicted endoxylanase-like genes (Simpson et al. 2003) in GH10, while papaya has 5 putative genes and tomato 10. Fewer genes for xyloglucan endotransglycosylase/hydrolase (GH16, XET, or XTH) are found in papaya with 16 than the 33 in Arabidopsis and 38 in tomato. Of the 38 in tomato, fifteen are predominately expressed in fruit development and ripening (The Tomato Genome Consortium 2012). In Arabidopsis, there are 51 β-1,3-glucanases (GH17) and only 36 genes so far found in papaya. Multiple copies of β-galactosidase (GH35) are found in papaya, Arabidopsis, and tomato, 10 copies in papaya, 18 in Arabidopsis, and 17 in tomato. Papaya has 21 pectin lyase (PL 1) genes, while Arabidopsis has 26 and tomato 23. The gene number in papaya for starch degradation is also fewer than in Arabidopsis (Table 14.4). Seven genes for α-amylase (GH13) are found in papaya versus 10 in Arabidopsis while there are 7 and 9, respectively, for β-amylase (GH14). In conclusion, the fewer number of cell wall degrading genes in papaya make it a simpler model system to study the network of genes involved in cell wall rearrangement that plays a crucial role in plant development.
Fruit Ripening
Skin Chlorophyll Degradation
The pathway of chlorophyll breakdown during fruit ripening is expected to be similar to that occurring during senescence and involves the removal of the phytol residue and the central Mg by chlorophyllase and a dechelatase (Barry 2009). The product of these two activities is pheophorbide a, which is the substrate for pheophorbide a oxygenase (PAO) and red chlorophyll catabolite reductase (RCCR) (Hortensteiner 2006). Proteomic analysis indicated that PAO located in plastid inner envelope membrane and RCCR was found in chloroplasts and mitochondria (Kleffmann et al. 2004; Yao and Greenbreg 2006). The breakdown product from PAO and RCCR is then exported from the degenerating chloroplast for further breakdown in the cytoplasm. The predicted Mg dechelatase gene has not been identified (Kunieda et al. 2005).
One chlorophyllase (CpCLH) gene is predicted in the papaya genome versus two genes in Arabidopsis thaliana and three in Brassica oleracea (Hortensteiner 2006). The papaya CpCLH protein has a predicted chloroplast transit peptide of 21 amino acids and has 60 % homology to B. oleracea BoCLH3 protein and 61 % to AtCLH2. The CpCLH has a 2Fe–2S ferredoxin, iron-sulfur binding site and is expressed in papaya based on ESTs. A magnesium chelatase subunit gene with three ESTs is predicted in papaya. This gene has 91 % identity with soybean (Paull et al. 2008).
PAO genes have been identified by functional genomics. A single PAO gene is predicted for papaya (CpPAO). The predicted gene is expressed based upon EST data and has a 72 aa chloroplast transit peptide. CpPAO was similar to the Arabidopsis AtPAO gene, first described as accelerated cell death (ACD1) in Arabidopsis. ACD1 has homology to tomato lethal leaf spot 1-like protein and a putative cell death suppressor protein in Oryza sativa. The PAO genes of all three species contain the predicted iron-sulfur binding domain (Paull et al. 2008). There are reports on stay-green gene, SGR, and its role in regulating the upstream process of PAO gene (Aubry et al. 2008) resulting in stay-green phenotype. Recently Hu et al. (2011) reported LeSGR gene identification and its expression during tomato fruit ripening. However, no similarity was found to any predicted papaya gene sequences to Arabidopsis stay-green or LeSGR.
As with a single gene in Arabidopsis, described as ACD2 (Wüthrich et al. 2000; Mach et al. 2001), a single RCCR gene is predicted in papaya (CpRCCR). The papaya CpRCCR protein has a predicted chloroplast transit peptide of 61 amino acids, and ESTs indicate it is expressed. RCCR has slightly greater identity (74 %) to the tomato RCCR (Pruzinska et al. 2007) than to the ArabidopsisACD2 (71 %).
Carotenoid Biosynthesis
Carotenoids serve several functions in plants (Cunningham and Gantt 1998). During fruit ripening as chlorophyll is degraded, the underlying carotenoids are unmasked, and de novo biosynthesis occurs in the papaya mesocarp chromoplasts. Carotenoids are derived from the 5-carbon compound isopentenyl pyrophosphate (IPP). The C40 backbone of all carotenoids is assembled from two C20 geranylgeranyl pyrophosphate (GGPP) molecules which in turn are each derived from four C5 IPP molecules; see volatile production discussion that follows.
Synthesis of phytoene from two GGPP molecules is the first step in the carotenoid-specific biosynthesis pathway and is catalyzed by phytoene synthase (PSY). PSY is a key regulator in carotenoid biosynthesis and has been found to be the rate-limiting enzyme in ripening tomato fruits, canola seeds, and marigold flowers (Bramley et al. 1992; Fraser et al. 1994; Hirschberg 2001). PSY is expected to have close membrane association as phytoene is lipid soluble and is localized, along with its subsequent end products, inside the chloroplasts and chromoplasts (Cunningham and Gantt 1998). Additionally, two forms of PSY, a chromoplast- and chloroplast-specific form, have been found in tomato: PSY-1 and PSY-2, respectively (Cunningham and Gantt 1998), with different tissue expression and only PSY-1 controls fruit tissue pigmentation (The Tomato Genome Consortium 2012). Desaturation of phytoene into z-carotene and lycopene is mediated by phytoene desaturase (PDS) and ζ-carotene desaturase (ZDS), respectively. This desaturation converts the colorless phytoene into the pink-hued lycopene. Like PSY, the desaturases PDS and ZDS are closely associated with the plastid membrane but are not integral membrane proteins (Schledz et al. 1996). Plastid transit peptides were detected for PSY, PDS, ZDS, and LCY-b from the papaya genome predicted genes. The published sequences for both PDS and ZDS appear to be lacking part of the leader sequences and do not start with a methionine required by the transit peptide prediction software. When the predicted peptide sequences for PSY and ZDS from the papaya genome are used, both of which start with methionine, transit peptides are predicted.
Cyclization of lycopene via lycopene e-cyclase (LCY-e) or lycopene b-cyclase (LCY-b) results in a α- or β-carotene, respectively. Yamamoto (1964) showed that 59.3 % of the total carotenoids in yellow-fleshed papaya are comprised of b-carotene or its derivatives, i.e., cryptoxanthin, indicating the b-ring cyclization pathway for synthesis of the yellow-pigmented carotenoids. Yellow fruits contain only trace amounts of lycopene (Schweiggert et al. 2011), while lycopene predominates in red papaya (51 % of total carotenoids). Tubular plastids are abundant in yellow papaya with larger crystalloid structures present in red papaya chromoplasts.
The family of lycopene cyclases in plants shows evidence of multiple gene duplication events and divergence of catalytic function (Blas et al. 2010). Plant lycopene cyclases share a common phylogenetic origin with bacterial crtY and crtL lycopene cyclases. Plant and bacterial lycopene cyclases are polypeptides of ~400 aa; however, the plant lycopene cyclases have an additional 100 aa N-terminal transit sequence. Five regions of conserved amino acid sequence have been identified: one putative dinucleotide-binding region and four motifs of unknown function (Armstrong and Hearst 1996; Cunningham and Gantt 1998).
Several papaya genes that encode enzymes in the carotenoid biosynthesis pathway have been previously identified: phytoene synthase (PSY), phytoene desaturase (PDS), ζ-carotene desaturase (ZDS), and a chloroplast-specific lycopene β-carotene (LCY-b) (Paull et al. 2008; Blas et al. 2010). BLASTn search of an EST database generated from a multiple-tissue type cDNA library supports the activity of these single genes in papaya. Putative papaya homologs were identified for lycopene ε-cyclase (LCY-e), β-ring carotene hydroxylase (CRTR), and zeaxanthin epoxidase (ZE) based on amino acid sequences obtained from GenBank (Paull et al. 2008) and cloning of a chromoplast-specific lycopene α-cyclase, CpCYC-b (Blas et al. 2010). Expression of these putative papaya carotenoid biosynthesis genes was supported by EST data in NCBI that include fruit EST (Devitt et al. 2006).
Sugar Accumulation
Photosynthates are transported into fruits mainly as sucrose with some fruits accumulating starch that is broken down to sugars during ripening while other fruit such as papaya depending upon a continued supply of sucrose for sweetness when ripe. During early fruit growth the sucrose is thought to be unloaded through the symplast pathway involving SS (sucrose synthase) and SPS (sucrose phosphate synthase) activities and metabolized in respiration and used for growth (Patrick 1997; Sturm and Tang 1999; Zhang et al. 2006). The activities of SS and SPS and their mRNA levels parallel the increase in growth. After fruit growth stops, sucrose accumulation is thought to involve a cell wall invertase to cleave the sucrose arriving via the phloem to hexoses, thus maintaining the sucrose source to sink gradient. The hexoses are then taken up into the fruit cells and the vacuole via hexose transporters (Caspari et al. 1994; Patrick 1997; Wu et al. 2004; Zhang et al. 2004, 2006).
Sugars, mainly as sucrose, begin to accumulate in papaya fruit about 110 days after anthesis during the last 28–42 days of fruit development (Chan 1979; Zhou and Paull 2001). Flesh total soluble solids can be as low as 5 % and up to 19 % (Table 14.1). The fact that invertase gene expression (Zhou et al. 2003; Zhu et al. 2003) and protein expression (Nogueira et al. 2012) and sugar (hexose) transporters (Sangwanangkul and Paull 2005) are upregulated and the protein detected just before and during sugar accumulation suggests the participation of both invertases and hexose transporter activities in fruit sugar accumulation.
Invertase (INV) is responsible for hydrolyzing sucrose into fructose and glucose and can be found in the cytoplasm, vacuole, and apoplast (Sturm 1999). The family is classified into acid, alkaline, and cell wall invertases (Tymowska-Lalanne and Kreis 1998; Ji et al. 2005; Bocock et al. 2008). Arabidopsis has one cell wall invertase and one acid invertase (Tsuchisaka et al. 2007). In tomato, 16 cell wall invertase sequences have been found (Fridman and Zamir 2003), while only 2 cell wall invertases are found in the papaya genome, CpCWINV1 and CpCWINV2 (Paull et al. 2008). These two papaya cell wall invertases have the GH32 domain, and the secretory peptide occupied the first 26 amino acids. CpCWINV1 has 100 % homology in sequence with the published papaya invertase (Zhou et al. 2003; Zhu et al. 2003). The second cell wall invertase (CpCWIN2) had high homology to the Arabidopsis cell wall INV1. A possible third papaya acid invertase CpCWINV3 has the required GH32 domain, but no signal peptide and no papaya ESTs are published. This acid invertase prediction showed moderate homology with coffee invertase and Arabidopsis cell wall invertase. Another predicted acid invertase had less than 20 % peptide sequence homology with Japan pear and Arabidopsis acid invertases.
Sucrose synthase (SUS) catalyzes the reversible conversion of sucrose and UDP to UDP-glucose and fructose and is only found in the cytoplasm (Martin et al. 1993; Baud et al. 2004). In Arabidopsis, five known sucrose synthases and another predicted sucrose synthase are found (Baud et al. 2004) with AtSUS1 having 67–72 % homology to sucrose synthase genes from other species. Citrus is reported to have three SUS genes (Komatsu et al. 2002), and papaya had four predicted SUS genes (CpSUS1 to CpSUS4) (Paull et al. 2008). All the predicted papaya SUS genes had sucrose synthase and glycosyltransferase (GT4) domains. The CpSUS1 has 99 % homology with the reported partial sequence of SUS in papaya and 90 % homology with that of citrus and 85 and 87 % to ArabidopsisAtSUS1 and AtSUS3, respectively (Paull et al. 2008). CpSUS2 showed similarity with citrus SUS2 85 % homology to AtSUS3, while CpSUS3 had high homology with onion (E-value 0.0) and tobacco (E-value 0.0). The fourth papaya sucrose gene (CpSUS4) had high homology with AtSUS5. Another SUS gene was predicted in papaya with a GT4 domain and sucrose synthase domains, but the sequence was not complete and only about half the expected peptide length.
Three sucrose phosphate synthase (SPS) genes were predicted in papaya (CpSPS1, CpSPS2, CpSPS3). All had GT4 and sucrose synthase domains (Paull et al. 2008). The CpSPS1 and CpSPS2 showed homology with the sucrose synthase in citrus and Arabidopsis. In higher plants, three families of sucrose phosphate synthase are described, and the functional differences between the families are not obvious (Langenkamper et al. 2002). Four putative SPS genes occur in Arabidopsis, two genes belong to Family A and one gene each belongs to Family B and Family C. The CpSPS1 had 87 % homology to citrus SPS and 72 % to Arabidopsis’s AtSPS1F. The second papaya SPS, CpSPS2, was similar to ArabidopsisAtSPS2F with a homology of 77 and 80 % to the citrus SPS. The AtSPS4F gene in Arabidopsis had a homology of 73 % with the CpSPS3 in papaya.
Papaya contains at least four hexose transporter genes (CpHT1 to CpHT4). In contrast to papaya, tomato contains three named loci (HT1, HT2, and HT3) and seven putative loci (Gear et al. 2000; Dibley et al. 2005); Arabidopsis contains four named loci (SGB1, TMT1, TMT2, TMT3) and five putative loci (Büttner 2007). A fifth papaya hexose transporter was indicated, but the genomic sequence of the ORF is incomplete. Papaya ESTs are found for two of the predicted hexose transporters CpHT2 and CpHT3 (Paull et al. 2008). The number of introns varied widely; CpHT1, CpHT2, CpHT3, and CpHT4 had 2, 12, 11, and 1, respectively. CpHT3 had a 60 % chance of being localizing to the plastid. The remaining papaya hexose transporters did not show specific localization or export. Papaya contains at least three incomplete hexose transporter genes or pseudogenes that had only 7–11 of the 12 expected transmembrane domains while having domains for MFS and sugar transporter. One of the pseudogenes had four ESTs identified. Two of the three pseudogenes had secretory sequences for the endoplasmic reticulum.
Respiration
Plant respiration differs from most animal respiration in possessing a number of additional components including (a) the presence of an alternative oxidase to cytochrome c oxidase that is cyanide insensitive, (b) an internal rotenone-insensitive NAD(P)H non-proton-pumping dehydrogenase, and (c) an external NAD(P)H non-proton-pumping dehydrogenases (Vanlerberghe and McIntosh 1997; Mackenzie and McIntosh 1999; Elhafez et al. 2006). Alternate oxidase (AOX) is a terminal quinol oxidase that is non-proton pumping and transfers an electron to oxygen while dissipating the energy as heat (McDonald 2008). AOX is resistant to cyanide (Henry and Nyns 1975) and sensitive to salicylhydroxamic acid (SHAM) (Schonbaum et al. 1971). AOX activity is taxonomically widespread and found in all kingdoms (McDonald 2008). In dicots, two types of AOX, AOX1 and AOX2, are found. Monocots seem to possess only AOX1 (Considine et al. 2001) which has been associated with a number of physiological functions such as thermogenesis in sacred lotus during flowering (Watling et al. 2006). Other roles include balancing carbon metabolism and electron transport, as may happen during climacteric fruit ripening (Theologis and Laties 1978; Considine et al. 2001), control of reactive oxygen species generation, O2 scavenging, and resistance to toxins and pathogenicity (Moore et al. 2002; McDonald 2008). Though AOX1 and AOX2 are present in multigene families in most plants (McDonald 2008), only two genes for alternative oxidase AOX1 were predicted for papaya, CpAOX1 and CpAOX2. The predicted CpAOX1 protein had a predicted signal peptide of 22 amino acids, two transmembrane regions, and was supported by finding papaya ESTs. The two characteristic AOX conserved cysteine residues occurred at Cys 105 and Cys 155, and two introns similar to Oryza sativa AOX were present. The predicted CpAOX1 gene has 79 and 71 % identity to the two Arabidopsis genes Q9ZRT8 and Q39219, respectively. CpAOX2 similarly has two transmembrane regions and no secretory sequence is predicted, though EST data suggested it is expressed. CpAOX2 has 88 % identity to Vigna unguiculata AOX (Q93X12) and 82 % with Arabidopsis (O22049).
Plant mitochondria can oxidize NADH and NADPH without proton pumping (Melo et al. 2004; Geisler et al. 2007). These dehydrogenases are referred to as Type II NAD(P)H (Michalecka et al. 2003; Melo et al. 2004). Type II dehydrogenases operate in parallel to Type I proton-pumping multi-subunit complex I. Both types are found in the electron transfer chains of several bacteria and in fungal and plant mitochondria. Type II dehydrogenases are found on the external (NDB) and internal (NDA) faces of the inner mitochondrial membrane and transfer electrons from NAD(P)H to quinone. It has been proposed that there are four distinct types of NAD(P)H dehydrogenases: two on either side of the inner membrane. One was to oxidize NADH and the other for NADPH (Roberts et al. 1995; Melo et al. 2004). However, Rasmusson et al. (1999) reported two Type II dehydrogenases for potato located on inner and outer surfaces of the inner mitochondrial membrane. Only three open reading frames (ORF) with similarity to Type II dehydrogenase genes were found in the papaya genome. The ORF for an internal NAD(P)H dehydrogenase, CpNDA1, had 67 % identity to ArabidopsisNDA2. A papaya EST was found for CpNDA1. One CpNDB gene was also predicted, CpNDB1, that had 79 % identity to ArabidopsisNDB4. A mitochondrial transit peptide was predicted for the CpNDB1 protein but not for CpNDA1. All had predicted ORFs motifs for the pyridine-redox superfamily.
Ethylene Synthesis
Arabidopsis and papaya show evolutionary similarity in the number of genes associated with ethylene synthesis, receptors, and response pathways. Papaya had four S-adenosyl-l-methionine synthases (SAMS), while 12 are reported for Arabidopsis (Yamagami et al. 2003) and 8 in tomato (Nakatsuka et al. 1998). Two of the papaya sequences from different regions of the papaya genome had the closest BLASTp homology to Catharanthus roseus SAM-2 (Paull et al. 2008). Unlike other SAMs, which do not have introns, one of these sequences appeared to have three. This may be a problem with the protein prediction, since corresponding gaps also appeared in the amino acid alignment with C. roseus SAM-2. A tBLASTn search using the assembled nucleotide sequence showed no match between amino acids 97 and 102, plus one large region of low homology between amino acids 254–328; otherwise, there was very good homology with many SAMs genes.
The 11 tomato 1-aminocyclopropane-1-carboxylic acid synthase (ACS) (LeACS) genes differ in tissue-specific expression, developmental control, and ethylene induction (Nakatsuka et al. 1998; Alba et al. 2005; The Tomato Genome Consortium 2012). The papaya genome was predicted to contain seven ACS genes, each with aminotransferase I and II domains which were predicted to be an ACS domain (Paull et al. 2008). Two other sequences translatable to the aminotransferases I and II domains were found; however, the closest BLASTp matches to these two sequences was not annotated as ACS, and their homology to non-ACS aminotransferases was high and was not included in the papaya ACS list. Three sequences matched papaya sequences already deposited in GenBank, including CpACS1 and CpACS2 (98 and 98 % identity, respectively). All seven putative ACS sequences contained the conserved dodecapeptide binding site (Yip et al. 1990), although, one had an Asn substitution for the key Lys that bound either PLP or the 2-aminobutyrate of adenosylmethionine. The possible existence of nonfunctional sequences with ACS homology in the papaya genome would not be novel; Arabidopsis has at least one nonfunctional gene (AtACS1) shown to encode a protein with no enzyme activity (Tsuchisaka et al. 2007) that is nevertheless included in the gene family. In addition, AtACS3 is not even transcribed, and AtACS10 and AtACS12 encode aminotransferase domains but have no ACS activity; none of these are included in the ACS family (Yamagami et al. 2003).
At a minimum, three ACO genes were predicted in papaya (Paull et al. 2008), with six additional sequences that showed partial homology to ACO-like genes or genes with the 2-oxoglutarate FeII oxygenase domain. This domain encompasses more than ACO’s, but it is the motif that describes ACO’s. As with the ACS, papaya has two well-studied CpACO genes, with multiple GenBank representatives. The CpACO genes identified (Paull et al. 2008) reflect BLASTp matches to CpACO1 and CpACO2 from Carica papaya, plus a translated supercontig with 79 % identity to a poplar protein shown to have ACO activity (Andersson-Gunneras et al. 2003). The sequence that matched CpACO1 lacks the first 83 amino acids from ACO1, due to sequencing ambiguities, but otherwise matched the remaining 235 amino acids. All three sequences retained the three conserved subdomains of ACOs and 2-oxoglutarate FeII oxygenases (Trentmann and Kende 1995), as well as the HxD site for enzymatic activity. CpACOs share the motif KxxR within the conserved C-terminal site that was identified as essential for Petunia hybrida ACO1 enzyme activity (Yoo et al. 2006). The protein encoded on CpACO3 differs at this site by three amino acids, which is more than any of the ACOs from 24 different plants, including papaya (LPKEPRFR versus the consensus QAKEPRFE). Five other predicted models had between 42 and 71 % homology to Arabidopsis proteins with 2-oxoglutarate FeII oxygenase domains, but these Arabidopsis proteins are not clearly involved in ACO activity.
Ethylene Response
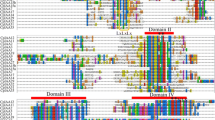
Papaya ripening has both ethylene insensitive and ethylene sensitive components (Chen and Paull 2003; Manenoi and Paull 2007) as has been found for tomato (Picton et al. 1993). At least three ethylene receptor genes (CpETR1 to CpETR3) were predicted in the papaya genome (Table 14.5 and Fig. 14.1). All three have the expected protein domains, though CpETR1 had no receiver domain (Paull et al. 2008). CpETR1 was homologous (100 %) to the papaya receptor cDNA sequence deposited by the late Dr. Hamid Lazan and his group (Che Husin et al. 2000) from Malaysia. This sequence has 78 % homology with the Arabidopsis ERS1 (Paull et al. 2008). Arabidopsis ETR1 has the greatest homology with CpETR2 (86 %). Based upon gene and protein structure, both CpETR1 and CpETR2 would fall into the ethylene receptor subfamily 1 (Hua et al. 1998) with CpETR3 being in subfamily 2 (Table 14.5 and Fig. 14.1). Subfamily 1 receptors maybe required for most ethylene responses (Wang et al. 2003). Originally, we reported four two-component ethylene receptors (Ming et al. 2008); however, a more thorough analysis indicated that one of the previously reported CpETRs lacked the expected GAF domain and a full histidine domain (Wang et al. 2003; Hall et al. 2007). This partial sequence is a 139 aa-long peptide with 75 % homology to the apple ethylene receptor. A fifth potential gene was short (224 aa) and only had a DUF623 domain of unknown function and low homology to the nearest ethylene receptor of Litchi chinensis. Papaya’s three predicted ethylene receptors were fewer than the five ETRs found in Arabidopsis (Schaller and Kieber 2002) and the six in tomato (Klee and Tieman 2002). A seventh pseudogene has been reported in tomato with no expression data (The Tomato Genome Consortium 2012). Since ethylene receptors seem to be a negative regulator of action (Hua and Meyerowitz 1998), degradation plays a significant role in the control of ripening (Kevany et al. 2007). The relatively small complement of papaya ethylene receptors means that fewer interactions may occur between receptors so that their role in ripening should be more easily discerned.
Predicted papaya ethylene receptors, structure and phylogenetic relationship with known Arabidopsis ethylene receptors. The whole genome shotgun sequence accession number in NCBI (WGS Accession), coding sequence for amino acids (CDS), the number of introns in the nucleotide sequence (introns), amino acids (aa), presence of discrete portion of protein possessing its own function and the superfamily (domain), similarity in peptide sequence with another species (homology), extent the sequences were invariant (identity), E-value was the expectation value for homology, and the number of papaya expressed sequence tags (EST) detected of at least 500 bases and 99 % identity [reproduced with kind permission from Springer Science+Business Media from Paull et al. (2008)]
Ethylene receptors are disulfide-linked dimers and ethylene binding involves a copper cofactor (Rodríguez et al. 1999). The gene RAN (response to antagonist) plays a role in making the ethylene receptor apoprotein functional by transporting a copper ion to the ethylene receptor site in the membrane-spanning regions. As with Arabidopsis, only one RAN homolog was found in papaya (Paull et al. 2008), and CpRAN had 76 % homology to Arabidopsis RAN1.
The ethylene response pathway after ethylene binding involves a RAF-related kinase CTR1 that has a role in the negative response by forming a complex with the receptor (Hua and Meyerowitz 1998). Ethylene binding inhibits CTR1 kinase activity (Ouaked et al. 2003) and thus relieves the repression of the ethylene response pathway (Alonso et al. 2003; Alonso and Stepanova 2004). A signal is then transmitted from the positive regulator EIN2 to EIN3/EILs and induces transcription of ethylene response factors (ERF). AtCTR1 is one of the six Arabidopsis MAPK kinases and three LeCTR1-like genes in tomato (Frye et al. 2001; Adams-Phillips et al. 2004a; The Tomato Genome Consortium 2012) with no evidence that more than one (AtCTR1, LeCTR1) being involved in the ethylene signal transduction pathway. At least one CpCTR1 gene was found in the papaya genome that had 56 % homology with LeCTR1. A second, incomplete CTR sequence was also found (Paull et al. 2008). One possible CpEIN2 gene was found; although the nucleotide and amino acid sequence were short (Paull et al. 2008), The alignments with CTR1-like kinase and the protein kinase family had fewer gaps, and about one hundred amino acid matches. Four possible EIN3/EIL1 genes were also found, with two (CpEIL1, CpEIN3) having high EIN2/EIL homologies to published sequences, and these were expressed as papaya ESTs. Arabidopsis has nine EIN3 and EIN3-like genes (Binder et al. 2007), and tomato has at least five (Stepanova and Alonso 2005).
Eight ERF genes were found with high homology, and another 12 predicted ERF-like sequences that had low homology matches or had no significant protein domains. One hundred and twenty-two ethylene-responsive binding factors in the AP2/ERF superfamily have been found in Arabidopsis (Nakano et al. 2006). AtERF1 falls into group IX with 17 members and 8 in group VIII. Using the conserved 60 amino acid sequences for AP2/ERF (Nakano et al. 2006), an additional 92 potential genes with this sequence were found in papaya which suggests more ERF may be present in the papaya genome.
Laticifers and Protease
The papaya fruit, as well as other aerial parts of the plant, has a dense network of laticifers (Roth and Clausnitzer 1972). The articulated laticifers begin as a column of cells that form vessel-like structures that retain the usual organelles. The milky latex is about 85 % water with the remaining 15 % being composed of 25 % insoluble matter of unknown composition. The soluble fraction contains carbohydrates (~10 %), salts (~10 %), lipids (~5 %), and biomolecules mainly proteins (~40 %) (El Moussaoui et al. 2001). The biomolecules include cysteine proteinases (papain, chymopapain), cystatin, β-1,3-glucanase, chitinase, lipases, and other proteins. The latex is thought to function as an induced defense mechanism (Salas et al. 2008; Shindo and Van Der Hoorn 2008). The latex is harvested from large fruited green varieties by scratching the skin, allowing the exuded latex to coagulate and dry, then collecting the dried latex by scraping. The latex is purified and its proteolytic activity is used for meat tenderization and chillproofing of beer (El Moussaoui et al. 2001). During fruit ripening some of the laticifers break and release latex under the cuticle disrupting this barrier and increasing fruit water loss rate (Paull and Chen 1989).
Four cysteine proteinases account for 80 % of the enzyme fraction (El Moussaoui et al. 2001). The proteinases are papain, chymopapain, caricain (proteinase omega), and glycyl endopeptidase (proteinase IV). Papaya proteinases are synthesized as proenzymes with a signal sequence. The prosequence is cleaved and proteinase activated. All four proteinases are members of the peptidase C1A subfamily of cysteine proteinases. Papain has been most intensively studied (El Moussaoui et al. 2001) although it is a minor component (5–8 %) of the endopeptidases in papaya latex (Baines and Brocklehurst 1982; Azarkan et al. 2003). One gene for a propapain (CpPAPA) precursor was predicted. The encoded protein had 26 amino acids ER secretory sequence and 95 % identity to the published papaya cDNA sequence (Paull et al. 2008). A papaya EST was found for CpPAPA.
A single papaya chymopapain gene (CpCHYP) was predicted that had 99 % identity to chymopapain isoforms I, III, and V and 100 % to chymopapain isoforms II and IV (Paull et al. 2008). Chymopapain is distinguished from papain by the proteolytic activity remaining after papain is removed (Jansen and Balls 1941). The five prochymopapain isoforms (I–V) were identified from sequenced leaf cDNAs (Taylor et al. 1999); all the isoforms have a free cysteine at position 251. The translated isoform cDNAs differ in one or two amino acid substitutions. At position 222, a cysteine is replaced by a tyrosine in isoforms III and V, while at position 266, valine is replaced by phenylalanine in isoforms II, III, and V. These single amino acid substitutions require only a single base change. The predicted CpCHYP protein had the nine amino acids reported for isoforms III–V. It is possible that the chymopapain isoforms differ because of errors in PCR, sequencing, or translation. The CpCHY protein, possibly isoform III, had 66 ESTs. A chymopapain isoform III EST was also reported by Devitt et al. (2006).
A number of other proteinases have been reported in papaya latex (El Moussaoui et al. 2001; Azarkan et al. 2006; Shindo and Van Der Hoorn 2008). Two caricain genes were predicted (proteinase omega) and one glycyl endopeptidase gene (Paull et al. 2008). The predicted gene numbers are in agreement with those reported (El Moussaoui et al. 2001), though one of the caricain gene cDNAs has not been deposited in GenBank. The CpCARP1 was 100 % identical to the described sequence, although it is only 130 amino acids long due to incomplete sequencing of the WGS. A glutamine cyclotransferase (glutamine cyclase) gene was predicted in papaya (CpGTR) as previously reported from papaya latex (Oberg et al. 1998; El Moussaoui et al. 2001). The predicted papaya gene (Paull et al. 2008) was longer than the predicted 288 amino acid peptide from the cDNA in GenBank though analysis suggested intron boundaries may have not been accurately predicted. One cystatin (CpCYST) and two Kunitz-type trypsin inhibitors (CpTINH1, CpTINH2) were predicted in the papaya genome. The cystatin had the I25A domain and was classified as member of the CY superfamily. Papaya ESTs were found for both the cystatin and trypsin inhibitors (Paull et al. 2008).
At least 27 β-1,3-glucanase proteins (GH17) were predicted in papaya, and activity has been detected in papaya latex (El Moussaoui et al. 2001). In addition to β-1,3-glucanase, chitinase II (GH19) has been reported for papaya latex. A gene with 61 % identity to the partial cDNA (P81241) was found in the papaya genome (Paull et al. 2008).
Other than the genes encoding proteinases and those laticifer proteins described previously, the genes encoding other proteins potentially found in papaya latex have not been well described. A search of the papaya genome for latex-associated protein genes found six genes including one for caspase reported as a rubber latex-abundant protein and lysophospholipase. In addition, a number of potential latex genes were found for which predicted protein had allergen domains. Othman and Nuraziyan (2010) reported the specific expression of subtilase in papaya mesocarp that reached the highest level at ripening stage. Recently, there was a report on a new phospholipase D (CpPLD1) in papaya latex (Abdelkafi et al. 2012). The sequence alignment showed four conserved regions (I–IV) defined by most of the PLD superfamily.
Volatile Production
All plant parts emit volatiles, which have multiple functions. Of the many roles in plants, ecological interactions such as defense against herbivores, pathogens, and as attractants for animals to disperse pollen and seeds are presumed to be principal functions (Pichersky and Gershenzon 2002; Phillips et al. 2006). Additionally, volatiles as components of flavor and aroma perception contribute substantially to the utility of plant parts as human foodstuffs. Odorants are volatile and interact with human olfactory reception (Buck and Axel 1991), but they must be somewhat lipophilic as well as water soluble, possess adequate vapor pressure, and occur in sufficient concentration for olfactory receptor interaction. At least 166 compounds have been identified in papaya fruit volatiles (Flath and Forrey 1977; Flath et al. 1990; MacLeod and Pieris 1983; Pino et al. 2003). The most commonly identified papaya volatiles have been methyl butanoate, ethyl butanoate, 3-methyl-1-butanol, and 1-butanol. The esters of lower fatty acids are considered to contribute much to the typical papaya flavor (Pino et al. 2003). The amounts and relative content of volatiles have been shown to vary with the stage of ripeness (Katague and Kirch 1965; Flath et al. 1990), for example, linalool production increases nearly 400-fold with only a sevenfold increase in benzyl isothiocyanate during ripening (Flath et al. 1990). 2-Ethyl-1-hexanol is found specifically in green fruit, while ethyl octanoate is found only in fully ripe fruit (Fuggate et al. 2010). When the fruit has reached the edible ripe stage, butanol, 3-methylbutanol, benzyl alcohol, and α-terpineol are at their maximum concentrations (Almora et al. 2004). One of the two volatiles that most closely resembled those of papayas is linalool, a product of the plastid synthesis pathway. The other papaya volatile is methyl benzoate, described as having papaya qualities on odor assessment (MacLeod and Pieris 1983). The sweaty odor quality of some papaya cultivars is due probably to the production of methyl butanoate (MacLeod and Pieris 1983). The Hawaii variety had very little methyl butanoate (0.06 %) (Flath and Forrey 1977), while the Sri Lankan variety had 48.3 % (MacLeod and Pieris 1983). In addition, benzyl isothiocyanate contributes a pungent off-odor. The amount of each volatile component varies both with cultivar and locality (MacLeod and Pieris 1983; Franco and Rodriguez 1993). No difference in volatile profiles is found in papaya fruit during on-tree and postharvest ripening (Fuggate et al. 2010).
Most volatile esters are described as fruity (Burdock 2002), and the most likely precursors are amino acids and lipids. The precursors of volatile esters, with branched alkyl chain, are valine, isoleucine, and other amino acids from threonine (Yabumoto et al. 1977; Newcomb et al. 2006). Aliphatic esters and alcohols are produced from free fatty acids such as linoleic and linolenic (Baldwin et al. 2000). The pathways come together in the formation of aldehydes that are reduced to alcohols by alcohol dehydrogenase (Speirs et al. 1998) and ester formation by alcohol acyltransferase (Fellman et al. 2000). The mesocarp alcohol dehydrogenase activity mesocarp increases dramatically during the early ripening stages, while alcohol acetyltransferase is active throughout ripening (Fuggate et al. 2010).
Single genes were found in the papaya genome for all the enzymes in the biosynthesis from threonine to 3-keto-3-methylvalerate (Paull et al. 2008). At least three branched chain amino transferase genes were found that could convert 3-keto-3-methylvalerate via isoleucine to 3-oxo-methylpentanoic acid. The next step to 2-methylbutanal involves pyruvate decarboxylase, and three genes encoding this enzyme were predicted from the genome data; all were predicted to have the characteristic domains. Four branched chain alpha-ketoacid dehydrogenases (alcohol dehydrogenases) were predicted with three that had papaya EST. The interconversion between 3-methyl butanol and 2-methylbutyl acetate possibly involves a single predicted alcohol acyltransferase gene and four predicted carboxylesterases (Paull et al. 2008).
Straight chain ester biosynthesis commonly occurs from fatty acids with linoleic acid being a common precursor. The first enzyme is lipoxygenase of which nine were predicted in papaya with another possible partial sequence found (Paull et al. 2008). Except for two of the predicted lipoxygenase, all had papaya ESTs. The predicted gene for the previously described papaya lipoxygenase (CpLOX1) had eight ESTs and a chloroplast transit peptide. The other CpLOXs did not have predicted chloroplast transit peptides, suggesting they may be located in the cytoplasm (Paull et al. 2008).
One papaya hydroperoxide lyase gene (P450 superfamily member) was found and no isomerase was predicted (Paull et al. 2008). Hex-3-enal to hex-2-enal isomerase has been reported to be unstable and no sequences are in the gene databases. The dehydrogenation of hex-3-enal to hex-3-enoic acid by aldehyde dehydrogenase could involve upwards of five genes. Two of the aldehydrogenases have predicted mitochondrial transit peptides and may be involved in volatile production. A number of alcohol dehydrogenase genes were predicted that could convert hex-3-enal to hex-3-enol (Paull et al. 2008).
Two volatiles with fruity/floral properties are 2-phenylacetaldehyde and 2-phenylethanol derived from phenylalanine. The first enzyme in the pathway is a bifunctional phenylacetaldehyde synthase possibly combining decarboxylation-amine oxidation leading to phenylacetaldehyde (Kaminaga et al. 2007). The last step is the reduction of 2-phenylacetaldehyde to 2-phenylethanol. The reduction involves NADPH and 2-phenylacetaldehyde reductase (Tieman et al. 2007). Genes were predicted in papaya for both steps in biosynthesis (Paull et al. 2008). Two predicted phenylacetaldehyde synthase (CpPALDS1, CpPALDS2) genes were found having high homology to the deposited gene sequences for rose and petunia (Paull et al. 2008). A single gene with moderate homology (48 %) to the tomato gene was predicted for the reduction to 2-phenylethanol (Tieman et al. 2007).
Terpenoids, widely distributed in lichens, algae, and higher plants, represent the most diverse family of natural products, as there are over 40,000 different structures identified thus far. Many terpenoids are nonvolatile and constitute important elements of plant functional processes, i.e., plant growth regulation, redox reactions, membrane structure, and photosynthesis (Bohlmann et al. 1998; Croteau et al. 2000; Aubourg et al. 2002). Volatile and flavor compounds from essential oils of various terpene classes are produced in leaves and fruit, while carotenoids give the fruit skin and flesh its unique color. Biosynthesis occurs through two different pathways: the acetate-mevalonate pathway in the cytoplasm (Bohlmann et al. 1998) and the non-mevalonate pathway in the plastids (Lichtenthaler 1999; 2000). The mevalonate pathway leads to sesquiterpenes and triterpenes (sterols), while monoterpenes, diterpenes, tetraterpenes (carotenoids), and polyterpenes are formed in the plastids.
Genes were predicted for most of the steps in terpene biosynthesis occurring both in the plastids and in the cytoplasm (Paull et al. 2008). Two 1-deoxy-d-xylulose-5-phosphate (DXP) synthases were predicted with the shorter predicted peptide lacking a chloroplast transit peptide. Another partial DXP synthase, one-third the length of the expected peptide, was found with 78 % homology to the rubber TPP enzyme domain. A full-length linalool synthase gene was predicted with two partial sequences. No geranylgeranyl pyrophosphate (GGPP) synthase was found that had a chloroplast transit peptide. A short sequence was found that has low homology (61 %, 105 aa) to GGPP synthase that catalyzes the farnesyl diphosphate to GGPP conversion.
Cytoplasmic terpene synthesis from mevalonate to dimethylally diphosphate has a single gene for each step (Paull et al. 2008). Two geranyl diphosphate genes were predicted with significant homology to published sequences. Two full-length squalene synthases were predicted along with two partial sequences that had high homology to a tomato homolog and Panax quinquefolius sequence.
The cleavage of carotenoids derived from the terpene synthesis pathway can lead to the production of volatiles. Carotenoid cleavage can occur at any conjugated double bonds by various enzymes such as CCD and NCEDs (discussed previously in the Fruit Growth section) to form an aldehyde or ketone in each product. The products include abscisic acid, strigolactone, and various aroma compounds (Vogel et al. 2008). While Arabidopsis contains nine CCD, five of which are directly involved in abscisic acid synthesis, the other CCDs lead to volatiles and nonvolatile apocarotenoids (Simkin et al. 2004), with one CCD able to cleave multiple substrates at different positions (Vogel et al. 2008). Only one CpCCD was predicted for papaya and another partial CCD ORF was found. Tomato contains two closely related CCDs, LeCCD1A and LeCCD1B, that generate the aldehydes and ketones including geranyl acetone, pseudoionone, and β-ionone (Simkin et al. 2004). CpCCD may serve a similar role in papaya ripening and generate ketone volatiles such as 6-methyl-5-hepten-2-one in tomatoes (Vogel et al. 2008) and as detected in papaya fruit volatiles (Flath et al. 1990).
Plant Growth Regulator Related Genes and Fruit Ripening
A list of papaya homologs to functionally characterized Arabidopsis plant growth regulator (PGR) synthesis, reception, response, and degradation genes (Quecini et al. 2007; Michael et al. 2008; Peng et al. 2009) was generated using BLASTp. Papaya has fewer genes in most PGR categories, and changes in ~150 microarray probes have been evaluated during ripening (Ming et al. 2012). Two of three ethylene receptors are upregulated, while the receptor previously reported to be expressed in papaya mesocarp (Che Husin et al. 2000) was not expressed in either of the ripening stages tested. One ACC synthase gene’s expression declined and another increased threefold. Most auxin-related genes declined with upregulation of auxin response factors (ARF8), three SAURs, and two auxin-binding proteins. GA-related genes were mostly downregulated. Two cytokinin oxidases and a zeatin 4-glucosyltransferase were upregulated. Most brassinosteroid-related genes were downregulated. Genes associated with ABA showed little change. These results indicate that the changes in PGR during ripening show varying response and potential positive and negative interactions (Wu and Paull, 2012, unpublished data).
Fruit Ripening and Evolution
Fleshy fruit ripening is a complex gene-controlled process, involving molecular, physiological, and biochemical events that require developmental and temporal coordination and regulation (Fig. 14.2). Fruit ripening changes in gene clusters such as for cell wall separation and degradation, carotenoid synthesis, chlorophyll breakdown, and altered organic acid metabolism affect texture, color, taste, and aroma (Giovannoni 2001, 2004; Alexander and Grierson 2002; Kevany et al. 2007). Although our understanding of ripening processes has progressed significantly over the last 30 years, the initiation and coordination of ripening are only partially understood. Variation between species and cultivars does occur in coordination, timing, and regulation of ripening, but similar networks of regulatory genes are expected to be expressed.
Similarities between senescence, abscission, and fruit ripening in the networks of genes involved in molecular and biological processes (modules) in ultimate developmental stages that lead to final death of the organ. Papaya is a unique model system in which all processes can be sampled and gene expression determined
The premise that genes expressed in different species during fruit ripening may be conserved and regulated in similar ways (Adams-Phillips et al. 2004b; Brummell et al. 2004; Barry and Giovannoni 2007; Seymour et al. 2008) is strengthened by the similarities in the ripening processes in fleshy fruit originating from different tissues. The edible fruit tissues vary widely, from the floral receptacle (strawberry) to fused clustered flowers (pineapple), the entire pericarp (tomato), the exocarp and mesocarp (apple, peach), the mesocarp only (papaya), the endocarp (citrus), and aril tissue arising from the funicle (litchi, durian) or the seed coat (rambutan). Dehiscence does not occur in most fleshy fruit but is common in arillate fruits (akee, durian). In durian, similar cell wall changes and biochemical activities occur in the thick woody pericarp as occurs in ripening and dry fruit dehiscence but not in the non-climacteric edible aril. The genes associated with softening of the Arabidopsis valve margin during dehiscence occur in abscission and are similar to the softening that occurs in fleshy fruit ripening (Roberts et al. 2000; Seymour and Manning 2002; Rose et al. 2004; Brummell 2006). Fleshy fruit expansion is probably not directly associated with ripening since many fruits are not fleshy and undergo ripening.
A key unanswered question in plant biology is how fleshy fruit ripening and associated softening evolved seemingly independently throughout the higher plant families. Fleshy fruits, with their mutually beneficial interaction of providing nutrition to animals and improved seed dispersal, have arisen independently in different families, have disappeared and reappeared, are not evolutionarily conserved, and show no clear association with phylogeny (Knapp 2002; Givnish et al. 2005; Lorts et al. 2008). This suggests few constraints on fruit evolution from the ancestral dry follicle (Roth 1977; Knapp 2002) with only limited changes needed in the interconnected networks of clustered genes. Genome-wide changes that would have led to fleshy fruit in connected branches of the angiosperm phylogenetic tree did not occur. Either developmental processes or modules are recruited and adapted to new functions with the original transcription factors and hormones remaining in place. Alternatively, transcription factors and hormones have been recruited by preexisting developmental processes. A similar process has apparently occurred in gymnosperms; Lovisetto et al. (2012) showed that MAD-box genes are involved in the formation of fruit-like structure and suggested that the same gene types have been recruited in phylogenetically distant species to make fleshy structures that also have different anatomical origins. The regulation and gene expression that occurs in fleshy fruit ripening is more likely associated with the recruiting of a more ancient developmental process (e.g., senescence) (Thomas et al. 2009). This recruitment could have occurred via adaptation to new functions with new interactions developed between the networks regulating the spatial-temporal expression of the various regulatory modules controlling senescence-like processes (Fig. 14.1).
Prospects
The genome of papaya (372 Mbp) is three times larger than that of Arabidopsis (125 Mbp) and two-fifths the size of the tomato genome (The Tomato Genome Consortium 2012). Papaya is predicted to code for 24,746 genes, which is 10–20 % fewer than in Arabidopsis (Ming et al. 2008, 2012) and 35 % fewer than the estimated 31,760 genes in tomato (The Tomato Genome Consortium 2012). Unlike the tomato genome, the papaya genome is largely euchromatic. The lower gene number in papaya may reflect that, unlike Arabidopsis, the papaya genome has not undergone recent whole-genome duplication and has had fewer opportunities for fractionation of linkage arrangements by post-duplication gene loss. This suggests that gene structure, function, and arrangement in papaya may resemble the ancestral angiosperm more closely than do previously sequenced angiosperms. Comparison of the five sequenced clade members suggests a minimal angiosperm gene set of 13,311. Fewer introns have been found in papaya genes than in their orthologs in Arabidopsis (8,319 versus 11,718) and rice (6,874 versus 7,011). Approximately 2,000 transcription factors in over 60 families have been identified in Arabidopsis, with papaya having fewer members in the majority of families, but a significantly higher number of predicted MADS-box proteins (171 versus 141). The comparison of gene expression and activity during fruit ripening suggests that the same regulatory pathways and similar controls occur. The lack of whole genome duplication and reductions in most papaya gene families and biosynthetic pathways (Ming et al. 2012) makes papaya a valuable tool for the study of the complex regulatory networks active in fruit ripening. For example, fewer gene numbers for ethylene synthesis, receptors, and in the ethylene response pathways may provide insights as to how ripening is controlled.
Many major questions remain about the regulation and systems involved in leaf senescence and abscission and fruit ripening. What are the primary cues for the initiation of these processes? What regulators or gene clusters exert the earliest control over signal perception and response networks? Why do some organs senescence, abscise, and ripen in response to a given stimulus whereas others do not? What genes control ethylene-independent and ethylene-dependent pathways, and how is the process regulated and how do the network pathways interact? Global expression analysis and functional genomics can provide the foundation to addressing these system network questions.
References
Abdelkafi S, Abousalham A, Fendri I, Ogata H, Barouh N, Fouquet B, Scheirlinckx F, Villeneuve P, Carrière F (2012) Identification of a new phospholipase D in Carica papaya latex. Gene 499:243–249
Adams-Phillips L, Barry C, Kannan P, Lecercq J, Bouzayen M, Giovannoni JJ (2004a) Ctr1-mediated ethylene signal transduction in tomato is encoded by a multigene family whose members display distinct regulatory features. Plant Mol Biol 54:387–404
Adams-Phillips L, Barry C, Giovannoni J (2004b) Signal transduction systems regulating fruit ripening. Trends Plant Sci 9:331–338
Alba R, Payton P, Fei Z, McQuinn R, Debbie P, Martin GB, Tanksley SD, Giovannoni JJ (2005) Transcriptome and selected metabolite analysis reveal multiple points of ethylene control during tomato fruit development. Plant Cell 17:2954–2965
Alexander L, Grierson D (2002) Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot 53:2039–2055
Alferez F, Zacarias L (1999) Interaction between ethylene and abscisic acid in the regulation of citrus fruit maturation. In: Kanellis AK, Chang C, Klee H, Blecker AB, Pech JC, Grierson D (eds) Biology and biotechnology of the plant hormone ethylene. II. Kluwer Academic, Amsterdam, pp 183–184
Almora K, Pino JA, Hernandez M, Duarte C, Gonzalez J, Roncal E (2004) Evaluation of volatiles from ripening papaya (Carica papaya L., var. Maradol roja). Food Chem 86:127–130
Alonso JM, Stepanova AN (2004) The ethylene signaling pathway. Science 306:1513–1515
Alonso JM, Stepanova AN, Solano R, Wisman E, Ferrari S, Ausubel FM, Ecker JR (2003) Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. Proc Natl Acad Sci USA 100:2992–2997
Andersson-Gunneras S, Hellgren JM, Bjorklund S, Regan S, Moritz T, Sundberg B (2003) Asymmetric expression of a poplar ACC oxidase controls ethylene production during gravitational induction of tension wood. Plant J 34:339–349
Armstrong GA, Hearst JE (1996) Carotenoids 2: genetics and molecular biology of carotenoid pigment biosynthesis. FASEB J 10:228–237
Asha VA, Sane AP, Sane PN (2007) Multiple forms of α-expansin genes are expressed during banana fruit ripening and development. Postharvest Biol Technol 45:184–192
Aubourg S, Lecharny A, Bohlmann J (2002) Genomic analysis of the terpenoid synthase (AtTPS) gene family of Arabidopsis thaliana. Mol Genet Genomics 267:730–745
Aubry S, Mani J, Hörtensteiner S (2008) Stay-green protein, defective in Mendel’s green cotyledon mutant, acts independent and upstream of pheophorbide a oxygenase in the chlorophyll catabolic pathway. Plant Mol Biol 67:2443–2456
Auldridge ME, Block A, Vogel JT, Dabney-Smith C, Mila I, Bouzayen M, Magallanes-Lundback M, DellaPenna D, McCarty DR, Klee HJ (2006a) Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J 45:982–993
Auldridge M, McCarty DR, Klee HJ (2006b) Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr Opin Plant Biol 9:315–321
Azarkan M, El Moussaoui A, van Wuytswinkel D, Dehon G, Looze Y (2003) Fractionation and purification of the enzymes stored in the latex of Carica papaya. J Chromatogr B 790:229–238
Azarkan M, Dibiani R, Goormaghtigh E, Raussens V, Baeyens-Volant D (2006) The papaya Kunitz-type trypsin inhibitor is a highly stable β-sheet glycoprotein. Biochim Biophys Acta Proteins Proteomics 1764:1063–1072
Baines BS, Brocklehurst K (1982) Characterization of papaya peptidase A as a cysteine proteinase of Carica papaya L. with active center properties that differ from those of papain by using 2,2′-dipyridyl disulfide and 4-chloro-7-nitrobenzofurazan as reactivity probes. Biochem J 205:205–211
Baldwin EA, Scott JW, Shewmaker CK, Schuch W (2000) Flavor trivia and tomato aroma: biochemistry and possible mechanisms for control of important aroma components. Hortscience 35:1013–1022
Barry CS (2009) The stay-green revolution: recent progress in deciphering the mechanisms of chlorophyll degradation in higher plants. Plant Sci 176:325–333
Barry C, Giovannoni JJ (2007) Ethylene and fruit ripening. J Plant Growth Regul 26:143–159
Barry CS, Llop-Tous MI, Grierson D (2000) The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiol 123:979–986
Bartley GE, Ishida BK (2003) Developmental gene regulation during tomato fruit ripening and in-vitro sepal morphogenesis. BMC Plant Biol 3, 4. http://www.biomedcentral.com/1471-2229/3/4
Baud S, Vaultier MN, Rochat C (2004) Structure and expression profile of the sucrose synthase multigene family in Arabidopsis. J Exp Bot 55:397–409
Biale JB (1964) Growth, maturation, and senescence in fruits. Science 146:880–888
Binder BM, Walker JM, Gagne JM, Emborg TJ, Hemmann G, Bleecker AB, Vierstra RD (2007) The Arabidopsis EIN3 binding F-Box proteins EBF1 and EBF2 have distinct but overlapping roles in ethylene signaling. Plant Cell 19:509–523
Blas AL, Ming R, Liu Z, Veatch OJ, Paull RE, Moore PH, Yu Q (2010) Cloning of papaya chromoplast specific lycopene â-cyclase, CpCYC-b, controlling fruit flesh color reveals conserved microsynteny and a recombination hotspot. Plant Physiol 152:2013–2022
Blas AL, Yu Q, Veatch OJ, Paull RE, Moore PH, Ming R (2012) Genetic mapping of quantitative trait loci controlling fruit size and shape in papaya. Mol Biol 29:457–466
Bocock PN, Morse AM, Dervinis C, Davis JM (2008) Evolution and diversity of invertase genes in Populus trichocarpa. Planta 227:565–576
Bohlmann J, Meyer-Gauen G, Croteau R (1998) Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci USA 95:4126–4133
Booker J, Auldridge ME, Wills S, McCarty DR, Klee HJ, Leyser O (2004) MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr Biol 14:1–20
Bramley P, Teulieres C, Blain I, Bird C, Schuch W (1992) Biochemical characterization of transgenic tomato plants in which carotenoid synthesis has been inhibited through the expression of antisense RNA to pTOM5. Plant J 2:343–349
Bruinsma J, Paull RE (1984) Respiration during postharvest development of soursop fruit. Annona muricata L. Plant Physiol 76:131–138
Brummell DA (2006) Cell wall disassembly in ripening fruit. Funct Plant Biol 33:103–119
Brummell DA, Harpster MH, Civello PM, Palys JM, Bennett AB, Dunsmuir P (1999) Modification of expansin protein abundance in tomato fruit alters softening and cell wall polymer metabolism during ripening. Plant Cell 11:2203–2216
Brummell DA, Dal Cin V, Crisosto CH, Labavitch JM (2004) Cell wall metabolism during maturation, ripening and senescence of peach fruit. J Exp Bot 55:2029–2039
Buck L, Axel R (1991) A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65:175–187
Burdock GA (2002) Fenaroli’s handbook of flavor ingredients, 4th edn. CRC, Boca Raton
Burg SP, Burg EA (1965) Relationship between ethylene production and ripening in bananas. Bot Gaz 126:200–204
Büttner M (2007) The monosaccharide transporter(-like) gene family in Arabidopsis. FEBS Lett 581:2318–2324
Carpita N, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with physical properties of the walls during growth. Plant J 3:1–30
Caspari T, Will A, Opekarova M, Sauer N, Tanner W (1994) Hexose/H+ symporters in lower and higher plants. J Exp Biol 196:483–491
Chan HT (1979) Sugar composition of papaya during development. Hortscience 14:140–141
Chan YK, Paull RE (2007) Papaya Carica papaya L., Caricaceae. In: Janick J, Paull RE (eds) Encyclopedia of fruit and nuts. CABI, Wallingford, pp 237–247
Che Husin NM, Ali ZM, Lazan H (2000) Isolation and characterization of carERS1, an ethylene receptor cDNA clone from ripening papaya fruit. Trans Malaysian Soc Plant Physiol 9:136–138
Chen NJ, Paull RE (2003) Endoxylanase expressed during papaya fruit ripening: purification, cloning and characterization. Funct Plant Biol 30:433–441
Choi D, Cho DT, Lee Y (2006) Expansins: expanding importance in plant growth and development. Physiol Plant 126:511–518
Cong B, Tanksley SD (2006) FW2.2 and cell cycle control in developing tomato fruit: a possible example of gene co-option in the evolution of a novel organ. Plant Mol Biol 62:867–880
Considine MJ, Daley DO, Whelan J (2001) The expression of alternative oxidase and uncoupling protein during fruit ripening in Mango. Plant Physiol 126:1619–1629
Cosgrove DJ (1999) Enzymes and other agents that enhance cell wall extensibility. Annu Rev Plant Physiol Plant Mol Biol 50:391–417
Cosgrove DJ (2001) Wall structure and wall loosening. A look backwards and forwards. Plant Physiol 125:131–134
Cosgrove DJ (2007) Expansin home page. https://homes.bio.psu.edu/expansins/. Accessed 30 Dec 2011
Coutinho PM, Henrissat B (1999) Carbohydrate-active enzymes: an integrated database approach. In: Gilbert HJ, Davies G, Henrissat B, Svensson B (eds) Recent advances in carbohydrate bioengineering. The Royal Society of Chemistry, Cambridge, pp 3–12
Croteau R, Lutchan TM, Lewis NG (2000) Natural products (secondary metabolites). In: Buchanan B, Gruissem W, Jones R (eds) Biochemistry and molecular biology of plants. Amer Soc Plant Physiol, Rockville, pp 1250–1318
Cunningham FX Jr, Gantt E (1998) Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 49:557–583
Devitt LC, Sawbridge T, Holton TA, Mitchelson K, Dietzgen RG (2006) Discovery of genes associated with fruit ripening in Carica papaya using expressed sequence tags. Plant Sci 170:356–363
Dibley SJ, Gear ML, Xiao Y, Rosche EG, Offler CE, McCurdy DW, Patrick JW (2005) Temporal and spatial expression of hexose transporters in developing tomato (Lycopersicon esculentum) fruit. Funct Plant Biol 32:777–785
El Moussaoui A, Nijs M, Paul C, Wintjens R, Vincentelli J, Azarkan M, Looze Y (2001) Revisiting the enzymes stored in the laticifers of Carica papaya in the context of their possible participation in the plant defence mechanism. Cell Mol Life Sci 58:556–570
Elhafez D, Murcha MW, Clifton R, Soole KL, Day DA, Whelan J (2006) Characterization of mitochondrial alternative NAD(P)H dehydrogenases in Arabidopsis: intraorganelle location and expression. Plant Cell Physiol 47:43–54
Fellman JK, Miller TW, Mattison DS, Mattheis JP (2000) Factors that influence biosynthesis of volatile flavor compounds in apple fruits. Hortscience 35:1026–1033
Flath RA, Forrey RR (1977) Volatile components of papaya (Carica papaya L., Solo variety). J Agric Food Chem 25:103–109
Flath RA, Light DM, Jang EB, Mon TR, John JO (1990) Headspace examination of volatile emissions form ripening papaya (Carica papaya L., Solo variety). J Agric Food Chem 38:1060–1063
Franco MRB, Rodriguez ADB (1993) Volatile components of two pawpaw cultivars. Arquivos Biol Tecnol 36:613–632
Frary A, Nesbitt C, Frary A, Grandillo S, van der Knaap E, Cong B, Liu J, Meller J, Elber R, Alpert KB, Tanksley SD (2000) fw2.2: a quantitative trait locus key to the evolution of tomato fruit size. Science 289:85–88
Fraser PD, Truesdale M, Bird C, Schuch W, Bramley PM (1994) Carotenoid biosynthesis during tomato fruit development. Plant Physiol 105:405–413
Fridman M, Zamir D (2003) Functional divergence of a syntenic invertase gene family in tomato, potato, and Arabidopsis. Plant Physiol 131:603–609
Fry SC (2004) Primary cell wall metabolism: tracking the careers of wall polymers in living plant cells. New Phytol 161:641–675
Frye CA, Tang D, Innes RW (2001) Negative regulation of defense responses in plant by conserved MAPKK kinase. Proc Natl Acad Sci USA 98:373–378
Fuggate P, Wongs-Aree C, Noichinda S, Kanlayanarat S (2010) Quality and volatile attributes of attached and detached ‘Pluk Mai Lie’ papaya during fruit ripening. Sci Hortic 126:120–129
Gear ML, McPhillips ML, Patrick JW, McCurdy DW (2000) Hexose transporters of tomato: molecular cloning, expression analysis and functional characterization. Plant Mol Biol 44:687–697
Geisler DA, Broselid C, Hederstedt L, Rasmusson AG (2007) Ca2+-binding and Ca2+-independent respiratory NADH and NADPH dehydrogenases of Arabidopsis thaliana. J Biol Chem 282:28455–28464
Giovannoni J (2001) Molecular biology of fruit maturation and ripening. Annu Rev Plant Physiol Plant Mol Biol 52:725–749
Giovannoni JJ (2004) Genetic regulation of fruit development and ripening. Plant Cell 16:S170–S180
Givnish TJ, Pires JC, Graham SW, McPherson MA, Prince LM, Patterson TB, Rai HS, Roalson EH, Evans TM, Hahn WJ, Millam KC, Meerow AW, Molvray M, Kores PJ, O'Brien HE, Hall JC, Kress WJ, Sytsma KJ (2005) Repeated evolution of net venation and fleshy fruits among monocots in shaded habitats confirms a priori predictions: evidence from an ndhF phylogeny. Proc R Soc B Biol Sci 272:1481–1490
Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pages V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, Bouwmeester H, Becard G, Beveridge CA, Rameau C, Rochange SF (2008) Strigolactone inhibition of shoot branching. Nature 455:189–194
Gussman CD, Goffredz JC, Gianfagna TJ (1993) Ethylene production and fruit-softening rates in several apple fruit ripening variants. Hortscience 28:135–137
Hall BP, Shakeel SN, Schaller GE (2007) Ethylene receptors: ethylene perception and signal transduction. J Plant Growth Regul 26:118–130
Henrissat B, Coutinho PM, Davies GJ (2001) A census of carbohydrate-active enzymes in the genome of Arabidopsis thaliana. Plant Mol Biol 47:55–72
Henry MF, Nyns EJ (1975) Cyanide-insensitive respiration. An alternative mitochondrial pathway. Sub-Cell Biochem 4:1–65
Hirschberg J (2001) Carotenoid biosynthesis in flowering plants. Curr Opin Plant Biol 4:210–218
Hortensteiner S (2006) Chlorophyll degradation during senescence. Annu Rev Plant Biol 57:55–77
Hu Z, Deng L, Yan B, Pan Y, Luo M, Chen X, Hu T, Chen G (2011) Silencing of the LeSGR1 gene in tomato inhibits chlorophyll degradation and exhibits a stay-green phenotype. Biol Plant 55:27–34
Hua J, Meyerowitz EM (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94:261–271
Hua J, Sakai H, Nourizadeh S, Chen QG, Bleecker AB, Ecker JR, Meyerowitz EM (1998) EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell 10:1321–1332
Ito PJ, Zee F, Hamilton RA, Paull R (1990) Genetic resources in tropical fruit and nut crops. Acta Hortic 269:125–130
Jansen EF, Balls AK (1941) Chymopapain: a new crystalline proteinase from papaya latex. J Biol Chem 137:459–460
Ji X, Van den Ende W, Van Laere A, Cheng S, Bennett J (2005) Structure, evolution, and expression of the two invertase gene families of rice. J Mol Evol 60:615–634
Kaminaga Y, Schnepp J, Peel G, Kish CM, Ben-Nissan G, Weiss D, Orlova I, Lavie O, Rhodes D, Wood K, Porterfield DM, Cooper AJL, Schloss JV, Pichersky E, Vainstein A, Dudareva N (2007) Plant phenylacetaldehyde synthase is a bifunctional homotetrameric enzyme that catalyzes phenylalanine decarboxylation and oxidation. J Biol Chem 281:23357–23366
Katague DB, Kirch ER (1965) Chromatographic analysis of volatile components of papaya fruits. J Pharm Sci 54:891–894
Kevany B, Taylor M, Dal Cin V, Klee HJ (2007) Ethylene receptor degradation controls the timing of ripening in tomato fruit. Plant J 51:458–467
Klee H, Tieman D (2002) The tomato ethylene receptor gene family: form and function. Physiol Plant 115:336–341
Kleffmann T, Russenberger D, Zychlinski A, Christopher W, Sjolander K, Gruissem W, Baginsky S (2004) The Arabidopsis thaliana chloroplast proteome reveals pathway abundance and novel protein functions. Curr Biol 14:354–362
Knapp S (2002) Tobacco to tomatoes: a phylogenetic perspective on fruit diversity in the Solanaceae. J Exp Bot 53:2001–2022
Komatsu A, Moriguchi T, Koyama K, Omura M, Akihama T (2002) Analysis of sucrose synthase genes in citrus suggests different roles and phylogenetic relationships. J Exp Bot 53:61–71
Ku HM, Doganlar S, Chen KY, Tanksley S (1999) The genetic basis of pear-shaped tomato fruit. TAG 9:844–850
Ku HM, Grandillo G, Tanksley SD (2000a) fs8.1, a major QTL, sets the pattern of tomato carpel shape well before anthesis. TAG 101:873–878
Ku HM, Vision T, Liu JP, Tanksley SD (2000b) Comparing sequenced segments of the tomato and Arabidopsis genomes: Large-scale duplication followed by selective gene loss creates a network of synteny. Proc Natl Acad Sci USA 97:9121–9126
Kunieda T, Amano T, Shioi Y (2005) Search for chlorophyll degradation enzyme, Mg-dechelatase, from extracts of Chenopodium album with native and artificial substrates. Plant Sci 169:177–183
Langenkamper G, Fung RWM, Newcomb RD, Atkinson RG, Gardner RC, MacRae EA (2002) Sucrose phosphate synthase genes in plants belong to three different families. J Mol Evol 54:322–332
Lelievre JM, Latche A, Jones B, Bouzayen M, Pech JC (1997) Ethylene and fruit ripening. Physiol Plant 101:727–739
Li Y, Darley CP, Ongaro V, Fleming A, Schipper O, Baldauf SL, McQueen-Mason SJ (2002) Plant expansins are a complex multigene family with an ancient evolutionary origin. Plant Physiol 128:854–864
Lichtenthaler HK (1999) The 1-deoxy-d-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 50:47–65
Lichtenthaler HK (2000) Non-mevalonate isoprenoid biosynthesis: Enzymes, genes and inhibitors. Biochem Soc Trans 28:785–789
Lippman Z, Tanksley SD (2001) Dissecting the genetic pathway to extreme fruit size in tomato using a cross between the small-fruited wild species Lycopersicon pimpinellifolium and L. esculentum var. Giant Heirloom. Genetics 158:413–422
Liu J, Eck JV, Cong B, Tanksley SD (2002) A new class of regulatory genes underlying the cause of pear-shaped tomato fruit. Proc Natl Acad Sci USA 99:13302–13306
Lorts CM, Briggeman T, Sang T (2008) Evolution of fruit types and seed dispersal. A phylogenetic and ecological snapshot. J Syst Evol 46:396–404
Lovisetto A, Guzzo F, Tadiello A, Toffali K, Favretto A, Casadoro G (2012) Molecular analyses of MADS-Box genes trace back to gymnosperms the invention of fleshy fruits. Mol Biol Evol 29:409–419
Mach JM, Castillo AR, Hoogstraten R, Greenberg JT (2001) The Arabidopsis accelerated cell death gene ACD2 encodes red chlorophyll catabolite reductase and suppresses the spread of disease symptoms. Proc Natl Acad Sci USA 98:771–776
Mackenzie S, McIntosh L (1999) Higher plant mitochondria. Plant Cell 11:571–586
MacLeod AJ, Pieris NM (1983) Volatile components of papaya (Carica papaya L.) with particular reference to glucosinolate products. J Agric Food Chem 31:1005–1008
Manenoi A, Paull RE (2007) Papaya fruit softening, endoxylanase gene expression, protein and activity. Physiol Plant 131:470–480
Manenoi A, Bayogan ERV, Thumdee S, Paull RE (2007) Utility of 1-methylcyclopropene as a papaya postharvest treatment. Postharvest Biol Technol 44:55–62
Marchler-Bauer A, Anderson JB, Cherukuri PF, DeWeese-Scott C, Geer LY, Gwadz M, He S, Hurwitz DI, Jackson JD, Ke Z, Lanczycki CJ, Liebert CA, Liu C, Lu F, Marchler GH, Mullokandov M, Shoemaker BA, Simonyan V, Song JS, Thiessen PA, Yamashita RA, Yin JJ, Zhang D, Bryant SH (2005) CDD: a conserved domain database for protein classification. Nucleic Acids Res 33:192–196
Martin T, Frommer WB, Salanoubat M, Willmitzer L (1993) Expression of an Arabidopsis sucrose synthase gene indicates a role in metabolization of sucrose both during phloem loading and in sink organs. Plant J 4:367–377
McDonald AE (2008) Alternative oxidase: an inter-kingdom perspective on the function and regulation of this broadly distributed ‘cyanide-resistant’ terminal oxidase. Funct Plant Biol 35:535–552
McQueen-Mason S, Durachko DM, Cosgrove DJ (1992) Two endogenous proteins that induce cell wall expansion in plants. Plant Cell 4:1425–1433
Melo AMP, Bandeiras TM, Teixeira M (2004) New insights into type II NAD(P)H:quinone oxidoreductases. Microbiol Mol Biol Rev 68:603–616
Michael TP, Breton G, Hazen SP, Priest H, Mockler TC, Kay SA, Chory J (2008) A morning-specific phytohormone gene expression program underlying rhythmic plant growth. PLoS Biol 6:e225
Michalecka AM, Svensson AS, Johansson FI, Agius SC, Johanson U, Brennicke A, Binder S, Rasmusson AG (2003) Arabidopsis genes encoding mitochondrial type II NAD(P)H dehydrogenases have different evolutionary origin and show distinct responses to light. Plant Physiol 133:642–652
Ming R, Hou S, Feng Y, Yu Q, Dionne-Laporte A, Saw JH, Senin P, Wang W, Ly BV, Lewis KLT, Salzberg SL, Feng L, Jones MR, Skelton RL, Murray JE, Chen C, Qian W, Shen J, Du P, Eustice M, Tong E, Tang H, Lyons E, Paull RE, Michael TP, Wall K, Rice D, Albert H, Li Wang M, Zhu YJ, Schatz M, Nagarajan N, Agbayani R, Guan P, Blas A, Man Wai C, Ackerman CM, Ren Y, Liu C, Wang J, Wang J, Na JK, Shakirov EV, Haas B, Thimmapuram J, Nelson D, Wang X, Bowers JE, Gschwend AR, Delcher AL, Singh R, Suzuki JY, Tripathi S, Neupane K, Wei H, Irikura B, Paidi M, Jiang N, Zhang W, Presting G, Windsor A, Navajas-Pérez R, Torres MJ, Feltus FA, Porter B, Li Y, Burroughs AM, Luo MC, Liu L, Christopher DA, Mount SM, Moore PH, Sugimura T, Jiang J, Schuler MA, Friedman V, Mitchell-Olds T, Shippen DE, dePamphilis CW, Palmer JD, Freeling M, Paterson AH, Gonsalves D, Wang L, Alam M (2008) Genome of the transgenic tropical fruit tree papaya (Carica papaya Linnaeus). Nature 452:991–996
Ming R, Yu Q, Moore PH, Paull RE, Zhu YJ, Schuler MA, Jiang J, Paterson AH (2012) Genome of papaya, a fast growing tropical fruit tree. Tree Genet Genomics 8:445–462
Moore AL, Albury MS, Crichton PG, Affourtit C (2002) Function of the alternative oxidase: is it still a scavenger? Trends Plant Sci 7:478–481
Nakano T, Suzuki K, Fujimura T, Shinshi H (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140:411–432
Nakasone HY, Paull RE (1998) Papaya. In: Tropical fruit. CAB International, Wallingford, pp 239–269
Nakatsuka A, Murachi S, Okunishi H, Shiomi S, Nakano R, Kubo Y, Inaba A (1998) Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, of 1-aminocyclopropane-1-carboxylase oxidase and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol 118:1295–1305
Newcomb RD, Crowhurst RN, Gleave AP, Rikkerink EHA, Allan AC, Beuning LL, Bowen JH, Kim E, Jamieson R, Janssen BJ, Laing WA, McArtney S, Nain B, Ross GS, Snowden KC, Souleyre EJF, Walton EF, Yauk YK (2006) Analyses of expressed sequence tags from apple. Plant Physiol 141:147–166
Nogueira SB, Labate CA, Gozzo FC, Pilau EJ, Nascimento FM, Lajolo JRO (2012) Proteomic analysis of papaya fruit ripening using 2DE-DIGE. J Proteomics 75:1428–1439
Oberg KA, Ruysschaert JM, Azarkan M, Smolders N, Zerhouni S, Wintjens R, Amrani A, Looze Y (1998) Papaya glutamine cyclase, a plant enzyme highly resistant to proteolysis, adopts an all-b conformation. Eur J Biochem 258:214–222
Othman R, Nuraziyan A (2010) Fruit-specific expression of papaya subtilase gene J. Plant Physiol 167:131–137
Othman R, Chong HL, Choo TS, Mohd Ali Z (2011) Three b-galactosidase cDNA clones related to fruit ripening in papaya (Carica papaya). Acta Physiol Plant 33:2301–2310
Ouaked F, Rozhon W, Lecourieux D, Hirt H (2003) A MAPK pathway mediates ethylene signaling in plants. EMBO J 22:1282–1288
Park BH, Karpinets TV, Syed MH, Leuze MR, Uberbacher EC (2010) CAZymes Analysis Toolkit (CAT): web-service for searching and analyzing carbohydrate-active enzymes in a newly sequenced organism using CAZy database. Glycobiology 20:1574–1584. http://cricket.ornl.gov/cgi-bin/cat.cgi
Patrick JW (1997) Phloem unloading: sieve element unloading and post-sieve element transport. Annu Rev Plant Physiol Plant Mol Biol 48:191–222
Paull RE, Chen NJ (1983) Postharvest variation in cell wall-degrading enzymes of papaya (Carica papaya L.) during fruit ripening. Plant Physiol 72:382–385
Paull RE, Chen NJ (1989) Waxing and plastic wraps influence water loss from papaya fruit during storage and ripening. J Am Soc Hortic Sci 114:937–942
Paull R, Irikura B, Wu PF, Turano H, Chen N, Blas A, Fellman J, Gschwend A, Wai CM, Yu Q, Presting G, Alam M, Ming R (2008) Fruit development, ripening and quality related genes in the papaya genome. Trop Plant Biol 1:246–277
Peng Z-Y, Zhou X, Li L, Yu X, Li H, Jiang Z, Cao G, Bai M, Wang X, Jiang C, Lu H, Hou X, Qu L, Wang Z, Zuo J, Fu X, Su Z, Li S, Guo H (2009) Arabidopsis hormone database: a comprehensive genetic and phenotypic information database for plant hormone research in Arabidopsis. Nucleic Acids Res 37:D975–D982
Phillips DR, Rasbery JM, Bartel B, Matsuda SPT (2006) Biosynthetic diversity in plant triterpene cyclization. Curr Opin Plant Biol 9:305–314
Pichersky E, Gershenzon J (2002) The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol 5:237–243
Picton S, Gray J, Barton S, AbuBakar U, Lowe A, Grierson D (1993) cDNA cloning and characterisation of novel ripening-related mRNAs with altered patterns of accumulation in the ripening inhibitor (rin) tomato ripening mutant. Plant Mol Biol 23:193–207
Pino JA, Almora K, Marbot R (2003) Volatile components of papaya (Carica papaya L., Maradol variety) fruit. Flavour Fragrance J 18:492–496
Pruzinska A, Anders I, Aubry S, Schenk A, Tapernoux-Luthe E, Muller T, Krautler B, Hortensteiner S (2007) In vivo participation of red chlorophyll catabolite reductase in chlorophyll breakdown. Plant Cell 19:369–387
Qiu YX, Nishina MS, Paull RE (1995) Papaya fruit growth, calcium uptake and fruit ripening. J Am Soc Hortic Sci 120:246–253
Quecini V, Torres GAM, Rosa Jr VEd, Gimenes MA, Machado JBdM, Figueira AvdO, Benedito V, Targon MLPN, Cristofani-Yaly M (2007) In silico analysis of phytohormone metabolism and communication pathways in citrus transcriptome. Genet Mol Biol 30:713–733
Rasmusson AG, Svensson AS, Knoop V, Grohmann L, Brennicke A (1999) Homologues of yeast and bacterial rotenone-insensitive NADH dehydrogenases in higher eukaryotes: two enzymes are present in potato mitochondria. Plant J 20:79–87
Roberts TH, Fredlund KM, Moller IM (1995) Direct evidence for the presence of two external NAD(P)H dehydrogenases coupled to the electron transport chain in plant mitochondria. FEBS Lett 373:307–309
Roberts JA, Whitelaw CA, Gonzalez-Carranza ZH, McManus MT (2000) Cell separation processes in plants - models, mechanisms and manipulation. Ann Bot 86:223–235
Rodrigo MJ, Marcos JF, Alférez F, Mallent MD, Zacarías L (2003) Characterization of Pinalate, a novel Citrus sinensis mutant with a fruit-specific alteration that results in yellow pigmentation and decreased ABA content. J Exp Bot 54:727–738
Rodrigo MJ, Alquezar B, Zacarias L (2006) Cloning and characterization of two 9-cis-epoxycarotenoid dioxygenase genes, differentially regulated during fruit maturation and under stress conditions, from orange (Citrus sinensis L. Osbeck). J. Exp Bot 57:633–643
Rodríguez FI, Esch JJ, Hall AE, Binder BM, Schaller GE, Bleecker AB (1999) A copper cofactor for the ethylene receptor ETR1 from Arabidopsis. Science 283:996–998
Ronse de Craene LP, Haston E (2006) The systematic relationships of glucosinolate-producing plants and related families: a cladistic investigation based on morphological and molecular characters. Bot J Linnean Soc 151:453–494
Rose JKC, Lee HH, Bennett AB (1997) Expression of a divergent expansin gene is fruit-specific and ripening regulated. Proc Natl Acad Sci USA 94:5955–5960
Rose JKC, Cosgrove DJ, Albersheim P, Darvill AG, Bennett AB (2000) Detection of expansin proteins and activity during tomato fruit ontogeny. Plant Physiol 123:1583–1592
Rose JKC, Saladié M, Catalá C (2004) The plot thickens: new perspectives of primary cell wall modification. Curr Opin Plant Biol 7:296–301
Roth I (1977) Fruits of angiosperms. Gebrüder Borntraeger, Berlin
Roth I, Clausnitzer I (1972) Desarrollo y anatomía del fruto y la semilla de Carica papaya L. (Lechosa). Acta Bot Venezuela 7:187–206
Salas CE, Gomes MTR, Hernandez M, Lopes MTP (2008) Plant cysteine proteinases: evaluation of the pharmacological activity. Phytochemistry 69:2263–2269
Sampedro J, Lee Y, Carey RE, de Pamphilis C, Cosgrove DJ (2005) Use of genomic history to improve phylogeny and understanding of births and deaths in a gene family. Plant J 44:409–419
Sampedro J, Carey RE, Cosgrove DJ (2006) Genome histories clarify evolution of the expansin superfamily: new insights from the poplar genome and pine ESTs. J Plant Res 119:11–21
Sangwanangkul P, Paull RE (2005) The role of hexose transporter in sugar accumulation of papaya fruit during maturation and ripening. Acta Hortic 740:313–316
Schaller GE, Kieber JJ (2002) Ethylene. In: Somerville C, Meyerowitz E (eds) The Arabidopsis book. American Society of Plant Biologists, Rockville
Schledz M, al-Babili S, von Lintig J, Haubruck H, Rabbani S, Kleinig H, Beyer P (1996) Phytoene synthase from Narcissus pseudonarcissus: functional expression, galactolipid requirement, topological distribution in chromoplasts and induction during flowering. Plant J 10:781–792
Schonbaum GR, Bonner WD Jr, Storey BT, Bahr JT (1971) Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol 47:124–128
Schwab W, Davidovich-Rikanati R, Lewinsohn E (2008) Biosynthesis of plant-derived flavor compounds. Plant J 54:712–732
Schweiggert RM, Steingass CB, Heller A, Esquivel P, Reinhold C (2011) Characterization of chromoplasts and carotenoids of red- and yellow-fleshed papaya (Carica papaya L.). Planta 234:1031–1044
Seymour GB, Manning K (2002) Genetic control of fruit ripening. In: Knee MM (ed) Fruit quality and its biological basis. Academic, Sheffield, pp 253–274
Seymour G, Poole M, Manning K, King GJ (2008) Genetics and epigenetics of fruit development and ripening. Curr Opin Plant Biol 11:58–63
Shindo T, Van Der Hoorn RAL (2008) Papain-like cysteine proteases: key players at molecular battlefields employed by both plants and their invaders. Mol Plant Pathol 9:119–125
Simkin A, Schwartz S, Auldridge M, Taylor M, Klee HJ (2004) The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles B-ionone, pseudoionone and geranylacetone. Plant J 40:882–892
Simpson DJ, Fincher GB, Huang AH, Cameron-Mills V (2003) Structure and function of cereal and related higher plant (1-4)-β-xylan endohydrolases. J Cereal Sci 37:111–127
Smith DL, Gross KC (2000) A family of at least seven ß-galactosidase genes is expressed during tomato fruit development. Plant Physiol 123:1173–1183
Soh CP, Ali ZM, Lazan H (2006) Characterization of an alpha-galactosidase with potential relevance to ripening related texture changes. Phytochemistry 67:242–254
Speirs J, Lee E, Holt K, Yong-Duk K, Scott NS, Loveys B, Schuch W (1998) Genetic manipulation of alcohol dehydrogenase levels in ripening tomato fruit affects the balance of some flavor aldehydes and alcohols. Plant Physiol 117:1047–1058
Stepanova AN, Alonso JM (2005) Ethylene signaling pathway. Sci STKE 276:cm3. doi:10.1126/stke.2762005cm3
Sturm A (1999) Invertases. Primary structures, functions, and roles in plant development and sucrose partitioning. Plant Physiol 121:1–8
Sturm A, Tang G-Q (1999) The sucrose-cleaving enzymes of plants are crucial for development, growth and carbon partitioning. Trends Plant Sci 4:401–407
Taylor MAJ, Al-Sheikh M, Revell DF, Sumner IG, Connerton IF (1999) cDNA cloning and expression of Carica papaya prochymopapain isoforms in Escherichia coli. Plant Sci 145:41–47
Theologis A, Laties GG (1978) Respiratory contribution of the alternate path during various stages of ripening in avocado and banana fruits. Plant Physiol 62:249–255
Thomas H, Huang L, Young M, Ougham H (2009) Evolution of plant senescence. BMC Evol Biol 9:163
Thumdee S, Manenoi A, Chen NJ, Paull RE (2010) Papaya fruit softening: role of hydrolases. Trop Plant Biol 3:98–109
Tieman DM, Loucas HM, Kim JY, Clark DG, Klee HJ (2007) Tomato phenylacetaldehyde reductases catalyze the last step in the synthesis of the aroma volatile 2-phenylethanol. Phytochemistry 68:2660–2669
Tomato Consortium (2012) The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485:635–641
Trentmann SM, Kende H (1995) Analysis of Arabidopsis cDNA that shows homology to the tomato E8 cDNA. Plant Mol Biol 29:161–166
Tsuchisaka A, Yamagami T, Alonso JM, Ecker JR, Theologis A (2007) The Arabidopsis 1-aminocyclopropane-1-carboxylic acid synthase (ACS) gene family. In: Proceedings of the 18th international conference on Arabidopsis research, 2007. TAIR Publication 501722104
Tymowska-Lalanne Z, Kreis M (1998) Expression of the Arabidopsis thaliana invertase gene family. Planta 207:259–265
Vanlerberghe GC, McIntosh L (1997) Alternative oxidase: from gene to function. Annu Rev Plant Physiol Plant Mol Biol 48:703–734
Vogel JT, Tan B-C, McCarty DR, Klee HJ (2008) The carotenoid cleavage dioxygenase 1 enzyme has broad substrate specificity, cleaving multiple carotenoids at two different bond positions. J Biol Chem 283:11364–11373
Wang W, Hall AE, O’Malley R, Bleecker AB (2003) Canonical histidine kinase activity of the transmitter domain of the ETR1 ethylene receptor from Arabidopsis is not required for signal transmission. Proc Natl Acad Sci USA 100:352–357
Watling JR, Robinson SA, Seymour RS (2006) Contribution of the alternative pathway to respiration during thermogenesis in flowers of the sacred lotus. Plant Physiol 140:1367–1373
Wu G-L, Zhang X-Y, Zhang L-Y, Pan Q-H, Shen Y-Y, Zhang D-P (2004) Phloem unloading in developing walnut fruit is symplasmic in the seed pericarp and apoplasmic in the fleshy pericarp. Plant Cell Physiol 45:1461–1470
Wüthrich KL, Bovet L, Hunziker PE, Donnison IS, Hörtensteiner S (2000) Molecular cloning, functional expression and characterisation of RCC reductase involved in chlorophyll catabolism. Plant J 21:189–198
Xiao H, Jiang N, Schaffner E, Stockinger EJ, van der Knaap E (2008) A retrotransposon-mediated gene duplication underlies morphological variation of tomato fruit. Science 319:1527–1530
Yabumoto K, Yamaguchi M, Jennings WG (1977) Biosynthesis of some volatiles constituents of `muskmelon Cucumis melo. Chem Mikrobiol Technol Lebensraum 5:53–56
Yamagami T, Tsuchisaka A, Yamada K, Haddon WF, Harden LA, Theologis A (2003) Biochemical diversity among the 1-amino-cyclopropane-1-carboxylate synthase isozymes encoded by the Arabidopsis gene family. J Biol Chem 278:49102–49112
Yamamoto HY (1964) Comparison of the carotenoids in yellow- and red-fleshed Carica papaya. Nature 201:1049–1050
Yao N, Greenbreg JT (2006) Arabidopsis ACCELERATED CELL DEATH2 modulates programmed cell death. Plant Cell 18:397–411
Yip WK, Dong JG, Kenny JW, Thompson GA, Yang SF (1990) Characterization and sequencing of the active site of 1-aminocyclopropane-1-carboxylate synthase. Proc Natl Acad Sci USA 87:7930–7934
Yoo A, Seo YS, Jung JW, Sung SK, Kim WT, Lee W, Yang DR (2006) Lys296 and Arg299 residues in the C-terminus of MD-ACO1 are essential for a 1-aminocyclopropane-1-carboxylate oxidase enzyme activity. J Struct Biol 156:407–420
Zeevart JAD, Creelman RA (1988) Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol Plant Mol Biol 39:439–473
Zhang L-Y, Peng Y-B, Pelleschi-Travier S, Fan Y, Lu Y-F, Lu Y-M, Gao X-P, Shen Y-Y, Delrot S, Zhang D-P (2004) Evidence for apoplasmic phloem unloading in developing apple fruit. Plant Physiol 135:574–586
Zhang X-Y, Wang X-L, Wang X-F, Xia G-H, Pan Q-H, Fan R-C, Wu F-Q, Yu X-C, Zhang D-P (2006) A shift of phloem unloading from symplasmic to apoplasmic pathway is involved in developmental onset of ripening in grape berry. Plant Physiol 142:220–232
Zhou LL, Paull RE (2001) Sucrose metabolism during papaya (Carica papaya) fruit growth and ripening. J Am Soc Hortic Sci 126:351–357
Zhou L, Chen CC, Ming R, Christopher DA, Paull RE (2003) Apoplastic invertase and its enhanced expression and post-translational control during papaya fruit maturation and ripening. J Am Soc Hortic Sci 128:628–635
Zhu YJ, Qiu XH, Moore PH, Borth W, Hu J, Ferreira S, Albert HH (2003) Systemic acquired resistance induced by BTH in papaya. Physiol Mol Plant Pathol 63:237–248
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Paull, R.E., Wu, P., Chen, N.J. (2014). Genomics of Papaya Fruit Development and Ripening. In: Ming, R., Moore, P. (eds) Genetics and Genomics of Papaya. Plant Genetics and Genomics: Crops and Models, vol 10. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-8087-7_14
Download citation
DOI: https://doi.org/10.1007/978-1-4614-8087-7_14
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-8086-0
Online ISBN: 978-1-4614-8087-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)