Abstract
To examine the hypothesis that the relationship between minute ventilation (VE) and deoxygenation from the intercostal space (IC) would be steady regardless of exercise protocols, if an increase in O2 consumption of the accessory respiratory muscles with an increase of VE brings about deoxygenation in IC, we measured the relationship between VE and O2 saturation in IC (SO2IC) during a constant-load exercise test (CET), and the relationship was compared with that during a ramp incremental exercise test (RIET). Six male subjects performed RIET. On a different day, the subjects performed a moderate and heavy CET (CET_MOD and CET_HVY, respectively). SO2IC decreased from the start of both CET_MOD and CET_HVY and changed little from 2 min. Moreover, SO2IC was significantly lower during CET_HVY than during CET_MOD. In comparison between RIET and CET_HVY at the similar VE level, SO2IC was significantly higher during CET_HVY than RIET. These results suggest that the decrease in SO2IC was caused not only by an increase in O2 consumption in IC with an increase in VE but also by a decrease in O2 supply.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Intercostal Space (IC)
- Ramp Incremental Exercise Test (RIET)
- Constant-load Exercise Test (CET)
- Accessory Respiratory Muscles
- Deoxygenation
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
During incremental cycling exercise, deoxygenation, measured by near-infrared spectroscopy (NIRS), in the working muscle (the vastus lateralis) was attenuated at high intensity [1–4], and in previous studies, it was thought to be related to metabolism of the respiratory and accessory respiratory muscles [1]. Deoxygenation from the intercostal space (IC) hyperbolically increased until exhaustion. The deoxygenation was thought to be because the recruitment of the accessory respiratory muscles increased the O2 consumption. However, since the NIRS data reflect the balance of O2 supply and consumption [5, 6], deoxygenation was not always induced by an increase of O2 consumption but also by a decrease of O2 supply.
During constant-load exercise, minute ventilation (VE) increases gradually from the start of exercise [7]. However, little is known about deoxygenation in IC. If deoxygenation in IC was led by an increase in O2 consumption, it could be hypothesized that deoxygenation was controlled by the amplitude of VE, independent of exercise protocols. However, if deoxygenation was different between protocols despite the same VE, deoxygenation would be influenced not only by the change of O2 consumption but also by O2 supply.
The purpose of this study was to examine deoxygenation in IC during constant-load exercise and to compare between deoxygenation during constant-load exercise with that during incremental exercise at the similar VE level.
2 Methods
2.1 Subjects
Six active male volunteers participated in this study (age, 22 ± 1 years; height, 170.5 ± 5.7 cm; body mass, 67.3 ± 12.0 kg; mean ± S.D.). Before participation in the experiment, all procedures and any potential risks were explained to each subject and an informed consent document was signed. This study was approved by the local ethics committee, and all work was performed in accordance with the Declaration of Helsinki.
2.2 Experiment Design
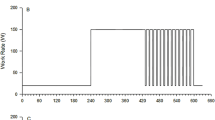
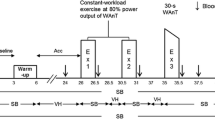
The subjects performed a ramp incremental exercise test (RIET). The protocol followed 0-W exercise for 1 min and increased at a ramp rate of 20 W · min−1 to exhaustion. Ventilatory threshold (VT) calculated by a V-slope method [8] and peak O2 uptake (VO2peak) were evaluated. On a different day, the subjects performed a moderate (CET_MOD; the intensity was 90 % of VT) and heavy (CET_HVY; the intensity was the middle between VT and VO2peak) constant-load exercise test. The protocol followed a warm-up exercise (the same intensity as CET_MOD) for 3 min and 0-W exercise for 6 min and performed CET_MOD and CET_HVY exercise for 6 min separated by 0-W exercise for 6 min. The warm-up exercise was performed to meet the metabolic balance before CET_MOD and CET_HVY exercise. An electromagnetically braked cycle ergometer (Strength Ergo 8, Fukuda Denshi, Japan) was utilized; the seat and handle height remained constant for each subject, and the pedal frequency was maintained at 60 rpm.
2.3 Measurements
VE and O2 uptake (VO2) were determined breath by breath by a computerized metabolic cart (AE300S, Minato, Japan). Expiratory flow measurements were performed by a mass flow sensor. VE was normalized by the peak of VE during RIET (%VEpeak − RIET). O2 saturation in IC (SO2IC) was measured with near-infrared spatially resolved spectroscopy (NIRO-200, Hamamatsu photonics, Japan). The optode was placed on the interion between the seventh intercostal space and the anterior axillary line. The source-detector distance was 4 cm.
2.4 Statistical Analyses
All data represented as means ± S.D. Statistical analyses were performed using the statistical package SPSS for Windows (version 19.0; SPSS, Chicago, IL). SO2IC during CET_MOD and CET_HVY were compared by two-way ANOVA, with time and intensity as main effects. The Dunnett post hoc test was performed to examine time changes, and the paired t-test was used to compare between CET_MOD and CET_HVY (Fig. 20.1). SO2IC during RIET, CET_MOD, and CET_HVY were compared by the paired t-test, at the similar %VEpeak − RIET (Fig. 20.2). Values of P < 0.05 were considered significant.
3 Results
At VO2peak during RIET, VE, VO2, and SO2IC were 126 ± 22 L · min−1, 51.7 ± 9.1 mL · kg−1 · min−1, and 54.6 ± 14.0 %, respectively.
VE increased progressively as exercise time increased and was significantly higher during CET_HVY than CET_MOD. In contrast, SO2IC decreased from the start of both CET_MOD and CET_HVY and changed little from 2 to 6 min (Fig. 20.1). The amplitude of SO2IC was significantly higher during CET_HVY than CET_MOD. The final values of VE, %VEpeak − RIET, VO2, and SO2IC were 59.1 ± 4.6 L · min−1, 46.1 ± 6.9 %, 32.3 ± 6.1 mL · kg−1 · min−1, and 68.9 ± 7.3 % during CET_MOD and 103 ± 19 L · min−1, 79.3 ± 11.5 %, 47.9 ± 6.9 mL · kg−1 · min−1, and 61.3 ± 10.2 % during CET_HVY, respectively.
In comparison with the similar %VEpeak − RIET between RIET (48.3 ± 3.3 %) and CET_MOD at the peak of %VEpeak − RIET (48.4 ± 3.2 %), SO2IC tend to be higher during CET_MOD than RIET, but not significant (Fig. 20.2a). On the other hand, in comparison between RIET (81.0 ± 12.2 %) and CET_MOD at the peak of %VEpeak − RIET (81.0 ± 11.7 %), SO2IC was significantly higher during CET_HVY than RIET (Fig. 20.2b).
4 Discussion
We examined SO2IC kinetics during constant-load exercise and compared SO2IC at similar %VEpeak − RIET between RIET and CET. The main findings of this study are that SO2IC was little changed over 2 min during both CET_MOD and CET_HVY and that the level of SO2IC was different between RIET and CET_HVY despite similar %VEpeak − RIET. These findings suggest that the decrease of SO2IC is not induced by increased O2 consumption of the accessory respiratory muscles under the measurement area. In this study, neither blood flow nor O2 supply in IC was measured, but a decrease in them would mainly result in a decrease in SO2IC.
O2 saturation is thought to represent the balance between O2 supply and consumption. We believe that O2 consumption in the respiratory and accessory respiratory muscles was increased because a rise of VE led to high work of breathing [9]. However, it would be unlikely that the increase in O2 consumption caused deoxygenation in these muscles, at least the intercostal muscles. Rather, we assume that SO2IC was more related to O2 supply changes, which could be effected by sympathetic nerve activity-induced vasoconstriction, which was seen in organs and resting muscles during high-intensity exercise [10, 11]. Blood flow in working muscle was relatively maintained or increased by vasodilation effects such as NO [12], but blood flow and O2 supply in resting muscles were decreased [11]. Although neither autonomic nerve activity nor blood flow in the resting muscle was measured in this study, previous studies have reported that during high-intensity exercise, the resting muscle deoxygenation increased gradually [13], similar to SO2IC in this study. Hence, in our study where O2 saturation in IC and the resting muscle were simultaneously monitored, these values were similar during both incremental and decremental exercise (unpublished data). Furthermore, an isocapnic hyperpnea at rest did not deoxygenate IC [14]. Therefore, it was unclear why SO2IC showed different kinetics between protocols, but it was likely that the change was more related to the O2 supply than to the O2 consumption.
In summary, the present study demonstrated that SO2IC during CET_HVY did not change despite VE increasing and that SO2IC during CET_HVY was different from that during RIET in the same VE. These results imply that a decrease in SO2IC is caused by a decrease of the O2 supply, rather than an increase of the O2 consumption.
References
Legrand R, Marles A, Prieur F, Lazzari S, Blondel N, Mucci P (2007) Related trends in locomotor and respiratory muscle oxygenation during exercise. Med Sci Sports Exerc 39(1):91–100
Osawa T, Kime R, Hamaoka T, Katsumura T, Yamamoto M (2011) Attenuation of muscle deoxygenation precedes EMG threshold in normoxia and hypoxia. Med Sci Sports Exerc 43(8):1406–1413
Osawa T, Kime R, Katsumura T, Yamamoto M (2010) Effects of acute hypoxia on the inflection point of muscle oxygenation. Adv Exp Med Biol 662:385–390
Wilson JR, Mancini DM, McCully K, Ferraro N, Lanoce V, Chance B (1989) Noninvasive detection of skeletal muscle underperfusion with near-infrared spectroscopy in patients with heart failure. Circulation 80(6):1668–1674
Ferrari M, Mottola L, Quaresima V (2004) Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol 29:463–487
Hamaoka T, McCully KK, Quaresima V, Yamamoto K, Chance B (2007) Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J Biomed Opt 12(6):062105
Kowalchuk JM, Rossiter HB, Ward SA, Whipp BJ (2002) The effect of resistive breathing on leg muscle oxygenation using near-infrared spectroscopy during exercise in men. Exp Physiol 87(5):601–611
Beaver WL, Wasserman K, Whipp BJ (1986) A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 60(6):2020–2027
Vogiatzis I, Athanasopoulos D, Habazettl H et al (2009) Intercostal muscle blood flow limitation in athletes during maximal exercise. J Physiol 587(Pt 14):3665–3677
Osada T, Katsumura T, Hamaoka T et al (1999) Reduced blood flow in abdominal viscera measured by Doppler ultrasound during one-legged knee extension. J Appl Physiol 86(2):709–719
Shiroishi K, Kime R, Osada T, Murase N, Shimomura K, Katsumura T (2010) Decreased muscle oxygenation and increased arterial blood flow in the non-exercising limb during leg exercise. Adv Exp Med Biol 662:379–384
Boushel R (2010) Muscle metaboreflex control of the circulation during exercise. Acta Physiol (Oxf) 199(4):367–383
Ogata H, Arimitsu T, Matsuura R, Yunoki T, Horiuchi M, Yano T (2007) Relationship between oxygenation in inactive biceps brachii muscle and hyperventilation during leg cycling. Physiol Res 56(1):57–65
Guenette JA, Vogiatzis I, Zakynthinos S et al (2008) Human respiratory muscle blood flow measured by near-infrared spectroscopy and indocyanine green. J Appl Physiol 104(4):1202–1210
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this paper
Cite this paper
Osawa, T., Kime, R., Fujioka, M., Osada, T., Murase, N., Katsumura, T. (2013). O2 Saturation in the Intercostal Space During Moderate and Heavy Constant-Load Exercise. In: Van Huffel, S., Naulaers, G., Caicedo, A., Bruley, D.F., Harrison, D.K. (eds) Oxygen Transport to Tissue XXXV. Advances in Experimental Medicine and Biology, vol 789. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-7411-1_20
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7411-1_20
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-7256-8
Online ISBN: 978-1-4614-7411-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)