Abstract
The association of hydrocephalus and the Chiari malformations has been described from the time of Hans Chiari’s initial report in 1891. Whether hydrocephalus is the cause of or the result of hindbrain herniation remains a subject of long-standing controversy, but recent advances in vascular and volumetric imaging may eventually provide definitive information to settle the debate. Though there is a wide range of hindbrain herniations that fall under the Chiari rubric, most authors would agree that coexisting hydrocephalus should be managed with CSF diversion first, either by shunting or endoscopic ventriculostomy, before consideration is given to posterior fossa decompression. It is crucial to keep in mind that pseudotumor cerebri may also present with symptoms that mimic those seen in Chiari malformation, making it critical that the neurosurgeon strive to differentiate these two groups prior to surgical intervention for optimal outcome.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hydrocephalus
- Chiari malformation
- Myelomeningocele
- Syringomyelia
- Endoscopic third ventriculostomy
- Pseudotumor cerebri
Introduction and Historical Background
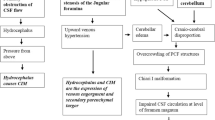
The association of hydrocephalus and the Chiari malformations has been described from the time of Hans Chiari’s initial report in 1891 [1]. The pathophysiology of Chiari-associated hydrocephalus has nonetheless been controversial, with several hypotheses proposed to explain its pathophysiology. In his original manuscript, Chiari postulated that tonsillar herniation resulted from supratentorial pressure due to concomitant hydrocephalus [1], suggesting that brain herniation was in fact secondary to intrinsic hydrocephalus. This initial explanation was cogent and quite popular and still provides the rationale for CSF diversion as a primary treatment of Chiari-associated hydrocephalus. The Dutch surgeon van Houweninge Graftdijk proposed a converse theory in 1932 [2], whereby the foramina of the fourth ventricle, herniated into the upper end of the spinal canal, act as a valvular obstruction and precipitate hydrocephalus. He advocated surgical correction of the hindbrain herniation in order to widen the space and allow for better flow of CSF. More recent advances in cranial imaging and volumetric analysis have provided some detail to this theory, linking tonsillar herniation to a disorder of the paraxial mesoderm with underdevelopment of the occipital somites and secondary hypoplasia of the occipital bone, leading to overcrowding of the vascular and neural structures within the posterior cranial fossa [3, 4]. This combination of factors may lead to impaired CSF absorption and flow due to hindbrain distortion, decreased cisterns, and anomalies of the venous circulation, resulting in hydrocephalus. Whether hydrocephalus is the cause of or the result of hindbrain herniation is quite relevant, as it may dictate the surgeon’s approach to a management strategy that is perceived to lead to the best clinical outcome for each individual patient. Given the wide range of hindbrain anomalies that fall under the Chiari rubric, each Chiari subtype will be discussed separately.
Chiari I
Epidemiology and Clinical Presentation
Reported incidence rates of hydrocephalus in cases of Chiari I malformation (CMI) range from 0 to 9.6 % [4–6], and it may often be associated with concomitant syringomyelia [4]. In addition to classic hindbrain symptoms of CMI, symptoms of hydrocephalus and resultant elevated ICP may include headaches, vomiting, papilledema, and enlarging head circumference in infants. Magnetic resonance imaging is the modality of choice for diagnosis of both entities and also allows evaluation of the spinal cord to rule out associated syringomyelia. In patients being considered for ETV, MRI also provides necessary anatomic detail of the third ventricle, basilar artery, and prepontine space (Fig. 24.1). Threshold values of ventricular enlargement necessary for a diagnosis of hydrocephalus are not well defined in the literature, making clinical diagnosis of elevated ICP a crucial component of the decision-making process.
Management and Outcomes
As mentioned before, some controversy exists regarding whether hydrocephalus should be considered the cause of the CMI hindbrain herniation or rather the effect of obstruction at the level of the fourth ventricular outlet or even abnormal CSF absorption at the level of the posterior fossa cisterns [3]. This controversy notwithstanding, it is generally accepted that in cases of CMI, hydrocephalus should be managed by adequate CSF diversion before consideration is given to suboccipital decompression [7–10].
Ventriculoperitoneal shunt placement has long been the mainstay of treatment for CMI-associated hydrocephalus. While there are no published studies looking specifically at the durability of VPS for CMI-associated hydrocephalus, the complication and infection rates for shunt placement are not insignificant, especially in the pediatric population [11, 12].
Recent studies have reported relative success of ETV in the management of CMI-related HCP [13–24]. The two largest series [22, 23] reported an 87–94 % (28 of 31) early success rate with two failures due to late (>1 year) stoma closure and in one case of a previously shunted patient. Together, the two series reported no mortality or clinically significant complications and syringomyelia resolution or improvement in 8 of 11 patients. Of note, despite similar success rates in management of HCP, the two groups reported very different rates of patients going on to require subsequent posterior fossa decompression for persistent CMI symptomatology (0 vs. 37.5 %). Whether this was due to different mean ages of their populations (15.2 vs. 31.9) or criteria for operative decompression is unclear. Nonetheless, both groups advocate ETV as the procedure of choice in the management of hydrocephalus associated with CMI, both for hydrocephalus control and treatment of hindbrain herniation symptoms, including syringomyelia.
Prospective, randomized studies are required to validate this encouraging preliminary data.
Chiari II Malformation
Epidemiology and Clinical Presentation
The Chiari II malformation (CMII) almost always occurs in patients born with neural tube defects, most commonly myelomeningocele or encephalocele. Criteria for diagnosis include the elongation and caudal migration of the cerebellar vermis, brainstem, and fourth ventricle into the upper cervical canal, as well as a host of other cerebral anomalies (Fig. 24.2). Associated findings may include tectal beaking, basilar invagination, colpocephaly, low-lying torcular, skull anomalies, and syringomyelia (40–95 %) [25]. Incidence of clinical hydrocephalus requiring CSF diversion varies from 40 % in prenatally closed groups [26] to 52–90 % in postnatally closed series [27–33]. This wide variation in reported incidence is likely a reflection of different patient populations, health systems, and criteria for diagnosis and intervention.
Symptoms of hydrocephalus in infants with CMII may include bulging fontanelle, split cranial sutures, and leakage from the myelomeningocele closure site. Of note, hydrocephalus may also worsen symptoms referable to the CMII, including lower cranial neuropathies, swallowing dysfunction, and stridor. Numerous methods exist to quantify ventriculomegaly, including calculation of the ratio of biventricular diameter to biparietal diameter [34], frontal-occipital horn ratio, and ventricular index, though no published studies to date have clearly delineated the best measure.
Management and Outcomes
As in CMI, management of hydrocephalus or verification of a working shunt should always precede suboccipital decompression, even in the setting of brainstem symptomatology (i.e., stridor, dysphagia, sleep apnea) or worsening syringomyelia. Criteria for CSF diversion have historically been somewhat variable, though efforts have been made to standardize indications to better compare outcomes across multiple institutions [26]. Traditionally, ventriculoperitoneal shunting has been the most common procedure used for treatment of hydrocephalus associated with CMII and myelomeningocele. However, shunt complication rates and death in children with myelomeningocele may be higher than in children requiring shunt for other reasons [35–38]. Some authors have suggested that infective complications of shunting may have a greater impact on cognitive development than the hydrocephalus [39] and that children with myelomeningocele who do not require shunt placement have better survival [40, 41] and higher IQ [42] than those who have undergone shunt placement. These studies are limited by their retrospective nature and potential bias, and prospective studies are required to elucidate the appropriate threshold for intervention given the known risks of shunting. In children with mild ventriculomegaly and no signs or symptoms of increased intracranial pressure, potentially improved brain development must be weighed against the known risks of CSF diversion in this population. Based on preliminary data suggesting improved neuropsychological scores 6 months following shunt insertion [43], prospective studies are under way to evaluate ventricular size and neurocognitive outcome.
More recently, endoscopic third ventriculostomy has been proposed as an acceptable alternative to ventriculoperitoneal shunting in children with myelomeningocele [44–49], with acknowledged lower success rates in infants and children with a previously placed shunt [46–48, 50]. The addition of choroid plexus cauterization to ETV has also been investigated, based on extensive experience with children in developing countries [51–54], where cost and medical access preclude ventriculoperitoneal shunt placement. Long-term follow-up in this cohort demonstrated similar neurocognitive outcomes in the ETV/CPC and VP shunt groups [52]. It remains unclear whether these very promising findings are directly translatable to infants in developed countries.
Chiari III
Chiari malformation type III (CM III) is an extremely rare entity characterized by herniation of the posterior fossa contents through a low occipital and/or upper cervical osseous defect [1, 55, 56], estimated to account for 0.64–4 % of all Chiari malformations [57, 58]. Published series report a high incidence of associated hydrocephalus, syringomyelia, and tethered cord syndrome [25, 55, 56]. Associated hydrocephalus has traditionally been managed with ventriculoperitoneal shunt placement, and the rarity of CM III limits the availability of published data regarding long-term shunt survival or alternative CSF diversion.
Pseudotumor Cerebri and the Chiari Malformation
Tonsillar descent secondary to lumboperitoneal shunting for pseudotumor cerebri (PTC) is quite common, well described in the literature [59], and will not be treated further here. However, there has been much disagreement regarding the nature of the association between PTC and primary Chiari malformation. PTC classically presents with headaches, visual changes, elevated intracranial pressure measured on lumbar puncture in the lateral decubitus position, and no evidence of hydrocephalus or intracranial pathology. It is most often observed in obese women of childbearing age, though it can also be seen secondary to certain medications (tetracycline, minocycline, vitamin A, corticosteroids, lithium, and oral contraceptives) and in the setting of venous sinus thrombosis [60]. The source of the controversy lies in two observations. First, several groups have described an increased prevalence of cerebellar ectopia in patients with PTC. Sinclair described a series of 156 cases of PTC noting an overall incidence of 2.7 %, significantly higher than the 0.77 % rate previously reported in the general population [61]. Banik observed a 24 % rate of inferior tonsillar displacement in patients with PTC, with 10 % fulfilling criteria for Chiari malformation (>5 mm) [62]. Of note, all patients with tonsillar descent were female and obese.
Second, several groups have described the effectiveness of CSF shunting in patients with recurrent Chiari symptoms following posterior fossa decompression. Fagan reported a series of 15 patients with post-Chiari PTC, defined as recurrence of Chiari-like symptoms after decompression, elevated lumbar CSF pressure in the absence of meningitis or ventriculomegaly, and transient resolution of symptoms following lumbar CSF drainage [63]. All patients were evaluated with CSF flow studies at the foramen magnum as well as lumbar puncture to rule out infection/aseptic meningitis and to evaluate intracranial pressure. Those found to have increased ICP underwent lumboperitoneal shunting, with significant symptom resolution in 7/9 (78 %) pediatric patients and in 0/6 adult patients [63]. Bejjani reported a series of six adult patients with similar recurrence of Chiari-like symptoms following posterior fossa decompression and found significant improvement in all following either shunting or repeat LP with acetazolamide [64].
Whether the association is real or coincidental is unclear. Some authors argue that they are two pathophysiologically distinct entities with overlapping clinical presentation, specifically headaches and tonsillar descent [65]. Others suggest that the entities may actually share a similar pathophysiology, namely, increased intracranial contents, engorged brain with venous hypertension, decreased intracranial volume, and mechanical obstruction of CSF outflow at the foramen magnum with a common end result of altered compliance and disturbed neural hydrodynamics [62, 64, 66]. Definitive resolution of the issue will require more detailed imaging and prospective studies of larger populations.
Practically speaking, given the similar demographics, clinical presentation, and increased incidence of tonsillar ectopia in patients with pseudotumor cerebri, it is critical that the neurosurgeon strive to differentiate these two groups during clinical evaluation prior to surgery for optimal outcome. Patients with atypical headaches, obesity, relevant medication exposure, visual changes, and papilledema should be most closely examined to better differentiate between the two diagnoses. Detailed fundoscopic exam, MRI cine studies to visualize CSF flow at the foramen magnum, and lumbar puncture may be considered in these complex patients to evaluate intracranial pressure and also determine if the patient responds symptomatically to CSF drainage. Those patients with evidence of intracranial hypertension and symptomatic improvement following lumbar puncture will benefit from CSF diversion instead of posterior fossa decompression.
A Special Note About Predicting Success of ETV in the Chiari I and II Populations
Major factors that predict the success of ETV include age, etiology of hydrocephalus, and the presence or absence of a shunt preoperatively [48, 67–79]. In 2009, Kulkarni et al. used these factors to develop a model to predict the probability of ETV success in the treatment of childhood hydrocephalus: the Endoscopic Third Ventriculostomy Success Score [48] (Fig. 24.3). By assigning a score to age range, etiology, and shunt history, an overall score is calculated that predicts the likelihood of successful ETV at 6 months post-procedure and was found to closely approximate success. Since that time, there have been several publications using the ETVSS. The Canadian Pediatric Neurosurgery Group evaluated a multicenter cohort of children newly diagnosed with hydrocephalus and evaluated the risk of failure between ETV and VPS for high-, moderate-, and low-ETVSS group [80]. For all groups, the risk of ETV failure became progressively lower compared with shunt failure with increasing time from surgery. In the high-ETVSS group, the risk of ETV failure was lower than shunt failure soon after surgery. For all the rest, the risk of ETV failure only became lower than shunt failure 3–6 months out from surgery. Kulkarni, Riva-Cambrin, and Browd then applied the ETVSS to several well-known published series of patients on whom ETV was performed for various reasons. The overall mean-predicted ETVSS was 58 %, and the actual ETV success rate was 59 %, showing excellent predictive capability of the model [46]. Two articles were published in November of 2011 in Journal of Neurosurgery: Pediatrics intended to further validate the ETVSS [45, 81]. Both single-institution series showed excellent predictive capability of the ETVSS in separate analyses.
As reviewed earlier, hydrocephalus related to the CMI is relatively rare. Therefore, ETVs for hydrocephalus due to CMI make up only a small part of most single-center and multicenter ETV series. Within the etiology portion of the ETVSS, the same predictive capability is apportioned to CMI (“other”) as those etiologies that traditionally have a high rate of success: aqueductal stenosis and tectal tumors. Despite the initial success in published series reviewed above, this may very well be an overestimation of the capability of ETV to adequately treat hydrocephalus in patients with CMI, or this may be clinically accurate. Additional series continue to accrue [22, 23], and future adjustments to the model may be necessary.
Performance of an ETV for hydrocephalus related to spina bifida, however, is becoming more common. Based on the current ETVSS model, there is less likelihood of success in patients with this etiology than with CMI. Due to the large number of patients with post-infectious hydrocephalus, Warf, Mugamba, and Kulkarni modified the existing ETVSS in order to apply it to children seen at the CURE Children’s Hospital of Uganda (CCHU) [78]. As a result, the CCHU ETVSS for use in the field predicted ETV success to a much higher degree by taking into consideration age, etiology, and the degree of choroid plexus cauterization (CPC). A substantial number of ETVs in this population were performed with choroid plexus cauterization. In a very interesting finding and one that was independent of CPC and age, regression analysis revealed that the odds of ETV success were 2.25 times greater in spina bifida patients compared with other etiologies. Further validation studies may shed more light on this relationship and again may contribute to a further honing of the model.
Conclusion
The Chiari malformations have long been associated with hydrocephalus. Controversy remains, however, regarding which entity precedes the other, though most authors agree the pathophysiology is multifactorial. Though hydrocephalus is relatively rare in the setting of the CMI, most agree that CSF diversion should be performed prior to suboccipital decompression. In addition to traditional shunting, emerging data suggest that ETV is effective in a large majority of these patients and may improve hindbrain compression symptoms and syringomyelia. Hydrocephalus is much more common in the CMII–myelomeningocele population, though controversy remains regarding the proportion of these patients that will ultimately require CSF diversion. As in the Chiari I population, adequate CSF drainage should be ensured prior to surgical decompression, even in the setting of hindbrain compression symptoms or syringomyelia. Though traditional shunting remains the most common treatment for these patients, ETV has emerged as an acceptable alternative, especially in experienced centers when combined with choroid plexus cauterization. Prospective studies of both entities are ongoing, examining diagnostic criteria, neuropsychological outcomes, and preferred CSF diversion technique in these challenging populations.
References
Chiari H. Uber Veranderungen des Kleingirns infolge von Hydrocephalie des Grosshirns. Dtsch Med Wochenschr. 1891;17:1172–5.
van Houweninge Graftdijk C. Over hydrocephalus. Leiden: Eduard Ijdo; 1932.
Di Rocco C, Frassanito P, Massimi L, Peraio S. Hydrocephalus and Chiari type I malformation. Childs Nerv Syst. 2011;27(10):1653–64.
Milhorat TH, Chou MW, Trinidad EM, et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44(5):1005–17.
Badie B, Mendoza D, Batzdorf U. Posterior fossa volume and response to suboccipital decompression in patients with Chiari I malformation. Neurosurgery. 1995;37(2):214–8.
Tubbs RS, Beckman J, Naftel RP, et al. Institutional experience with 500 cases of surgically treated pediatric Chiari malformation type I. J Neurosurg Pediatr. 2011;7(3):248–56.
Osuagwu FC, Lazareff JA, Rahman S, Bash S. Chiari I anatomy after ventriculoperitoneal shunting: posterior fossa volumetric evaluation with MRI. Childs Nerv Syst. 2006;22(11):1451–6.
Grabb PA, Mapstone TB, Oakes WJ. Ventral brain stem compression in pediatric and young adult patients with Chiari I malformations. Neurosurgery. 1999;44(3):520–7; discussion 527–8.
Schijman E, Steinbok P. International survey on the management of Chiari I malformation and syringomyelia. Childs Nerv Syst. 2004;20(5):341–8.
Tubbs RS, Lyerly MJ, Loukas M, Shoja MM, Oakes WJ. The pediatric Chiari I malformation: a review. Childs Nerv Syst. 2007;23(11):1239–50.
Drake JM, Kestle JR, Milner R, et al. Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery. 1998;43(2):294–303; discussion 303–5.
Vernet O, Rilliet B. Late complications of ventriculoatrial or ventriculoperitoneal shunts. Lancet. 2001;358(9293):1569–70.
Decq P, Le Guerinel C, Sol JC, Brugieres P, Djindjian M, Nguyen JP. Chiari I malformation: a rare cause of noncommunicating hydrocephalus treated by third ventriculostomy. J Neurosurg. 2001;95(5):783–90.
Ersahin Y, Gokcay A. Acquired Chiari I malformation changes postendoscopic third ventriculostomy. Pediatr Neurosurg. 2002;36(1):54.
Fukuhara T, Vorster SJ, Luciano MG. Risk factors for failure of endoscopic third ventriculostomy for obstructive hydrocephalus. Neurosurgery. 2000;46(5):1100–9; discussion 1109–11.
Kandasamy J, Kneen R, Gladstone M, Newman W, Mohamed T, Mallucci C. Chiari I malformation without hydrocephalus: acute intracranial hypertension managed with endoscopic third ventriculostomy (ETV). Childs Nerv Syst. 2008;24(12):1493–7.
Metellus P, Dufour H, Levrier O, Grisoli F. Endoscopic third ventriculostomy for treatment of noncommunicating syringomyelia associated with a Chiari I malformation and hydrocephalus: case report and pathophysiological considerations. Neurosurgery. 2002;51(2):500–3; discussion 503–4.
Mohanty A, Suman R, Shankar SR, Satish S, Praharaj SS. Endoscopic third ventriculostomy in the management of Chiari I malformation and syringomyelia associated with hydrocephalus. Clin Neurol Neurosurg. 2005;108(1):87–92.
Nishihara T, Hara T, Suzuki I, Kirino T, Yamakawa K. Third ventriculostomy for symptomatic syringomyelia using flexible endoscope: case report. Minim Invasive Neurosurg. 1996;39(4):130–2.
Suehiro T, Inamura T, Natori Y, Sasaki M, Fukui M. Successful neuroendoscopic third ventriculostomy for hydrocephalus and syringomyelia associated with fourth ventricle outlet obstruction. Case report. J Neurosurg. 2000;93(2):326–9.
Teo C, Nakaji P, Serisier D, Coughlan M. Resolution of trigeminal neuralgia following third ventriculostomy for hydrocephalus associated with Chiari I malformation: case report. Minim Invasive Neurosurg. 2005;48(5):302–5.
Massimi L, Pravata E, Tamburrini G, et al. Endoscopic third ventriculostomy for the management of Chiari I and related hydrocephalus: outcome and pathogenetic implications. Neurosurgery. 2011;68(4):950–6.
Hayhurst C, Osman-Farah J, Das K, Mallucci C. Initial management of hydrocephalus associated with Chiari malformation type I-syringomyelia complex via endoscopic third ventriculostomy: an outcome analysis. J Neurosurg. 2008;108(6):1211–4.
Buxton N, Jaspan T, Punt J. Treatment of Chiari malformation, syringomyelia and hydrocephalus by neuroendoscopic third ventriculostomy. Minim Invasive Neurosurg. 2002;45(4):231–4.
Soleau S, Tubbs RS, Oakes JW. Chiari malformations. In: Albright AL, Pollack IF, Adelson PD, editors. Principles and practice of pediatric neurosurgery. 2nd ed. New York: Thieme; 2008. p. 217–32.
Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364(11):993–1004.
Arynchyna A, Shannon C, Ditty B, Oakes JW, Blount J, Wellons J. Applying the MOMS shunting criteria to a single institutional experience. Austin: AANS/CNS Section on Pediatric Neurological Surgery; 2011.
Chakraborty A, Crimmins D, Hayward R, Thompson D. Toward reducing shunt placement rates in patients with myelomeningocele. J Neurosurg Pediatr. 2008;1(5):361–5.
Bowman RM, McLone DG, Grant JA, Tomita T, Ito JA. Spina bifida outcome: a 25-year prospective. Pediatr Neurosurg. 2001;34(3):114–20.
Rintoul NE, Sutton LN, Hubbard AM, et al. A new look at myelomeningoceles: functional level, vertebral level, shunting, and the implications for fetal intervention. Pediatrics. 2002;109(3):409–13.
Swank M, Dias L. Myelomeningocele: a review of the orthopaedic aspects of 206 patients treated from birth with no selection criteria. Dev Med Child Neurol. 1992;34(12):1047–52.
Talamonti G, D’Aliberti G, Collice M. Myelomeningocele: long-term neurosurgical treatment and follow-up in 202 patients. J Neurosurg. 2007;107(5 Suppl):368–86.
Dias M, McLone DG. Myelomeningocele. In: Albright AL, Pollack IF, Adelson PD, editors. Principles and practice of pediatric neurosurgery. 2nd ed. New York: Thieme; 2008. p. 338–66.
O’Hayon BB, Drake JM, Ossip MG, Tuli S, Clarke M. Frontal and occipital horn ratio: a linear estimate of ventricular size for multiple imaging modalities in pediatric hydrocephalus. Pediatr Neurosurg. 1998;29(5):245–9.
Steinbok P, Irvine B, Cochrane DD, Irwin BJ. Long-term outcome and complications of children born with meningomyelocele. Childs Nerv Syst. 1992;8(2):92–6.
Acakpo-Satchivi L, Shannon CN, Tubbs RS, et al. Death in shunted hydrocephalic children: a follow-up study. Childs Nerv Syst. 2008;24(2):197–201.
Iskandar BJ, Tubbs S, Mapstone TB, Grabb PA, Bartolucci AA, Oakes WJ. Death in shunted hydrocephalic children in the 1990s. Pediatr Neurosurg. 1998;28(4):173–6.
Tuli S, Drake J, Lamberti-Pasculli M. Long-term outcome of hydrocephalus management in myelomeningoceles. Childs Nerv Syst. 2003;19(5–6):286–91.
McLone DG. Central nervous system infections as a limiting factor in the intelligence of children with myelomeningocele. Pediatrics. 1982;70(3):338–42.
Davis BE, Daley CM, Shurtleff DB, et al. Long-term survival of individuals with myelomeningocele. Pediatr Neurosurg. 2005;41(4):186–91.
Tuli S, Tuli J, Drake J, Spears J. Predictors of death in pediatric patients requiring cerebrospinal fluid shunts. J Neurosurg. 2004;100(5 Suppl Pediatrics):442–6.
Mapstone TB, Rekate HL, Nulsen FE, Dixon Jr MS, Glaser N, Jaffe M. Relationship of CSF shunting and IQ in children with myelomeningocele: a retrospective analysis. Childs Brain. 1984;11(2):112–8.
Mataro M, Poca MA, Sahuquillo J, et al. Cognitive changes after cerebrospinal fluid shunting in young adults with spina bifida and assumed arrested hydrocephalus. J Neurol Neurosurg Psychiatry. 2000;68(5):615–21.
Kadrian D, van Gelder J, Florida D, et al. Long-term reliability of endoscopic third ventriculostomy. Neurosurgery. 2008;62 Suppl 2:614–21.
Naftel RP, Reed GT, Kulkarni AV, Wellons JC. Evaluating the Children’s Hospital of Alabama endoscopic third ventriculostomy experience using the Endoscopic Third Ventriculostomy Success Score: an external validation study. J Neurosurg Pediatr. 2011;8(5):494–501.
Kulkarni AV, Riva-Cambrin J, Browd SR. Use of the ETV Success Score to explain the variation in reported endoscopic third ventriculostomy success rates among published case series of childhood hydrocephalus. J Neurosurg Pediatr. 2011;7(2):143–6.
Kulkarni AV, Drake JM, Kestle JR, Mallucci CL, Sgouros S, Constantini S. Predicting who will benefit from endoscopic third ventriculostomy compared with shunt insertion in childhood hydrocephalus using the ETV Success Score. J Neurosurg Pediatr. 2010;6(4):310–5.
Kulkarni AV, Drake JM, Mallucci CL, Sgouros S, Roth J, Constantini S. Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. J Pediatr. 2009;155(2):254–9. e251.
Drake JM, Kulkarni AV, Kestle J. Endoscopic third ventriculostomy versus ventriculoperitoneal shunt in pediatric patients: a decision analysis. Childs Nerv Syst. 2009;25(4):467–72.
Kulkarni AV, Warf BC, Drake JM, Mallucci CL, Sgouros S, Constantini S. Surgery for hydrocephalus in sub-Saharan Africa versus developed nations: a risk-adjusted comparison of outcome. Childs Nerv Syst. 2010;26(12):1711–7.
Warf BC. Hydrocephalus associated with neural tube defects: characteristics, management, and outcome in sub-Saharan Africa. Childs Nerv Syst. 2011;27(10):1589–94.
Warf B, Ondoma S, Kulkarni A, et al. Neurocognitive outcome and ventricular volume in children with myelomeningocele treated for hydrocephalus in Uganda. J Neurosurg Pediatr. 2009;4(6):564–70.
Warf BC, Campbell JW. Combined endoscopic third ventriculostomy and choroid plexus cauterization as primary treatment of hydrocephalus for infants with myelomeningocele: long-term results of a prospective intent-to-treat study in 115 East African infants. J Neurosurg Pediatr. 2008;2(5):310–6.
Warf BC. Endoscopic third ventriculostomy and choroid plexus cauterization for pediatric hydrocephalus. Clin Neurosurg. 2007;54:78–82.
Castillo M, Quencer RM, Dominguez R. Chiari III malformation: imaging features. AJNR Am J Neuroradiol. 1992;13(1):107–13.
Isik N, Elmaci I, Silav G, Celik M, Kalelioglu M. Chiari malformation type III and results of surgery: a clinical study: report of eight surgically treated cases and review of the literature. Pediatr Neurosurg. 2009;45(1):19–28.
Cama A, Tortori-Donati P, Piatelli GL, Fondelli MP, Andreussi L. Chiari complex in children – neuroradiological diagnosis, neurosurgical treatment and proposal of a new classification (312 cases). Eur J Pediatr Surg. 1995;5 Suppl 1:35–8.
Dyste GN, Menezes AH, VanGilder JC. Symptomatic Chiari malformations. An analysis of presentation, management, and long-term outcome. J Neurosurg. 1989;71(2):159–68.
Chumas PD, Armstrong DC, Drake JM, et al. Tonsillar herniation: the rule rather than the exception after lumboperitoneal shunting in the pediatric population. J Neurosurg. 1993;78(4):568–73.
Degnan AJ, Levy LM. Pseudotumor cerebri: brief review of clinical syndrome and imaging findings. AJNR Am J Neuroradiol. 2011;32(11):1986–93.
Meadows J, Kraut M, Guarnieri M, Haroun RI, Carson BS. Asymptomatic Chiari type I malformations identified on magnetic resonance imaging. J Neurosurg. 2000;92(6):920–6.
Banik R, Lin D, Miller NR. Prevalence of Chiari I malformation and cerebellar ectopia in patients with pseudotumor cerebri. J Neurol Sci. 2006;247(1):71–5.
Fagan LH, Ferguson S, Yassari R, Frim DM. The Chiari pseudotumor cerebri syndrome: symptom recurrence after decompressive surgery for Chiari malformation type I. Pediatr Neurosurg. 2006;42(1):14–9.
Bejjani GK, Cockerham KP, Rothfus WE, Maroon JC, Maddock M. Treatment of failed adult Chiari malformation decompression with CSF drainage: observations in six patients. Acta Neurochir (Wien). 2003;145(2):107–16; discussion 116.
Sinclair N, Assaad N, Johnston I. Pseudotumour cerebri occurring in association with the Chiari malformation. J Clin Neurosci. 2002;9(1):99–101.
Bejjani GK. Association of the Adult Chiari Malformation and Idiopathic Intracranial Hypertension: more than a coincidence. Med Hypotheses. 2003;60(6):859–63.
Warf BC. Hydrocephalus in Uganda: the predominance of infectious origin and primary management with endoscopic third ventriculostomy. J Neurosurg. 2005;102:1–15.
Balthasar AJ, Kort H, Cornips EM, Beuls EA, Weber JW, Vles JS. Analysis of the success and failure of endoscopic third ventriculostomy in infants less than 1 year of age. Childs Nerv Syst. 2007;23:151–5.
Boschert J, Hellwig D, Krauss JK. Endoscopic third ventriculostomy for shunt dysfunction in occlusive hydrocephalus: long-term follow up and review. J Neurosurg. 2003;98:1032–9.
Buxton N, Macarthur D, Robertson I, Punt J. Neuroendoscopic third ventriculostomy for failed shunts. Surg Neurol. 2003;60:201–3; discussion 203–4.
Drake JM. Endoscopic third ventriculostomy in pediatric patients: the Canadian experience. Neurosurgery. 2007;60:881–6; discussion 881–6.
Elgamal EA, El-Dawlatly AA, Murshid WR, El-Watidy SM, Jamjoom ZA. Endoscopic third ventriculostomy for hydrocephalus in children younger than 1 year of age. Childs Nerv Syst. 2011;27(1):111–6.
Hader WJ, Walker RL, Myles ST, Hamilton M. Complications of endoscopic third ventriculostomy in previously shunted patients. Neurosurgery. 2008;63:ONS168–74; discussion ONS174–65.
Marton E, Feletti A, Basaldella L, Longatti P. Endoscopic third ventriculostomy in previously shunted children: a retrospective study. Childs Nerv Syst. 2010;26:937–43.
Navarro R, Gil-Parra R, Reitman AJ, Olavarria G, Grant JA, Tomita T. Endoscopic third ventriculostomy in children: early and late complications and their avoidance. Childs Nerv Syst. 2006;22:506–13.
Ogiwara H, Dipatri Jr AJ, Alden TD, Bowman RM, Tomita T. Endoscopic third ventriculostomy for obstructive hydrocephalus in children younger than 6 months of age. Childs Nerv Syst. 2010;26:343–7.
Siomin V, Cinalli G, Grotenhuis A, Golash A, Oi S, Kothbauer K, et al. Endoscopic third ventriculostomy in patients with cerebrospinal fluid infection and/or hemorrhage. J Neurosurg. 2002;97(3):519–24.
Warf BC, Mugamba J, Kulkarni AV. Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus in Uganda: report of a scoring system that predicts success. J Neurosurg Pediatr. 2010;5:143–8.
Wellons 3rd JC, Tubbs RS, Banks JT, Grabb B, Blount JP, Oakes WJ. Long-term control of hydrocephalus via endoscopic third ventriculostomy in children with tectal plate gliomas. Neurosurgery. 2002;51:63–7; discussion 67–8.
Kulkarni AV, Drake JM, Mallucci CL, Sgouros S, Constantini S. Predicting who will benefit from endoscopic third ventriculostomy compared with shunt insertion in childhood hydrocephalus using the ETV Success Score. J Neurosurg Pediatr. 2010;6:310–5.
Durnford AJ, Kirkham FJ, Mathad N, Sparrow OC. Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus: validation of a success score that predicts long-term outcome. J Neurosurg Pediatr. 2011;8(5):489–93.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Johnston, J.M., Wellons, J.C. (2013). The Chiari Malformations and Hydrocephalus. In: Tubbs, R., Oakes, W. (eds) The Chiari Malformations. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6369-6_24
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6369-6_24
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6368-9
Online ISBN: 978-1-4614-6369-6
eBook Packages: MedicineMedicine (R0)