Abstract
Merlin, encoded by the NF2 tumor-suppressive gene, has been established through genetic studies in both Drosophila and mice as an important upstream regulator of the Hippo-Yap pathway. Recently, biochemical studies have identified Angiomotin and Angiomotin-like proteins as major interacting partners for both Merlin and Yap. The exact mechanisms of how Merlin and Angiomotin regulate Hippo signaling remain undetermined. In this chapter, we will summarize past findings and discuss controversies and remaining questions regarding the roles of Merlin and Angiomotin in Hippo signaling and tumorigenesis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Neurofibromatosis Type 2 and Merlin
Neurofibromatosis type 2 is an inherited disorder with an incidence of approximately 1 in 30,000 births, caused by germ line mutations of the NF2 gene, which is located on chromosome 22q12. The disease is characterized mainly by the development of bilateral Schwann cell tumors of the eighth cranial nerve. Mutations and loss of heterozygosity (LOH) of the NF2 locus have been detected at high frequency in various tumors of the nervous system, including schwannomas, meningiomas, and ependymomas, indicative of classical tumor suppressor gene pattern (Gusella et al. 1996, 1999). In further support of a role for NF2 in tumor suppression, mice heterozygous for an Nf2 mutation are predisposed to a wide variety of tumors, while mice with both Nf2 alleles inactivated specifically in Schwann cells develop schwannomas and Schwann cell hyperplasia (McClatchey et al. 1998; Giovannini et al. 1999, 2000).
The NF2 tumor suppressor gene encodes a 69-kDa protein called Merlin (Moesin, ezrin, and radixin-like protein). Merlin contains an N-terminal FERM domain that comprises three subdomains organized into a cloverleaf-like structure (Shimizu et al. 2002; Pearson et al. 2000), followed by a coiled-coil domain and a charged C-terminal tail. The NF2 allele is alternatively spliced resulting in two predominate forms of the Merlin protein (isoform 1 and 2) that differ at the extreme C-terminus. Several studies indicated that Merlin forms intramolecular associations between the C-terminal tail and the FERM domain, transitioning between “open” and “closed” conformation (Sher et al. 2012; Gutmann et al. 1999; Sherman et al. 1997). While it has been long thought that the “closed” state represents the active/growth-suppressive form of Merlin, recent studies have cast doubt on this model (Sher et al. 2012; Hennigan et al. 2010; Lallemand et al. 2009a; Schulz et al. 2010). In particular, a study using Merlin mutants that adopt open or closed forms demonstrated that the open form is the active form of the protein (Sher et al. 2012).
A number of factors have been shown to regulate Merlin activity, including phosphorylation on serine 518 in the C-terminal tail. This phosphorylation is induced by the small G-proteins, Rac1 and Cdc42, and mediated by the immediate Rac/Cdc42 effectors—the p21-activated kinases (Paks) (Kissil et al. 2002; Xiao et al. 2002). In addition, it was shown that cAMP-dependent kinase (PKA) also phosphorylates Merlin at serine 518 (Alfthan et al. 2004). An additional level of regulation is provided by the myosin phosphatase MYPT1-PP1, which dephosphorylates Merlin at serine 518 (Jin et al. 2006). Finally, AKT was shown to phosphorylate Merlin at serine 10, threonine 230, and serine 315, promoting its proteosomal degradation (Tang et al. 2007; Laulajainen et al. 2011).
2 Merlin Localization and Function
Merlin is localized predominantly to membrane periphery within cells. As cells reach confluence, Merlin is recruited to cell junctions, most likely through interactions with α-catenin (adherens junctions, AJs) or Amot (tight junctions, TJs), where it is thought to coordinate the establishment of intercellular contacts with concomitant inhibition of proliferative signaling (Curto et al. 2007; Rangwala et al. 2005; Lallemand et al. 2003; Morris and McClatchey 2009; Gladden et al. 2010; Yi et al. 2011). In vitro and in vivo studies using different experimental systems have yielded conflicting results on whether or not Merlin is required for the assembly or maintenance of cell junctions (Lallemand et al. 2003; Morris and McClatchey 2009; Gladden et al. 2010; Houshmandi et al. 2009; Lallemand et al. 2009b; McLaughlin et al. 2007; Flaiz et al. 2009; Okada et al. 2005). Nevertheless, the localization of Merlin to cell junctions appears to be critical for its tumor-suppressive function, as patient-derived mutations that impair Merlin’s junctional localization render the protein inactive (Lallemand et al. 2003; Gutmann et al. 2001; Stokowski and Cox 2000; Deguen et al. 1998).
While Merlin has also been shown to have nuclear functions (Li et al. 2010), the vast majority of evidence implicates Merlin in mediating contact-dependent inhibition of cell proliferation from the cell membrane, by coupling signals initiated through cell–cell interactions with regulation of growth regulatory pathways, including the Ras and Rac, Src, mTOR, and Hippo-Yap pathways (Huson et al. 2011). In particular, numerous studies have linked Merlin to the Ras and Rac signaling pathways (Fig. 2.1) (Yi et al. 2011; Okada et al. 2005; Kaempchen et al. 2003; Morrison et al. 2007; Wong et al. 2012; Zhou et al. 2011a; Hennigan et al. 2012; Bosco et al. 2010). The Ras and Rac protein families are small G-proteins that function as molecular switches cycling between an “ON” state when bound to GTP and an “OFF” state when bound to GDP. They are tightly regulated by various groups of proteins, including GEFs (Guanine Exchange Factors), which promote binding of small GTPases to GTP, and GAPs (GTPase Activating Proteins) that promote the hydrolysis of GTP to GDP. The transition from an “ON” to an “OFF” state is regulated by various stimuli, including growth-factor receptors and integrins (Burridge and Wennerberg 2004). Ras is a well-documented oncogene that is mutated in a significant number of cancers. However, the roles of the Rac family of proteins in cancer have not been fully elucidated. Both Ras and Rac protein families are master regulators of diverse signaling pathways that control the shape, motility, and growth of cells. These are processes that often go awry in cancer. While activating Rac mutations have not been found in tumors, there is strong evidence that Rac plays a crucial role in the regulation of signaling cascades downstream of Ras. One of the main mechanisms demonstrated is through the phosphorylation of c-Raf (serine 338) and MEK1 (serine 298) by Paks following Rac1 activation by Ras, which is required for the sustained activation of the MAPK signaling by Ras (Fig. 2.1) (Sun et al. 2000; Howe and Juliano 2000; Diaz et al. 1997; Frost et al. 1997; King et al. 1998; Vadlamudi et al. 2000).
Merlin has been shown to regulate mitogenic signaling at multiple levels. Recent studies suggested that loss of Merlin leads to accumulation of receptor tyrosine kinases (RTKs) at the cell surface, possibly due to defects in receptor trafficking (Lallemand et al. 2009b; Ammoun et al. 2008; Maitra et al. 2006), or by sequestering them to microdomains of the plasma membrane (Curto et al. 2007; Morris and McClatchey 2009; Cole et al. 2008). Downstream of RTKs, Merlin was shown to also inhibit the activation of the small GTPases Ras and Rac1 (Okada et al. 2005; Morrison et al. 2007). Many Ras-controlled pathways are upregulated in human schwannomas (Ammoun et al. 2008) and mechanistic studies suggested that Merlin regulates Ras signaling by disrupting a Grb2-SOS-ERM-Ras complex, leading to lower levels of activated Ras (Morrison et al. 2007). Previous work by multiple groups demonstrated that Merlin functions to prevent Rac1-mediated activation of Paks by interfering with the binding of activated Rac1 to Pak1 (Kissil et al. 2003; Xiao et al. 2005; Hirokawa et al. 2004). Merlin also acts upstream of Rac1, as expression of dominant-active Rac1 as well as dominant-active Pak prevents Merlin from inhibiting Ras-induced activation of MAPK signaling (Morrison et al. 2007). Recent work added insights into the mechanisms of how Merlin functions upstream of Rac1 and Ras-MAPK signaling through inhibition of Rich1, a Rac1/Cdc42 GAP (see below) (Yi et al. 2011). In addition, Merlin was shown to inhibit contact-dependent recruitment of active Rac1 to the plasma membrane in endothelial cells (Okada et al. 2005).
Genetic studies in both flies and mice demonstrated that the Hippo-Yap pathway is a key effector pathway mediating the tumor-suppressive function of Merlin (Zhang et al. 2010; Hamaratoglu et al. 2006). In this chapter, we will discuss our current understanding of how Merlin and its major interacting partners, the Angiomotins, modulate the Hippo-Yap pathway. The reader is referred to other reviews for details relating to Merlin’s regulation of other signaling pathways (Li et al. 2012a; Zhou and Hanemann 2012).
3 The Angiomotins: Novel Merlin Interacting Proteins
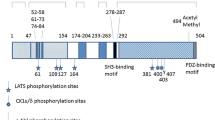
While numerous proteins have been identified as merlin-interacting proteins over the past decade, it is the Angiomotins that have been directly implicated in the tumor-suppressive function of Merlin as well as regulation of the Hippo-Yap pathway (Yi et al. 2011; Varelas et al. 2010; Zhao et al. 2011; Wang et al. 2011a; Chan et al. 2011; Paramasivam et al. 2011; Oka et al. 2012). As members of the Motin protein family, Angiomotin (Amot), Angiomotin-like 1 (AmotL1), and Angiomotin-like 2 (Amotl2) are characterized by a conserved N-terminal glutamine-rich domain, followed by a coiled-coil domain and a C-terminal PDZ-binding motif (Fig. 2.2) (Bratt et al. 2002). Angiomotin, the founding member of the Motin family has two major splice forms (p80 and p130 isoforms) and was originally isolated as an Angiostatin binding protein that mediates the anti-migratory properties of Angiostatin (Bratt et al. 2005; Troyanovsky et al. 2001). Interestingly, all members of the Motin family appear to associate with TJs through binding to the TJ-associated Patj/Mupp1 proteins (Wells et al. 2006; Sugihara-Mizuno et al. 2007; Ernkvist et al. 2009).
The Angiomotins have been extensively studied in the context of angiogenesis during development. Knocking down or deleting Motin family members individually or in combination results in defects in endothelial cell polarization, migration, and proliferation of various severities, suggesting that these proteins have overlapping functions in promoting angiogenesis (Ernkvist et al. 2009; Huang et al. 2007; Aase et al. 2007; Garnaas et al. 2008; Zheng et al. 2009; Wang et al. 2011b). The roles of Amot in mediating endothelial cell polarization and migration require its C-terminal PDZ binding motif, which mediates its indirect association with Syx, a RhoA-specific GEF, via Patj/Mupp1 (Ernkvist et al. 2009; Garnaas et al. 2008). In addition, Amot was shown to preferentially bind to mono-phosphorylated phosphatidylinositols and mediate endocytic recruitment of Patj/Mupp1 and Syx (Heller et al. 2010; Wu et al. 2011). It has been suggested that Amot might coordinate cell migration and junctional remodeling by trafficking Syx together with TJ proteins Patj/Mupp1 through endocytic vesicles to the leading edge of migrating endothelial cells, leading to focal activation of RhoA at the leading edge (Ernkvist et al. 2009; Wu et al. 2011).
In addition to its role in regulating local activity of RhoA, multiple studies demonstrated that Amot inhibits Rac1 and Cdc42 activities by bindings to and inhibiting the function of Rich1, a Rac1/Cdc42 GAP localized to TJs and AJs in epithelial cells (Yi et al. 2011; Wells et al. 2006). We previously showed that Merlin, through competitive binding to Amot, releases Rich1 from an Amot-inhibitory complex, allowing Rich1 to inactivate Rac1, ultimately leading to attenuation of Rac1 and Ras/MAPK signaling (Yi et al. 2011). Moreover, Merlin mutants that carry mutations found in NF2 patients showed diminished binding capacities to Amot and were unable to dissociate Rich1 from Amot or inhibit MAPK signaling (Yi et al. 2011). The depletion of Amot in Nf2 -/- Schwann cells attenuated the Ras-MAPK signaling pathway, impeded cellular proliferation in vitro and tumorigenesis in vivo (Yi et al. 2011). Consistent with our findings, Amot and AmotL2 were later reported to promote MAPK signaling and cell proliferation in human mammary epithelial cells, human umbilical vein endothelial cells, and zebrafish embryos (Wang et al. 2011b; Ranahan et al. 2010). Similarly, knockdown of AmotL1 was shown to decrease pERK and pAKT levels in MCF10A cells (Wang et al. 2011a). Interestingly, the same study found that silencing of AmotL2 has opposite effects on pERK and pAKT levels (Wang et al. 2011a). Further investigation will be required to reconcile these findings with previous reports.
Finally, the Angiomotins were recently identified as major binding partners for Yap (Varelas et al. 2010; Zhao et al. 2011; Wang et al. 2011a; Chan et al. 2011; Paramasivam et al. 2011; Oka et al. 2012). We will discuss in detail below the various mechanisms that have been proposed of how the Angiomotins intercept with Hippo-Yap signaling and their relationship to Merlin.
4 The Hippo-Yap Signaling Pathway
The Hippo-Yap pathway is described in great detail in other chapters of this book. Briefly, this pathway was initially characterized in flies and shown to play a role in organ size control. Subsequent studies indicate that the pathway is a mediator of cell contact inhibition and tumor suppression (Ota and Sasaki 2008; Zeng and Hong 2008). The pathway is composed of a core kinase cascade, in which the Mst1/2 kinases (Hippo in flies) in complex with scaffold protein WW45 (Salvador in flies) phosphorylate Lats1/2 kinases (Warts in flies) and their adaptor protein, Mob1 (Mats in flies). Phosphorylated Lats1/2 in turn phosphorylate Yap (Yorkie in flies), a transcriptional co-activator. The phosphorylation of Yap not only prevents it from entering into the nucleus but also primes it for ubiquitination and degradation by the proteasome (Zhao et al. 2007, 2010). Upon dephosphorylation likely by PP1A (Wang et al. 2011c; Liu et al. 2011), Yap translocates into the nucleus where it complexes with Tead (Scalloped in flies) and other transcription factors to drive the expression of pro-proliferative or anti-apoptotic genes (Hong and Guan 2012). A series of recent studies have demonstrated that, akin to what has been observed in flies, the mammalian Hippo pathway also regulates organ size, particularly of the liver. Inducible overexpression of Yap in adult mouse liver results in rapid and reversible increase in liver size (Camargo et al. 2007; Dong et al. 2007). Comparable hepatomegaly phenotypes were observed when Mst1/2, WW45 and Nf2 were ablated specifically in the liver (Zhang et al. 2010; Zhou et al. 2009, 2011b; Benhamouche et al. 2010; Song et al. 2010; Lee et al. 2010; Lu et al. 2010). Finally, increased Yap activity appears to be a common occurrence in human hepatocellular carcinoma (Zhou et al. 2009; Li et al. 2012b; Xu et al. 2009; Zender et al. 2006).
5 Merlin and Hippo-Yap Signaling
Merlin and another FERM domain protein, Expanded, were first identified as upstream regulators of Hippo signaling through genetic screens in Drosophila (Hamaratoglu et al. 2006). Merlin regulates the expression and localization of Yap in mammalian cells in a manner similar to what has been observed in flies (Zhao et al. 2007; Striedinger et al. 2008; Yokoyama et al. 2008). It was reported that majority of human meningioma and mesothelioma samples, which are frequently associated with loss of NF2, exhibited elevated Yap expression in the nuclei, indicative of abnormal Yap activation (Zhao et al. 2007; Striedinger et al. 2008; Baia et al. 2012; Sekido 2011). Knockdown of Yap was shown to rescue the hyperproliferative phenotype of NF2-deficient meningioma and mesothelioma cells, whereas hepatomegaly/tumorigenesis phenotypes associated liver-specific Nf2 deletion in mice were largely suppressed by concomitant heterozygous deletion of Yap or overexpression of a dominant-negative Tead (Zhang et al. 2010; Striedinger et al. 2008; Baia et al. 2012; Mizuno et al. 2012; Liu-Chittenden et al. 2012). These studies thus validated the Yap/Tead transcriptional complexes as major downstream effectors of Merlin/NF2 in growth regulation in mammals.
The exact mechanisms of how Merlin regulates Hippo signaling and Yap activity remain to be elucidated. In Drosophila, Merlin was shown to form an apical complex with Kibra and together they activate Sav and Wts, leading to Yki phosphorylation and inactivation (Yu et al. 2010; Genevet et al. 2010; Baumgartner et al. 2010). Yeast two hybrid and biochemical studies indicated that Merlin directly binds to Sav through its N-terminal FERM domain and Kibra through its C-terminal half (Yu et al. 2010). Interestingly, the physical and functional interactions between Merlin, Kibra, and WW45 (the mammalian counterpart of Sav) appear to be conserved in mammalian cells (Yu et al. 2010).
In addition to Hippo signaling components, Merlin has been reported to function as a linker between the AJ-associated catenin complex and the TJ-associated Par3 complex by directly binding to α-catenin and Par3 (Gladden et al. 2010). Interestingly, two recent studies demonstrated that Yap also associates with α-catenin through 14-3-3 in a phosphorylation-dependent manner (Silvis et al. 2011; Schlegelmilch et al. 2011). Besides the catenin complex, both Merlin and Yap interact with TJ-associated Crumbs-Pals1-Patj polarity complex via direct binding to distinct domains of the Angiomotins (Yi et al. 2011; Varelas et al. 2010). Taken together, it is tempting to speculate that Merlin together with the junctional complexes may promote Hippo signaling and Yap inactivation by assembling signaling platforms where Lats1/2 kinases, in response to signals from the junctional complexes, phosphorylate and inactivate Yap (Fig. 2.3).
Schematic representation of putative signaling complexes assembled by Merlin at the cell junctions. In green are proteins previously implicated as having growth/tumor-suppressive functions, in red proteins implicated as having pro-proliferative functions and in purple proteins with yet to be defined functions
6 Angiomotin and Hippo-Yap Signaling
Recent work has also implicated the Angiomotin family members in the regulation of Hippo-Yap signaling (Varelas et al. 2010; Zhao et al. 2011; Wang et al. 2011a; Chan et al. 2011; Paramasivam et al. 2011; Oka et al. 2012). At least under conditions used for immunoprecipitation, the Angiomotins are arguably one of the strongest binding partners for Yap, as evidenced by four independent studies identifying them as major Yap-associated proteins (Varelas et al. 2010; Zhao et al. 2011; Wang et al. 2011a; Chan et al. 2011). The interactions between the Angiomotins and Yap are mediated through PPXY motifs within the N-terminal regions of the Angiomotins and the conserved WW domains of Yap (Zhao et al. 2011; Wang et al. 2011a; Chan et al. 2011). Interestingly, several other proteins including Lats1/2 also bind to the WW domains of Yap through their PPXY motifs (Chen and Sudol 1995; Strano et al. 2001; Komuro et al. 2003; Hao et al. 2008; Espanel and Sudol 2001). Therefore, it would be interesting to test whether Angiomotins may regulate their interactions with Yap.
Largely based on overexpression of the Angiomotins in HeLa and MCF7 cells, which do not appear to express these proteins endogenously, several recent studies suggested that the Angiomotins function as negative regulators of Yap and its paralog Taz by sequestering them in the cytoplasm (Zhao et al. 2011; Wang et al. 2011a; Chan et al. 2011). It should be noted, however, that exogenously expressed Angiomotins in these cells form mainly cytoplasmic aggregates, instead of localizing to the cell junctions (Zhao et al. 2011; Wang et al. 2011a; Chan et al. 2011). This raises the question of whether these approaches accurately reflect the physiological functions of the Angiomotins. Significantly, stable overexpression of Amot-p130 in MDCK cells, where it is correctly targeted to TJs, leads to increased localization of endogenous Yap not only to the junctions but also within the nucleus (Zhao et al. 2011). Loss of function studies by knocking down members of the Motin family in cells that express endogenously at least one of three members of the family demonstrated that silencing of AmotL2 but not AmotL1 increased the localization of Yap and Taz to the nucleus and induced cellular transformation in MDCK and MCF10A cells (Zhao et al. 2011; Wang et al. 2011a; Chan et al. 2011). In addition, knockdown of AmotL2 or AmotL1 appeared to have opposite effects on MAPK signaling in MCF10A cells (Wang et al. 2011a). Notably, the observed effect of AmotL2 silencing on MAPK signaling in MCF10A cells was in contrast to another knockdown study in zebrafish embryos and HUVEC cells (Wang et al. 2011a, 2011b). It is possible that these discrepancies are due to tissue- or cell-type-specific functions of different members of the Motin family. Further studies will be necessary to clarify these differences.
In addition to controlling Yap subcellular localization, the Angiomotins have been reported to promote Yap phosphorylation and diminish transcription of two known Yap target genes, CTGF and Cyr61 (Zhao et al. 2011; Wang et al. 2011a; Paramasivam et al. 2011). One study suggested that the Angiomotins bind to and enhance the kinase activity of Lats1/2, thereby increasing Yap phosphorylation (Paramasivam et al. 2011). One potential caveat is that this study was performed primarily with overexpressed Angiomotins tagged at their C-terminus, which may mask their C-terminal PDZ binding domains (Fig. 2.2) and hinder their binding to partners such as Patj/Mupp1, thus potentially interfering with the normal functions of the Angiomotins. Given that other studies have shown that the Angiomotins can regulate Yap activity independent of Hippo signaling, the precise functions of the Motin family members in Hippo signaling remain to be defined.
Conflicting data also exist regarding to the roles of the Motin family in tumorigenesis. We previously showed that Amot functions downstream of Merlin as a positive regulator of Rac1 and MAPK signaling in vitro and is required for tumorigenesis following Merlin/Nf2 loss in vivo, using an orthotopic model of NF2 (Yi et al. 2011). This finding of a pro-proliferative role for Amot was corroborated by two other studies in mammary epithelial cells and zebrafish embryos (Wang et al. 2011b; Heller et al. 2010). Other reports suggested that knockdown of AmotL2 leads to cellular transformation in MDCK and MCF10A cells in vitro (Zhao et al. 2011; Wang et al. 2011a). It is possible that this is a reflection of different roles carried out by members of the Motin family and/or a choice of model system used in the studies. The availability of knockout and transgenic mouse models in which components of the Hippo-Yap pathway have been targeted will no doubt facilitate the definition of the roles the Motin family plays in tumorigenesis (Zhang et al. 2010; Camargo et al. 2007; Dong et al. 2007; Zhou et al. 2009, 2011b; Benhamouche et al. 2010; Song et al. 2010; Lee et al. 2010; Lu et al. 2010; Schlegelmilch et al. 2011). For example, the role of Amot in tumorigenesis could be addressed, in vivo, by combining the Amot conditional knockout allele (Shimono and Behringer 2003) with knockout or transgenic alleles of Hippo-Yap pathway components in a tractable system such as the liver and examining the effects of Amot knockout on hepatomegaly and tumorigenesis phenotypes caused by perturbance of Merlin or the Hippo-Yap pathway. These studies in a physiological system should help define the relationship between Merlin, the Motin family, and the Hippo-Yap pathway.
References
Aase K, et al. Angiomotin regulates endothelial cell migration during embryonic angiogenesis. Genes Dev. 2007;21(16):2055–68.
Alfthan K, et al. Cyclic AMP-dependent protein kinase phosphorylates merlin at serine 518 independently of p21-activated kinase and promotes merlin-ezrin heterodimerization. J Biol Chem. 2004;279(18):18559–66.
Ammoun S, et al. Dissecting and targeting the growth factor-dependent and growth factor-independent extracellular signal-regulated kinase pathway in human schwannoma. Cancer Res. 2008;68(13):5236–45.
Baia GS, Caballero OL, Orr BA, Lal A, Ho JS, Cowdrey C, et al. Yes-associated protein 1 is activated and functions as an oncogene in meningiomas. Mol Cancer Res. 2012;10(7):904–13.
Baumgartner R, et al. The WW domain protein Kibra acts upstream of Hippo in Drosophila. Dev Cell. 2010;18(2):309–16.
Benhamouche S, et al. Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev. 2010;24(16):1718–30.
Bosco EE, et al. NF2-deficient cells depend on the Rac1-canonical Wnt signaling pathway to promote the loss of contact inhibition of proliferation. Oncogene. 2010;29(17):2540–9.
Bratt A, et al. Angiomotin belongs to a novel protein family with conserved coiled-coil and PDZ binding domains. Gene. 2002;298(1):69–77.
Bratt A, et al. Angiomotin regulates endothelial cell-cell junctions and cell motility. J Biol Chem. 2005;280(41):34859–69.
Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116(2):167–79.
Camargo FD, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17(23):2054–60.
Chan SW, et al. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J Biol Chem. 2011;286(9):7018–26.
Chen HI, Sudol M. The WW domain of Yes-associated protein binds a proline-rich ligand that differs from the consensus established for Src homology 3-binding modules. Proc Natl Acad Sci U S A. 1995;92(17):7819–23.
Cole BK, et al. Localization to the cortical cytoskeleton is necessary for Nf2/merlin-dependent epidermal growth factor receptor silencing. Mol Cell Biol. 2008;28(4):1274–84.
Curto M, et al. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol. 2007;177(5):893–903.
Deguen B, et al. Impaired interaction of naturally occurring mutant NF2 protein with actin-based cytoskeleton and membrane. Hum Mol Genet. 1998;7(2):217–26.
Diaz B, et al. Phosphorylation of Raf-1 serine 338-serine 339 is an essential regulatory event for Ras-dependent activation and biological signaling. Mol Cell Biol. 1997;17(8):4509–16.
Dong J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130(6):1120–33.
Ernkvist M, et al. The Amot/Patj/Syx signaling complex spatially controls RhoA GTPase activity in migrating endothelial cells. Blood. 2009;113(1):244–53.
Espanel X, Sudol M. Yes-associated protein and p53-binding protein-2 interact through their WW and SH3 domains. J Biol Chem. 2001;276(17):14514–23.
Flaiz C, et al. Altered adhesive structures and their relation to RhoGTPase activation in merlin-deficient Schwannoma. Brain Pathol. 2009;19(1):27–38.
Frost JA, et al. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J. 1997;16(21):6426–38.
Garnaas MK, et al. Syx, a RhoA guanine exchange factor, is essential for angiogenesis in Vivo. Circ Res. 2008;103(7):710–6.
Genevet A, et al. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev Cell. 2010;18(2):300–8.
Giovannini M, et al. Schwann cell hyperplasia and tumors in transgenic mice expressing a naturally occurring mutant NF2 protein. Genes Dev. 1999;13(8):978–86.
Giovannini M, et al. Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes Dev. 2000;14(13):1617–30.
Gladden AB, et al. The NF2 tumor suppressor, Merlin, regulates epidermal development through the establishment of a junctional polarity complex. Dev Cell. 2010;19(5):727–39.
Gusella JF, et al. Neurofibromatosis 2: loss of merlin’s protective spell. Curr Opin Genet Dev. 1996;6(1):87–92.
Gusella JF, et al. Merlin: the neurofibromatosis 2 tumor suppressor. Biochim Biophys Acta. 1999;1423(2):M29–36.
Gutmann DH, Haipek CA, Hoang Lu K. Neurofibromatosis 2 tumor suppressor protein, merlin, forms two functionally important intramolecular associations. J Neurosci Res. 1999;58(5):706–16.
Gutmann DH, Hirbe AC, Haipek CA. Functional analysis of neurofibromatosis 2 (NF2) missense mutations. Hum Mol Genet. 2001;10(14):1519–29.
Hamaratoglu F, et al. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8(1):27–36.
Hao Y, et al. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283(9):5496–509.
Heller B, et al. Amot recognizes a juxtanuclear endocytic recycling compartment via a novel lipid binding domain. J Biol Chem. 2010;285(16):12308–20.
Hennigan RF, et al. Fluorescence resonance energy transfer analysis of merlin conformational changes. Mol Cell Biol. 2010;30(1):54–67.
Hennigan RF, et al. The NF2 tumor suppressor regulates microtubule-based vesicle trafficking via a novel Rac, MLK and p38(SAPK) pathway. Oncogene. 2012 [Epub ahead of print].
Hirokawa Y, et al. A clue to the therapy of neurofibromatosis type 2: NF2/merlin is a PAK1 inhibitor. Cancer J. 2004;10(1):20–6.
Hong W, Guan KL. The YAP and TAZ transcription co-activators: key downstream effectors of the mammalian Hippo pathway. Semin Cell Dev Biol. 2012;23(7):785–93.
Houshmandi SS, et al. The neurofibromatosis 2 protein, merlin, regulates glial cell growth in an ErbB2- and Src-dependent manner. Mol Cell Biol. 2009;29(6):1472–86.
Howe AK, Juliano RL. Regulation of anchorage-dependent signal transduction by protein kinase A and p21-activated kinase. Nat Cell Biol. 2000;2(9):593–600.
Huang H, et al. Amotl2 is essential for cell movements in zebrafish embryo and regulates c-Src translocation. Development. 2007;134(5):979–88.
Huson SM, et al. Back to the future: proceedings from the 2010 NF Conference. Am J Med Genet A. 2011;155A(2):307–21.
Jin H, et al. Tumorigenic transformation by CPI-17 through inhibition of a merlin phosphatase. Nature. 2006;442(7102):576–9.
Kaempchen K, et al. Upregulation of the Rac1/JNK signaling pathway in primary human schwannoma cells. Hum Mol Genet. 2003;12(11):1211–21.
King AJ, et al. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature. 1998;396(6707):180–3.
Kissil JL, et al. Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J Biol Chem. 2002;277(12):10394–9.
Kissil JL, et al. Merlin, the product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol Cell. 2003;12(4):841–9.
Komuro A, et al. WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. J Biol Chem. 2003;278(35):33334–41.
Lallemand D, et al. NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev. 2003;17(9):1090–100.
Lallemand D, Saint-Amaux AL, Giovannini M. Tumor-suppression functions of merlin are independent of its role as an organizer of the actin cytoskeleton in Schwann cells. J Cell Sci. 2009a;122(Pt 22):4141–9.
Lallemand D, et al. Merlin regulates transmembrane receptor accumulation and signaling at the plasma membrane in primary mouse Schwann cells and in human schwannomas. Oncogene. 2009b;28(6):854–65.
Laulajainen M, et al. Multistep phosphorylation by oncogenic kinases enhances the degradation of the NF2 tumor suppressor merlin. Neoplasia. 2011;13(7):643–52.
Lee KP, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107(18):8248–53.
Li W, et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell. 2010;140(4):477–90.
Li W, et al. Merlin: a tumour suppressor with functions at the cell cortex and in the nucleus. EMBO Rep. 2012a;13:204–15.
Li H, et al. Deregulation of Hippo kinase signalling in human hepatic malignancies. Liver Int. 2012b;32(1):38–47.
Liu CY, et al. PP1 cooperates with ASPP2 to dephosphorylate and activate TAZ. J Biol Chem. 2011;286(7):5558–66.
Liu-Chittenden Y, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26(12):1300–5.
Lu L, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107(4):1437–42.
Maitra S, et al. The tumor suppressors Merlin and Expanded function cooperatively to modulate receptor endocytosis and signaling. Curr Biol. 2006;16(7):702–9.
McClatchey AI, et al. Mice heterozygous for a mutation at the Nf2 tumor suppressor locus develop a range of highly metastatic tumors. Genes Dev. 1998;12(8):1121–33.
McLaughlin ME, et al. The Nf2 tumor suppressor regulates cell-cell adhesion during tissue fusion. Proc Natl Acad Sci U S A. 2007;104(9):3261–6.
Mizuno T, et al. YAP induces malignant mesothelioma cell proliferation by upregulating transcription of cell cycle-promoting genes. Oncogene. 2012;31:5117–22.
Morris ZS, McClatchey AI. Aberrant epithelial morphology and persistent epidermal growth factor receptor signaling in a mouse model of renal carcinoma. Proc Natl Acad Sci U S A. 2009;106(24):9767–72.
Morrison H, et al. Merlin/neurofibromatosis type 2 suppresses growth by inhibiting the activation of Ras and Rac. Cancer Res. 2007;67(2):520–7.
Oka T, Schmitt AP, Sudol M. Opposing roles of angiomotin-like-1 and zona occludens-2 on pro-apoptotic function of YAP. Oncogene. 2012;31(1):128–34.
Okada T, Lopez-Lago M, Giancotti FG. Merlin/NF-2 mediates contact inhibition of growth by suppressing recruitment of Rac to the plasma membrane. J Cell Biol. 2005;171(2):361–71.
Ota M, Sasaki H. Mammalian Tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development. 2008;135(24):4059–69.
Paramasivam M, et al. Angiomotin family proteins are novel activators of the LATS2 kinase tumor suppressor. Mol Biol Cell. 2011;22(19):3725–33.
Pearson MA, et al. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell. 2000;101(3):259–70.
Ranahan WP, et al. The adaptor protein AMOT promotes the proliferation of mammary epithelial cells via the prolonged activation of the extracellular signal-regulated kinases. Cancer Res. 2010;71(6):2203–11.
Rangwala R, et al. Erbin regulates mitogen-activated protein (MAP) kinase activation and MAP kinase-dependent interactions between Merlin and adherens junction protein complexes in Schwann cells. J Biol Chem. 2005;280(12):11790–7.
Schlegelmilch K, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144(5):782–95.
Schulz A, et al. Merlin inhibits neurite outgrowth in the CNS. J Neurosci. 2010;30(30):10177–86.
Sekido Y. Inactivation of Merlin in malignant mesothelioma cells and the Hippo signaling cascade dysregulation. Pathol Int. 2011;61(6):331–44.
Sher I, et al. The tumor suppressor merlin controls growth in its open state, and phosphorylation converts it to a less-active more-closed state. Dev Cell. 2012;22(4):703–5.
Sherman L, et al. Interdomain binding mediates tumor growth suppression by the NF2 gene product. Oncogene. 1997;15(20):2505–9.
Shimizu T, et al. Structural basis for neurofibromatosis type 2. Crystal structure of the merlin FERM domain. J Biol Chem. 2002;277(12):10332–6.
Shimono A, Behringer RR. Angiomotin regulates visceral endoderm movements during mouse embryogenesis. Curr Biol. 2003;13(7):613–7.
Silvis MR, et al. Alpha-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci Signal. 2011;4(174):ra33.
Song H, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci U S A. 2010;107(4):1431–6.
Stokowski RP, Cox DR. Functional analysis of the neurofibromatosis type 2 protein by means of disease-causing point mutations. Am J Hum Genet. 2000;66(3):873–91.
Strano S, et al. Physical interaction with Yes-associated protein enhances p73 transcriptional activity. J Biol Chem. 2001;276(18):15164–73.
Striedinger K, et al. The neurofibromatosis 2 tumor suppressor gene product, merlin, regulates human meningioma cell growth by signaling through YAP. Neoplasia. 2008;10(11):1204–12.
Sugihara-Mizuno Y, et al. Molecular characterization of angiomotin/JEAP family proteins: interaction with MUPP1/Patj and their endogenous properties. Genes Cells. 2007;12(4):473–86.
Sun H, et al. Regulation of the protein kinase Raf-1 by oncogenic Ras through phosphatidylinositol 3-kinase, Cdc42/Rac and Pak. Curr Biol. 2000;10(5):281–4.
Tang X, et al. Akt phosphorylation regulates the tumour-suppressor merlin through ubiquitination and degradation. Nat Cell Biol. 2007;9(10):1199–207.
Troyanovsky B, et al. Angiomotin: an angiostatin binding protein that regulates endothelial cell migration and tube formation. J Cell Biol. 2001;152(6):1247–54.
Vadlamudi RK, et al. Regulatable expression of p21-activated kinase-1 promotes anchorage-independent growth and abnormal organization of mitotic spindles in human epithelial breast cancer cells. J Biol Chem. 2000;275(46):36238–44.
Varelas X, et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev Cell. 2010;19(6):831–44.
Wang W, Huang J, Chen J. Angiomotin-like proteins associate with and negatively regulate YAP1. J Biol Chem. 2011a;286(6):4364–70.
Wang Y, et al. Angiomotin-like2 gene (amotl2) is required for migration and proliferation of endothelial cells during angiogenesis. J Biol Chem. 2011b;286(47):41095–104.
Wang P, et al. PP1A-mediated dephosphorylation positively regulates YAP2 activity. PLoS One. 2011c;6(9):e24288.
Wells CD, et al. A Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell. 2006;125(3):535–48.
Wong HK, et al. Merlin/NF2 regulates angiogenesis in schwannomas through a Rac1/semaphorin 3F-dependent mechanism. Neoplasia. 2012;14(2):84–94.
Wu C, et al. Rab13-dependent trafficking of RhoA is required for directional migration and angiogenesis. J Biol Chem. 2011;286(26):23511–20.
Xiao GH, et al. p21-activated Kinase Links Rac/Cdc42 Signaling to Merlin. J Biol Chem. 2002;277(2):883–6.
Xiao GH, et al. The NF2 tumor suppressor gene product, merlin, inhibits cell proliferation and cell cycle progression by repressing cyclin D1 expression. Mol Cell Biol. 2005;25(6):2384–94.
Xu MZ, et al. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115(19):4576–85.
Yi C, et al. A tight junction-associated Merlin-angiomotin complex mediates Merlin’s regulation of mitogenic signaling and tumor suppressive functions. Cancer Cell. 2011;19(4):527–40.
Yokoyama T, et al. YAP1 is involved in mesothelioma development and negatively regulated by Merlin through phosphorylation. Carcinogenesis. 2008;29(11):2139–46.
Yu J, et al. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell. 2010;18(2):288–99.
Zender L, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125(7):1253–67.
Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell. 2008;13(3):188–92.
Zhang N, et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19(1):27–38.
Zhao B, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21(21):2747–61.
Zhao B, et al. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 2010;24(1):72–85.
Zhao B, et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25(1):51–63.
Zheng Y, et al. Angiomotin-like protein 1 controls endothelial polarity and junction stability during sprouting angiogenesis. Circ Res. 2009;105(3):260–70.
Zhou L, Hanemann CO. Merlin, a multi-suppressor from cell membrane to the nucleus. FEBS Lett. 2012;586(10):1403–8.
Zhou D, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16(5):425–38.
Zhou L, et al. Merlin-deficient human tumors show loss of contact inhibition and activation of Wnt/beta-catenin signaling linked to the PDGFR/Src and Rac/PAK pathways. Neoplasia. 2011a;13(12):1101–12.
Zhou D, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci U S A. 2011b;108(49):E1312–20.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Yi, C., Kissil, J. (2013). Merlin and Angiomotin in Hippo-Yap Signaling. In: Oren, M., Aylon, Y. (eds) The Hippo Signaling Pathway and Cancer. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6220-0_2
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6220-0_2
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6219-4
Online ISBN: 978-1-4614-6220-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)