Abstract
The treatment of esophagogastric cancer has been rapidly evolving in the past decade. New cytotoxic drugs and targeted agents have been integrated in the therapeutic paradigm. To better understand the tumor biology and to better utilize targeted agents, genetic alterations in esophagogastric cancer have been actively explored. For example, Her2/Neu amplification and expression were observed in gastric and gastroesophageal (GE) junction cancers. Combination of trastuzumab with cytotoxic chemotherapy has demonstrated a survival advantage in patients with Her2/Neu positive gastric cancer. However, the prognosis of advanced esophagogastric cancer remains poor. This is largely attributed to the tumor heterogeneity and poorly understood tumor biology. This article provides a summary of potential genetic targets and the role of novel targeted agents in the treatment of esophagogastric cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Esophagogastric cancer is a heterogeneous disease. Histologically, it includes squamous cell carcinoma and adenocarcinoma. Traditionally, these two histologies are treated very similarly. Most clinical protocols include both histologies. However, the etiology and prognosis are very different between squamous cell carcinoma and adenocarcinoma. Anatomically, adenocarcinoma is usually located at the distal one-third of the esophagus and frequently involves GE junction with extending into the stomach. The disease is mainly caused by acid reflux, Barrett’s esophagus, high fat diet and obesity [1–3]. Squamous cell carcinoma, however, involves the upper third of the esophagus and is usually related to tobacco and alcohol consumption. Epidemiologically, adenocarcinoma is more prevalent in Western world [4, 5]. The incidence of squamous cell carcinoma is declining while the incidence of adenocarcinoma is rising [6]. Thus, these two histologies should be considered two distinct disease entities. With new targeted agents available on the market, better understanding the molecular pathologenesis of squamous cell carcinoma and adenocarcinoma of the esophagus is at urge. Incorporating targeted agents to the therapeutic paradigm would potentially allow us to have better patient selection and to tailor therapy based on tumor genetics.
Targeting Epidermal Growth Factor Receptor (EGFR) Family Pathway
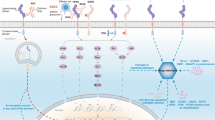
Epidermal growth factor receptor family consists of EGFR (ErbB1), Her2/Neu (ErbB2), ErbB3 and ErbB4 [7–9]. Upon binding of the ligand, the receptor undergoes dimerization and activation of its intrinsic tyrosine kinase activity resulting in initiation of down stream signaling cascade [10]. The activation of EGFRs ultimately leads to cell proliferation, differentiation or even transformation. Targeting EGFR with monoclonal antibody or small typrosine kinase inhibitors has been demonstrated clinically efficacious in solid tumors such as non-small cell lung cancer, head and neck cancer and colorectal cancers either alone or in combination with chemotherapy or radiotherapy. In head and neck cancers, cetuximab potentiates the effect of radiotherapy with a significant survival benefit [11]. More importantly, targeted agents allow us better understanding the tumor biology and tailoring therapy to individual patient. For example, non-small cell lung cancer harboring specific EGFR mutations are particularly sensitive to gefitinib therapy [12]. Patients with such mutations usually demonstrated a higher response rate and longer survival. Like conventional chemotherapy, drug resistance remains a problem. Such resistance to gefitinib was identified [13].
Expression or overexpression of EGFR family members has been described in esophageal and gastric cancers. Overexpression of EGFR has been detected in majority of esophagogastric cancers ranging from 18 to 90% [14–16]. This overexpression is generally associated with a more clinically aggressive disease and a poor prognosis [17–20]. In addition, the EGFR overexpression may cause treatment resistance such as radio resistance in esophageal cancer [21]. Cetuximab, a chimeric monoclonal antibody against the extracellular domain of EGFR, was evaluated in esophageal and gastric cancers. Single agent cetuximab has minimal activity in patients with metastatic esophageal and gastric cancers [22, 23]. However, addition cetuximab to chemotherapy may augment the efficacy of cytotoxic drugs. Pinto and colleagues investigated the activity of cetuximab in combination with cisplatin and decetaxel in patients with advanced GE junction or gastric cancers for first line therapy [24]. The authors showed that a 41.2% response rate was achieved. The median overall survival was 9 months and median time to progression was 5 months. Comparing to the historically data, the addition of cetuximab may have a small improvement of response rate but not overall survival.
Several studies have assessed cetuximab in the preoperative chemoradiation setting and demonstrated feasibility and safety. Ruhstaller et al. published a phase IB/II study (SAKK75/06) using cetuximab with chemotherapy and chemoradiation in both squamous cell carcinoma and adenocarcinoma of the esophagus [25]. A total of 28 patients entered the study. All patients were treated with 2 cycles of induction chemotherapy with cisplatin and docetaxel. Subsequently, patients were allocated into two cohorts. One cohort received weekly cisplatin and cetuximab with radiation while the other cohort received weekly decetaxel, cisplatin and cetuximab with radiation. R0 resection was performed in 25 patients. The study demonstrated that addition of cetuximab with chemoradiation is feasible. An impressive 86% (95% CI, 57–98%) pathological complete response (pCR) or near complete response were achieved in patients with adenocarcinoma. A 64% (95% CI, 31–89%) pCR or near pCR were observed in squamous cell carcinoma. De Vita and colleagues reported another approach using oxaliplatin and cetuximab in a single arm phase II study [26]. Patients in the study were treated with induction chemotherapy of FOLFOX4 and cetuximab followed by cetuximab concurrent with radiotherapy (50 Gy). A 27% pCR was reached. RTOG phase III study is under the way to evaluate cisplatin, paclitaxel and radiation with or without cetuximab in adenocarinoma or squamous cell carcinoma of the esophagus (www.clinicaltrials.gov). The role of cetuximab in the preoperative chemoradiation will be further delineated.
Erlotinib is a small molecule inhibiting EGFR function. Ilson et al. reported a phase II study of erlotinib in patients with advanced previously treated adenocarcinoma and squamous cell carcinoma of the esophagus [27]. Eighty percent of patients in the study had tumors overexpressing EGFR. The partial response rate is low (8%). Time-to-progression was longer in squamous cell histology than that of adenocarcinoma. Thus, it is worthwhile to further evaluate this agent in squamous histology.
Her2/neu is a receptor tyrosine kinase that belongs to EGFR family. Her2/neu gene amplification (FISH) and protein overexpression are found in approximately 20–25% of breast cancers and are predictive of poor prognosis [28]. Trastuzumab, a humanized IgG1 monoclonal antibody to Her2/neu, showed clinical activity when used as a single agent or in combination with chemotherapy in Her2/neu positive breast cancers. Similar to breast cancer, roughly 19–22% of gastric or GE junction cancers have amplification and overexpression of Her2/neu [29–31]. Unlike in breast cancers, Her2/neu as a prognostic marker is less consistent. For example, some studies showed that the expression and amplification were frequently associated with nodal metastasis, advanced stages, distant metastasis and intestinal histology [31, 32]. On the other hand, Shah and co-workers found that Her2/neu expression is a favorable prognostic factor in an univariate analysis but not in the multivariate analysis [33]. Hence, Her2/neu is not an independent prognostic biomarker.
ToGA study is by far the largest phase III study evaluating the activity of trastuzumab in advanced gastric and GE junction cancer [30]. A total of 3,803 patients were screened for the study. The study tested 3,665 patients for Her2/neu expression by both fluorescence in-situ hybridization (FISH) and immunohistochemistry staining (IHC). Eight hundred and ten patients were positive for either FISH or IHC. Finally, a total of 594 patients were randomized 1:1 to receive either chemotherapy (cisplatin, 5FU) alone or trastuzumab with chemotherapy. The median overall survival (OS) was 13.8 months in the trastuzumab arm and 11.1 months in the chemotherapy arm (HR 0.74; 95% CI 0.6–0.91; p = 0.0046). Progression-free survival was also superior in the trastuzumab arm (6.7 months vs. 5.5 months; HR 0.71; 95% CI 0.59–0.85; p = 0.0002). Further analysis showed that nearly all patients with FISH positive/IHC 1+ above tumors benefited from trastuzumab. However, a small proportion of patients that are FISH positive but IHC negative had minimal benefit from trastuzumab (HR 0.92; 95% CI 0.48–1.76). About 15 patients whose tumors were IHC 3+ but FISH negative had no benefit from trastuzumab. The discrepancies of these results could be due to testing error or bias. In addition, these two groups contained very small number of patients that did not have sufficient statistical power to draw further conclusions. The ToGA study is the first phase III study to incorporate a targeted agent in treating gastric and esophageal cancers. Assessing Her2/neu expression in patients with metastatic gastric or GE junction cancers became a new standard practice. Transtuzumab in combination with different chemotherapy regimens such as oxaliplatin and capecitabine (CAPOX) is also under investigation. Currently, RTOG has launched a phase III study using carboplatin, paclitaxel with or without trastuzumab concurrent with radiotherapy in the preoperative setting (www.clinicatrial.gov). Disease-free survival and pCR will be assessed. The results of these clinical studies are awaited.
Lapatinib, a small molecular inhibitor to Her2/neu and EGFR, has demonstrated good clinical activity in breast cancer. Recently, Iqbal et al. published a Southwest Oncology Group (SWOG) study using lapatinib as a single agent in unselected metastatic gastric cancer patients [34]. The study enrolled a total of 47 patients. A 11% partial response rate and 23% stable disease were reported. Combination of lapatinib with various chemotherapies is being explored.
Targeting Angiogenesis Pathway
Anti-angiogenesis therapy has been proven to be effective in many solid tumors. Bevacizumab, a humanized monoclonal antibody against vascular endothelial growth factor (VEGF), has been used to treat many solid tumors including colorectal cancer, breast cancer, renal cell carcinoma and hepatocellular carcinoma [35–39]. In colorectal cancer, addition of bevacizumab to chemotherapy results in a better response rate, progression-free survival and overall survival [36].
Like in other solid tumors, a higher level of VEGF was also found in the surgical specimen of esophagogastric cancer specimens [40–43]. The higher level of VEGF expression is associated with a more aggressive clinical course, nodal metastasis, higher TNM staging and poor prognosis. Based on these preclinical findings and clinical success in other tumors, bevacizumab was first evaluated in several small single arm studies. Shah and co-workers showed that combination of bevaizumab, irinotecan and cisplatin resulted in 65% response rate and 12.3 overall median survival rate [44]. Recently, Shah and colleagues reported another single arm phase II study using bevacizumab with modified DCF (decetaxel, cisplatin and 5FU) [45]. The study demonstrated a median overall survival of 16.8 months. During the subgroup analysis, a 85% response rate was achieved in GE junction and proximal gastric cancers. A 56% response rate was reached for distal and body gastric cancers. However, diffuse type gastric cancer has much lower response rate (38%). Despite these encouraging data, the efficacy of bevacizumab was disappointing in the phase III study. AVAGAST (avastin for advanced gastric cancer) study is a double-blind, randomized, placebo-controlled phase III study of cisplatin and capecitabine with or without bevacizumab as first line therapy in advanced gastric or GE junction cancers [46]. A total of 774 patients were enrolled in the study. The study subjects were randomized to chemotherapy with placebo or chemotherapy with bevacizumab. The overall response rate was significantly higher with the addition of bevacizumab (38% vs. 29.5%, p = 0.0121). The progression-free survival is significantly longer in the bevacizumab arm (6.7 months in bevacizumab arm vs. 5.3 months in the placebo arm; p = 0.0037). However, there was no difference in overall survival between the two study arms (HR = 0.87, p = 0.1002).
Bevacizumab was also evaluated in preoperative chemoradiation to explore the feasibility, safety and preliminary efficacy. Ilson et al. presented their study of bevacizumab, irinotecan and cisplatin with radiotherapy in localized gastroesophageal cancer [47]. Preliminary analysis showed that it is safe and feasible. Delayed wound-healing was not observed. Other approaches such as using bevacizumab in the perioperative chemotherapy are under evaluation. MAGIC study offered preioperative chemotherapy ECF in operable gastric and GE junction cancer. The study demonstrated survival benefit for this approach [48]. Because of the success of MAGIC trial, integrating bevacizumab in perioperative chemotherapy is currently being evaluated in a phase III study (ST03). Preliminary data showed reasonable safety without increasing surgical risks significantly [49]. Other anti-angiogenesis agents (ramucuryumab and apatinib) in the second or third line therapy are under the way (www.clinicaltrials.gov). Multi-tyrosine kinase inhibitors with anti-angiogenic effects including sorafenib and sunitinib are being actively evaluated [50–52]. To better select patients and to stratify patients in future clinical trials, several biomarkers have been explored to predict the efficacy of bevacizumab. These markers include VEGF level, circulating endothelial progenitor cells or circulating endothelial cells [38, 53–58]. However, the results are rather disappointing. More researches are needed to better understand the role angiogenesis in tumor development and progression. Furthermore, the mechanism of these anti-angiogenic agents needs to be further defined.
Other Potential Targets
In addition to these two major pathways, other potential molecular targets were assessed. Cyclooxygenase-2 (COX-2) pathway has been the center of attention in cancer development and progression several years ago. It has been shown that COX-2 inhibitors can reduce the risks for esophageal, gastric and colorectal cancers [59–61]. COX-2 is frequently upper regulated in gastrointestinal tumors [62, 63]. Through cross talk with other signaling pathways, COX-2 has been shown to activate NFκB and EGFR or to inactive tumor suppressor gene, APC [64–67]. Celecoxib, a COX-2 inhibitor, has been assessed with chemotherapy in both advanced and localized disease [68, 69]. However, it is difficult to obtain conclusive results in these small studies. Because of the recently recognized cardiac complications of COX-2 inhibitors, further utilization of celecoxib in gastroesophageal cancers and other tumor types has been placed on hold.
PI3 kinase/AKT/mTOR pathways are important in regulating cell proliferation and transformation. These pathways frequently cross talk with other receptor tyrosine kinase mediated signal cascade. Hilebrandt and co-workers from M.D. Anderson examined the genetic variations of PI3 kinase/PTEN/AKT/mTOR pathway in 210 patients with respectable esophageal cancer [70]. The authors demonstrated that certain single nucleotide polymorphism (SNP) is associated with higher risk of recurrence and resistance to chemoradiation. For example, patients with SNP AKT1:rs892119 variation has much poor prognosis comparing to wild type AKT (median survival of 12 months for one or two AKT variant; median survival of 42 months for the wild type). The poor survival for the AKT1:rs892119 was not affected by different chemotherapy agents such as 5FU or cisplatin. Clinically, everolimus, a mTOR inhibitor, is being actively assessed in advanced gastric cancer [71].
Perhaps, the most intriguing target is “cancer stem cells” that was first explored in hematological malignancies. The esophageal stem cells have similar characteristics to other cancer stem cells. They are slow growing, self-renewal and a high proliferative potential triggered by wound healing process [72]. Characterization of esophageal stem cells, however, is rather inconsistent in the literature. Kalabis et al. described that the esophageal basal epithelial cells with self-renewal properties are CD34+ [73]. Other markers including CD133, adenosine triphosphate-binding cassette superfamily G 2 (ABCG2), CD44 and Musashi-1 have been described in the literature [74–76]. It is not clear what the clinical implications are when these markers are detected in clinical specimens or cell lines. Although there is a great interest to target stem cells in cancer therapy, such approach is largely hindered by poorly defined biology of these cells.
Conclusions
Esophagogastric cancer remains a public health problem worldwide. Over the past decade, the treatment of esophagogastric cancer has been evolving rapidly. These include surgical techniques and development of new cytotoxic agents as well as targeted therapy. Recent introduction of trastuzumab in advanced disease brought a new era of personalized medicine in managing esophagogastric cancers. However, more researches are required to better understand the tumor biology and the mechanism of action of targeted agents. Only through these mechanistic explorations, potential predictive and prognostic biomarkers could be identified. These biomarkers will allow us to have better patient selection, better stratification and better trial designs.
References
Solaymani-Dodaran M, Logan RF, West J, Card T, Coupland C. Risk of oesophageal cancer in Barrett’s oesophagus and gastro-oesophageal reflux. Gut. 2004;53(8):1070–4.
Altorki NK, Oliveria S, Schrump DS. Epidemiology and molecular biology of Barrett’s adenocarcinoma. Semin Surg Oncol. 1997;13(4):270–80.
Spechler SJ, Robbins AH, Rubins HB, Vincent ME, Heeren T, Doos WG, et al. Adenocarcinoma and Barrett’s esophagus. An overrated risk. Gastroenterology. 1984;87(4):927–33.
Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17(1):2–9. Epub 2006/12/23.
Siewert JR, Ott K. Are squamous and adenocarcinomas of the esophagus the same disease. Semin Radiat Oncol. 2007;17(1):38–44. Epub 2006/12/23.
American Cancer Society: estimated new cases by sex for all sites, U.S. 2007.
Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110(6):669–72.
Burgess AW, Cho HS, Eigenbrot C, Ferguson KM, Garrett TP, Leahy DJ, et al. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003;12(3):541–52. Epub 2003/10/07.
Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5(4):317–28. Epub 2004/04/20.
Yarden Y, Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–78. Epub 1988/01/01.
Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–78. Epub 2006/02/10.
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39. Epub 2004/05/01.
Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352(8):786–92. Epub 2005/02/25.
Takehana T, Kunitomo K, Suzuki S, Kono K, Fujii H, Matsumoto Y, et al. Expression of epidermal growth factor receptor in gastric carcinomas. Clin Gastroenterol Hepatol. 2003;1(6):438–45. Epub 2004/03/16.
Tokunaga A, Onda M, Okuda T, Teramoto T, Fujita I, Mizutani T, et al. Clinical significance of epidermal growth factor (EGF), EGF receptor, and c-erbB-2 in human gastric cancer. Cancer. 1995;75(Suppl 6):1418–25. Epub 1995/03/15.
Albanell J, Rojo F, Baselga J. Pharmacodynamic studies with the epidermal growth factor receptor tyrosine kinase inhibitor ZD1839. Semin Oncol. 2001;28(5 Suppl 16):56–66. Epub 2001/11/14.
Kitagawa Y, Ueda M, Ando N, Ozawa S, Shimizu N, Kitajima M. Further evidence for prognostic significance of epidermal growth factor receptor gene amplification in patients with esophageal squamous cell carcinoma. Clin Cancer Res. 1996;2(5):909–14. Epub 1996/05/01.
Gibault L, Metges JP, Conan-Charlet V, Lozac’h P, Robaszkiewicz M, Bessaguet C, et al. Diffuse EGFR staining is associated with reduced overall survival in locally advanced oesophageal squamous cell cancer. Br J Cancer. 2005;93(1):107–15. Epub 2005/06/30.
Wilkinson NW, Black JD, Roukhadze E, Driscoll D, Smiley S, Hoshi H, et al. Epidermal growth factor receptor expression correlates with histologic grade in resected esophageal adenocarcinoma. J Gastrointest Surg. 2004;8(4):448–53. Epub 2004/05/04.
Gibson MK, Abraham SC, Wu TT, Burtness B, Heitmiller RF, Heath E, et al. Epidermal growth factor receptor, p53 mutation, and pathological response predict survival in patients with locally advanced esophageal cancer treated with preoperative chemoradiotherapy. Clin Cancer Res. 2003;9(17):6461–8. Epub 2003/12/26.
Liang K, Ang KK, Milas L, Hunter N, Fan Z. The epidermal growth factor receptor mediates radioresistance. Int J Radiat Oncol Biol Phys. 2003;57(1):246–54. Epub 2003/08/12.
Gold PJ, Goldman B, Iqbal S, Leichman LP, Zhang W, Lenz HJ, et al. Cetuximab as second-line therapy in patients with metastatic esophageal adenocarcinoma: a phase II Southwest Oncology Group Study (S0415). J Thorac Oncol. 2010;5(9):1472–6. Epub 2010/07/16.
Chan JA, Blaszkowsky LS, Enzinger PC, Ryan DP, Abrams TA, Zhu AX, et al. A multicenter phase II trial of single-agent cetuximab in advanced esophageal and gastric adenocarcinoma. Ann Oncol. 2011;22(6):1367–73. Epub 2011/01/11.
Pinto C, Di Fabio F, Barone C, Siena S, Falcone A, Cascinu S, et al. Phase II study of cetuximab in combination with cisplatin and docetaxel in patients with untreated advanced gastric or gastro-oesophageal junction adenocarcinoma (DOCETUX study). Br J Cancer. 2009;101(8):1261–8. Epub 2009/09/24.
Ruhstaller T, Pless M, Dietrich D, Kranzbuehler H, von Moos R, Moosmann P, et al. Cetuximab in combination with chemoradiotherapy before surgery in patients with resectable, locally advanced esophageal carcinoma: a prospective, multicenter phase IB/II Trial (SAKK 75/06). J Clin Oncol. 2011;29(6):626–31. Epub 2011/01/06.
De Vita F, Orditura M, Martinelli E, Vecchione L, Innocenti R, Sileni VC, et al. A multicenter phase II study of induction chemotherapy with FOLFOX-4 and cetuximab followed by radiation and cetuximab in locally advanced oesophageal cancer. Br J Cancer. 2011;104(3):427–32. Epub 2011/01/20.
Ilson DH, Kelsen D, Shah M, Schwartz G, Levine DA, Boyd J, et al. A phase 2 trial of erlotinib in patients with previously treated squamous cell and adenocarcinoma of the esophagus. Cancer. 2011;117(7):1409–14. Epub 2011/03/23.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82. Epub 1987/01/09.
Sato-Kuwabara Y, Neves JI, Fregnani JH, Sallum RA, Soares FA. Evaluation of gene amplification and protein expression of HER-2/neu in esophageal squamous cell carcinoma using Fluorescence In Situ Hybridization (FISH) and immunohistochemistry. BMC Cancer. 2009;9:6. Epub 2009/01/09.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97. Epub 2010/08/24.
Yan SY, Hu Y, Fan JG, Tao GQ, Lu YM, Cai X, et al. Clinicopathologic significance of HER-2/neu protein expression and gene amplification in gastric carcinoma. World J Gastroenterol. 2011;17(11):1501–6. Epub 2011/04/08.
Begnami MD, Fukuda E, Fregnani JH, Nonogaki S, Montagnini AL, Da Costa Jr WL, et al. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 Are predictors of poor outcome. J Clin Oncol. 2011;29:3030–6.
Shah MA, Janjigian YY, Pauligk C, et al. Prognostic significance of human epidermal growth factor-2 (HER2) in advanced gastric cancer: a U.S. and European international collaborative analysis. J Clin Oncol. 2011;29:(suppl; abstr 4014).
Iqbal S, Goldman B, Fenoglio-Preiser CM, Lenz HJ, Zhang W, Danenberg KD, et al. Southwest oncology group study S0413: a phase II trial of lapatinib (GW572016) as first-line therapy in patients with advanced or metastatic gastric cancer. Ann Oncol. 2011;22:2610–5.
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–50. Epub 2006/12/15.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42. Epub 2004/06/04.
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–76. Epub 2007/12/28.
Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349(5):427–34. Epub 2003/08/02.
Bukowski RM, Kabbinavar FF, Figlin RA, Flaherty K, Srinivas S, Vaishampayan U, et al. Randomized phase II study of erlotinib combined with bevacizumab compared with bevacizumab alone in metastatic renal cell cancer. J Clin Oncol. 2007;25(29):4536–41. Epub 2007/09/19.
Tanaka T, Ishiguro H, Kuwabara Y, Kimura M, Mitsui A, Katada T, et al. Vascular endothelial growth factor C (VEGF-C) in esophageal cancer correlates with lymph node metastasis and poor patient prognosis. J Exp Clin Cancer Res. 2010;29:83. Epub 2010/06/30.
Kozlowski M, Naumnik W, Niklinski J, Milewski R, Dziegielewski P, Laudanski J. Vascular endothelial growth factor C and D expression correlates with lymph node metastasis and poor prognosis in patients with resected esophageal cancer. Neoplasma. 2011;58(4):311–9. Epub 2011/04/28.
Maeda K, Chung YS, Takatsuka S, Ogawa Y, Onoda N, Sawada T, et al. Tumour angiogenesis and tumour cell proliferation as prognostic indicators in gastric carcinoma. Br J Cancer. 1995;72(2):319–23. Epub 1995/08/01.
Juttner S, Wissmann C, Jons T, Vieth M, Hertel J, Gretschel S, et al. Vascular endothelial growth factor-D and its receptor VEGFR-3: two novel independent prognostic markers in gastric adenocarcinoma. J Clin Oncol. 2006;24(2):228–40. Epub 2005/12/14.
Shah MA, Ramanathan RK, Ilson DH, Levnor A, D’Adamo D, O’Reilly E, et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2006;24(33):5201–6. Epub 2006/11/23.
Shah MA, Jhawer M, Ilson DH, Lefkowitz RA, Robinson E, Capanu M, et al. Phase II study of modified docetaxel, cisplatin, and fluorouracil with bevacizumab in patients with metastatic gastroesophageal adenocarcinoma. J Clin Oncol. 2011;29(7):868–74. Epub 2010/12/30.
Kang Y, Ohtsu A, Van Cutsem E, et al. AVAGAST: a randomized, double-blind, placebo-controlled, phase III study of first-line capecitabine and cisplatin plus bevacizumab or placebo in patients with advanced gastric cancer (AGC). J Clin Oncol. 2010;28:18s (suppl; abstr LBA4007).
Ilson D, Bains M, Rizk N, et al. Phase II trial of preoperative bevacizumab (Bev), irinotecan (I), cisplatin (C), and radiation (RT) in esophageal adenocarcinoma: preliminary safety analysis. J Clin Oncol. 2009;27(15s). suppl; abstr 4573 2009.
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20.
Okines AF, Langley R, Cafferty FH, et al. Preliminary safety data from a randomized trial of perioperative epirubicin, cisplatin plus capecitabine (ECX) with or without bevacizumab (B) in patients (pts) with gastric or oesophagogastric junction (OGJ) adenocarcinoma. J Clin Oncol. 2010;28(15s) (suppl; abstr 4019).
Bang YJ, Kang YK, Kang WK, Boku N, Chung HC, Chen JS, et al. Phase II study of sunitinib as second-line treatment for advanced gastric cancer. Invest New Drugs. 2011;29:1449–58. Epub 2010/05/13.
Sun W, Powell M, O’Dwyer PJ, Catalano P, Ansari RH, Benson III AB. Phase II study of sorafenib in combination with docetaxel and cisplatin in the treatment of metastatic or advanced gastric and gastroesophageal junction adenocarcinoma: ECOG 5203. J Clin Oncol. 2010;28(18):2947–51.
Kim C, Lee JL, Choi YH, Kang BW, Ryu MH, Chang HM, et al. Phase I dose-finding study of sorafenib in combination with capecitabine and cisplatin as a first-line treatment in patients with advanced gastric cancer. Invest New Drugs. 2012;30:306–15. Epub 2010/09/15.
Dowlati A, Gray R, Sandler AB, Schiller JH, Johnson DH. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab—an Eastern cooperative oncology group study. Clin Cancer Res. 2008;14(5):1407–12. Epub 2008/03/05.
Kopetz S, Hoff PM, Morris JS, Wolff RA, Eng C, Glover KY, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol. 2010;28(9):453. Epub 2009/12/17.
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–7. Epub 1997/02/14.
Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7(11):1194–201. Epub 2001/11/02.
Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319(5860):195–8. Epub 2008/01/12.
Furstenberger G, von Moos R, Lucas R, Thurlimann B, Senn HJ, Hamacher J, et al. Circulating endothelial cells and angiogenic serum factors during neoadjuvant chemotherapy of primary breast cancer. Br J Cancer. 2006;94(4):524–31. Epub 2006/02/02.
Abnet CC, Freedman ND, Kamangar F, Leitzmann MF, Hollenbeck AR, Schatzkin A. Non-steroidal anti-inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta-analysis. Br J Cancer. 2009;100(3):551–7. Epub 2009/01/22.
Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356(21):2131–42. Epub 2007/05/25.
Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10(5):501–7. Epub 2009/05/05.
Rigas B, Goldman IS, Levine L. Altered eicosanoid levels in human colon cancer. J Lab Clin Med. 1993;122(5):518–23. Epub 1993/11/01.
Wu WK, Sung JJ, Yu L, Li ZJ, Chu KM, Cho CH. Constitutive hypophosphorylation of extracellular signal-regulated kinases-1/2 and down-regulation of c-Jun in human gastric adenocarcinoma. Biochem Biophys Res Commun. 2008;373(2):330–4. Epub 2008/06/24.
Wu WK, Sung JJ, Lee CW, Yu J, Cho CH. Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: an update on the molecular mechanisms. Cancer Lett. 2010;295(1):7–16. Epub 2010/04/13.
Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med. 2002;8(3):289–93. Epub 2002/03/05.
Pai R, Nakamura T, Moon WS, Tarnawski AS. Prostaglandins promote colon cancer cell invasion; signaling by cross-talk between two distinct growth factor receptors. FASEB J. 2003;17(12):1640–7. Epub 2003/09/06.
Boolbol SK, Dannenberg AJ, Chadburn A, Martucci C, Guo XJ, Ramonetti JT, et al. Cyclooxygenase-2 overexpression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Res. 1996;56(11):2556–60. Epub 1996/06/01.
Govindan R, McLeod H, Mantravadi P, Fineberg N, Helft P, Kesler K, et al. Cisplatin, fluorouracil, celecoxib, and RT in resectable esophageal cancer: preliminary results. Oncology (Williston Park). 2004;18(14 Suppl 14):18–21. Epub 2005/02/03.
Enzinger PC, Mamon H, Choi N, et al. Phase II cisplatin, irinotecan, celecoxib and concurrent radiation therapy followed by surgery for locally advanced esophageal cancer. 2004 Gastrointestinal Cancer Symposium, abstract 35.
Hildebrandt MA, Yang H, Hung MC, Izzo JG, Huang M, Lin J, et al. Genetic variations in the PI3K/PTEN/AKT/mTOR pathway are associated with clinical outcomes in esophageal cancer patients treated with chemoradiotherapy. J Clin Oncol. 2009;27(6):857–71. Epub 2009/01/24.
Yamada Y, Doi T, Muro K, et al. Multicenter phase II study of everolimus in patients with previously treated metastatic gastric cancer: main results. Gastrointestinal cancers symposium, San Francisco January 15–17, 2009.
Lavker RM, Sun TT. Epidermal stem cells: properties, markers, and location. Proc Natl Acad Sci U S A. 2000;97(25):13473–5. Epub 2000/11/23.
Kalabis J, Oyama K, Okawa T, Nakagawa H, Michaylira CZ, Stairs DB, et al. A subpopulation of mouse esophageal basal cells has properties of stem cells with the capacity for self-renewal and lineage specification. J Clin Invest. 2008;118(12):3860–9. Epub 2008/11/27.
Hang D, Dong HC, Ning T, Dong B, Hou DL, Xu WG. Prognostic value of the stem cell markers CD133 and ABCG2 expression in esophageal squamous cell carcinoma. Dis Esophagus. 2012;25:638–44. Epub 2012/01/13.
Zhao JS, Li WJ, Ge D, Zhang PJ, Li JJ, Lu CL, et al. Tumor initiating cells in esophageal squamous cell carcinomas express high levels of CD44. PLoS One. 2011;6(6):e21419. Epub 2011/07/07.
Bobryshev YV, Freeman AK, Botelho NK, Tran D, Levert-Mignon AJ, Lord RV. Expression of the putative stem cell marker musashi-1 in Barrett’s esophagus and esophageal adenocarcinoma. Dis Esophagus. 2010;23(7):580–9. Epub 2010/05/13.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Jiang, Y. (2013). Impact of Genetic Targets on Cancer Therapy in Esophagogastric Cancer. In: El-Deiry, W. (eds) Impact of Genetic Targets on Cancer Therapy. Advances in Experimental Medicine and Biology, vol 779. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6176-0_3
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6176-0_3
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6175-3
Online ISBN: 978-1-4614-6176-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)