Abstract
New exciting discoveries have emerged in the field of aggressive lymphomas. A better understanding of the biology of lymphomas has been translated into clinical practice, and new drugs are emerging as a hope for better disease control. In this chapter, we review the new therapies for aggressive lymphomas, like proteasome inhibitors, B-cell receptor signaling inhibitors (Syk, Burton’s tyrosine kinase and protein kinase C inhibitors) and mammalian targets of rapamycin. Moreover, a concise review of the new monoclonal antibodies (MoAbs) and their new targets is presented. Immunomodulatory drugs like lenalidomide also have shown potential benefit in patients with aggressive lymphomas. Although more studies are necessary, these drugs will probably be incorporated in the management of aggressive lymphomas, with less toxic, targeted therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
The hematological malignancies have long been in the forefront of development of cancer therapies. The association of new discoveries in genetics and molecular biology has contributed to a better understanding of cancer and consequent treatment innovations. Chronic myeloid leukemia (CML) remains a paradigm of the foresaid. From the Philadelphia chromosome, the first chromosome-specific abnormality related to cancer [1], to the consequent discovery of the BCR-ABL, a fusion protein with abnormal kinase activity responsible for the proliferation of myeloid cells [2], a new era in cancer biology has emerged. Not long, patients with CML could benefit from these discoveries: imatinib (Gleevec®), a selective inhibitor of tyrosine kinase, has been the first approved molecular targeted therapy in cancer and is now considered standard therapy for patients with CML [3].
New exciting discoveries also emerged in the field of lymphomas. Rituximab (Rituxan®), a chimeric monoclonal antibody targeted at the CD20 epitope present in B cells, has brought to attention the role of immunotherapy in cancer treatment and is now considered essential in the treatment of most B-cell lymphomas [4]. New monoclonal antibodies (MoAb) have been developed for other malignancies, such as acute myeloid leukemia and renal and pulmonary cancer [5, 6]. Conjugation of a radionuclide with MoAbs introduced the conception of radioimmunotherapy, now available for treatment of selected patients with lymphoma [7].
In the past, aggressive lymphomas were considered an obscure disease with a dismal prognosis. Recent discoveries have shed new light, and aggressive lymphomas are now a treatable and curable disease.
New Insights in the Biology of Aggressive Lymphomas
In the past, the diagnosis of lymphoma and cancer in general relied mostly on morphologic features. However, the findings of recurrent cytogenetic abnormalities and consequent molecular alterations, urged the need of more refined diagnosis. Mantle cell lymphoma, for example, is now recognized as a molecular-defined lymphoma, with over expression of cyclin D1, a consequence of the t(11;14) present in malignant cells [8]. In contrast, the diagnosis of diffuse large B-cell lymphoma (DLBCL), the most common subtype of lymphoma, remains confined to morphological and immunohistochemical findings. Consequently, it is reasonable to conclude that different types of DLBCL, with diverse molecular alterations and clinical behavior, are diagnosed and treated the same.
An area of great interest in recent research is the attempt to divide different subtypes of DLBCL [9]. Recently, great interest have been directed to subtypes of aggressive DLBCL-associated MYC rearrangements. Overexpression of c-MYC drives cell proliferation and the expression of other genes involved in cell growth, and patients with DLBCL with c-MYC expression usually have high-grade morphologic features that morphologically resemble Burkitt lymphoma (BL) [10]. A subgroup of patients also presents concurrent translocations involving the BCL-2 gene. This subtype of lymphomas, so-called “double hit” DLBCL, usually present with very aggressive disease, with bone marrow involvement, elevated lactate dehydrogenase levels, and multiple extranodal sites [11, 12]. Patients seldom respond to chemotherapy, and those who achieve a response subsequently suffer an early relapse [13]. Studies are being conducted to evaluate the role of intensified chemotherapy in this subset of patients.
Gene profile expression studies have contributed to a better understanding and separation of at least two subtypes of DLBCL: germinal center B-cell (GCB) and activated B-cell (ABC) lymphomas [14]. When the clinical outcome of the cases was examined, cases with the GBC signature had a significantly better prognosis than the cases that expresses the ABC signature [15]. Although the initial studies were conducted in the era pre-rituximab, subsequent studies confirmed the prognostic value of the GCB-cell signature [16]. Subsequent studies have focused on the genes expressed in the GCB or ABC subtypes. High expression of a proliferation signature and low expression of major histocompatibility complex signature are associated with poor survival. Interestingly, studies focusing on the lymph node stromal signature disclosed a potential prognostic value [17]. High expression of stromal signatures associated with extracellular matrix deposition and tissue inflammation (stromal I signature) is associated with better outcome than cases with high expression of signatures associated with angiogenesis (stromal II signature) [18]. Other studies on subsets of DLBCL based on site of presentation also show different patterns of gene expression.
Although these studies have shown the potential of gene expression to define oncogenic pathways, it has yet to be translated in clinical practice. However, new studies have shown that a subset of DLBCL may benefit from a specific therapy. For instance, the Biocoral study, a post hoc study of biological variables of the CORAL study, has shown that patients with relapsed DLBCL with GCB phenotype have better outcomes when treated with R-DHAP instead of R-ICE [19].
Imaging in Aggressive Lymphomas
The traditional approach to treatment monitoring through imaging has relied on anatomical changes assessing tumor size before and after treatment. This approach has proven through time to be rather problematic. The rate of structural regression of tumors after chemotherapy depends on several factors in the host, as well as the amount of fibrosis present in the tumor. Moreover, the response criteria universally adopted are somewhat arbitrary, and a consistent correlation between tumor response and patient survival has not been demonstrated.
Over 50 years ago, Otto Heinrich Warburg discovered that malignant cancers ferment glucose to lactic acid much more rapidly than most normal cells [20]. The latter, known as the “Warburg effect,” is the basis for the use of fluorine-18 fluorodeoxyglucose ([18F]FDG) positron emission tomography (PET-CT) for the staging and treatment monitoring of a variety of cancers [21–23]. The first reports of PET-CT for lymphoma imaging were published more than 20 years ago, and during the last decade, PET-CT has been introduced into all of the steps of lymphoma management.
In aggressive lymphomas, PET-CT has proved to be highly sensitive in determining sites of disease [24, 25]. Several studies have demonstrated the superiority of PET-CT for monitoring treatment of aggressive NHL [24–26]. Patients with a negative PET-CT after treatment have better progression-free survival [27]. However, the role of interim PET-CT in DLBCL patients is controversial, with studies showing conflicting results [28, 29]. Outside clinical trials, interim PET-CT is not recommended in patients with DLBCL.
Recent trials are now focusing on PET-CT-tailored strategies. The Groupe d’Etude des Lymphomes de l’Adulte (GELA) is currently conducting a phase III trial of salvage autologous stem cell transplantation (ASCT) if PET results are positive after two cycles of R-CHOP [30]. In the same manner, the British Columbia Cancer Agency is testing four cycles of R-ICE if PET results are positive after four cycles of R-CHOP [31]. The Alberta Cancer Board also conducted a trial of PET-CT-guided treatment and preliminary results suggest that high-dose therapy with R-DICEP and ASCT may improve EFS for poor prognosis DLBCL with interim positive PET-CT [32].
New Treatments in Aggressive Lymphomas
The development of new drugs as well as incorporation of drugs already developed for other malignancies shows promising results in aggressive results.
Proteasome Inhibitors
The proteasome is a multi-subunit protease complex responsible for the elimination of intracellular proteins tagged for degradation [33]. The ubiquitin-proteasome pathway controls protein function through the degradation of polyubiquitinated intracellular proteins, involved in cell cycle regulation, transcription factor activation, and apoptosis [34, 35]. Diverse types of cancer cells undergo apoptosis in response to proteasome inhibition, and many proteins involved in lymphomagenesis are regulated by the proteasome pathway, including cyclins, NF-κB and p53 [36–38].
NF-κB is a family of proteins that control genes implicated in cell activation, proliferation, and apoptosis [39]. Several studies have implicated increased NF-κB activity in different lymphomas, such as MALT lymphomas [40], peripheral T-cell lymphoma [41], and activated B cell-like DLBCL [42]. Bortezomib (Velcade®), a 20S proteasome subunit inhibitor approved for the treatment of relapsed refractory multiple myeloma, has also shown activity in NHL [43]. A phase II trial of 155 pretreated patients with MCL receiving bortezomib (PINNACLE) reported an ORR of 31%, with 14 patients with CR/Cru [44]. Based on this trial, bortezomib is now approved for patients with relapsed/refractory MCL. The role of bortezomib in association with standard chemotherapy for untreated patients with MCL is being evaluated.
Bortezomib has also been studied in patients with DLBCL, with modest activity as a single agent. The association of bortezomib with standard R-CHOP (BR-CHOP) therapy was evaluated in a phase II prospective trial with 76 patients with DLBCL and MCL (40 patients with DLBCL). BR-CHOP resulted in an intent-to-treat response of 88% [45]. The addition of bortezomib in salvage therapy of patients with DLBCL has also been evaluated. Relapsed patients with ABC subtype of DLBCL were treated with bortezomib in combination with dose-adjusted EPOCH chemotherapy. The ORR was 83%, with an OS of 10.8 months [46]. Two prospective trials (LYM2034 and PYRAMID) are assessing the role of bortezomib combined with chemotherapy in upfront therapy of patients with non-GCB DLBCL.
B-Cell Receptor Signaling Inhibitors
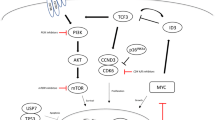
B-cell receptor (BCR) plays an important role in normal B-cell development. Progenitor B cells only differentiate into mature B cells if they express a functional BCR, and those B cells that fail to express a BCR undergo apoptosis. Antigen BCR ligation activates a family of tyrosine kinases, named Scr protein kinases. These tyrosine kinases consequently recruit Syk, another tyrosine kinase responsible for activation of several other molecules, including protein kinase C (PKC), phosphatidyl-inositol-3-kinase (PI3K) and AKT (also known as protein kinase B) [47]. In DLBCL, a chronically “tonic signaling” has been observed and gene expression profiling studies have shown that some DLBCL have overexpression of the BCR, indicating a dependence on this signaling pathway [48]. New drugs targeting the BCR signaling pathway are being developed.
Syk Inhibitors
In vitro studies have confirmed the presence of deregulated Syk pathway in DLBCL [49]. Furthermore, in vitro inhibition of Syk induced apoptosis of DLBCL cell lines. Syk inhibitors are now testing the possible therapeutic effect of these drugs.
Fostamatinib disodium (R406), an oral ATP-competitive Syk inhibitor, is the first to be tested in clinical trials. In preclinical studies, R406 inhibited the proliferation and induced apoptosis of DLBCL lines with tonic BCR signaling, especially lines with high expression of cell-surface immunoglobulin [48]. A phase I/II trial including 68 patients with relapsed NHL (23 DLCBL) showed promising results, including a 22% clinical response in DLBCL, with four partial responses and one complete response [50]. Adverse events were mild and included fatigue, gastrointestinal symptoms, and myelosuppression. Further studies both as single agent and in combination with other drugs are being conducted. Moreover, it has been demonstrated that some patients with peripheral T-cell lymphoma also have a deregulated Syk pathway [51], and studies are conducted with fostamatinib in these patients.
Bruton Tyrosine Kinase Inhibitors
Bruton agammaglobulinemia, an X-linked agammaglobulinemia first described in 1952, is caused by mutations in the gene coding for Bruton tyrosine kinase (BTK). Patients with Bruton agammaglobulinemia have profound hypogammaglobulinemia, with fewer than 1% of the normal number of B cells, with an immature phenotype [52].
Another approach to interrupting BCR signaling is with BTK inhibitors. BTK is a component of the BCR signaling pathway and is downstream of Syk. BTK is critical to the maturation of pre-B cells to differentiating mature B cells [52]. Findings suggest that BTK may be an essential transducer of signals that govern immunoglobulin rearrangement events and re-expression of the RAG gene products. BTK signaling may also regulate the survival of immature B cells that have performed a successful Ig light chain rearrangement, as well as governing the entrance of B cells in the follicular center [53].
Targeting BTK is a promising new therapeutic approach in NHL. Ibrutinib (PCI-32765), an oral irreversible BTK inhibitor, has been tested in a phase Ib/II follow-up trial in patients with relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma and showed an ORR of 70%, in a 10 month follow-up [54]. An interim analysis of a phase II study in 48 patients with relapsed/refractory mantle cell lymphoma showed an overall response rate of 67%. Interestingly, patients exposed to bortezomib had higher rates of response (75% vs. 58%) [55].
Ibrutinib has also been tested in patients with relapsed/refractory ABC DLBCL. In an interim analysis of a phase I/II study, eight patients received ibrutinib in a fixed dose of 560 mg daily for 35 days. Two patients achieved CR, and other three patients achieved stable disease (SD), showing that ibrutinib has clinical activity in aggressive lymphomas [56].
PKC Inhibitors
The PKC family is composed of four main members: conventional PKCs (α, β and γ), novel PKCs, atypical PKCs, and PKC-related kinases. PKC-β protein is a critical component of the BCR signaling pathway and is related to cell survival by activation of the NF-κB complex [57]. PCK-β has also been related in VEGF-mediated angiogenesis [58]. Since both NF-κB and VEGF are implicated with DLBCL, PKC-β is an attractive target for development of new drugs.
Enzastaurin is an oral ATP-competitive selective inhibitor of PKC-b that also targets the PI3K-AKT pathway and has shown proapoptotic, antiproliferative, and antiangiogenic activities in several cancer lines [59]. A phase II trial including 55 patients with relapsed DLBCL disclosed a safe toxicity profile, with rare hematological toxicities. The primary endpoint was freedom from progression after two cycles, and 22% of patients achieved this endpoint, including three patients achieving CR lasting more than 20 months [60]. A phase III trial is testing enzaustarin in combination with R-CHOP for first-line treatment of patients with DLBCL. Enzastaurin maintenance is also being tested in high-risk DLBCL patients achieving a CR after R-CHOP treatment [61].
Bryostatin 1 (B-1) is a modulator of the PKC family and has shown antitumor activity in vitro. An immunomodulatory component is also present, with neutrophil and immune cell activation. Studies in patients with CLL or indolent NHLs are being conducted, exploring the effect of B-1 in combination with other cytotoxic agents [62].
Heat Shock Protein Inhibitors
Heat shock proteins (HSP) are cytosolic molecules responsible for chaperoning multiple proteins necessary for cell signaling, proliferation, and survival [63]. In the presence of cellular stress, an increase in the expression of HSP occurs, and HSP bind to client proteins protecting them from degradation and preserving cells from apoptosis [64].
HSP90, a member of the HSP family, is overexpressed in cancer cells and may be involved in the survival advantage of these cells. Moreover, HSP90 is overexpressed in many NHL subsets, including DLBCL, and is related to histological transformation of indolent lymphomas [65]. Geldanamycin (17-allylamino-17-demethoxygeldanamycin [17-AAG]) is the first HSP90 inhibitor tested in humans. It has shown to induce cell cycle arrest and apoptosis through the downregulation of cyclin-dependent kinase 1 and AKT and the activation of caspase 9 in MCL cell lines [66]. A number of phase I trials with 17-AAG and similars have shown some activity in melanoma [67], myeloma [68], or breast cancer [69]. Phase I studies in patients with relapsed or refractory NHL are ongoing.
Mammalian Target of Rapamycin Inhibitors
Mammalian target of rapamycin (mTOR) is a kinase involved in regulation of proliferation, cell growth, and apoptosis. The activation of the PI3K-AKT-mTOR pathway promotes the translation of proteins that regulate cell cycle, in particular the eukaryotic initiation factor 4E-binding protein 1 and the p70S6 kinase, responsible for the translation of cyclin D1, c-MYC, and Stat3 proteins, all involved in the pathogenesis of NHL [70]. The PI3K pathway is constitutively activated in the majority of B-cell lymphomas, as manifested by phosphorylation of S6K and 4E-BP1 [71]. Moreover, mTOR inhibitors can decrease the expression of cyclin D1 and cyclin/cyclin-dependent kinases, an interesting property for the treatment of MCL [72].
Two rapamycin analogs, temsirolimus and everolimus, have proved to produce growth inhibition in a broad range of tumor models, including lymphoma. Rapamycin and temsirolimus have demonstrated antitumor activity in vitro against a variety of lymphoma cell lines, especially in MCL cells [73]. In a phase II trial with 35 patients with relapsed/refractory MCL, temsirolimus was delivered as a weekly 250 mg intravenous infusion, and showed an ORR of 38%, including 1 CR [74]. The most significant myelosuppression was thrombocytopenia, and the study was repeated with additional patients receiving 25 mg temsirolimus intravenously every week. An ORR of 41% was observed, with lower incidence of thrombocytopenia.
A phase III trial, comparing temsirolimus with investigator’s choice of conventional chemotherapy for patients with relapsed/refractory MCL showed better PFS and ORR in patients treated with temsirolimus [75]. In another phase II trial, temsirolimus was combined with rituximab in 71 patients with MCL exposed to rituximab. The ORR was 48%, with 20% (14 of 71) complete responses, and 28% (20 of 71) partial responses [76]. A phase I trial for new, untreated MCL patients is being conducted, combining rituximab, temsirolimus, and cladribine for patients not transplant eligible.
Everolimus, an oral mTORC1 inhibitor, has also shown antitumor effects. Preclinical studies have shown that everolimus inhibits phosphorylation of mTOR substrates and induces G1 arrest [77]. Moreover, everolimus can sensitize MCL cells to cytotoxic agents, including doxorubicin and bortezomib. Hodgkin lymphoma cells also show activation of the PI3K pathway and are sensitive to everolimus [78]. A phase II trial evaluated everolimus in 37 patients with refractory/relapsed aggressive NHL, including 20 patients with DLBCL and 14 with MCL. The ORR was 32%, with a median duration of response of 3.1 months [79]. Everolimus was well tolerated, despite mild hematological toxicity. Subsequent studies have demonstrated positive results in patients with CLL/SLL [80] and Waldenstrom macroglobulinemia [81]. mTOR inhibitors also have activity in relapsed, aggressive lymphomas. In a phase II study, 47 patients with relapsed DLBCL were treated with single-agent everolimus with an ORR of 30%. Interestingly, an ORR of 63% was observed in relapsed T-cell NHL [82].
In conclusion, the mTOR inhibitors temsirolimus and everolimus have modest single-agent activity in NHL, CLL/SLL, Hodgkin lymphoma, and Waldenstrom macroglobulinemia. These agents are now being tested in larger single-agent studies as consolidation after induction therapy for DLBCL, as treatment for new untreated Waldenstrom macroglobulinemia, relapsed Hodgkin lymphoma, and in combination with chemoimmunotherapy for untreated MCL.
Histone Deacetylase Inhibitors
Histones are small basic proteins that form the nucleosome core by binding to DNA. Histone acetylation is relevant in diverse cellular mechanisms, including chromatin assembly, DNA repair, and gene expression. In a general way, histone acetylation is linked to a relaxed chromatin status, with activation of transcriptional activity. Histone deacetylation, however, causes condensation of the chromatin and repression of transcriptional activity [83]. An imbalance in these mechanisms is involved in the pathogenesis of different tumors. In NHL, the frequent translocation affecting the BCL6 gene activates the HDAC-containing complex and inhibits transcription and differentiation of germinal center B cells [84].
Several histone deacetylase inhibitors (HDAC) are available for treatment of hematological malignancies. Vorinostat (Zolinza®, SAHA) is a HDAC inhibitor approved for the treatment of relapsed cutaneous T-cell lymphomas [85]. A phase II trial of vorinostat in 18 patients with relapsed DLBCL showed modest activity, with only one patient achieving CR [86].
MGCD0103 (mocetinostat) is an oral HDAC inhibitor under study. An interim analysis of a phase II trial in 50 patients with relapsed/refractory DLCBL and FL reported an ORR of 23.5% in DLBCL, with one CR and three PR [87]. Mocetinostat is also being evaluated for patients with HL. Panobinostat, a novel HDAC, inhibits proliferation and induces apoptosis in tumor cell lines. Interestingly, a synergic effect with mTOR inhibitors has been demonstrated [88], and phase I/II trials of panobinostat associated with everolimus are being conducted for patients with relapsed/refractory NHL.
Immunomodulatory Drugs
Immunomodulatory drugs (IMiDs) are a group of compounds structurally and functionally related to thalidomide, an oral sedative with anti-inflammatory properties. Thalidomide interferes with tumor microenvironment and inhibits tumor necrosis factor TNF-a through degradation of its mRNA, as well as IL-6, IL-1, E-selectin, L-selectin, and GM-CSF [89, 90]. Thalidome also stimulates T-cell lymphocytes, inducing proliferation, cytokine production, and cytotoxic activity and upregulates natural killer (NK) cell activity [91]. Furthermore, thalidomide exhibits antiangiogenenic properties, by decreasing expression of vascular endothelial growth factor (VEGF) [92].
Lenalidomide (Revlimid®), a less toxic and more potent IMiD, is extensively used in multiple myeloma [93, 94], and recent clinical studies show promising results in NHL. A phase II trial of single-agent lenalidomide included 49 patients with aggressive NHL, including MCL and DLBCL. The ORR was 35% in all patients and 28% in DLBCL patients [95]. Patients with MCL also showed responses with lenalidomide in combination with rituximab [96]. A phase I/II trial combining lenalidomide with standard R-CHOP (R2-CHOP) in aggressive lymphoma patients has shown a secure toxicity profile, with no major effects or hematological recovery delays. Moreover, for 30 patients evaluable for response, the overall and complete response rate was 100% and 83%, respectively [97]. Lenalidomide may also have distinct activity depending on the GBC/ABC phenotype. In a retrospective study of patients treated with salvage lenalidomide, the overall response rate in patients with non-GCB subtype was superior to the GCB phenotype [98].
Novel Monoclonal Antibodies
Since the introduction of rituximab in the treatment of lymphomas, research has focused on the development of novel monoclonal antibodies. Strategies have been divided in two different groups: improving anti-CD20 activity and development of new targets for immunotherapy.
New Anti-CD20 MoAbs
Ofatumumab (Arzerra®), a fully human IgG anti-CD20 antibody, targets a different epitope within the CD20 molecule, responsible for different therapeutic properties. Compared with rituximab, ofatumumab binds in a region closer to cell membrane, improving binding stability [99]. Moreover, stronger complement-dependent cell lysis (CDC) is observed with ofatumumab, with similar antibody-dependent cellular toxicity (ADCC) [100]. Ofatumumab was tested as monotherapy in patients with FL in a phase I/II trial, with ORR of 13% and an ORR of 22% in patients refractory to rituximab [101]. These encouraging results led to a trial in heavily pretreated patients with relapsed/refractory aggressive B-cell NHL, most of them preexposed to rituximab. Overall response rates reached 11% (three complete responses and six partial responses) [102].
Veltuzumab is also a second-generation of anti-CD20 MoAbs, developed to improve the efficacy of rituximab. Veltuzumab is a humanized antibody with structural differences compared with rituximab and shows similar in vitro CDC and ADCC properties, with a slower dissociation from the CD20 epitope. Moreover, veltuzumab has shown enhanced tumor B-cell depletion compared with rituximab. A recent phase I/II study in patients with relapsed/refractory different subtypes of NHL showed an ORR of 44 and 27% CR [103]. Interestingly, in the DLBCL patients, a PR of 43% was observed, all of them previously treated with R-CHOP.
Obinutuzumab (GA101), a humanized anti-CD20, has specific modifications in the Fc and hinge regions, leading to a high binding affinity to a distinct CD20 epitope and significant increase in FcgRIII receptor binding. These particular alterations result in a superior ADCC and reduced CDC activity. Moreover, obinutuzumab is thought to increase signaling in target cells, activating apoptotic pathways. A superior antitumor activity of GA101 over rituximab has been suggested by studies in animal models of lymphoma [104]. A phase I/II trial of GA101 in relapsed patients with NHL, mostly FL, showed a safe toxicity profile, with ORR of 58% in 12 patients (three CRs and four PRs). In a study with 40 relapsed/refractory aggressive lymphoma patients, an ORR of 30% was observed in DLBCL patients [105].
New Targeted MoAbs
Epratuzumab is a humanized IgG1 anti-CD22 antibody, a protein expressed on the membrane of normal and malignant B cells. CD22 is internalized when it interacts with its natural ligand, and its precise role in B cells is unclear. Epratuzumab shows ADCC and CDC activities, and a phase I/II trial with epratuzumab associated with rituximab showed impressive results in patients with indolent NHL, including CR in patients previously exposed to rituximab. A multicenter phase II trial combining epratuzumab with standard R-CHOP was conducted in patients with DLBCL. Seventy-eight eligible patients were treated with ER-CHOP, with ORR of 95 and 73% CR [106].
Galiximab is a new chimeric IgG MoAb directed against CD80, with synergy effects with rituximab. CD80 is a T-cell co-stimulatory molecule expressed in normal and malignant B cells, and cross-linking of CD80 induces caspase-dependent apoptosis in lymphoma cell lines. Clinical activity of galiximab has been proved mostly in FL, with phase I/II study showing ORR of 64% when associated with rituximab [107].
SGN-40 (dacetuzumab) is also a new and promising MoAb. CD40 is a critical molecule of the TNF family, involved in normal B-cell activation, proliferation, and differentiation. Preclinical studies in human lymphoma cells have shown ADCC activity, as well as cell growth inhibition and apoptosis promotion. After a phase I trial with 35 patients with NHL reporting a safe profile and clinical activity [108], two phase II trials are ongoing with patients with relapsed DLBCL, either as single agent or in combination with chemotherapy. Hence, a phase 1 clinical trial of SGN-40 in combination with rituximab and gemcitabine showed multiple responses in patients with relapsed DLBCL [109].
Blinatumomab is a bi-specific antibody targeting CD19 (B-cell marker) and CD3 (a T-cell engager). Upon binding and engaging both cells, the B cell is stimulated to growth arrest and apoptosis, and T cell is driven to proliferate. In a phase I study with 12 patients with lymphoma, 11 patients had an objective response, and at least half of the responders remained in response at 1 year out of therapy [110].
Inotuzumab ozogamicin is an antibody against CD22 conjugated with calicheamicin, a cytotoxic agent. In a phase I study, responses of 39% were observed in patients with lymphoma, including a 15% response in DLBCL patients. A combination of rituximab with inotuzumab ozogamicin has been tested in patients with recurrent DLBCL, with an ORR of 80%. However, in rituximab-refractory patients, the ORR was much lower (20%) [111].
Conclusion
It has been said that normal lymphocyte differentiation is, in some sense, a disaster waiting to happen [112]. The consequence of this “disaster” is the outbreak of clonal, unorganized, and rapid multiplying malignant B cells. For many years, the use of drugs with cytotoxic effect remained the principle of lymphoma therapy. Outstanding results were achieved with this strategy in some NHL, and a relatively significant proportion of patients could be cured. However, toxicity and late side effects compromised the majority of patients.
Modern oncology is one of the most fascinating areas or research in present years. Knowledge from basic sciences has progressively been translated in clinical outsets. Less toxic, targeted therapy has emerged as a promising approach in lymphoma patients. The understanding of molecular pathways involved in the pathogenesis and clinical behavior of lymphomas has served as foundation for new drug development. Many patients, including the considered nonfit ones, may rely on these new drugs for disease control.
References
Nowell PC, Hungerford DA (1960) A minute chromosome in human chronic granulocytic leukemia. Science 132:1497
Lugo TG, Pendergast AM, Muller AJ, Witte ON (1990) Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science 247:1079–1082
Druker BJ, Talpaz M, Resta D et al (2001) Efficacy and safety of a specific inhibitor of the Bcr-Abl tyrosine kinase in chronic myeloid leukemia. N Engl J Med 344:1031–1037
Coiffier B, Haioun C, Ketterer N et al (1998) Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood 92:1927–1932
Sievers EL, Larson RA, Stadtmauer EA, the Mylotarg Study Group et al (2001) Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol 19:3244–3254
Scott AM, Wolchok JD, Old LJ (2012) Antibody therapy of cancer. Nat Rev Cancer 12(4):278–287
Wiseman GA, Gordon LI, Multani PS et al (2002) Ibritumomab tiuxetan radioimmunotherapy for patients with relapsed or refractory non-Hodgkin’s lymphoma and mild thrombocytopenia: a phase II multicenter trial. Blood 99:4336–4342
Jaffe ES, Harris NL, Stein H, Vardiman JW (eds) (2008) World Health Organization classification of tumors: tumours of the haematopoietic and lymphoid tissues. International Agency for Research on Cancer (IARC) Press, Lyon, France, pp 17–44
Au WY, Horsman DE, Gascoyne RD, Viswanatha DS, Klasa RJ, Connors JM (2004) The spectrum of lymphoma with 8q24 aberrations: a clinical, pathological and cytogenetic study of 87 consecutive cases. Leuk Lymphoma 45:519–528
Kramer MH, Hermans J, Wijburg E, Philippo K, Geelen E, van Krieken JH et al (1998) Clinical relevance of BCL2, BCL6, and MYC rearrangements in diffuse large B-cell lymphoma. Blood 92:3152–3162
Le Gouill S, Talmant P, Touzeau C, Moreau A, Garand R, Juge-Morineau N et al (2007) The clinical presentation and prognosis of diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC rearrangement. Haematologica 92:1335–1342
Kanungo A, Medeiros LJ, Abruzzo LV, Lin P (2006) Lymphoid neoplasms associated with concurrent t(14;18) and 8q24/c-MYC translocation generally have a poor prognosis. Mod Pathol 19:25–33
Niitsu N, Okamoto M, Hirano M (2009) Clinical features and prognosis of de novo diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC translocations. Leukemia 23:777–783
Alizadeh AA, Eisen MB, Davis RE et al (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503–511
Rosenwald A, Wright G, Chan WC et al (2002) The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 346:1937–1947
Friedberg JW (2011) New strategies in diffuse large B-cell lymphoma: translating findings from gene expression analyses into clinical practice. Clin Cancer Res 17(19):6112–6117
Orsborne C, Byers R (2011) Impact of gene expression profiling in lymphoma diagnosis and prognosis. Histopathology 58(1):106–127
Lenz G, Wright G, Dave SS et al (2008) Lymphoma/leukemia molecular profiling project. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med 359(22):2313–2323
Thieblemont C, Briere J, Mounier N et al (2011) The germinal center/activated B-cell subclassification has a prognostic impact for response to salvage therapy in relapsed/refractory diffuse large B-cell lymphoma: a bio-CORAL study. J Clin Oncol 29(31):4079–4087
Warburg O, Geissler AW, Lorenz S (1967) [On growth of cancer cells in media in which glucose is replaced by galactose]. Hoppe Seylers Z Physiol Chem 348(12):1686–1687
Histed SN, Lindenberg ML, Mena E, Turkbey B, Choyke PL, Kurdziel KA (2012) Review of functional/anatomical imaging in oncology. Nucl Med Commun 33(4):349–361
Miller JC, Fischman AJ, Aquino SL, Blake MA, Thrall JH, Lee SI (2007) FDG-PET CT for tumor imaging. J Am Coll Radiol 4(4):256–259
Blodgett TM, Meltzer CC, Townsend DW (2007) PET/CT: form and function. Radiology 242(2):360–385
Cheson BD (2011) Role of functional imaging in the management of lymphoma. J Clin Oncol 29(14):1844–1854
Cheson BD, Pfistner B, Juweid ME et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25(5):579–586
Juweid ME, Stroobants S, Hoekstra OS et al (2007) Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol 25(5):571–578
Spaepen K, Stroobants S, Dupont P et al (2001) Prognostic value of positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose ((18F)FDG) after first line chemotherapy in non-Hodgkin’s lymphoma: is (18)FDG-PET a valid alternative to conventional diagnostic method? J Clin Oncol 19(2):4141–4149
Spaepen K, Stroobants S, Dupont P et al (2002) Early restaging positron emission tomography with (18)-fluorodeoxyglucose predicts outcome in patients with aggressive non-Hodgkin lymphoma. Ann Oncol 13(9):1356–1363
Itti E, Lin C, Dupuis J et al (2009) Prognostic value of interim 18F-FDG PET in patients with diffuse large B-cell lymphoma: SUV-based assessment at 4 cycles of chemotherapy. J Nucl Med 50(4):527–533
A study of two associations of rituximab and chemotherapy, with a PET-driven strategy, in lymphoma (LNH2007-3B). ClinicalTrials.gov. http://www.clinicaltrials.gov/ct2/show/NCT00498043. (2007) Last accessed on 07 Jan 2011.
Tailoring treatment for B cell non-Hodgkin’s lymphoma based on PET scan results mid treatment. ClinicalTrials.gov. http://www.clinicaltrials.gov/ct2/show/NCT00324467. (2006) Last accessed 09 Jan 2010.
Stewart DA, Duggan P, Bahlis NJ et al (2009) Interim restaging FDG-PET/CT to guide use of high dose sequential induction therapy with rituximab-dose-intensive cyclophosphamide, etoposide, cisplatin (RDICEP) and rituximab-carmustine, etoposide, cytarabine, melphalan (RBEAM) and autologous stem cell transplantation (ASCT) for poor prognosis diffuse large b-cell lymphoma (DLBCL). ClinicalTrials. Gov Identifier: NCT00530179. Blood 114:3414 (ASH Annual Meeting Abstracts)
Bhaumik SR, Malik S (2008) Diverse regulatory mechanisms of eukaryotic transcriptional activation by the proteasome complex. Crit Rev Biochem Mol Biol 43:419–433
Frankland-Searby S, Bhaumik SR (2012) The 26S proteasome complex: an attractive target for cancer therapy. Biochim Biophys Acta 1825(1):64–76
Sterz J, von Metzler I, Hahne JC et al (2008) The potential of proteasome inhibitors in cancer therapy. Expert Opin Investig Drugs 17(6):879–895
Voorhees PM, Dees EC, O’Neil B, Orlowski RZ (2003) The proteasome as a target for cancer therapy. Clin Cancer Res 9:6316–6325
Adams J, Palombella VJ, Sausville EA et al (1999) Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res 59:2615–2622
Salvat C, Aquaviva C, Jariel-Encontre I et al (1999) Are there multiple proteolytic pathways contributing to c-Fos, c-Jun and p53 protein degradation in vivo? Mol Biol Rep 26:45–51
Karin M, Cao Y, Greten FR, Li ZW (2002) NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2:301–310
Hosokawa Y, Seto M (2004) Nuclear factor kappaB activation and antiapoptosis in mucosa-associated lymphoid tissue lymphoma. Int J Hematol 80(3):215–223
Martínez-Delgado B, Cuadros M, Honrado E et al (2005) Differential expression of NF-kappaB pathway genes among peripheral T-cell lymphomas. Leukemia 19(12):2254–2263
Calado DP, Zhang B, Srinivasan L et al (2010) Constitutive canonical NF-κB activation cooperates with disruption of BLIMP1 in the pathogenesis of activated B cell-like diffuse large cell lymphoma. Cancer Cell 18(6):580–589
Goy A, Younes A, McLaughlin P et al (2005) Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin’s lymphoma. J Clin Oncol 23:667–675
Fisher RI, Bernstein SH, Kahl BS et al (2006) Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol 24(30):4867–4874
Ruan J, Martin P, Furman RR, Lee SM, Cheung K, Vose JM, Lacasce A, Morrison J, Elstrom R, Ely S, Chadburn A, Cesarman E, Coleman M, Leonard JP (2011) Bortezomib plus CHOP-rituximab for previously untreated diffuse large B-cell lymphoma and mantle cell lymphoma. J Clin Oncol 29(6):690–697
Dunleavy K, Pittaluga S, Czuczman MS, Dave SS, Wright G, Grant N, Shovlin M, Jaffe ES, Janik JE, Staudt LM, Wilson WH (2009) Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood 113(24):6069–6076
Gururajan M, Jennings CD, Bondada S (2006) Cutting edge: constitutive B cell receptor signaling is critical for basal growth of B lymphoma. J Immunol 176:5715–5719
Chen L, Monti S, Juszczynski P et al (2008) SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood 111:2230–2237
Chen L, Juszczynski P, Takeyama K, Aguiar RC, Shipp MA (2006) Protein tyrosine phosphatase receptor-type O truncated (PTPROt) regulates SYK phosphorylation, proximal B-cell receptor signaling, and cellular proliferation. Blood 108:3428–3433
Friedberg JW, Sharman J, Sweetenham J et al (2010) Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 115:2578–2585
Feldman AL, Sun DX, Law ME et al (2008) Overexpression of Syk tyrosine kinase in peripheral T-cell lymphomas. Leukemia 22:1139–1143
Vihinen M, Mattsson PT, Smith CI (2000) Bruton tyrosine kinase (BTK) in X-linked agammaglobulinemia (XLA). Front Biosci 5:D917–D928
Winer ES, Ingham RR, Castillo JJ (2012) PCI-32765: a novel Bruton’s tyrosine kinase inhibitor for the treatment of lymphoid malignancies. Expert Opin Investig Drugs 21(3):355–361
Byrd JC, Blum KA, Burger JA et al (2011) Activity and tolerability of the Bruton’s tyrosine kinase (Btk) inhibitor PCI-32765 in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): interim results of a phase Ib/II study. J Clin Oncol 29(suppl):6508
Wang L, Martin P, Blum KA et al (2011) The Bruton’s tyrosine kinase inhibitor PCI-32765 is highly active as single-agent therapy in previously-treated mantle cell lymphoma (MCL): preliminary results of a phase II trial. Blood 118:442
Staudt LM, Dunleavy K, Buggy JJ et al (2011) The Bruton’s tyrosine kinase (Btk) inhibitor PCI-32765 modulates chronic active BCR signaling and induces tumor regression in relapsed/refractory ABC DLBCL. Blood 118:2716
Goekjian PG, Jirousek MR (2001) Protein kinase C inhibitors as novel anticancer drugs. Expert Opin Investig Drugs 10:2117–2140
McMahon G (2000) VEGF receptor signaling in tumor angiogenesis. Oncologist 5(Suppl 1):3–10
Ma S, Rosen ST (2007) Enzastaurin. Curr Opin Oncol 19:590–595
Robertson MJ, Kahl BS, Vose JM et al (2007) Phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 25:1741–1746
Hagemeister FB (2010) Maintenance and consolidation strategies in non-Hodgkin’s lymphoma: a review of the data. Curr Oncol Rep 12(6):395–401
Kortmansky J, Schwartz GK (2003) Bryostatin-1: a novel PKC inhibitor in clinical development. Cancer Invest 21(6):924–936
Whitesell L, Lindquist SL (2005) HSP90 and the chaperoning of cancer. Nat Rev Cancer 5:761–772
Jolly C, Morimoto RI (2000) Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst 92:1564–1572
Valbuena JR, Rassidakis GZ, Lin P et al (2005) Expression of heat-shock protein-90 in non-Hodgkin’s lymphomas. Mod Pathol 18:1343–1349
Georgakis GV, Li Y, Younes A (2006) The heat shock protein 90 inhibitor 17-AAG induces cell cycle arrest and apoptosis in mantle cell lymphoma cell lines by depleting cyclin D1, Akt, Bid and activating caspase 9. Br J Haematol 135:68–71
Solit DB, Chiosis G (2008) Development and application of Hsp90 inhibitors. Drug Discov Today 13:38–43
Richardson PG, Chanan-Khan A, Lonial S, Krishman A, Carroll M, Cropp GF, Kersey K, Abitar M, Johnson RG, Hannah AL et al (2007) Tanespimycin (T) + bortezomib (BZ) in multiple myeloma (MM): confirmation of the recommended dose using a novel formulation. Blood 110:1165, ASH Annual Meeting Abstracts
Modi S, Stopeck AT, Gordon MS, Mendelson D, Solit DB, Bagatell R, Ma W, Wheler J, Rosen N, Norton L et al (2007) Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: a phase I dose-escalation study. J Clin Oncol 25:5410–5417
Paez J, Sellers WR (2003) PI3K/PTEN/AKT pathway. A critical mediator of oncogenic signaling. Cancer Treat Res 115:145–167
Wlodarski P, Kasprzycka M, Liu X et al (2005) Activation of mammalian target of rapamycin in transformed B lymphocytes is nutrient dependent but independent of Akt, mitogen-activated protein kinase/extracellular signal-regulated kinase, insulin growth factor-I, and serum. Cancer Res 65:7800–7808
Bertoni F, Zucca E, Cotter FE (2004) Molecular basis of mantle cell lymphoma. Br J Haematol 124:130–140
Bjornsti MA, Houghton PJ (2004) The TOR pathway: a target for cancer therapy. Nat Rev Cancer 4:335–348
Witzig TE, Geyer SM, Ghobrial I et al (2005) Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol 23:5347–5356
Hess G, Herbrecht R, Romaguera J et al (2009) Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol 27:3822–3829
Ansell SM, Tang H, Kurtin P et al (2009) A phase II study of temsirolimus (CCI-779) in combination with rituximab in patients with relapsed or refractory mantle cell lymphoma [Abstract]. Blood 114:166
Haritunians T, Mori A, O’Kelly J, Luong QT, Giles FJ, Koeffler HP (2007) Antiproliferative activity of RAD001 (everolimus) as a single agent and combined with other agents in mantle cell lymphoma. Leukemia 21:333–339
Jundt F, Raetzel N, Muller C et al (2005) A rapamycin derivative (everolimus) controls proliferation through down-regulation of truncated CCAAT enhancer binding protein beta and NF- {kappa}B activity in Hodgkin and anaplastic large cell lymphomas. Blood 106:1801–1807
Reeder CB, Gornet MK, Habermann TM et al (2007) A phase II trial of the oral mTOR inhibitor everolimus (RAD001) in relapsed aggressive non-Hodgkin lymphoma (NHL). Blood 110:Abstract 121
Zent CS, LaPlant BR, Johnston PB et al (2010) The treatment of recurrent/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) with everolimus results in clinical responses and mobilization of CLL cells into the circulation. Cancer 116:2201–2207
Ghobrial IM, Gertz M, Laplant B et al (2010) Phase II trial of the oral mammalian target of rapamycin inhibitor everolimus in relapsed or refractory Waldenstrom macroglobulinemia. J Clin Oncol 28:1408–1414
Witzig TE, Habermann TM, Reeder C et al (2009) A phase II trial of the oral mtor inhibitor everolimus in relapsed non-Hodgkin’s lymphoma and Hodgkin disease [Abstract]. Haematologia 94(Suppl 2):436
Menhert JM, Kelly WK (2007) Histone deacetylase inhibitors: biology and mechanism of action. Cancer J 13:23–29
Shaffer AL, Yu X, He Y et al (2000) BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity 13:199–212
Olsen EA, Kim YH, Kuzel TM et al (2007) Phase IIB multicenter trial of Vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol 25:3109–3115
Crump M, Coiffier B, Jacobsen ED et al (2008) Phase II trial of oral vorinostat (suberoylanilide hydroxamic acid) in relapsed diffuse large-B-cell lymphoma. Ann Oncol 19:964–969
Crump M, Andreadis C, Assouline S et al (2008) Treatment of relapsed or refractory non-Hodgkin lymphoma with the oral isotype-selective histone deacetylase inhibitor MGCD0103: interim results from a phase II study. J Clin Oncol 26(15 suppl):Abstract 8528
Lemoine M, Derenzini E, Buglio D, Medeiros LJ, Davis RE, Zhang J, Ji Y, Younes A (2012) The pan-deacetylase inhibitor panobinostat induces cell death and synergizes with everolimus in Hodgkin lymphoma cell lines. Blood 26:119(17):4017–4025
Corral LG, Muller GW, Moreira AL et al (1996) Selection of novel analogs of thalidomide with enhanced tumor necrosis factor alpha inhibitory activity. Mol Med 2:506–515
Corral LG, Haslett PA, Muller GW et al (1999) Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol 163:380–386
Haslett PA, Corral LG, Albert M et al (1998) Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J Exp Med 187:1885–1982
Gupta D, Treon SP, Shima Y et al (2001) Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia 15:1950–1961
Weber D, Chen C, Niesviyky R et al (2006) Lenalidomide plus high-dose dexamethasone versus dexamethasone alone for relapsed or refractory multiple myeloma (MM): results of North American phase III study (MM-009). J Clin Oncol 24:427s
Dimopoulos MA, Spenser A, Attal M et al (2005) Study of lenalidomide plus dexamethasone versus dexamethasone alone in relapsed or refractory multiple myeloma (MM): results of phase III study (MM-010). Proc Am Soc Hematol 106:6
Witzig TE, Vose JM, Zinzani PL et al (2011) An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma. Ann Oncol 22(7):1622–1627
Wang M, Fayad L, Hagemeister F et al (2007) A phase I/II study of lenalidomide in combination with rituximab in relapsed/refractory mantle cell lymphoma with early evidence of efficacy. J Clin Oncol 25(18 suppl):Abstract 8030
Nowakowski GS, Reeder CB, LaPlant B et al (2011) Combination of lenalidomide with R-CHOP (R2CHOP) as an initial therapy in aggressive lymphomas: a phase I/II study. J Clin Oncol 29(suppl):Abstract 8015
Hernandez-Ilizaliturri FJ, Deeb G, Zinzani PL et al (2011) Higher response to lenalidomide in elapsed/refractory diffuse large B-cell lymphoma in nongerminal center B-cell-like than in germinal center B-cell-like phenotype. Cancer 117(22):5058–5066
Nightingale G (2011) Ofatumumab: a novel anti-CD20 monoclonal antibody for treatment of refractory chronic lymphocytic leukemia. Ann Pharmacother 45(10):1248–1255
Sanford M, McCormack PL (2010) Ofatumumab. Drugs 70(8):1013–1019
Czuczman MS, Fayad L, Delwail V et al (2012) Ofatumumab monotherapy in rituximab-refractory follicular lymphoma: results from a multicenter study. Blood 119(16):3698–3704
Coiffier B, Bosly A, Wu KL et al (2010) Ofatumumab monotherapy for treatment of patients with relapsed/progressive diffuse large B cell lymphoma: results from a multicenter phase II study. Blood 116:Abstract 3955
Morschhauser F, Leonard JP, Fayad L et al (2009) Humanized anti-CD20 antibody, veltuzumab, in refractory/recurrent non-Hodgkin’s lymphoma: phase I/II results. J Clin Oncol 27(20):3346–3353
Mössner E, Brünker P, Moser S et al (2010) Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 115(22):4393–4402
Cartron G, Thieblemont C, Solal-Celigny P et al (2010) Promising efficacy with the new anti-CD20 antibody GA101 in heavily pre-treated NHL patients—first results from a phase II study in patients with relapsed/refractory DLBCL and MCL. Blood 116(21):2878 (ASH Annual Meeting Abstracts)
Micallef IN, Maurer MJ, Wiseman GA et al (2011) Epratuzumab with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy in patients with previously untreated diffuse large B-cell lymphoma. Blood 118(15):4053–4061
Bhat S, Czuczman MS (2010) Galiximab: a review. Expert Opin Biol Ther 10(3):451–458
Advani R, Forero-Torres A, Furman RR et al (2009) Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin’s lymphoma. J Clin Oncol 27(26):4371–4377
Forero-Torres A, Bartlett NL, Nasta SD et al (2009) A phase 1b clinical trial of dacetuzumab in combination with rituximab and gemcitabine: multiple responses observed in patients with relapsed diffuse large b-cell lymphoma. Blood 114:586 (ASH Annual Meeting Abstracts)
Nagorsen D, Zugmaier G, Viardot A et al (2009) Confirmation of safety, efficacy and response duration in non-Hodgkin lymphoma patients treated with 60 μg/m2/d of BiTE® antibody Blinatumomab. Blood 114:2723 (ASH Annual Meeting Abstracts)
Dang NH, Smith MR, Offner F et al (2009) Anti-CD22 Immunoconjugate inotuzumab ozogamicin (CMC-544) + rituximab: clinical activity including survival in patients with recurrent/refractory follicular or ‘aggressive’ lymphoma. Blood 114:584 (ASH Annual Meeting Abstracts)
Shaffer AL, Rosenwald A, Staudt LM (2002) Lymphoid malignancies: the dark side of B-cell differentiation. Nat Rev Immunol 2(12):920–932
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Perini, G.F., Fayad, L.E. (2013). Future Directions in Aggressive Lymphomas. In: Quesenberry, P., Castillo, J. (eds) Non-Hodgkin Lymphoma. Cancer Drug Discovery and Development. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-5851-7_10
Download citation
DOI: https://doi.org/10.1007/978-1-4614-5851-7_10
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-5850-0
Online ISBN: 978-1-4614-5851-7
eBook Packages: MedicineMedicine (R0)