Opinion Statement
Burkitt lymphoma (BL) is highly curable, and prompt institution of therapy is critical to achieving optimal outcomes. Although current “standard” approaches are very effective in disease eradication, treatment-related toxicity makes optimal delivery of curative therapy a challenge, especially in older and immunocompromised individuals. Reduced intensity approaches with fewer toxic complications have been the focus of some recent studies. A critical question is if they can replace “standard” approaches by maintaining high curability with improved tolerability. Additionally, new molecular insights in BL biology suggest that in the future, “targeted therapy” approaches may be feasible using small molecule inhibitors and novel strategies. Recently, a new category of aggressive lymphoma named “high-grade B-cell lymphoma (HGBL) with MYC and BCL2 and/or BCL6 translocations” has been recognized. This category overlaps clinically and biologically with BL and has an inferior prognosis compared to most B-cell lymphomas, and the optimal approach to its management remains, as yet, undefined. In this review, we discuss the current landscape of BL treatment including recent results with low-intensity regimens and also consider current approaches to HGBL. We also explore how recently elucidated novel biological insights in BL biology may shape future therapeutic directions including the use of novel cellular therapy approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Burkitt lymphoma (BL) is a rare and highly aggressive mature B-cell neoplasm that is characterized by a rapid clinical course and high fatality rate if left untreated [1, 2]. Traditionally, BL has been classified into three variants based upon clinical and epidemiological characteristics. While the “endemic” variant, described by Denis Burkitt in 1958, predominantly affects young children in malaria-endemic Africa and is Epstein-Barr virus (EBV) infection driven, “immunodeficiency-associated” BL is most often diagnosed in patients with HIV or, less frequently, in patients with a congenital immunodeficiency or in the post organ transplant setting [3,4,5]. By contrast, “sporadic” BL occurs worldwide and has a peak incidence in adolescents and young adults. Although the hallmark of all three variants is a reciprocal translocation of the MYC proto-oncogene, recent sequencing studies have identified other recurrently mutated genes that contribute to lymphomagenesis through a complex interplay of mutational, transcriptional, and epigenetic mechanisms [6,7,8•]. Despite high cure rates in children and young adults with intense, short-cycle chemotherapy, toxicity frequently limits the use of these regimens in immunosuppressed and elderly patients. Recently, reduced intensity regimens have shown good efficacy with acceptable toxicity [9, 10]. Additionally, new insights into the molecular biology of BL have opened up the possibility of clinical trials that investigate the addition of small molecule inhibitors to conventional platforms. The World Health Organization Classification of Lymphoid Tumors recently defined a new group of aggressive lymphomas with an inferior outcome called “high-grade B-cell lymphoma (HGBL) with MYC and BCL2 and/or BCL6 rearrangements” [11]. These tumors have many clinical and pathologic characteristics that overlap with BL, and this new categorization is helpful as we attempt to define optimal approaches to manage them.
Pathology and Molecular Genetics of Burkitt Lymphoma and High-Grade B-Cell Lymphomas
All three subtypes of BL are morphologically similar and comprised of medium-sized lymphoid cells that grow in a monotonous pattern. The tumor cells have a high nuclear to cytoplasmic ratio with round nuclei that contain dispersed chromatin in which multiple small nucleoli are visible. The cytoplasm is deeply basophilic and includes typical lipid-laden vacuoles. The very high proliferation rate of the tumor cells is reflected in numerous mitotic figures and a proliferation rate by Ki-67 or MIB-1 staining at or above 95%. An accompanying high rate of apoptosis results in the classical histological image of a “starry sky” appearance due to scattered macrophages. BL cells typically express (mature) B cell-associated antigens, including CD19, CD20, CD22, and CD79a, as well as surface immunoglobulins with light chain restriction, and germinal center (GC) markers CD10, CD38, and BCL-6. CD21, the EBV receptor, is expressed on EBV-associated BL cells [12, 13]. The tumor cells are usually negative for CD5, CD23, BCL-2, and terminal deoxynucleotidyl transferase (TdT).
EBV is identified in virtually all cases of endemic BL but only detected in up to 25–40% of sporadic or immunodeficiency-associated BL (Table 1) [4]. Cases of endemic BL are mostly limited to areas holo-endemic for Plasmodium falciparum infection, suggesting that EBV and P. falciparum have cooperating roles in lymphomagenesis [10, 14, 15]. Furthermore, various studies have shown that P. falciparum not only facilitates EBV infection but also can induce EBV reactivation [16]. Although EBV may transform B lymphocytes in vitro, most BL cells only express EBV nuclear antigen 1 (EBNA1), a latent viral protein, and two Epstein-Barr encoded RNAs (EBERs), which have not clearly been identified as oncogenic [17]. Interestingly, a recent study suggested that tumor EBV status defines a specific BL phenotype irrespective of geographic origin [18••]. EBV-positive tumors were found to have a higher expression of activation-induced cytidine deaminase (AICDA), which under normal circumstances induces hypervariable region mutation and class switch recombination. In contrast, aberrant AICDA activity is thought to promote double strand breaks, especially between the heavy chain constant regions, and, therefore, can result in translocations and mutations. As expected, EBV-positive tumors were shown to have a higher mutational burden, albeit fewer mutations in putative drivers. Although the exact mechanism of cooperation between EBV and P. falciparum remains to be elucidated, genomic instability may be a differentiating factor between EBV positive and negative BL.
The MYC proto-oncogene encodes a transcription factor, which controls many biological processes in the cell, including proliferation, growth, apoptosis, and differentiation [19]. Not surprisingly, MYC has been found to be overexpressed or activated in more than fifty percent of human cancers. For over 40 years, it has been recognized that the pathognomonic hallmark of BL is a reciprocal translocation involving the MYC locus on chromosome 8q24 to any of three immunoglobulin (IG) loci [6, 19,20,21,22]. In 75% of cases, the translocation partner of MYC involves the IG heavy chain locus on chromosome 14 at band q32. In the remainder, MYC is juxtaposed to one of the IG light chain loci, either K or λ, by rejoining at 2p12 or 22q11. Regardless, in all three events, MYC is placed under direct regulation of the strong IG enhancer, resulting in constitutive expression of MYC. Importantly, a recent study highlighted the complexity that underlies MYC deregulation in BL, which was found to be dependent on location of the breakage point, the presence of additional mutations in the MYC gene, and concomitant activation of other oncogenes through juxtaposition of IG loci as a result of enhancer hijacking [8•, 23]. While this study has emphasized the central role of deregulated MYC in BL pathogenesis, studies with transgenic mice expressing the MYC gene under control of the IG heavy chain promotor (Eμ-Myc) have shown that these mice do not develop B cell lymphomas that faithfully represent human BL [24]. Moreover, apparently healthy individuals have been described who have a low number of lymphoid cells in the blood and bone marrow with the t(8;14)(q24;q32) [25]. Based on these findings, the current understanding is that in addition to deregulated MYC, additional oncogenic drivers need to be present for the development of BL [26•].
In the last decade, high-throughput sequencing approaches have brought more insight into the molecular biology underlying Burkitt lymphomagenesis by implicating several genes and signaling pathways that cooperate with deregulated MYC. A crucial finding in Burkitt lymphomagenesis was the synergy that was observed between MYC and phosphoinositide-3-kinase (PI3K) signaling (Fig. 1) [27, 28]. Subsequent studies identified recurrent mutations in genes that would enhance B cell survival as a result of tonic BCR-dependent PI3K signaling, including TCF3, ID3, and PTEN [7]. In particular, activating mutations in TCF3 and/or loss-of-function mutations in its negative regulator ID3 have been identified in a high percentage of BL cases, including up to 70% of sporadic and immunodeficiency-associated variants as well as in up to 40% of the endemic variant. In addition to stimulating B cell survival, the transcription factor TCF3 is highly expressed in the dark zone of germinal centers (GCs), where it is responsible for GC function and promoting cell proliferation. Finally, TCF3 is also able to promote B cell proliferation directly, by stimulation of its down-stream target cyclin D3 (CCND3). Recurrent mutations in CCND3 have been identified in up to 38% of cases, which results in protein stabilization that drives cell cycle progression through cyclin-dependent kinase 6 (CDK6). While many studies have implicated other genes and pathways in Burkitt lymphomagenesis—most notably apoptosis pathways (TP53, USP7, CDKN2A), epigenetic regulation (ARID1A, SMARCA4, KMT2D), GPCR signaling (GBA13, RHOA, P2RY8), and transcriptional regulation (DDX3X, FBX011)—it has been postulated that most mutated genes and aberrant signaling pathways only affect a few crucial cellular functions, including cell proliferation and survival, BCR signaling, and epigenetic regulation [7, 18••, 29, 30]. Due to this relative simplicity, targeting these specific abnormalities with small molecule inhibitors might identify treatment strategies that are more effective and less toxic than current regimens.
Molecular mechanism of Burkitt lymphomagenesis. Transcriptional deregulation of MYC plays a central role in the development of Burkitt lymphoma. In addition to the growth-promoting effects of deregulated MYC, several cooperating genetic alterations have been identified that are associated with tonic BCR-dependent PI3K signaling, cell cycle progression, inhibition of apoptosis, and aberrant epigenetic regulation. Several classes of small molecule inhibitors have been developed, or are currently in development, that can target these cooperating signaling pathways as shown above.
The 2016 revision of the World Health Organization (WHO) Classification of Lymphoid Tumors included a new provisional entity “Burkitt-like lymphoma with 11q aberration.” This subset of MYC translocation-negative aggressive B-cell lymphoma closely resembles BL morphologically, phenotypically, and by gene expression profiling (GEP), but MYC rearrangements are lacking (Table 1) [31]. This entity has been described in both immunocompetent individuals and post-transplant immunocompromised patients [32, 33]. These lymphomas are characterized by recurrent chromosome 11q aberration, including proximal gains (11q23) and telomeric losses (11q24-25) [32, 33]. Compared with BL, these lymphomas tend to have more complex karyotypes, absence of ID3 mutations, a lower expression of MYC, a certain degree of cytological pleomorphism, occasionally a follicular pattern, and frequently a nodal presentation. Despite the presence of complex genomic rearrangements, these lymphomas have excellent survival rates and should be studied in prospective trials [34].
In addition to the above provisional entity, the 2016 revision of the WHO Lymphoid Tumor Classification also included a new category of HGBL. These cases are distinct from diffuse large B-cell lymphoma (DLBCL) or BL but with many overlapping clinical and pathologic characteristics to the parent entities. Most cases in this new category are classified as “high-grade B-cell lymphoma with translocations involving MYC, BCL-2, and/or BCL-6” (Fig. 2) [31]. Many of these cases were previously identified as “Burkitt-like” or “high-grade,” and entities that were called “double-hit” or “triple-hit” are now included in here. It is important to identify these rearrangements and to accurately diagnose these patients, as many studies have associated them with a more aggressive course, poor response to conventional chemo-immunotherapy, and high risk of central nervous system (CNS) involvement [35]. There is controversy regarding the prognostic impact of these combinations of genomic aberrations, but a recent large-scale study in DLBCL identified that the presence of an IG partner gene for MYC was the most significant negative prognostic factor [36,37,38,39•]. It should also be noted that rarely some HGBL cases do not have translocations but high-grade morphology and these have been classified as HGBL-NOS.
Graphic representation of the new category of aggressive B-cell lymphomas “high-grade B-Cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements” described in the 2016 revision to the WHO Classification of Tumors of Hematopoietic and Lymphoid Tumors (2008). Most cases with MYC and BCL2 rearrangements are of GCB origin, whereas most cases with BCL6 rearrangements are of ABC origin. This category includes double-hit (DH) lymphomas, which involve MYC and BCL2 or MYC and BCL6, as well as triple hit (TH) lymphomas that involve MYC, BCL2, and BCL6. When translocated, MYC may have an IG or non-IG partner gene with the former being associated with an inferior outcome.
Clinical Presentation and Epidemiology
The three variants of BL each have a distinct clinical presentation, although borderline cases exist [1, 40]. Endemic BL is the most common pediatric cancer in sub-Saharan Africa, where it accounts for 30–50% of all childhood cancers with an estimated incidence of 5–10 cases per 100,000 people per year [20]. The peak incidence lies between the ages of 4 and 7 years, and patients within this age group often present with the characteristic symptom of a rapidly growing tumor affecting the jaw or facial bones. However, other sites can be involved as well, including the gonads, kidney, the mesentery, and retroperitoneum. Sporadic BL, in contrast, is diagnosed throughout the world and is the most common type of BL in North America and Western Europe. It is rarely encountered with an annual incidence of 2–3 cases per 1,000,000 people. The median age at diagnosis is 30 years, and patients commonly present with abdominal involvement, especially of the ileocecal region, but extra-nodal localization including the bone marrow, gonads, and kidneys is seen as well. The immunodeficiency variant of BL occurs in patients with a weakened immune system, often in the context of advanced HIV infection. In this population, BL has an annual incidence rate of 6 cases per 1000 patients [5]. Interestingly, where the incidence of non-BL NHL increases with lower CD4 cell counts, BL rarely occurs below 50 CD4 cells/mm3 [41]. Most patients with this variant present with abdominal nodal disease, although extensive bone marrow involvement is common as well.
A full lymphoma work-up should be performed at presentation, including a mandatory bone marrow biopsy. In all three variants, and especially with advanced disease, leptomeningeal central nervous system (CNS) involvement is common, and evaluation for CNS localization is required in all new cases. Clinical evidence of spontaneous tumor lysis syndrome might be present, even before treatment has started. Burkitt-type acute lymphocytic leukemia (ALL-L3), also called mature B-ALL, is thought of as a different manifestation of the disease, with a clinical presentation that is similar to other subtypes of B-ALL. Its surface markers, cytogenetic aberrations, and molecular genetics are, however, identical to BL. It is critical to distinguish BL from non-BL HGBL with a MYC rearrangement, as CHOP-based therapy is considered inadequate for treatment of the former. In rare cases where distinction of the two entities is challenging, GEP may be helpful [42]. Clinical staging in adults is performed according to the Ann Arbor lymphoma classification, whereas staging of children and adolescents often utilizes the St. Jude/Murphy staging systems. The single most important prognostic factor is tumor burden, which is also directly reflected by serum LDH, uric acid levels, and stage of the disease. Other poor prognostic factors include age >40 years at diagnosis, black race, and poor performance status [43].
Current Treatment Strategies in Adult Burkitt Lymphoma
Despite its clinically aggressive behavior, BL is recognized as one of the most curable lymphomas due to its sensitivity to chemotherapy, especially alkylating agents and antimetabolites. The first described treatment regimens mimicked pediatric ALL protocols and consisted of dose-intense combination chemotherapy delivered over a prolonged period of time [44]. However, relapses occurred frequently as a result of treatment interruptions and new strategies were developed. These new strategies also administered multi-agent chemotherapy in a dose-intense fashion but now as part of short-term rotational regimens to minimize treatment delays and maintain serum drug concentrations [45]. Most of the regimens used currently for the treatment of adult BL were modeled based on experiences with these strategies in pediatric patients and adults [46]. These regimens typically have a backbone of cyclophosphamide, doxorubicin, and vincristine, often following an initial pre-treatment phase in which steroids and low-dose cyclophosphamide are administered. In addition to intrathecal administration, high systemic doses of methotrexate and cytarabine are often incorporated due to their ability to penetrate the blood-brain barrier. Although no gold standard treatment for adult patients with BL exists, the regimens that are most frequently cited include original or modified CODOX-M/IVAC +/- R, hyper-CVAD-R, CALGB10002, dose-adjusted EPOCH-R, and the European LMB and BFM/GMALL protocols. Because of the intensity, severe treatment-related toxicity is frequently observed, especially in elderly and immunocompromised individuals. Even with a pre-treatment phase as prophylaxis for the development of tumor lysis syndrome, clinical evidence of tumor lysis is common, and there is a low threshold for institution of aggressive hydration, allopurinol, and, if necessary, rasburicase. Granulocyte colony-stimulating factor is typically administered.
A study that laid the foundation for the regimen that is most frequently used in the USA was developed by Magrath and colleagues at the NCI in the early 1990s (Table 2) [47]. In the study, 41 previously untreated patients with BL, 20 adults, and 21 children were stratified into low-risk (n=7) and high-risk (n=34) groups based on tumor extent and serum LDH levels at presentation. Low-risk patients were treated with 3 cycles of CODOX-M (cyclophosphamide, doxorubicin, vincristine, and methotrexate), whereas high-risk patients received a total of 4 cycles of alternating regimens of CODOX-M and IVAC (ifosfamide, etoposide, high-dose cytarabine, and IT methotrexate). With this new regimen, an event-free survival (EFS) in these patients was reported of 92% at 2 years and beyond. Adults and children were shown to have a similar prognosis, although a low median age in the “adult” group might have contributed to this. Severe myelosuppression was universal, as well as a high incidence of septicemia in 22.1% of cycles. In a subsequent international phase II study, Mead et al. confirmed the high cure rate of the CODOX-M/IVAC regimen in 52 adult patients with BL with a median age of 35 years [48]. However, with 2-year EFS and OS in low-risk patients (n=12) of 83% and 82%, respectively, and 2-year EFS and OS in high-risk patients (n=40) of 60% and 70%, respectively, this suggested an inferior outcome for adult patients treated with this strategy in comparison to children and young adults. Importantly, high treatment-related toxicity was observed again, particularly severe myelosuppression and grade 3 and 4 mucositis. Several groups have tried over the years to modify the CODOX-M/IVAC protocol in such a way to maintain treatment efficacy, while at the same time improving tolerability. A follow-up study by Mead et al. investigated a dose reduction of methotrexate to 3 g/m2 [49]. Fifty-three patients with a median age of 37 years were stratified as either low- or high-risk and assigned to treatment with either 3 cycles of dose-modified CODOX-M or alternating cycles of dose-modified CODOX-M and IVAC. Reported outcomes included a 2-year PFS of 64% (95% CI, 51–77%) and a 2-year OS of 67% (95% CI, 54–80%), indicating similar efficacy of the regimen despite dose adjustment of methotrexate. Importantly, a reduction in toxicity was reported, although less older patients were enrolled in the study then initially desired. Follow-up studies confirmed the efficacy of the dose-modified regimen in older adults as well as in patients with HIV-associated BL [50, 51]. Further improvement was made by addition of the CD20 monoclonal antibody, rituximab, to the regimen. Retrospective analysis of a cohort of 80 adult patients with BL treated with CODOX-M/IVAC with or without rituximab showed that rituximab-containing therapy significantly decreased the recurrence rate and in addition showed a trend in favor of improved 3-year PFS (74% versus 61%) and 3-year OS (77% versus 66%) [52]. Since then, other groups have evaluated the addition of rituximab to dose-modified CODOX-M/IVAC [53, 54]. It should be noted that the addition of rituximab to chemotherapy has been controversial in the treatment of pediatric Burkitt lymphoma, but recently the US Children’s Oncology Group demonstrated that it not only improved PFS but also OS in high-risk, pediatric/adolescent mature B-cell NHL, most of which were BL [55].
The hyper-CVAD regimen was developed at MD Anderson in the early 1990s for the treatment of Burkitt-type adult ALL (ALL-L3) after successful results with a similar regimen in a pediatric population [56]. In the study, 26 consecutive patients with ALL-L3 received a total of 8 cycles of hyper-fractionated CVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone) alternated with courses of high-dose methotrexate and high-dose cytarabine. The 3-year OS was 49% (±11%) with a subgroup analysis revealing a 3-year OS for patients <60 years of 77% (n=14) but only 17% for patients >60 years of age (n=12). Five induction deaths were reported. A subsequent prospective, phase II study evaluated the addition of rituximab to the hyper-CVAD regimen in 31 adult patients with BL or ALL-L3 [57]. Median age at diagnosis was 46 years, and 29% of patients were older than 60 years. Results were impressive with a 3-year EFS and OS of 80% and 89%, respectively. No induction deaths were noted, and nine elderly patients achieved CR with a 3-year OS of 89%. In a similar manner, the Cancer and Leukemia Group B (CALGB) modified the German Multicenter ALL Group (GMALL) regimen for pediatric ALL-L3 in the CALGB9251 study, thereby combining multi-agent chemotherapy with prophylactic cranial radiotherapy [58]. Ninety-two patients with BL were enrolled and received alternating cycles of ifosfamide, methotrexate, vincristine, cytarabine, etoposide, and dexamethasone with cyclophosphamide, methotrexate, vincristine, doxorubicin, and dexamethasone. In addition, the first cohort of patients (n=52) received CNS-directed treatment consisting of 12 doses of IT methotrexate, cytarabine, and dexamethasone in combination with a total of 2400 cGy of cranial irradiation. The second cohort (n=40) only received 6 doses of IT triple chemotherapy. Although treatment responses were similar in both cohorts with a 2-year EFS of 52% in patients of cohort 1 and 45% in patients of cohort 2, significant neurotoxicity was reported in cohort 1. Authors concluded that less extensive CNS prophylaxis adequately controlled disease, while at the same time sparing patients the risk of neurotoxicity. The subsequent CALGB10002 phase II study investigated the addition of rituximab to the CALGB9251 regimen without cranial irradiation [59]. Treatment of 103 patients with BL resulted in an EFS and OS of 74% and 78%, respectively. Besides these regimens developed in the USA, European groups have described various promising strategies as well, most notably the French LMB and German BFM/GMALL protocols. A modification of the pediatric LMB89 study investigated a risk-adapted approach in 72 adult patients with BL with a median age of 33 years [60]. Risk group A received only induction chemotherapy consisting of cyclophosphamide, vincristine, doxorubicin, and prednisone. Group B received 5 courses of chemotherapy including high doses of methotrexate and cytarabine followed by a consolidation and limited maintenance phase. Patients in group C received eight courses of chemotherapy including higher doses of methotrexate and cytarabine as well as extended maintenance chemotherapy. Two-year EFS and OS were reported to be 65% and 70%, respectively. A follow-up study evaluated the same risk-adapted regimen in 257 adult patients with BL but this time with or without addition of rituximab [61]. Patients in the rituximab group achieved a better 3-year EFS (75%; 95% CI, 66–82%) and 3-year OS (83%; 95% CI, 75–88%) than those in the non-rituximab group (EFS: 62%; 95% CI, 53–70% and OS: 70%; 95% CI, 62–78%). The BFM/GMALL protocol also used a risk-adaptive approach with treatment consisting of six 5-day chemotherapy cycles of high-dose methotrexate and cytarabine, cyclophosphamide, etoposide, ifosfamide, prednisone, and triple intrathecal therapy [62]. In addition, rituximab was given before each cycle and twice as maintenance. A total of 363 patients were treated with a median age of 42 years, with patients over 55 years receiving a reduced regimen. Five-year PFS and OS were 71% and 80%, respectively, although significance differences were observed between adolescents, adults, and elderly (OS rate 90%, 84%, and 62%, respectively).
Most adults with BL that can tolerate intense treatment can be successfully treated with these approaches, including CODOX-M/IVAC +/- R, hyper-CVAD-R, or CALGB10002. However, treatment-related toxicity is a significant concern, especially in older adults and HIV-positive patients. In an attempt to address the challenge of high toxicity with “standard approaches,” the NCI group evaluated a reduced intensity approach and investigated EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in patients with BL due to its efficacy in DLBCL and hypothetical ability to overcome high tumor proliferation [63]. In an initial study of 30 patients with BL, two regimens were tested: a standard, dose-adjusted EPOCH-R regimen in 19 HIV-negative patients (DA-EPOCH-R) and a short course combination with a double dose of rituximab in 11 HIV-positive patients (SC-EPOCH-RR group). The overall median age of patients was 33 years, and 40% were 40 years of age or older. After a median follow-up of 86 months in the DA-EPOCH-R group and 73 months in the SC-EPOCH-RR group, freedom from progression (FFP) and OS were 95% and 100%, respectively. These results led to the development of an NCI Intergroup Study of DA-EPOCH-R in 113 patients across 22 centers—the median age of patients was 49 years, and 62% were 40 years or over [64, 65••]. Patients were stratified into high-risk (87%) and low-risk based on clinical characteristics, and at a median follow-up time of close to 6 years, EFS and OS were 84.5% and 87%, respectively. While outcomes were similar across different age groups and irrespective of HIV status, CNS involvement portended a poor prognosis suggesting that this sub-group should be a focus when developing novel strategies for the disease [53]. An international, phase III, prospective, multi-center trial is now underway comparing CODOX-M/IVAC-R with DA-EPOCH-R for patients with high-risk BL. Recently, a large retrospective study looked at outcomes of BL from 30 US cancer centers over a decade and included 641 patients [66]. While outcomes were superior in academic versus community settings, collectively outcomes were inferior to those observed in clinical trials.
Treatment Recommendations for Adult BL
High-dose approaches such as CODOX-M/IVAC with rituximab are tolerated in adolescents and young adults with BL and can be considered one of the standard options for this group. When using these regimens, risk-adapted therapy has been shown to be effective when patients are stratified into low- and high-risk groups and receive different strategies accordingly. Less intensive strategies such as DA-EPOCH-R are highly effective in both low- and high-risk patients of all age groups and may be considered an additional standard. For older or immunosuppressed patients, as well as those with comorbidities, CODOX-M/IVAC-like approaches are very poorly tolerated, which limits delivery of optimal therapy, and, therefore, less toxic strategies such as EPOCH-R should be considered. Patients with CNS involvement at diagnosis have significantly inferior outcomes with these approaches, and new strategies are needed.
There is a paucity of data in patients with relapsed or refractory BL, and most approaches are associated with very short survivals [67, 68]. A recent retrospective study found an overall response rate (ORR) of only 39% to salvage chemotherapy with a median OS of just 2.8 months for this group highlighting the urgent need for new treatment approaches [69]. Salvage regimens for chemo-sensitive disease include hyper-CVAD re-induction or high-dose cytarabine for cytarabine-naïve patients. Alternatively, R-ICE (ifosfamide, carboplatin, etoposide with rituximab) or R-GDP (gemcitabine, dexamethasone, cisplatin with rituximab) has been proposed and can be used for patients with prior cytarabine exposure. Patients who achieve remission should be considered for some form of transplantation.
Treatment Approach for High-Grade B-Cell Lymphomas with MYC, BCL2, and/or BCL6 translocations
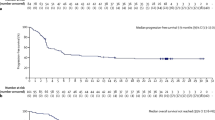
At the present time, it is recommended that all aggressive large B-cell lymphomas should be evaluated for MYC, BCL2, and BCL6 translocation status (“double-hit” and “triple-hit”) by FISH testing. Should FISH testing not be widely available, a reasonable approach may be to limit testing to germinal center B-cell like (GCB) lymphomas that overexpress MYC and BCL2 by IHC [35]. Considering the high risk of CNS involvement in this subset of patients, high consideration should be given to performing a lumbar puncture to exclude leptomeningeal disease. Several retrospective studies have now shown that patients with HGBL with translocations involving MYC, BCL2, and/or BCL6 do poorly with standard therapy (R-CHOP) [70, 71]. Additionally, retrospective studies have also shown an improved outcome when more intensive regimens are used, and this prompted the National Comprehensive Cancer Network (NCCN) to recommend “more intensive” approaches than R-CHOP (version 4.2018). Currently, the optimal “intensive” strategy for HGBL is undefined, but most advocate for regimens used in BL with the expectation that they may better overcome high tumor proliferation and other adverse biological factors associated with this entity. Tolerability must be carefully considered, as most patients are elderly. The NCI/US intergroup completed the first prospective single-arm study of DA EPOCH-R therapy in 53 patients with MYC rearranged aggressive B-cell lymphomas, and at close to 5-year follow-up, the 48-month EFS and OS were 71% and 77%, respectively, and in those patients with “double hit” lymphomas, EFS and OS were 73.4% and 82%, respectively [72••]. The HOVON group prospectively evaluated lenalidomide in combination with R-CHOP in 82 patients with MYC-rearranged HGBL and showed 2-year EFS and OS of 63% and 73%, respectively [73].
Future Directions and Conclusions
Though the outcome for children and adolescent and young adult (AYA) groups diagnosed with BL is excellent following “standard” BL regimens, treatment-associated toxicity (even with the incorporation of reduced intensity regimens for low-risk disease) curtails the routine use of “standard” approaches in many adult groups. Alternative, new paradigms that maintain high curability with manageable toxicity are needed and are currently being successfully developed. The WHO’s recent distinguishing of the HGBLs with MYC and BCL2 and/or BCL6 rearrangements from other B-cell lymphomas is helpful in that it separates out this group with an inferior prognosis and lays the foundation for investigating novel strategies in this subset. While MYC is the elusive target for drug development in BL and MYC-rearranged aggressive lymphomas, the identification of other genes and signaling pathways that cooperate with deregulated MYC paves the way for targeting these alterations with small molecule inhibitors [29, 74•]. Considering the synergy between MYC and PI3K signaling, targeting the PI3K/AKT/mTOR pathway with inhibitors of PI3K, AKT, or mTOR is a rational strategy. Dual-CDK4/6 inhibitors may be a beneficial approach to target tumors with a mutated CCND3 gene or increased oncogenic activation of CCND3 due to upstream alterations. A high number of BL tumors harbor mutations in genes that encode for proteins that are part of the SWI/SNF chromatin remodeling complex, such as ARID1A and SMARCA4, and a recent study suggested that these are sensitive to BET bromodomain inhibitors [75]. Interestingly, BET inhibition has also been found to potently suppress MYC gene expression, suggesting a synergistic treatment effect [76]. Various cellular therapy approaches are currently under investigation in BL and MYC-driven HGBL. Anti-CD19 chimeric antigen receptor (CAR) T cell therapy for BL is a promising development that is emphasized with a first case report showing CR in a patient with refractory BL [77, 78•, 79]. Moving forward, as new approaches demonstrate good efficacy in the relapsed and refractory setting, they can be incorporated into up-front BL investigation with the goal to improve upon current standards with less reliance on very toxic agents.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Molyneux EM, Rochford R, Griffin B, et al. Burkitt’s lymphoma. Lancet. 2012;379(9822):1234–44.
Blum KA, Lozanski G, Byrd JC. Adult Burkitt leukemia and lymphoma. Blood. 2004;104(10):3009–20.
Burkitt D. A sarcoma involving the jaws in African children. Br J Surg. 1958;46(197):218–23.
Brady G, MacArthur GJ, Farrell PJ. Epstein-Barr virus and Burkitt lymphoma. J Clin Pathol. 2007;60(12):1397–402.
Guech-Ongey M, Simard EP, Anderson WF, et al. AIDS-related Burkitt lymphoma in the United States: what do age and CD4 lymphocyte patterns tell us about etiology and/or biology? Blood. 2010;116(25):5600–4.
Dalla-Favera R, Bregni M, Erikson J, Patterson D, Gallo RC, Croce CM. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982;79(24):7824–7.
Schmitz R, Young RM, Ceribelli M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012;490(7418):116–20.
• Lopez C, Kleinheinz K, Aukema SM, et al. Genomic and transcriptomic changes complement each other in the pathogenesis of sporadic Burkitt lymphoma. Nat Commun. 2019;10(1):1459 Whole genome and transcriptome sequencing of 39 cases of sporadic BL reveals the mutational landscape, structural variants and mutational processes that underly sporadic BL pathogenesis.
Dunleavy K. Approach to the diagnosis and treatment of adult Burkitt’s lymphoma. J Oncol Pract. 2018;14(11):665–71.
Gopal S, Gross TG. How I treat Burkitt lymphoma in children, adolescents, and young adults in sub-Saharan Africa. Blood. 2018;132(3):254–63.
Alsharif R, Dunleavy K. Burkitt lymphoma and other high-grade b-cell lymphomas with or without MYC, BCL2, and/or BCL6 rearrangements. Hematol Oncol Clin North Am. 2019;33(4):587–96.
Picker LJ, Weiss LM, Medeiros LJ, Wood GS, Warnke RA. Immunophenotypic criteria for the diagnosis of non-Hodgkin’s lymphoma. Am J Pathol. 1987;128(1):181–201.
Jaffe ES, Pittaluga S. Aggressive B-cell lymphomas: a review of new and old entities in the WHO classification. Hematology Am Soc Hematol Educ Program. 2011;2011:506–14.
Neri A, Barriga F, Inghirami G, et al. Epstein-Barr virus infection precedes clonal expansion in Burkitt’s and acquired immunodeficiency syndrome-associated lymphoma. Blood. 1991;77(5):1092–5.
van den Bosch CA. Is endemic Burkitt’s lymphoma an alliance between three infections and a tumour promoter? Lancet Oncol. 2004;5(12):738–46.
Chene A, Donati D, Guerreiro-Cacais AO, et al. A molecular link between malaria and Epstein-Barr virus reactivation. PLoS Pathog. 2007;3(6):e80.
Kuppers R. B cells under influence: transformation of B cells by Epstein-Barr virus. Nat Rev Immunol. 2003;3(10):801–12.
•• Grande BM, Gerhard DS, Jiang A, et al. Genome-wide discovery of somatic coding and noncoding mutations in pediatric endemic and sporadic Burkitt lymphoma. Blood. 2019;133(12):1313–24 Whole genome and transcriptome sequencing of BL cases reveals that EBV status defines a specific BL phenotype, irrespective of geographic location, with particular molecular properties and distinct pathogenic mechanisms that could be targeted with small molecule inhibitors.
Gabay M, Li Y, Felsher DW. MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb Perspect Med. 2014;4(6).
Zech L, Haglund U, Nilsson K, Klein G. Characteristic chromosomal abnormalities in biopsies and lymphoid-cell lines from patients with Burkitt and non-Burkitt lymphomas. Int J Cancer. 1976;17(1):47–56.
Cai Q, Medeiros LJ, Xu X, Young KH. MYC-driven aggressive B-cell lymphomas: biology, entity, differential diagnosis and clinical management. Oncotarget. 2015;6(36):38591–616.
Ott G, Rosenwald A, Campo E. Understanding MYC-driven aggressive B-cell lymphomas: pathogenesis and classification. Blood. 2013;122(24):3884–91.
Pelicci PG, Knowles DM 2nd, Magrath I, Dalla-Favera R. Chromosomal breakpoints and structural alterations of the c-myc locus differ in endemic and sporadic forms of Burkitt lymphoma. Proc Natl Acad Sci U S A. 1986;83(9):2984–8.
Ramezani-Rad P, Rickert RC. Murine models of germinal center derived-lymphomas. Curr Opin Immunol. 2017;45:31–6.
Janz S, Potter M, Rabkin CS. Lymphoma- and leukemia-associated chromosomal translocations in healthy individuals. Genes Chromosom Cancer. 2003;36(3):211–23.
• Wagener R, Seufert J, Raimondi F, et al. The mutational landscape of Burkitt-like lymphoma with 11q aberration is distinct from that of Burkitt lymphoma. Blood. 2019;133(9):962–6 Describes the new WHO provisional entity of “Burkitt-like lymphoma with 11q aberration” and shows how the molecular landscape of this subtype differs from other forms of BL.
Sander S, Calado DP, Srinivasan L, et al. Synergy between PI3K signaling and MYC in Burkitt lymphomagenesis. Cancer Cell. 2012;22(2):167–79.
Srinivasan L, Sasaki Y, Calado DP, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139(3):573–86.
Schmitz R, Ceribelli M, Pittaluga S, Wright G, Staudt LM. Oncogenic mechanisms in Burkitt lymphoma. Cold Spring Harb Perspect Med. 2014;4(2).
Love C, Sun Z, Jima D, et al. The genetic landscape of mutations in Burkitt lymphoma. Nat Genet. 2012;44(12):1321–5.
Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–90.
Salaverria I, Martin-Guerrero I, Wagener R, et al. A recurrent 11q aberration pattern characterizes a subset of MYC-negative high-grade B-cell lymphomas resembling Burkitt lymphoma. Blood. 2014;123(8):1187–98.
Ferreiro JF, Morscio J, Dierickx D, et al. Post-transplant molecularly defined Burkitt lymphomas are frequently MYC-negative and characterized by the 11q-gain/loss pattern. Haematologica. 2015;100(7):e275–9.
Havelange V, Ameye G, Theate I, et al. The peculiar 11q-gain/loss aberration reported in a subset of MYC-negative high-grade B-cell lymphomas can also occur in a MYC-rearranged lymphoma. Cancer Gene Ther. 2016;209(3):117–8.
Friedberg JW. How I treat double-hit lymphoma. Blood. 2017;130(5):590–6.
Niitsu N, Okamoto M, Miura I, Hirano M. Clinical features and prognosis of de novo diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC translocations. Leukemia. 2009;23(4):777–83.
Landsburg DJ, Falkiewicz MK, Petrich AM, et al. Sole rearrangement but not amplification of MYC is associated with a poor prognosis in patients with diffuse large B cell lymphoma and B cell lymphoma unclassifiable. Br J Haematol. 2016;175(4):631–40.
Kuhnl A, Cunningham D, Counsell N, et al. Outcome of elderly patients with diffuse large B-cell lymphoma treated with R-CHOP: results from the UK NCRI R-CHOP14v21 trial with combined analysis of molecular characteristics with the DSHNHL RICOVER-60 trial. Annals of oncology: official journal of the European Society for Medical Oncology. 2017;28(7):1540–6.
• Rosenwald A, Bens S, Advani R, et al. Prognostic significance of MYC rearrangement and translocation partner in diffuse large b-cell lymphoma: a study by the Lunenburg Lymphoma Biomarker Consortium. J Clin Oncol. 2019;37(35):3359–68 Cohort study validating the negative prognostic impact of a MYC-rearrangement in DLBCL, especially when MYC is translocated to an IG partner.
Magrath I. Epidemiology: clues to the pathogenesis of Burkitt lymphoma. Br J Haematol. 2012;156(6):744–56.
Dunleavy K, Wilson WH. How I treat HIV-associated lymphoma. Blood. 2012;119(14):3245–55.
Dave SS, Fu K, Wright GW, et al. Molecular diagnosis of Burkitt’s lymphoma. N Engl J Med. 2006;354(23):2431–42.
Mukhtar F, Boffetta P, Risch HA, et al. Survival predictors of Burkitt’s lymphoma in children, adults and elderly in the United States during 2000-2013. Int J Cancer. 2017;140(7):1494–502.
De Vita VT, Jr., Hubbard SM, Longo DL. The chemotherapy of lymphomas: looking back, moving forward--the Richard and Hinda Rosenthal Foundation award lecture. Cancer Res. 1987;47(22):5810–24.
McMaster ML, Greer JP, Greco FA, Johnson DH, Wolff SN, Hainsworth JD. Effective treatment of small-noncleaved-cell lymphoma with high-intensity, brief-duration chemotherapy. J Clin Oncol. 1991;9(6):941–6.
Dunleavy K, Gross TG. Management of aggressive B-cell NHLs in the AYA population: an adult vs pediatric perspective. Blood. 2018;132(4):369–75.
Magrath I, Adde M, Shad A, et al. Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J Clin Oncol. 1996;14(3):925–34.
Mead GM, Sydes MR, Walewski J, et al. An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt’s lymphoma: results of United Kingdom Lymphoma Group LY06 study. Ann Oncol. 2002;13(8):1264–74.
Mead GM, Barrans SL, Qian W, et al. A prospective clinicopathologic study of dose-modified CODOX-M/IVAC in patients with sporadic Burkitt lymphoma defined using cytogenetic and immunophenotypic criteria (MRC/NCRI LY10 trial). Blood. 2008;112(6):2248–60.
Lacasce A, Howard O, Lib S, et al. Modified magrath regimens for adults with Burkitt and Burkitt-like lymphomas: preserved efficacy with decreased toxicity. Leuk Lymphoma. 2004;45(4):761–7.
Wang ES, Straus DJ, Teruya-Feldstein J, et al. Intensive chemotherapy with cyclophosphamide, doxorubicin, high-dose methotrexate/ifosfamide, etoposide, and high-dose cytarabine (CODOX-M/IVAC) for human immunodeficiency virus-associated Burkitt lymphoma. Cancer. 2003;98(6):1196–205.
Barnes JA, Lacasce AS, Feng Y, et al. Evaluation of the addition of rituximab to CODOX-M/IVAC for Burkitt’s lymphoma: a retrospective analysis. Ann Oncol. 2011;22(8):1859–64.
Noy A, Lee JY, Cesarman E, et al. AMC 048: modified CODOX-M/IVAC-rituximab is safe and effective for HIV-associated Burkitt lymphoma. Blood. 2015;126(2):160–6.
Mohamedbhai SG, Sibson K, Marafioti T, et al. Rituximab in combination with CODOX-M/IVAC: a retrospective analysis of 23 cases of non-HIV related B-cell non-Hodgkin lymphoma with proliferation index >95%. Br J Haematol. 2011;152(2):175–81.
Minard-Colin V, Auperin A, Pillon M, et al. Rituximab for high-risk, mature B-cell non-Hodgkin’s lymphoma in children. N Engl J Med. 2020;382(23):2207–19.
Thomas DA, Cortes J, O'Brien S, et al. Hyper-CVAD program in Burkitt’s-type adult acute lymphoblastic leukemia. J Clin Oncol. 1999;17(8):2461–70.
Thomas DA, Faderl S, O'Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106(7):1569–80.
Rizzieri DA, Johnson JL, Niedzwiecki D, et al. Intensive chemotherapy with and without cranial radiation for Burkitt leukemia and lymphoma: final results of Cancer and Leukemia Group B Study 9251. Cancer. 2004;100(7):1438–48.
Rizzieri DA, Johnson JL, Byrd JC, et al. Improved efficacy using rituximab and brief duration, high intensity chemotherapy with filgrastim support for Burkitt or aggressive lymphomas: cancer and Leukemia Group B study 10 002. Br J Haematol. 2014;165(1):102–11.
Divine M, Casassus P, Koscielny S, et al. Burkitt lymphoma in adults: a prospective study of 72 patients treated with an adapted pediatric LMB protocol. Ann Oncol. 2005;16(12):1928–35.
Ribrag V, Koscielny S, Bosq J, et al. Rituximab and dose-dense chemotherapy for adults with Burkitt’s lymphoma: a randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387(10036):2402–11.
Hoelzer D, Walewski J, Dohner H, et al. Improved outcome of adult Burkitt lymphoma/leukemia with rituximab and chemotherapy: report of a large prospective multicenter trial. Blood. 2014;124(26):3870–9.
Dunleavy K, Pittaluga S, Shovlin M, et al. Low-intensity therapy in adults with Burkitt’s lymphoma. N Engl J Med. 2013;369(20):1915–25.
Dunleavy K, Roschewski M, Abramson J, et al. Risk-adapted therapy in adults with burkitt lymphoma: updated results of a Multi-Center Prospective Phase II Study of DA-EPOCH-R. Haematological Oncology. 2017;35(Proc Lugano Lymphoma Meeting 2017).
•• Roschewski M, Dunleavy K, Abramson JS, et al. Multicenter study of risk-adapted therapy with dose-adjusted EPOCH-R in adults with untreated Burkitt lymphoma. J Clin Oncol. 2020;38(22):2519–29 Phase II study showing that risk-adapted, dose-adjusted EPOCH-R is effective in adult BL, regardless of age or HIV status, and is well tolerated.
Evens AM, Danilov AV, Jagadeesh D, et al. Burkitt lymphoma in the Modern Era: real world outcomes and prognostication across 30 US cancer centers. Blood. 2020.
Sweetenham JW, Pearce R, Taghipour G, Blaise D, Gisselbrecht C, Goldstone AH. Adult Burkitt’s and Burkitt-like non-Hodgkin’s lymphoma--outcome for patients treated with high-dose therapy and autologous stem-cell transplantation in first remission or at relapse: results from the European Group for Blood and Marrow Transplantation. J Clin Oncol. 1996;14(9):2465–72.
Simkins A, Dunleavy K. Tackling Burkitt when it’s back. Blood. 2020;135(14):1078–80.
Short NJ, Kantarjian HM, Ko H, et al. Outcomes of adults with relapsed or refractory Burkitt and high-grade B-cell leukemia/lymphoma. Am J Hematol. 2017;92(6):E114–7.
Savage KJ, Johnson NA, Ben-Neriah S, et al. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood. 2009;114(17):3533–7.
Dunleavy K. Double-hit lymphomas: current paradigms and novel treatment approaches. Hematology Am Soc Hematol Educ Program. 2014;2014(1):107–12.
•• Dunleavy K, Fanale MA, Abramson JS, et al. Dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in untreated aggressive diffuse large B-cell lymphoma with MYC rearrangement: a prospective, multicentre, single-arm phase 2 study. Lancet Haematol. 2018;5(12):e609–17 Phase II study showing that dose-adjusted EPOCH-R is able to produce durable remissions in patients with MYC-rearranged aggressive B-cell lymphoma and should be considered for the treatment of these diseases.
Chamuleau MED, Burggraaf CN, Nijland M, et al. Treatment of patients with MYC rearrangement positive large B-cell lymphoma with R-CHOP plus lenalidomide: results of a multi-center HOVON phase II trial. Haematol. 2020;105(12):2805–12.
• Tomska K, Kurilov R, Lee KS, et al. Drug-based perturbation screen uncovers synergistic drug combinations in Burkitt lymphoma. Sci Rep. 2018;8(1):12046 Study that describes drug response profiling in BL revealing not only potential synergistic combinations but also that targeting CDK and BET could provide a novel vulnerability.
Shorstova T, Marques M, Su J, et al. SWI/SNF-compromised cancers are susceptible to bromodomain inhibitors. Cancer Res. 2019;79(10):2761–74.
Delmore JE, Issa GC, Lemieux ME, et al. BET bromodomain inhibition as a therapeutic strategy to target c-myc. Cell. 2011;146(6):904–17.
Enblad G, Karlsson H, Gammelgard G, et al. A Phase I/IIa Trial using CD19-targeted third-generation CAR T cells for lymphoma and leukemia. Clin Cancer Res. 2018;24(24):6185–94.
• Avigdor A, Shouval R, Jacoby E, et al. CAR T cells induce a complete response in refractory Burkitt Lymphoma. Bone Marrow Transplant. 2018;53(12):1583–5 Case-report of a patient with relapsed BL that was treated with salvage CAR T-cell therapy resulting in a deep response.
Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-Cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–44.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed equally.
Corresponding author
Ethics declarations
Conflict of Interest
Coen J. Lap declares that he has no conflict of interest.
Samah Nassereddine declares that she has no conflict of interest.
Kieron Dunleavy has received compensation for service as a consultant from Daiichi Sankyo, Pharmacyclics, Amgen/AbbVie, AstraZeneca, MorphoSys, Genmab, Genentech, and Janssen.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Leukemia
Rights and permissions
About this article
Cite this article
Lap, C.J., Nassereddine, S. & Dunleavy, K. Novel Biological Insights and New Developments in Management of Burkitt Lymphoma and High-Grade B-Cell Lymphoma. Curr. Treat. Options in Oncol. 22, 60 (2021). https://doi.org/10.1007/s11864-021-00857-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s11864-021-00857-w