Abstract
This entry treats sources of only ionizing radiation, such as electrons, protons, high-energy photons, neutrons, and similar radiations that have the ability to cause ionization, either directly or indirectly, and, thus, to induce chemical and physical changes along their passages through materials. Not included are sources of relatively lower frequency electromagnetic radiation from radio waves to ultraviolet light.
This chapter was originally published as part of the Encyclopedia of Sustainability Science and Technology edited by Robert A. Meyers. DOI:10.1007/978-1-4419-0851-3
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Definition of the Subject
This entry treats sources of only ionizing radiation, such as electrons, protons, high-energy photons, neutrons, and similar radiations that have the ability to cause ionization, either directly or indirectly, and, thus, to induce chemical and physical changes along their passages through materials. Not included are sources of relatively lower frequency electromagnetic radiation from radio waves to ultraviolet light.
Characterization of sources requires characterization of radiations as well. Many sources encountered are radioactive isotopes of elements in the periodic table. These sources, as they decay radioactively, emit ionizing radiation. Some of the ionizing radiation is in the form of alpha particles, gamma rays, and x-rays, all characteristically monoenergetic in nature. Some is in the form of beta particles, distributed in energy but with well-defined maxima. Other sources are not directly associated with radioisotopes. X-ray machines and accelerators release ionizing radiation generally distributed in energy but with some monoenergetic components. The radiation belts surrounding the earth are composed of electrons and protons distributed in energy. Solar radiation and galactic cosmic rays are ionizing radiations widely distributed in type and energy. The nuclear fission process results in prompt emission of gamma rays and neutrons very widely distributed in energy. Fission also yields an extremely wide range of fission products, which radioactively decay over long periods of time.
Characterization of sources, to be meaningful, also begs discussion of radiation doses and radiation effects. There are acute effects accompanying high exposures. There are also known carcinogenic effects of human exposure and suspected mutational and hereditary effects. Therefore, a portion of this entry is devoted to examination of health effects associated with exposure to ionizing radiation.
This entry is divided into two parts. In the first several sections, the quantitative technical characterization of physical processes that produce ionizing radiation are reviewed. In latter sections, a qualitative examination is given of the various types of radiation sources encountered in the environment, workplace, laboratory, or medical facility.
Introduction
Throughout our lives, ionizing radiation is ever present, though rarely sensed. Radioactive sources are present in the food we eat, in the water we drink, and in the air we breathe. Most of these sources have but brief sojourns in our bodies, but some are taken up in bone and permanently retained. These sources, isotopes of elements in the periodic table, decay radioactively, emitting ionizing radiation most often in the form of gamma and x-rays, alpha particles, beta particles, and electrons. The ionization taking place in the body accounts for biological effects, good and bad.
Radiation also reaches us from sources outside our bodies. Radioactivity is present in our soils and minerals, and in our construction materials. Electromagnetic radiation of all wavelengths, including radio waves, microwaves, radar, and light, of both man-made and natural origins, constantly, bombard us. Photons are far more prevalent in number than atoms in our universe; for every nucleon there are about 109 photons. Cosmic rays and the subatomic debris they create during interactions in the atmosphere also impinge on us. Neutrinos from fusion reactions in our sun reach us in such numbers that tens of billions per second pass through every square centimeter of our skin. Most of this radiation, for example, neutrinos and radio waves, fortunately, passes harmlessly through us. Other radiation such as light and longer wavelength electromagnetic radiation usually interacts harmlessly with our tissues. However, shorter wavelength electromagnetic radiation, for example, ultraviolet light, x-rays and gamma rays, and charged particles produced by nuclear reactions can cause various degrees of damage to our cells.
The types and sources of radiation just described may be naturally occurring, may be a legacy of the era of nuclear weapons testing, or may be a result of human enterprise, for example, uranium in coal ash, radium in mine tailings, medical wastes, and fission or activation products in wastes from nuclear power production. All vary with latitude, longitude, and altitude – even on a small scale.
There are also population groups especially affected, but in different ways, by exposure to ionizing radiation from many sources. First among these in importance are patients and providers of medical radiation exposures. In the USA, as of 2006, collective effective doses to patients accruing from medical exposure, about 900,000 person-Sv annually, amount to almost half the total for the entire population. Computed tomography and nuclear medicine procedures dominate the exposure, with fluoroscopy and radiology accounting for about 230,000 person-Sv annual effective dose. Interventional fluoroscopy, while of great value to the patient, contributes in a major way to provider dose. Brachytherapy and beam therapy using photons, electrons, and protons lead to high therapeutic radiation exposures to patients, of course. Modern beam therapy utilizes exquisite beam shielding techniques to minimize doses to off-target patient tissues. Of all occupational groups, medical workers are greatest in number and accrue the highest collective doses, namely, 549 person-Sv annually in the USA. However, recordable individual worker annual doses are about 0.75 mSv as compared to 1.87 mSv for the fewer workers in commercial nuclear power.
Another population group consists of astronauts and aviation flight crews especially in high-altitude, international flights. Radiation from solar and galactic sources lead to high occupational radiation exposures. For astronauts, the life-threatening risk of solar-flare radiation exposures is a major concern.
Radiation sources used in industry affect another significant occupational group. Industrial radiography using x-ray, gamma-ray, or even neutron sources is an important component of occupational exposure. Other sources find wide use in measurement devices and sensor appliances.
For radiation to produce biological damage, it must first interact with tissue to alter molecular bonds and change the chemistry of the cells. Likewise, for radiation to produce damage in structural and electrical materials, it must cause interactions that disrupt crystalline and molecular bonds. Such radiation must be capable of creating ion–electron pairs and is termed ionizing radiation. Fast-moving charged particles, such as alpha particles, beta particles, and fission fragments, can directly ionize matter. Neutral particles, such as photons and neutrons, cannot interact directly with the electrons of the matter through they pass; rather they cause interactions that transfer some of their energy to charged secondary particles, which in turn produce ionization as they slow.
Radiation-Producing Reactions
Origins of Ionizing Radiation
Ionizing radiation is invariably the consequence of physical reactions, involving subatomic particles, at the atomic or nuclear level. The possible radiation-producing reactions are many, and usually, although not always, involve altering the configuration of neutrons and protons in an atomic nucleus or the rearrangement of atomic electrons about a nucleus. These reactions can be divided into two categories:
Radioactive decay. In the first type of radiation-producing reaction, the nucleus of an atom spontaneously changes its internal arrangement of neutrons and protons to achieve a more stable configuration. In such spontaneous radioactive transmutations, ionizing radiation is almost always emitted. The number of known different atoms, each with a distinct combination of Z and A is about 3,200. Of these, only 266 are stable and 65 are long-lived radioisotopes all of which are found in nature. The remaining nuclides have been made by humans and are radioactive with lifetimes much shorter than the age of the solar system. Both naturally occurring and manufactured radionuclides are the mostly commonly encountered sources of ionizing radiation.
Binary reactions. The second category of radiation-producing interactions involves two impinging atomic or subatomic particles that react to form one or more reaction products. Examples include neutrons interacting with nuclei of atoms, or photons interacting with nuclei or atomic electrons. Many binary reactions, in which an incident subatomic particle x strikes an atom or nucleus X, produce only two reaction products, typically a residual atom or nucleus Y and some subatomic particle y. These binary two-product reactions are often written as X(x,y)Y, for example, \(\ _{7}^{14}{\text{N}}\left( {\alpha, p} \right)_{8}^{17}{\text{O}} \).
Energetics of Radiation-Producing Reactions
In any nuclear reaction, total energy must be conserved. The total energy (kinetic plus rest-mass energy) of the initial particles must equal the total energy of the final products, that is, \( \sum\nolimits_i \left[ {{E_i} + {m_i}{c^2}} \right] = \sum\nolimits_i \left[ {E_i^{\prime} + m_i^\prime {c^2}} \right] \), where E i (E′ i ) is the kinetic energy of the ith initial (final) particle with a rest mass m i (m′ i ), and c is the speed of light.
Any change in the total kinetic energy of particles before and after the reaction, ΔE, must be accompanied by an equivalent change in the total rest mass of the particles before and after the reaction, Δm, that are related by Einstein’s famous equation ΔE = Δmc 2. To quantify this change in the kinetic or rest-mass energies, a so-called Q-value is defined as
The Q value of a nuclear reaction may be either positive or negative. If the rest masses of the reactants exceed the rest masses of the products, the Q value of the reaction is positive with the decrease in rest mass being converted into a gain in kinetic energy. Such a reaction is exoergic. Radioactive decay is such a spontaneous exoergic nuclear reaction in which the Q-value energy is converted into the kinetic energy of the products.
Conversely, if Q is negative, the reaction is endoergic. For this case, kinetic energy of the initial particles is converted into rest-mass energy of the reaction products. The kinetic energy decrease equals the rest-mass energy increase. Such reactions cannot occur unless the colliding particles have at least a certain amount of kinetic energy, the so-called threshold energy for the reaction. For the binary, two-product reaction X(x,y)Y, the threshold kinetic energy of x incident on a stationary X is, neglecting Coulombic barrier effects, given approximately by
In any reaction, linear momentum must also be conserved. Thus, the momentum of the reaction products must equal that of the reactants. For two-product nuclear reactions, conservation of linear momentum requires that the products, depending on their recoil directions, have very definite amounts of kinetic energy. By contrast, for reactions with three or more products, there is no unique division of the reaction energy, and the products generally have a continuous distribution of kinetic energies.
Physical Characterization of Sources
The most fundamental type of source is a point source. Clearly, no real source can have zero size, but a real source can be approximated as a point source provided (1) that the volume is sufficiently small, that is, with dimensions much smaller than the dimensions of the attenuating medium between source and detector, and (2) that there is negligible interaction of radiation with the matter in the source volume. The second requirement may be relaxed if source characteristics are modified to account for source self-absorption and other source–particle interactions.
In general, a point source may be characterized as depending on energy, direction, and time. In almost all cases, time is not treated as an independent variable because the time delay between a change in the source and the resulting change in the radiation field is usually negligible. Therefore, the most general characterization of a point source used here is in terms of energy and direction. Most radiation sources treated in shielding practice are isotropic, so that source characterization requires only knowledge of energy dependence. Radioisotope sources are certainly isotropic, as are fission sources and capture gamma-ray sources.
A careful distinction must be made between the activity of a radioisotope and its source strength. Activity is precisely defined as the expected number of atoms undergoing radioactive transformation per unit time. It is not defined as the number of particles emitted per unit time. Decay of two very common laboratory radioisotopes illustrate this point. Each transformation of 60Co, for example, results in the emission of two gamma rays, one at 1.173 MeV and the other at 1.333 MeV. Each transformation of 137Cs, accompanied by a transformation of its decay product 137mBa, results in emission of a 0.662-MeV gamma ray with probability 0.85.
The SI unit of activity is the becquerel (Bq), equivalent to one transformation per second. In medical and health physics, radiation source strengths are commonly calculated on the basis of accumulated activity, Bq s. Such time-integrated activities account for the cumulative number of transformations in some biological entity during the transient presence of radionuclides in the entity. Of interest in such circumstances is not the time-dependent dose rate to that entity or some other nearby region, but rather the total dose accumulated during the transient. Similar practices are followed in dose evaluation for reactor transients, solar flares, nuclear weapons, and so on.
Radiation sources may be distributed along a line, over an area, or within a volume. Source characterization requires, in general, spatial and energy dependence. Occasionally, it is necessary to include angular dependence. This is especially true for effective area sources associated with computed angular flows across certain planes. Energy dependence may be discrete, such as for radionuclide sources, or continuous, as for bremsstrahlung or fission neutrons and photons.
Radioactivity
Radioactive Decay Dynamics
The decay of a radioactive nuclide is a stochastic phenomenon. The time an individual radionuclide decays cannot be predicted; rather, only the probability of decay in a specified time interval can be predicted. The rate at which a sample of a large number of identical radionuclides decays is determined by the radioactive decay constant λ for the nuclide. This constant is the probability, per unit time, that a radionuclide decays in an infinitesimal time interval. That λ is constant for a given radionuclide species implies that the expected number of radionuclides, N(t), at time t is N(t) = N(0)e −λt, where N(0) is the initial number of radionuclides in the sample. The exponential decay of radionuclides is sometimes called the radioactive decay law.
Generally, the number of radionuclides in a sample is not of interest. Rather the activity A(t) or rate at which a radionuclide sample decays, dN(t)/dt, is desired since this quantity determines the rate of radiation emission from the sample. From the radioactive decay law, it is found that \( {{{{\text{d}}N(t)}} \left/ {{{\text{d}}t = \lambda N(t)}} \right.} \equiv {{A}}(t) \), so that the activity of a radionuclide sample also decays exponentially, that is, A(t) = A(0)e −λt. The standard unit of activity is the becquerel (Bq) equal to one radioactive decay per second. The traditional unit is the curie (Ci) = 3.7 × 1010 Bq (approximately the activity of 1 g of 226Ra).
The rate at which a radioactive sample decays is commonly described by its half-life T 1/2. The half-life is the time required for half of the sample to decay, or, equivalently, for the sample activity to halve. From the radioactive decay law, it is found \( {T_{{{1} \left/ {2} \right.}}} = { \ln }\;{{2} \left/ {\lambda } \right.} \simeq {{{0.{693}}} \left/ {\lambda } \right.} \).
Types of Radioactive Decay
There are several types of spontaneous changes (or transmutations) that can occur in radioactive nuclides. In each transmutation, the nucleus of the parent atom \( _Z^A{\text{P}} \) is altered in some manner and one or more particles of radiation are emitted. If the number of protons in the nucleus is changed, then the number of orbital electrons in the daughter atom D must subsequently also be changed, either by releasing an electron to or absorbing an electron from the ambient medium. The most commonly encountered types of radioactive decay are
-
Gamma decay (γ) \( _Z^A{{\text{P}}^* } \to_Z^A{\text{P}} + \gamma:\) An excited nucleus decays (usually within 10−8 s) to its ground state by the emission of one or more gamma photons. The excited parent is often the product of radioactive decay or a binary nuclear reaction.
-
Isomeric transition (IT) \( _Z^{Am}{{\text{P}}^* } \to {}_Z^A{\text{P}} + \gamma :\) This is a special case of gamma decay, in which the excited parent has a lifetime much greater than usual nuclear lifetimes (10−8 s), ranging from seconds to thousands of years. Such a long-lived excited nucleus is said to be metastable and is called an isomer.
-
Internal conversion (IC) \( _Z^A{{\text{P}}^* } \to {[_Z^A{\text{P}}]^{ + 1}} + {{\text{e}}^{-} } \): The excitation energy of a nucleus is used to eject an orbital electron (usually a K-shell electron).
-
Alpha decay (α) \( _Z^A{\text{P}} \to {}_{Z - 2}^{Z - 4}{\text{D}} + \alpha \): An α particle is emitted leaving the daughter with 2 fewer neutrons and 2 fewer protons than the parent. The transition often is to an excited nuclear state of the daughter which decays by emission of one or more gamma photons.
-
Beta decay (β −)\( _Z^A{\text{P}} \to {}_{Z + 1}^A{\text{D}} + {\beta^{-} } + \bar{\nu } \): In effect, a neutron in the nucleus decays to a proton. An electron (β −) and antineutrino (\( \bar{\nu } \)) are emitted, which share the decay energy. The daughter is often produced in an excited nuclear state and subsequently emits gamma photons.
-
Positron decay (β +)\( _Z^A{\text{P}} \to {}_{Z - 1}^A{\text{D}} + {\beta^{+} } + \nu \): In effect, a proton in the nucleus changes into a neutron. A positron (β +) and neutrino (ν) are emitted, which share the decay energy. If the daughter is produced in an excited state, gamma decay results. The emitted positron, after slowing in the ambient medium, annihilates with an ambient electron producing two 0.511-MeV gamma rays.
-
Electron capture (EC) \( _Z^A{\text{P}} \to {}_{Z - 1}^A{{\text{D}}^* } + \nu \): An orbital electron is absorbed by the nucleus, converts a nuclear proton into a neutron, emits a neutrino (ν), and, generally, leaves the nucleus in an excited state, which decays by the emission of one or more gamma photons.
-
Spontaneous fission (SP) \( {_Z^A{\text{P}} \to {}_{{Z_H}}^{{A_H}}{{\text{D}}_H} + {}_{{Z_L}}^{{A_L}}{{\text{D}}_L} + n({}_0^1{\text{n}}) + m(\gamma )} \): A heavy nucleus spontaneously splits or fissions into a heavy (H) and light (L) fission fragment. The fission fragments are produced in highly excited nuclear states and decay by prompt neutron and gamma photon emission within 10−13 s of the fission event, releasing, on the average, n neutrons and m γ photons. The resulting fission products are usually radioactive and undergo a chain of β − decays releasing several delayed gamma photons and beta particles until a stable nucleus is reached. In some instances, ternary rather than binary fission takes place, releasing a light product such as tritium.
Many radionuclides decay by more than a single decay mechanism. For example, electron capture is always in competition with positron decay. An example of a radionuclide that decays by three mechanisms is 64Cu whose decay scheme is shown in Fig. 13.1.
The radioactive decay scheme for 64Cu per decay, on average a beta particle of maximum energy 0.579 MeV is emitted with a probability of 0.385, a positron of maximum energy 0.653 MeV is emitted with a probability of 0.176, and a gamma ray of energy 1.346 MeV is emitted with a probability of 0.005. Source: NUDAT 2.5, National Nuclear Data Center, Brookhaven National Laboratory
In any radioactive decay that alters the proton number Z, electron rearrangements necessarily result. The resulting cascade of orbital electrons to lower energy levels results in emission of x-rays and, in competition, ejection of what are called Auger electrons.
Naturally Occurring Radionuclides
Singly Occurring Primordial Radionuclides
Of the many radioactive species present when the earth was formed, only those very few with half-lives comparable to the age of the earth remain in the environment. Of these few primordial radionuclides not belonging to a decay chain, only 40K and 87Rb contribute significantly to human exposure. Of minor consequence are the nuclides 138La, 147Sm, and 176Lu. The radionuclide 87Rb has a half-life of 4.8 × 1010 years and decays by beta-particle emission. In the human body, its main impact is on bone-surface cells. The radionuclide 40K is a major contributor to human exposure from natural radiation. Present in an isotopic abundance of 0.0118%, it has a half-life of 1.227 × 109 years, decaying both by electron capture and beta-particle emission. Annual human doses are about 140 μGy to bone surface, 170 μGy on average to soft tissue, and 270 μGy to red marrow [1]. 40K also contributes in a major way to external exposure. The population-average specific activity of the nuclide in soil is 420 Bq/kg [2]. Based on a soil density of 1,600 kg/m3, and dose conversion factors from [3], 40K in the soil contributes 120 μSv effective dose annually.
Decay Series of Terrestrial Origin
Two actinide decay series, identified by the long-lived parents 238U and 232Th contribute appreciably to human exposure to natural radiation. Another series headed by 235U contributes very little. Members of the two important series are listed in Table 13.1. Many of the radionuclides in these series decay by emission of alpha particles with energies from 4 to 6 MeV. Others in the series emit beta particles accompanied by gamma rays. With long-lived parent radionuclides and short-lived daughter products, the chain might be thought to exist in a state of secular equilibrium, that is, each component having the same decay rate per unit volume of the host medium. However, some of the chain members are more soluble than others, and some are gaseous. Thus, unless the host medium is a rigid solid, such as granite, decay rates are far from equilibrium state.
Ingestion of elements in the uranium and thorium decay chains is unavoidable. Table 13.2 illustrates the consequences in terms of the committed effective dose incurred by ingestion but perhaps experienced long thereafter.
The portions of the series headed by the gases 220Rn and 222Rn are of special importance in public health. The gases escape from soil and rock into the atmosphere and into the airspace within homes. Their daughter products, some of which emit alpha particles, may be inhaled, with risk of radiation damage to radiation-sensitive cells in the lungs potentially leading to lung cancer. 222Rn and its daughters ordinarily present a greater hazard than 220Rn (thoron) and its daughters, largely because the much shorter half-life of 220Rn makes decay more likely prior to release into the atmosphere. Globally, the mean annual effective dose equivalent due to 222Rn daughters is about 1 mSv (100 mrem) while that due to 220Rn daughters is estimated to be about 0.2 mSv (20 mrem).
Cosmogenic Radionuclides
Cosmic-ray interactions with constituents of the atmosphere, sea, or earth, but mostly with the atmosphere, lead directly to radioactive products. Capture of secondary neutrons produced in primary interactions of cosmic rays, leads to the formation of many more radionuclides. Of the nuclides produced in the atmosphere, only 3H, 7Be, 14C, and 22Na contribute appreciably to human radiation exposure.
Over the past century, combustion of fossil fuels and the emission of carbon dioxide not containing 14C has diluted the cosmogenic content of 14C in the environment. Moreover, since World War II, artificial introduction of 14C, 3H, and other nuclides into the environment by human activity has been significant, especially as a result of atmospheric nuclear tests. Consequently, these radionuclides no longer exist in natural equilibria in the environment.
The tritium3H nuclide is produced mainly from interactions of neutrons with nitrogen and oxygen. Tritium has a half-life of 12.3 years and, upon decay, releases one low-energy beta particle of mean energy 5.7 keV. Tritium exists in nature almost exclusively in water form (HTO) but, in foods, may be partially incorporated into organic compounds. The nuclide 14C is produced mainly from the interactions of neutrons with nitrogen in the atmosphere. It exists in the atmosphere as CO2, but the main reservoir is the ocean. It has a half-life of 5,700 years and decays by beta particle emission of mean energy 49.5 keV.
7Be is also produced by cosmic ray interactions with nitrogen and oxygen in the atmosphere. It decays by electron capture, 10.4% of which events yield a 0.478-MeV gamma ray. 22Na decays by positron emission (90%) and electron capture (10%). The positron emission yields two annihilation photons as well as a positron of mean energy 215 keV. Both the positron emission and electron capture yield a 1.275-MeV gamma ray. Table 13.3 lists natural inventories, atmospheric concentrations, and effective doses to populations. Note that 1 PBq = 1015 Bq.
Sources of Neutrons
Fission Neutrons
Many heavy nuclides fission after the absorption of a neutron, or even spontaneously, producing several energetic fission neutrons. Almost all of the fast neutrons produced from a fission event are emitted within 10−14 s of the fission event, and are called prompt neutrons. Generally, less than 1% of the total fission neutrons are emitted as delayed neutrons, which are produced by the neutron decay of fission products at times up to many seconds or even minutes after the fission event. As the energy of the neutron which induces the fission in a heavy nucleus increases, the average number of fission neutrons also increases. For example, the fission of 235U by a thermal neutron (average energy 0.025 eV) produces, on the average, 2.43 fission neutrons. A fission caused by a 10-MeV neutron, by contrast, yields 3.8 fission neutrons. For 239Pu, fission by thermal or 10 MeV neutrons yield 2.87 or 4.2 neutrons. The fission of 238U is induced only by fast neutrons, a 10-MeV neutron yielding 3.9 fission neutrons.
Since the advent of fission reactors, many transuranic isotopes have been produced in significant quantities. Many of these isotopes have appreciable spontaneous fission probabilities, and consequently they can be used as very compact sources of fission neutrons. For example, 1 g of 252Cf releases 2.3 × 1012 neutrons per second, and very intense neutron sources can be made from this isotope, limited in size only by the need to remove the fission heat through the necessary encapsulation. Almost all spontaneously fissioning isotopes decay much more frequently by α emission than by fission.
The energy dependence of the fission neutron spectrum has been investigated extensively, particularly for the important isotope 235U. All fissionable nuclides produce prompt-fission neutrons with energy frequency distributions that go to zero at low and high energies, reaching a maximum at about 0.7 MeV, and have an average energy of about 2 MeV. The fraction of prompt fission neutrons emitted per unit energy about E, χ(E), can be described quite accurately by a Watt distribution
where the parameters a, b, and c depend on the fissioning isotope. For example, a = 0.5535 MeV, b = 1.0347 MeV, and c = 1.6214 MeV−1 for thermal-neutron fission of 235U, whose fission-neutron spectrum is often used as an approximation for other fissioning isotopes.
Fusion Neutrons
Neutrons can be produced as products of nuclear reactions in which energetic charged particles hit target atoms. Most such reactions require accelerators to produce the energetic charged particles and, hence, such neutrons are to be encountered only near accelerator targets.
One major exception to the insignificance of charged-particle-induced reactions are those in which light elements fuse exoergically to yield a heavier nucleus and which are accompanied quite often by the release of energetic neutrons. The resulting fusion neutrons are usually the major source of radiation to be shielded against. The two neutron-producing fusion reactions of most interest in the development of thermonuclear fusion power are
When these reactions are produced by accelerating one nuclide toward the other, the velocity of the center of mass must first be added to the center-of-mass neutron velocity before determining the neutron energy in the laboratory coordinate system. In most designs for fusion power, the velocity of the center of mass is negligible, and the concern is with monoenergetic 2.45- or 14.1-MeV fusion neutrons. The 14.1-MeV fusion neutrons are also produced copiously in a thermonuclear explosion.
A beam of relatively low-energy deuterons (100–300 keV) incident on a deuterium or tritium target can produce a significant number of thermonuclear neutrons. Thus, these D–D or D–T reactions are used in relatively compact accelerators, called neutron generators, in which deuterium ions are accelerated through a high voltage (100–300 kV) and allowed to fall on a thick deuterium- or tritium-bearing target. Typically, in such devices, a 1-mA beam current produces up to 109 14-MeV neutrons per second from a thick tritium target.
Photoneutrons
A gamma photon with energy sufficiently large to overcome the neutron binding energy (about 7 MeV in most nuclides) may cause a (γ,n) reaction. Very intense and energetic photoneutron production can be realized in an electron accelerator where the bombardment of an appropriate target material with the energetic electrons produces intense bremsstrahlung (see “Sources of X-rays”) with a distribution of energies up to that of the incident electrons. The probability a photon will cause a (γ,n) reaction increases with the photon energy, reaching a maximum over a broad energy range of approximately 20–23 MeV for light nuclei (A≲40) and 13–18 MeV for medium and heavy nuclei. The peak energy of this broad (often called giant) nuclear resonance can be approximated by 80 A −1/3 MeV for A > 40. The width of the resonance varies from about 10 MeV for light nuclei to 3 MeV for heavy nuclei. Consequently, in medical or accelerator facilities that produce photons with energies above about 15 MeV, neutron production in the surrounding walls can lead to a significant neutron field.
However, the gamma photons produced in radioactive decay of fission and activation products in nuclear reactors generally have energies too low, and most materials have a photoneutron threshold too high for photoneutrons to be of concern. Only for the light elements 2H, 6Li, 7Li, 9Be, and 12C are the thresholds for photoneutron production sufficiently low that these secondary neutrons may have to be considered. In heavy-water- or beryllium-moderated reactors, the photoneutron source may be very appreciable, and the neutron field deep within an hydrogenous shield is often determined by photoneutron production in deuterium, which constitutes about 0.015 atom percent of the hydrogen. Capture gamma photons arising from neutron absorption have particularly high energies, and thus may also cause a significant production of energetic photoneutrons.
The photoneutron mechanism can be used to create laboratory neutron sources by mixing intimately a beryllium or deuterium compound with a radioisotope that decays with the emission of high-energy photons. Alternatively, the encapsulated radioisotope may be surrounded by a beryllium- or deuterium-bearing shell. A common reactor photoneutron source is an antimony–beryllium mixture, which has the advantage of being rejuvenated by exposing the source to the neutrons in the reactor to transmute the stable 123Sb into the required 124Sb isotope (half-life of 60.2 days).
One very attractive feature of such (γ,n) sources is the nearly monoenergetic nature of the neutrons if the photons are monoenergetic. However, in large sources, the neutrons may undergo significant scattering in the source material and thereby degrade the nearly monoenergetic nature of their spectrum. These photoneutron sources generally require careful use because of their inherently large photon emission rates. Because nominally only one in a million high-energy photons actually interacts with the source material to produce a neutron, these sources generate gamma rays that are of far greater biological concern than are the neutrons.
Alpha-Neutron Sources
Many compact laboratory neutron sources use energetic alpha particles from various radioisotopes (emitters) to induce (α,n) reactions in appropriate materials (converters). Although a large number of nuclides emit neutrons if bombarded with alpha particles of sufficient energy, the energies of the alpha particles from radioisotopes are capable of penetrating the potential barriers of only the lighter nuclei.
Of particular interest are those light isotopes for which the (α,n) reaction is exoergic (Q > 0) or, at least, has a low threshold energy. For endoergic reactions (Q > 0), the threshold alpha energy is − Q (1 + 4/A). Thus, for an (α,n) reaction to occur, the alpha particle must (1) have enough energy to overcome the repulsive Coulombic force field of the nucleus, and (2) exceed the threshold energy for the reaction. Converter materials used to make practical (α,n) sources include lithium, beryllium, boron, carbon, fluorine, and sodium.
The converter nuclides 18O and 19F are responsible for neutron production in many areas of the nuclear fuel cycle. Alpha particles emitted by uranium and plutonium range between 4 and 6 MeV in energy and can cause (α,n) neutron production when in the presence of oxygen or fluorine. In particular, (α,n) neutrons often dominate the spontaneous fission neutrons in UF6 or in aqueous mixtures of uranium and plutonium such as found in nuclear waste.
A neutron source can be fabricated by mixing intimately a light converter element, such as lithium or beryllium, with a radioisotope that emits energetic alpha particles. Most of the practical alpha emitters are actinide elements, which form intermetallic compounds with beryllium. Such a compound, for example, PuBe13, ensures both that the emitted alpha particles immediately encounter converter nuclei, thereby producing a maximum neutron yield, and that the radioactive actinides are bound into the source material, thereby reducing the risk of leakage of the alpha-emitting component.
The neutron yield from an (α,n) source varies strongly with the converter material, the energy of the alpha particle, and the relative concentrations of the emitter and converter elements. The degree of mixing between converter and emitter and the size, geometry, and source encapsulation may also affect the neutron yield. For example, a 239Pu/Be source has an optimum neutron yield of about 60 neutrons per 106 primary alpha particles.
The energy distributions of neutrons emitted from (α,n) sources are continuous below some maximum neutron energy with definite structure at well-defined energies determined by the energy levels of the converter and the excited product nuclei. The use of the same converter material with different alpha emitters produces similar neutron spectra with different portions of the same basic spectrum accentuated or reduced as a result of the different alpha-particle energies. Average energies of neutrons typically are several MeV. For example, the neutrons produced by a 239Pu/Be source have an average energy of 4.6 MeV.
Activation Neutrons
A few highly unstable nuclides decay by the emission of a neutron. The delayed neutrons associated with fission arise from such decay of the fission products. However, there are nuclides other than those in the fission-product decay chain which also decay by neutron emission. Only one of these nuclides, 17N, is of importance in nuclear reactor situations. This isotope is produced in water-moderated reactors by an (n,p) reaction with 17O (threshold energy, 8 MeV). The decay of 17N by beta emission (half-life 4.4 s) produces 17O in a highly excited state, which in turn decays rapidly by neutron emission. Most of the decay neutrons are emitted within ±0.2 MeV of the most probable energy of about 1 MeV, although neutrons with energies up to 2 MeV may be produced.
Spallation Neutron Sources
In a spallation neutron source, pulses of very energetic protons (up to 1 GeV), produced by an accelerator, strike a heavy metal target such as mercury or liquid bismuth. Such an energetic proton when it strikes a target nucleus “spalls” or knocks out neutrons. Additional neutrons boil off as the struck nucleus heats up. Typically, 20–30 neutrons are produced per spallation reaction. These pulses of neutrons are then slowed down or thermalized by passing them through cells filled with water, or even liquid hydrogen if very slow neutrons are needed.
Sources of Gamma Photons
Radioactive Decay
Radioactive sources serve a wide variety of purposes in educational, medical, research, industrial, governmental, and commercial activities. The radionuclides in these sources almost always leave their decay daughters in excited nuclear states whose subsequent transitions to lower-energy states usually result in the emission of one or more gamma photons.
Prompt Fission Photons
The fission process produces copious gamma photons either within the first 6 × 10−8 s after the fission event (the prompt fission gamma photons) or from the subsequent decay of the fission products. These photons are of extreme importance in the shielding and gamma-heating calculations for a nuclear reactor. Consequently, much effort has been directed toward determining their nature.
Most investigations of prompt fission gamma photons have centered on the thermal-neutron-induced fission of 235U. For this nuclide, it has been found that the number of prompt fission photons is 8.13 ± 0.35 photons per fission over the energy range 0.1–10.5 MeV, and the energy carried by this number of photons is 7.25 ± 0.26 MeV per fission. The energy spectrum of prompt gamma photons from the thermal fission of 235U between 0.1 and 0.6 MeV is approximately constant at 6.6 photons MeV−1 fission−1. At higher energies, the spectrum falls off sharply with increasing energy. The measured energy distribution of the prompt fission photons can be represented by the following empirical fit over the range 0.1–10.5 MeV:
where E is in MeV and N(E) is in units of photons MeV−1 fission−1.
Investigation of 233U, 239Pu, and 252Cf indicates that the prompt fission photon energy spectra for these isotopes resembles very closely that for 235U, and hence for most purposes, it is reasonable to use the 235U spectrum for other fissioning isotopes.
Fission-Product Photons
With the widespread application of nuclear fission, an important concern is the consideration of the very long lasting gamma activity produced by the decay of fission products.
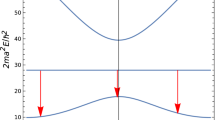
In the fission process, most often two fragments are produced (binary fission) with a distribution in mass shown in Fig. 13.2. About 0.3% of the time, a third light fragment is produced (ternary fission), most often 3H. As seen in Fig. 13.2, the mass distribution or fission-product chain yield is bimodal, with many products having atomic mass number around 95 and around 140. Among the former are the important long-lived radionuclide 90Sr, several isotopes of the halogen bromine, and various isotopes of the noble gas krypton. Among the heavy fragments are the important long-lived radionuclide 137Cs, radioisotopes of halogen iodine, notably 131I, and isotopes of the noble gas xenon. The fission-products are neutron-rich and decay almost exclusively by β − emission, often forming long decay chains. From the range of mass numbers produced (see Fig. 13.2), about 100 different decay chains are formed. An example of a short chain is
The total gamma-ray energy released by the fission product chains is comparable to that released as prompt fission gamma photons. The gamma-ray energy release rate declines rapidly in the time after fission. About three-fourths of the delayed gamma-ray energy is released in the first 1,000 s after fission. In most calculations involving spent nuclear fuel, the gamma activity at several months or even years after removal of fuel from the nuclear reactor is of interest and only the long-lived fission products need be considered.
It has been found that the gamma energy released from fission products is relatively independent of the energy of the neutrons causing the fissions. However, the gamma-ray energy released and the photon energy spectrum depend significantly on the fissioning isotope, particularly in the first 10 s after fission. Generally, fissioning isotopes having a greater proportion of neutrons to protons produce fission-product chains of longer average length, with isotopes richer in neutrons and hence with greater available decay energy. Also, the photon energy spectrum generally becomes less energetic as the time after fission increases.
For very approximate calculations, the energy spectrum of delayed gamma photons from the fission of 235U, at times up to about 500 s, may be approximated by the proportionality N(E) ∼e −1.1E, where N(E) is the delayed gamma yield (photons MeV−1 fission−1) and E is the photon energy in MeV. The time dependence for the total gamma photon energy emission rate F(t) (MeV s−1 fission−1) is often described by the simple decay formula F(t) = 1.4 t −1.2, 10 s < t < 107 s, where t is in seconds. More complicated (and accurate) expressions for F(t) have been obtained from fits to experimental data; but for preliminary calculations the simpler result is usually adequate. It is observed that both 235U and 239Pu have roughly the same total gamma-ray-energy decay characteristics for up to 200 days after fission, at which time 235U products begin to decay more rapidly until at 1 year after fission, the 239Pu gamma activity is about 60% greater than that of 235U.
For accurate calculations involving fission products, the variation with time after fission of the energy spectra of the photons must be taken into account. Often the energy spectra are averaged over discrete energy intervals and the energy emission rate in each energy group is considered as a function of time after fission. Computer codes, based on extensive libraries of radionuclide data, have been developed to compute the abundances and decay rates of the hundreds of fission-product radionuclides. An example of such calculations is shown in Fig. 13.3.
Capture Gamma Photons
The compound nucleus formed by neutron absorption is initially created in a highly excited state with excitation energy equal to the kinetic energy of the incident neutron plus the neutron binding energy, which averages about 7 MeV. The decay of this nucleus, usually within 10−12 s, and usually by way of intermediate states, typically produces several energetic photons. Generally, the probability a neutron causes an (n,γ) reaction is greatest for slow-moving thermal neutrons, that is, neutrons whose speed is in equilibrium with the thermal motion of the atoms in a medium. At high energies, it is more likely that a neutron scatters, thereby losing some of its kinetic energy, and then slows toward thermal energies.
Capture photons may be created intentionally by placing a material with a high thermal-neutron (n,γ) cross section in a thermal neutron beam. The energy spectrum of the resulting capture gamma photons can then be used to identify trace elements in the sample. More often, however, capture gamma photons are an undesired secondary source of radiation.
Inelastic Scattering Photons
The excited nucleus formed when a neutron is inelastically scattered decays to the ground state within about 10−14 s, with the excitation energy being released via one or more photons. Because of the constraints imposed by the conservation of energy and momentum in all scattering interactions, inelastic neutron scattering cannot occur unless the incident neutron energy is greater than (A + 1)/A times the energy required to excite the scattering nucleus to its first excited state. Except for the heavy nuclides, neutron energies above about 0.5 MeV are typically required for inelastic scattering.
The detailed calculation of secondary photon source strengths from inelastic neutron scattering requires knowledge of the fast-neutron fluence, the inelastic scattering cross sections, and spectra of resultant photons, all as functions of the incident neutron energy. The cross sections and energy spectra of the secondary photons depend strongly on the incident neutron energy and the particular nuclide. Such inelastic scattering data are known only for the more important structural and shielding materials, and even the known data require extensive data libraries. Fortunately, in most situations, these secondary photons are of little importance compared to the capture photons. Although inelastic neutron scattering is usually neglected with regard to its secondary-photon radiation, it is a very important mechanism in the attenuation of fast neutrons, better even than elastic scattering in some cases.
Activation Photons
For many materials, absorption of a neutron produces a radionuclide with a half-life ranging from a fraction of a second to many years. The radiation produced by the subsequent decay of these activation nuclei may be very significant for materials that have been exposed to large neutron fluences, especially structural components in a reactor or accelerator. Many radionuclides encountered in research laboratories, medical facilities, and industry are produced as activation nuclides from neutron absorption in some parent material (see Table 13.4). Such nuclides decay, usually by beta emission, leaving the daughter nucleus in an excited state, which usually decays quickly to its ground state with the emission of one or more gamma photons. Thus, the apparent half-life of the photon emitter is that of the parent (or activation nuclide), while the number and energy of the photons are characteristic of the nuclear structure of the decay daughter.
Although most activation products of concern in shielding problems arise from neutron absorption, there is one important exception in water-moderated nuclear reactors. The 16O in the water can be transmuted to 16 N in the presence of fission neutrons by an (n,p) reaction with a threshold energy of 9.6 MeV. 16N decays with a 7.4-s half-life emitting gamma photons of 6.13 and 7.10 MeV (yields of 0.69 and 0.05 per decay). This gamma-ray source is very important in coolant channels of power reactors.
Positron Annihilation Photons
Positrons, generated either from the positron decay of radionuclides or from pair production interactions induced by high-energy photons, slow down in matter within about 10−10 s and are subsequently annihilated with electrons. With rare exception, the rest-mass energy of the electron and positron is emitted in the form of two annihilation photons, each of energy m e c 2 (= 0.511 MeV).
Sources of X-rays
The interaction of photons or charged particles with matter leads inevitably to the production of secondary x-ray photons. The x-rays in many applications have energies ≲100 keV, and hence are easily attenuated by any shield adequate for the primary radiation. Consequently, the secondary x-rays are often completely neglected in analyses involving higher-energy photons. There are many cases, though, when the energies of x-rays and Auger electrons must be accounted for as well as those of the x-rays. An example is the evaluation of radiation dose to the total body, or an organ of the body, after a radionuclide intake. This is a situation in which the source and receiver volumes may be the same.
There are important situations in which x-ray production is the only source of photons. To estimate the intensity, energies, and doses from the x-ray photons, it is necessary to understand how the x-rays are produced and some characteristics of the production mechanisms. There are two principal methods whereby secondary x-ray photons are generated: the rearrangement of atomic electron configurations leads to characteristic x-rays, and the deflection of charged particles in the nuclear electric field results in bremsstrahlung.
Characteristic X-rays and Fluorescence
The electrons around a nucleus are arranged in shells or layers, each of which can hold a maximum number of electrons. The two electrons in the innermost shell (K shell) are the most tightly bound, the six electrons in the next shell (L shell) are the next most tightly bound, and so on outward for the M, N,… shells. If the normal electron arrangement around a nucleus is altered, say by ejection of an inner electron, the electrons begin a complex series of transitions to vacancies in the inner shells (thereby acquiring higher binding energies) until the unexcited state of the atom is achieved. In each electronic transition, the difference in binding energy between the final and initial states is either emitted as a photon, called a characteristic x-ray, or given up to another electron which is ejected from the atom, called an Auger electron. The discrete electron energy levels and the transition probabilities between levels vary with the Z number of the atom, and thus the characteristic x-rays provide a unique signature for each element.
The number of x-rays with different energies is greatly increased by the multiplicity of electron energy levels available in each shell (1, 3, 5, 7, … distinct energy levels for the K, L, M, N, … shells, respectively). To identify the various characteristic x-rays for an element, many different schemes have been proposed. One of the more popular uses the letter of the shell whose vacancy is filled together with a numbered Greek subscript to identify a particular electron transition (e.g., K α1 and L γ5).
Production of Characteristic X-Rays
There are several methods whereby atoms may be excited and characteristic x-rays produced. A photoelectric absorption leaves the absorbing atom in an ionized state. If the incident photon energy is sufficiently greater than the binding energy of the K-shell electron, which ranges from 14 eV for hydrogen to 115 keV for uranium, it is most likely (80–100%) that a vacancy is created in the K shell and thus that the K series of x-rays dominates the subsequent secondary radiation. These x-ray photons produced from photoelectric absorption are often called fluorescent radiation and are widely used to identify trace elements in a sample by bombarding the sample with low-energy photons from a radioactive source or with x-rays from an x-ray machine and then observing the induced fluorescent radiation.
Characteristic x-rays can also arise following the decay of a radionuclide. In the decay process known as electron capture, an orbital electron, most likely from the K shell, is absorbed into the nucleus, thereby decreasing the nuclear charge by one unit. The resulting K-shell vacancy then gives rise to the K series of characteristic x-rays. A second source of characteristic x-rays which occurs in many radionuclides is a result of internal conversion. Most daughter nuclei formed as a result of any type of nuclear decay are left in excited states. This excitation energy may be either emitted as a gamma photon or transferred to an orbital electron which is ejected from the atom. Again, it is most likely that a K-shell electron is involved in this internal conversion process.
X-Ray Energies
To generate a particular series of characteristic x-rays, an electron vacancy must be created in an appropriate electron shell. Such vacancies are created only when sufficient energy is transferred to an electron in that shell so as to allow it to break free of the atom or at least be transferred to an energy level above all the other electrons. The characteristic x-rays emitted when electrons fill a vacancy in a shell always have less energy than that required to create the vacancy. The most energetic x-rays arise from an electron filling a K-shell vacancy and, since the binding energy of K-shell electrons increases with the atomic number Z, the most energetic x-rays are K-shell x-rays from heavy atoms. For example, the K α x-ray energy varies from only 0.52 keV for oxygen (Z = 8) to 6.4 keV for iron (Z = 26) to 98 keV for uranium (Z = 92). By comparison, the L series of x-rays for uranium occurs at energies around 15 keV. Thus, in most shielding situations, only the K series of x-rays from heavy elements are sufficiently penetrating to be of concern.
X-Ray Yields
The fluorescent yield of a material is the fraction of the atoms with a vacancy in an inner electron shell that emit an x-ray upon the filling of the vacancy. The fluorescent yield increases dramatically with the Z number of the atom. For example, the fluorescent yield for vacancies in the K shell increases from 0.0069 for oxygen (Z = 8) to 0.97 for uranium (Z = 92). Thus, the secondary fluorescent radiation is of more concern for heavy materials.
Bremsstrahlung
A charged particle gives up its kinetic energy either by collisions with electrons along its path or by photon emission as it is deflected, and hence accelerated, by the electric fields of nuclei. The photons produced by the deflection of the charged particle are called bremsstrahlung (literally, “braking radiation”). For a given type of charged particle, the ratio of the rate at which the particle loses energy by bremsstrahlung to that by ionizing and exciting the surrounding medium is
where E is in MeV, m e is the electron mass, and M is the mass of the charged particle. From this result, it is seen that bremsstrahlung is more important for high-energy particles of small mass incident on high-Z material. In shielding situations, only electrons (m e /M = 1) are ever of importance for their associated bremsstrahlung. All other charged particles are far too massive to produce significant amounts of bremsstrahlung. Bremsstrahlung from electrons, however, is of particular radiological interest for devices that accelerate electrons, such as betatrons and x-ray tubes, or for situations involving radionuclides that emit only beta particles.
Energy Distribution of Bremsstrahlung
The energy distribution of the photons produced by the bremsstrahlung mechanism is continuous up to a maximum energy corresponding to the maximum kinetic energy of the incident charged particles. The exact shape of the continuous bremsstrahlung spectrum depends on many factors, including the energy distribution of the incident charged particles, the thickness of the target, and the amount of bremsstrahlung absorbed in the target and other masking material.
For monoenergetic electrons of energy E o incident on a target thick compared to the electron range, the number of bremsstrahlung photons of energy E, per unit energy and per incident electron, emitted as the electron is completely slowed down can be approximated by the Kramer distribution
where \( k \simeq 0.0007\;{\text{Me}}{{\text{V}}^{ - 1}} \) is a normalization constant. The fraction of the incident electron’s kinetic energy that is subsequently emitted as bremsstrahlung can then be calculated from this approximation as kZE o, which is usually a small fraction. For example, about 10% of the energy of a 2-MeV electron, when stopped in lead, is converted into bremsstrahlung.
Angular Distribution of Bremsstrahlung
The angular distribution of bremsstrahlung is generally quite anisotropic and varies with the incident electron energy. Bremsstrahlung induced by low-energy electrons \( (\lesssim100\;{\text{keV}}) \) is emitted over a relatively broad range of directions around the direction of the incident electron. As the electron energy increases, the direction of the peak intensity shifts increasingly toward the forward direction until, for electrons above a few MeV, the bremsstrahlung is confined to a very narrow forward beam. The angular distribution of radiation leaving a target is very difficult to compute since it depends on the target size and orientation. For thin targets, the anisotropy of the bremsstrahlung resembles that for a single electron–nucleus interaction, while for thick targets multiple electron interactions and photon absorption in the target must be considered.
X-ray Machines
The production of x-ray photons as bremsstrahlung and fluorescence occurs in any device that produces high-energy electrons. Devices that can produce significant quantities of x-rays are those in which a high voltage is used to accelerate electrons, which then strike an appropriate target material. Such is the basic principle of all x-ray tubes used in medical diagnosis and therapy, industrial applications, and research laboratories.
Although there are many different designs of x-ray sources for different applications, most designs for low-to-medium voltage sources \( (\lesssim 180\;{\text{kV}}) \) place the electron source (cathode) and electron target (anode) in a sealed glass tube. The glass tube acts as both an insulator between the anode and cathode and a chamber for the necessary vacuum through which the electrons are accelerated. The anodes of x-ray tubes incorporate a suitable metal upon which the electrons impinge and generate the bremsstrahlung and characteristic x-rays. Most of the electron energy is deposited in the anode as heat rather than being radiated away as x-rays, and thus heat removal is an important aspect in the design of x-ray tubes. Tungsten is the most commonly used target material because of its high atomic number and because of its high melting point, high thermal conductivity, and low vapor pressure. Occasionally, other target materials are used when different characteristic x-ray energies are desired. For most medical and dental diagnostic units, voltages between 40 and 150 kV are used, while medical therapy units may use 6–150 kV for superficial treatment or 180 kV to 50 MV for treatment requiring very penetrating radiation.
The energy spectrum of x-ray photons emitted from an x-ray tube has a continuous bremsstrahlung component up to the maximum electron energy, that is, the maximum voltage applied to the tube. If the applied voltage is sufficiently high as to cause ionization in the target material, there will also be characteristic x-ray lines superimposed on the continuous bremsstrahlung spectrum. Absorbing filters are used to minimize low-energy x-rays, which are damaging to skin. As the beam filtration increases, the low-energy x-rays are preferentially attenuated and the x-ray spectrum hardens and becomes more penetrating. These phenomena are illustrated in Fig. 13.4. Calculated exposure spectra of x-rays are shown for the same operating voltage but for two different amounts of beam filtration. As the filtration increases, lower energy x-rays are preferentially attenuated; the spectrum hardens and becomes more penetrating. Readily apparent in these spectra are the tungsten K α1 and K α2 characteristic x-rays.
Measured photon spectra from a Machlett Aeromax x-ray tube (tungsten anode) operated at a constant 140 kV potential. This tube has an inherent filter thickness of 2.50-mm aluminum equivalent and produces the spectrum shown by the thick line. The addition of an external 6-mm aluminum filter hardens the spectrum shown by the thin line. Both spectra are normalized to unit area. Data are from [6]
The characteristic x-rays may contribute a substantial fraction of the total x-ray emission. For example, the L-shell radiation from a tungsten target is between 20% and 35% of the total energy emission when voltages between 15 and 50 kV are used. Above and below this voltage range, the L component rapidly decreases in importance. However, even a small degree of filtering of the x-ray beam effectively eliminates the low-energy portion of the spectrum containing the L-shell x-rays. The higher-energy K-series x-rays from a tungsten target contribute a maximum of 12% of the x-ray emission from the target for operating voltages between 100 and 200 kV.
Synchrotron Photons
When a charged particle moving in a straight line is accelerated by deflecting it in an electromagnetic field, the perturbation in the particle’s electric field travels away from the particle at the speed of light and is observed as electromagnetic radiation (photons). Such is the origin of bremsstrahlung produced when fast electrons (beta particles) are deflected by the electric field of a nucleus.
This same mechanism can be used to produce intense photon radiation by deflecting an electron beam by magnetic fields. In a special accelerator called a synchrotron, highly relativistic electrons are forced to move in a circular path inside a storage ring by placing bending magnets along the ring. Photons are emitted when the beam is accelerated transversely by (1) the bending magnets (used to form the circular electron beam), and by (2) insertion device magnets such as undulators, wigglers, and wavelength shifters.
Because the electrons are very relativistic, the synchrotron radiation is emitted in a very narrow cone in the direction of electron travel as they are deflected. Undulators cause the beam to be deflected sinusoidally by a weak oscillatory magnetic field, thereby producing nearly monochromatic photons. By contrast, a wiggler uses a strong oscillatory magnetic field which, because of relativistic effects, produces distorted sinusoidal deflections of the electron beam and synchrotron radiation with multiple harmonics, that is, a line spectrum. If very strong magnetic fields are used, many harmonics are produced that merge to yield a continuous spectrum ranging from the infrared to hard x-rays. By placing undulators or wigglers at a specific location in the storage ring, very intense and narrowly collimated beams of photons with energies up to a few keV can be produced to use, for example, in x-ray diffraction analysis.
Cosmic Rays, Solar Radiation, and Trapped Radiation Belts
The earth is subjected continuously to radiation with sources in our sun and its corona, from sources within our galaxy, and from sources beyond our galaxy. In addition, surrounding the earth are belts of trapped particles with solar origins. Radiation reaching the earth’s atmosphere consists of high-energy electrons and atomic nuclei. Hydrogen nuclei (protons) constitute the major component, with heavier atoms decreasing in importance with increasing atomic number. The highest energy particles originate in our galaxy and more distant galaxies and are referred to as galactic cosmic radiation (GCR). Cascades of nuclear interactions in the atmosphere give rise to many types of secondary particles. Of much lower energy are particles of solar origin, which are highly variable in time and are associated with solar activity. Sources of these particles are sometimes associated with solar flares but are more generally identified with solar particle events (SPE) or coronal mass ejections (CME). The number of solar flare events and SPE emissions fluctuate with the 11-year cycle associated with solar activity. GCR intensity is modulated by SPE emissions, being minimal when solar activity is maximal.
Galactic Cosmic Radiation
At the earth’s surface, cosmic radiation dose rates are largely due to muons and electrons. The intensity and angular distribution of galactic radiation reaching the earth is affected by the earth’s magnetic field and perturbed by magnetic disturbances generated by solar flare activity. Consequently, at any given location, cosmic ray doses may vary in time by a factor of 3. At any given time, cosmic ray dose rates at sea level may vary with geomagnetic latitude by as much as a factor of 8, being greatest at the pole and least at the equator. Cosmic ray dose rates also increase with altitude. At geomagnetic latitude 55°N, for example, the absorbed dose rate in tissue approximately doubles with each 2.75 km (9,000 ft) increase in altitude, up to 10 km (33,000 ft). Fig. 13.5 illustrates the relative importance, in terms of dose rate, for cosmic rays and their reaction products in the atmosphere. Cosmic ray dose rates affecting populations vary strongly with latitude. Table 13.5 describes this variation. The outdoor and indoor average effective dose rates for space radiation in the most heavily populated urban areas in the USA are 45 and 36 nSv/h [7]. Population averaged annual doses are about the same in the northern and southern hemispheres, and globally amount to 31 nSv/h for charged particles plus 5.5 nSv/h for neutrons. GCR energies span a vast range and there is no way of shielding astronauts from GCR effects. On the earth’s surface, the GCR presence results in a source of steady low-dose-rate radiation.
As a result of nuclear reactions of cosmic rays with constituents of the atmosphere, secondary neutrons, protons, and pions, mainly, are produced. Subsequent pion decay results in electrons, photons, and muons. Muon decay, in turn, leads to secondary electrons, as do scattering interactions of charged particles in the atmosphere. Cosmic ray debris that reaches the surface of the earth consists mainly of muons and electrons with a few neutrons. Except for short-term influences of solar activity, galactic cosmic radiation has been constant in intensity for at least several thousand years. The influence of solar activity is cyclical and the principal variation is on an 11-year cycle. The geomagnetic field of the earth is responsible for limiting the number of cosmic rays that can reach the atmosphere thus accounting for the strong effect of latitude on cosmic-ray dose rates.
Solar Particle Events
Both SPE and CME emissions are mainly hydrogen and helium nuclei, that is, protons and alpha particles, predominantly the former. Electrons are thought to be emitted as well, but with energies less than those of protons by a factor equal to the ratio of the rest masses. Energy spectra are highly variable, as are temporal variations of intensity. A typical course of events for a flare is as follows. Gamma and x-ray emission takes place over about 4 h as is evidenced by radio interference. The first significant quantities of protons reach the earth after about 15 h and peak proton intensity occurs at about 40 h after the solar eruption.
Solar particle events are closely related to solar flares associated with sunspots with intense magnetic fields linking the corona to the solar interior. CME emissions are not directly connected to flares, but originate in the corona driven by the energy of the sun’s magnetic field. While of too low energy to contribute to radiation doses at the surface of the earth, these radiations, which fluctuate cyclically with an 11-year period, perturb earth’s magnetic field and thereby modulate galactic cosmic-ray intensities with the same period. Maxima in solar flare activity lead to minimal GCR intensity. SPE and CME emissions, in comparison to galactic cosmic rays, are of little significance as a hazard in aircraft flight or low orbital space travel. On the other hand, these radiations present considerable, life-threatening risk to personnel and equipment in space travel outside the earth’s magnetic field. Protection of astronauts in space missions beyond low-earth orbit is addressed in [8].
Trapped Radiation Belts
Released continuously from the sun, as an extension of the corona, is the solar wind, a plasma of low-energy protons and electrons. The solar wind does not present a radiation hazard, even in interplanetary space travel. However, it does affect the interplanetary magnetic field and the shape of the geomagnetically trapped radiation belts. These radiation belts are thought to be supplied by captured solar-wind particles and by decay into protons and electrons of neutrons created by interactions of galactic cosmic rays in the atmosphere. The trapped radiation can present a significant hazard to personnel and equipment in space missions.
The earth’s geomagnetically trapped radiation belts are also known as Van Allen belts in recognition of James A. Van Allen and his coworkers who discovered their existence in 1958. There are two belts. The inner belt consists of protons and electrons, the protons being responsible for radiation doses in the region. The outer belt consists primarily of electrons. The particles travel in helical trajectories determined by the magnetic field surrounding the planet. They occur at maximum altitude at the equator and approach the earth most closely near the poles. At the equator, the inner belt extends to about 2.8 earth radii. The center of the outer belt is at about 5 earth radii. The solar wind compresses the trapped radiation on the sunny side of the earth and the compression is enhanced by solar flare activity. In the earth’s shadow, the belts are distended as the solar wind sweeps the magnetosphere outward. In a plane through the earth, perpendicular to the earth–sun axis, the proton and electron belts are maximum in intensity at altitudes of about 3,000 and 18,000 km, respectively.
In the southern Atlantic Ocean, there is an eccentricity of the geomagnetic field with respect to the earth’s center, and magnetic field lines dip closer to earth. This region, the South Atlantic Anomaly, is the primary source of radiation exposure to astronaut crew members in low-altitude and low-inclination missions. Radiation protection guidance for low-earth orbit missions is found in [9] and [10].
Radiation Sources Used in Human Activities
Life on earth is continually subjected to radiation of natural origin. Exposure is from sources outside the body, arising from cosmic radiation and radionuclides in the environment, and from sources inside the body, arising from ingested or inhaled radionuclides retained in the body. Natural sources are the major contributors to human radiation exposure and represent a reference against which exposure to man-made sources may be compared. Table 13.6 summarizes radiation doses to man resulting from natural sources. Listed in the table are both doses to individual organs or tissues of the body and the effective dose equivalent, which is a composite dose weighted by the relative radiation sensitivities of many organs and tissues of the body.
Since the early 1980s, there has been negligible change in exposure to naturally occurring radiation, an increase estimated from 3 to 3.1 mSv annually. However, in the USA, medical diagnostic exposures have increased by a factor of 5.5 by 2006, an increase from 0.53 to 3 mSv annually. Of the 3 mSv total, 1.47 mSv is from computed tomography (CT) scans, 0.43 mSv for interventional fluoroscopy, 0.77 mSv for nuclear medicine procedures, and 0.33 mSv for conventional radiography and fluoroscopy (10).
Sources in Medicine
Very shortly after their discoveries at the end of the nineteenth century, radium and x-rays were used for medical purposes – radium sources being concentrated from natural materials and x-rays being generated using new technology. These were the only radiation sources seeing significant use until the 1930s, when research into nuclear fission began and when high-energy particle accelerators were developed for nuclear research. In the first half of the twentieth century, x-rays revolutionized diagnostic medicine. In the second half, accelerator radiation and radionuclides produced by accelerators and nuclear reactors established radiography, radiation therapy, and nuclear medicine, both diagnostic and therapeutic, as mature medical sciences. Table 13.4 lists the radioisotopes commonly used in medicine and industry. Some of these radionuclides are produced in nuclear reactors, either as products of fission or as products of neutron absorption. Nuclei of these isotopes are rich in neutrons and tend to decay by emission of negative beta particles, thereby becoming more positive in charge and more stable. Other isotopes are produced in accelerators. These generally have nuclei deficient in neutrons and tend to decay either by emission of a position or capture of an electron, either process leaving the nucleus more negative and more stable.
There are three broad categories of medical procedures resulting in human radiation exposure: (1) diagnostic x-ray examinations, including mammography and computed tomographic (CT) scans, (2) diagnostic nuclear medicine, and (3) radiation therapy.
Diagnostic X-Rays
Of all the radiation exposures to the general public arising from human activity, the greatest is due to medical procedures, and collective exposures from diagnostic x-rays dominate all other medical exposures. Also, the population subgroup receiving diagnostic x-rays is not small. In the USA, about 250 million medical x rays are delivered annually, as are about 70 million CT scans. About 900 thousand persons receive radiation therapy annually [2, 7].
Diagnostic Nuclear Medicine
Internally administered radionuclides are used medically for imaging studies of various body organs and for non-imaging studies such as thyroid uptake and blood volume measurements. Such uses present hazards for both patients and medical staff. Radiopharmaceuticals are also used for in vitro studies such as radioimmunoassay measurements and thus are of potential hazard to medical staff. Frequencies of procedures, while steadily increasing, vary widely from country to country. As of 2000, in industrialized countries, about 10–40 examinations involving radiopharmaceuticals are carried out annually per 1,000 population. In developing countries, annual frequencies are on the order of 0.2–2 examinations per 1,000 population. In the USA in 2006, for example, some 18 million radionuclide administrations were performed annually for diagnostic purposes [2, 7].
Radiation Therapy
There are three broad categories of radiation therapy– teletherapy, brachytherapy, and therapy using administered radiation sources. Teletherapy involves external beams from sources such as sealed 60Co sources, x-ray machines, and accelerators that generate electron, proton, neutron, or x-ray beams. Brachytherapy involves sources placed within body cavities (intracavitary means) or placed directly within tumor-bearing tissue (interstitial means). In the USA, Europe, and Japan, the frequencies for teletherapy and brachytherapy procedures exceed 2,000 annually per million population.
Thyroid disorders, including cancer, for many years have been treated by 131I, usually by oral administration. Introduced about 1980, in association with the development of techniques for producing monoclonal antibodies, were new cancer diagnosis and treatment methodologies called radioimmunoimaging and radioimmunotherapy. The therapy involves administration of large doses of antibodies tagged with radionuclides and selected to bind with antigens on the surfaces of tumor cells. Imaging involves administration of very much smaller doses, with the goal of detecting the presence of tumor cells using standard camera and scanner imaging techniques. Imaging requires the use of radionuclides such as 99mTc, which emit low-energy gamma rays. Therapy involves the use of radionuclides emitting beta particles and electrons, with minimum emission of gamma rays, thus limiting radiation exposure, to the extent possible, to tumor cells alone. Among radionuclides used in radioimmunotherapy are 75Se, 90Y, 111In, 125I, 186Re, and 191Os.
Occupational Medical Exposure
A world survey conducted by the United Nations for the years 1990–1994 reports the annual average effective dose 1.39 mSv to some 550,000 workers receiving measurable doses (2.3 million total). Of this group, the greatest number were involved in diagnostic radiology (350,000 at 1.34 mSv). Overall, exposures were in the range of 0.9–1.7 mSv annually [2].
Accelerator Sources
The earliest particle accelerators were the x-ray tubes of the late nineteenth century. Indeed, the radio and television (cathode-ray) tubes of the twentieth century are low-voltage electron accelerators. As electrons beams are stopped, x-rays are produced, inadvertently in the case of radio tubes, and deliberately in the case of x-ray generators.
Modern charged-particle accelerators date from the early 1930s, when Cockroft and Walton in England, and Lawrence and Livingston in America developed particle accelerators for research purposes using beams of electrons or ions. Over the years, steady advances have been made in types of accelerators, in the energies of the particles accelerated, and in the magnitude of the current carried by the charged particle beams. Accelerators continue to serve at the frontiers of atomic and nuclear physics as well as the materials sciences. Moreover, accelerators play an ever more important role in diagnostic and therapeutic medicine and in industrial production processes such as radiography, analysis of materials, radiation processing, and radioisotope production.
Particle accelerators may be classified technologically as direct (potential drop) accelerators and indirect (radio-frequency, plasma) accelerators. Among the former are the Van de Graaff and Cockroft-Walton devices. Among the latter are linear accelerators, betatrons, cyclotrons, and synchrotrons. In the linear accelerator, the particles travel in straight lines, accelerated by the electric fields along their paths. In cyclic accelerators, magnets are used to direct particles into approximately circular paths, along which they may pass through the same accelerating electric fields many times along their paths. The ultimate energies reached by the accelerated particles have increased from about 106 eV in the accelerators of the 1930s to 1012 eV in modern research accelerators.
By their very nature and function, accelerators are intense radiation sources. In certain applications, accelerated beams of charged particles are extracted from accelerators and directed onto external receivers. Medical applications and radiation processing see this use of accelerators. In other applications, charged particle beams impinge on internal target receivers designed to act as desired sources of secondary radiations such as x-rays or neutrons. In all cases, beams are stopped by targets within which secondary x-rays, neutrons, and other particles such as mesons may be produced as undesirable but unavoidable by-product radiations. Radiation shielding integral with the accelerator as well as structural radiation shielding surrounding the accelerator are necessary for personnel protection.
The production of secondary radiations arises mainly from three phenomena, direct nuclear reactions of ions or electron with accelerator components, electromagnetic cascades, and hadronic cascades. Among the secondary radiations are neutrons, which in turn may be absorbed in accelerator and structural materials thereby leading to capture gamma rays and radioactive reaction products.
Representative of the direct nuclear reactions are those of relatively low-energy proton or deuteron beams in light-element targets. A popular method of generating energetic neutrons, for example, involves interactions of deuterons accelerated to 150 keV with tritium atoms in a target. The resulting reaction, 3H(d,n)4He, produces an approximately isotropic and monoenergetic source of 14-MeV neutrons. Other such reactions include 3H(p,n)3He, 2H(d,n)3He, and 7Li(p,n)7Be.
The electromagnetic cascade involves exchanges of the kinetic energy of an electron to electromagnetic energy of multiple photons in the bremsstrahlung process, followed by creation of the rest-mass and kinetic energies of an electron–positron pair in the pair-production process experienced by the photons. As the positrons and electrons lose kinetic energy radiatively, more photons are produced, and the cascade continues. The cascade is quenched when photons have insufficient energy to generate the rest masses of the electron–positron pair and when electron radiative energy losses fall below collisional energy losses.
In high-energy electron or proton accelerators, hadronic cascades may be produced when particles collide with atoms in the accelerator target or, inadvertently, with some other accelerator component, giving rise to many reaction products, including pions, kaons, protons, and neutrons. There is also exchange with the electromagnetic cascade via photodisintegration reactions and by production of energetic gamma rays upon decay of π 0 mesons. Propagation of the hadronic cascade occurs through reactions of the secondary protons and neutrons, and is especially important for nucleon energies of 150 MeV or greater. The cascade process produces most of the induced radioactivity at high-energy accelerators. Many reaction-product nuclei are in highly excited nuclear states and relax by emission of neutrons, whose subsequent absorption leads, in many cases, to radioactive by-products.
Water, plastics, and oils in the radiation environs of high-energy accelerators yield 7Be and 11C. Aluminum yields these same radionuclides plus 18F, 22Na, and 24Na. Steel, stainless steel, and copper yield all the aforementioned, plus a very wide range of radionuclides, especially those of V, Cr, Mn, Co, Fe, Ni, Cu, and Zn. Neutron absorption in structural concrete also leads to a wide range of radionuclides, among which 24Na is a major concern. This nuclide has a half-life of 15 h and, in each decay, emits high-energy beta particles and gamma rays.
Industrial Isotope Sources
Radionuclides used in industry contribute very little to collective population doses, although individual occupational exposures may be significant. The largest sources are those used in radiography, typically comprising 10–100 Ci of 192Ir, 137Cs, 170Tm, or 60Co. Borehole logging is accomplished using somewhat lower activity gamma-ray sources and neutron sources such as mixtures of plutonium, americium, or californium with beryllium. Much lower activity sources, often 90Sr − 90Y beta-particle sources, are used for various instrumentation and gaging applications.
There are many consumer products containing radiation sources. While these sources are very weak and no one individual receives significant radiation exposure, many persons are involved. For example, the soil, water supplies, and building materials contain low concentrations of naturally occurring radionuclides. Electronic devices emit very low levels of x-rays, and devices ranging from luminous timepieces to smoke detectors contain weak radiation sources. Even the use of tobacco exposes smokers to alpha particles from naturally occurring 210Po in the tobacco leaf.
Various modern technologies have led to human radiation exposures in excess of those which would have occurred in the absence of the technologies. For example, the mining of coal and other minerals and their use is responsible for increased releases of naturally occurring radionuclides to the environment. World production of coal is about 4 billion tonnes annually. About 70% is used in generation of electricity, the balance mainly in domestic heating and cooking. Coal contains 40K, and the 238U and 232Th decay chains in widely varying concentrations. Depending on the nature of combustion, radionuclides are partitioned between fly ash and bottom ash. The smaller-sized fly ash particles are more heavily enriched with radionuclides, particularly 210Pb and 210Po. The average ash content of coal is about 10% by weight, but may be as high as 40%. Efficiency of ash removal in power plants is quite variable– from only 80% removal to as much as 99% removal, the average being about 97.5%. In domestic use of coal, as much as 50% of the total ash is released into the atmosphere. In terms of doses to individual tissues, the main impact of atmospheric releases during combustion is the dose to bone surface cells accruing from inhalation of 232Th present in the downwind plumes of particulates from plants.
Annually, some 1.4 billion tonnes of phosphate rock are mined and processed for use in production of fertilizers and phosphoric acid. By-product (phospho)gypsum finds wide use in the construction industry. The USA produces about 38% of the phosphate rock, the former Soviet Union 19%, and Morocco 14%. Sedimentary phosphate rock contains high concentrations of radionuclides in the 238U decay chain. Most airborne radioactivity releases are associated with dust produced in strip mining, grinding, and drying of the ore. Utilization of the phosphates leads to both internal and external radiation exposure, the greatest exposure resulting from use of by-product gypsum in construction.
The Nuclear Power Industry
In mid-2008, there were 439 nuclear power plants operating around the world, with a total electrical generating capacity of 372,000 MW. An additional 42 nuclear plants were under construction in 15 countries. Most of the generating capacity consisted of pressurized-water and boiling-water reactors, which use ordinary water as coolant. Gas-cooled reactors, heavy-water reactors, light-water graphite reactors, and sodium-cooled reactors provided the balance of the capacity.
Nuclear Power Reactors
The fission process and production of neutrons associated with reactor operation lead to a wide array of radioactive fission products and activation products arising from neutron absorption. Moreover, large quantities of uranium and plutonium are fissioned in modern nuclear power plants (typically 3 kg/day) to produce the thermal energy needed to produce electricity. Consequently, large quantities of fission products are produced and accumulate in the fuel. Also contained within the fuel are actinides produced by cumulative neutron absorption in uranium, thorium, and plutonium fuels. The actinides are characterized by spontaneous fission in competition with alpha-particle decay, and require sequestration to the same degree as the fission products.
One way of categorizing the generated radionuclides is by their physical–chemical behavior, namely, (1) noble gases (2) 3H and 14C, (3) halogens, and (4) particulates. These divisions are based on the relative ease of isolation of the radionuclides from airborne effluents. The noble gases include the many isotopes of the krypton and xenon fission products as well as the activation product 41Ar. These elements cannot be removed from a gas stream by filtration. Halogens include the many isotopes of the bromine and iodine fission products. If they are present in a gas stream, they are likely to be in a chemical form unsuitable for filtration, and effective removal requires adsorption on a material such as activated charcoal. Other radionuclides and the halogens in ionic form may be removed from a gas stream by filtration. In aqueous liquids, the particulates may be isolated by evaporation, filtration, or ion exchange. The halogens, unless in ionic form, cannot be isolated by evaporation or filtration, nor can noble gases. Special cases are 3H in the form of tritiated water and 14C as carbon dioxide. The tritium can be isolated only with very great difficulty, and CO2 removal requires chemical treatment.
There are two sources of radionuclides in reactor coolant, leakage from defective fuel rods and activation products produced by neutron interactions in the coolant itself or with fuel and structure in contact with the coolant. Activation product sources are inevitable, and include a number of radionuclides which may be produced in the coolant. For example, 16N is produced as a result of neutron interactions with oxygen, 41Ar as a result of neutron absorption in naturally occurring argon in the atmosphere and 3H as a result of neutron absorption in deuterium and, especially in pressurized-water reactors, by neutron-induced breakup of 10B. Of course, in a sodium-cooled fast reactor, activation of natural sodium to short-lived 24Na is an important consideration for in-plant radiation protection. Other activation products include isotopes of iron, cobalt, chromium, manganese, and other constituents of structural and special-purpose alloys. The radionuclides are leached into the coolant stream. They then may be adsorbed on surfaces or trapped as particulates in the boundaries of coolant streams within the plant, only later to be resuspended in the coolant. These sources can be minimized by carefully specifying the alloy and trace-element concentrations in plant components.
Uranium Mining and Fuel Fabrication
In the preparation of new fuel for nuclear reactors, the radiation sources encountered are natural sources associated with the uranium and thorium decay chains.
The principal release of radiation sources associated with uranium mining, underground or open pit, is release of natural 222Rn to the atmosphere. Airborne particulates containing natural uranium daughter products also arise from open pit mining and from ore crushing and grinding in the milling process. Mill tailings can also become a long-term source of radiative contamination due to wind and water erosion, leaching, and radon release, the degree depending on the tailing-stabilization program followed. Mining and milling operations are generally conducted in remote areas, and liquid releases containing dissolved uranium daughter products are of little impact on human populations.
The product of milling is U3O8 “yellow cake” ore concentrate. In this phase of the nuclear fuel cycle, the concentrate is purified and most often converted to UF6 for enrichment in 235U via gaseous diffusion or centrifuge processes. Prior to fuel fabrication, the uranium is converted to the metallic or the ceramic UO2 form suitable for use in fuel elements. Large quantities of uranium depleted in 235U are by-products of the enrichment process. Under current practice, the depleted uranium is held in storage as being potentially valuable for use in breeder reactors. In this stage of the nuclear fuel cycle, there are relatively minor liquid and gaseous releases of uranium and daughter products to the environment.
Fuel Reprocessing
As nuclear fuel reaches the end of its useful life in power generation, there remain within the fuel recoverable quantities of uranium and plutonium which may be extracted for reuse in the fuel reprocessing stage of the nuclear fuel cycle. Whether or not the fuel is reprocessed is governed by economic and political considerations. Among the former are costs of reprocessing as compared to costs of mining, milling, conversion, and enrichment of new stocks of uranium. Among the latter are concerns over the potential diversion of plutonium to nuclear-weapons use.
In the reprocessing of oxide fuels, the spent fuel is first dissolved in nitric acid. Plutonium and uranium are extracted into a separate organic phase from which they are ultimately recovered and converted into the oxide form. The aqueous phase containing fission and activation products is then neutralized and stored in liquid form pending solidification and ultimate disposal. Because one reprocessing plant may serve scores of power plants, inventories of radionuclides in process may be very great and extraordinary design features and safety procedures are called for. Because of the time delays between removal of fuel from service and reprocessing, concerns are with only relatively long-lived radionuclides, notably 3H, 14C, 85Kr, 90Sr, 106Ru, 129I, 134Cs, and 137Cs.
During the dissolution step of reprocessing, all the 85Kr, the bulk of the 14C (as CO2), and portions of the 3H and 129I appear in a gas phase. This gas is cleaned, dried, and released through a tall stack to the atmosphere. All the 85Kr is thus released; however, the major part of the 3H is removed in the drying process and the bulk of the 129I and 14C is removed by reaction with caustic soda. The 14C may then be precipitated and held as solid waste. Depending on the degree of liquid-effluent cleanup, some of the 129I and other fission products subsequently may be released to the environment.
Waste Storage and Disposal
Wastes generated in the nuclear fuel cycle fall into the broad categories of high-level wastes (HLW) and low-level wastes (LLW). The former, comprising unprocessed spent fuel or liquid residues from fuel reprocessing, accounts for only about 1–5% of the waste volume, but about 99% of the waste activity. The latter is comprised of in-reactor components, filter media, ion-exchange resins, contaminated clothing and tools, and laboratory wastes. For the most part, LLW consists of short-lived beta-particle and gamma-ray emitters. Wastes of low specific activity, but containing long-lived alpha-particle emitters, for example, 239Pu, require special handling more in the nature of that required for HLW.
In the USA, fuel elements from commercial reactors are presently not processed. By the year 2000, the cumulative spent fuel reached about 16,000 m3, amounting to 40,000 t of uranium and fission products. Most of this spent fuel will be stored at the plant sites where it is generated which are primarily in eastern states.
Nuclear Power and Occupational Exposure
Table 13.7 summarizes a United Nations survey of occupational exposures as well as public exposures based on nuclear power operations in the 1990s. Improvements continue to be made and US occupational annual committed occupational doses have by 2007 declined from about 3 to 1.1 person Sv per GWy(e).
Nuclear Explosives
Large fractions of radioactive debris from atmospheric nuclear weapons tests are distributed globally, and the radionuclides remain in the biosphere indefinitely. The hazard is better characterized by the long-term dose commitment than by the dose rate at any instant and location. The fusion and fission energy released in a nuclear-weapon explosion is usually measured in units of megatons (Mt). One megaton refers to the release of 1015 cal of explosive energy– approximately the amount of energy released in the detonation of 106 metric tons of TNT. The quantity of fission products produced in a nuclear explosion is proportional to the weapon fission yield. For a 1-Mt weapon fission yield, there must be the complete fissioning of about 56 kg of uranium or plutonium. The quantities of 3H and 14C, which are produced in the atmosphere by interactions of high-energy fission neutrons, are also proportional to the weapon fusion yield. There are several fusion reactions used in thermonuclear devices, with a 1-Mt weapon fusion yield requiring, for example, the fusion of 7.4 kg of tritium with 4.9 kg of deuterium.
The disposition of weapon debris may be divided into three categories, local fallout, tropospheric fallout, and stratospheric fallout. Local fallout, comprising as much as 50% of the debris and consisting of large particles, is defined as that deposited within 100 miles of the detonation site. Depending on detonation altitude and weather conditions, a portion of the weapon’s debris is injected into the stratosphere and a portion remains in the troposphere. These two atmospheric regions are separated by the tropopause (about 16 km altitude at the equator and 9 km at the poles). Temperature decreases with elevation in the troposphere. This hydrodynamically unstable condition leads to the development of convective weather patterns superimposed upon generally westerly winds. In the stratosphere, temperature is more nearly constant or, in equatorial regions, even rises with elevation. Vertical convective motion is relatively slight and the tropical temperature inversion restricts transfer of material in the stratosphere from hemisphere to hemisphere.
Debris in the troposphere is distributed in longitude but remains within a band of about 30° of latitude. The mean lifetime of radioactive debris in the troposphere is about 30 days and tropospheric fallout is important for radionuclides with half-lives of a few days to several months. Over the years, the bulk of the radioactive debris from weapons tests has been injected into the stratosphere in the northern hemisphere and at altitudes less than 20 km. Mechanisms for transfer of the debris to the troposphere and thence to fallout on the earth’s surface are complex. At elevations less than 20 km, the half-life for transfer of aerosols between hemispheres is about 60 months, while the half-life for transfer to the troposphere is only about 10 months, with little material crossing the tropopause in equatorial regions. Consequently, the bulk of the fallout from any one test occurs over the hemisphere of injection and in temperate regions. In terms of the megatons of fission energy, in the period prior to 1980, 78% of the debris was injected into the stratosphere– 70% into the northern hemisphere and 8% into the southern.
Eight radionuclides contribute significantly to the committed effective dose equivalent to the population. These are 137Cs, 131I, 14C, 239Pu, 90Sr, 106Ru, 144Ce, and 3H. Because of its long half-life, 5,730 years, the commitment from 14C extends over many human generations. The collective effective dose equivalent commitment into the indefinite future due to weapons tests to date is equivalent to about four extra years of exposure of the current world population to natural background radiation.
High-level radioactive wastes generated in the USA in the production of nuclear weapons have accumulated for decades. The wastes are stored at three sites, one in the state of Washington, one in Idaho, and one in South Carolina. The approximately 9,000 t of waste has a volume of 380,000 cubic meters and there are plans to dispose of this waste in a repository used also for disposal of spent fuel for nuclear power plants.
Future Directions
Accelerators
Research, materials processing, and teletherapy accelerators employ higher and higher energy beams and beam currents. The production of secondary radiations associated with beam interactions with targets and structure lead to radiation sources of new types and increased magnitudes. Proton-beam accelerators grow in use for specialized radiation therapy. Synchrotrons deliver beams of protons with energies up to 250 MeV, with increased demands for new and better radiation surveillance, shielding, and dosimetry. Minimization of radiation damage to accelerator components calls for more precise beam simulation in the design process as well as more robust components.
Space Activities
In low-earth orbit, galactic cosmic rays (GCR) properties are well known except for short-term enhancements caused by solar particle events (SPE), especially in polar regions [10]. Consequences may be important in extravehicular activities (EVA) outside the Space Shuttle or International Space Station. There is a need for real-time measurement of instantaneous absorbed dose and effective dose rates as well as cumulative doses. Improved modeling of the space environment is needed so that longer-term predictions may be of conditions in orbit.
For activities beyond low-earth orbit, there is also a need for improved forecasting capabilities [8] for SPE. There is also a need for development and validation of space radiation transport codes, accounting for neutrons, protons, light and heavy ions, mesons, and electromagnetic cascades. Radiation spectrometers are needed for combined measurements of neutron doses and doses from high-energy charged particles. As to biological effects, there is need for more sophisticated risk assessment methodology – at one extreme addressing late somatic and carcinogenic effects – at the other extreme addressing thresholds for symptoms affecting mission requirements, namely, central nervous system effects, dermal and immune issues.
Nuclear Power
Extending the operating lives of nuclear power plants brings on the need for increased attention to maintenance, corrosion control, and surveillance of piping and components. Similarly, extending the in-core operating life of individual fuel assemblies places intense demands on design, manufacturing, and quality assurance. Likewise, greater fuel “burnup” requires increased attention to actinide source inventories and secondary neutron production in spent fuels. Plant operating lives of 60 or more years need support of strong technical and manufacturing infrastructure.
No doubt the future will also see new generations of plants operating with radically advanced designs, with breeding capability, and using mixed 235U/239Pu fuels as well as fuels utilizing the 232Th fuel cycle. These changes will bring on new design and operational challenges as well as the continued support of the physics community in broadening the evaluated nuclear data files (ENDF) and the evaluated nuclear structure data files (ENSDF).
Methods of storage and disposal of spent nuclear fuel continues to involve a complex mixture of technical, economic, political, and emotional issues. Resolution of the political and emotional issues seems to be the more demanding challenge.
Medical Applications
Challenges in nuclear medicine vary from nation to nation, but a recent survey [4] identified a number of universal concerns.
Hybrid imaging, employing dual use of CT, PET, MRI, and SPECT methods, introduces new combinations of radiation sources – positron emitters for PET, x-rays for CT, and gamma ray emitters for SPECT. Accounting for these mixed sources brings new challenges in facility design, treatment planning, patient and staff protection, as well as management of wastes. The same is true for radionuclides used in nuclear-medicine therapy such as delivery of radionuclides to malignant tumor cells using monoclonal antibody and related techniques.
In many countries, there is a need for improvement of domestic medical radionuclide production to alleviate the shortage of accelerator- and nuclear reactor–produced medical radionuclides available for research, diagnosis, and treatment. Finally, improvements in detector technology, image reconstruction algorithms, and advanced data processing techniques are needed to facilitate translation from research laboratory to the clinic.
Industrial and Commercial Activities
Sources of ionizing radiation find use in a very broad array of applications, examples being radiography and tracer techniques in manufacturing and density and moisture gauging in highway construction. Sources find their way into the home via 241Am in smoke detectors and into public buildings via 3H in exit signs. There is a very sad history of injury and death caused by the loss, abandonment, and theft of such sources. Better control of inventory, use, storage, and disposal of such sources is badly needed as well as better oversight by regulatory bodies.
Abbreviations
- Absorbed dose:
-
A general term for the energy transferred from radiation to matter. Specifically, the absorbed dose is the amount of energy absorbed in a unit mass of matter from ionizing radiation. Units are the gray (Gy) and rad, respectively, equivalent to 1 J/kg and 100 ergs/g. Thus, 1 Gy equals 100 rad.
- Activity:
-
The decay rate (expected number of nuclear transformations per unit time) in a radioactive sample. Units are the becquerel (Bq) equal to one decay per second, and the curie (Ci) equal to 3.7 × 1010 decays per second.
- Alpha particle:
-
The nucleus of a 4He atom, composed of two neutrons and two protons and denoted by α.
- Committed dose:
-
The dose equivalent accumulated over the rest of a person’s life following the ingestion or inhalation of radioactive material into the body.
- Coulomb force:
-
The electrostatic force between two charges. It is proportional to the product of the charges and inversely proportional to the square of the distance between them. The force is attractive if the charges are of opposite sign, and repulsive if of like sign.
- Dose equivalent:
-
A measure of the health risk associated with the absorption of radiation locally in the human body. It equals the local absorbed dose multiplied by a quality factor to correct for the relative biological effect associated with different radiations. Units are the sievert (Sv) or rem.
- Effective dose:
-
An overall measure of the risk of cancer or hereditary illness associated with radiation exposure. It is a weighted average dose to multiple organs and tissues of the body, with weighting over both quality factor and the relative sensitivity of each organ or tissue. Units are the sievert (Sv) or rem for the dose in grays and rads, respectively.
- Hadron:
-
A subatomic particle that reacts via strong nuclear forces. Hadrons include mesons (e.g., pions and kaons) and baryons (e.g., protons and neutrons). Hadrons do not include bosons (e.g., photons) and leptons (e.g., electrons, muons, and neutrinos).
- Meson:
-
A subatomic particle, a subclass of hadrons, composed of an even number of other subatomic particles called quarks. Most important are the pi meson (pion) and K meson (kaon).
- Nuclide:
-
A term used to refer to a particular atom or nucleus with a specific neutron number N and atomic (proton) number Z. The nuclide with N neutrons and Z protons and electrons is denoted as \( _Z^A{\text{X}} \) where X is the chemical symbol (determined by Z) and A = Z + N is the mass number. If the nuclide is radioactive, it is called a radionuclide.
- Photon:
-
A quantum of electromagnetic radiation with energy E = hν where h is Planck’s constant and ν is the frequency. Photons produced by changes in the structure of a nucleus are called gamma photons, and those produced by atomic electron rearrangement are called x-rays.
- Positron:
-
The antiparticle of the electron with the same mass m e but with a positive charge equal in magnitude to the negative charge of the electron. A positron, denoted by β +, quickly after its formation annihilates with an ambient electron converting the two electron masses into two photons each with energy m e c 2 = 0.511 MeV.
Bibliography
Primary Literature
UN (1982) Sources and effects of ionizing radiation. Reports of the United Nations Scientific Committee on the effects of atomic radiation. United Nations, New York
UN (2000) Sources and effects of ionizing radiation. Reports of the United Nations Scientific Committee on the effects of atomic radiation. United Nations, New York
Eckerman KF, Ryman JC (1993) External exposure to radionuclides in air, water, and soil. Federal guidance report 12, EPA-402-R-93-081. U.S. Environmental Protection Agency, Washington, DC
National Research Council (2007) Advancing nuclear medicine through innovation. Committee on State of the Science of Nuclear Medicine. National Academy of Sciences, Washington, DC
World Nuclear Association (2006) Radioisotopes in industry (Information paper). www.world-nuclear.org/info/inf56.html
Fewell TR, Shuping RE, Hawkins KR (1981) Handbook of computed tomography and X-ray spectra. Report HHS (FDA) 81-8162
NCRP (2009) Ionizing radiation exposure of the population of the United States. Report no 160. National Council on Radiation Protection and Measurements, Bethesda
NCRP (2006) Information needed to make radiation protection recommendations for space missions beyond low-earth orbit. Report no 153. National Council on Radiation Protection and Measurements, Bethesda
NCRP (2000) Radiation protection guidance for activities in low-earth orbit. Report no 132. National Council on Radiation Protection and Measurements, Bethesda
NCRP (2002) Operational radiation safety program for astronauts in low-earth orbit. Report no 142. National Council on Radiation Protection and Measurements, Bethesda
NCRP (1987) Exposure of the population in the United States and Canada from natural background radiation. Report no 94. National Council on Radiation Protection and Measurements, Bethesda
Books and Reviews
Cohen BL (1986) A national survey of 222Rn in U.S. homes and correlating factors. Health Phys 51:175–183
Cohen BL, Shah RS (1991) Radon levels in United States homes by states and counties. Health Phys 60:243–259
Eisenbud M (1987) Environmental radioactivity, 3rd edn. Academic, Orlando
Faw RE, Shultis JK (1999) Radiological assessment: sources and doses. American Nuclear Society, La Grange Park
Firestone RB, Shirley VS (eds) (1996) Table of isotopes, 8th edn. Wiley-Interscience, Malden
Haffner JW (1967) Radiation and shielding in space. Academic, New York
Glasstone S, Dolan PJ (eds) (1977) The effects of nuclear weapons. United States Departments of Energy and Defense, Washington, DC
ICRP (1987) Radiation dose to patients from radiopharmaceuticals. ICRP publication 53. Annals of the ICRP 18(1–4). International Commission on Radiological Protection, Pergamon Press, Oxford
Kocher DC (1981) Radioactive decay tables. Report DOE/TIC-11026. National Technical Information Service, Springfield
NAS (1971) Radioactivity in the marine environment. Report of the panel on radioactivity in the marine environment. Committee on Oceanography, National Research Council, National Academy of Sciences, Washington, DC
NAS (1988) Health risks of radon and other internally deposited alpha-emitters. Report of the BEIR Committee [The BEIR-IV Report]. National Research Council, National Academy of Sciences, Washington, DC
NAS (1990) Health effects of exposure to low levels of ionizing radiation. Report of the BEIR Committee [The BEIR-V Report]. National Research Council, National Academy of Sciences, Washington, DC
NCRP (1975) Natural background radiation in the United States. Report no 45. National Council on Radiation Protection and Measurements, Washington, DC
NCRP (1977) Radiation protection design guidelines for 0.1–100 MeV particle accelerator facilities. Report no 51. National Council on Radiation Protection and Measurements, Bethesda
NCRP (1984) Exposures from the Uranium Series with Emphasis on Radon and its Daughters. Report no 77. National Council on Radiation Protection and Measurements, Washington, DC
NCRP (1987a) Ionizing radiation exposure of the population of the United States. Report no 93. National Council on Radiation Protection and Measurements, Washington, DC
NCRP (1987b) Radiation exposure of the U.S. population from consumer products and miscellaneous sources. Report no 95. National Council on Radiation Protection and Measurements, Bethesda
NCRP (1989a) Exposure of the U.S. population from diagnostic medical radiation. Report no 100. National Council on Radiation Protection and Measurements, Bethesda
NCRP (1989b) Exposure of the U.S. population from occupational radiation. Report no 101. National Council on Radiation Protection and Measurements, Bethesda
NCRP (1989c) Radiation protection for medical and allied health personnel. Report no 105. National Council on Radiation Protection and Measurements, Bethesda
NCRP (2003) Radiation protection for particle accelerator facilities. Report no 144. National Council on Radiation Protection and Measurements, Bethesda
Shultis JK, Faw RE (2000) Radiation shielding. American Nuclear Society, La Grange Park
Slaback LA Jr, Birky B, Schleien B (1997) Handbook of health physics and radiological health. Lippincott Williams & Wilkins, Hagerstown
UN (1977/1982/1988/1993/2000) Report of the United Nations Scientific Committee on the effects of atomic radiation. United Nations, New York
Weber DA, Eckerman KF, Dillman LT, Ryman JC (1989) MIRD: radionuclide data and decay schemes. Society of Nuclear Medicine, New York
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Faw, R.E., Shultis, J.K. (2013). Radiation Sources. In: Tsoulfanidis, N. (eds) Nuclear Energy. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-5716-9_13
Download citation
DOI: https://doi.org/10.1007/978-1-4614-5716-9_13
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-5715-2
Online ISBN: 978-1-4614-5716-9
eBook Packages: EnergyEnergy (R0)