Abstract
The past few years have seen the development of a suite of extended epidemic models that take into account the “active” nature of individuals and/or population. Many models start from the natural premise that individuals are not “passive” but, on the contrary, receive and process information about potential or ongoing epidemics. Therefore, risk perception and behaviour change play a major role in shaping and changing the outcome of an epidemic. Incorporating such aspects into classical epidemic models poses many challenges. First of all, there are many open questions about how information is generated, its availability locally and globally, its routes of dissemination and diminishing returns of “old” information. All these factors lead to a significantly extended state space with many more variables and parameters compared to standard epidemic models. Thus, apart from issues around measuring and quantifying risk perception and/or behaviour change driven by information, a major modelling challenge revolves around model complexity. More precisely, how to achieve an optimal balance between model accuracy and tractability. In this chapter, starting from a pairwise model that accounts for the concurrent spread of an epidemic and information, modelling complexity and results are discussed by (1) evaluating the effectiveness of various information generating and transmitting mechanisms followed by (2) the deconstruction of the pairwise model to a simpler variant and by (3) discussing concrete modelling alternatives (i.e., pairwise and effective degree models for dynamic networks) and potential future modelling trends in the area of coupled models of human behaviour and disease transmission.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Information Transmission
- Epidemic Model
- Basic Reproduction Number
- Alternative Modelling Approach
- Pairwise Model
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Classical epidemic models operate on the assumption of a “passive” population where neither individuals nor population can absorb and process information arising from a potential or an ongoing epidemic [1, 4]. This is a strong and unrealistic assumption since for most diseases (e.g., sexually transmitted infections (STIs), SARS, pandemic influenza, childhood diseases) the heightened risk of an outbreak or ongoing epidemic leads to a series of measures aimed at preventing or limiting the negative impact of an epidemic. These can be broadly divided into two classes: (1) information generation and dissemination and (2) concrete measures taken by individuals or groups to prevent infection or limit onward spread. While measures of the first type can range from centrally driven population-wide campaigns and dissemination of news via different mass-media to more local interactions in acquaintance circles, measures of the second type include concrete pre-emptive or reactive measures such as vaccination [2], limiting exposure via altering contact patterns [9], seeking early treatment or taking medication (e.g., antiviral drugs) [6].
In this context and probably against intuition, information is not always beneficial. For example, the risk associated with vaccination has been documented to lead to limited uptake to the extent where herd-immunity thresholds have been breached [2]. To capture such counterintuitive effects various game theoretical models have been developed to combine epidemic dynamics with risk perception and model different strategies of vaccine uptake or avoidance [18]. Such models first and foremost are determined by the type of disease, e.g., SIS (susceptible-infected-susceptible) or different versions of it for STIs [17] and SIR (susceptible-infected-recovered) for childhood disease and influenza, and these are then modified to account for information generation and transmission together with modelling the benefits or penalties of having the information and choosing to act on or ignore it. For example, for STIs, Chen [3] formulated an economic/game-theoretic epidemic model to capture the interplay between the quality of information and its availability, the prevalence of the infection and disease dynamics.
A more traditional, population dynamics type approach has been proposed by Funk et al. [7] where information about the disease generates awareness which in turn can lead to discounted infection rates. The model is based on an extended version of the SIR combined with results based on individual-based simulation. As a result of the added complexity imposed by the awareness, the S, I and R classes were further divided to reflect both disease and awareness status. They have found that for the compartmental ODE model the spread of awareness has no effect on the basic reproduction number R 0 but leads to a reduction in the number of infecteds. The consideration of the same model on theoretical network models has revealed that if the disease transmission is not too fast, the generation and transmission of awareness can stop the outbreak, i.e., R 0 < 1.
In this chapter a coupled model of information and disease transmission in the context of STIs [10, 17] is revisited and this model is used to discuss issues around the efficacy of various information generating and transmitting mechanisms and modelling complexity. The results and discussion from the analysis of the model are followed by a deconstruction of the model into its simpler components by first relaxing assumptions about the population contact structure and then about the way in which information is generated and transmitted. This helps to pinpoint and discuss model assumptions along with identifying alternative modelling approaches which account for evolving or adaptive contact networks that link naturally to aspects around the concurrent spread of disease and information.
2 Model
The model presented here is based on that formulated by Hatzopoulos et al. [10] but with some new aspects on interpretation of results and calculation of R 0. This pairwise model captures both disease and information transmission where evolution equations are written down for the expected number of individuals of various types which in turn depend on the expected number of pairs. The dependency of singles on pairs and then of pairs on triples is curtailed by using a closure relation where triples are approximated in terms of singles and pairs [11]. This framework allows us to take the population contact structure explicitly into account and thus produce an accurate description of the problem where, as an added advantage, multiple routes of information transmission (e.g., local and global) can also be accounted for.
Following on from [10, 12], individuals can be divided into one of five different classes that specify the individuals’ status with respect to disease and information. These are susceptible non-responsive (S nr ), susceptible responsive (S r ), infected non-responsive (I nr ), infected responsive (I r ) and in treatment (T). The term responsiveness denotes the willingness to act or respond to information and is key in trying to avoid infection or halting further spread [17]. The important components of the model relate to the generation and transmission of information as well as the benefits of possessing and acting on information. In the model, information or responsiveness is generated in three ways: (1) \(I_{nr} \rightarrow I_{r}\) as a result of symptoms, (2) \(I_{x} \rightarrow T\), where x ∈ { nr, r}, as a result of being diagnosed and moving to the treatment class and (3) \(X_{nr} \rightarrow X_{r}\), where X ∈ { S, I} as a result of global information transmission. While the first two are intuitive, the latter is used to model the effect of mass-media campaigns which act as a single-source of information with its strength and duration often linked to the prevalence of infection in the population. Information transmission is possible in two different ways: (1) local or individual to individual and (2) mean-field. While information dissemination locally captures circles of close friends or acquaintances, the mean-field type transmission accounts for a less clear-cut interaction at the population level, often centrally lead or orchestrated. Many of these mechanisms of information generation and transmission pathways can be easily linked to various ways in which information is disseminated in real life. The model also accounts for the depreciation or decay of responsiveness over time and this is achieved by allowing \(X_{r} \rightarrow X_{nr}\)-type transitions at rate d X , where X ∈ { S, I}. The main benefits of being informed and responding to information amount to reduced susceptibility, reduced infectivity and/or faster recovery if infected. To keep the model as general as possible, all the above factors are accounted for, but their presence or absence will be determined by the precise modelling context and should be used accordingly. The full suite of transitions are given in Table 1.
3 Results
Using the pairwise model (for a sample, see group of equations given in Eq. (1)), the efficacy of different information generating and transmitting mechanisms in slowing or stopping disease spread is investigated. Results are followed by a close scrutiny of model complexity including a model deconstruction and discussion around alternative modelling directions in the area of modelling the concurrent spread of disease and information.
3.1 Pairwise Model: Impact and Efficacy of Different Information Generating and Transmitting Mechanisms
Pairwise ODE models [11] represent an improvement upon standard compartmental models as they allow us to capture the local nature of contacts. They also interpolate with success between simple and full simulation models allowing for some degree of analytical tractability and transparency. Similarly to contact tracing models [5], for information transmission it is important to represent the network of contacts and be able to represent the formation of clusters of responsive individuals that are difficult to capture via simple compartmental models. The resulting pairwise model has 20 equations, 5 for singles and 15 for pairs (see Eq. (1) for a sample). The dimensionality of the system can be further reduced by taking into account that the population is closed and that all pairs add up to \(\langle k\rangle N\), where \(\langle k\rangle\) is the average node degree and N is the population size. The sample equations are:
To integrate the equations numerically, the standard closure proposed in [11] is used. This amounts to approximating all triples in terms of singles and pairs with the general closure relation given by
This approximation closes the system and numerical integration can be performed. Parameter values are based on those used in [10] and, for simplicity, it is assumed that \(\alpha _{s} = \alpha _{i} = \alpha \), \(d_{s} = d_{i} = d\) and \(\delta _{s} = \delta _{i} = \delta \). λs are binary and are used to switch on and off various information transmission routes. The system exhibits three qualitatively different behaviours: (1) neither disease nor responsiveness can spread, (2) only responsiveness spreads and a state of endemic-responsiveness is reached and (3) both responsiveness and infection are endemic.
The system accounts for disease transmission through a static network of contacts with information generated either by individuals in treatment or by those that are infected and likely to self-diagnose. The model accounts for three different routes of responsiveness transmission. The first overlaps completely with the disease transmission route, while the second and third account for mean-field and global transmission of information, respectively. The analysis compares the potential of different sources and pathways of information generation and transmission to reduce prevalence or stop infection. These desirable outcomes can be achieved due to fractions of the population moving to the responsive class. As a result, these informed individuals will experience decreases in their levels of susceptibility and infectivity and a faster recovery if infected.
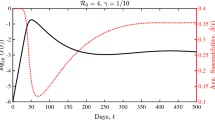
The system is seeded with a small number of individuals of type I nr and S r and then it is numerically integrated to identify the smallest or critical rate that will lead to the desired prevalence level I eq = 0. 01. This is repeated for a range of τ values to determine the relative capacities of α, ω and δ to deliver a state of low infection prevalence. A value of p = 0. 9 was used as this approaches a worse-case scenario limit whereby no information is generated by the individuals themselves through past experience. This setup allows us to examine the effects of α, ω and δ in relative isolation (peer-to-peer transmission at rate α relies on the presence of informed or responsive individuals via self-diagnosis or via treatment). According to Fig. 1a, contact-based transmission of information is by far the most potent pathway to generating a responsive population. Similarly to disease transmission, every receiver of information (I nr or S nr ) immediately becomes a transmitter, in contrast to global transmission of information that acts in isolation and remains singular at all times. The mean-field type transmission of information, not shown in Fig. 1a, is equally effective and produces an outcome that is similar to the contact-based transmission case, especially if the network is densely connected. For smaller values of \(\langle k\rangle\), and as expected, the mean-field transmission performs better than the purely contact-based but with small differences.
(a) Critical information rates resulting in a prevalence of I eq = 0. 01 as a function of the per-contact infection transmission rate τ (computed via the pairwise model). For τ > 0. 42 and in the absence of information, the prevalence equilibrates at I eq > 0. 01. At and beyond this point different amounts of each information rate are needed to lower the prevalence to I eq = 0. 01. In this case, the effect of each transmission route is investigated in the absence of all others. Solid and thick solid line correspond to αc and ωc, respectively. The four dashed lines represent δc for different values of the population inertia parameter \(K = \left [0,5,20,100\right ]\) increasing from right towards the left. The values for αc are denoted on the righty axis and all the others on the lefty axis. (b) The effect of combining different sources of information. On the top left panel the endemic infection prevalence is shown for a range of α and τ values. In the remaining panels, for each combination of α and τ, either global information or self-diagnosis or both are added with the same constant rate equal to 12. Other parameters are p = 0. 9, \(\sigma _{s} = \sigma _{i} = 0.5\), σ r = 2, γ nr = 2, \(\gamma _{r} = \sigma _{r}\gamma _{nr}\), d = γ r , N = 104, and \(\langle k\rangle = 6\), from [10]. © Elsevier Science
The transition to the responsive class due to media exposure is assumed to happen at a rate given by the function
where in this chapter, n = 1 at all times. The efficacy of global information (acting on I nr or S nr ) strongly depends on the value of the K which controls the growth of G( ⋅, ⋅), for low prevalence the function grows like \(\frac{1} {K}{(I_{nr} + I_{r})}^{n}\). The parameter K can be thought of as a measure of population’s willingness in responding to information. Populations that resist behavioural change correlate with high values of K, and if it could be measured or inferred could act as an indicator for the quality of global information campaigns. For example, high values of K will simply translate to observing vanishingly small returns from global information campaigns. The critical rates for self-diagnosis are at best similar to those for global information, especially for diseases with low transmissibility. As is the case for global information, self-diagnosis lags behind the front of infection and will only produce benefits once infected individuals are present. This is made even worse given that ω can only act on I nr .
Information generation depends heavily on the precise type of the disease. The self-diagnosis rate correlates directly with the disease being symptomatic. Diseases with mild symptoms or the slow generation of new sources of information transmission requires an efficient peer-to-peer communication and a population which is responsive and is ready to adapt. Finally, where the population’s behavioural inertia is high, self-diagnosis can be more effective than global information dissemination. This is illustrated by comparing the appropriate curves in Fig. 1a. The figure also shows that as τ increases it is less and less likely that information generation and/or transmission can prevent an epidemic. More precisely at large but finite values of τ, the rates of information generation and/or transmission needed to prevent the spread will tend to unfeasibly large values.
In reality information generation and transmission will not operate singly. Mass-media campaigns increase awareness which can bring forth behavioural change. Infected members of the population are likely to learn from experience and share their knowledge with their acquaintances. The model presented here is able to accommodate these elements as it is shown in Fig. 1b for various combinations of α, δ and ω. As indicated, the α and δ combination is the most effective, while the combination of all three is capable of preventing a significant proportion of epidemics, especially for large τ. Contact-based transmission of information is by far the most efficient as it generates new information transmitters. When epidemics are successfully halted, the responsive and non-responsive susceptible individuals form clusters that can resist infection invasions [10]. Such desirable endemic steady states, with no disease but with informed and/or aware individuals, provide an illustration of optimal dissemination of information that can prevent disease invasion and calculating the basic reproduction number at such an equilibrium can provide valuable insight into how disease, information and contact structure interact and determine the outcome of potential invasions. The basic reproduction number for such a setup, and with taking into account the heterogeneity in individuals’ connectivity, can be written as
where \(W_{x}^{k} = kS_{x}^{k}/\langle k\rangle N\) is the probability that an initial index case chosen uniformly at random reaches an individual of type \(S_{x}^{k}\) with \(x,y \in \{ nr,r\}\) and \(k,l \in \{ k_{min},\ldots,k_{max}\}\) with minimal an maximal nodal degree. \(P(S_{y}^{l}\vert I_{x}^{k})\) incapsulates the neighbourhood composition, e.g., the extent to which non-responsive or responsive individuals are surrounded by responsive individuals. The final component, \(P(I_{x}^{k} \rightarrow S_{y}^{l})\), simply denotes the probability of infection being passed across a link with infectious and susceptible individuals of different types and can be challenging to compute, see [10]. In this individual-based framework, R 0 involves the information generation and transmission components and provides a better representation when compared to simple ODE models and it can be used to explore the optimal arrangement that minimises the likelihood of an outbreak [8, 10].
3.2 Model Deconstruction
The pairwise model above can be deconstructed by relaxing the assumption about the mixing pattern in the population. Furthermore, applying it strictly under the assumption of asymptomatic disease [17] (i.e., ω = 0), the model becomes [12]:
where \(g_{x}(\cdot,\cdot ) = \delta _{x}(i_{nr} + i_{r})/(k + (i_{nr} + i_{r}))\) with x ∈ { s, i}. A standard dynamical system analysis of the model above reveals two steady states ((1, 0, 0, 0, 0) and \((1 - s_{0} = d_{s}/\alpha _{s}),s_{0},0,0,0\)) with two important threshold parameters (\(R_{0}^{r} = \alpha _{s}/d_{s}\) for the responsiveness and \(R_{0}^{i} = \beta _{nr}/\gamma _{nr}\) for the disease) and an analytical relation between the two determining the bifurcation picture of the system. In summary, the trivial disease-free steady state is locally stable if and only if \(R_{0}^{r} < 1\) and \(R_{0}^{i} < 1\) and the non-trivial disease-free steady state is locally stable if and only if \(R_{0}^{r} > 1\) and
This is illustrated in Fig. 2a and highlights that information at the right level can prevent an epidemic. However, it is important to note that in this simplified model, \(R_{0}^{i}\) does not depend on the information, as it was the case in [7]. This means that information cannot halt an epidemic at the onset but it can do so once information generation and transmission is quick started. In an SIR model this amounts to always experiencing a small epidemic whereas for an SIS model, the system can be driven back to full susceptibility and with a proportion of the population “infected” by awareness or responsiveness.
(a) Illustration of the long-term behaviour of the system as a function of \(R_{0}^{r}\) and \(R_{0}^{i}\) for increasing values of \(\alpha _{i} = 0.05\,j\,(j = 0,1,2,3,4)\) and \(d_{i} = 1/(52\,\,\,\text{ weeks})\) with other parameters being \(\gamma _{nr} = 1/(26\,\,\,\text{ weeks})\), \(\gamma _{r} = 1/(13\,\,\,\text{ weeks})\) and β r = γ nr . (b) The parameter space is divided into three regions: the disease-free is the only stable equilibrium (above the transcritical curve), one unstable (disease-free) and a stable endemic equilibria co-exist (below the transcritical curve and outside the Hopf bifurcation island) and, finally, a Hopf island with a stable limit cycle and unstable disease-free and endemic equilibria. Parameter values are as follows: N = 100, \(\langle k\rangle = 10\), \(\alpha _{SS} = 0.004\), α SI = 0. 005, α II = 0, ω SS = 0. 005, ω II = 0 and γ = 1. 0
3.3 Alternative Modelling Approaches: Pairwise Models for Evolving Contact Structures
The principal aim of any pre-emptive or reactive interventions is to reduce the number of those affected by the disease. The reduction in onward spread can be achieved by either limiting or reducing the number of potentially infectious contacts, in network language amounting to cutting links, or keeping the connectivity but reducing the susceptibility and infectivity level as well as the typical time that an infectious individual spends in the population. While this last component cannot be modelled by the alteration of the network of contacts, the former aspects can be modelled by the explicit alteration of the connectivity pattern of the population. Evolving or adaptive networks have already been studied in terms of simple epidemic models where susceptible individuals break links to infected neighbours and reconnect to other susceptibles [9]. This model can be regarded as an implicit model of information generation and transmission where the action of individuals of certain type can lead to curtailing an epidemic. A generalisation of the model proposed by Gross et al. in terms of an SIS-based pairwise models can be written as:
where, α AB and ω AB represent the rate at which AB-type links are created and cut (A, B ∈ { S, I}). This system is closed in the same way as the initial pairwise model (see Eq. (2)) and the system lends itself to a bifurcation type analysis in order to determine full system behaviour [13, 19]. The main outcome is illustrated in Fig. 2b, where the cutting of [SI]-type links can halt the spread of the epidemic. While the precise mechanism of information generation and transmission is not explicit, this model is attractive in that it shows a qualitatively similar behaviour to result from models with explicit information components yet it keeps a good degree of transparency and analytical tractability. Moreover, for most diseases avoiding infection will involve a significant amount of contact reduction or limitation and hence a necessity of capturing evolving contact patterns. Previous models, explicitly including aspects of information transmission, have recognised the importance of also explicitly modelling contact and overlap between the disease and information transmitting contact [8, 10] but have not considered a changing network structure. Future research may shed light on whether the static network approach with information modelled explicitly or dynamic networks with or without explicit contact are more suitable and whether these should be used in combination or singly. As more data becomes available, model fitting techniques may lead to better understanding as to how link cutting and creation rates behave and whether these need to be considered as time dependent functions of prevalence, including delays. Dynamic network models of this type could be also refined to include aspects such as the propensity of informed individuals to seek early treatment and thus limiting further onward spread.
4 Discussion: Impact of Information and Modelling Outlook
In reference to the first pairwise model, Eq. (1), the numerical analysis suggests that contact-based transmission is always more efficient in lowering prevalence when compared to global information dissemination. Preliminary results also highlight that increasing individual specific heterogeneity in σ (while keeping the same mean) leads to lower prevalence as a large number of nodes, with small values of σ, are almost completely immune or unable to transmit infection. A similar observation holds for α, where a high proportion of individuals with weak potential to transmit the information will result in higher prevalence. The discrepancy between the impact of peer-to-peer and population-wide transmission of information on epidemic outcomes has important public health implications as illustrated in the United Kingdom’s early AIDS epidemic, which was concentrated largely among men who have sex with men (MSM). Informal campaigns within the male homosexual community can be dated to early 1983. This was prior to dissemination in the gay press (1983–4) and much earlier then the wider government sponsored campaigns of 1986–7. It is estimated that HIV transmission peaked around 1983 among MSM [16], followed by a rapid decrease which limited the size of the HIV epidemic in the UK. The population-wide campaigns of 1986–7 were however associated with less dramatic changes in STI diagnosis.
On the modelling side further progress and model refinement can be made by looking at ODE-based models that have been developed for approximating epidemic transmission on static and dynamic networks. For example, the pairwise models presented above do not take into account heterogeneity in the connectivity of individuals. This can be circumvented by using heterogenous pairwise models [5] where variables are determined by both disease status and number of links (e.g., [S k ] - susceptible nodes with k contacts and \([S_{k}I_{l}]\) - pairs linking a susceptible with k contacts to and infected with l contacts). Obviously, this will increase the number of equations and including information explicitly may make the model difficult to analyse. In such cases, adaptive network models, where information is included implicitly, may be more desirable. A recently proposed novel approach that comes to meet this demand and also accounts for degree heterogeneity is the so-called effective-degree type model developed by Lindquist et al. [14] and extended further for dynamic networks by Marceau et al. [15] and Taylor et al. [20]. Here, a “smart” choice of variables, with equations formulated in terms of the expected number of S si and I si (susceptibles and infecteds with s susceptibles and i infectious neighbours, respectively), leads to further modelling flexibility and more accurate bookkeeping of nodes and the status of their contacts. However, this approach also relies on a closure and raises further questions about the performance of various approximate models when compared to true simulation. The large spectrum of modelling approaches coupled with the natural tendency to increase model accuracy can easily lead to overly complex models that are not transparent and difficult to analyse and thus we advocate a modelling approach that aims for a good balance between capturing key mechanisms and tractability.
References
Anderson, R.M., May, R.M.: Infectious Diseases of Humans: Dynamics and Control. Oxford University Press, Oxford (1991)
Buonomo, B., D’Onofrio, A., Lacitignola, D.: Math. Biosci. 216, 9–16 (2008)
Chen, F.H.: Math. Biosci. 217, 125–133 (2009)
Diekmann, O., Heesterbeek, J.A.P.: Mathematical Epidemiology of Infectious Diseases: Model Building, Analysis and Interpretation. Wiley, Chichester (2000)
Eames, K.T.D., Keeling, M.J.: Proc. Natl. Acad. Sci. USA 99, 13330–13335 (2002)
Ferguson, N.M., Cummings, D.A.T., Fraser, C., Cajka, J.C., Cooley, P.C., Burke, D.S.: Nature 442, 228–452 (2006)
Funk, S., Gilad, E., Watkins, C., Jansen, V.A.A.: Proc. Natl. Acad. Sci. USA 106, 6872–6877 (2009)
Funk, S., Gilad, E., Jansen, V.A.A.: J. Theor. Biol. 264, 501–509 (2010)
Gross, T., Dommar D’Lima, C.J., Blasius, B.: Phys. Rev. Lett. 96, 208701 (2006)
Hatzopoulos, V., Taylor, M., Simon, P.L., Kiss, I.Z.: Math. Biosci. 231, 197–209 (2011)
Keeling, M.J.: Proc. R. Soc. Lond. B 266, 859–867 (1999)
Kiss, I.Z., Cassell, J., Recker, M., Simon, P.L.: Math. Biosci. 225, 1–10 (2010)
Kiss, I.Z., Berthouze, L., Taylor, T.J., Simon, P.L.: Proc. Roy. Soc. A 468, 1332–1355 (2012)
Lindquist, J., Ma, J., van den Driessche, P., Willeboordse, F.H.J.: Math. Biol. 62, 143–164 (2011)
Marceau, V., Noël, P.-A., Hébert-Dufresne, L., Allard, A., Dubé, L.J.: Phys. Rev. E 82, 036116 (2010)
Nicoll, A., Hughes, G., Donnelly, M., Livingstone, S., De Angelis, D., Fenton, K., Evans, B., Nöel Gill, O., Catchpole, M.: Sex. Transm. Inf. 77, 242–247 (2001)
Oh, M.K., Grimley, D.M., Merchant, J.S., Brown, P.R., Cecil, H.: Hook III, E.W.: J. Adoles. Health 31, 40–47 (2002)
Reluga, T.C., Galvani, A.P.: Math. Biosci. 230, 67–78 (2011)
Szabó, A., Simon P.L., Kiss, I.Z.: Differ. Equ. Appl. 4, 277–296 (2012)
Taylor, M., Taylor, T.J., Kiss, I.Z.: Phys. Rev. E. 85 016103 (2012)
Acknowledgements
I.Z. Kiss acknowledge support from EPSRC (EP/H001085/1).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Kiss, I.Z. (2013). Incorporating Human Behaviour in Epidemic Dynamics: A Modelling Perspective. In: Manfredi, P., D'Onofrio, A. (eds) Modeling the Interplay Between Human Behavior and the Spread of Infectious Diseases. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-5474-8_8
Download citation
DOI: https://doi.org/10.1007/978-1-4614-5474-8_8
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-5473-1
Online ISBN: 978-1-4614-5474-8
eBook Packages: Mathematics and StatisticsMathematics and Statistics (R0)