Abstract
Abiotic stresses such as drought, cold, heat, and salinity are major factors that limit plant growth and development and account for a major loss in crop productivity worldwide. Thus, engineering plants for tolerance towards such environmental menaces is the prime concern for crop improvement programs. Engineering osmoprotectants biosynthetic pathway is considered as one among many successful approaches taken for crop improvement under adverse conditions. Osmoprotectants or compatible solutes are thought to act by stabilizing membranes and proteins and maintaining osmotic potential in the cell during stresses. Although not all crop plants are able to synthesize these special molecules, many stress tolerant plants are shown to accumulate them under stress conditions. This group primarily includes proline, glycine betaines, ectoine, trehalose, and polyols. However, many attempts have been made at engineering plant system with genes from biosynthetic pathways of osmoprotectants; these transgenic plants have shown different tolerance levels, because of many metabolic limitations. Thus, a more elaborate and wholesome approach may be required to look past the current scenario. This chapter will encompass the potential role of osmoprotectants in plant stress adaptation and the possibilities for crop improvement.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The world human population is constantly rising and is expected to reach eight billion by 2025 and 8.9 billion by 2050. Hence, there is an urgent need to double the world food production to feed eight billion people by 2025 (FAO 2008). This is even more challenging to meet such huge demand in the current context of climate variability, particularly extreme temperature and unusual rainfall. It has been estimated that approximately 70 % of yield reduction is direct result of abiotic stresses alone (Acquaah 2007; Lobell and Field 2007).
One approach to increase crop production is to develop stress tolerant crops by transferring gene(s) for the adaptive traits from the tolerant species to the crops. However, through conventional breeding, this process has only been partially successful, partly because of poorly described traits and transfer of unavoidable genes during crossing (Yeo and Flowers 1989). Furthermore, complexity of stress tolerance trait, low genetic variance of yield component under stress and lack of efficient selection techniques make it more difficult to produce such stress resistant germplasms (Ribaut et al. 1996, 1997; Frova et al. 1999).
In contrast to traditional breeding, genetic engineering appears to be an attractive alternative with respect to the possibility of direct introduction of single or multiple genes into crops for betterment (Holmberg and Bülow 1998; Smirnoff 1998). Among various abiotic stresses, drought, salinity, and temperature (low and high) are the major factors that primarily limit plant growth and productivity and the common effect that all these factors impose on plant is osmotic stress.
In response to such stress, certain plants, marine algae, bacteria, and few other organisms synthesize and accumulate various low molecular weight organic compounds known as osmoprotectants or osmolytes or compatible solutes (Johnson et al. 1968; Yancey et al. 1982; Serraj and Sinclair 2002). Though, many crops lack the ability to synthesize some specific osmoprotectants found in stress tolerant organisms, ectopic expression of osmoprotectants is reported to be functional in several crop plants.
These osmoprotectants have been one of the favorite targets for genetic engineering for many years. Many crops are engineered using osmoprotectants like mannitol, glycine betaine, and trehalose, though the level of tolerance exhibited by these engineered crops varies greatly (Sheveleva et al. 1997; Huang et al. 2000). In this chapter, we elaborate the role of these osmoprotectants in stress tolerance including constraints and prospects of their use in metabolic engineering.
2 Osmoprotectants
Osmoprotectants are low molecular weight organic compounds primarily accumulated in response to osmotic stresses in diverse taxa including plants (Yancey et al. 1982). These are highly soluble compounds carrying no net charge at physiological pH and are nontoxic even at high concentrations. These molecules increase the osmotic pressure in the cytoplasm, thereby maintaining driving gradient for both water uptake and turgor pressure. Apart from osmotic adjustment, these compounds are reported to function as scavengers of reactive oxygen species (ROS), having chaperone-like activity and help in metabolic detoxification (Serraj and Sinclair 2002). In addition, osmoprotectants play an essential role in stabilizing proteins and membranes during oxidative damage by stress-induced ROS outburst (Yancey 1994; Bohnert and Jensen 1996).
Chemically they fall into three major groups viz. amino acids (e.g., Proline), quaternary ammonium compounds (e.g., glycine betaine), polyols and sugars (mannitol, d-ononitol, trehalose, fructans) (Yancey 1994). Among these osmoprotectants, proline, glycine betaine, and mannitol are commonly found in plants. In plant cells, osmoprotectants are primarily accumulated in cytosol and chloroplast but are also reported to be distributed in few other organelles.
2.1 Polyols
Polyols such as glycerol, mannitol, and sorbitol are straight chain metabolites and cyclic polyols like inositols, pinitol have been shown to accumulate in evolutionary diverse organisms in response to dehydration, salinity, and osmotic stress.
2.1.1 Mannitol and Sorbitol
Mannitol is a hexitol sugar alcohol and widely distributed in nature including more than 100 species of vascular plants. Mannitol is known to serve as a major carbon source in many organisms (Stoop et al. 1996). The mannitol biosynthetic pathway in higher plants starts with the isomerization of fructose-6-phosphate to mannose-6-phosphate by mannose-6-phosphate isomerase (M6PI, EC 5.3.1.8) which is then converted to mannitol-1-phosphate by mannose-6-phosphate reductase (M6PR, EC 1.1.1.224) (Loescher et al. 1992). In the final step, mannitol-1-phosphate is acted upon by mannose-1-phosphate phosphatase (M1PP, EC 3.1.3.22) to release free mannitol (Fig. 9.1a). In E. coli, mannitol is catabolized by the enzyme mannitol-1-phosphate dehydrogenase (MtlD, EC 1.1.1.17) in a reversible reaction whereas when expressed in transgenic tobacco it functions anabolically and synthesizes mannitol (Tarczynski et al. 1993).
Polyol biosynthetic pathways. [a] Mannitol biosynthesis: HPI hexose phosphate isomerase; MTLD mannitol-1-phosphate dehydrogenase; M1PP mannitol-1-phosphate phosphatase. [b] Sorbitol biosynthesis: S6PDH sorbitol-6-phosphate dehydrogenase; S6PP sorbitol-6-phosphate phosphatase. [c] Myo-Inositol biosynthesis: MIPS myo-inositol-1-phosphate synthase; IMP inositol monophosphatase; IMT inositol methyltransferase; OE ononitol epimerase
Initially Tarczynski et al. (1993) demonstrated transgenic plants engineered for MtlD from E. coli in tobacco and Arabidopsis result in salinity tolerant phenotype. Targeted mannitol biosynthesis in chloroplasts with the help of an amino terminal transit peptide in tobacco resulted in increased tolerance to methyl violagen-induced oxidative stress and a better photosynthetic efficiency in transgenics, which was attributed to their increased ROS scavenging capacity (Shen et al. 1997b). The gene MtlD has also been engineered in economically important plants with substantial results, e.g., Sorghum transgenics overexpressing this gene were found to perform better under salt stress and demonstrated an overall better growth in comparison to control (Maheswari et al. 2010).
Another report of mannitol engineering in potato (Solanum tuberosum L.) revealed enhanced NaCl tolerance in both in vitro and in hydroponic culture, where transgenic plants were shown to retain more fresh weight than wild-type plants during salt stress (Rahnama et al. 2011). In addition to these, a series of experiments demonstrate transgenic eggplants expressing mtlD gene to be tolerant not only towards abiotic stress but biotic stress as well since they demonstrated increased resistance towards three fungal wilts caused by Fusarium oxysporum, Verticillium dahlia and Rhizoctonia solani under both in vitro and in vivo conditions (Prabhavathi et al. 2002; Prabhavathi and Rajam 2007).
Sorbitol is a sugar alcohol accumulated in higher plants especially in Rosaceae (Bieleski 1982). In microorganisms (Zymomonas mobilis), sorbitol biosynthesis requires a one step reaction catalyzed by the enzyme glucose-fructose oxidoreductase (GFOR, EC 1.1.99.28) from glucose and fructose. While in higher plants, NADP-dependent sorbitol-6-phosphate dehydrogenase (S6PDH, EC 1.1.1.200) catalyzes the key step conversion of glucose-6-phosphate to sorbitol-6-phosphate, which is later converted into sorbitol by sorbitol-6-phosphate phosphatase (S6PP, EC 3.1.3.50) (Fig. 9.1b). Many plants use it as a major photosynthetic product which is translocated from mature leaves to growing tissues such as fruits and young leaves (Webb and Burley 1962; Bieleski and Redgwell 1985). Studies show that transgenic tobacco plants over expressing Stpd1 gene coding for S6PDH from apple accumulate higher amounts of sorbitol and were found to be phenotypically altered with necrotic lesions on the leaves. This was explained on the basis of higher concentration of sorbitol interfering with inositol biosynthesis and leading to osmotic imbalance (Sheveleva et al. 1998).
2.1.2 Inositol and Derivatives
Inositols and their derivatives are a functionally important class of compounds required for normal growth of cells. These inositols are cyclohexane hexitols and exist in nine isomeric forms, out of which myo-inositol is the most favored form in nature. The two step inositol biosynthetic pathway is the only de novo pathway for inositol synthesis and an out branch of the central glycolytic pathway. This inositol biosynthetic pathway is highly conserved throughout the biological kingdom where the rate limiting enzyme myo-inositol-1-phosphate synthase (MIPS, EC 5.5.1.4) catalyzes the conversion of glucose-6-phosphate to myo-inositol-1-phosphate and subsequently myo-inositol-1-phosphate is converted to free myo-inositol by the enzyme myo-inositol mono phosphatase (IMP, EC 3.1.3.25) (Fig. 9.1c). Free inositol can be further channelized to other physiologically significant pathways and produce various inositol derivatives (Loewus and Murthy 2000; Stevenson et al. 2000).
These inositols are required for normal growth and development, membrane biogenesis along with the roles of their phosphorylated derivatives as phosphorus store and as a secondary messenger in signal transduction pathways (Loewus and Murthy 2000). In addition to this, inositol and its derivatives such as pinitol, galactinol and other raffinose series oligosaccharides have been found to act as osmoprotectants and provide protection against abiotic stresses like salt and osmotic stress (Taji et al. 2002). Inositol is also utilized by the cell for the synthesis of molecules like stachyose and verbose which are carbohydrate stores for the cells and are stress induced in some species (Bohnert et al. 1995).
The very first plant gene for MIPS was isolated from Spirodela polyrrhiza and was shown to be spatially upregulated during ABA-induced morphogenic responses (Smart and Fleming 1993). The gene was further overexpressed in Arabidopsis and the plants were shown to contain fourfold increase in myo-inositol content (Smart and Flores 1997). Paul and Cockburn (1989) demonstrated that Mesembryanthemum crystallinum (Ice plant) could tolerate upto 400 mM NaCl by accumulating an inositol derivative pinitol which accounts for around two third of the soluble carbohydrate content. The osmotic adjustment of this particular plant under such stress was thus attributed to its high level of pinitol. Further, coordinated induction of myo-inositol-1-phosphate synthase with inositol methyl transferase (IMT1) in ice plant was shown, resulting in tenfold accumulation of free inositol during salt stress condition. However, no such response was observed in Arabidopsis thaliana during similar stresses, which indicates a remarkable difference in the regulation of gene expression between halophytes and glycophytes (Ishitani et al. 1996). Tobacco plants expressing McIMT1 gene accumulated increased amounts of d-ononitol and were shown to be less inhibited in growth and photosynthetic carbon fixation than wild-type plants in salt and drought stress condition (Sheveleva et al. 1997).
A novel salt tolerant MIPS (PINO1) from Porteresia coarctata has been reported and it’s over expression in tobacco plant results in better growth and photosynthetic efficiency than control plants under high salinity stress (Majee et al. 2004). In a follow up study, it was shown that functional over expression of this gene could confer salt tolerance to a wide variety of organisms from bacteria to crop plants (Das Chatterjee et al. 2006). Later on, it has also been shown that co expression of PINO1 and McIMT1 allowed the transgenic tobacco plants to perform better under salt stress in comparison to expression of PINO1 or McIMT1 alone (Patra et al. 2010).
Recently, two divergent genes (CaMIPS1 and CaMIPS2) encoding MIPS have been reported in chickpea and CaMIPS2 has been shown to be highly induced under dehydration stress and provides better stress tolerance to transformed yeast under high salt and temperature stress (Kaur et al. 2008).
2.2 Trehalose
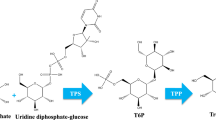
Trehalose is a nonreducing disaccharide (1,1 α-d glucopyranosyl, α-d-glucopyranoside) found in various organisms including bacteria, algae, fungi, yeast, insects, and some plants (Miranda et al. 2007; Elbein et al. 2003). Besides being a carbohydrate reserve, trehalose protects organisms against several physical and chemical stresses (Van Laere 1989; Wiemken 1990; Eleutherio et al. 1993). Trehalose is synthesized in a two step process in bacteria and yeast, first reaction catalyzed by trehalose-6-phosphate synthase (TPS, EC 2.4.1.15) forming trehalose-6-phosphate from UDP-glucose and glucose-6-phosphate; in second reaction trehalose-6-phosphate phosphatase (TPP, EC 3.1.1.12) converts trehalose-6-phosphate to trehalose (Goddijn and Van Dun 1999) (Fig. 9.2). In E. coli, these TPS and TPP enzymes have been shown to be encoded by genes OtsA and OtsB, where as Saccharomyces cerevisiae have evolved a trehalose synthase complex which includes a TPS (Tps1) and a TPP (Tps2) along with a regulatory subunit TSL (Tps3). In Arabidopsis thaliana, a family of TPS genes with 11 members including trehalose-6-phosphate synthase exists with a subfamily of TPPs (Leyman et al. 2001).
Trehalose is having a unique water absorption capacity which protects the macromolecules from desiccation-induced damage (Rontein et al. 2002). During dehydration, trehalose has been thought to replace water molecules and thereby prevent protein denaturation and membrane fusion (Clegg 1985). It has been shown that trehalose along with other compounds like glycine betaine, proline, and mannitol is active in scavenging ROS (both hydrogen peroxide and superoxide anion) in a concentration-dependent manner (Zhu 2001; Luo et al. 2008). A significant amount of trehalose has been found in two resurrection plants Myrothamnus flabellifolia and Sporobolus stapfianus (Phillips et al. 2002) where trehalose is thought to prevent intracellular structural damage due to anhydrobiosis (Lunn 2007).
Trehalose metabolism and its engineering in plants for stress tolerance has been an area of immense interest. But studies in tobacco and potato plants (Holmström et al. 1996; Romero et al. 1997; Goddijn et al. 1997; Goddijn and Van Dun 1999; Paul et al. 2001) with a constitutive over expression of yeast or bacterial TPS and TPP genes have shown undesirable effects like stunted growth and abnormal metabolism. Later on, transgenic rice plants were generated using a fusion construct of coding regions of OtsA and OtsB (with TPS and TPP activity respectively) with either stress inducible (ABA) or tissue specific (rice rbcs) promoter. The phenotypically normal and fertile transgenic rice was achieved with an increased amount of trehalose with increased tolerance to a variety of stresses like salt, drought and low temperature. Transgenic plants also showed increased photosynthetic capacity (Garg et al. 2002). The over expression of trehalose-6 phosphate synthase (AtTps1) using 35S promoter in Arabidopsis led to significant dehydration tolerance without affecting its morphological traits (Avonce et al. 2004). The level of tolerance provided by these transgenic plants did not correlate well with amount of trehalose accumulated, signifying the other roles of trehalose apart from osmoprotection (Iordachescu and Imai 2008).
2.3 Proline
Proline, an imino acid, is one of the most common compatible osmolyte with high water solubility and stable conformation. It is an essential component of cellular and metabolic events and also responsible for osmotic adjustment in cell (Yancey 2005). Apart from plants, the accumulation of proline has been observed in bacteria, protozoa, algae, and marine invertebrates (McCue and Hanson 1990; Delauney and Verma 1993).
In plants, the biosynthesis of proline can occur via glutamate or ornithine pathway. Glutamate is the primary precursor for proline synthesis in osmotically stressed out and nitrogen deficient cells, while at higher levels of available nitrogen, the ornithine pathway is followed (Delauney et al. 1993). Biosynthetic pathway from glutamate to proline involves two important enzymes l-Δ1-pyrroline-5-carboxylate synthetase (P5CS, EC 2.7.2.11) and l-Δ1-pyrroline-5-carboxylate reductase (P5CR, EC 1.5.1.2). First glutamate is converted to glutamic-γ-semialdehyde (GSA) and L-Δ1-pyrroline-5-carboxylate (P5C) by the action of P5CS, and then P5CR catalyzes the conversion of P5C to l-proline (Fig. 9.3). The level of proline in plants is controlled by degradation or metabolism of proline, where ProDH (proline dehydrogenase, EC 1.5.1.12) oxidizes proline to P5C in plant mitochondria and finally P5C dehydrogenase (P5CD, EC 1.5.1.12) converts P5C to l-glutamate (Boggess and Koeppe 1978; Elthon and Stewart 1981). In normal conditions, this oxidation pathway is followed whereas, under salt and water stress such proline degradation pathway is inhibited, as a result proline level increases (Delauney and Verma 1993; Peng et al. 1996).
Increased cellular proline content is reported to stabilize protein structure and protect cellular functions possibly by scavenging ROS under osmotic stress. Proline may also serve as a source of organic nitrogen, carbon, and energy during recovery from stress (Tyagi and Sairam 2004). This molecule is also involved in maintaining osmotic balance in the cell under dehydration conditions (Singh et al. 1972; Wyn Jones and Storeys 1978). During stress, higher proline content helps in maintaining the NADP+/NADPH ratio in the cell (Hare and Cress 1997). In E. coli, proline has been shown to be a potent osmoprotectant as proline over-producing mutant of E. coli was found to possess increased osmotolerance and enhanced stability of proteins and membranes in low water and high temperature conditions (Csonka et al. 1988).
Transgenic plants or mutants raised in several studies demonstrate metabolism and accumulation of proline and its importance for development and survival of plants in various adverse environmental conditions (Hong et al. 2000; Mattioli et al. 2008; Szekely et al. 2008). Over expression of moth bean P5CS in rice, wheat and in carrot cell lines conferred enhanced tolerance to salt stress (Zhu et al. 1998; Sawahel and Hassan 2002; Han and Hwang 2003). Various studies revealed upregulation of P5CS in Oryza sativa and Arabidopsis thaliana exposed to salt, dehydration, and ABA (Yoshiba et al. 1995; Igarashi et al. 1997). Tolerance to freezing and high salinity was established in antisense transgenic Arabidopsis plants carrying AtProDH encoding proline dehydrogenase, resulting in higher proline accumulation (Nanjo et al. 1999).
Studies have shown that P5CS is feedback inhibited by proline (Hu et al. 1992). A correlation between induction of P5CS gene and accumulation of proline has been found in Arabidopsis thaliana under abiotic stress (Savouré et al. 1995), but this feed back regulation of P5CS is relieved in plants under stress conditions, so as to accumulate more proline for combating disturbance in osmotic balance. In a study of transgenic tobacco plants, over expressing wild-type P5CS from Vigna aconitifolia and P5CSF1298 (a mutated P5CS, where feedback inhibition was removed through site directed mutagenesis) were used to compare proline level. Tobacco plant over expressing mutated P5CS accumulated almost twofold more proline than that of transgenic plants expressing wild-type P5CS (Kishor et al. 1995; Verma 1999).
2.4 Polyamines
Polyamines are small organic compounds with two or more primary amino groups, found in all eukaryotic cells. Putrescine (Put, a diamine), spermidine (Spd, a triamine), and spermine (Spm, a tetramine) are the major polyamines found in plants involved in various processes such as cell proliferation, growth, morphogenesis, differentiation, and programmed cell death (Yamaguchi et al. 2007; Alcázar et al. 2010a). In addition, several uncommon polyamines such as homospermidine, 1,3-diaminopropane, cadaverine, and canavalamine have been reported across the kingdoms of life (Minguet et al. 2008). Polyamines occur in free or conjugated forms either with phenolic compounds or macromolecules such as proteins and nucleic acids. Polycationic nature of polyamines at physiological pH is attributed for their biological activity (Gill and Tuteja 2010).
Polyamines play an important role in several plant developmental processes such as cell division, embryogenesis (Bastola and Minocha 1995), fruit ripening (Mehta et al. 1997, 2002), root growth (Watson et al. 1998), tuber development (Kumar et al. 1996; Rafart-Pedros et al. 1999), floral initiation, floral development, and stem elongation (Gerats et al. 1988; Masgrau et al. 1997; Hanzawa et al. 2000; Panicot et al. 2002).
Putrescine, spermidine, spermine, and cadaverine accumulation is well studied under abiotic stress conditions and has been reported in many plant species (Evans and Malmberg 1989; Alcázar et al. 2006, 2010b). Putrescine in plants is either directly synthesized from ornithine by ornithine decarboxylase (ODC, EC 4.1.1.17) or from arginine via N-carbamoylputrescine and agmatine. Arginine conversion requires the enzymes arginine decarboxylase (ADC, EC 4.1.1.19), N-carbamoylputrescine amidohydrolase (CPA, EC 3.5.1.53) and agmatine deiminase (ADI, EC 3.5.3.12) (Urano et al. 2003). Putrescine is further converted into spermidine and consequently to spermine by spermidine or spermine synthases (SPDS, EC 2.5.1.16; SPMS, EC 2.5.1.22) by the addition of an aminopropyl moiety from decarboxylated S-adenosylmethionine generated by S-adenosylmethionine decarboxylase (SAMDC, EC 4.1.1.50) (Fig. 9.4). S-adenosylmethionine is also the precursor of an important source of ethylene, aminocyclopropane carboxylic acid, thus metabolism of polyamine and ethylene is coupled together, which has significance in stress response (Zapata et al. 2004).
The less common polyamine cadaverine is the product of direct decarboxylation of lysine (Bakhanashvili et al. 1985). In Arabidopsis, genes involved in polyamine synthesis were identified as ADC, SAMDC, SPDS, SPMS (Urano et al. 2003), CPA (Piotrowski et al. 2003) and ADI (Janowitz et al. 2003). Beside their possible effects on the osmotic adjustment, polyamines are also involved in stomata closure by regulating voltage-dependent inward K+ channels in the plasma membrane of guard cells (Liu et al. 2000). In addition polyamines are known to be components of the cellular antioxidant system and are usually regarded as scavengers of hydroxyl radicals. Cadaverine via hydroxyl radical-generating system inhibits DNA oxidative degradation in vitro (Kuznetsov et al. 2007). Putrescine, spermidine, and spermine act as hydroxyl radical scavengers in a dose-dependent manner. In addition spermine or spermidine was shown to quench singlet oxygen at higher concentrations (Das and Misra 2004). Transgenic approaches helped to generate plants expressing polyamine biosynthetic enzymes such as ADC, ODC, SAMDC, SPDS, ACC (1-amino cyclopropane-1-carboxylic acid) synthase and ACC oxidase, with enhanced environmental stress tolerance (Gill and Tuteja 2010; Rubén et al. 2010).
2.5 Ectoine
Ectoine (1,4,5,6-tetrahydro-2-methyl-4-pyrimidine carboxylic acid), a common solute of aerobic heterotrophic bacteria (Kempf and Bremer 1998; Galinski 1995; Severin et al. 1992; Kalyuzhnaya et al. 2001), was first discovered as an osmoprotectant in the halophilic bacterium Ectothiorhodospira halochloris (Galinski et al. 1985). Ectoines constitute a class of small molecule chaperones (SMCs), which accumulate to high intracellular concentrations without affecting the cellular functions and prevent the misfolding of proteins and other labile macromolecules from environmental stresses (Marina et al. 2008). This organic solute can either be synthesized de novo or taken up from the environment when available (Galinski and Trüper 1994; Kempf and Bremer 1998). The exact mechanisms of protein stabilization by ectoines are poorly understood, but they are believed to aid in hydration of proteins with solvent molecules (Kanapathipillai et al. 2005). Ectoine is synthesized from aspartate semialdehyde which is converted to l-2,4-diaminobutyrate (DABA) by l-2,4-diaminobutyric acid transaminase (EctB, EC 2.6.1.76). After that, DABA is acetylated to form Nγ-acetyl-l-2,4-diaminobutyrate (Nγ-acetyl-DABA) by l-2,4-diaminobutyric acid acetyltransferase (EctA, EC 2.3.1.178) (Fig. 9.5). The final step is the cyclization of Nγ-acetyl-DABA to form ectoine by the action of ectoine synthase (EctC, EC 4.2.1.108) (Reshetnikov et al. 2011).
The ectABC gene cluster involved in the biosynthesis of ectoine has been isolated from Chromohalobacter salexigens (Cánovas et al. 1997), Marinococcus halophilus (Louis and Galinski 1997), and Halomonas elongata (Göller et al. 1998). Functional expression of Marinococcus halophilus ectoine biosynthetic pathway genes in E. coli resulted in enhanced tolerance to salt (Louis and Galinski 1997). Plants transformed with ectoine biosynthesis genes from Halomonas elongata demonstrated enhanced tolerance to mannitol and NaCl (Nakayama et al. 2000; Moghaieb et al. 2006, 2011).
2.6 Glycine Betaine
Glycine betaine, a quaternary ammonium compound is widely distributed in microorganisms, higher plants and animals and one of the most common betaines found in plants (Rhodes and Hanson 1993). In many halotolerant plants, glycine betaine is reported to accumulate in plastids (Allard et al. 1998) and higher levels of glycine betaine correlates with higher level of stress tolerance (McNeil et al. 1999). Glycine betaine has diverse functions in plant cell such as stabilization of the quaternary structure of enzyme, proteins, and maintenance of membrane integrity under salt, cold, and heat stress (Sakamoto and Murata 2000).
The biosynthetic pathway in most plants follows the conversion of choline to glycine betaine in two oxidation steps via the intermediate betaine aldehyde. The first reaction is catalyzed by choline monooxygenase (CMO, EC 1.14.15.7) that converts choline to betaine aldehyde hydrate thus spontaneously forming betaine aldehyde which is acted upon by betaine aldehyde dehydrogenase (BADH, EC 1.2.1.8) to form glycine betaine, whereas in Arthrobacter spp. only one enzyme, choline oxidase (CO, EC 1.1.3.17) is required (Ikuta et al. 1977) (Fig. 9.6).
In higher plants, both these enzymes are localized in stroma of chloroplast (Lerma et al. 1988; Rathinasabapathi et al. 1997). Glycine betaine, when engineered in plants or exogenously applied provides sufficient tolerance to a variety of abiotic stresses (Sakamoto and Murata 2001, 2002). Transgenics generated in rice and tomato using choline oxidase (codA) targeting both chloroplast and cytosol have shown that the accumulation of glycine betaine in chloroplast is more efficient in providing stress tolerance than accumulation of glycine betaine in cytosol (Sakamoto et al. 1998; Chen and Murata 2002; Park et al. 2004, 2007). The photosynthetic machinery was found to be protected against salt and cold stresses in transgenic rice expressing codA with no negative effects on growth and development (Alia et al. 1998; Sakamoto et al. 1998). Interestingly, the codA over expressing Arabidopsis produced more flowers, siliques, and seeds than wild-type plant when grown under normal conditions (Park et al. 2004). Most of the plants are vulnerable to abiotic stress in their reproductive stage and it has been observed that accumulation of glycine betaine in reproductive organs can effectively protect the various organs from the damaging effect of stress and increase the crop yield (Park et al. 2004; Quan et al. 2004). Microarray studies in Arabidopsis reveals that exogenous application of glycine betaine also enhances the expression of other genes that are directly or indirectly involved in stress tolerance such as genes for ROS scavenging enzymes, transcription factors, ferric reductase, and membrane trafficking components (Einset et al. 2007).
3 Mechanism of Stress Tolerance
Osmoprotectants generally localize in cytoplasm following osmotic stress, though the mechanism by which these molecules provide tolerance under stress is not clearly understood (Ramanjulu and Bartels 2002). These osmoprotectants are thought to counteract osmotic imbalance by reducing cell’s osmotic potential and thereby maintaining turgor pressure under conditions of low water potential and high ionic strength (Pathan et al. 2004). They also function to protect or replace the water shell around proteins (Yancey et al. 1982; Stoop et al. 1996) and stabilize protein complexes and membranes (Murata et al. 1992; Papageorgiou and Murata 1995). The accumulation of these osmolytes in overexpressing transgenic plants is too low to provide protection by the way of osmotic mass action alone (Sheveleva et al. 1997; Sakamoto et al. 1998; Huang et al. 2000). Apart from this, investigators have also revealed some alternative modes of stress protection offered by these osmoprotectants like scavenging of ROS and chaperon like activities that protect protein structure (Shen et al. 1997b; Serraj and Sinclair 2002). Table 9.1 summarizes the specific functions of some common osmoprotectants under abiotic stress.
4 Metabolic Engineering for Osmoprotectant Synthesis
Genetic transformation technology enables us to achieve gene transfer in precise and predictable manner. Hence genetic engineering approaches would be useful to manipulate these osmoprotectants biosynthetic pathways for accumulating such molecules that act by scavenging ROS, reducing lipid peroxidation, maintaining protein structure and functions (Hare et al. 1998; McNeil et al. 1999; Diamant et al. 2001; Yamada et al. 2005). The physiological and agricultural implications of metabolic engineering of plants for osmoprotectant biosynthesis have been thoroughly reviewed and analyzed (Jain and Selvaraj 1997; Nelson et al. 1998; Bohnert and Sheveleva 1998; Yeo 1998). Table 9.2 summarizes different transgenics developed using genes involved in osmoprotectant biosynthesis for abiotic stress tolerance.
5 Constraints in Path of Metabolic Engineering
It has been observed that out of many transgenics developed for higher osmoprotectant accumulation, only a few succeeded due to metabolic constraints, a few are enlisted here:
-
1.
Transgenes used for transforming a plant were of non-plant origin, mainly bacterial, while plants have their own genes for osmoprotectant synthesis. Use of plant origin genes can aid in overcoming this hurdle (Hanson et al. 1994).
-
2.
Two major factors that generally limit the accumulation of osmoprotectants in transgenic plants are the availability of endogenous substrate and transport of osmolytes across the membranes (Nuccio et al. 1998, 2000; McNeil et al. 2000; Huang et al. 2000).
-
3.
Some of the metabolic pathways are very rigid from flux point of view; they oppose the flux redistribution which arises due to over expression of transgene for metabolite biosynthesis (Stephanopoulos and Vallino 1991; Fernie et al. 2002).
-
4.
Metabolic flux of the transgenics developed using constitutive promoter remains diverted all the time and there by affects plant’s growth and development. Employing tissue specific and stress inducible promoters may support in balancing metabolic flux (Nelson et al. 1998; Russell et al. 1998; Garg et al. 2002).
-
5.
Over expression of transgene may lead to diversion of metabolic flux from primary metabolism and therefore this can give rise to undesirable consequences (Sheveleva et al. 1998; Bohmert et al. 2000; Roessner et al. 2001; Garg et al. 2002) or it may lead to feedback effects on engineered pathway (Fernie et al. 2002; Regierer et al. 2002).
-
6.
Cells may recognize the over expressed metabolite as non-self and may degrade it using endogenous machinery (Goddijn et al. 1997) or the host may lack regulatory control upon the over expressing enzyme (Trethewey 2004).
-
7.
Over accumulation of various compatible solutes (mannitol, sorbitol, and trehalose) in transgenic plants have shown some harmful side effects (Karakas et al. 1997; Sheveleva et al. 1998; Yeo et al. 2000).
-
8.
Studies show that osmolytes have minor impact on cellular water retention or osmotic adjustment in comparison to stabilization and protection of cellular components (Blum et al. 1996; Konstantinova et al. 2002; Turner et al. 2007).
-
9.
Transgenic plants engineered for over expression of osmoprotectant synthesis gene could not be assessed rigorously for their stress tolerance potential (Bhatnagar-Mathur et al. 2008).
6 Conclusion
The avenues and possibilities of plants engineered for osmoprotectants has been an area of consistent research for plant scientists and have been reviewed extensively in Bohnert et al. 1995; Nuccio et al. 1999; Rathinasabapathi 2000; Chen and Murata 2002. Although the mode of action of these diverse categories of osmoprotectants might be overlapping, it is still a mystery as to what triggers the accumulation of different osmolytes under different stress conditions. Additionally, the protection offered by these molecules is still under speculation as whether it is a result of a better osmotic adjustment of the cell under stressful situations or they have some deeper impacts on the cellular system coping with stress. Among many attempts made at installing genes for osmoprotectant biosynthesis in plants, only a moderate level of stress tolerance has been achieved in controlled stress conditions and no significant performance has been reported from the field trials if any.
The past has nevertheless shown us that the way forward now is to first understand the comprehensive roles of these molecules in relieving stress in the cellular system along with the implications of over expressing these genes in terms of energy efficiency and channelization of metabolic flux away from physiologically important pathways.
7 Future Prospects
Considering the multigenicity of stress tolerance trait, transgenics developed through single gene insertions are inefficient in providing sustainable stress tolerance to crop plants. Therefore, it is important to carefully identify regulatory factors, which affect expression of key genes following any abiotic stress. Use of these regulatory factors like stress inducible transcription factor in transforming any crop plant may lead to regulation of many genes involved in stress response and thereby impart tolerance to multiple stresses. The overall functional analysis of transgenics made for different osmoprotectants may help us to select key regulatory genes for developing multiple stress tolerant crop varieties. So far, attempts for developing stress tolerant transgenics are restricted mostly to model plants, therefore focus on crop plants is the need of the hour.
Though in some cases, it has been reported that modification of compatible solute machinery could lead to no benefit in terms of yield under stress, therefore further research is necessary in order to genetically manipulate tissue specific and stress inducible osmoprotection in crop plants as these transgenics will be more efficient in abiotic stress tolerance without much affecting the metabolic flux. Broad stress tolerant genotypes may be generated by combining different strategies involved in enhancing stress tolerance, like stress-related genes, and their regulatory transcription factors.
Defining the exact mechanism of action of osmolyte and the specific macromolecules being targeted will lead to further improvement in metabolic engineering of osmoprotectants. Identification and characterization of novel osmoprotectants from stress tolerant crop varieties will also aid in achieving this objective.
Therefore, after analyzing these prospects it can be safely concluded that there exists a lot of scope in crop improvement using osmoprotectants but further developments will demand extensive evaluation of stress tolerance potential of these transgenic crops as there is much difference between controlled lab and field conditions.
Abbreviations
- ROS:
-
Reactive oxygen species
- M6PI:
-
Mannose-6-phosphate isomerase
- M6PR:
-
Mannose-6-phosphate reductase
- M1PP:
-
Mannose-1-phosphate phosphatase
- MtlD:
-
Mannitol-1-phosphate dehydrogenase
- NAD:
-
Nicotinamide adenine dinucleotide
- GFOR:
-
Glucose-fructose oxidoreductase
- S6PDH:
-
Sorbitol-6-phosphate dehydrogenase
- NADP:
-
Nicotinamide adenine dinucleotide phosphate
- S6PP:
-
Sorbitol-6-phosphate phosphatase
- Stpd1 :
-
Gene encoding sorbitol-6-phosphate dehydrogenase
- MIPS:
-
myo-Inositol-1-phosphate synthase
- IMP:
-
Inositol monophosphatase
- ABA:
-
Abscisic acid
- PINO1:
-
Porteresia coarctata inositol-1-phosphate synthase
- TPS:
-
Trehalose-6-phosphate synthase
- TPP:
-
Trehalose-6-phosphate phosphatase
- OtsA :
-
E. coli gene encoding TPS
- OtsB :
-
E. coli gene encoding TPP
- P5CS:
-
l-Δ1-pyrroline-5-carboxylate synthetase
- P5CR:
-
l-Δ1-pyrroline-5-carboxylate reductase
- ProDH:
-
Proline dehydrogenase
- P5C:
-
l-Δ1-pyrroline-5-carboxylate
- Put:
-
Putrescine
- Spd:
-
Spermidine
- Spm:
-
Spermine
- ODC:
-
Ornithine decarboxylase
- ADC:
-
Arginine decarboxylase
- CPA:
-
N-carbamoylputrescine amidohydrolase
- SPDS:
-
Spermidine synthases
- SPMS:
-
Spermine synthases
- SAMDC:
-
S-adenosylmethionine decarboxylase
- SMCs:
-
Small molecule chaperones
- DABA:
-
l-2,4-diaminobutyrate
- EctB:
-
l-2,4-diaminobutyric acid transaminase
- EctA:
-
l-2,4-diaminobutyric acid acetyltransferase
- EctC:
-
Ectoine synthase
- CMO:
-
Choline monooxygenase
- BADH:
-
Betaine aldehyde dehydrogenase
- codA :
-
Gene encoding choline oxidase
References
Abebe T, Guenzi AC, Martin B, Cushman JC (2003) Tolerance of mannitol-accumulating transgenic wheat to water stress and salinity. Plant Physiol 131:1748–1755
Acquaah G (2007) Principles of plant genetics and breeding. Blackwell Publishing, Oxford, UK
Alcázar R, Marco F, Cuevas JC, Patrón M, Ferrando A, Carrasco P, Tiburcio AF, Altabella T (2006) Involvement of polyamines in plant response to abiotic stress. Biotechnol Lett 28:1867–1876
Alcázar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C, Carrasco P, Tiburcio AF (2010a) Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231:1237–1249
Alcázar R, Planas J, Saxena T, Zarza X, Bortolotti C, Cuevas JC, Bitrián M, Tiburcio AF, Altabella T (2010b) Putrescine accumulation confers drought tolerance in transgenic Arabidopsis plants over-expressing the homologous Arginine decarboxylase 2 gene. Plant Physiol Biochem 48(7):547–552
Alia HH, Chen THH, Murata N (1998) Transformation with a gene for choline oxidase enhances the cold tolerance of Arabidopsis during germination and early growth. Plant Cell Environ 21:232–239
Allard F, Houde M, Krol M, Ivanov A, Huner NPA, Sarhan F (1998) Betaine improves freezing tolerance in wheat. Plant Cell Physiol 39:1194–1202
Almeida AM, Silva AB, Arajo SS, Cardoso LA, Santos DM, Torn JM, Silva JM, Paul MJ, Fevereiro PS (2007) Responses to water withdrawal of tobacco plants genetically engineered with the AtTPS1 gene: a special reference to photosynthetic parameters. Euphytica 154:113–126
Altabella T, Tiburcio AF, Ferrando A (2009) Plant with resistance to low temperature and method of production thereof. Spanish patent application WO2010/004070
Anoop N, Gupta AK (2003) Transgenic indica rice cv IR-50 over-expressing Vigna aconitifolia delta (1)-pyrroline-5-carboxylate synthetase cDNA shows tolerance to high salt. J Plant Biochem Biotechnol 12:109–116
Avonce N, Leyman B, Mascorro-Gallardo JO, Van Dijck P, Thevelein JM, Iturriaga G (2004) The Arabidopsis trehalose-6-P synthase AtTPS1 gene is a regulator of glucose, abscisic acid, and stress signaling. Plant Physiol 136:3649–3659
Bakhanashvili M, Icekson I, Apelbaum A (1985) Cadaverine formation by specific lysine decarboxylation in Pisum sativum seedlings. Plant Cell Rep 4(6):297–299
Bastola DR, Minocha SC (1995) Increased putrescine biosynthesis through transfer of mouse ornithine decarboxylase cDNA in carrot promotes somatic embryogenesis. Plant Physiol 109:63–71
Bhatnagar-Mathur P, Vadez V, Sharma KK (2008) Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects. Plant Cell Rep 27:411–424
Bieleski RL (1982) Sugar alcohols. In: Loewus FA, Tanner W (eds) Plant carbohydrates I: intracellular carbohydrates, vol 13A, Encyclopedia of Plant physiology. Springer, New York, pp 158–192
Bieleski RL, Redgwell RJ (1985) Sorbitol versus sucrose as photosynthesis and translocation products in developing apricot leaves. Aust J Plant Physiol 12:657–668
Blum A, Munns R, Passioura JB, Turner NC (1996) Genetically engineered plants resistant to soil drying and salt stress: how to interpret osmotic relations? Plant Physiol 110:1051–1053
Boggess SF, Koeppe DE (1978) Oxidation of proline by plant mitochondria. Plant Physiol 62:22–25
Bohmert K, Balbo I, Kopka J, Mittendorf V, Nawrath C, Poirier Y, Tischendorf G, Trethewey RN, Willmitzer L (2000) Transgenic Arabidopsis plants can accumulate polyhydroxybutyrate to up to 4 % of their fresh weight. Planta 211:841–845
Bohnert HJ, Jensen RG (1996) Strategies for engineering water stress tolerance in plants. Trends Biotechnol 14:89–97
Bohnert HJ, Sheveleva E (1998) Plant stress adaptations- making metabolism move. Curr Opin Plant Biol 1:267–274
Bohnert HJ, Nelson DE, Jensen RG (1995) Adaptations to environmental stresses. Plant Cell 7:1099–1111
Cánovas D, Vargas C, Iglesias-Guerra F, Csonka LN, Rhodes D, Ventosa A, Nieto JJ (1997) Isolation and characterization of salt-sensitive mutants of the moderate halophile Halomonas elongata and cloning of the ectoine synthesis genes. J Biol Chem 272:25794–25801
Capell T, Bassie L, Christou P (2004) Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc Natl Acad Sci U S A 101:9909–9914
Carolina C, Francisco ACM (2005) Tomato abiotic stress enhanced tolerance by trehalose biosynthesis. Plant Sci 169:75–82
Chen THH, Murata N (2002) Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr Opin Plant Biol 5:250–257
Chen WP, Li PH, Chen THH (2000) Glycinebetaine increases chilling tolerance and reduces chilling-induced lipid peroxidation in Zea mays. Plant Cell Environ 23:609–618
Cheng L, Zou YJ, Ding SL, Zhang JJ, Yu XL, Cao JS, Lu G (2009) Polyamine accumulation in transgenic tomato enhances the tolerance to high temperature stress. J Integr Plant Biol 51:489–499
Chiang YC, Stushnoff C, McSay AE, Jones ML, Bohnert H (2005) Overexpression of Mannitol-1-phosphate dehydrogenase increases mannitol accumulation and adds protection against chilling injury in Petunia. J Am Soc Horti Sci 30:605–610
Clegg JS (1985) The physical properties and metabolic status of Artemia cysts at low water contents: the water replacement hypothesis. In: Leopold AC (ed) Membranes, metabolism and dry organisms. Cornell University Press, New York, pp 169–187
Csonka LN, Gelvin SB, Goodner BW, Orser CS, Siemieniak D, Slightom JL (1988) Nucleotide sequence of a mutation in the proB gene of Escherichia coli that confers proline overproduction and enhanced tolerance to osmotic stress. Gene 64:199–205
Das Chatterjee A, Goswami L, Maitra S, Dastidar KG, Ray S, Majumder AL (2006) Introgression of a novel salt tolerant L myo-inositol 1 phosphate synthase from Porteresia coarctata (Roxb.) Tateoka (PcINO1) confers salt tolerance to evolutionary diverse organisms. FEBS Lett 580:3980–3988
Das KC, Misra HP (2004) Hydroxyl radical scavenging and singlet oxygen quenching properties of polyamines. Mol Cell Biochem 262:127–133
De Ronde JA, Spreeth MH, Cress WA (2000) Effect of antisense-pyrroline-5-carboxylate reductase transgenic soybean plants subjected to osmotic and drought stress. Plant Growth Regul 32:13–26
De Ronde JA, Cress WA, Krüger GHJ, Strasser RJ, Van Staden J (2004) Photosynthetic response of transgenic soybean plants, containing an Arabidopsis P5CR gene, during heat and drought stress. J Plant Physiol 161:1211–1224
Delauney AJ, Verma DPS (1993) Proline biosynthesis and osmoregulation in plants. Plant J 4:215–223
Delauney AJ, Hu CA, Kishor BP, Verma DP (1993) Cloning of ornithine delta amino transferase cDNA from Vigna aconitifolia by transcomplementation in Escherichia coli and regulation of proline biosynthesis. J Biol Chem 268:18673–18678
Diamant S, Eliahu N, Rosenthal D, Goloubinoff P (2001) Chemical chaperones regulate molecular chaperones in vitro and in cells under combined salt and heat stresses. J Biol Chem 276:39586–39591
Einset J, Nielsen E, Connolly EL, Bones A, Sparstad T, Winge P, Zhu JK (2007) Membrane-trafficking RabA4c involved in the effect of glycinebetaine on recovery from chilling stress in Arabidopsis. Physiol Plant 130:511–518
Elbein AD, Pan YT, Pastuszak I, David Carroll D (2003) New insights on trehalose: a multifunctional molecule. Glycobiology 13:17–27
Eleutherio ECA, Araujo PS, Panek AD (1993) Protective role of trehalose during heat stress in Saccharomyces cerevisiae. Cryobiology 30:591–596
Elthon TE, Stewart CR (1981) Sub-mitochondrial location and electron transport characteristics of enzymes involved in proline oxidation. Plant Physiol 67:780–784
Evans PT, Malmberg RL (1989) Do polyamines have roles in plant development? Annu Rev Plant Physiol Plant Mol Biol 40:235–269
FAO (2008) Soaring food prices: facts, perspectives, impacts and actions required. Document HLC/08/INF/1 prepared for the High-level conference on world food security: the challenges of climate change and bioenergy, June 3–5, Rome, pp. 3–5
Fernie A, Willmitzer L, Trethewey R (2002) Sucrose to starch: a transition in molecular plant physiology. Trends Plant Sci 7:35–41
Frova C, Krajewski P, Di Fonzo N, Villa M, Sari-Gorla M (1999) Genetic analysis of drought tolerance in maize by molecular markers. I. Yield components. Theor Appl Genet 99:280–288
Galinski EA (1995) Osmoadaptation in bacteria. Adv Microb Physiol 37:273–328
Galinski EA, Trüper HG (1994) Microbial behavior in salt-stressed ecosystems. FEMS Microbiol Rev 15:95–108
Galinski EA, Pfeiffer HP, Trüper HG (1985) 1,4,5,6,-Tetrahydro-2-methyl-4-pyrimidinecarboxylic acid, a novel cyclic acid from halophilic phototrophic bacteria of the genus Ectothiorhodospira. Eur J Biochem 149:135–139
Garg AK, Kim JK, Owens TG, Ranwala AP, Choi YC, Kochian LV, Wu RJ (2002) Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc Natl Acad Sci U S A 99:15898–15903
Gerats AGM, Kaye C, Collins C, Malmberg ML (1988) Polyamine levels in Petunia genotypes with normal and abnormal floral morphologies. Plant Physiol 86:390–393
Gill SS, Tuteja N (2010) Polyamines and abiotic stress tolerance in plants. Plant Signal Behav 51:26–33
Goddijn OJM, Van Dun K (1999) Trehalose metabolism in plants. Trends Plant Sci 4:315–319
Goddijn OJM, Verwoerd TC, Voogd E, Krutwagen RWHH, De Graaf PTHM, Poels J, Van Dun K, Ponstein AS, Damm B, Pen J (1997) Inhibition of trehalase activity enhances trehalose accumulation in transgenic plants. Plant Physiol 113:181–190
Göller K, Ofer A, Galinski EA (1998) Construction and characterization of an NaCl-sensitive mutant of Halomonas elongata impaired in ectoine biosynthesis. FEMS Microbiol Lett 161:293–300
Han KH, Hwang CH (2003) Salt tolerance enhanced by transformation of a P5CS gene in carrot. J Plant Biotechnol 5:149–153
Hanson AD, Rathinasabapathi B, Rivoal J, Burnet M, Dillon DO, Gage DA (1994) Osmoprotective compounds of the Plumbaginaceae: a natural experiment in metabolic engineering of stress tolerance. Proc Natl Acad Sci U S A 91:306–310
Hanzawa Y, Takahashi T, Michael AJ, Burtin D, Long D, Pineiro M, Coupland G, Komeda Y (2000) ACAULIS5, an Arabidopsis gene required for stem elongation, encodes a spermine synthase. EMBO J 19:4248–4256
Hare PD, Cress WA (1997) Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul 21:79–102
Hare PD, Cress WA, Van Staden J (1998) Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ 21:535–553
Hayashi H, Alia Mustardy L, Deshnium P, Ida M, Murata N (1997) Transformation of Arabidopsis thaliana with the codA gene for choline oxidase: accumulation of glycinebetaine and enhanced tolerance to salt and cold stress. Plant J 12:133–142
Hideki N, Kazuya Y, Hisayo O, Yoshikatsu M, Atsuhiko S (2000) Ectoine, the compatible solute of Halomonas elongata, confers hyperosmotic tolerance in cultured tobacco cells. Plant Physiol 122:1239–1247
Hmida-Sayari A, Gargouri-Bouzid R, Bidani A, Jaoua L, Savouré A, Jaou S (2005) Over expression of 1-pyrroline-5-carboxylate synthetase increases proline production and confers salt tolerance in transgenic potato plants. Plant Sci 169:746–752
Holmberg N, Bülow L (1998) Improving stress tolerance in plants by gene transfer. Trends Plant Sci 3:61–66
Holmström KO, Mantyala E, Welin B, Mandal A, Palva TE, Tunnela O, Londsborough J (1996) Drought tolerance in tobacco. Nature 379:683–684
Holmström KO, Somersalo S, Mandal A, Palva TE, Welin B (2000) Improved tolerance to salinity and low temperature in transgenic tobacco producing glycinebetaine. J Exp Bot 51:177–185
Hong Z, Lakkineni K, Zhang Z, Verma DPS (2000) Removal of feedback inhibition of delta1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol 122:1129–1136
Hoshida H, Tanaka Y, Hibino T, Hayashi Y, Tanaka A, Takabe T (2000) Enhanced tolerance to salt stress in transgenic rice that over expresses chloroplast glutamine synthetase. Plant Mol Biol 43:103–111
Hu CA, Delauney AJ, Verma DP (1992) A bifunctional enzyme (delta1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proc Natl Acad Sci U S A 89:9354–9358
Hu L, Lu H, Liu Q, Chen X, Jiang X (2005) Over expression of mtlD gene in transgenic Populus tomentosa improves salt tolerance through accumulation of mannitol. Tree Physiol 25:1273–1281
Huang J, Hirji R, Adam L, Rozwadowski KL, Hammerlindl JK, Keller WA, Selvaraj G (2000) Genetic engineering of glycinebetaine production toward enhancing stress tolerance in plants: metabolic limitations. Plant Physiol 122:747–756
Hur J, Hong Jong K, Lee C-H, An G (2004) Stress-inducible OsP5CS2 gene is essential for salt and cold tolerance in rice. Plant Sci 167:417–426
Igarashi Y, Yoshiba Y, Sanada Y, Wada K, Yamaguchi-Shinozaki K, Shinozaki K (1997) Characterization of the gene for delta1-pyrroline-5-carboxylate synthetase and correlation between the expression of the gene and salt tolerance in Oryza sativa L. Plant Mol Biol 33:857–865
Ikuta S, Mamura S, Misaki H, Horiuti Y (1977) Purification and characterization of choline oxidase from Arthrobacter globiformis. J Biochem 82:1741–1749
Iordachescu M, Imai R (2008) Trehalose biosynthesis in response to abiotic stresses. J Integr Plant Biol 50(10):1223–1229
Ishitani M, Majumder AL, Bornhouser A, Michalowski CB, Jensen RG, Bohnert HJ (1996) Coordinate transcriptional induction of myo-inositol metabolism during environmental stress. Plant J 9:537–548
Jain RK, Selvaraj G (1997) Molecular genetic improvement of salt tolerance in plants. Biotechnol Annu Rev 3:245–267
Jang I, Oh S, Seo J, Choi WB, Song SI, Kim CH, Kim YS, Seo HS, Choi YD, Nahm BH, Kim JK (2003) Expression of a bifunctional fusion of the Escherichia coli genes for Trehalose-6-Phosphate synthase and Trehalose-6-Phosphate phosphatase in transgenic rice plants increases trehalose accumulation and abiotic stress tolerance without stunting growth. Plant Physiol 131:516–524
Janowitz T, Kneifel H, Piotrowski M (2003) Identification and characterization of plant agmatine iminohydrolase, the last missing link in polyamine biosynthesis of plants. FEBS Lett 544:258–261
Johnson MK, Johnson EJ, MacElroy RD, Speer HL, Bruff BS (1968) Effects of salts on the halophilic alga Dunaliella viridis. J Bacteriol 95:1461–1468
Kalyuzhnaya MG, Khmelenina VN, Eshinimaev BT, Suzina NE, Nikitin D, Solonin A, Lin JL, McDonald IR, Murrell JC, Trotsenko YA (2001) Taxonomic characterization of new alkaliphilic and alkali tolerant methanotrophs from soda lakes of the Southeastern transbaikal region and description of Methylomicrobium buryatense sp. Syst Appl Microbiol 24:166–176
Kanapathipillai M, Lentzen G, Sierks M, Park CB (2005) Ectoine and hydroxyectoine inhibit aggregation and neurotoxicity of Alzheimer’s β-amyloid. FEBS Lett 579:4775–4780
Karakas B, Ozias-Akins P, Stushnoff C, Suefferheld M, Rieger M (1997) Salinity and drought tolerance of mannitol-accumulating transgenic tobacco. Plant Cell Environ 20:609–616
Karim S, Aronsson H, Ericson H, Pirhonen M, Leyman B, Welin B, Palva ET, Patrick VD, Kjell-Ove H (2007) Improved drought tolerance without undesired side effects in transgenic plants producing trehalose. Plant Mol Biol 64:371–386
Kasukabe Y, He LX, Nada K, Misawa S, Ihara I, Tachibana S (2004) Over expression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol 45:712–722
Kaur H, Shukla RK, Yadav G, Chattopadhyay D, Majee M (2008) Two divergent genes encoding L-myo-inositol 1-phosphate synthase1 (CaMIPS1) and 2 (CaMIPS2) are differentially expressed in chickpea. Plant Cell Environ 31:1701–1716
Kempf B, Bremer E (1998) Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch Microbiol 170:319–330
Kishor PBK, Hong Z, Miao G-H, Hu CAA, Verma DPS (1995) Over expression of delta-1-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol 108:1387–1394
Kochetov AV, Titov SE, Kolodyazhnaya YS, Komarova ML, Koval VS, Makarova NN, Il’yinskyi YY, Trifonova EA, Shumny VK (2004) Tobacco transformants bearing antisense suppressor of proline dehydrogenase gene are characterized by higher proline content and cytoplasm osmotic pressure. Russ J Genet 40:216–218
Konstantinova T, Pavanova D, Atanassov A, Djilianov D (2002) Freezing tolerant tobacco, transformed to accumulate osmoprotectants. Plant Sci 163:157–164
Kumar A, Taylor MA, Mad Arif SA, Davies HV (1996) Potato plants expressing antisense and sense SAMDC transgenes show altered levels of polyamines and ethylene: antisense plants display abnormal phenotypes. Plant J 9:147–158
Kumar S, Dhingra A, Daniell H (2004) Plastid-expressed Betaine aldehyde dehydrogenase gene in carrot cultured cells, roots and leaves confers enhanced salt tolerance. Plant Physiol 135:2843–2854
Kumria R, Rajam MV (2002) Ornithine decarboxylase transgene in tobacco affects polyamines, in vitro morphogenesis and response to salt stress. J Plant Physiol 159:983–990
Kuznetsov Vl V, Shevyakova NI (1999) Proline under stress conditions: biological role, metabolism, and regulation. Russ J Plant Physiol 46:321–336
Kuznetsov V, Shorina M, Aronova E, Stetsenko L, Rakitin V, Shevyakova N (2007) NaCl and ethylene-dependent cadaverine accumulation and its possible protective role in the adaptation of the common ice plant to salt stress. Plant Sci 172:363–370
LaRosa PC, Rhodes D, Rhodes JC, Bressan RA, Csonka LN (1991) Elevated accumulation of proline in NaCl-adapted tobacco cells is not due to altered D1-pyrroline-5-carboxylate reductase. Plant Physiol 96:245–250
Lerma C, Hanson AD, Rhodes D (1988) Oxygen-18 and deuterium labeling studies of choline oxidation by spinach and sugar beet. Plant Physiol 88:695–702
Leyman B, Dijck PV, Thevelein JM (2001) An unexpected plethora of trehalose biosynthetic genes in Arabidopsis thaliana. Trends Plant Sci 6:510–513
Lilius G, Holmberg N, Bülow L (1996) Enhanced NaCl stress tolerance in transgenic tobacco expressing bacterial choline dehydrogenase. Biotechnology 14:177–180
Liu Q, Zhang Y, Shouyi C (2000) Plant protein kinase genes induced by drought, high salt and cold stresses. Chin Sci Bull 45:1153–1157
Lobell DB, Field CB (2007) Global scale climate-crop yield relationships and the impacts of recent warming. Environ Res Lett 2:014002
Loescher WH, Tyson RH, Everard JD, Redgwell RJ, Bieleski RL (1992) Mannitol synthesis in higher plants: evidence for the role and characterization of a NADPH-dependent mannose-6-phosphate reductase. Plant Physiol 98(4):1396–1402
Loewus FA, Murthy PN (2000) Myo-inositol metabolism in plants. Plant Sci 150:1–19
Louis P, Galinski EA (1997) Characterization of genes for the biosynthesis of the compatible solute ectoine from Marinococcus halophilus and osmoregulated expression in Escherichia coli. Microbiology 143:1141–1149
Lunn JE (2007) Gene families and evolution of trehalose metabolism in plants. Funct Plant Biol 34:550–563
Luo Y, Li WM, Wang W (2008) Trehalose: protector of antioxidant enzymes or reactive oxygen species scavenger under heat stress? Environ Exp Botany 63:378–384
Maheswari M, Varalaxmi Y, Vijayalakshmi A, Yadav SK, Sharmila P, Venkateswarlu B, Vanaja M, PardhaSardhi P (2010) Metabolic engineering using mtlD gene enhances tolerance to water deficit and salinity in sorghum. Biol Plant 54:647–652
Majee M, Maitra S, Dastidar KG, Pattnaik S, Chatterjee A, Hait NC, Das KP, Majumder AL (2004) A novel salt tolerant L myo-inositol-1-phosphate synthase from Porteresia coarctata (Roxb) Tateoka, a halophytic wild rice. J Biol Chem 279:28539–28552
Mani S, Van de Cotte B, Van Montagu M, Verbruggen N (2002) Altered levels of proline dehydrogenase cause hypersensitivity to proline and its analogs in Arabidopsis. Plant Physiol 128:73–83
Marina M, Maiale SJ, Rossi FR, Romero MF, Rivas EI, Gárriz A, Ruiz OA, Pieckenstain FL (2008) Apoplastic polyamine oxidation plays different roles in local responses of tobacco to infection by the necrotrophic fungus Sclerotinia sclerotiorum and the biotrophic bacterium Pseudomonas viridiflava. Plant Physiol 147(4):2164–2178
Masgrau C, Altabella T, Farrás R, Flores D, Thompson AJ, Besford RT, Tiburcio AF (1997) Inducible over expression of oat arginine decarboxylase in transgenic tobacco plants. Plant J 11:465–473
Mattioli R, Marchese D, D’Angeli S, Altamura MM, Costantino P, Trovato M (2008) Modulation of intracellular proline levels affects flowering time and inflorescence architecture in Arabidopsis. Plant Mol Biol 66:277–288
McCue KF, Hanson AD (1990) Drought and salt tolerance: towards understanding and application. Trends Biotechnol 8:358–362
McNeil SD, Nuccio ML, Hanson AD (1999) Betaines and related osmoprotectants. Targets for metabolic engineering of stress resistance. Plant Physiol 120:945–949
McNeil SD, Rhodes D, Russell BL, Nuccio ML, Shachar-Hill Y, Andrew D, Hanson AD (2000) Metabolic model identifies key constraints on an engineered glycinebetaine synthetic pathway in tobacco. Plant Physiol 124:153–162
Mehta RA, Handa A, Li N, Mattoo AK (1997) Ripening activated expression of S-adenosylmethionine decarboxylase increases polyamine levels and influences ripening in transgenic tomato fruits (abstract no.134). Plant Physiol 114:S-44
Mehta RA, Cassol T, Li N, Ali N, Handa AK, Mattoo AK (2002) Engineered polyamine accumulation in tomato enhances phytonutrient content, juice quality, and vine life. Nat Biotechnol 20:613–618
Minguet EG, Vera-Sirera F, Marina A, Carbonell J, Blazquez MA (2008) Evolutionary diversification in polyamine biosynthesis. Mol Biol Evol 25:2119–2128
Miranda JA, Avonce N, Suarez R, Thevelein JM, Dijck PV, Iturriaga GA (2007) A bifunctional TPS-TPP enzyme from yeast confers tolerance to multiple and extreme abiotic-stress conditions in transgenic Arabidopsis. Planta 226:1411–1421
Moghaieb REA, Tanaka N, Saneoka H, Hussein HA, Yousef SS, Ewada MAF, Aly MAM, Fujika K (2000) Expression of betaine aldehyde dehydrogenase gene in transgenic tomato hairy roots leads to the accumulation of glycinebetaine and contributes to the maintenance of the osmotic potential under salt stress. Soil Sci Plant Nutr 46:873–883
Moghaieb REA, Tanaka N, Sanoeka H, Murooka Y, Ono H, Morikawa H, Nakamura A, Nguyen NT, Suwa R, Fujita K (2006) Characterization of salt tolerance in ectoine-transformed tobacco plants (Nicotiana tabaccum): photosynthesis, osmotic adjustment, and nitrogen partitioning. Plant Cell Environ 29:173–182
Moghaieb REA, Nakamura A, Saneoka H, Fujita K (2011) Evaluation of salt tolerance in ectoine-transgenic tomato plants (Lycopersicon esculentum) in terms of photosynthesis, osmotic adjustment, and carbon partitioning. GM Crops 2–1:58–65
Mohanty C, Kathuria H, Ferjani A, Sakamoto A, Mohanty P, Murata N, Tyagi AK (2003) Transgenics of an elite indica rice variety Pusa Basmati 1 harbouring the codA gene are highly tolerant to salt stress. Theor Appl Genet 106:51–57
Molinari HBC, Marur CJ, Filho JCB, Kobayashi AK, Pileggi M, Junior RPL, Pereira LFP, Vieira LGE (2004) Osmotic adjustment in transgenic citrus rootstock Carrizo citrange (Citrus sinensis Osb. Poncirus trifoliate L. Raf.) overproducing proline. Plant Sci 167:1375–1381
Molinari HBC, Marur CJ, Daros E, Freitas de Campos MK, Portela de Carvalho JFR, Filho JCB, Pereira LFP, Vieira LGE (2007) Evaluation of the stress-inducible production of proline in transgenic sugarcane (Saccharum spp.): osmotic adjustment, chlorophyll fluorescence and oxidative stress. Physiol Planta 130:218–229
Murata N, Mohanty PS, Hayashi H, Papageorgiou GC (1992) Glycinebetaine stabilizes the association of extrinsic proteins with the photosynthetic oxygen-evolving complex. FEBS Lett 296:187–189
Nakayama H, Yoshida K, Ono H, Murooka Y, Shinmyo A (2000) Ectoine, the compatible solute of Halomonas elongata, confers hyperosmotic tolerance in cultured tobacco cells. Plant Physiol 122:1239–1247
Nanjo T, Kobayashi M, Yoshiba Y, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (1999) Antisense suppression of proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana. FEBS Lett 461:205–210
Nelson DE, Shen B, Bohnert HJ (1998) Salinity tolerance-mechanisms, models and the metabolic engineering of complex traits. Genet Eng 20:153–176
Nuccio ML, Russell BL, Nolte KD, Rathinasabapathi B, Gage DA, Hanson AD (1998) The endogenous choline supply limits glycinebetaine synthesis in transgenic tobacco expressing choline monooxygenase. Plant J 16:487–496
Nuccio ML, Rhodes D, McNeil SD, Hanson AD (1999) Metabolic engineering of plants for osmotic stress resistance. Curr Opin Plant Biol 2:128–134
Nuccio ML, McNeil SD, Ziemak MJ, Hanson AD, Jain RK, Selvaraj G (2000) Choline import into chloroplasts limits glycine betaine synthesis in tobacco: analysis of plants engineered with a chloroplastic or a cytosolic pathway. Metab Eng 2:300–311
Panicot M, Masgrau C, Borrell A, Cordeiro A, Tiburcio AF, Altabella T (2002) Effects of putrescine accumulation in tobacco transgenic plants with different expression of oat arginine decarboxylases. Physiol Planta 114:281–287
Papageorgiou GC, Murata N (1995) The unusually strong stabilizing effects of glycinebetaine on the structure and function in the oxygen-evolving photosystem II complex. Photosyn Res 44:243–252
Park EJ, Jeknic Z, Sakamoto A, DeNoma J, Yuwansiri R, Murata N, Chen THH (2004) Genetic engineering of glycinebetaine synthesis in tomato protects seeds, plants, and flowers from chilling damage. Plant J 40:474–487
Park EJ, Jeknic Z, Pino MT, Murata N, Chen THH (2007) Glycinebetaine accumulation in chloroplasts is more effective than that in cytosol in protecting transgenic tomato plants against abiotic stress. Plant Cell Environ 30:994–1005
Pathan MS, Subudhi PK, Courtois B, Nguyen HT (2004) Molecular dissection of abiotic stress tolerance in sorghum and rice. In: Nguyen HT, Blum A (eds) Physiology and biotechnology integration for plant breeding. Marcel Dekker, New York, pp 525–569
Patra B, Ray S, Richter A, Majumder AL (2010) Enhanced salt tolerance of transgenic tobacco plants by co-expression of PcINO1 and McIMT1 is accompanied by increased level of myo-inositol and methylated inositol. Protoplasma 245(1–4):143–152
Paul MJ, Cockburn W (1989) Pinitol, a compatible solute in Mesembryanthemum crystallinum L. J Exp Bot 40:1093–1098
Paul M, Pellny T, Goddijn OJM (2001) Enhancing photosynthesis with sugar signals. Trends Plant Sci 6:197–200
Peng Z, Lu Q, Verma DPS (1996) Reciprocal regulation of D1-pyrroline-5-carboxylate synthetase and proline dehydrogenase genes control levels during and after osmotic stress in plants. Mol Gen Genet 253:334–341
Phillips JR, Oliver MJ, Bartels D (2002) Molecular genetics of desiccation and tolerant systems. In: Black M, Pritchard H (eds) Desiccation and survival in plants: drying without dying. CAB International, Wallingford, pp 319–341
Pilon-Smits EAH, Terry N, Sears T, Kim H, Zayed A, Hwang S, van Dun K, Voogd E, Verwoerd TC, Krutwagen RWHH, Goddijn OJM (1998) Trehalose-producing transgenic tobacco plants show improved growth performance under drought stress. J Plant Physiol 152:525–532
Piotrowski M, Janowitz T, Kneifel H (2003) Plant C-N Hydrolases and the identification of a plant N-Carbamoylputrescine amidohydrolase involved in polyamine biosynthesis. J Biol Chem 278(3):1708–1712
Prabhavathi V, Rajam MV (2007) Mannitol-accumulating transgenic eggplants exhibit enhanced resistance to fungal wilts. Plant Sci 173:50–54
Prabhavathi S, Yadav JS, Kumar PA, Rajam MV (2002) Abiotic stress tolerance in transgenic eggplant (Solanum melongena L.) by introduction of bacterial mannitol phosphodehydrogenase gene. Molecular Breed 9:137–147
Prasad KVSK, Saradhi PP (2004) Enhanced tolerance to photoinhibition in transgenic plants through targeting of glycinebetaine biosynthesis into the chloroplasts. Plant Sci 166:1197–1212
Quan R, Shang M, Zhang H, Zhao Y, Zhang J (2004) Improved chilling tolerance by transformation with betA gene for the enhancement of glycinebetaine synthesis in maize. Plant Sci 166:141–149
Rafart-Pedros A, Mac Leod MR, Ross HA, McRae D, Tiburcio AF, Davies HD, Taylor M (1999) Manipulation of the S-adenosylmethionine decarboxylase transcript level in potato tubers. Over-expression leads to an increase in tuber number and a change in tuber size distribution. Planta 209:153–160
Rahnama H, Vakilian H, Fahimi H, Ghareyazie B (2011) Enhanced salt stress tolerance in transgenic potato plants (Solanum tuberosum L.) expressing a bacterial mtlD gene. Acta Physiol Plant 33:1521–1532
Ramanjulu S, Bartels D (2002) Drought and dessication-induced modulation of gene expression in plants. Plant Cell Environ 25:141–151
Rathinasabapathi B (2000) Metabolic engineering for stress tolerance: installing osmoprotectant synthesis pathways. Ann Bot 86:709–716
Rathinasabapathi B, Burnet M, Russell BL, Gage DA, Liao PC, Nye GJ, Scott P, Golbeck JH, Hanson AD (1997) Choline monooxygenase, an unusual iron-sulphur enzyme catalyzing the first step of glycinebetaine synthesis in plants: prosthetic group characterization and cDNA cloning. Proc Natl Acad Sci U S A 94:3454–3458
Regierer B, Fernie AR, Springer F, Perez-Melis A, Leisse A, Koehl K, Willmitzer L, Giegenberger P, Kossmann J (2002) Starch content and yield increase as a result of altering adenylate pools in transgenic plants. Nat Biotechnol 20:1256–1260
Reshetnikov AS, Khmelenina VN, Mustakhimov II, Trotsenko YA (2011) Genes and enzymes of ectoine biosynthesis in halotolerant methanotrophs. Methods Enzymol 495:15–30
Rhodes D, Hanson AD (1993) Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu Rev Plant Physiol Plant Mol Biol 44:357–384
Ribaut JM, Hosington DA, Deitsch JA, Jiang C, Gonzalez-de-Leon D (1996) Identification of quantitative trait loci under drought conditions in tropical maize. 1. Flowering parameters and the anthesis-silking interval. Theor Appl Genet 92:905–914
Ribaut JM, Jiang C, Gonzalez-de-Leon D, Edmeades GO, Hosington DA (1997) Identification of quantitative trait loci under drought conditions in tropical maize, 2. Yield components and marker assisted selection strategies. Theor Appl Genet 94:887–896
Roessner U, Luedemann A, Brust D, Fiehn O, Linke T, Willmitzer L, Fernie AR (2001) Metabolic profiling allows comprehensive phenotyping of genetically or environmentally modified plant systems. Plant Cell 13:11–29
Romero C, Belles JM, Vaya JL, Serrano R, Culianez-Macia FA (1997) Expression of the yeast trehalose-6-phosphate synthase gene in transgenic tobacco plants: pleiotropic phenotypes include drought tolerance. Planta 201:293–297
Ronan S, Hirokazu T, Hideko N, Laszlo M, Chen THH, Norio M (2003) Enhanced formation of flowers in salt-stressed Arabidopsis after genetic engineering of the synthesis of glycinebetaine. Plant J 36:165–176
Rontein D, Basset G, Hanson AD (2002) Metabolic engineering of osmoprotectant accumulation in plants. Metab Eng 4:49–56
Roosens NH, Bitar FA, Loenders K, Angenon G, Jacobs M (2002) Over expression of ornthine-d-aminotransferase increases proline biosynthesis and confers osmotolerance in transgenic plants. Mol Breed 9:73–80
Roy M, Wu R (2001) Arginine decarboxylase transgene expression and analysis of environmental stress tolerance in transgenic rice. Plant Sci 160:869–875
Roy M, Wu R (2002) Overexpression of S-adenosylmethionine decarboxylase gene in rice increases polyamine level and enhances sodium chloride-stress tolerance. Plant Sci 163:987–992
Rubén A, Teresa A, Francisco M, Cristina B, Matthieu R, Csaba K, Pedro C, Antonio FT (2010) Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231:1237–1249
Ruidang Q, Shang M, Zhang H, Zhao Y, Zhang J (2004) Improved chilling tolerance by transformation with betA gene for the enhancement of glycinebetaine synthesis in maize. Plant Sci 166:141–149
Russell BL, Rathinasabapathi B, Hanson AD (1998) Osmotic stress induces expression of choline monooxygenase in sugar beet and amaranth. Plant Physiol 116:859–865
Sakamoto A, Murata N (2000) Genetic engineering of glycinebetaine synthesis in plants: current status and implications for enhancement of stress tolerance. J Exp Bot 51:81–88
Sakamoto A, Murata N (2001) The use of bacterial choline oxidase, a glycinebetaine-synthesizing enzyme, to create stress-resistant transgenic plants. Plant Physiol 125:180–188
Sakamoto A, Murata N (2002) The role of glycinebetaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ 25:163–171
Sakamoto A, Murata A, Murata N (1998) Metabolic engineering of rice leading to biosynthesis of glycinebetaine and tolerance to salt and cold. Plant Mol Biol 38:1011–1019
Savouré A, Jaoua S, Hua XJ, Ardiles W, Van MM, Verbruggen N (1995) Isolation, characterization and chromosomal location of a gene encoding the delta 1-pyrroline-5-carboxylate synthetase in Arabidopsis thaliana. FEBS Lett 372:13–19
Sawahel WA, Hassan AH (2002) Generation of transgenic wheat plants producing high levels of the osmoprotectant proline. Biotechnol Lett 24:721–725
Serraj R, Sinclair TR (2002) Osmolyte accumulation: can it really help increase crop yield under drought conditions? Plant Cell Environ 25:333–341
Severin J, Wohlfarth A, Galinski EA (1992) The predominant role of recently discovered tetrahydropyrimidines for the osmoadaptation of halophilic eubacteria. J Gen Microbiol 138:1629–1638
Shen B, Jensen RG, Bohnert HJ (1997a) Increased resistance to oxidative stress in transgenic plants by targeting mannitol biosynthesis to chloroplasts. Plant Physiol 113:1177–1183
Shen B, Jensen RG, Bohnert HJ (1997b) Mannitol protects against oxidation by hydroxyl radicals. Plant Physiol 115:527–532
Shen B, Hohmann S, Jensen RG, Bohnert HJ (1999) Roles of sugar alcohols in osmotic stress adaptation. Replacement of glycerol by mannitol and sorbitol in yeast. Plant Physiol 121:45–52
Sheveleva EV, Chmara W, Bohnert HJ, Jensen RG (1997) Increased salt and drought tolerance by D-ononitol production in transgenic Nicotiana tabaccum L. Plant Physiol 115:1211–1219
Sheveleva EV, Marquez S, Chmara W, Zegeer A, Jensen RG, Bohnert HJ (1998) Sorbitol-6-phosphate dehydrogenase expression in transgenic tobacco: high amounts of sorbitol lead to necrotic lesions. Plant Physiol 117:831–839
Sickler CM, Edwards GE, Kiirats O, Gao Z, Loescher W (2007) Response of mannitol-producing Arabidopsis thaliana to abiotic stress. Funct Plant Biol 34:382–391
Singh TN, Aspinall D, Paleg LG (1972) Proline accumulation and varietal adaptability to drought in barley: a potential metabolic measure of drought resistance. Nat New Biol 236:188–190
Smart CC, Fleming AJ (1993) A plant gene with homology to D-myo-inositol-3 phosphate synthase is rapidly and spatially upregulated during an abscisic acid induced morphogenic response in Spirodela polyrrhiza. Plant J 4:279–293
Smart CC, Flores S (1997) Overexpression of d-myo-inositol-3-phosphate synthase leads to elevated levels of inositol in Arabidopsis. Plant Mol Biol 33:811–820
Smirnoff N (1998) Plant resistance to environmental stress. Curr Opin Biotechnol 9:214–219
Stephanopoulos G, Vallino JJ (1991) Network rigidity and metabolic engineering in metabolite overproduction. Science 252:1675–1681
Stevenson JM, Perera IY, Heilmann I, Persson S, Boss WF (2000) Inositol signaling and plant growth. Trends Plant Sci 5:252–258
Stoop JMH, Williamson JD, Pharr DM (1996) Mannitol metabolism in plants: a method for coping with stress. Trends Plant Sci 1:139–144
Su J, Wu R (2004) Stress-inducible synthesis of proline in transgenic rice confers faster growth under stress conditions than that with constitutive synthesis. Plant Sci 166:941–948
Su J, Hirji R, Zhang L, He C, Selvaraj G, Wu R (2006) Evaluation of the stress-inducible production of choline oxidase in transgenic rice as a strategy for producing the stress-protectant glycinebetaine. J Exp Bot 57:1129–1135
Szekely G, Abraham E, Cseplo A, Rigo G, Zsigmond L, Csiszar J, Ayaydin F, Strizhov N, Jasik J, Schmelzer E, Koncz C, Szabados L (2008) Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J 53:11–28
Taji T, Ohsumi C, Iuchi S, Seki M, Kasuga M, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K (2002) Important roles of drought and cold inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J 29:417–426
Tang W, Peng X, Newton RJ (2005) Enhanced tolerance to salt stress in transgenic loblolly pine simultaneously expressing two genes encoding mannitol-1-phosphate dehydrogenase and lucitol-6-phosphate dehydrogenase. Plant Physiol Biochem 43:139–146
Tarczynski MC, Jensen RG, Bohnert HJ (1993) Stress protection of transgenic tobacco by production of the osmolyte mannitol. Science 259:508–510
Thomas JC, Sepahi M, Arendall B, Bohnert HJ (1995) Enhancement of seed germination in high salinity by engineering mannitol expression in Arabidopsis thaliana. Plant Cell Environ 18:801–806
Trethewey RN (2004) Metabolite profiling as an aid to metabolic engineering in plants. Curr Opin Plant Biol 7:196–201
Turner NC, Shahal A, Berger JD, Chaturvedi SK, French RJ, Ludwig C, Mannur DM, Singh SJ, Yadava HS (2007) Osmotic adjustment in chickpea (Cicer arietinum L.) results in no yield benefit under terminal drought. J Exp Bot 58:187–194
Tyagi A, Sairam RK (2004) Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci 86(3):407–420
Urano K, Yoshiba Y, Nanjo T, Igarashi A, Seki M, Sekiguchi F, Yamaguchi-Shinozaki K, Shinozaki K (2003) Characterization of Arabidopsis genes involved in biosynthesis of polyamines in abiotic stress responses and developmental stages. Plant Cell Environ 26:1917–1926
Van Laere A (1989) Trehalose, reserve and/or stress metabolite? FEMS Microbiol Rev 63(3):201–210
Verdoy D, Coba De La Pena T, Redondo FJ, Lucas MM, Pueyo JJ (2006) Transgenic Medicago truncatula plants that accumulate proline display nitrogen-fixing activity with enhanced tolerance to osmotic stress. Plant Cell Environ 29:1913–1923
Verma DPS (1999) Osmotic stress tolerance in plants: role of proline and sulfur metabolisms. In: Shinozaki K, Yamaguchi-Shinozaki K (eds) Molecular responses to cold, drought, heat and salt stress in higher plants. Austin, RG Landes, pp 153–168
Waie B, Rajam MV (2003) Effect of increased polyamine biosynthesis on stress responses in transgenic tobacco by introduction of human S-adenosylmethionine gene. Plant Sci 164:727–734
Watson MB, Emory KK, Piatak RM, Malmberg RL (1998) Arginine decarboxylase (polyamine synthesis) mutants of Arabidopsis thaliana exhibit altered root growth. Plant J 13:231–239
Webb KL, Burley JWA (1962) Sorbitol translocation in apple. Science 137:766
Wen XP, Ban Y, Inoue H, Matsuda N, Moriguchi T (2009) Aluminum tolerance in a spermidine synthase-overexpressing transgenic European pear is correlated with the enhanced level of spermidine via alleviating oxidative status. Environ Exp Bot 66:471–478
Wi SJ, Park KY (2002) Antisense expression of carnation cDNA encoding ACC synthase or ACC oxidase enhances polyamine content and abiotic stress tolerance in transgenic tobacco plants. Mol Cells 13:209–220
Wi SJ, Kim WT, Park KY (2006) Over expression of carnation S-adenosylmethionine decarboxylase gene generates a broad-spectrum tolerance to abiotic stresses in transgenic tobacco plants. Plant Cell Rep 25:1111–1121
Wiemken A (1990) Trehalose in yeast, stress protectant rather than reserve carbohydrate. J Gen Microbiol 58:209–217
Wu LQ, Fan ZM, Guo L, Li YQ, Zhang WJ, Qu LJ, Chen ZL (2003) Over-expression of an Arabidopsis delta-OAT gene enhances salt and drought tolerance in transgenic rice. Chin Sci Bull 48:2594–2600
Wyn Jones RG, Storeys R (1978) Salt stress and comparative physiology in the Gramineaell. Glycine betaine and proline accumulation in two salt and water stressed barley cultivar. Aust J Plant Physiol 5:817–829
Yamada M, Morishita H, Urano K, Shiozaki N, Yamaguchi-Shinozaki K, Shinozaki K, Yoshiba Y (2005) Effects of free proline accumulation in petunias under drought stress. J Exp Bot 56:1975–1981
Yamaguchi K, Takahashi Y, Berberich T, Imai A, Takahashi T, Michael AJ, Kusano T (2007) A protective role for the polyamine spermine against drought stress in Arabidopsis. Biochem Biophys Res Commun 352:486–490
Yancey PH (1994) Compatible and counteracting solutes. In: Strange K (ed) Cellular and molecular physiology of cell volume regulation. CRC, Boca Raton, pp 81–109
Yancey PH (2005) Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 208:2819–2830
Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN (1982) Living with water stress: evolution of osmolyte systems. Science 217:1214–1222
Yang X, Liang Z, Lu C (2005) Genetic engineering of the biosynthesis of glycinebetaine enhances photosynthesis against high temperature stress in transgenic tobacco plants. Plant Physiol 138:2299–2309
Yeo AR (1998) Molecular biology of salt tolerance in the context of whole-plant physiology. J Exp Bot 49:915–929
Yeo AR, Flowers TJ (1989) Selection for physiological characters—examples from breeding for salt tolerance. In: Flowers TJ, Jackson MB, Jones HG (eds) Plants under stress: biochemistry, physiology and ecology and their application to plant improvement. Cambridge University Press, Cambridge, pp 217–234
Yeo ET, Kwon HB, Han SE, Lee JT, Ryu JC, Byun MO (2000) Genetic engineering of drought-resistant potato plants by introduction of the trehalose-6-phosphate synthase (TPS1) gene from Saccharomyces cerevisiae. Mol Cells 10:263–268
Yi-Guo S, Bao-Xing D, Wan-Ke Z, Jin-Song Z, Shou-Yi C (2002) AhCMO, regulated by stresses in Atriplex hortensis, can improve drought tolerance in transgenic tobacco. Theor Appl Genet 105:815–821
Yoshiba Y, Kiyosue T, Katagiri T, Ueda H, Mizoguchi T, Yamaguchi-Shinozaki K, Wada K, Harada Y, Shinozaki K (1995) Correlation between the induction of a gene for delta 1-pyrroline-5-carboxylate synthetase and the accumulation of proline in Arabidopsis thaliana under osmotic stress. Plant J 7:751–760
Zapata PJ, Serrano M, Pretel MT, Amoros A, Botella MA (2004) Polyamines and ethylene changes during germination of different plant species under salinity. Plant Sci 167:781–788
Zhifang G, Loescher WH (2003) Expression of a celery mannose-6-phosphate reductase in Arabidopsis thaliana enhances salt tolerance and induces biosynthesis of both mannitol and a glucosyl-mannitol dimer. Plant Cell Environ 26:275–283
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Zhu B, Su J, Chang M, Verma DPS, Fan YL, Wu R (1998) Overexpression of a D1-pyrroline-5-carboxylate synthetase gene and analysis of tolerance to water-and salt-stress in transgenic rice. Plant Sci 139:41–48
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Saxena, S.C., Kaur, H., Verma, P., Petla, B.P., Andugula, V.R., Majee, M. (2013). Osmoprotectants: Potential for Crop Improvement Under Adverse Conditions. In: Tuteja, N., Singh Gill, S. (eds) Plant Acclimation to Environmental Stress. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-5001-6_9
Download citation
DOI: https://doi.org/10.1007/978-1-4614-5001-6_9
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-5000-9
Online ISBN: 978-1-4614-5001-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)