Abstract
Stingless bee pot-honey is a valuable product with a long tradition of harvest and consumption. The differences found among meliponine honeys with respect to their physicochemical composition, sugar content, and floral origin depend not only on the geographic region but also on the stingless bee species kept for honey production. Tropical habitats are frequently shared by several dozen meliponine species and, consequently, diet overlap in terms of food plants used is considerable. Competition and the selective pressure to maximise food collection shaped a rich variety of foraging-related traits among the stingless bees. In the present chapter, we want to give a brief overview of this diversity, discussing the importance of morphological traits (tongue length, body colour, and body size) for the separation of fundamental food niches among the Meliponini. In contrast to a species’ fundamental niche, which is delimited by the morphological and physiological characteristics of an organism, the food niche realised by a species is determined through the interactions with other organisms that share the same fundamental food niche. Here, differences in foraging strategy among the stingless bees with regard to aggression, recruitment ability, and recruitment precision influence dominance relationships at a feeding site and, thus, are important factors concerning the partitioning of resources.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
When thinking about bees and flowers, frequently an image of a balmy spring-meadow where honey bees, and sometimes maybe a bumble bee, peacefully buzz from flower to flower almost automatically pops up in our minds. Yet, as so often, nature is much more realistic than our soft-focus-lens imagination, for there is tough competition for available food in the insects’ world. Thus, our romantic summer-meadow is far from being an amicable place, but rather resembles a free cold buffet at which all invited and uninvited guests, each one equipped with his/her particular little vicious tricks and strategies, struggle to get the major portion.
Due to the rich diversity of both flowering plants and flower-visiting insects, the tropics have been an ideal evolutionary playground to develop a spectacular diversity of plant–insect, plant–plant, and insect–insect interactions, governed by the continuous struggle for survival and successful reproduction. Plants, on the one hand, have evolved a fascinating variety of floral shapes, flowering traits, and phenological strategies in order to prevail in the inter- and intraspecific competition for pollinators (Bawa 1983; Frankie et al. 1983; Waser 1983; Caruso 2000). Flower-visiting insects, on the other hand, have developed a no less impressive diversity of strategies and mechanisms aiming at maximising the exploitation of floral foraging bonanzas (Johnson 1983; Roubik 1989; Goulson 1999).
In virtually all tropical habitats, the most abundant flower visitors are bees, in particular the eusocial corbiculate bees: the stingless bees (Apidae, Meliponini), bumble bees (Apidae, Bombini), and honey bees (Apidae, Apini) (Roubik 1989; Bawa 1990; Biesmeijer and Slaa 2006). In contrast to solitary insects, which collect food for their individual and direct fitness, foragers of social insect colonies gather food to guarantee the successful rearing of the brood and to satisfy the energetic demands of all non-foraging colony members (Wilson 1971; Michener 1974; Jarau and Hrncir 2009). The survival of a bee colony, therefore, largely depends upon the success of the foragers in collecting carbohydrates (usually nectar) and proteins (usually pollen). Both these food items are stored within the nest to insure a constant food supply, thus preventing potentially fatal colony-weakening during periods of resource scarcity.
Most stingless bees are generalist foragers, and even those species with a relatively low niche breath usually collect at a wide array of food plants (Wilms et al. 1996; Ramalho 2004; Biesmeijer and Slaa 2006). Thus, and due to the fact that tropical habitats are frequently shared by several dozen meliponine species, diet overlap in terms of food sources used is considerable. The generalised utilisation of common resources among stingless bees results in both interference and scramble competition between species, which reduces not only the foraging efficiency at food patches but also diminishes the pollen and nectar harvest of colonies (Johnson 1983; Johnson and Hubbell 1974; Roubik 1980; Roubik et al. 1986; Wilms and Wiechers 1997; Biesmeijer et al. 1999a; Nagamitsu and Inoue 2005; Maia-Silva et al. 2010a). Thus, selective pressure to maximise food collection led to the evolution of a rich variety of foraging strategies among meliponine bees that differ according to variation in different foraging-related traits, among them morphology, foraging strategy, aggressiveness, and recruitment efficiency (Lindauer and Kerr 1958; Johnson 1983; Roubik 1989; Biesmeijer et al. 1999a; Biesmeijer and Slaa 2004; Nieh 2004; Willmer and Stone 2004; Nagamitsu and Inoue 2005; Barth et al. 2008; Hrncir 2009; Jarau 2009). With the present chapter, we want to give a brief overview of some of this diversity found among stingless bees shaped by the competition for food.
2 Food Niches
If we want to understand the diet breath of stingless bees, why they collect at particular plant species while ignoring others, we need to differentiate between a species’ fundamental food niche and its realised food niche (Biesmeijer and Slaa 2006). The fundamental niche, on the one hand, is the ecological niche occupied by a species in the absence of competitors. Its breath is determined by both the morphological and physiological characteristics of the respective organism. A species’ realised niche, on the other hand, is determined through the interactions with other organisms and, thus, depends on the competitor-community of the respective habitat. In the following, we discuss some morphological traits, tongue length, body colour, and size, which putatively play a major role for the separation of fundamental food niches among stingless bees. Further, concerning the realised food niche, we discuss how differences in foraging strategy with regard to aggression, recruitment ability, and recruitment precision may influence dominance relationships at a feeding site and, thus, the partitioning of resources.
3 The Fundamental Food Niche: Tongue Length as Predictor of Flower Choice
A major trait for the segregation of stingless bee species in order to reduce competition for food is their morphology. At least since Charles Darwin (1859) it has become clear that the body shape of a bee species is adapted to the plants at which it collects floral resources. In “The Origin of Species” (1859), Darwin wrote: “The tubes of the corollas of the common red and incarnate clovers (Trifolium pratense and incarnatum) do not on a hasty glance appear to differ in length; yet the hive-bee [honey bee; authors’ note] can easily suck the nectar out of the incarnate clover, but not out of the common red clover, which is visited by humble-bees [bumble bees; authors’ note] alone; so that whole fields of the red clover offer in vain an abundant supply of precious nectar to the hive-bee”. More recent, detailed studies investigating possible correlations between bee morphology and flower choice corroborate Darwin’s observations indicating in both stingless bees and bumble bees a morphological matching between tongue length and corolla depth of the preferentially visited flowers (Heinrich 1976; Pleasants 1983; Harder 1985; Johnson 1986; Nagamitsu and Inoue 1998) (Fig. 13.1). Yet, as demonstrated for bumble bees, the relationship between glossa length and corolla depth is not a straight one: long-tongued bees are able to collect nectar at flowers with both long and short corollas, whereas short-tongued species are restricted to shallow flowers. Consequently, species with a long glossa, hypothetically, have access to nectar in a greater diversity of food plants (larger fundamental food niche breath) than those with a short glossa (Heinrich 1976; Harder 1985; Johnson 1986).
Bee morphology, nectar feeding, and illegitimate flower-visits. Since floral morphology determines the accessibility to floral resources, stingless bees with different tongue length should specialise on different plant species. (a) Example of bee tongue-flower-matching: Trigona spinipes collecting nectar at Waltheria rotundifolia (Malvaceae). (b) Example of an illegitimate flower-visit: Melipona subnitida collecting nectar at Tarenaya spinosa (Capparaceae), which is pollinated by bats. (c) Flowers of Tarenaya spinosa: note the protruding stamina. (d) Example of nectar robbing: Trigona spinipes collecting nectar through a hole at the flower-base of Hibiscus sp. (Malvaceae). Photos: M. Hrncir
Increasing corolla depth raises the energetic costs of foraging due to an increase in probing time. Probing time comprises, in essence, two components: access time, which increases linearly with corolla depth, and ingestion time, which increases with corolla depth only in those flowers that are deeper than the bee’s glossa due to a reduced nectar uptake rate (Harder 1983, 1985). Thus, given that bee species with long tongues have the choice to collect nectar from flowers with both shallow and long corollas, why should they bother feeding at deep flowers, thereby unnecessarily increasing their foraging costs? In an investigation of 13 bumble bee-visited plant species, Harder (1985) demonstrated that the average 12-h sugar production was positively correlated with corolla depth. This elevated offer of sugar, and, consequently, energetic gain, putatively is the crucial incentive for bees to visit deep-flower plants as long as the net energetic profit of nectar collection remains positive. Thus, when available, bees should preferentially feed from flowers with corollas approximately as deep as their glossae (Harder 1985).
The high sugar reward of several deep flowers certainly is interesting for most nectar-feeding animals, and several species evolved strategies to circumvent the elevated energetic costs associated with probing time. Several bee species, for instance, easily enter the flowers designed for larger animals, such as bats or humming birds, without even getting anywhere close to the plant’s reproductive units (Heard 1999) (Fig. 13.1). The extremists among these illegitimate flower-visitors are bees who steal nectar and pollen by entering the flowers through piercing or biting (Wille 1963; Inouye 1980; Roubik 1982) (Fig. 13.1). Among the Meliponini, species of the genus Trigona have brought this larceny-technique to perfection. Through goal-directed mass-recruitment, these bees are able to activate a large number of nestmates to profitable food patches and, subsequently, defend them against other flower-visitors. Thus, after perforating a flower, and recruiting additional foragers to the food source, the bees aggressively dominate the flower patch, repelling other bees or even hummingbirds through joint attacks. The detrimental effect of these robbers for the plants, therefore, is not so much the damaging of the floral structures, but the fact that they prevent potentially effective pollinators from visiting the flower (Roubik 1982; Heard 1999).
4 The Fundamental Food Niche: Body Colour, Body Size, and Thermal Tolerance
In addition to the, since Darwin well-established, relation between flower morphology and bee tongues, two morphological traits, related to thermal tolerance, are considered responsible for the spatio-temporal separation of niches among bee species: body size and colouration (Biesmeijer et al. 1999a, b; Pereboom and Biesmeijer 2003).
Tropical and subtropical bees, such as the Meliponini, are constrained by high ambient temperatures and heat production when foraging (Heinrich 1993; Biesmeijer et al. 1999a; Pereboom and Biesmeijer 2003). Due to the production of excess temperature when flying, many bees are exposed to the danger of overheating, some even operating close to their lethal limit. In full sunlight, generally, small bees heat up and cool down more rapidly than large bees (Fig. 13.2), but, in contrast to large bees, they will not attain excessively high body temperatures due to an elevated convective heat loss (Digby 1955; Pereboom and Biesmeijer 2003) (Fig. 13.2). Large species, therefore, run a higher risk of overheating than small species when foraging in sunshine. Here, body coloration comes into play. Physically, temperature excess and overheating are proportional to absorptivity (radiation absorbed by an object). Consequently, since absorptivity is lower for light than for dark colours (pale-coloured insects: 63–86%; dark-coloured insects: 71–117%Footnote 1; Digby 1955), pale-coloured bees heat up more slowly in full sunlight than dark-coloured bees (Digby 1955; Pereboom and Biesmeijer 2003) (Fig. 13.2).
The importance of body size and colouration for heat gain and heat loss of stingless bee foragers. Scatter plots showing the correlation between body temperature (thorax width) and temperature excess (maximum difference between thoracic and ambient temperature) (a) as well as passive cooling/heating (cooling constant K) (b) of 24 species of stingless bees. Light-coloured bees (grey-filled circles) have a lower temperature excess and cool down (and warm up) less rapidly than dark bees (black-filled circles) of similar size. Dashed lines indicate linear regressions for light-coloured and dark-coloured bees (data from Pereboom and Biesmeijer 2003)
Stingless bees show both a spatial and temporal segregation concerning sunlit flower-patches in compliance with the thermal characteristics assigned to body size and colouration (Fig. 13.3). Meliponine species of similar size, but differing in body colour, partition patches of the same floral resource according to sunlight incidence.Footnote 2 In consequence of differential evaporation, inter-patch differences in illumination result in more concentrated nectar in sunlit flower patches as compared to shaded patches (Willmer and Corbet 1981; Biesmeijer et al. 1999a, b). Consequently, light-coloured Meliponini, which favour sunlit patches, collect more concentrated nectar from the same plant species and at the same time of day as do dark-coloured species that prefer the shaded patches (Biesmeijer et al. 1999b) (Fig. 13.4).
Spatial niche differentiation among stingless bee species differing in body colouration. (a) Under clear sky-conditions, foragers of the light-coloured Melipona beecheii (grey bars) preferentially collect at sunlit patches whereas the dark-coloured M. costaricensis (black bars) prefer food patches in the shade. (b) Under changing weather conditions, foragers of M. costaricensis react immediately with respect to their patch preference in response to switches from sunny to cloudy weather or vice versa (data from Biesmeijer et al. 1999a)
Sugar concentration of nectars collected by stingless bee species differing in body colouration. Light-coloured Melipona beecheii (grey-filled bars and squares) collect nectars of significantly higher sugar concentration than dark-coloured M. costaricensis (black-filled bars and squares). (a) Percentage of foragers returning with loads of the respective sugar concentration. (b) Variation of sugar concentration (mean ± 1 SD) of nectar collected in the course of a day. (c) Sugar concentration (mean + 1 SD) of nectar of different botanic origin obtained from foragers at the nest entrance. [1] Oyedaea verbesinoides (Asteraceae); [2] Vernonia patens (Asteraceae); [3] Bidens squarrosa (Asteraceae); [4] Type 11; [5] cf. Heliocarpus (Malvaceae); [6] Hyptis capitata (Lamiaceae); [7] Serjania sp. (Sapindaceae); [8] Mikania micrantha (Asteraceae); [9] Bravaisia integerrima (Acanthaceae); [10] Schlegelia parviflora (Schlegeliaceae); [11] cf. Celtis (Cannabaceae); [12] Type 9; [13] Type 16; [14] Type 42; [15] Type 50 (data from Biesmeijer et al. 1999b). Photos: M. Hrncir
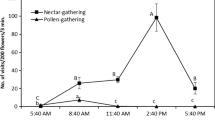
Concerning the temporal partitioning of floral resources among bee species, it has been repeatedly demonstrated that large Meliponini start foraging earlier during the day than smaller species (Fig. 13.5). The first stingless bees to initiate foraging early in the morning are species of the genus Melipona, some of which start their activity even before sunrise and at low ambient temperatures (de Bruijn and Sommeijer 1997; Pereboom and Biesmeijer 2003; Teixiera and Campos 2005; Maia-Silva et al. 2010a, b). Their capacity to fly at low temperatures is putatively related to their larger body size as compared to other stingless bee species. Due to their elevated mass of thoracic flight muscles (responsible for heat production), large species are capable of attaining ideal flight temperatures even at low ambient temperatures (Heinrich 1993), and can initiate foraging long before the small species warmed up sufficiently. Concerning the onset of flight activity, body colouration might play a decisive role for smaller species, since dark-coloured bees absorb thermal radiation more efficiently (Digby 1955) and, consequently, heat up quicker than light-coloured species (Fig. 13.5).
Temporal niche differentiation among stingless bee species differing in body size and colouration. (a–d) Foraging of big, dark-coloured Melipona quadrifasicata (a), big, pale-coloured M. scutellaris (b), and small, dark-coloured Scaptotrigona aff. depilis (c) at mass-flowering Eugenia uniflora. (d) Onset, maximum, and end of the foraging processes are influenced by body size and colouration of the respective bee species. Data collected in August 2009 at the campus of the University of São Paulo in Ribeirão Preto, Brazil (given are the proportions of bees returning to colonies with pollen loads relative to the maximum number of foragers; data are presented as mean ± 1 SD of 12 observations per species; CMS and MH, unpublished data). (e) Onset of foraging in nine stingless bee species differing in body size (given as intertegular width); MQ, Melipona quadrifasciata; MB, Melipona bicolor, PH, Partamona helleri; SX, Scaptotrigona xanthotricha; NT, Nannotrigona testaceicornis; PD, Plebeia droryana; FV, Frieseomelitta varia; FS, Friesella schrottkyi; PL, Plebeia lucii. Note earlier foraging start of dark-coloured PH compared to the similar-sized, light-coloured SX (data from Teixiera and Campos 2005). Photos: M. Hrncir
5 The Realised Food Niche: Aggression and Dominance at a Feeding Site
Stingless bee colonies are, in essence, sessile. Consequently, both the food sources available in space and time and the presence of potential competitors are determined by the nest’s location. In bee assemblages, competition for food putatively is strongest among coexisting colonies of the same species and among species of the same genus, which tend to be similar in body size, colony size, and foraging strategy, and, therefore, tend to have similar fundamental food niches (Biesmeijer and Slaa 2006). In these cases, common resources might be shared either through spatio-temporal differences in foraging activity among congeneric species (see above) or through scramble competition.
Consistent with the idea of limiting similarity (MacArthur and Levins 1967), eusocial bee assemblages in the tropics tend to consist largely of species from different genera. Even so, food niches overlap, and there is strong association among several coexisting taxa with respect to food sources used (Biesmeijer and Slaa 2006). Here, differences in foraging strategies and underlying recruitment mechanisms between different genera might be important factors concerning the partitioning of common resources.
In stingless bees, foraging strategies can be described in terms of three basic foraging traits: recruitment ability (solitary or group foraging), individual aggressiveness (present or absent), and local enhancement in heterospecific encounters (attraction or avoidance) (Biesmeijer and Slaa 2004). Among the possible combinations of these traits, a highly successful strategy is aggressive group foraging, as found in several Trigona and Oxytrigona species (Nagamitsu and Inoue 1997; Johnson 1983; Slaa 2003). These mass-recruiting aggressive species form dense forager groups through local enhancement, and attack everything at or near the exploited food patch. Consequently, these bees “extirpate” less aggressive group foragers or solitary foraging species at the food patch, and, thus, monopolise clumped and rich resources (Johnson and Hubbell 1974, 1975; Johnson 1983; Biesmeijer and Slaa 2004; Lichtenberg et al. 2010). However, due to a low independent scouting activity, aggressive mass-recruiters have a limited capacity of discovering new food sources or even neighbouring food patches independently (Hubbell and Johnson 1978; Biesmeijer and Slaa 2004).
Although aggressiveness can lead to dominance at a food patch, it should not be used as a direct measure for dominance. Rather, dominance should be interpreted as the suppression or exclusion of one species or individual by another (Johnson and Hubbell 1974; Lichtenberg et al. 2010). In solitarily foraging animals, like many vertebrates, larger and stronger species, or individuals within a species, tend to dominate at a feeding site. In social insects, however, the strength often lies in numbers. When a large group of foragers of a single colony arrives at a feeding site, other species are often at a loss due to the sheer fact that they cannot find a free spot to land and feed (Johnson 1983; Biesmeijer et al. 1999a; Hrncir 2009; Lichtenberg et al. 2010). Consequently, non-aggressive mass-recruiters, such as Scaptotrigona, Partamona, and some Trigona species, are able to numerically dominate rich clumped patches, excluding other species even without aggressive interactionsFootnote 3 (Johnson 1983; Biesmeijer and Slaa 2004; Lichtenberg et al. 2010). Scrambler species that forage individually or in small groups, therefore, would need to move to less disputed, often poorer feeding sites or, alternatively, arrive at rich patches ahead of the mass-recruiting species.
6 The Realised Food Niche: First Come First Served
Many medium-sized, unaggressive Meliponini share similar floral resources (Biesmeijer and Slaa 2006) and, therefore, experience scramble competition when foraging. Scramble competition among colonies is highest at rich clumped food sources, such as mass flowering plants (Biesmeijer and Slaa 2006), which produce a large amount of new flowers each day over a short period of time (“big-bang” or “mass-flowering” strategy) (Augspurger 1980; Bawa 1983). Within plant populations, in general, mass-flowering individuals of a species bloom synchronously. Slight differences in the onset of flowering among individuals result in an extended blooming period on the population level (Bawa 1983). Mass-flowering plants, therefore, offer a great opportunity for colonies to hoard large amounts of food within a short period of time, and represent the predominant source of both nectar and pollen for stingless bees, contributing up to 90% of the annual nutritional input into the colonies (Wilms et al. 1996; Wilms and Wiechers 1997; Ramalho 2004).
Fully grown mass-flowering trees are usually too big to be monopolised by a single colony of mass-recruiting bees (aggressive or unaggressive). Individual or group-foraging scramblers, consequently, can exploit such kind of resource virtually undisturbed (Biesmeijer and Slaa 2006). The situation, however, might be different with small mass-flowering trees or shrubs, which can be easily defended by aggressive colonies (Johnson and Hubbell 1975) or numerically dominated by non-aggressive mass-recruiters (Johnson 1983). Here, in order to be able to profit from such foraging bonanzas, non-aggressive scramblers that forage individually or in small groups should get to the food patch prior to others, or as long as the population density of potential competitors is low.
An important trait that allows bees to arrive at a food patch ahead of competitors is their capability to learn both the position of a potential collecting site and the time of resource availability (Johnson 1983; Biesmeijer and Slaa 2004; Schorkopf et al. 2004; Murphy and Breed 2008). Food-patch-experienced foragers, consequently, arrive at familiar feeding sites far quicker than inexperienced bees, which still have to search for it. So far, however, few studies investigated the time–place–memory of stingless bees (Biesmeijer and Slaa 2004). An important topic for future research, therefore, is to investigate whether the capacity to memorise the spatio-temporal characteristics of food sources differs among species with fundamentally different foraging strategies (aggressive mass-recruiters, unaggressive mass-recruiters, group-foraging scramblers, solitary scramblers, insinuators).
For group-foraging bees, a second parameter important for the efficient exploitation of resources is recruitment velocity (Jarau et al. 2003). Here, we have to distinguish, in essence, between mass-recruiting species (aggressive and unaggressive) and species that forage in small groups. The strategy of mass-recruiting species relies on the rapid mobilisation of a huge number of foragers to one particular feeding site. In aggressive mass-recruiters, the overwhelming multitude of recruits extirpates other species at a feeding site and, subsequently, defends this patch against other aggressive colonies (Hubbell and Johnson 1978; Johnson 1983). Through similar fast and goal-oriented recruitment, unaggressive mass-recruiters are able to dominate food patches numerically, thereby diminishing exploitative competition by other scramblers or even keeping off aggressive species (see footnote 3). In contrast to mass-recruiters, the strategy of unaggressive scrambler colonies that forage in small groups, such as Melipona or Nannotrigona species, relies on a quick mobilisation of all available recruits, yet without indicating the position of a particular food patch. Due to this lack of vector information, the foraging force spreads out over the surroundings to find any patch that carries the odour that has been brought back to the colony by successful scouts (Hubbell and Johnson 1978; Jarau et al. 2000; Slaa 2003; Biesmeijer and Slaa 2006; Hrncir 2009). Thus, when excluded from one feeding site by a mass-recruiting species (aggressive or unaggressive), the colonies are still able to profit from a rich food source by switching their foraging focus to less disputed patches (Hubbell and Johnson 1978; Johnson 1983; Biesmeijer and Slaa 2006).
Based on the differences in necessity to guide the foraging force to a specific food patch, recruitment strategies should differ between mass-recruiters and scramblers that forage in small groups with respect to the information about the exact position of a feeding site (important for mass-recruiters, useless for unaggressive scramblers) but not necessarily concerning the velocity of mobilising the foraging force. So far, few meliponine species have been analysed in detail concerning their recruitment strategies. In both mass-recruiters (Scaptotrigona aff. depilis) and unaggressive scramblers that forage in small groups (Melipona spp., Nannotrigona testaceicornis), the temporal pattern of thoracic vibrations generated by recruiting scouts within the nest is related to the profitability of a food source (Fig. 13.6). These vibrations, putatively, are an alerting signal, activating the foraging force (Hrncir 2009). Although these nest-internal recruitment signals are similar for mass-recruiters and small-group-scramblers, only the mass-recruiting species have been shown to be able to guide recruits to a specific food patch (aggressive mass-recruiters: Trigona corvina, T. hyalinata, T. spinipes; unaggressive mass-recruiters: Geotrigona mombuca, Scaptotrigona aff. depilis, S. postica, S. mexicana, Trigona recursa). In contrast to honey bees, which indicate the position of a feeding site through their waggle dance (Grüter and Farina 2009), mass-recruiting stingless bees achieve this goal-directed recruitment through species- or even colony-specific pheromone trails or pheromone marks at and near the feeding site (Jarau 2009; Stangler et al. 2009; Jarau et al. 2010; Schorkopf et al. 2011).
Activation signals of stingless bees. The nest-internal recruitment signals of stingless bees, the thoracic vibrations, are directed at the fast activation of additional foragers. The temporal pattern of the foragers’ pulsed vibrations is influenced by the value of the visited food source. Increasing energetic gains at the food patch result in longer pulses (PD), shorter intervals (ID), and, consequently, an increasing duty cycle (DC = PD/[PD + ID]). Increasing energetic costs, by contrast, result in shorter pulses, longer intervals, and a decreasing duty cycle (figure adapted from Hrncir 2009)
7 Concluding Remarks
Stingless bee pot-honey is a valuable product with a long tradition of harvest and consumption (Camargo and Posey 1990; Crane 1999). A large diversity of stingless bee species is kept by meliponiculturists all over Latin America to provide this precious gold. The differences found among meliponine honeys with respect to their physiochemical composition, sugar content, and floral origin depend not only on the geographic region where it has been harvested but also on the stingless bee species being used for honey production (Barth 1989; Souza et al. 2006; see related chapters in this book).
Tropical habitats are frequently shared by several dozen meliponine species. Consequently, diet overlap in terms of food sources used is considerable. The selective pressure to maximise food collection led to the evolution of a rich variety of foraging-related traits among the stingless bees. In our chapter, we wanted to give a brief overview of this diversity, discussing the importance of morphological characteristics (tongue length, body colour, and body size) for the separation of fundamental food niches among the Meliponini. In contrast to a species’ fundamental niche, which is delimited by the morphological and physiological characteristics of an organism, the food niche realised by a species is determined through the interactions with other organisms that share the same fundamental food niche. Here, differences in foraging strategy among the stingless bees with regard to aggression, recruitment ability, and recruitment precision influence dominance relationships at a feeding site and, thus, are important factors concerning the partitioning of resources.
To be sure, our overview is far from being complete, since our description of the foraging strategies used by stingless bees almost entirely omitted the unaggressive solitary foragers, often very small species that remain competitive through an “insinuation strategy” (Johnson 1983). These insinuators fly off a food patch when threatened by dominant species, yet they quickly return to the same site or nearby flowers and continue feeding as if indifferent to the aggressors (Biesmeijer and Slaa 2006). Several of these insinuator species, like Tetragonisca angustula or Frieseomelitta varia, are bees important for meliponiculture (Souza et al. 2006). Yet, knowledge about the foraging strategies of the small Meliponini is rather poor, probably because the large bees, like Melipona spp., and the aggressive ones, like Trigona spp., are more attractive to scientists.
Notes
- 1.
The explanation for this apparent absorptivity in excess of 100% probably lies in the site of absorption. Heat produced is carried away by conduction and convection to the air, and by conduction to the underlying body of the insect and to the other cooling surfaces (radiation being very slight). Where the surface is highly absorbing, the heat is produced at the surface where it will readily be carried away; but where the surface absorbs little of the heat, more radiation will be available for absorption throughout the thickness of the thorax. In this case, as cooling is only at the outer surface, the inside will be hotter than the outside” (Digby 1955, pp 287–288).
- 2.
- 3.
Johnson (1983) described a situation in which two non-aggressive mass-recruiters, Partamona orizabaensis (as Trigona testacea) and Scaptotrigona mexicana (as Trigona mexicana), numerically dominated the inflorescences of a Bactris palm tree. Although both these scrambler species did not exclude each other from the food patch, insinuators (small, unaggressive, and mostly solitarily foraging bees, such as many Plebeia species) did not find space to land at the inflorescences. More surprisingly, even an aggressive group-foraging species, Trigona silvestriana, was competitively outnumbered by the scrambling mass of bees and, consequently, left the patch (Johnson 1983).
References
Augspurger CK. 1980. Mass-flowering in a tropical shrub (Hybanthus prunifolius): influence on pollinator attraction and movement. Evolution 34:475–488.
Barth OM. 1989. O Pólen no mel brasileiro. Editora Luxor; Rio de Janeiro, Brazil. 151 pp.
Barth FG, Hrncir M, Jarau S. 2008. Signals and cues in the recruitment behavior of stingless bees (Meliponini). Journal of Comparative Physiology A 194:313–327.
Bawa KS. 1983. Patterns of flowering in tropical plants. pp. 394–410. In Jones CE, Little RJ, eds. Handbook of Experimental Pollination Biology. Van Nostrand Reinhold Company Inc.; New York-NY, USA. 558 pp.
Bawa KS. 1990. Plant-pollinator interactions in tropical rain forests. Annual Review of Ecology, Evolution, and Systematics 21:399–422.
Biesmeijer JC, Slaa EJ. 2004. Information flow and organization of stingless bee foraging. Apidologie 35:143–157.
Biesmeijer JC, Slaa EJ. 2006. The structure of eusocial bee assemblages in Brazil. Apidologie 37:240–258.
Biesmeijer JC, Richter JAP, Smeets MJP, Sommeijer MJ. 1999a. Niche differentiation in nectar-collecting stingless bees: the influence of morphology, floral choice and interference competition. Ecological Entomology 24:380–388.
Biesmeijer JC, Smeets MJAP, Richter JAP, Sommeijer MJ. 1999b. Nectar foraging by stingless bees in Costa Rica: botanical and climatological influences on sugar concentration of nectar collected by Melipona. Apidologie 30:43–55.
Camargo JMF, Posey DA. 1990. O conhecimento dos Kayapó sobre as abelhas sociais sem ferrão (Meliponidae, Apidae, Hymenoptera): notas adicionais. Boletim do Museu do Paraense Emílio Goeldi, Série Zoologia 6:17–42.
Caruso CM. 2000. Competition for pollination influences selection on floral traits of Ipomopsis aggregata. Evolution 54:1546–1557.
Crane E. 1999. The World History of Beekeeping and Honey Hunting. Routledge; New York, USA. 682 pp.
Darwin C. 1859. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. John Murray; London, UK. 502 pp.
de Bruijn LLM, Sommeijer MJ. 1997. Colony foraging in different species of stingless bees (Apidae, Meliponinae) and the regulation of individual nectar foraging. Insectes Sociaux 44:35–47.
Digby PSB. 1955. Factors affecting the temperature excess of insects in sunshine. Journal of Experimental Biology 32:279–298.
Frankie GW, Haber WA, Opler PA, Bawa KS. 1983. Characteristics and organization of the large bee pollination system in the Costa Rican dry forest. pp. 411–447. In Jones CE, Little RJ, eds. Handbook of Experimental Pollination Biology. Van Nostrand Reinhold Company Inc.; New York-NY, USA. 558 pp.
Goulson D. 1999. Foraging strategies of insects for gathering nectar and pollen, and implications for plant ecology and evolution. Perspectives in Plant Ecology, Evolution and Systematics 2:185–209.
Grüter C, Farina W. 2009. The honeybee waggle dance: can we follow the steps? Trends in Ecology and Evolution 24:242–247.
Harder LD. 1983. Flower handling efficiency of bumble bees: morphological aspects of probing time. Oecologia 57:274–280.
Harder LD. 1985. Morphology as a predictor of flower choice by bumble bees. Ecology 66:198–210.
Heard TA. 1999. The role of stingless bees in crop pollination. Annual Review of Entomology 44:183–206.
Heinrich B. 1976. Resource partitioning among some eusocial insects: bumblebees. Ecology 57:874–889.
Heinrich B. 1993. The Hot-Blooded Insects. Springer; Berlin, Heidelberg, New York. 601 pp.
Hrncir M. 2009. Mobilizing the foraging force - mechanical signals in stingless bee recruitment. pp. 199–221. In Jarau S, Hrncir M, eds. Food Exploitation by Social Insects - Ecological, Behavioral, and Theoretical Approaches. CRC-Press, Taylor & Francis Group; Boca Raton-FL, USA. 348 pp.
Hubbell SP, Johnson LK. 1978. Comparative foraging behavior of six stingless bee species exploiting a standardized resource. Ecology 59:1123–1136.
Inouye DW. 1980. The terminology of floral larceny. Ecology 61:1251–1253.
Jarau S. 2009. Chemical communication during food exploitation in stingless bees. pp. 223–249. In Jarau S, Hrncir M, eds. Food Exploitation by Social Insects - Ecological, Behavioral, and Theoretical Approaches. CRC-Press, Taylor & Francis Group; Boca Raton-FL, USA. 348 pp.
Jarau S, Hrncir M. 2009. Social insects and the exploitation of food sources: concluding thoughts. pp. 323–330. In Jarau S, Hrncir M, eds. Food Exploitation by Social Insects – Ecological, Behavioral, and Theoretical Approaches. CRC-Press, Taylor & Francis Group; Boca Raton-FL, USA. 348 pp.
Jarau S, Hrncir M, Zucchi R, Barth FG. 2000. Recruitment behavior in stingless bees, Melipona scutellaris and M. quadrifasciata. I. Foraging at food sources differing in direction and distance. Apidologie 31:81–91.
Jarau S, Hrncir M, Schmidt VM, Zucchi R, Barth FG. 2003; Effectiveness of recruitment behavior in stingless bees (Apidae, Meliponini). Insectes Sociaux 50:365–374.
Jarau S, Dambacher J, Twele R, Aguilar I, Francke W, Ayasse M. 2010. The trail pheromone of a stingless bee, Trigona corvina (Hymenoptera, Apidae, Meliponini), varies between populations. Chemical Senses 35:593–601.
Johnson LK. 1983. Foraging strategies and the structure of stingless bee communities in Costa Rica. pp. 31–58. In Jaisson P, ed. Social Insects in the Tropics 2. Université Paris-Nord; Paris, France. 252 pp.
Johnson RA. 1986. Intraspecific resource partitioning in the bumble bees Bombus ternarius and pennsylvanicus. Ecology 67:133–138.
Johnson LK, Hubbell SP. 1974. Aggression and competition among stingless bees: Field studies. Ecology 55:120–127.
Johnson LK, Hubbell SP. 1975. Contrasting foraging strategies and coexistence of two bee species on a single resource. Ecology 56:1398–1406.
Lichtenberg EM, Imperatriz-Fonseca VL, Nieh JC. 2010. Behavioral suites mediate group-level foraging dynamics in communities of tropical stingless bees. Insectes Sociaux 57:105–113.
Lindauer M, Kerr WE. 1958. Die gegenseitige Verständigung bei den stachellosen Bienen. Zeitschrift für vergleichende Physiologie 41:405–434.
MacArthur RH, Levins R. 1967. The limiting similarity, convergence, and divergence of coexisting species. American Naturalist 101:377–385.
Maia-Silva C, Aleixo KP, Imperatriz-Fonseca VL, Hrncir M. 2010a. Competição por exploração entre duas espécies de abelhas sem ferrão (Melipona quadrifasciata, M. scutellaris): a influência do tamanho corporal na estratégia de forrageamento. CD-ROM. In Anais do XXVIII. Encontro Anual de Etologia e II. Simpósio Latino-americano de Etologia, Alfenas-MG, Brazil.
Maia-Silva C, de Souza DA, da Silva CI, Hrncir M, Imperatriz-Fonseca VL. 2010b. Forrageamento de três espécies de abelhas sem ferrão (Melipona quadrifasciata, Melipona scutellaris, Scaptotrigona aff. depilis) durante a floração em massa de Eugenia uniflora (pitanga). p. 493. In Anais do IX Encontro sobre Abelhas, Ribeirão Preto-SP, Brazil.
Michener CD. 1974. The Social Behavior of the Bees. Harvard University Press; Cambridge-MA, USA. 404 pp.
Murphy CM, Breed MD. 2008. Time-place learning in a Neotropical stingless bee, Trigona fulviventris Guérin (Hymenoptera: Apidae). Journal of the Kansas Entomological Society 81:73–76.
Nagamitsu T, Inoue, T. 1997. Aggressive foraging of social bees as a mechanism of floral resource partitioning in an Asian tropical rainforest. Oecologia 110:432–439.
Nagamitsu T, Inoue T. 1998. Interspecific morphological variation in stingless bees (Hymenoptera: Apidae, Meliponinae) associated with floral shape and location in an Asian tropical rainforest. Entomological Science 1:189–194.
Nagamitsu T, Inoue T. 2005. Floral resource utilization by stingless bees (Apidae, Meliponini). pp. 73–88. In Roubik DW, Sakai S, Karim AAH, eds. Pollination Ecology and the Rain Forest - Sarawak Studies. Springer Verlag; Berlin, Heidelberg, New York. 336 pp.
Nieh JC. 2004. Recruitment communication in stingless bees (Hymenoptera, Apidae, Meliponini). Apidologie 35:159–182.
Pereboom JJM, Biesmeijer JC. 2003. Thermal constraints for stingless bee foragers: the importance of body size and coloration. Oecologia 137:42–50.
Pleasants JM. 1983. Structure of plant and pollinator communities. pp. 375–393. In Jones CE, Little RJ, eds. Handbook of Experimental Pollination Biology. Van Nostrand Reinhold Company Inc.; New York-NY, USA. 558 pp.
Ramalho M. 2004. Stingless bees and mass flowering trees in the canopy of Atlantic Forest: a tight relationship. Acta Botanica Brasilica 18:37–47.
Roubik DW. 1980. Foraging behavior of competing Africanized honeybees and stingless bees. Ecology 61:836–845.
Roubik DW. 1982. The ecological impact of nectar-robbing bees and pollinating hummingbirds on a tropical shrub. Ecology 63:354–360.
Roubik DW. 1989. Ecology and natural history of tropical bees. Cambridge University Press; New York, USA. 514 pp.
Roubik DW, Moreno JE, Vergara C, Wittmann D. 1986. Sporadic food competition with the African honey bee: projected impact on neotropical social bees. Journal of Tropical Ecology 2:97–111.
Schorkopf DLP, Zucchi R, Barth FG. 2004. Memory of feeding time. p. 643. In Proceedings of the 8th International Conference on Tropical Bees and VI Encontro sobre Abelhas, Ribeirão Preto-SP, Brazil.
Schorkopf DLP, Morawetz L, Bento JMS, Zucchi R, Barth FG. 2011. Pheromone paths attached to the substrate in meliponine bees: helpful but not obligatory for recruitment success. Journal of Comparative Physiology A 197:755–764.
Slaa EJ (2003) Foraging ecology of stingless bees: from individual behaviour to community ecology. Doctoral thesis, Utrecht University; Utrecht, The Netherlands. 181 pp.
Souza B, Roubik D, Barth O, Heard T, Enríquez E, Carvalho C, Villas-Bôas J, Marchini L, Locatelli J, Persano-Oddo L, Almeida-Muradian L, Bogdanov S, Vit P. 2006. Composition of stingless bee honey: setting quality standards. Interciencia 31:867–875.
Stangler ES, Jarau S, Hrncir M, Zucchi R, Ayasse M. 2009. Identification of trail pheromone compounds from the labial glands of the stingless bee Geotrigona mombuca. Chemoecology 19:13–19.
Teixiera LV, Campos FNM. 2005. Início da atividade de vôo em abelhas sem ferrão (Hymenoptera, Apidae): influência do tamanho da abelha e da temperatura ambiente. Revista Brasileira de Zoociências 7:195–202.
Waser NM. 1983. Competition for pollination and floral character differences among sympatric plant species: a review of evidence. pp. 277–293. In Jones CE, Little RJ, eds. Handbook of Experimental Pollination Biology. Van Nostrand Reinhold Company Inc.; New York, NY, USA. 558 pp.
Wille A. 1963. Behavioral adaptations of bees for pollen collecting from Cassia flowers. Revista de Biologia Tropical 11:205–210.
Willmer PG, Corbet SA. 1981. Temporal and mircroclimatic partitioning of the floral resources of Justicia aurea amongst a concourse of pollen vectors and nectar robbers. Oecologia 51:67–78.
Willmer PG, Stone GN. 2004. Behavioral, ecological, and physiological determinants of the activity patterns of bees. Advances in the Study of Behavior 34:347–466.
Wilms W, Wiechers B. 1997. Floral resource partitioning between native Melipona bees and the introduced Africanized honey bee in the Brazilian Atlantic rain forest. Apidologie 28:339–355.
Wilms W, Imperatriz-Fonseca, VL, Engels W. 1996. Resource partitioning between highly eusocial bees and possible impact of the introduced Africanized honey bee on native stingless bees in the Brazilian Atlantic rainforest. Studies on Neotropical Fauna and Environment 31:137–151.
Wilson, EO. 1971. The Insect Societies. Belknap Press of Harvard University Press; Cambridge, MA, USA. 548 pp.
Acknowledgements
We would like to thank Rubens Teixeira de Queiroz for identifying the flowers in Fig. 13.1, and four anonymous reviewers for valuable comments on the manuscript. The authors were financially supported by grants of the Brazilian science foundations, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Grant 304722/2010-3 to MH), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, bolsa doutorado to CMS).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Hrncir, M., Maia-Silva, C. (2013). On the Diversity of Foraging-Related Traits in Stingless Bees. In: Vit, P., Pedro, S., Roubik, D. (eds) Pot-Honey. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-4960-7_13
Download citation
DOI: https://doi.org/10.1007/978-1-4614-4960-7_13
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-4959-1
Online ISBN: 978-1-4614-4960-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)