Abstract
Nanotechnology is the control of matter at dimensions between 1 and 100 nm. This field is expected to play an important role in molecular diagnostics, by enabling new methods based on the unique properties of nanometer-scale materials. Nanomaterials display enhanced optical, thermal, magnetic, electrical, and chemical behavior compared to bulk materials and single atoms or molecules of similar composition. In addition, they interact differently with molecules in the same size range, such as proteins and nucleic acids. Thus, nanomaterials may improve assay sensitivity, specificity, speed, capacity for multiplexing, affordability, portability, and ease of use. Materials produced by nanotechnology include nanoparticles, biobarcodes, dendrimers, nanowires, nanocantilevers, nanopores, and nanofluidic devices. Many of these structures can be functionalized with biorecognition molecules to create specific molecular diagnostic probes. Nanoparticles such as quantum dots and gold nanoparticles produce distinct fluorescent or light scattering patterns, which can be controlled by varying particle size or composition. In appropriate combinations, functionalized nanoparticle probes have been utilized in quantitative multiplexed assays, including histopathologic studies of fixed tissues. Nanowires and nanocantilevers have been employed in highly multiplexed biosensors. Nanofluidic devices include nanoelectromechanical (lab-on-a-chip) systems that perform multiple complex laboratory analyses in a compact point-of-care platform. Multifunctional nanostructures create the potential to combine molecular diagnostics with imaging and therapeutics. Thus, nanomaterials are likely to play an increasing role in early disease detection, genomic technologies, in vivo diagnosis, and personalized, predictive medicine.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 What is Nanotechnology?
-
The National Nanotechnology Initiative (http://nano.gov) states that “Nanotechnology is the understanding and control of matter at the nanoscale, at dimensions between approximately 1 and 100 nm, where unique phenomena enable novel applications. Encompassing nanoscale science, engineering, and technology, nanotechnology involves imaging, measuring, modeling, and manipulating matter at this length scale”
-
Nanoscale materials that are useful for diagnostic medicine exhibit physical, chemical, and biological properties that differ from the properties of bulk materials and single atoms or molecules
-
The properties of nanoscale materials can be varied in a controlled manner, depending on size, shape, and composition
-
Compared to other forms or sizes of the same material, nanoscale materials are often stronger; conduct heat or electricity better; have different magnetic properties; become more chemically reactive; have different optical properties; or interact differently with biomolecules in the same size range, such as proteins (1–20 nm) and nucleic acids (2.5 nm in diameter)
-
-
Nanotechnology is not simply the study of naturally occurring nanoscale materials. Rather, it is the controlled production and manipulation of nanoscale materials, designed with specific size, shape, and composition to produce intended physical, chemical, and biological properties
-
There are two main approaches to producing nanoscale materials: top-down fabrication and bottom-up synthesis
-
Top-down fabrication reduces larger pieces of material down to the nanoscale, using methods such as controlled etching, elimination, and layering, which are similar to common processes in the semiconductor industry
-
Compared to bottom-up synthesis, the top-down approach is relatively fast and inexpensive, but tends to require larger amounts of starting materials, produce excess discarded waste, and result in higher defect rates
-
-
Bottom-up synthesis creates nanoscale materials by building up from atomic- and molecular-scale components, through directed assembly or spontaneous self-assembly
-
Compared to top-down fabrication, the bottom-up approach is more precise, but tends to be expensive, time-consuming, and difficult to scale up for commercial applications
-
-
-
Regardless of the approach to fabrication, nanotechnology results in materials with one or more dimension between 1 and 100 nanometers (nm). For example
-
Nanoparticles measure 1–100 nm in all three size dimensions. Nanospheres are equal in all dimensions, while nanorods are greater in length than width
-
Nanowires, nanotubes, or nanocantilevers measure 1–100 nm in cross-sectional diameter. Length may be larger than nanoscale
-
Nanoporous materials and nanofluidic devices contain openings or channels measuring 1–100 nm. These features may be contained within structures larger than nanoscale
-
2 What Is the Likely Role of Nanotechnology for Molecular Diagnostics?
-
The aim of researchers in this field is to develop molecular diagnostic assays with novel performance characteristics, based on the unique properties of nanoscale materials. Nanotechnology has potential to improve performance of existing diagnostic platforms or create fundamentally novel diagnostic platforms
-
Characteristics that can be improved by nanotechnology include diagnostic sensitivity and specificity, analytical sensitivity (including single molecule detection), speed, multiplexing, affordability, portability, and ease of use
-
Nanoscale materials exhibit high surface area per volume and thus provide a large surface for chemical reactions or conjugation of biorecognition molecules, such as nucleic acids, antibodies, ligands, or aptamers
-
These features are important for rapid biochemical reactions with high sensitivity and specificity
-
-
Nanoparticles exhibit many unique optical properties related to their size and composition
-
They can be designed to generate intense, nonoverlapping optical detection signals, such as fluorescence or Raman light scattering
-
Nanoparticles conjugated to biorecognition molecules are used for ultrasensitive, multiplexed bioassays
-
Excitation and emission in the near-infrared (IR) or IR spectrum is possible, which reduces background signal from blood, body fluids, or tissues
-
-
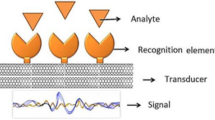
Nanocantilevers or nanowires that are conjugated to biorecognition molecules transduce specific binding of biomarkers into electrical or optical signals
-
These structures are useful for label-free detection of biomarkers in platforms such as microarrays and point-of-care devices
-
-
Nanoporous materials permit passage of specific biomolecules, based on molecular composition and shape
-
When linked to appropriate sensors (e.g., electrical), nanopores are used to detect and sequence specific DNA species or detect and probe the function of biomarkers at single molecule sensitivity
-
-
Nanomaterials have been combined with micro- or nanofluidic channels, and electrical or magnetic sensors, to create portable “lab-on-a-chip” platforms
-
Nanotechnologies have great potential for integrating molecular diagnostics with in vivo molecular imaging and therapy
-
Nanoscale materials are of appropriate size and structure for multiple functional attachments (e.g., detection signals and therapeutic agents), as well as for targeted delivery to anatomical sites
-
-
Based on the novel features of nanoscale materials, nanotechnology is widely expected to play an important role in early disease detection and personalized, predictive medicine
3 Types of Nanotechnology with Diagnostic Applications
-
Numerous nanotechnologies have been developed, for use in a diverse range of biomedical applications. Key examples are listed in Table 12.1. For additional information on a more complete listing of nanotechnologies useful for biomedicine, please refer to “Further Reading” at the end of the chapter
3.1 Nanoparticles
-
Nanoparticles can be synthesized from a variety of materials, with sufficient purity and uniformity for biomedical applications. They are usually fabricated with the bottom-up approach. Nanoparticles with great diagnostic potential generate a measurable signal (such as optical, electronic, or magnetic), which is controlled by varying particle size, shape, or composition
-
Diagnostically useful nanoparticles include semiconductor quantum dots, gold nanoparticles and nanorods, surface-enhanced Raman scattering (SERS) gold nanoparticles, and biobarcodes, among others
3.1.1 Quantum Dots (QDs)
-
Structure
-
QDs are semiconductor nanocrystals used as fluorescent labels in bioassays. The electronic and optical characteristics of QDs are highly dependent on nanocrystal size; nanocrystal shape and composition can also affect these properties
-
In electronic terms, the excitons of QDs are confined in all three spatial dimensions, with sizes smaller than the exciton Bohr radius. This “quantum confinement” creates properties intermediate between bulk semiconductors and individual molecules
-
Typical QDs used in diagnostic assays are colloidal inorganic nanocrystals with diameters ranging from 2 to 8 nm. They are often produced as core/shell structures, with a cadmium selenide core capped by a zinc sulfide shell (CdSe/ZnS)
-
Unmodified QDs are neither water-soluble nor biocompatible, but they can be coated, for example, with silica or an amphiphilic substance, such as polyethylene glycol, for use in bioassays
-
The optical emission of a QD is determined by its band gap, which describes the energy difference between the excited state and resting state of an electron in the nanomaterial
-
Small nanocrystals generally display large band gaps. Thus, smaller QDs emit bluer (higher-energy) fluorescence, while larger QDs emit redder (lower-energy) fluorescence
-
-
Biocompatible QDs can be conjugated to antibodies, oligonucleotides, or aptamers, or coated with streptavidin, for specific binding to biomarker targets in molecular diagnostic assays (Fig. 12.1)
-
-
Optical signal
-
QDs exhibit several optical properties that are advantageous for bioassays, compared with conventional organic fluorescent dyes (Fig. 12.1)
-
QDs emit strong fluorescence, with high quantum yields and molar extinction coefficients 10–50 times larger than organic dyes, making them much brighter in fluorescent assays
-
QD fluorescence intensity is highly stable in comparison to organic dyes, which can rapidly lose fluorescence through irreversible chemical changes. This photostability reduces the effect of photobleaching on assay performance and permits archiving of specimens
-
QDs exhibit size-tunable fluorescence emission color. QDs are manufactured under controlled conditions to specify precise sizes, resulting in different species with different fluorescent properties
-
Researchers have extended the emission wavelength into the near infrared (NIR) (650–950 nm) to take advantage of the improved tissue penetration and reduced background, which is of particular value for assays of whole blood and tissues, including histopathology
-
QDs exhibit broad excitation and narrow emission spectra. Therefore, multiple QDs can be excited with a single wavelength, and their emissions can be resolved with relative ease. These properties make QDs well suited for multiplexed assays
-
QDs have been embedded in various combinations in larger structures, such as microbeads, to create optical barcodes. Theoretically, one million barcodes are possible by combining six resolvable QD emission colors and ten intensity levels for each color. Beads embedded with specific barcodes are conjugated to unique biorecognition molecules for very high-level multiplexed bioassays
-
-
Biomedical applications
-
Applications include in vitro diagnostic assays such as immunofluorescence microscopy, flow cytometry, and multiplexed barcoding assays. Since QDs are typically composed of toxic heavy metals, the applicability to in vivo diagnostics is limited
-
Immunofluorescence microscopy of cells and formalin-fixed paraffin-embedded tissues has been pioneered by the group of Shuming Nie at Emory University, among others (Figs. 12.2 and 12.3)
-
The capacity for high-order multiplexed analysis using a single excitation source is valuable for protein expression profiling of cancer and other complex diseases
-
Signals from multiple QDs are detected with standard fluorescence microscopy and can be easily resolved and quantified with spectral imaging techniques
-
Effects of tissue autofluorescence are reduced by use of QDs that emit at red or near-infrared wavelengths
-
Data are more quantitative than standard immunofluorescence or immunohistochemistry
-
While recent advances in computerized image analysis and multispectral imaging have increased the potential of quantitative multiplexed analysis using bright-field immunohistochemistry, the capacity to develop systems for high-order multiplexed analysis with precise quantification is expected to be greater with QDs
-
Table 12.2 shows a comparison of quantum dot immunofluorescence with standard immunohistochemistry. In the majority of pathology departments, routine tests are designed to analyze one biomarker semiquantitatively per microscope slide
-
-
Multiplexed QD immunofluorescence has been used for expression profiling of prostate cancer and identifying rare Reed–Sternberg cells in lymph nodes (Liu et al. 2010a, b)
-
-
QDs have been use as fluorescent probes for flow cytometry
-
Compared to standard organic fluorescent dyes, signals are stronger and more stable
-
Fewer excitation wavelengths can produce a larger number of resolvable fluorescent signals
-
Table 12.3 shows a comparison of QD fluorescence with the properties of standard organic fluorophores
-
-
-
The Nie group achieved multicolor optical barcoding by embedding different-sized QDs into porous silica and polystyrene microbeads at precisely controlled ratios
-
The QD-tagged beads were microscopically and spectroscopically uniform and reproducible, yielding bead identification accuracies as high as 99.99%
-
When conjugated to nucleic acid probes, the beads were able to detect and distinguish DNA species in multiplexed assays (Gao and Nie 2005)
-
-
Luminescent semiconductor quantum dot (QD). (Left) Structure of typical CdSe/ZnS QD. (Top right) Size-tunable fluorescent emission of QDs. Note narrow emission spectra. (Bottom right) Fluorescence from aqueous suspensions of QDs of increasing size, after excitation with a near-UV lamp (From S Nie laboratory)
Sequential QD staining. Two primary antibodies from two animal species are used to detect two tissue antigens. After washing, secondary antibody QD conjugates are applied. The specimen is washed and the procedure is repeated with primary antibodies for two other antigens followed by secondary antibody QD conjugates with distinct emission (Adapted from Liu et al. 2010b)
3.1.2 Gold Nanoparticles
-
Structure
-
The synthesis of colloidal gold nanoparticles ranging from 3 to 100 nm in size is very reproducible. Particles are stable and their surfaces can be modified by a variety of chemical reactions to create specific binding or signaling properties
-
Gold nanospheres in the range of 20–120 nm appear bright red, due to efficient light scattering and surface plasmon resonance (SPR)
-
Gold nanorods have distinct properties of tissue penetration and light absorption or scattering, based on their aspect ratio (length of the major axis divided by width of the minor axis)
-
-
Biomedical applications
-
Gold nanoparticles are used in many clinical bioassays based on colorimetry, fluorescence, and light scatter. Gold nanoparticles are nontoxic, in contrast to QDs, which could expand their potential for in vivo applications
-
Point-of-care lateral flow (immunochromatographic) tests represent an early and very common diagnostic application for 20–120 nm gold nanoparticles
-
In a standard lateral flow assay, the analyte is applied to a solid substrate (such as a nitrocellulose strip) and flows in a single direction via capillary action
-
During lateral flow, the analyte is bound by a colored detection reagent (such as gold nanoparticle bound to antibody), and the complex migrates until encountering zone(s) on the substrate that are pretreated with a capture molecule (such as a capture antibody)
-
Capture of the complex results in a line that can be detected visually or with an instrument
-
Gold nanoparticles and dyed latex particles are the most commonly used detection reagents in lateral flow assays. Gold nanoparticles have several advantages over latex
-
They are smaller and have higher diffusion rates, allowing them to mix well with the analyte during lateral flow and readily penetrate pores in the solid substrate
-
They pack very densely at the capture line, resulting in greater visibility
-
Using appropriate instrumentation, optical signals can be quantified more precisely than by simple visualization
-
FDA-cleared point-of-care lateral flow tests based on gold nanoparticles are available commercially, such as rapid tests for pregnancy or specific IgE against common allergens
-
-
-
Gold nanoparticles functionalized with nucleic acids have also been applied in commercial benchtop microarray platforms
-
Gold nanoparticles are amplified by chemical deposition of silver and detected and quantified by measuring evanescent wave-induced light scatter
-
This method was reported to be several orders of magnitude more sensitive than Cy3-based fluorescence microarray analysis
-
The gold nanoparticle microarray platform is utilized in FDA-cleared assays for respiratory viruses, inherited hypercoagulable states, and warfarin resistance (Buchan et al. 2011)
-
-
3.1.3 Surface-Enhanced Raman Scattering (SERS) Gold Nanoparticles
-
Structure
-
SERS nanoparticles are optical detection tags consisting of Raman-active reporter molecules adsorbed on a gold nanoparticle core (40–50 nm), surrounded by a biocompatible shell (Fig. 12.4)
-
Absorption of reporter molecule on gold (or other noble metal) nanoparticle results in surface enhancement of Raman light scattering, in some cases by more than one billion times
-
The shell is usually comprised of silica or polyethylene glycol. It prevents aggregation, retains the reporter on the nanoparticle, and serves as a conjugation site for biorecognition molecules
-
-
Optical signal
-
SERS nanoparticles offer the potential for extremely bright, photostable signals, with potential for high-level multiplexing. The theory of surface-enhanced Raman scattering is described below
-
Raman scattering is inelastic scattering of photons from a molecule, caused by interaction of incident light with molecular vibrations
-
The vibrational modes available to a molecule are influenced by its unique chemical bond structure. Thus, a molecule is characterized by a unique Raman spectral signature, comprised of multiple narrow spectral peaks (Fig. 12.4)
-
The vast majority of light scattering is elastic (Rayleigh scattering), with scattered photons characterized by the same frequency and wavelength as incident photons
-
Rare photons undergo inelastic Raman scattering, when incident light interacts with chemical bonds to excite molecules from ground state to a virtual vibrational energy state
-
Raman-scattered photons are characterized by shifted frequency and wavelength compared to incident light, usually of lower energy (Fig. 12.4)
-
Since unenhanced Raman scattering is a relatively rare event, signals are weak and difficult to resolve from the dominant Rayleigh scattering
-
-
Raman scattering is enhanced by many orders of magnitude when molecules are adsorbed on a rough noble metal surface, such as a gold nanoparticle
-
Raman enhancement is mainly due to surface plasmon resonance
-
In contrast to unenhanced Raman spectra, SERS spectra are sufficiently intense to serve as detection signals for biomedical assays
-
-
-
SERS nanoparticles have many optical properties that are useful for bioassays
-
Signals are intense and stable, with a high signal-to-noise ratio, providing high analytical sensitivity
-
Surface enhancement results in optical emission that is stronger and more resistant to photobleaching than organic fluorophores or even QDs (Qian et al. 2008)
-
Excitation can be achieved with red or near-infrared wavelengths, which minimizes background autofluorescence from biological materials
-
-
Raman spectra are quantitative and specific to the unique chemical bonds of the reporter molecule, resulting in high specificity and capacity for multiplexing
-
Raman spectra cover vibrational energies from 300 to 5,000 cm–1 and are characterized by narrow line widths that can be easily resolved in multiplexed signals (Fig. 12.4)
-
-
SERS nanoparticle shells are readily conjugated to a variety of biorecognition molecules such as antibodies, aptamers, ligands, and nucleic acids
-
Multiplexed assays are developed using a panel of SERS nanoparticles, conjugated to distinct biorecognition molecules, and distinguished by specific Raman reporter molecules
-
-
-
Clinical applications
-
SERS nanoparticles have been used for multiplexed immunoassays, tumor targeting, and detection of circulating tumor cells
-
Natan et al. developed a multiplexed lateral flow assay for respiratory viruses using silica-encapsulated SERS nanoparticles. Assay signals were resolved with a Raman spectrometer applicable for benchtop or point-of-care testing
-
SERS nanoparticles improved virus detection sensitivity, quantification, and assay reproducibility compared to standard lateral flow tests
-
-
The same group devised a homogeneous SERS-based cell detection assay for rapid quantification of circulating tumor cells in whole blood (Sha et al. 2008)
-
Magnetic beads and silica-encapsulated SERS nanoparticles were conjugated to antibodies against distinct cancer biomarkers, for cell capture and detection in homogenous assays
-
Near-infrared excitation permitted use of the SERS nanoparticles in whole blood
-
The reaction was quantitative, with a limit of detection of 50 tumor cells/mL
-
-
The Nie group has described a polyethylene glycol-encapsulated SERS nanoparticle assay for circulating tumor cells (Wang et al. 2011)
-
SERS nanoparticles conjugated to epidermal growth factor (EGF) were incubated with circulating tumor cells and admixed leukocytes, isolated from whole blood by density-gradient centrifugation
-
Cells were washed, pelleted, and assayed by infrared light excitation (785 nm)
-
Raman signals were analyzed with a handheld Raman spectrometer
-
-
The reported limit of detection was 5–50 circulating tumor cells/mL of whole blood
-
In a clinical trial, circulating tumor cells were identified in 17 of 19 patients with EGF receptor-positive head and neck squamous cell carcinoma
-
-
The Nie group targeted EGF receptor-positive tumor cells in vitro and in vivo, using polyethylene glycol-encapsulated SERS nanoparticles conjugated to anti-EGF receptor antibody (Fig. 12.5)
-
-
Surface-enhanced Raman scattering gold nanoparticles. (Top left) Encapsulated SERS nanoparticle. (Top right) Raman spectra of reporter molecules. Each spectral signature (A–E) contains multiple narrow peaks with reproducible relative peak size. Total area under peaks is proportional to amount of reporter. Each spectrum has one or more unique peaks. Deconvolution of complex spectral data is capable of resolving signals in quantitative multiplexed assays. (Bottom) Schematic diagram of SERS phenomenon
In vitro tumor cell targeting with SERS nanoparticles. The targeted SERS agent consisted of a gold nanoparticle core, Raman reporter, and polyethylene glycol shell, conjugated to anti-EGF receptor (EGFR) antibody. Experimental tumor cells (EGFR-positive) were treated with the targeted SERS agent, washed, and analyzed by Raman spectroscopy. The SERS spectrum from treated cells was identical to the pure SERS nanoparticle (compare red and black spectra on right). No SERS signal was obtained from negative controls, including experimental cells treated with control SERS agent (no conjugated antibody) and control cells (EGFR-negative) treated with targeted SERS agent (black, blue, and green spectra on right). Targeting was also achieved when tumor cells were implanted in mice and SERS agents were injected in the tail vein (not shown) (From Qian et al. 2008, with permission)
3.1.4 Dendrimers
-
Structure
-
Dendrimers are branched, polymeric nanomaterials with symmetric and ordered architecture. They are synthesized in a highly controlled, sequential process to produce nanoparticles with near perfect monodispersity
-
Dendrimers can be grown using a divergent process (where all of the branches grow exponentially from the core) or in a convergent process (where individual branches, or dendrons, are grown separately and then attached to the core during a final step)
-
Dendrimer size is largely controlled by its generation, which refers to the number of branch points moving radially from the core to the periphery (G1, G2, G3, etc.)
-
A wide variety of materials can be used in dendrimer synthesis, providing versatile material properties which can be tuned for the intended application
-
One important property of dendrimers is multivalency, resulting from the highly branched architecture. Multivalency results in a number of points for the attachment of detection agents, imaging agents, and targeting molecules or drugs, for the production of multifunctional biomedical tools (Fig. 12.6)
-
-
Biomedical applications
-
Dendrimers have been used for drug and gene delivery and can be modified with imaging moieties to serve as nanoparticle contrast agents for bioimaging
-
Wiener et al. developed a new class of MRI contrast agent using dendrimers as a carrier for gadolinium
-
PAMAM dendrimers ranging from 8.5 to 140 kDa in size were produced and used to chelate gadolinium ions in a nanocomplex
-
The nanoparticle contrast agents showed up to six-fold enhancement of the relaxivity per Gd(III) ion in comparison to standard chelates and had significantly increased half-lives in vivo, making them ideal for in vivo imaging applications
-
-
Kukowsla–Latallo et al. have synthesized a dendrimer-based carrier for the targeted delivery of therapeutics for cancer treatment
-
A G5 PAMAM dendrimer was synthesized to produce a nanoparticle <5 nm in diameter with approximately 100 functional groups on the surface
-
Folic acid and methotrexate were conjugated to the nanoparticle for targeting functionality and therapeutic effect, respectively
-
Targeted nanocarriers showed increased antitumor activity and reduced toxicity in comparison to free drug
-
-
3.1.5 Biobarcodes
-
The biobarcode assay was developed by the Chad Mirkin group at Northwestern University. It is an ultrasensitive, enzyme-free method to detect biomarkers including proteins and nucleic acids
-
The analytical sensitivity for nucleic acid detection is comparable to polymerase chain reaction, and for protein detection exceeds that of ELISA by several orders of magnitude
-
-
Assay system
-
In a biobarcode assay, the biomarker is detected using a sandwich method
-
Biomarker is captured by magnetic microparticles conjugated to biorecognition molecules
-
Biomarker is sandwiched by gold nanoparticles conjugated to detection biorecognition molecules, as well as a high density of “barcode” DNA sequences, which are designed to specifically represent the biomarker of interest and amplify the detection signal (Fig. 12.7)
-
For nucleic acid detection, the biorecognition molecules can be complementary nucleic acid probes (unrelated to the barcode)
-
For protein detection, the biorecognition molecules can be antibodies, ligands, or aptamers
-
Biomarkers sandwiched by the magnetic microparticle and barcoded nanoparticle are separated from solution in a magnetic field
-
Barcode DNA is dehybridized from the gold nanoparticles and measured by conventional methods such as scanned microarrays or quantitative PCR
-
The presence of numerous DNA barcodes per sandwich structure amplifies the detection signal massively, resulting in extremely high detection sensitivity
-
Assays for many biomarkers can be combined for high-level multiplexed profiling, by utilizing unique biobarcodes for each biomarker
-
-
-
Biomedical applications
-
The biobarcode system has been modified for simple desktop and point-of-care assays, as well as complex multiplexed analysis
-
The Mirkin group developed a biobarcode assay for PSA with a reported analytical sensitivity of 330 fg/ml, several orders of magnitude more sensitive than commercial immunoassays
-
In a clinical trial of patients following radical prostatectomy, the biobarcode assay detected minute residual PSA in all patients and identified patients with rising PSA at an earlier point than the commercial immunoassays (Thaxton et al. 2009)
-
-
Biobarcode assays for nucleic acids have demonstrated comparable sensitivity to PCR without enzymatic amplification of target sequence
-
Biobarcode assay. The biobarcode assay is useful for ultrasensitive, nonamplified detection of nucleic acid or protein targets. Two types of particle are used to detect biomarker. The first is a magnetic microparticle with recognition elements for biomarker (e.g., antibody). The second is a gold nanoparticle with a second recognition agent, which forms a sandwich around the biomarker target; this gold nanoparticle is also conjugated to a large number of oligonucleotide barcodes used for enhanced signal. After reaction with biomaterial containing the biomarker target, a magnetic field is used to isolate the sandwich structures, and a reducing agent is used to release the barcode strands. The barcode strands are identified and quantified using standard microarray or scanometric methods
3.2 Nanowires and Nanocantilevers
-
Nanowires and nanocantilevers have been used as label-free signal transduction systems for biomarker detection
3.2.1 Nanowires
-
Structure
-
Nanowires are materials with diameter in the nanoscale range and aspect ratios (length to width) of 1,000 or more
-
Nanowires have been fabricated from silicon, carbon nanotubes, conducting polymers, and other materials
-
They are functionalized for bioassays by conjugation to biorecognition molecules
-
Nanowires exhibit electrical properties that are not seen in bulk materials because electrons undergo quantum confinement in the lateral dimension
-
Silicon nanowire biosensors undergo a change in electrical conductance when bound by target biomarker because the binding event causes a change in chemical potential, producing a field-effect gate upon the nanowire
-
Detection sensitivity of nanowire biosensors depends on solution ionic strength. Desalting is required for samples with a high ionic strength to optimize sensitivity
-
-
Biomedical applications
-
A high density of functionalized nanowires can be combined in array formats for multiplexed analysis
-
Nanowire assays have been used for multiplexed detection of cancer biomarkers at femtomolar concentrations in undiluted serum
-
3.2.2 Nanocantilevers
-
Structure
-
Cantilevers function as mechanical sensors, which are tethered at one end and free at the other
-
Devices used for biomedical diagnostics typically consist of an array of cantilevers in the nanoscale, each conjugated to a specific biorecognition molecule (Fig. 12.8)
-
Nanocantilevers are deformed or deflected when bound by their target biomarker
-
This movement can be detected optically or electronically and transformed into electrical current for data analysis and biomarker measurement
-
-
Biomedical applications
-
A high density of nanowire or nanocantilever biosensors can be combined in electronically addressed arrays
-
In theory, large-scale circuits can be constructed in microfluidic environments, enabling the rapid measurement of large number of biomolecules from a minute sample
-
Nanocantilever device. The cantilevers are flexible nanoscale beams built using semiconductor lithographic techniques. A large number of nanocantilevers are constructed as part of a larger diagnostic device, which can be used for high-order multiplexed detection of protein, DNA, RNA, and other biomarkers
3.3 Nanofluidic Materials
-
Devices with nanoscale channels can be designed to process fluid samples for complex diagnostic analyses
-
Nanofluidic devices are generally fabricated using top-down approaches, similar to those used for the machining of microelectromechanical systems (MEMS)
-
Nanofluidic devices are often referred to as nanoelectromechanical systems (NEMS). Like MEMS, they are characterized by high accuracy and precision
-
Many examples of NEMS integrate nanoelectronic transistors with mechanical pumps or motors
-
For biomedical purposes, NEMS can serve as biological and chemical sensors
-
Total analysis systems (TAS) at the micro- or nanoscale (sometimes referred to as “lab-on-a-chip” devices) are capable of performing many analytical functions in small, self-contained units applicable to point-of-care testing of minute specimens
-
Functions include sample introduction, sample processing (such as cell lysis or dilution), analyte separation (such as electrophoresis or chromatography), and analyte detection
-
-
-
Compared with microfluidic devices, nanofluidic technologies permit novel analytical approaches by interacting with fluids at the molecular scale, with high surface areas for chemical processes
-
By controlling size and surface chemistry, nanochannel devices can be designed to perform diagnostically useful processes, with minimal requirements for sample volume (in the picoliter range)
-
By fabricating nanochannels that are sensitive to the structure or sequence of biomolecules, the devices can designed for the following functions in diagnostic assays
-
Regulated transport of analytes
-
Highly selective filtering and enrichment of specific biomolecules
-
Directed chemical reactions between diffusing reagents
-
Detection of specific nucleic acid sequences
-
-
-
Nanoporous materials can be used similarly for highly selective fractionation and binding of diagnostically important molecules from complex specimens
-
Glass and silicon nanopores have been used to selectively fractionate complex protein mixtures from biological fluids such as serum
-
In some studies, fractionated proteins were analyzed by proteomic techniques for biomarker discovery
-
-
Sequence-specific nanopores linked to electrical sensors have been applied for sensitive and specific detection of DNA species of interest
-
4 Other Clinical Applications of Nanotechnology
4.1 Molecular Imaging
-
Multifunctional nanostructures for biomedical imaging combine contrast agent with functionality that targets the agent to a desired anatomical location. These agents have potential for emerging in vivo diagnostic assays
-
Gold nanoparticles coated with polyethylene glycol have been described as radiographic contrast agents with high signal and long circulation time
-
Paramagnetic iron oxide nanoparticles have been proposed as MRI contrast agents with uniquely high contrast at low concentrations
-
Other nanoscale materials have been used as carriers for a variety of imaging agents, including buckyballs (hollow spherical carbon molecules, or fullerenes); modified recombinant adenoviruses; dendrimers (spherical, monodisperse, repetitively branched molecules with high degree of symmetry); and liposomes (spherical vesicles composed of a lipid bilayer membrane, usually containing an aqueous core)
-
Nanoscale imaging reagents have several favorable features
-
High signal with low background
-
Blood circulation times that are longer than current contrast agents
-
Generally low toxicity
-
Functionalization (for example, with biorecognition molecules) that targets agent to specific tissue
-
An important limitation of nanoscale imaging agents is accumulation in the reticuloendothelial system
-
4.2 Drug Delivery and Therapy
-
Similar to imaging agents, nanoscale materials have been utilized to deliver therapeutic agents to diseased tissues
-
Nanoparticle albumin-bound (nab™) technology employs drugs that are encapsulated in negatively charged, monodisperse albumin nanoparticles (50–150 nm)
-
The nanoparticle drugs pass through leaky capillaries in diseased tissue and may be delivered specifically by receptor-mediated transcytosis and binding to secreted protein acidic rich in cysteine (SPARC) on the surface of target cells
-
Nanoparticle albumin-bound paclitaxel is the first agent of this class and is FDA-approved for treatment of breast cancer
-
-
Liposomes can be designed for temperature- or pH-sensitivity by formulation with lipids of different fatty acid chain lengths, to permit controlled release of their contents
-
Chemotherapy drugs loaded or attached nanoparticle serve as therapeutic agents
-
The collaborative groups of Shuming Nie and Dong Shin have developed ternary complexes comprised of a self-assembling heparin-based nanoparticle, targeting ligand (such as folate or EGF), and a chemotherapeutic or imaging agent, for treatment of head and neck and other cancers
-
5 Summary of Key Points for Nanotechnology in Molecular Diagnostics
-
Nanotechnology is the understanding and control of matter at the nanoscale, at dimensions between approximately 1 and 100 nm, where unique phenomena enable novel applications
-
Compared to bulk materials and single atoms or molecules, nanoscale materials display unique optical or magnetic properties, electrical conductivity, chemical reactivity, and other features
-
Nanomaterials interact differently with biomolecules in the same size range, such as proteins (1–20 nm) and nucleic acids (2.5 nm in diameter)
-
Important examples of nanotechnology include nanoparticles, nanowires, nanocantilevers, nanopores, and nanofluidic devices
-
-
The aim of nanodiagnostics to develop assays with novel performance, based on the unique properties of nanomaterials. Nanotechnology has potential to improve diagnostic sensitivity and specificity, analytical sensitivity, speed, multiplexing, affordability, portability, and ease of use
-
Based on the novel features of nanomaterials, nanotechnology is expected to profoundly affect laboratory medicine and molecular diagnostics, playing an important role in early disease detection, genomic technologies, in vivo diagnosis, and personalized, predictive medicine
Further Reading
Azzazy HM, Mansour MM, Kazmierczak SC. Nanodiagnostics: a new frontier for clinical laboratory medicine. Clin Chem. 2006;52:1238–46.
Azzazy HM, Mansour MM, Kazmierczak SC. From diagnostics to therapy: prospects of quantum dots. Clin Biochem. 2007;40:917–27.
Buchan BW, Peterson JF, Cogbill CH, et al. Evaluation of a microarray-based genotyping assay for the rapid detection of cytochrome P450 2C19 *2 and *3 polymorphisms from whole blood using nanoparticle probes. Am J Clin Pathol. 2011;136:604–8.
Cheng MM, Cuda G, Bunimovich YL, et al. Nanotechnologies for biomolecular detection and medical diagnostics. Curr Opin Chem Biol. 2006;10:11–9.
Gao X, Nie S. Quantum dot-encoded beads. Methods Mol Biol. 2005;303:61–71.
Hu Y, Fine DH, Tasciotti E, et al. Nanodevices in diagnostics. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3:11–32.
Jain KK. Applications of nanobiotechnology in clinical diagnostics. Clin Chem. 2007;53:2002–9.
Kukowska–Latallo JF, Candido KA, Cao Z, et al. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005;65:5317–24.
Liu J, Lau SK, Varma VA, et al. Multiplexed detection and characterization of rare tumor cells in Hodgkin’s lymphoma with multicolor quantum dots. Anal Chem. 2010a;82:6237–43.
Liu J, Lau SK, Varma VA, et al. Molecular mapping of tumor heterogeneity on clinical tissue specimens with multiplexed quantum dots. ACS Nano. 2010b;4:2755–65.
Nam JM, Stoeva SI, Mirkin CA. Bio-bar-code-based DNA detection with PCR-like sensitivity. J Am Chem Soc. 2004;126:5932–3.
Qian X, Peng XH, Ansari DO, et al. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat Biotechnol. 2008;26:83–90.
Sha MY, Xu H, Natan MJ, Cromer R. Surface-enhanced Raman scattering tags for rapid and homogeneous detection of circulating tumor cells in the presence of human whole blood. J Am Chem Soc. 2008;130:17214–5.
Thaxton CS, Nam JM, Mirkin CA. PCR-like sensitivity for proteins with bio-bar-code amplification. Discov Med. 2003;3:58–60.
Thaxton CS, Elghanian R, Thomas AD, et al. Nanoparticle-based bio-barcode assay redefines “undetectable” PSA and biochemical recurrence after radical prostatectomy. Proc Natl Acad Sci USA. 2009;106:18437–42.
Wang X, Qian X, Beitler JJ, et al. Detection of circulating tumor cells in human peripheral blood using surface-enhanced Raman scattering nanoparticles. Cancer Res. 2011;71:1526–32.
Wiener EC, Brechbiel MW, Brothers H, et al. Dendrimer-based metal chelates: a new class of magnetic resonance imaging contrast agents. Magn Reson Med. 1994;1:1–8.
Xing Y, Chaudry Q, Shen C, et al. Bioconjugated quantum dots for multiplexed and quantitative immunohistochemistry. Nat Protoc. 2007;2:1152–65.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this entry
Cite this entry
Young, A.N., Kairdolf, B.A. (2013). Nanotechnology in Molecular Diagnostics. In: Cheng, L., Zhang, D., Eble, J. (eds) Molecular Genetic Pathology. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-4800-6_47
Download citation
DOI: https://doi.org/10.1007/978-1-4614-4800-6_47
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-4799-3
Online ISBN: 978-1-4614-4800-6
eBook Packages: MedicineReference Module Medicine