Abstract
Purpose and method: Monocyte chemotactic protein-1 (MCP-1) is a chemokine involved in the macrophage infiltration of tumor tissue. Tumor-associated macrophages (TAMs) are a population of mononuclear phagocytic cells that can have a complex function in tumor biology. The aim of this study was to determine the possible correlation between parenchymal MCP-1 expression and TAM level by immunohistochemical analysis of 97 invasive ductal breast carcinomas, not otherwise specified (NOS), and to investigate their relation with tumor size, histological grade, mitotic activity index (MAI) and lymph node status. Secondly, the MCP-1 mRNA was determined by reverse transcriptase–polymerase chain reaction (RT-PCR) in eight samples of normal breast tissue and 27 samples of invasive breast carcinomas and compared with TAMs. Results: MCP-1 immunoreactivity was present in tumor cells (17/97), but also in TAMs, fibroblasts and endothelial cells. The statistical analysis did not show a significant correlation between MCP-1 expression in tumoral epithelium and tumor size, histological grade, MAI, lymph node status or TAMs. The results of RT-PCR showed that, in all cases of breast carcinomas (27/27) and the majority of normal breast tissues (7/8), the number of detected MCP-1 cDNA copies was above the detection limit. However, carcinomas showed higher levels of MCP-1 mRNA than normal breast tissue. Nevertheless, the statistical analysis did not find a significant correlation between MCP-1 expression and macrophage infiltrations. Conclusion: These results indicate that MCP-1 is probably not the only and/or crucial factor involved in macrophage attraction to tumor locus in breast carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant cells and the components of tumoral stroma are interdependent. However, the progression of tumor, as well as patient’s outcome, is determined by the interaction of these two components. Tumor-associated macrophages (TAMs) are an important component of the tumoral stroma. Previously activated, macrophages can be cytotoxic to neoplastic cells in vivo (Zeigler-Heitbrock et al. 1988) but they can also facilitate tumor growth and dissemination by promoting tumor cell proliferation (Jonjić et al. 1998; Mantovani et al. 1986; Wu et al. 1993), fibrin deposition (Lorenzet et al. 1983), angiogenesis (Polverini and Laibovich 1984) and proteolytic activity (Mussoni et al. 1988). Since these cells are situated at the interference between the host and the tumor, understanding their biology is a prerequisite for possible therapeutic action. The number of TAMs in tumors of the same histological origin can vary (Evans 1972), and various tumors release different chemokines capable of inducing chemotactic migration of monocytes (Mantovani et al. 1986; Botazzi et al. 1983; Graves et al. 1989). One of these is monocyte chemotactic protein-1 (MCP-1), which is detected in many human tumors (Valković et al. 1998; Graves et al. 1992; Negus et al. 1995; Takeshima et al. 1994; Sato et al. 1995), and its chemo-attractive role in breast carcinoma is still questionable.
The recognition of biological characteristics of tumors is very important, since they can also have prognostic significance or they can be targeted for therapeutic treatment. The aim of this study was to determine MCP-1 expression immunohistochemically and by reverse transcriptase–polymerase chain reaction (RT-PCR) in tumor cells and to investigate whether this chemokine is responsible for the density of TAMs in ductal invasive carcinomas.
Materials and methods
Tissue and patients
Breast tissue was obtained from 124 surgical specimens of ductal invasive carcinomas not otherwise specified (NOS) submitted to the Department of Pathology, Medical Faculty of Rijeka. All carcinomas were histologically graded by Bloom and Richardson’s method modified by Elston and Ellis (1991). According to their size, tumors were grouped into two categories: those less than 2 cm and those more than 2 cm. In all cases, the mitotic activity index (MAI) was considered and tumors were divided in two groups: carcinomas with MAIs lower than 10 and higher than 10.
Determination of MCP-1 by immunohistochemical staining
A total number of 97 breast carcinomas was processed for immunohistochemical analysis. For the detection of MCP-1, samples were snap frozen and embedded in OCT (Miles, USA), and 6-μm sections were fixed in acetone. We used a monoclonal antibody (mAb) to MCP-1, clone 5D3-F7 (Peri et al. 1994) (obtained thanks to Alberto Mantovani, Istituto di Ricerche Farmacologiche Mario Negri, Milan, Italy) diluted to 1:100. The slides were incubated for 60 min at room temperature. Bound, primary, antibodies were detected by the standard avidin–biotin method using DAKO, LSAB kit alkaline phosphatase (DAKO, K 0682). The negative control in each tissue specimen consisted of the substitution of primary mAb with wash solution.
Two independent observers interpreted the slides; the results were compared and agreed upon. The expression of MCP-1 was determined as MCP-1 negative (no staining) or MCP-1-positive (positive staining in most tumor cells or focal positive staining with clear cytoplasmic staining).
Estimation of the MCP-1 mRNA
For this goal, RT-PCR was used. After excision, tissue from 27 ductal invasive breast carcinomas and eight normal breasts were frozen in liquid nitrogen and kept at −80° C. After mechanical homogenization by a EUROTURRAXR T 20 homogenizer (IKA, 2795101), the total RNA was extracted by TriPure Isolation Reagent (Boehringer, 1 667 165), according to the manufacturer’s instructions. One microgram of RNA was reverse transcribed using a First Strand cDNA Synthesis Kit for RT-PCR (AMV) (Roche, 1 483 188). Oligo p(dT) 15, provided in the kit, was used as a primer. The cDNA was diluted ten times and amplified by real-time PCR, using the LightCycler instrument (Roche, 2 011 468). LightCycler primer sets MCP-1 and human G-6-PDH (Search, GmbH) were used to amplify cDNA of MCP-1 and of housekeeping gene glucose-6-phosphate dehydrogenase (G-6-PDH), respectively. All other reagents were taken from LightCycler FastStart Master SybrRGreen I (Roche, 3 003 230). The quantity of the amplified cDNA was determined from standards provided in each primer set, according to the manufacturer’s protocol. The detection limit of the assays was 25 cDNA copies of respective standards. The MCP-1 mRNA expression was calculated as the number of MCP-1 cDNA copies per 100 copies of G-6-PDH mRNA.
Staining and estimation of TAMs
To visualize TAMs, single, representative, formalin-fixed, paraffin-embedded sections of invasive breast carcinoma, containing tumor parenchyma and stroma with mononuclear infiltrate, were stained with monoclonal CD68 antibodies using the standard immunoperoxidase avidin–biotin method (DAKO, clone KP1, M0814). All the sections were examined by two different methods. In tumor sections which were analyzed for immunohistochemical staining of MCP-1 the semiquantitative estimation was performed as follows: 1—no macrophages in tumoral stroma; 2—small and large foci of macrophages in tumoral stroma and 3—diffuse infiltration of TAMs in tumoral stroma.
In tumor sections that were analyzed for MCP-1 mRNA, quantitative estimation of TAMs was performed. Three areas with the highest density of macrophages were selected at low power. Macrophage count was performed under a magnification of x400 field, and the mean value was calculated (Goede et al. 1999; Hanada et al. 2000).
Statistical analysis
Statistical analysis was performed with SPSS PACKAGE, release 6.0 for PC. The chi-square test was performed to determine the statistical difference between parenchymal MCP-1 expression and other pathohistological parameters, including TAM level. The differences in numbers of cDNA copies for MCP-1 between breast carcinoma and normal breast tissue were calculated with a non-parametric Mann–Whitney U-test. The Spearman rank correlation coefficient analysis was applied to assess the association between pathobiological variables. A probability (P) level less than 0.05 was considered statistically significant.
Results
Immunohistochemical analysis
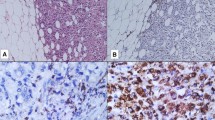
Monocyte chemotactic protein-1 immunoreactivity was present in 17/97 breast carcinomas. The staining was cytoplasmic, moderate or strong, diffused and homogenous in nature, confined to the tumor parenchyma, in most tumor cells, or focal. TAMs, fibroblasts and endothelial cells also exhibited MCP-1 immunoreactivity in the majority of tumors, and the number of positive stromal cells was highly variable, ranging from only a few to numerous cells, depending on the sections (Fig. 1). Immunoreactivity for MCP-1 in TAMs was strongly cytoplasmic, either granular or diffuse. Mostly, the positive cells were dispersed in the stroma or aggregated in small clusters around tumor cells. In a few cases, TAMs were found within the tumor nests. Fibroblasts and endothelial cells mostly exhibited faint and moderately diffused cytoplasmic staining. In cases where paraffin sections examined for CD68 immunoreactivity derived from the same tissue samples were used for MCP-1 immunolabeling, we could not observe macrophage aggregates near MCP-1-positive tumor cells. However, in many cases, we could see a correlation between angiogenic “hot-spots” and aggregates of TAMs.
Immunohistochemical analysis for MCP-1 on frozen sections of invasive ductal breast carcinoma NOS. a Tumor parenchyma is almost negative for MCP-1, while stromal cells such as fibroblasts, endothelial cells and macrophages are positive. b At higher magnification strong cytoplasmic staining can be observed in tumor-associated macrophages, while tumor cells are negative. c Moderately positive staining is observed in tumor parenchyma and strong cytoplasmic staining in tumor-associated macrophages
Statistical analysis did not show a significant correlation between MCP-1 expression in tumor epithelium and classical prognostic parameters of ductal carcinomas, such as tumor size (P=0.171), histological grade (P=0.583), MAI (P=0.526), and lymph node involvement (P=0.815). Furthermore, MCP-1 expression did not correlate with macrophage infiltration (P=0.736) (Table 1).
RT-PCR analysis
The result of RT-PCR showed that in all cases of breast carcinomas (27/27) and in the majority of the normal breast tissues (7/8), the number of detected MCP-1 cDNA copies were above the detection limit. The results and their descriptive statistical parameters are presented in Table 2. It can be observed that MCP-1 was significantly higher, statistically, in carcinomas than in normal breast tissue (P=0.001). Figure 2 shows the MCP-1 mRNA expression, calculated as the number of MCP-1 cDNA copies per 100 copies of G-6-PDH mRNA, in the malignant and non-malignant groups.
The number of TAMs within a single carcinoma was very inconsistent (mean value 49.2±22.3). Pearson’s coefficient of correlation between MCP-1 expression and macrophage infiltrations was 0.11 and was not statistically significant (P=0.586) (Fig. 3).
Discussion
Our previous results, as well as the results of other investigators, indicate a very impressive pro-tumoral role of macrophages in human malignant tumors, such as positive correlation between TAMs, proliferation and angiogenesis in invasive ductal breast carcinoma NOS (Jonjić et al. 1998; Valković et al. 2002). In addition, other authors have proved that TAMs contributed to proteolytic degradation of tumoral stroma (Pyke et al. 1993a, b). Thus, macrophages have numerous functions that can lead to tumor progression and dissemination (Bingle et al. 2002).
The basic prerequisite for TAMs’ pro-tumoral effects is their recruitment in neoplastic tissue. Chemokines facilitate leukocyte migration, and MCP-1 is one of the best-studied chemotactic molecules. By using immunohistochemistry, we demonstrated that parenchymal and stromal cells of human invasive breast carcinomas express MCP-1 (Valković et al. 1998). Furthermore, we wanted to explore the relationship between this chemokine and TAMs in ductal invasive breast carcinomas NOS, but there was no correlation between MCP-1 expression in tumor cells and macrophage infiltration, or between MCP-1 expression and standard prognostic factors such as tumor size, histological grade, MAI and lymph node involvement. We realized that immunohistochemical or in situ hybridization methods, used by us and some other authors (Negus et al. 1995), are excellent for the identification of cell types that produce MCP-1 but are not suitable as indicators of chemoattractant potential of MCP-1 in tumors. Namely, we showed that, in breast carcinoma, the tumoral parenchyma, but also different elements of the stroma, such as TAMs, fibroblasts, and endothelial cells, can express MCP-1 (Valković et al. 1998). The number of MCP-1-positive stromal cells in each section was highly variable, ranging from only a few to numerous cells, depending on the section. Furthermore, not only did the cell types and the number of positive cells vary, but the staining intensity was also inconsistent. Taking all this into consideration, it is almost impossible to create an objective semiquantitative model for the judgement of MCP-1 expression in tumors. Some authors used serum levels of this chemokine as a relevant parameter (Hefler et al. 1999), but this can also be dubious since, besides tumors, MCP-1 levels can also increase in other common diseases, such as rheumatoid arthritis (Hayashida et al. 2001), atherosclerosis (Kraemer 2000), and diabetes mellitus (Nomura et al. 2000). In addition, Inadera et al. found a significant increase in circulating levels of MCP-1 related to aging in a group of healthy Japanese (Inadera et al. 1999). In the present study we used RT-PCR to detect and quantify MCP-1 mRNA expression in ductal invasive breast carcinomas and normal breast tissue. We believe that this is a very good and objective method for determining the production of MCP-1, or more precisely their mRNA, in tumors. We found that all cases of breast carcinomas, but not all normal breast tissues, express MCP-1 mRNA. Generally, carcinomas showed higher levels of MCP-1 mRNA than normal breast tissues, which indicates a possible biological role of this chemokine in malignant breast tissue.
However, there was no positive correlation between MCP-1 and the number of TAMs. MCP-1 can regulate macrophage chemotaxis to the tumor locus but can also activate the expression of integrins during leukocyte–endothelial interaction or stimulate angiogenesis and angiostasis (Mackay 2001). To our knowledge, till now, only Ueno et al. have found a positive correlation between MCP-1 level in breast cancer extracts (measured by ELISA) and TAM accumulation, the level of some potent angiogenic factors [vascular endothelial growth factor (VEGF), interleukin-8, etc.] and poor prognosis (Ueno et al. 2000). In our previous study we found that VEGF might be an important factor in the recruitment of macrophages (Valković et al. 2002). These observations, together with the results from this study, indicate that MCP-1 is probably not the only and/or crucial factor involved in macrophage attraction to tumor locus in breast carcinoma.
References
Bingle L, Brown NJ, Lewis CE (2002) The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 196:254–256
Botazzi B, Polentarutti N, Acero R, Balsari A, Boraschi P, Ghezzi P, et al (1983) Regulation of the macrophage content of neoplasms by chemoattractants. Science 220:210–212
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast carcinoma. The value of histological grade in breast cancer. Experience from a large study with long-term follow-up. Histopathology 19:403–410
Evans R (1972) Macrophages in syngenic animal tumours. Transplantation 14:468–473
Goede V, Brogelli L, Ziche M, Augustin HG (1999) Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int J Cancer 82:756–770
Graves DT, Jiang YL, Williamson A, Valente AJ (1989) Identification of monocyte chemotactic activity produced by malignant cells. Science 245:1490–1492
Graves DT, Barnhill R, Galanopoulos T, Antoniades HN (1992) Expression of monocyte chemotactic protein-1 in human melanoma in vivo. Am J Pathol 140:9–14
Hanada T, Nakagawa M, Emoto A, Nomura T, Nasu N, Nomura Y (2000) Prognostic value of tumor-associated macrophage count in human bladder cancer. Int J Urol 7:263–269
Hayashida K, Nanki T, Girschich H, Yavuz S, Oshi T, Lipsky PE (2001) Synovial stromal cells from rheumatoid arthritis patients attract monocytes by producing MCP-1 and IL-8. Arthritis Res 3:118–126
Hefler L, Tempfer C, Heinze G, Mayerhofer K, Breitnech G, Leodolter S, et al (1999) Monocyte chemoattractant protein-1 serum levels in ovarian cancer patients. Br J Cancer 81:855–859
Inadera H, Egashira K, Takemoto M, Ouchi Y, Matsushima K (1999) Increase in circulating levels of monocyte chemoattractant protein-1 with aging. J Interferon Cytokine Res 19:1179–1182
Jonjić N, Valković T, Lučin K, Iternička Z, Krstulja M, Mustać E, et al (1998) Comparison of microvessel density with tumor associated macrophages in invasive breast carcinoma. Anticancer Res 18:3767–3770
Kraemer R (2000) Regulation of cell migration in atherosclerosis. Curr Atheroscler Rep 2:445–452
Lorenzet R, Peri G, Locati D, Allavena P, Colucci M, Semeraro N, et al (1983) Generation of procoagulant activity by mononuclear phagocytes: a possible mechanism contributing to blood clotting activation within malignant tissues. Blood 62:271–273
Mackay CR (2001) Chemokines: immunology’s high impact factors. Nat Immunol 2:95–101
Mantovani A, Ming WJ, Balotta C, Abdeljalil B, Botazzi B (1986) Origin and regulation of tumor-associated macrophages: the role of tumor-derived chemotactic factors. Biochem Biophys Acta 865:59–67
Mussoni L, Riganti M, Acero R, Erroi A, Conforti G, Mantovani A, et al (1988) Macrophages associated with murine tumours express plasminogen activator activity. Int J Cancer 41:227–230
Negus RP, Stamp GW, Relf MG, Burke F, Malik ST, Bernasconi S, et al (1995) The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J Clin Invest 95:2391–2396
Nomura S, Shouzu A, Omoto S, Nishikawa M, Fukuhara S (2000) Significance of chemokines and activated platelets in patients with diabetes. Clin Exp Immunol 121:437–444
Peri G, Milanes C, Matteucci C, Ruco L, Zhou D, Sozzani S, et al (1994) A new monoclonal antibody (5D3-F7) which recognizes human monocyte-chemotactic protein-1 but not related chemokines. Development of a sandwich ELISA and in situ detection of producing cells. J Immunol Methods 174:249–257
Polverini PJ, Laibovich SJ (1984) Induction of neovascularisation in vivo and endothelial proliferation in vitro by tumor associated macrophages. Lab Invest 51:635–642
Pyke C, Gream N, Ralfkiaer E, Ronne E, Hoyer Hansen G, Brunner N, et al (1993a) Receptor for urokinase is present in tumor-associated macrophages in ductal breast carcinoma. Cancer Res 53:1911–1915
Pyke C, Ralfkiaer E, Tryggvason K, Dano K (1993b) Messenger RNA for two type IV collagenases is located in stromal cells in human colon cancer. Am J Pathol 142:359–365
Sato K, Kuratsu JI, Takeshima H, Yoshimura T, Ushio Y (1995) Expression of monocyte chemoattractant protein-1 in meningioma. J Neurosurg 82:874–878
Takeshima H, Kuratsu J, Takeya M, Yoshimura T, Ushio Y (1994) Expression and localization of messenger RNA and protein for monocyte chemoattractant protein-1 in human malignant glioma. J Neurosurg 80:1056–1062
Ueono T, Toi M, Saji H, Muta M, Bando H, Kuroi K, et al (2000) Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis and survival in human breast cancer. Clin Cancer Res 6:3282–3289
Valković T, Lučin K, Krstulja M, Dobi-Babić R, Jonjić N (1998) Expression of monocyte chemotactic protein-1 in human invasive ductal breast carcinoma. Pathol Res Pract 194:335–340
Valković T, Dobrila F, Melato M, Sasso F, Jonjić N (2002) Correlation between vascular endothelial growth factor, angiogenesis and tumor associated macrophages in invasive ductal breast carcinoma. Virchows Arch 440:583–588
Wu S, Boyer CM, Whitaker RS, Berchuch A, Wienberg JB, Bast RCJ (1993) Tumor necrosis factor-alpha as an autocrine and paracrine growth factor for ovarian cancer: monokine induction of tumor cell proliferation and tumor necrosis factor-alpha expression. Cancer Res 53:1939–1944
Zeigler-Heitbrock HEL, Theil C, Haas JG, Moller A, Reith A, Muller G (1988) Tumor necrosis factor in human monocyte-mediated antitumor cytotoxicity. Nat Immun Cell Growth Regul 7:280–286
Acknowledgement
This work was supported by the Ministry of Science, Education and Sports of the Republic of Croatia (grant 0062060).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valković, T., Fučkar, D., Štifter, S. et al. Macrophage level is not affected by monocyte chemotactic protein-1 in invasive ductal breast carcinoma. J Cancer Res Clin Oncol 131, 453–458 (2005). https://doi.org/10.1007/s00432-004-0667-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-004-0667-3