Abstract
This chapter will primarily focus on the role of imaging in the evaluation of pituitary neoplasms (adenomas). Pituitary adenomas are usually benign indolent neoplasms. Although some may lead to symptoms due to hormonal hyperactivity with debilitating systemic consequences or from involvement of adjacent critical structures, many are asymptomatic and discovered incidentally. Adenomas arise from clonal mutations of somatic cells and their cause remains unknown. Oncogenes are not known to play a significant role in their genesis [1]. Definitive treatment involves any combination of medical and surgical intervention depending on the type of hormone they elaborate, their size, and local extent.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Key Points
-
Imaging is indicated in patients with signs and symptoms of pituitary hormonal excess or deficiency (moderate evidence).
-
MRI is the modality of choice in the initial investigation and follow-up of pituitary disorders (moderate evidence).
-
Dynamic MRI and SPGR sequences are useful adjuncts to conventional MRI in the diagnosis of pituitary microadenomas (limited evidence).
-
MRI is useful in the determination of tumor extent preoperatively (moderate evidence).
-
Inferior petrosal sinus sampling is highly sensitive for establishing the pituitary source for hypercortisolism when MRI is equivocal (moderate evidence).
2 Definition and Pathophysiology
This chapter will primarily focus on the role of imaging in the evaluation of pituitary neoplasms (adenomas). Pituitary adenomas are usually benign indolent neoplasms. Although some may lead to symptoms due to hormonal hyperactivity with debilitating systemic consequences or from involvement of adjacent critical structures, many are asymptomatic and discovered incidentally. Adenomas arise from clonal mutations of somatic cells and their cause remains unknown. Oncogenes are not known to play a significant role in their genesis [1]. Definitive treatment involves any combination of medical and surgical intervention depending on the type of hormone they elaborate, their size, and local extent.
3 Incidence/Prevalence
In a meta-analysis by Ezzat et al. [2], the overall prevalence of pituitary adenomas in autopsy and imaging series was found to be 16.7 %. McDowell et al. [1] estimate that adenomas are more common in females and also that males were more likely to present with larger tumors. They also identified a greater incidence in the African American population. About 10 % of MRI studies are estimated to reveal an incidental pituitary lesion or “incidentaloma” [3]. Incidentalomas are defined as circumscribed regions of decreased density/intensity on CT and MR scans, less than 10 mm in size. Less than 1 % of these are hormonally active.
4 Goals of Imaging
The overall goals of imaging are:
-
1.
To identify the presence of a sellar lesion and establish a differential diagnosis
-
2.
To map the extent of the lesion, with respect to the optic pathways and cavernous sinuses
-
3.
To enable monitoring of response to medical, surgical, or radiation therapy
5 Methodology
The search strategy employed both a MEDLINE search using PUBMED (National Library of Medicine, Bethesda) and Google Scholar for original research publications describing the diagnostic performance and effectiveness of different imaging modalities in the evaluation of pituitary disorders. The search covered the period January 1976 to February 2010. Different combinations of the following terms were employed: (1) Pituitary, (2) Sella, (3) MRI or CT or Venous sampling, and (4) Adenoma. Additional papers were identified by reviewing the reference list of selected publications. Only human studies in the English language literature were evaluated. The authors reviewed the titles and abstracts, and publications that were deemed relevant were then more thoroughly analyzed.
6 Discussion of Issues
6.1 Selection of Appropriate Imaging Strategy
6.1.1 Selection of Subjects for Initial Imaging
Summary
Imaging of the pituitary is indicated in patients who demonstrate laboratory evidence of pituitary dysfunction after secondary causes of pituitary hormonal excess or deficiency have been (moderate evidence). Imaging is also required to help diagnose the nature of pituitary disease, assess the effect of pituitary tumors on adjacent structures, guide surgical approach, and to enable appropriate monitoring of a lesion during the course of treatment.
Supporting Evidence
There is a general medical consensus for the need for pituitary imaging in patients with hyperprolactinemia. In an audit by Davies et al. [4], there was broad agreement on the need for imaging (MRI) in every patient with prolactin levels consistently above 1,000 mU/L to exclude an adenoma. It was also agreed that in patients with PRL above 6,000 mU/L, the presence of a prolactinoma can be assumed but that imaging was warranted to determine its size and extent and to enable monitoring during treatment. The same audit also revealed a lack of consensus on the appropriate strategy to evaluate Cushing’s disease (MRI vs. CT vs. CRH stimulation), but agreement existed that a series of dynamic tests was required. Guidelines issued by the Pituitary Society for the diagnosis and management of prolactinomas also recommend the use of gadolinium enhanced MRI after excluding potential secondary causes of hyperprolactinemia, including pregnancy. CT with contrast was deemed less effective in the diagnosis of small adenomas but was recommended if MRI was unavailable or contraindicated [5].
6.1.2 Selection of Imaging Strategy
Summary
MRI is superior to CT in the evaluation of the pituitary gland and of parasellar lesions (moderate evidence). Gadolinium-enhanced MRI is useful when unenhanced imaging fails to reveal a lesion. Dynamic MRI and SPGR sequences may be of use in equivocal cases (limited evidence). There is no standard dynamic MRI technique. Selective venous sinus sampling is a highly sensitive and specific technique for the establishment of a pituitary source of high ACTH when MRI fails to do so (moderate evidence).
Supporting Evidence
Several studies have evaluated the role of MRI and CT in the diagnosis of sellar lesions. Early studies demonstrated the superiority of unenhanced MRI [6, 7] over CT in the detection of adenomas and assessment of their parasellar extent. Peck et al. [8] demonstrated a sensitivity of 71 % for the detection of ACTH-producing microadenomas on a 1.5 T scanner. The use of a gadolinium contrast agent enabled identification of 10 out of 12 microadenomas in patients with Cushing’s disease in a series by Dwyer et al [9]. Escourolle et al. [10] also reported that MRI with contrast was able to identify ACTH producing microadenomas with a sensitivity of 69 %, compared to 50 % with CT. Conflicting reports do, however, exist in the early literature. In a study by Nichols et al. [11], MRI was found to be superior to CT in the determination of extrasellar extension but the two exams were equivalent in terms of overall lesion detection. Davis et al. [12], in a series of 13 microprolactinomas, found that unenhanced MRI was able to detect only 3, whereas CT was able to do so in 6 cases. Similar findings were reported by Pojunas et al. [13], who also compared unenhanced MRI with CT. However, the improved sensitivity of MRI today, which approaches 90 % [14], may be attributable to many factors, including the development of systems with higher field strength and higher signal-to-noise, superior spatial resolution, the use of gadolinium contrast agents, and an overall improvement in experience with image interpretation [14–16]. The unenhanced T1 weighted image, for many, remains the mainstay of adenoma detection. Gadolinium appears to confer a modest increase in sensitivity. For example, Dwyer et al., in a series of 12 patients with Cushing’s disease, found that MR with the use of gadolinium was able to detect a microadenoma in 2 additional patients out of 12, compared to unenhanced imaging [9].
False negative results with conventional MRI are usually due to the fact that some microadenomas remain isointense in signal to normal pituitary gland on precontrast and postcontrast sequences. The rationale behind the use of dynamic MRI techniques is that adenomas have dual blood supply from the hypothalamic-pituitary portal system and the meningohypophyseal branches of the internal carotid arteries and would, therefore, be expected to demonstrate a temporal difference in enhancement with respect to the normal gland during the administration of a bolus of contrast. Sakamoto et al. [17] described a dynamic technique that involved the acquisition of 7–10 SE images after rapid administration of a contrast bolus over a 20–30 s period. They observed that peak adenoma enhancement occurred 60–200 s after injection. Adenomas were best visualized during the early phases of injection, during which time they enhanced less than the normal gland. Dwyer et al. [9] warned of the variability in peak adenoma enhancement and cautioned that there was a period where imaging may not depict an adenoma due to enhancement identical with that of the normal gland. Kucharczyk et al. [18] described the use of a dynamic keyhole FSE MR technique where six sets of images were acquired through the gland. Three slices per glandular location are obtained at 11 s intervals, with a final set at 100 s. Dynamic studies revealed a lesion in 13/18 (sensitivity approximately 72 %) patients as opposed to 10/18 (sensitivity approximately 55 %) when compared to the conventional enhanced MRI [19, 20]. Rand et al. [20] concurred with these findings but used a slightly different technique, which obtained 10 slices per location, at 3 locations in the gland, at 11 s intervals. A different dynamic technique again was described by Bartynski et al. [19], where one-third of a dose of 0.1 mmol/kg contrast was infused over a 30 s period, followed by a dynamic scan for 160 s. During the course of the dynamic scan, the remainder of the contrast was administered and was followed by a coronal conventional SE sequence. In 42–47 % of the cases, the dynamic study was better than the standard postcontrast sequence in lesion detection. They also emphasized the value of analyzing both, as in 9 % of cases a lesion was seen only on the standard sequences. In a study using a half-dose protocol, Portocarrero et al. [21] were able to identify 100 % of ACTH producing microadenomas. In contrast to the above studies, no benefit was found in a dynamic technique in a series of 26 patients with ACTH dependant Cushing’s syndrome [22, 23]. They obtained nine sets of five images through the gland during injection of a bolus of contrast using a 1.0 T scanner. In their series of 21 patients with Cushing’s disease (14 surgically confirmed ACTH secreting microadenomas, 3 macroadenomas, and 4 glands which were surgically negative), conventional MRI was able to detect 8 out of 14 tumors with no false positives, whereas dynamic MR was able to detect 11 of 14 tumors but with 3 false positive cases. The above studies also reflect the variability in the literature with regard to the technique of dynamic imaging, if performed. Unfortunately, no consensus exists regarding what the optimal dynamic technique should be. Newer MR systems, with improved coils, better magnetic gradients, better magnetic homogeneity, and higher field strengths are capable of better temporal and spatial resolution. Further research to enable refinement and standardization of dynamic MRI and to validate it against standard postcontrast imaging would be of considerable importance. Dynamic studies appear to increase the sensitivity of MRI in the detection of microadenomas by a modest, but unquantifiable, degree and may be best utilized in cases where conventional MRI is negative in a patient in whom the presence of a pituitary lesion is strongly suspected on clinical and biochemical grounds.
The addition of a high-resolution 3-D MR technique, typified by the spoiled gradient echo (SPGR) sequence, to a standard pituitary MRI study has also been recommended by some authors [22, 23]. Patronas et al. [23], in a series of 50 patients with corticotroph adenomas, demonstrated an improved sensitivity of 80 % for a postcontrast SPGR sequence compared with the conventional postcontrast spin echo sequence (sensitivity 49 %), but also described a higher false-positive rate of 4 % compared to 2 % when compared to the conventional enhanced technique. According to Batista et al., this technique is especially of value in children and adolescents with Cushing’s disease [23]. In a series of 20 such patients, SPGR detected 18/28 adenomas (64 % sensitivity) but conventional contrast-enhanced imaging did so in only 5/28 (18 % sensitivity). The superiority of SPGR, according to the authors, may be due to its short acquisition time (which translates into less artifact), and superior spatial resolution (the ability to acquire 1 mm thin sections with no interslice gap). However, it is conceivable that in both of these studies the performance of the SPGR and conventional postcontrast spin echo sequence as a component of the same examination influenced the ability to detect microadenomas. The separation of the imaging times may result in the lesion being better depicted due to its inherent contrast enhancement characteristics as opposed to a superior performance for the sequence. Given that the SPGR sequence does not involve a significant increase in scan time, these sequences may be added to or used in place of a standard pituitary MR imaging protocol, when concern for a microadenoma, especially a corticotroph adenoma, exists.
6.1.3 The Role of Interior Petrosal Sinus Sampling
Cushing’s syndrome is produced by two broad categories associated with hypercortisolism – those that are dependent on ACTH and those that are not. The former includes ACTH hyperproduction from a pituitary adenoma and ectopic sources. The lateral includes functional adrenal adenomas and carcinoma. The two categories can be differentiated by measurement of plasma ACTH concentration before and after ovine corticotropin-releasing hormone (O-CRH) administration. The diagnosis of ACTH dependent Cushing syndrome can be problematic and the source of increased ACTH production difficult to determine by MRI and biochemical methods. In such cases, selective sampling of the pituitary venous effluent into the inferior petrosal sinuses, cavernous sinuses, or the internal jugular veins before and after O-CRH stimulation accurately localizes the source of ACTH production. Corrigan et al. [24] in 1977 demonstrated the feasibility of inferior petrosal sinus sampling (IPSS) as an accurate technique to localize the source of ACTH excess in a patient with Cushing syndrome. Oldfield et al. [25] were able to surgically confirm the laterality of a microadenoma in 7/10 patients with Cushing’s syndrome using IPSS. A sensitivity for IPSS of 95 % in the diagnosis of surgically confirmed Cushing’s disease was also reported in a series of 246 patients by Oldfield, Doppman et al. [26]. A similar result (92.2 % sensitivity, 90 % specificity) was reported for IPSS by Bonelli et al [27]. Jugular venous sampling was suggested as a safer alternative by Ilias et al. [28], but appeared to be less sensitive than IPSS (83 % compared to 94 %). Cavernous sinus sampling has also been suggested as an alternative, given the greater proximity of the cavernous sinus to the pituitary gland. Its sensitivity was found to be approximately 93 %, by Fujimura et al [29]. However, cavernous sinus sampling is more invasive and is not a routinely employed technique. The validity of IPSS as the gold standard in the confirmation of a pituitary origin of ACTH excess has been confirmed by several studies. The sensitivity and specificity of IPSS has ranged from 90 % to 100 % in most analyses [30]. Midgette et al. [31], in a cost benefit analysis, recommended that given its high cost, IPSS be reserved for those cases where the high-dose dexamethasone suppression test is negative.
6.2 How Is Preoperative Assessment of the Extent of a Sellar Lesion Best Performed?
Summary
MRI is superior to CT in the evaluation of the extent of sellar lesions (moderate evidence).The relationship of these lesions to the optic pathways is best assessed with MRI. Neither MRI nor CT is accurate enough in the estimation of cavernous sinus involvement, although MRI is superior. MRI is more specific than it is sensitive for cavernous sinus invasion. The only reliable sign of such invasion is circumferential encasement of the cavernous internal carotid artery by tumor. The value of MRI in the preoperative determination of adenoma consistency, information that is useful in surgical planning, is uncertain.
Supporting Evidence
The single most important feature that precludes complete resection of a macroadenoma is invasion of the cavernous sinuses. The difficulty in accurately determining whether the sinuses are involved lies in the inability of modern imaging methods to consistently demonstrate the gracile medial cavernous sinus wall. Differentiation between simple displacement and actual invasion is often difficult with imaging. In a small early series of 20 patients with macroadenomas, Nichols et al. [11] found that MRI was superior to CT in the assessment of extrasellar extension. In all 20 of these patients, unenhanced MRI was superior to CT in characterization of the extent of disease. MRI was found to be more specific (85.7 %) than sensitive (55 %) in a series of 30 patients by Scotti et al [32]. Cottier et al. [33] evaluated the performance of contrast enhanced MRI in 106 patients with macroadenomas. They evaluated the following features on coronal enhanced images: (1) total encasement of the ICA, (2) displacement of the ICA, (3) asymmetry of the cavernous sinuses, (4) non-depiction of the lateral, superior, inferolateral, and carotid sulcus venous compartments of the cavernous sinuses, (5) lateral bulging of the lateral dural wall, (6) non-depiction of the medial wall, (7) crossing of three intercarotid lines drawn along the medial and lateral walls and through the centers of the supraclinoid and cavernous segments of the ICA, (8) percentage of encasement of the ICA and, lastly, (9) pattern of lateral expansion relative to the ICA. All cases were correlated with surgical findings. The presence of normal gland interposed between the tumor and the cavernous sinus, depiction of a normal medial carotid sulcus venous component, encasement of less than 25 % of ICA circumference, and failure of tumor to pass the medial intercarotid line were features that had a 100 % negative predictive value. The only reliably specific sign, however, was encasement of greater than 67 % of the ICA circumference with a PPV of 100 %. Obliteration of the carotid venous sulcus compartment and crossing of the lateral intercarotid line by tumor demonstrated PPVs of 95 % and 85 %, respectively. The value of the lateral intercarotid line was also described by Knosp et al. [34], who reported invasion in 12/14 cavernous sinuses when the lateral carotid tangent was crossed by tumor. In a study of 103 patients, Vieira et al. [35] stated that the finding most specific for cavernous sinus invasion was encasement of greater than 30 % of the ICA circumference, as opposed to 67 % in Cottier’s series. The presence of normal gland between the tumor and sinus, demonstration of the carotid sulcus compartment and encasement of less than 25 % of the ICA excluded sinus invasion, findings consistent with those of Cottier et al. The use of higher field strength magnets may enable characterization of extrasellar extension with greater sensitivity. Wolfberger et al. [36] demonstrated an improved sensitivity and specificity of 83 % and 84 %, respectively, for invasion of the medial cavernous sinus wall on a 3.0 T system compared with 67 % and 58 % on 1.5 T systems.
The primary objective of transsphenoidal surgery is to remove as much tumor as is safely possible to achieve decompression of the optic chiasm. In about 5–14 % of cases, the fibrous consistency of tumors precludes their complete removal [37]. Failure of adequate tumor resection may warrant repeat surgery and/or radiotherapy. On the other hand, soft tumors may be amenable to aspiration. Knowledge of tumoral consistency is therefore useful information to possess preoperatively. Early approaches to using adenoma consistency with MRI involved using T2 weighted sequences, the theory being that fibrous adenomas would be expected to be hypointense on such sequences. However, findings have been conflicting with some authors reporting that hard tumors were hypointense on T2WI [38] while others have stated that the opposite was true [39]. Pierallini et al. [40] described the use of diffusion weighted imaging (DWI) to characterize adenoma consistency and found a significant correlation between tumor consistency and the determined apparent diffusion coefficient (ADC) values with softer tumors demonstrating lower values and appearing hyperintense on DWI. Boxerman et al. [37] in a recent study reported the converse, i.e., that the harder the tumor, the lower the ADC value. Suzuki et al. found no correlation between adenoma consistency and ADC values [41].
The role of intraoperative MRI in assessing the completeness of tumor resection has been studied by some authors [42, 43]. Intraoperative MRI was found to increase the completeness of tumor resection from 58 % to 82 % in a series of 85 patients with macroadenomas in whom complete removal was intended [42]. In a series of 23 patients with acromegaly, Fahlbusch et al. [43] reported that high-field-strength (1.5 T) intraoperative MRI enabled achievement of endocrine normalization from 33 % to 44 %. However, they also reported a false negative rate of 23 % with this technique and were unable to demonstrate residual tumor in 6/23 patients who had persistent growth hormone elevation after initial surgery. The authors argue that intraoperative MRI provided immediate quality control, eliminated the need for the 3-month wait period that is necessary for an artifact-free follow-up MRI to determine if residual tumor is present, and enabled immediate treatment planning with either surveillance, transcranial resection, or radiotherapy. However, the operational costs of intraoperative MRI remain high and no cost benefit analyses to support its routine use in this situation exist in the literature.
6.3 How Is Follow-up Imaging Evaluation of Adenomas Best Achieved?
Summary
Postoperative follow-up is best performed with contrast-enhanced MRI, with a first follow-up scan advocated by most authors 3–4 months after surgery (limited evidence). For follow-up imaging of nonsurgically treated incidentalomas no one particular imaging strategy exists.
Supporting Evidence
Recurrence after surgery is reportedly more likely to occur with functional ACTH adenomas and with hormonally silent adenomas compared to the other varieties [44]. Consensus appears to exist in the literature with regard to the use of MRI to follow up the postoperative sella. The optimal time to obtain a baseline is generally believed to be about 3–4 months after surgery, given that the appearance of the immediate postoperative pituitary gland may not be significantly different from its preoperative state [45–47]. Yoon et al. [48], however, advocate early follow-up with MRI within a week after surgery and found residual tumor in 22/83 patients, confirmed either by repeat surgery, biochemical abnormality, or demonstrable growth over serial MRI scans. Clinical practice guidelines issued by the Endocrine Society annual meeting in 2011, based on both systematic reviews of evidence and discussions through a series of conference calls and e-mails and one in-person meeting, recommend the initial evaluation of a patient with a pituitary incidentaloma to include laboratory screening for hormone hyper- and hyposecretion in all patients including those with and without symptoms. The measurement of a screening prolactin level was met with universal consensus. Nonsurgical follow-up was recommended with clinical assessments and functional testing for patients who do not meet criteria for surgical removal of a pituitary incidentaloma. As for follow-up imaging of nonsurgically treated incidentalomas, the task force considered that repeat scanning within the first year was warranted for all patients because despite the slow growth of most incidentalomas, some have a propensity to enlarge, and the true proliferative nature of incidentalomas is unknown. In the absence of growth, they recommend that the interval between MRI scans can be increased. The task force also believes that evidence did not support one particular algorithm for the frequency of follow-up imaging, but recommended repeating MRI every year in macroincidentalomas, every 1–2 years in microincidentalomas for the next 3 years, and then every other year for the next 6 years and gradually less frequently indefinitely so long as the lesion continues not to threaten the patient’s health. Some task force members also advocated imaging every 5 years [49].
8 Imaging Case Studies
Figures 27.1 through 27.4 highlight different case studies.
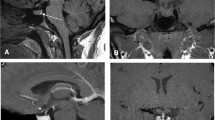
Coronal T2, T1, and postcontrast T1 weighted images in a patient with Cushing’s disease. Although a subtle right-sided contour deformity is suggested (arrows), a discrete adenoma is not demonstrable. An adenoma was, however, correctly lateralized to the right side by inferior petrosal sinus sampling (b)
Prediction of cavernous sinus invasion by MRI. (a) No cavernous sinus invasion. A thin rim of pituitary parenchyma (arrowhead) surrounds the tumor. MRI correctly predicted the absence of cavernous sinus invasion, which was confirmed at surgery. (b) Bilateral cavernous sinus invasion predicted correctly by MRI. Circumferential encasement of the cavernous ICA on the left (short arrows) and the presence of tumor in the right medial carotid sulcus venous compartment (arrow) enabled correct prediction of invasion bilaterally. (c) Involvement of the right carotid sulcus venous compartment representing cavernous sinus invasion correctly predicted by MRI (arrow). (d) Crossing of the right lateral intercarotid line by tumor (arrow). Invasion of the cavernous sinus was found at surgery. (e) False positive diagnosis of cavernous sinus invasion. The small focus of tumor extending beyond the lateral intercarotid line on the left was thought to represent cavernous sinus invasion preoperatively. At surgery, however, the medial cavernous sinus dural wall was merely pushed and not invaded
9 Future Research
-
Establish the true utility of dynamic MRI and develop a standardized technique.
-
Refine MRI techniques to enable an increase in the detection rate of small functional microadenomas.
-
Improve the sensitivity of MRI in the detection of cavernous sinus invasion.
-
Explore the role of advanced MRI techniques (diffusion, perfusion, and MR spectroscopic imaging) to enable preoperative assessment of tumoral consistency.
-
Determine the role of routine intraoperative MRI and evaluate its cost effectiveness.
References
McDowell B, Wallace R, Carnahan R, Chrischilles E, Lynch C, Schlechte J. Pituitary. 2011;14(1):23–30.
Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, et al. Cancer. 2004;101(3):613–19.
Frohman LA. Management of pituitary incidentalomas. In: Swearingen B, Biller BMK, editors. Diagnosis and management of pituitary disorders. Totowa: Humana Press; 2008. p. 399–409.
Davies MJ, Howlett TA. Clin Endocrinol. 1994;41(3):385–9.
Casanueva FF, Molitch ME, Schlechte JA, Abs R, Bonert V, Bronstein MD, et al. Clin Endocrinol. 2006;65(2):265–73.
Kucharczyk W, Davis DO, Kelly WM, Sze G, Norman D, Newton TH. Radiology. 1986;161(3):761–5.
Guy RL, Benn JJ, Ayers AB, Bingham JB, Lowy C, Cox TCS, et al. Clin Radiol. 1991;43(3):156–61.
Peck W, Dillon W, Norman D, Newton T, Wilson C. Am J Roentgenol. 1989;152(1):145–51.
Dwyer AJ, Frank JA, Doppman JL, Oldfield EH, Hickey AM, Cutler GB, et al. Radiology. 1987;163(2):421–6.
Escourolle H, Abecassis JP, Bertagna X, Guilhaume B, Pariente D, Derome P, et al. Clin Endocrinol. 1993;39(3):307–13.
Nichols DA, Laws ERJ, Houser WO, Abboud CF. Neurosurgery. 1988;22(2):380–5.
Davis P, Hoffman Jr J, Spencer T, Tindall G, Braun I. AJR Am J Roentgenol. 1987;148(4):797–802.
Pojunas K, Daniels D, Williams A, Haughton V. AJNR Am J Neuroradiol. 1986;7(2):209–13.
Elster AD. Radiology. 1993;187(1):1–14.
Erickson D, Erickson B, Watson R, Patton A, Atkinson J, Meyer F, et al. Clin Endocrinol. 2010;72(6):793–9.
Stadnik T, Stevenaert A, Beckers A, Luypaert R, Buisseret T, Osteaux M. Radiology. 1990;176(2):419–28.
Sakamoto Y, Takahashi M, Korogi Y, Bussaka H, Ushio Y. Radiology. 1991;178(2):441–5.
Kucharczyk W, Bishop J, Plewes D, Keller M, George S. AJR Am J Roentgenol. 1994;163(3):671–9.
Bartynski W, Lin L. AJNR Am J Neuroradiol. 1997;18(5):965–72.
Rand T, Lippitz P, Kink E, Huber H, Schneider B, Imhof H, et al. Eur J Radiol. 2002;41(2):131–5.
Portocarrero-Ortiz L, Bonifacio-Delgadillo D, Sotomayor-González A, Garcia-Marquez A, Lopez-Serna R. Pituitary. 2010;13(3):230–5.
Patronas N, Bulakbasi N, Stratakis CA, Lafferty A, Oldfield EH, Doppman J, et al. J Clin Endocrinol Metab. 2003;88(4):1565–9.
Batista D, Courkoutsakis NA, Oldfield EH, Griffin KJ, Keil M, Patronas NJ, et al. J Clin Endocrinol Metab. 2005;90(9):5134–40.
Corrigan DF, Schaaf M, Whaley RA, Czerwinski CL, Earll JM. N Engl J Med. 1977;296(15):861–2.
Oldfield EH, Chrousos GP, Schulte HM, Schaaf M, McKeever PE, Krudy AG, et al. N Engl J Med. 1985;312(2):100–3.
Oldfield EH, Doppman JL, Nieman LK, Chrousos GP, Miller DL, Katz DA, et al. N Engl J Med. 1991;325(13):897–905.
Bonelli FS, Huston III J, Carpenter PC, Erickson D, Young Jr WF, Meyer FB. AJNR Am J Neuroradiol. 2000;21(4):690–6.
Ilias I, Chang R, Pacak K, Oldfield EH, Wesley R, Doppman J, et al. J Clin Endocrinol Metab. 2004;89(8):3795–800.
Fujimura M, Ikeda H, Takahashi A, Ezura M, Yoshimoto T, Tominaga T. Neurolog Res. 2005;27:11–15.
Newell-Price J, Trainer P, Besser M, Grossman A. Endocr Rev. 1998;19(5):647–72.
Midgette AS, Aron DC. Am J Med Sci. 1995;309(3):162–70.
Scotti G, Yu C, Dillon W, Norman D, Colombo N, Newton T, et al. Am J Roentgenol. 1988;151(4):799–806.
Cottier J-P, Destrieux C, Brunereau L, Bertrand P, Moreau L, Jan M, et al. Radiology. 2000;215(2):463–9.
Knosp E, Steiner E, Kitz K, Matula C. Neurosurgery. 1993;33(4):610–18.
Vieira JJO, Cukiert A, Liberman B. Surg Neurol. 2006;65(2):130–5.
Wolfsberger S, Ba-Ssalamah A, Pinker K, Mlynárik V, Czech T, Knosp E, et al. J Neurosurg. 2004;100(2):278–86.
Boxerman JL, Rogg JM, Donahue JE, Machan JT, Goldman MA, Doberstein CE. Am J Roentgenol. 195(3):720–728.
Iuchi T, Saeki N, Tanaka M, Sunami K, Yamaura A. Acta Neurochir. 2010;140(8):779–86.
Snow RB, Johnson CE, Morgello S, Lavyne MH, Patterson RHJ. Neurosurgery. 1990;26(5):801–3.
Pierallini A, Caramia F, Falcone C, Tinelli E, Paonessa A, Ciddio AB, et al. Radiology. 2006;239(1):223–31.
Suzuki C, Maeda M, Hori K, Kozuka Y, Sakuma H, Taki W, et al. J Neuroradiol. 2007;34(4):228–35.
Nimsky C, Keller BV, Ganslandt O, Fahlbusch R. Neurosurgery. 2006;59(1):105–14.
Fahlbusch R, Keller BV, Ganslandt O, Kreutzer J, Nimsky C. Eur J Endocrinol. 2005;153(2):239–48.
Terada T, Kovacs K, Stefaneanu L, Horvath E. Endocr Pathol. 1995;6(4):301–10.
Rajaraman V, Schulder M. Surg Neurol. 1999;52(6):592–9.
Rodríguez O, Mateos B, de la Pedraja R, Villoria R, Hernando JI, Pastor A, et al. Neuroradiology. 1996;38(8):747–54.
Dina TS, Feaster SH, Laws Jr ER, Davis DO. AJNR Am J Neuroradiol. 1993;14:763–9.
Yoon P-H, Kim D-I, Jeon P, Lee S-I, Lee S-K, Kim S-H. AJNR Am J Neuroradiol. 2001;22:1097–100.
Practice Guidelines for Pituitary Incidentalomas, The Endocrine Society Annual Meeting. J Clin Endocrinol Metab. 2011;96(4):894–904.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this entry
Cite this entry
Raghavan, P., Phillips, C.D. (2013). Sellar Lesions: Evidence-Based Neuroimaging. In: Medina, L.S., Sanelli, P.C., Jarvik, J.G. (eds) Evidence-Based Neuroimaging Diagnosis and Treatment. Evidence-Based Imaging. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-3320-0_28
Download citation
DOI: https://doi.org/10.1007/978-1-4614-3320-0_28
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-3319-4
Online ISBN: 978-1-4614-3320-0
eBook Packages: MedicineReference Module Medicine