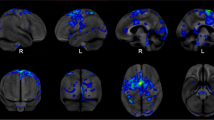
Abstract
Apraxia of speech (AOS) is a motor disorder that occurs as a result of impairment in the planning or programming of movements for speech production. It is typically associated with cerebrovascular events, although it can also occur in the context of neurodegeneration where its importance has typically been deemphasized to “just a component of a presenting syndrome.” Primary progressive aphasia (PPA) is such a syndrome in which AOS coexists with other linguistic deficits, typically agrammatic aphasia. Recently, however, AOS has been demonstrated to occur in a pure or isolated form, known as primary progressive apraxia of speech (PPAOS), reaffirming the importance of neurodegenerative AOS. Furthermore, anatomic and pathologic associations differ between AOS-dominant syndromes and PPA variants. Understanding the relationship between AOS-dominant variants, including PPAOS and PPA, and their relationship to movement disorders including corticobasal degeneration and progressive supranuclear palsy, is important and will be the focus of this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Apraxia of speech
- Primary progressive aphasia
- Tau
- Progressive supranuclear palsy
- Corticobasal degeneration
- Agrammatic aphasia
Part I Clinical
Apraxia of Speech
Apraxia of speech (AOS) is a type of motor speech disorder that occurs as a result of a defect in the planning and programming of speech production (Darley et al. 1975). Apraxia of speech has characteristics that are distinct from dysarthria, another type of motor speech disorder. Dysarthria, unlike AOS, reflects a defect of neuromuscular function (Darley et al. 1969). The salient features of AOS are sound production errors that may include any combination of distorted sound substitutions, additions, prolongations, and truncations. Articulation is effortful and is associated with groping and uncoordinated movements of buccolingual structures.
Apraxia of speech has been most commonly associated with cerebrovascular events in which the onset is acute and severity maximum. More recently, however, there has been increased interest in AOS that is progressive in nature (Duffy 2006, 2013). Progressive AOS (PAOS), unlike AOS due to cerebrovascular events, is characterized by a general worsening of AOS features; errors in speech production become more frequent and more severe over time (Table 13.1). As a result, verbal communication becomes more problematic. Ultimately, patients with PAOS become anarthric and are no longer able to verbally communicate.
Progressive Apraxia of Speech
Progressive AOS is one of the cardinal features of a neurodegenerative disorder and should be viewed in the same light as progressive memory loss or progressive gait impairment. Progressive AOS can co-occur with other cognitive and motor features and hence be a component of a neurodegenerative syndrome. In fact, it may be the most prominent component of the syndrome. Recently, it has been demonstrated that PAOS can be an isolated feature at presentation and hence can exist in a pure form in the absence of other cognitive and motor features (Josephs et al. 2012). When PAOS occurs in isolation, we used the term primary progressive apraxia of speech (PPAOS) (Duffy 2006; Josephs et al. 2012) (Table 13.1). Patients with PPAOS or PAOS, in which AOS is the most salient feature, will decline over time, and sometimes other cognitive and motor deficits will develop, resulting in the emergence of a variety of progressive clinical syndromes (Fig. 13.1). Most commonly, it appears that patients with PAOS or PPAOS later develop difficulty with balance and gait, aphasia, limb praxis, Parkinsonism, and eye movement abnormalities (Broussolle et al. 1992; Josephs et al. 2006a). In fact, it has been observed that some patients that initially presented with PAOS later develop features of progressive supranuclear palsy syndrome (PSPS) or corticobasal syndrome (CBS) (Josephs et al. 2006a; Josephs and Duffy 2008). Such patients tend to have limb coordination problems, may begin to fall, and may ultimately become wheel chair bound. Another subset of patients with dominant AOS or PPAOS develops agrammatic aphasia in written and spoken form (Josephs et al. 2006a). In such instances, the AOS remains more prominent than the aphasia.
demonstrates how patients presenting with apraxia of speech variants progress over time. AOS apraxia of speech, agPPA agrammatic variant of primary progressive aphasia, PAOS progressive apraxia of speech (i.e., AOS > > other cognitive and motor features), PPAOS primary progressive apraxia of speech (AOS only), CBS corticobasal syndrome, PSPS progressive supranuclear palsy syndrome
Syndromes Associated with Progressive Apraxia of Speech
Progressive apraxia of speech may coexist with other cognitive and motor deficits. In some instances, the PAOS is the most prominent feature of the presenting syndrome. In such instances, the chief complaint is usually that of speech problems; other presenting features are more subtle and at times may only have been identified during the neurological and/or speech and language evaluation. In other instances, when AOS coexists with other cognitive and/or motor deficits, the other features of the syndrome overshadow the AOS. Typically, when this occurs, the chief complaint is related to one of the other symptoms and the AOS tends to be mild. Two syndromes that are worth discussing in detail in which PAOS commonly occurs early in the disease course are primary progressive aphasia (PPA) and CBS.
Primary Progressive Aphasia
The term primary progressive aphasia (PPA) is used in situations in which there is a progressive disorder characterized by impairment of specific aspects of language function (Mesulam 1982, 2001). These include grammar, naming, single word comprehension, sentence comprehension, and repetition. Three subtypes of PPA are now recognized (Table 13.1).
Agrammatic Variant of PPA
The first subtype of PPA is the agrammatic variant (agPPA) (Gorno-Tempini et al. 2011). Agrammatic PPA is a syndrome in which the most salient feature is that of agrammatism in language production. This may be observed in spoken or written form. In agrammatic PPA, function words and articles may be conspicuously absent, e.g., “Man fishing with wife on dock. Boat goes by she waves to them.” Importantly, patients with agPPA commonly also exhibit PAOS (Ogar et al. 2005). Hence, agPPA is typically characterized by the presence of agrammatic aphasia of greater severity than the accompanying PAOS (Josephs et al. 2013a). Examination shows little deficits in naming and in sentence repetition for content words, as well as difficulty understanding complex sentence structures, e.g., “put the brown newspaper under the blue book in the middle drawer.” Importantly, given that PPA is a disorder of language, a diagnosis of PPA should only be made when language impairment, not AOS, is the most prominent feature of the syndrome (Mesulam 2003).
Semantic Variant of PPA
The second subtype of PPA is the semantic variant (svPPA) (Gorno-Tempini et al. 2011; Warrington 1975). In svPPA, the speech output is fluent and may even be excessively verbose. However, with more careful attention to what is actually being said, it becomes apparent that the patient is being circumloquacious in order to avoid certain words that no longer has specific meaning to the patient. Therefore, specific words may be absent in general conversation and replaced with more general words for the item or object being described, e.g., the patient may say animal instead of hyena or flower instead of hibiscus. There may also be reference to the fact that the patient no longer understands the meaning of certain words; the patient may no longer know what the word lapel means and hence will not be able to tell how a lapel is different from a collar. Examination shows poor naming and loss of single word meaning and comprehension (Hodges and Patterson 2007). Unlike in agPPA, AOS rarely, if ever, coexists with svPPA.
Logopenic Variant of PPA
The third subtype of PPA is the logopenic variant (lvPPA) (Gorno-Tempini et al. 2004, 2011). In lvPPA, the speech output may be fluent, but it may also be characterized by hesitancy as the patient pauses and searches for words. However, unlike in svPPA, patients with lvPPA can easily recognize the item when the name is provided by the examiner, and hence the patient has not lost word meaning. Memory loss is also often a common complaint in patients with lvPPA. Like in svPPA, patients with lvPPA perform poorly on naming tasks but there is no loss of word meaning. Examination also reveals poor repetition of sentences with loss of words or replacement of exact content words. One additional feature of lvPPA is the production of sound errors known as phonological or phonemic errors. With phonological errors, one sound is produced for another, e.g., sesipic instead of specific. Importantly, unlike in AOS, the sounds are not distorted. It can be difficult at times to distinguish AOS errors from phonological errors, resulting in patients with lvPPA being misdiagnosed as having PAOS and vice versa. Apraxia of speech rarely occurs in lvPPA.
Corticobasal Syndrome
Apraxia of speech and agrammatic aphasia, occurring together or separately, can be early features of corticobasal syndrome (CBS) (Josephs et al. 2006a; Josephs and Duffy 2008; Assal et al. 2012; Kertesz et al. 2005). As described in the CBS chapter, CBS is diagnosed in a patient presenting with asymmetric signs and symptoms indicative of cortical and basal ganglia dysfunction. Patients with CBS may present with asymmetric ideomotor apraxia, cogwheel or gegenhalten rigidity, bradykinesia, action myoclonus, dystonia, astereognosis, and agraphesthesia (Armstrong et al. 2013). Patients with CBS, who also have AOS, are more likely to show right limb asymmetry, i.e., the right side is the more affected side, and often also have agrammatic aphasia. In CBS in which AOS or agrammatic aphasia are present, the presenting complaint is usually that of limb dysfunction, as opposed to speech difficulties, although both may be mentioned as being experienced by the patient.
Overlap with Motor Neuron Disease
Although we have described these syndromes as being relatively pure, it needs to be recognized that these clinical syndromes can also be associated with motor neuron disease (MND) (Caselli et al. 1993; Coon et al. 2011; Czell et al. 2013; da Rocha et al. 2007) (Table 13.1). However, in such instances, the syndrome is also characterized by the presence of flaccid and spastic dysarthria. In fact, it is the dysarthrias that are most likely to be the dominant feature on examination, not the AOS nor the aphasia. Therefore, in the absence of flaccid and spastic, or just flaccid dysarthria, MND is unlikely to be identified. One large series identified patients with AOS that had MND (Duffy et al. 2007). However, all of the patients had coexisting spastic and flaccid dysarthria (Duffy et al. 2007). Motor neuron disease almost never coexists with lvPPA and only very rarely coexists with svPPA. In the latter case, a rare association of svPPA and pure upper MND, i.e., no anterior horn cell disease, has been described (Josephs et al. 2013b).
Movement Disorders Associated with Apraxia of Speech and Primary Progressive Aphasia
Apraxia of speech can be associated with movement disorders, although the movement disorder identified tends to be a syndrome as opposed to being an isolated feature. We previously discussed the association of AOS and CBS at presentation; patients with AOS may also show features of CBS and PSPS with disease progression. When this occurs, the patient is usually 5 years out from onset. It is rare, however, for the classic syndrome of PSPS to co-occur with AOS at presentation (Whitwell et al. 2013a). When PSPS features develop in patients that originally present with PPAOS or PAOS, they tend to be milder than what is typically observed in PSPS that starts off with balance problems and eye movement abnormalities. However, slowing of vertical eye movements, balance problems, and falls become more prevalent and severe over time in patients presenting with PPAOS or PAOS. Similarly, patients with PPAOS or PAOS may show the emergence of ideomotor apraxia after many years (Josephs et al. 2006a, 2012). Of the three PPA syndromes, agPPA is the one most likely to also develop features of CBS and PSPS over time (Kertesz et al. 2005). In fact, ideomotor apraxia has been shown to be more common in agPPA than lvPPA (Adeli et al. 2013). Parkinsonian features such as rigidity and bradykinesia are relatively common later on in the disease course in PPAOS and PAOS. In PPA, almost 30 % of patients present with at least one parkinsonian sign, such as bradykinesia, rigidity, tremor, postural instability, or gait disturbance (Kremen et al. 2011). Parkinsonian features are more common in agPPA than lvPPA and are almost absent in svPPA (Graff-Radford et al. 2012; Kremen et al. 2011). Dystonia is much less common but may also be observed later on in the disease course. Hyperkinetic movements can also occur, although resting tremor is rare. Asymmetric limb myoclonus can occur later on in the disease course in patients who later develop the CBS. Stereotypies and ticks have not been emphasized in PPA, PPAOS, or PAOS.
Part II Anatomy
Anatomy of Progressive Apraxia of Speech
Routine clinical studies in patients with PAOS have not been very helpful in identifying the anatomic correlate of PAOS. However, studies using research-based imaging techniques performed using groups of patients have demonstrated that PAOS is associated with the premotor cortex. Structural magnetic resonance imaging (MRI) studies using voxel-level techniques, such as voxel-based morphometry, in patients with PPAOS have shown focal patterns of grey matter atrophy involving the superior premotor and supplemental motor cortices (Josephs et al. 2012; Whitwell et al. 2013a). White matter atrophy is also observed in the premotor cortex, underlying regions of grey matter loss, but tends to also involve inferior premotor regions. Detailed analysis of white matter tracts using diffusion tensor imaging (DTI) has shown that PPAOS is associated with degeneration of premotor aspects of the superior longitudinal fasciculus and the body of the corpus callosum (Josephs et al. 2012). Hypometabolism on 18-F fluorodeoxyglucose positron emission tomography (FDG-PET) scans is also observed in the superior premotor cortices in patients with PPAOS (Fig. 13.2a), although hypometabolism on FDG-PET can be very mild, or even absent, in some patients (Josephs et al. 2012).
Imaging findings in patients with PPAOS overlap to some degree with those associated with PSPS, with both syndromes showing atrophy and hypometabolism in the premotor cortex and degeneration of the superior longitudinal fasciculus and body of the corpus callosum (Whitwell et al. 2011, 2013a; Knake et al. 2010; Josephs et al. 2008). However, in contrast to PPAOS, patients with PSPS typically show greater involvement of the prefrontal cortex and striking atrophy in the midbrain (Whitwell et al. 2013a). Mild midbrain atrophy has however been observed in patients with PPAOS, likely reflecting the fact that these patients often develop clinical features of PSPS (Whitwell et al. 2013a). In patients with both PAOS and agrammatic aphasia, where the AOS is the more dominant feature, VBM and FDG-PET studies show involvement of the superior premotor cortex extending into the middle and inferior premotor regions (Fig. 13.2b) (Josephs et al. 2013a). This finding suggests that the agrammatic aphasia component is associated with involvement of the inferior premotor cortex. Indeed, correlations have been observed between volume and metabolism in the inferior premotor cortex, particularly the pars triangularis which forms part of Broca’s area, and the severity of agrammatic aphasia (Whitwell et al. 2013b). In contrast, the severity of AOS has been shown to correlate to volume of the superior premotor cortex (Whitwell et al. 2013b). These findings show that AOS and agrammatic aphasia have distinct neuroanatomical underpinnings.
Anatomy of Primary Progressive Aphasia
Each of the three variants of PPA shows distinct regional abnormalities within the frontotemporal and parietal cortices. In agPPA, routine brain MRI scans reveal atrophy of the left inferior frontal lobe with widening of the left perisylvian fissure (Fig. 13.3a). Research studies demonstrate atrophy and hypometabolism on FDG-PET throughout the left premotor cortex (Fig. 13.3b) but also with involvement of the left insula, striatum, lateral temporal lobes, and often other regions in the left frontal and parietal lobes (Josephs et al. 2006a, 2010; Gorno-Tempini et al. 2004; Grossman et al. 2004; Rohrer et al. 2009). Patterns observed in patients with agPPA are typically more widespread than those observed in patients with PAOS. Relatively widespread patterns of white matter tract degeneration have also been observed in agPPA, involving the superior longitudinal fasciculus, corpus callosum, anterior cingulate, inferior frontal-occipital fasciculus, and temporal lobe tracts such as the uncinate fasciculus and inferior longitudinal fasciculus (Galantucci et al. 2011; Schwindt et al. 2013; Whitwell et al. 2010a; Grossman et al. 2013). White matter tract degeneration is typically asymmetric, with greater involvement of the left hemisphere. Patients with agPPA and parkinsonism can also show glucose hypometabolism in the bilateral dorsal and left ventral midbrain (Roh et al. 2010).
demonstrates the abnormalities that can be identified on MRI and FDG-PET in patients with the different variants of PPA. In (a), a patient with agPPA shows widening of the left perisylvian area, while in (b), FDG-PET shows premotor cortical hypometabolism, as well as hypometabolism in left temporal and even parietal cortices. On MRI (c) there is left > right anterior medial temporal lobe atrophy which is also seen on the FDG-PET scan (d) in a patient with svPPA. A patient with the lvPPA variant shows left parietal atrophy on MRI (e), while FDG-PET shows left temporal and parietal hypometabolism (f)
In svPPA, there is a distinct pattern of anterior medial temporal lobe atrophy that is easily detected with routine MRI scans (Fig. 13.3c). Atrophy typically targets the fusiform and inferior temporal gyri, although atrophy is also observed in the hippocampus, amygdala, entorhinal cortex, and middle temporal gyrus (Chan et al. 2001; Galton et al. 2001; Mummery et al. 2000). This pattern of abnormality is also observed on FDG-PET scans (Fig. 13.3d). White matter tract degeneration on DTI predominantly involves temporal lobe tracts, particularly the uncinate fasciculus and anterior regions of the inferior longitudinal fasciculus (Schwindt et al. 2013; Whitwell et al. 2010a; Acosta-Cabronero et al. 2012; Agosta et al. 2010; Zhang et al. 2009; Mahoney et al. 2013). The inferior longitudinal fasciculus has been shown to be involved to a greater degree in svPPA than both agPPA and lvPPA (Hodges and Patterson 2007; Assal et al. 2012). The left temporal lobe is usually affected more than the right (Chan et al. 2001; Galton et al. 2001; Mummery et al. 2000), although equal involvement of left and right temporal lobes can be observed, particularly later in the disease course when the right side has “caught up with” the left side. Patients with right greater than left sided atrophy and hypometabolism typically present with behavioral dyscontrol and loss of facial recognition (Chan et al. 2009; Edwards-Lee et al. 1997; Thompson et al. 2003; Josephs et al. 2009a), instead of PPA. Patients often also show atrophy and hypometabolism in the orbitofrontal cortices.
Patients with lvPPA also have distinct and characteristic patterns of atrophy, with routine head MRI showing atrophy of the left lateral temporal and parietal lobes (Fig. 13.3e). Group MRI studies and FDG-PET (Fig. 13.3f) show striking involvement of the left lateral temporal and parietal lobes but can also show involvement of the precuneus, frontal lobes, and even the occipital lobes (Gorno-Tempini et al. 2004; Josephs et al. 2010; Madhavan et al. 2013; Ridgway et al. 2012). The medial temporal lobe is usually relatively spared. The right temporal and parietal lobes may be abnormal but are nearly always less affected than the left. White matter tract degeneration can be observed on DTI affecting temporal and parietal tracts, including the left inferior longitudinal fasciculus, uncinate fasciculus, and superior longitudinal fasciculus (Armstrong et al. 2013; Graff-Radford et al. 2012).
Anatomy of Corticobasal Syndrome with Apraxia of Speech
The pattern of atrophy or hypometabolism observed in patients presenting with CBS and AOS is similar to the pattern observed in patients presenting with CBS without AOS. The same is true regardless of whether agrammatic aphasia is present. In both instances, i.e., AOS is absent or present, patients show involvement of the premotor cortex, often with extension into the prefrontal cortex and sometimes into the superior parietal lobe (Josephs et al. 2008; Grossman et al. 2004; Boxer et al. 2006; Huey et al. 2009; Whitwell et al. 2010b; Groschel et al. 2004). The striatum can also be heavily affected. Atrophy and hypometabolism are often asymmetric, involving the hemisphere contralateral to the side of the greatest affected limb (Whitwell et al. 2010b; Koyama et al. 2007; Soliveri et al. 1999). White matter tract degeneration is also asymmetric and involves frontoparietal association fibers as well as the body and splenium of the corpus callosum (Borroni et al. 2008). Therefore, patients presenting with CBS with AOS will show greater abnormalities, especially in prefrontal regions, compared to patients with PPAOS or PAOS that later develop features of CBS, at least earlier in the disease course.
Part III Pathology
Pathological Overview
Over the last decade, many investigators interested in AOS and PPA have assessed the pathological processes associated with these different syndromes (Josephs et al. 2005, 2006a, b; Mesulam 2001; Kertesz et al. 1994, 2005; Davies et al. 2005; Galton et al. 2000; Graff-Radford et al. 1990; Greene et al. 1996; Hodges et al. 2004; Knibb et al. 2006; Knopman et al. 1990; Lang 1992; Lippa et al. 1991; Wechsler et al. 1982). Unfortunately, it is very difficult to interpret some of these older studies since subjects were not separated by phenotype or syndrome and instead were all lumped as PPA, including those with PAOS and PPAOS. Regardless, these older studies demonstrated that the pathological processes associated with PPA were not homogeneous. Taken together, these studies demonstrated that many different pathologies, including Alzheimer’s disease (Kertesz et al. 2005; Galton et al. 2000; Greene et al. 1996; Knibb et al. 2006), accounted for PPA. Another problem with older studies was the fact that modern immunohistochemistry techniques were not performed, and even if they were performed, they were incomplete, at least compared to more recent studies.
With the advent of immunohistochemistry, and recent discoveries of different proteins that are associated with dementia in general, pathologists have a better grasp on the biochemistry and the pathological processes that are associated with these syndromes. Therefore, current classification first separates Alzheimer’s disease from non-Alzheimer’s disease pathologies. Non-Alzheimer’s disease pathologies are then further separated under the broad categories of synucleinopathies, tauopathies, and TDP-43 proteinopathies (Dickson 2003). Synucleinopathies are not discussed in this chapter but include multiple system atrophy and Lewy body diseases (Dickson 2003); Lewy bodies are rarely found in PPA patients (Caselli et al. 2002). Further subclassification of the tauopathies exists and includes diseases in which tau is the predominant abnormal protein identified histologically (Josephs et al. 2011). Therefore, tauopathies include progressive supranuclear palsy (PSP) (Hauw et al. 1994), corticobasal degeneration (CBD) (Dickson et al. 2002), Pick’s disease with Pick’s bodies (PiD) (Dickson 2001), and frontotemporal dementia with Parkinsonism linked to chromosome 17 (FTDP-17) (Ghetti et al. 2003). Similarly the TDP-43 proteinopathies are subclassified in types A–D (Mackenzie et al. 2011). For both tauopathies and TDP-43 proteinopathies, subclassification is based on the distribution of the inclusions, as well as the morphological appearances of the inclusions.
Clinicopathological Associations
There is no one-to-one relationship between the clinical syndromes described in this chapter and the pathologies discussed here. However, there are strong associations (Josephs et al. 2011) (Table 13.2). The clinical syndrome of PPAOS, for example, is strongly associated with tauopathies (Josephs et al. 2006a, 2011; Deramecourt et al. 2010). Although published studies are lacking, clinical evidence suggests that of the tauopathies that can be associated with PAOS and PPA, PPAOS is most strongly associated with PSP pathology, less so CBD and PiD (Josephs et al. 2006a). Similar to PPAOS, PAOS is also associated with tauopathies. However, PAOS may be more strongly associated with CBD pathology than PSP pathology (Josephs et al. 2006a). The three variants of PPA appear to also have a strong association with certain pathologies. The svPPA is strongly associated with TDP-43 proteinopathies and is almost always associated with type C pathology (Josephs et al. 2009b; Mackenzie et al. 2006; Snowden et al. 2007). The lvPPA is strongly associated with Alzheimer’s disease pathology (Mesulam et al. 2008). In fact, some investigators have argued not to include lvPPA in the discussion of AOS and PPA since the other variants are associated with non-Alzheimer’s disease pathologies, while lvPPA is associated with Alzheimer’s disease. Unfortunately, not all lvPPA patients have Alzheimer’s disease pathology (Mesulam et al. 2008) which is the counterargument to include lvPPA in such discussions. There is a small subset of lvPPA patients that shows TDP-43 proteinopathy, similar to svPPA (Mesulam et al. 2008). However, unlike svPPA that is associated with TDP-43 type C pathology, lvPPA when associated with TDP-43 pathology is associated with TDP-43 type A pathology (Josephs et al. 2009b). The most complicated syndromic pathologic association to explain is that of agPPA. Currently, it is unclear whether agPPA, as defined in this chapter, is strongly associated with any one pathology. In most cases, agPPA includes patients with and without AOS, and in such instances both tau and TDP-43 pathologies have been identified (Snowden et al. 2007; Mesulam et al. 2008). Weak evidence suggests that agPPA in which AOS is present may be more strongly associated with tauopathies, particularly CBD, while agPPA in which AOS is absent is more strongly associated with TDP-43 proteinopathy, especially TDP-43 type A. It is also worth mentioning that if any of the syndromes described in this chapter are associated with features of MND, then the underlying pathology is most likely to be that of TDP-43 type B (Josephs et al. 2009b, 2011; Mackenzie et al. 2006; Snowden et al. 2007). Therefore, in the presence of flaccid and spastic dysarthria, TDP type B pathology must be strongly suspected. Future studies are also necessary to assess clinicopathological associations in those PPA patients with parkinsonism.
Part IV Genetics
There are no genetic abnormalities that completely account for any one of the syndromes discussed in this chapter. However, there have been reports of patients with a genetic abnormality, namely, a mutation in the progranulin gene on chromosome 17, that have presented with PPA (Mesulam et al. 2007; Mukherjee et al. 2006; Rohrer et al. 2010; Kelley et al. 2009). Most of these reports did not further classify the PPA into one of the three variants. However, patients with progranulin gene mutations are almost always fluent and hence have features that overlap with svPPA and lvPPA. To our knowledge, there are no definitive, well-characterized patients with PPAOS or PAOS that have had a mutation in the progranulin gene. Recently, there have also been a handful of patients with PPA that have been found to have a repeat expansion in the C9ORF72 gene (Simon-Sanchez et al. 2012; Snowden et al. 2006, 2012; Mahoney et al. 2012). Other investigators with large cohorts of patients with C9ORF72 repeat expansions, however, have not identified patients with PPA (Boeve et al. 2012). There are no reports of the repeat expansion accounting for the PPAOS or PAOS phenotypes. Most patients with FTDP-17, i.e., with a mutation in the microtubule associated protein tau gene, have not commonly presented with any of the clinical syndromes discussed in this chapter. There are a few reports of patients presenting as svPPA, although it is unclear whether these patients were truly svPPA or whether they had a behavioral presentation in which naming was also affected as part of the behavioral syndrome (Pickering-Brown et al. 2008).
Summary
There are many different clinical syndromes in which a motor speech disorder or aphasia is the defining feature. In some instances, the motor speech disorders and aphasia co-occur. However, it is important not to lump all these features under the heading of PPA. In fact, the presence of AOS is strongly predictive of tau pathologies, the presence of flaccid and spastic dysarthrias of MND and TDP type B pathology, svPPA of TDP type C pathology, and lvPPA of Alzheimer’s disease. Understanding how to separate these clinical features and syndromes will have important prognostic value and future therapeutic benefit. Parkinsonism is frequent in agPPA and lvPPA, and further imaging and pathological studies are necessary to better characterize this feature.
References
Acosta-Cabronero J, Patterson K, Fryer TD, Hodges JR, Pengas G, et al. Atrophy, hypometabolism and white matter abnormalities in semantic dementia tell a coherent story. Brain. 2012;134:2025–35.
Adeli A, Whitwell JL, Duffy JR, Strand EA, Josephs KA. Ideomotor apraxia in agrammatic and logopenic variants of primary progressive aphasia. J Neurol. 2013;260:1594–600.
Agosta F, Henry RG, Migliaccio R, Neuhaus J, Miller BL, et al. Language networks in semantic dementia. Brain. 2010;133:286–99.
Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496–503.
Assal F, Laganaro M, Remund CD, Ragno Paquier C. Progressive crossed-apraxia of speech as a first manifestation of a probable corticobasal degeneration. Behav Neurol. 2012;25:285–9.
Boeve BF, Boylan KB, Graff-Radford NR, DeJesus-Hernandez M, Knopman DS, et al. Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain. 2012;135:765–83.
Borroni B, Garibotto V, Agosti C, Brambati SM, Bellelli G, et al. White matter changes in corticobasal degeneration syndrome and correlation with limb apraxia. Arch Neurol. 2008;65:796–801.
Boxer AL, Geschwind MD, Belfor N, Gorno-Tempini ML, Schauer GF, et al. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch Neurol. 2006;63:81–6.
Broussolle E, Tommasi M, Mauguiere F, Chazot G. Progressive anarthria with secondary parkinsonism: a clinico-pathological case report. J Neurol Neurosurg Psychiatry. 1992;55:577–80.
Caselli RJ, Windebank AJ, Petersen RC, Komori T, Parisi JE, et al. Rapidly progressive aphasic dementia and motor neuron disease. Ann Neurol. 1993;33:200–7.
Caselli RJ, Beach TG, Sue LI, Connor DJ, Sabbagh MN. Dement Geriatr Cogn Disord. 2002;14:55–8.
Chan D, Fox NC, Scahill RI, Crum WR, Whitwell JL, et al. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer’s disease. Ann Neurol. 2001;49:433–42.
Chan D, Anderson V, Pijnenburg Y, Whitwell J, Barnes J, et al. The clinical profile of right temporal lobe atrophy. Brain. 2009;132:1287–98.
Coon EA, Sorenson EJ, Whitwell JL, Knopman DS, Josephs KA. Predicting survival in frontotemporal dementia with motor neuron disease. Neurology. 2011;76:1886–93.
Czell D, Andersen PM, Neuwirth C, Morita M, Weber M. Progressive aphasia as the presenting symptom in a patient with amyotrophic lateral sclerosis with a novel mutation in the OPTN gene. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:138–40.
da Rocha AJ, Valerio BC, Buainain RP, Ferraz ME, da Silva CJ, et al. Motor neuron disease associated with non-fluent rapidly progressive aphasia: case report and review of the literature. Eur J Neurol. 2007;14:971–5.
Darley FL, Aronson AE, Brown JR. Differential diagnostic patterns of dysarthria. J Speech Hear Res. 1969;12:246–69.
Darley FL, Aronson AE, Brown JR. Motor speech disorders. Philadelphia: W.B. Saunders; 1975.
Davies RR, Hodges JR, Kril JJ, Patterson K, Halliday GM, et al. The pathological basis of semantic dementia. Brain. 2005;128:1984–95.
Deramecourt V, Lebert F, Debachy B, Mackowiak-Cordoliani MA, Bombois S, et al. Prediction of pathology in primary progressive language and speech disorders. Neurology. 2010;74:42–9.
Dickson DW. Neuropathology of Pick’s disease. Neurology. 2001;56:S16–20.
Dickson DW. Neurodegeneration: the molecular pathology of dementia and movement disorders. Basel: ISN Neuropath Press; 2003.
Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, et al. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61:935–46.
Duffy J. Apraxia of speech in degenerative neurologic disease. Aphasiology. 2006;20:511–27.
Duffy JR. Motor speech disorders: substrates, differential diagnosis, and management. St Louis: Mosby; 2013.
Duffy JR, Peach RK, Strand EA. Progressive apraxia of speech as a sign of motor neuron disease. Am J Speech Lang Pathol. 2007;16:198–208.
Edwards-Lee T, Miller BL, Benson DF, Cummings JL, Russell GL, et al. The temporal variant of frontotemporal dementia. Brain. 1997;120(Pt 6):1027–40.
Galantucci S, Tartaglia MC, Wilson SM, Henry ML, Filippi M, et al. White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain. 2011;134:3011–29.
Galton CJ, Patterson K, Xuereb JH, Hodges JR. Atypical and typical presentations of Alzheimer’s disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain. 2000;123(Pt 3):484–98.
Galton CJ, Patterson K, Graham K, Lambon-Ralph MA, Williams G, et al. Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology. 2001;57:216–25.
Ghetti B, Hutton M, Wszolek Z. Frontotemporal dementia and parkinsonism linked to chromosome 17 associated with tau gene mutations (FTDP-17T). In: Dickson D, editor. Neurodegeneration: the molecular pathology of dementia and movement disorders. Basel: ISN Neuropath Press; 2003. p. 86–102.
Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–46.
Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14.
Graff-Radford NR, Damasio AR, Hyman BT, Hart MN, Tranel D, et al. Progressive aphasia in a patient with Pick’s disease: a neuropsychological, radiologic, and anatomic study. Neurology. 1990;40:620–6.
Graff-Radford J, Duffy JR, Strand EA, Josephs KA. Parkinsonian motor features distinguish the agrammatic from logopenic variant of primary progressive aphasia. Parkinsonism Relat Disord. 2012;18:890–2.
Greene JD, Patterson K, Xuereb J, Hodges JR. Alzheimer disease and nonfluent progressive aphasia. Arch Neurol. 1996;53:1072–8.
Groschel K, Hauser TK, Luft A, Patronas N, Dichgans J, et al. Magnetic resonance imaging-based volumetry differentiates progressive supranuclear palsy from corticobasal degeneration. Neuroimage. 2004;21:714–24.
Grossman M, McMillan C, Moore P, Ding L, Glosser G, et al. What’s in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127:628–49.
Grossman M, Powers J, Ash S, McMillan C, Burkholder L, et al. Disruption of large-scale neural networks in non-fluent/agrammatic variant primary progressive aphasia associated with frontotemporal degeneration pathology. Brain Lang. 2013;127:106–20.
Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology. 1994;44:2015–9.
Hodges JR, Patterson K. Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol. 2007;6:1004–14.
Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol. 2004;56:399–406.
Huey ED, Pardini M, Cavanagh A, Wassermann EM, Kapogiannis D, et al. Association of ideomotor apraxia with frontal gray matter volume loss in corticobasal syndrome. Arch Neurol. 2009;66:1274–80.
Josephs KA, Duffy JR. Apraxia of speech and nonfluent aphasia: a new clinical marker for corticobasal degeneration and progressive supranuclear palsy. Curr Opin Neurol. 2008;21:688–92.
Josephs KA, Boeve BF, Duffy JR, Smith GE, Knopman DS, et al. Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase. 2005;11:283–96.
Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006a;129:1385–98.
Josephs KA, Petersen RC, Knopman DS, Boeve BF, Whitwell JL, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006b;66:41–8.
Josephs KA, Whitwell JL, Dickson DW, Boeve BF, Knopman DS, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging. 2008;29:280–9.
Josephs KA, Whitwell JL, Knopman DS, Boeve BF, Vemuri P, et al. Two distinct subtypes of right temporal variant frontotemporal dementia. Neurology. 2009a;73:1443–50.
Josephs KA, Stroh A, Dugger B, Dickson DW. Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathol. 2009b;118:349–58.
Josephs KA, Duffy JR, Fossett TR, Strand EA, Claassen DO, et al. Fluorodeoxyglucose F18 positron emission tomography in progressive apraxia of speech and primary progressive aphasia variants. Arch Neurol. 2010;67:596–605.
Josephs KA, Hodges JR, Snowden JS, Mackenzie IR, Neumann M, et al. Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol. 2011;122:137–53.
Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012;135:1522–36.
Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, et al. Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology. 2013a;81:337–45.
Josephs KA, Whitwell JL, Murray ME, Parisi JE, Graff-Radford NR, et al. Corticospinal tract degeneration associated with TDP-43 type C pathology and semantic dementia. Brain. 2013b;136:455–70.
Kelley BJ, Haidar W, Boeve BF, Baker M, Graff-Radford NR, et al. Prominent phenotypic variability associated with mutations in Progranulin. Neurobiol Aging. 2009;30:739–51.
Kertesz A, Hudson L, Mackenzie IR, Munoz DG. The pathology and nosology of primary progressive aphasia. Neurology. 1994;44:2065–72.
Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain. 2005;128:1996–2005.
Knake S, Belke M, Menzler K, Pilatus U, Eggert KM, et al. In vivo demonstration of microstructural brain pathology in progressive supranuclear palsy: a DTI study using TBSS. Mov Disord. 2010;25:1232–8.
Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Ann Neurol. 2006;59:156–65.
Knopman DS, Mastri AR, Frey 2nd WH, Sung JH, Rustan T. Dementia lacking distinctive histologic features: a common non-Alzheimer degenerative dementia. Neurology. 1990;40:251–6.
Koyama M, Yagishita A, Nakata Y, Hayashi M, Bandoh M, et al. Imaging of corticobasal degeneration syndrome. Neuroradiology. 2007;49:905–12.
Kremen SA, Mendez MF, Tsai P, Teng E. Extrapyramidal signs in the primary progressive aphasias. Am J Alzheimers Dis Other Demen. 2011;26:72–7.
Lang AE. Cortical basal ganglionic degeneration presenting with “progressive loss of speech output and orofacial dyspraxia”. J Neurol Neurosurg Psychiatry. 1992;55:1101.
Lippa CF, Cohen R, Smith TW, Drachman DA. Primary progressive aphasia with focal neuronal achromasia. Neurology. 1991;41:882–6.
Mackenzie IR, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, et al. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol (Berl). 2006;112:539–49.
Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122:111–3.
Madhavan A, Whitwell JL, Weigand SD, Duffy JR, Strand EA, et al. FDG PET and MRI in logopenic primary progressive aphasia versus dementia of the Alzheimer’s type. PLoS One. 2013;8:e62471.
Mahoney CJ, Beck J, Rohrer JD, Lashley T, Mok K, et al. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain. 2012;135:736–50.
Mahoney CJ, Malone IB, Ridgway GR, Buckley AH, Downey LE, et al. White matter tract signatures of the progressive aphasias. Neurobiol Aging. 2013;34:1687–99.
Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982;11:592–8.
Mesulam MM. Primary progressive aphasia. Ann Neurol. 2001;49:425–32.
Mesulam MM. Primary progressive aphasia–a language-based dementia. N Engl J Med. 2003;349:1535–42.
Mesulam M, Johnson N, Krefft TA, Gass JM, Cannon AD, et al. Progranulin mutations in primary progressive aphasia: the PPA1 and PPA3 families. Arch Neurol. 2007;64:43–7.
Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63:709–19.
Mukherjee O, Pastor P, Cairns NJ, Chakraverty S, Kauwe JS, et al. HDDD2 is a familial frontotemporal lobar degeneration with ubiquitin-positive, tau-negative inclusions caused by a missense mutation in the signal peptide of progranulin. Ann Neurol. 2006;60:314–22.
Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, et al. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45.
Ogar J, Slama H, Dronkers N, Amici S, Gorno-Tempini ML. Apraxia of speech: an overview. Neurocase. 2005;11:427–32.
Pickering-Brown SM, Rollinson S, Du Plessis D, Morrison KE, Varma A, et al. Frequency and clinical characteristics of progranulin mutation carriers in the Manchester frontotemporal lobar degeneration cohort: comparison with patients with MAPT and no known mutations. Brain. 2008;131:721–31.
Ridgway GR, Lehmann M, Barnes J, Rohrer JD, Warren JD, et al. Early-onset alzheimer disease clinical variants: multivariate analyses of cortical thickness. Neurology. 2012;79:80–4.
Roh JH, Suh MK, Kim EJ, Go SM, Na DL, Seo SW. Glucose metabolism in progressive nonfluent aphasia with and without parkinsonism. Neurology. 2010;75:1022–4.
Rohrer JD, Warren JD, Modat M, Ridgway GR, Douiri A, et al. Patterns of cortical thinning in the language variants of frontotemporal lobar degeneration. Neurology. 2009;72:1562–9.
Rohrer JD, Crutch SJ, Warrington EK, Warren JD. Progranulin-associated primary progressive aphasia: a distinct phenotype? Neuropsychologia. 2010;48:288–97.
Schwindt GC, Graham NL, Rochon E, Tang-Wai DF, Lobaugh NJ, et al. Whole-brain white matter disruption in semantic and nonfluent variants of primary progressive aphasia. Hum Brain Mapp. 2013;34:973–84.
Simon-Sanchez J, Dopper EG, Cohn-Hokke PE, Hukema RK, Nicolaou N, et al. The clinical and pathological phenotype of C9ORF72 hexanucleotide repeat expansions. Brain. 2012;135:723–35.
Snowden JS, Pickering-Brown SM, Mackenzie IR, Richardson AM, Varma A, et al. Progranulin gene mutations associated with frontotemporal dementia and progressive non-fluent aphasia. Brain. 2006;129:3091–102.
Snowden J, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol. 2007;114:31–8.
Snowden JS, Rollinson S, Thompson JC, Harris JM, Stopford CL, et al. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain. 2012;135:693–708.
Soliveri P, Monza D, Paridi D, Radice D, Grisoli M, et al. Cognitive and magnetic resonance imaging aspects of corticobasal degeneration and progressive supranuclear palsy. Neurology. 1999;53:502–7.
Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology. 2003;61:1196–203.
Warrington E. Selective impairment of semantic memory. Q J Exp Psychol. 1975;27:635–57.
Wechsler AF, Verity MA, Rosenschein S, Fried I, Scheibel AB. Pick’s disease. A clinical, computed tomographic, and histologic study with golgi impregnation observations. Arch Neurol. 1982;39:287–90.
Whitwell JL, Avula R, Senjem ML, Kantarci K, Weigand SD, et al. Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology. 2010a;74:1279–87.
Whitwell JL, Jack Jr CR, Boeve BF, Parisi JE, Ahlskog JE, et al. Imaging correlates of pathology in corticobasal syndrome. Neurology. 2010b;75:1879–87.
Whitwell JL, Master AV, Avula R, Kantarci K, Eggers SD, et al. Clinical correlates of white matter tract degeneration in progressive supranuclear palsy. Arch Neurol. 2011;68:753–60.
Whitwell JL, Duffy JR, Strand EA, Machulda MM, Senjem ML, et al. Neuroimaging comparison of primary progressive apraxia of speech and progressive supranuclear palsy. Eur J Neurol. 2013a;20:629–37.
Whitwell JL, Duffy JR, Strand EA, Xia R, Mandrekar J, et al. Distinct regional anatomic and functional correlates of neurodegenerative apraxia of speech and aphasia: an MRI and FDG-PET study. Brain Lang. 2013b;125:245–52.
Zhang Y, Schuff N, Du AT, Rosen HJ, Kramer JH, et al. White matter damage in frontotemporal dementia and Alzheimer’s disease measured by diffusion MRI. Brain. 2009;132:2579–92.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Josephs, K.A., Whitwell, J.L. (2014). Progressive Apraxia of Speech and Primary Progressive Aphasias. In: Merello, M., Starkstein, S. (eds) Movement Disorders in Dementias. Springer, London. https://doi.org/10.1007/978-1-4471-6365-7_13
Download citation
DOI: https://doi.org/10.1007/978-1-4471-6365-7_13
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-6364-0
Online ISBN: 978-1-4471-6365-7
eBook Packages: MedicineMedicine (R0)