Abstract
Introduction
Diagnosing corticobasal degeneration is often difficult on the basis of clinical symptoms and radiological images. We aimed to clarify the imaging findings of corticobasal degeneration syndrome (CBDS).
Methods
Included in the study were 16 patients (8 men, 8 women, 46–75 years old) with clinically diagnosed CBDS. We evaluated the patients’ symptoms and signs, and MR and single-photon emission CT (SPECT) imaging findings.
Results
All the patients had cerebral atrophy. Asymmetric cerebral atrophy was observed in 13 patients (81%) predominantly contralateral to the side clinically more affected. Atrophy in the cerebral peduncle was observed in seven patients. FLAIR images showed hyperintensity in the subcortical white matter in the frontoparietal lobes in the clinically more affected side in 14 patients, and in the rolandic region in 13 patients. Asymmetric hypoperfusion in the frontoparietal lobes on SPECT images was observed in all of the patients, and in the basal ganglia in 11 patients.
Conclusion
CBDS might be unique in showing hyperintensity in the subcortical white matter in the rolandic region on FLAIR images with asymmetric atrophy predominantly contralateral to the side clinically more severely affected. Asymmetric atrophy in the cerebral peduncle without signal abnormalities was also characteristic of CBDS. Atrophy in the midbrain tegmentum was also seen in patients with CBDS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Corticobasal degeneration (CBD) is a rare disease involving the cerebral cortices as well as extrapyramidal structures. Cortical signs usually consist of apraxia, cortical sensory loss, and involuntary movements, which have been referred to as alien hand. The extrapyramidal signs consist of various kinds of involuntary movements, such as parkinsonism or dystonia [1–3].
On clinical diagnosis, asymmetry of symptoms is noted in some patients with Parkinson’s disease (PD) and CBD, but not in those with progressive supranuclear palsy (PSP). PD is usually responsive to levodopa, while CBD is refractory to it. CBD shows cortical signs and late dementia. However, it is sometimes difficult to distinguish CBD from other diseases with parkinsonism, such as PD and PSP. Alzheimer’s disease (AD), frontotemporal dementia (FTD) and Lewy body disease are also difficult to distinguish from CBD, because CBD sometimes shows dementia [1–3].
Recently, Doran et al. [4] suggested that all clinically diagnosed cases of CBD should initially be labeled as “corticobasal degeneration syndrome” (CBDS) to emphasize that this is a diagnosis based on clinical phenotype, with the term acronym CBD being reserved for the specific neuropathological phenotype, which itself may show a variety of clinical presentations.
We describe here the clinical and imaging findings in 16 patients with CBDS. The purpose of this study was to clarify the imaging findings in CBDS.
Methods
Patients
Our institutional review board does not require us to seek approval for a retrospective study using routinely obtained clinical data. Patients’ informed consent was also not required.
All of the patients were admitted to our hospital from 1 January 1997 to 28 February 2006. Of 16 patients with CBDS examined, all fulfilled the criteria for the diagnosis of CBDS proposed by Boeve et al. [1]. The main criteria are insidious onset and progressive course, no identifiable cause, such as tumor or infarct, cortical dysfunction and extrapyramidal dysfunction. Cortical dysfunction is indicated by at least one of the following: focal and asymmetric ideomotor apraxia, alien limb phenomenon, cortical sensory loss, visual or sensory hemineglect, constructional apraxia, focal or asymmetric myoclonus, apraxia of speech/nonfluent aphasia. Extrapyramidal dysfunction is indicated by at least one of the following: focal or asymmetric appendicular rigidity lacking prominent and sustained L-dopa response, and focal or asymmetric appendicular dystonia. We excluded patients with complications of other neurological diseases or evidence of focal brain lesions on MR images.
MR acquisition
All studies were performed with a 1.5-T MR imaging unit. Imaging was performed up to four times in a single patient (mean 1.75 times) to give a total of 28 MR examinations performed. Axial T2-weighted (T2-W) and fluid-attenuated inversion recovery (FLAIR) images, and sagittal T1-weighted (T1-W) images were obtained in all patients. Surface scan imaging was performed in one patient. The parameters for the T2-W imaging were repeat time (TR)/echo time (TE) 4,000/99 ms, number of excitations (NEX) 2, field of view (FOV) 220 mm, slice thickness 5 mm with a slice gap of 1 mm; for the FLAIR imaging were TR/TE 10,002/152 ms, NEX 1, inversion time (TI) 2,200 ms or TR/TE 8,802/147.1 ms, NEX 1, TI 2,200 ms, slice thickness 5 mm with a gap of 1 mm; and for the T1-W imaging were TR/TE 400/10 ms, NEX 2, slice thickness 5 mm with a gap of 1 mm.
Brain perfusion study
Single-photon emission CT (SPECT) in most patients was performed using technetium-99m ethyl cysteinate dimer (ECD); in one patient, I-123 iodoamphetamine (IMP) was used. SPECT acquisition was performed on a dual head gamma camera. The regional cerebral blood flow was evaluated using time-activity data and brain hemispheres [5]. Imaging was performed one to three times in a single patient (mean 1.5 times) to give a total of 24 SPECT examinations performed. SPECT examination was usually performed after MR examination.
Two neuroradiologists (Y.A., N.Y.) who did not know the patients’ clinical information evaluated the MR images in relation to atrophy and signal intensity changes in the cerebral hemisphere, basal ganglia, midbrain tegmentum, corpus callosum, cerebral peduncle and pyramid. Atrophy in the midbrain tegmentum and corpus callosum was evaluated on sagittal T1-W images. Asymmetry of brain perfusion on SPECT was also evaluated. When the two failed to reach a consensus on a diagnosis, a radiologist (K.M.) who knew the patients’ information took part and a majority decision was reached.
Results
Clinical symptoms, signs and imaging findings are summarized in Tables 1 and 2. When two or more examinations were performed, the findings of the last clinical and imaging examinations are noted in the tables. The duration of the symptoms was 1 year to 14 years (mean 4.7 years). All the patients except one had focal or asymmetric rigidity with a dominant side. All of the patients but one had higher cortical dysfunctions with a dominant side.
MR images
All the patients had cerebral atrophy. Asymmetric cerebral atrophy was observed in 13 patients (81%) with the predominance contralateral to the side clinically more affected (Figs. 1, 2 and 3). In three patients, cerebral atrophy affected both sides equally. Atrophy in the posterior frontal and parietal lobes were always more severe than in the other lobes. Atrophy in the occipital lobe was rarely observed. Atrophy in the temporal and anterobasal frontal lobes was frequently observed, but was less severe than in the posterior frontal and parietal lobes.
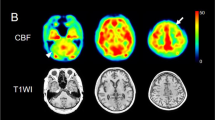
Patient 11: 65-year-old female with CBDS with right predominant rigidity, right pyramidal signs and right limb kinetic apraxia. a, b FLAIR (TR/TE/TI 8,802/147.1/2,200 ms) images show left frontoparietal cerebral atrophy. Hyperintense areas are apparent in the subcortical white matter in the left precentral gyrus (arrows) and less intensely in the postcental gyrus (arrowhead). c ECD-SPECT images show decreased perfusion in the left frontoparietal lobes and in the left putamen
Patient 16: 71-year-old male with CBDS with right rigidity and right apraxia. a FLAIR (TR/TE/TI 8,802/147.1/2,200 ms) image shows atrophy in the left frontoparietal lobes and faint hyperintense lesions in the subcortical white matter in the left precentral gyrus (arrow). b FLAIR image demonstrates subtle hyperintensities in the operculum (arrow). This image is the lowest section in which hyperintensities were observed (AAR anterior ascending ramus of the sylvian fissure, prC precentral sulcus, cs central sulcus, ics insular central sulcus). c, d T2-weighted (TR/TE 4,000/99 ms) images show unilateral atrophy in the left cerebral peduncle (c, arrow) and pyramid (d, arrow) without signal intensity changes. In this patient, unilateral atrophy in the cerebral peduncle is the most outstanding finding of the CBDS
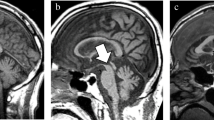
Patient 2: 62-year-old female with CBDS with left dominant rigidity, left pyramidal signs and right frontal and parietal lobe signs. a Surface anatomy scan (TR/TE 1,529/185 ms) image shows atrophy of the right precentral (prC) and postcentral (poC) gyri. b Midsagittal T1-weighted (TR/TE 400/10 ms) image shows atrophy of the corpus callosum (arrowhead) and midbrain tegmentum (arrow)
Atrophy in the cerebral peduncle was observed in seven patients in six of whom the atrophy was ipsilateral to the dominant atrophic cerebral hemisphere (Fig. 2c). All of the seven patients showed contralateral pyramidal signs. No signal intensity changes were noted in the cerebral peduncles. In patient 16, atrophy in the cerebral peduncle was the most outstanding imaging finding of CBDS (Fig. 2c). Atrophy in the pyramid ipsilateral to the clinically more affected cortices was observed in five patients (Fig. 2d). Four of the five patients also showed atrophy in the cerebral peduncle on the same side. Atrophy in the midbrain tegmentum was seen in eight patients (Fig. 3b). Among them, three patients showed vertical gaze palsy. Atrophy in the corpus callosum was observed in 15 patients (94%) (Fig. 3b).
On FLAIR images, increased signal intensity changes were noted in the frontal and/or parietal subcortical white matter in 14 patients (88%) (Figs. 1 and 2). The side of the hyperintense areas was ipsilateral to the clinically more affected cortices in 13 patients. In the contralateral sides, more subtle hyperintensities were occasionally observed. In the rolandic region, hyperintense lesions were observed in 13 patients. Hyperintense areas were more frequently observed in the upper sections in which the superior frontal sulcus was well visualized compared with the lower sections. The operculum was the lowest space in which hyperintense lesions were observed on FLAIR images (Fig. 2b). Motor aphasia with apraxia of speech, forced grasping reflex, and motor impersistence of eye closing and tongue protrusion were frontal signs. The MR images of seven of eight patients with the frontal signs showed hyperintensities in the frontal lobes. The MR images of two of four patients with parietal signs (cortical sensor loss and limb kinetic apraxia) showed hyperintensities in the parietal lobe.
No signal or volume changes in the basal ganglia were observed in our study. On T2-W images, no significant hypointense areas were noted in the motor cortices of the patients.
Brain perfusion
Asymmetric hypoperfusion was seen in the frontoparietal lobes on SPECT images in all patients (Fig. 1c), hypoperfusion in the basal ganglia, particularly the putamen, in 11 patients (69%), and hypoperfusion in the thalamus in 14 patients (88%). The side of the hypoperfusion was contralateral to the side more severely affected clinically. SPECT was generally more sensitive in detecting asymmetry than MR imaging. Hypoperfusion was seen in the cerebellar hemisphere in 10 patients (63%), on the side contralateral to the affected cerebral cortex.
Follow-up study
Patients 1, 2, 3, 6, 12 and 16 had two or more follow-up examinations.
Hyperintensities on FLAIR images became more prominent in patients 1, 2, 3, 12 and 16 a mean of 24 months (range 6–36 months) after the first examination. In patient 6, hyperintensities were not observed on the MR images of the examinations but were apparent at 6 months, and became more prominent over 2 years. The perfusion pattern was grossly unchanged in patients 1, 2, 3 and 6. In patient 12, asymmetric perfusion became apparent 31 months after the first examination.
Discussion
Asymmetric cortical atrophy was observed in 81% of the patients in our study, and it was a characteristic finding of CBDS, but not unique to CBDS [4]. In previous studies asymmetric cortical atrophy has also been observed in patients with AD, Pick disease [4, 6–8] and FTD. However, the anterior frontal and temporal lobe involvement was more distinctive in FTD [9].
Unilateral atrophy in the cerebral peduncle was observed in seven patients. None of the atrophy was accompanied by signal changes in the corticospinal tract (CST) in the cerebral peduncle. The side of atrophy was contralateral to pyramidal signs in all of the seven patients. In a review article, Boeve et al. [1] reported that asymmetric atrophy in the cerebral peduncle might be present in patients with CBDS. However, no images were presented. The cerebral peduncle consists of descending corticofugal fibers [10]. Neuronal degeneration in the posterior frontal and parietal lobes in CBD induces secondary degeneration in the corticospinal, corticobulbar and parietopontine fibers, and consequently there is atrophy in the cerebral peduncle on the affected side. The reason why signal intensity changes in the CST are not associated with atrophy in the cerebral peduncle is unknown. However, the slow progression of neuronal degeneration in CBD might be a reason. Recently, a histopathological study of the autopsy examinations of ten patients with CBD revealed constant and severe involvement of Betz cells in the primary motor cortex and degeneration of the CST [11]. Furthermore, Boelmans et al. [12] demonstrated CST atrophy in a patient with CBDS using tractography. Therefore, when unilateral atrophy in the cerebral peduncle without signal intensity changes in the CST is observed on MR images of patients with asymmetric parkinsonism, higher cortical dysfunction and dementia, CBDS might be a major consideration in the differential diagnosis.
Atrophy of the midbrain tegmentum was observed on sagittal images in eight patients. It was not uncommon that patients with CBDS developed supranuclear gaze palsies that were not distinguishable from those seen in PSP [1]. Atrophy of the midbrain tegmentum on MR images has also been observed in PSP patients [13, 14]. We did not find any difference in atrophy of the tegmentum between PSP and CBDS patients. Thus, atrophy of the midbrain tegmentum was not unique to PSP patients, but is also seen in CBDS patients.
Doi et al. [15] reported a patient with pathologically confirmed CBD. T2-W images of the patient showed focal atrophy of the bilateral frontal cortex and asymmetric regional hyperintensities of the subjacent white matter in the frontal lobe with greater prominence contralateral to the side more severely affected clinically. They concluded that these signal changes seemed to primarily reflect the progression of neuronal degeneration, especially the demyelination secondary to axonal loss or change. Although they described only one patient and used T2-W imaging, the hyperintensities in the subcortical white matter are consistent with our findings on FLAIR images. Moreover, Winkelmann et al. [16] reported two patients with CBDS in whom MR images demonstrated T2 elongation in the rolandic region.
Mirra and Hyman [17] note that the neuropathological features of CBD include an often asymmetric cortical atrophy, predominantly involving the perirolandic cortex, but also involving other cortical regions and the underlying white matter showing considerable loss of myelin and even axons. Therefore, we agree with the conclusions of Doi et al. [15] that these signal abnormalities reflect progression of neuronal degeneration, especially demyelination secondary to axonal loss or change. Moreover, we believe it is unique to CBDS that FLAIR images showed hyperintensities in the rolandic region with asymmetric atrophy with the predominance contralateral to the side clinically more severely affected. To the best of our knowledge, these hyperintensities have never been reported in patients with AD, PSP, FTD, Pick disease or Lewy body disease. We evaluated the hyperintensities in the cerebral subcortical white matter mainly on FLAIR images, because FLAIR images were more sensitive than T2-W images in showing lesions in the subcortical white matter.
Imaging in our study revealed that upper sections in the rolandic regions were affected more severely than the lower. Therefore, we speculated that the degeneration in CBD might be more severe in the upper parts in the rolandic regions than in the lower parts. The exact localization of apraxia and other cortical dysfunctions is a matter of controversy. Therefore, the location of cortical dysfunction had no clear correlation with the presence of hyperintensity. Hyperintensity in the subcortical white matter in the precentral gyri is also seen on MR images in amyotrophic lateral sclerosis (ALS) [18]. However, the signal changes are visualized on both sides, not in the asymmetric regions. Moreover, low signal intensity changes in the motor cortices on T2-W images [19] were not seen in our study. In chronic hepatic encephalopathy, hyperintensity in the subcortical white matter in the precentral gyri is also seen on T2-W images [20], but it is bilateral.
According to Kitagaki et al. [21] increased signal intensity changes in the frontotemporal white matter on T2- and proton density-weighted MR images are a useful diagnostic sign of FTD and distinguish this condition from AD. CBD, as well as FTD, Pick’s disease and PSP are tauopathies with tau protein expressed histopathologically. The signal intensity changes in the subcortical white matter in both CBDS and FTD might reflect similar pathological changes. Asymmetric hypoperfusion in the affected cortices was characteristic of CBDS but are not unique to CBDS. Generally, it could be more easily detected than asymmetric atrophy or hyperintensity. Therefore, when it is found, we should pay close attention to atrophy or subtle signal intensity changes in the frontoparietal lobes on the affected side on MR images. Hypoperfusion in the basal ganglia and thalamus ipsilateral to the affected cortices may be due to a remote effect, because no signal or volume change was noted on MR imaging. Hypoperfusion in the cerebellar hemisphere contralateral to the affected cerebral cortices also seems to be remote effect (crossed cerebellar diaschisis). These findings were found in most of the patients. However, we found no significant relationship with the clinical symptoms or MR imaging findings.
The major limitation of our study was that the diagnosis in the presented patients had not been proved by neuropathological studies, and it was possible that the clinical diagnosis of CBDS might have been in error. The clinical differentiation of CBDS from atypical AD, Pick’s disease, or PSP may often be difficult, and our patients might have been affected by these other diseases.
In conclusion, it was characteristic of CBDS and may be unique to CBDS that FLAIR images showed hyperintensity in the subcortical white matter in the rolandic region, with asymmetric atrophy with the predominance contralateral to the side clinically more severely affected. Moreover, in patients with asymmetric parkinsonism, higher cortical dysfunction and dementia, asymmetric atrophy in the cerebral peduncle without signal abnormalities in the CST was also characteristic of CBDS. Atrophy in the midbrain tegmentum was also seen in patients with CBDS.
References
Boeve BF, Lang AE, Litvan I (2003) Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol 54(Suppl 5):S15–S19
Lowe JS, Leigh N (2002) Corticobasal degeneration. In: Graham DI, Lantos PL (eds) Greenfield’s neuropathology, 7th edn, vol. 2. Arnold, London, pp 346–350
Shannon KM (2004) Corticobasal degeneration. In: Bradley WG, Daroff RB, Fenichel GM, Jankovic J (eds) Neurology in clinical practice, 4th edn, vol. 2. Butterworth Heinemann, Philadelphia, p 2142
Doran M, du Plessis DG, Enevoldson TP, Fletcher NA, Ghadiali E, Larner AJ (2003) Pathological heterogeneity of clinically diagnosed corticobasal degeneration. J Neurol Sci 216:127–134
Matsuda H, Yagishita A, Tsuji S, Hisada K (1995) A quantitative approach to technetium-99m ethyl cysteinate dimer; a comparison with technetium-99m hexamethylpropylene amine oxime. Eur J Nucl Med 22:633–637
Galton CJ, Patterson K, Xuereb JH, Hodges JR (2000) Atypical and typical presentations of Alzheimer’s disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain 123:484–498
Kaida K, Takeda K, Nagata N, Kamakura K (1998) Alzheimer’s disease with asymmetric parietal lobe atrophy: a case report. J Neurol Sci 160:96–99
Giannakopoulos P, Hof PR, Bouras C (1994) Alzheimer’s disease with asymmetric atrophy of the cerebral hemispheres: morphometric analysis of four cases. Acta Neuropathol (Berl) 88:440–447
Grossman RI, Yousem DM (2003) Neuroradiology: the requisites, 2nd edn. Mosby, Philadelphia, p 387
Parent A (1996) Carpenter’s human neuroanatomy, 9th edn. Williams & Wilkins, Baltimore, pp 452–456
Tsuchiya K, Murayama S, Mitani K, Oda T, Arima K, Mimura M, Nagura H, Haga C, Akiyama H, Yamanouchi H, Mizusawa H (2005) Constant and severe involvement of Betz cells in corticobasal degeneration is not consistent with pyramidal signs: a clinicopathological study of ten autopsy cases. Acta Neuropathol 109:353–366
Boelmans K, Kaufmann J, Bodammer N, Heinze HJ, Niehaus L (2006) Corticospinal tract atrophy in corticobasal degeneration. Arch Neurol 63:462–463
Yagishita A, Oda M (1996) Progressive supranuclear palsy: MRI and pathological findings. Neuroradiology 38(Suppl 1):S60–S66
Oba H, Yagishita A, Terada H, Barkovich AJ, Kutomi K, Yamauchi T, Furui T, Shimizu T, Uchigata M, Matsumura K, Sonoo M, Sakai M, Takada K, Harasawa A, Takeshita K, Kohtake H, Tanaka H, Suzuki S (2005) New and reliable MRI diagnosis for progressive supranuclear palsy. Neurology 64:2050–2055
Doi T, Iwasa K, Makifuchi T, Takamiri M (1999) White matter hyperintensities on MRI in a patient with corticobasal degeneration. Acta Neurol Scand 99:199–201
Winkelmann J, Auer DP, Lechner C, Elbel G, Trenkwalder C (1999) Magnetic resonance imaging findings in corticobasal degeneration. Mov Disord 14:669–673
Mirra SS, Hyman BT (2002) Corticobasal degeneration. In: Graham DI, Lantos PL (eds) Greenfield’s neuropathology, 7th edn, vol. 2. Arnold, London, pp 231–232
Hecht MJ, Fellner F, Fellner C, Hilz MN, Heuss D, Neundorfer B (2001) MRI-FLAIR images of the head show corticospinal tract alterations in ALS patients more frequently than T2-, T1- and proton-density-weighted images. J Neurol Sci 186:37–44
Oba H, Araki T, Ohtomo K, Monzawa S, Uchiyama G, Koizumi K, Nogata Y, Kachi K, Shiozawa Z, Kobayashi M (1993) Amyotrophic lateral sclerosis: T2 shortening in motor cortex at MR imaging. Radiology 189:843–846
Matsusue E, Kinoshita T, Ohama E, Ogawa T (2005) Cerebral cortical and white matter lesions in chronic hepatic encephalopathy: MR-pathologic correlations. AJNR Am J Neuroradiol 26:347–351
Kitagaki H, Mori E, Hirono N, Hirono N, Ikejiri Y, Ishii K, lmamura T, Ikeda M, Yamaji S, Yamashita H, Shimomura T, Nakagawa Y (1997) Alteration of white matter MR signal intensity in frontotemporal dementia. AJNR Am J Neuroradiol 18:367–378
Acknowledgement
We thank Shouki Takahashi, MD, for his advice.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest statement
We declare that we have no conflict of interest.
Rights and permissions
About this article
Cite this article
Koyama, M., Yagishita, A., Nakata, Y. et al. Imaging of corticobasal degeneration syndrome. Neuroradiology 49, 905–912 (2007). https://doi.org/10.1007/s00234-007-0265-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-007-0265-6