Abstract
Multiple organ dysfunction syndrome (MODS) occurs after a life-threatening primary insult, including severe infection, hypoxic-ischemic injury, or other serious injuries. It represents a continuum of physiological abnormalities rather than a distinct state (present or absent). Young age and chronic health conditions are the most important risk factors for the development of MODS. Increasing number of dysfunctional organs is correlated with mortality, greater use of resources, and prolonged stay in pediatric intensive care units. Severe insults converge towards a common systemic response resulting in organ dysfunctions, yet the underlying mechanism remains ill-defined. Acute illnesses may trigger severe inflammatory response resulting in cytokine liberation, activation of coagulation, development of shock and capillary leak. Most experimental therapies to date have focused on attenuating the initial inflammatory response with little benefits in humans. As the initial inflammatory storm subsides, relative immune suppression becomes a major contributor to the disease process. Consequently, MODS patients are highly vulnerable to nosocomial infections. Metabolic demands and neuroendocrine responses also follow a similar seesaw pattern of over-activation followed by a state of relative suppression. Therefore, MODS may emerge from the cumulative suppression of metabolic, neuroendocrine, and immune functions resembling a state of dormancy, hypothesized to be an evolutionary protective cellular mechanism in response to overwhelming injuries. Diagnosis of MODS should encourage physicians to uncover the underlying etiology that may require a specific therapy. The symptomatic management of organ dysfunctions must be carefully assessed in the context of systemic interactions with other failing organs. Although long term outcome data of critically ill children with MODS is limited, 60 % of survivors are reported to have a normal quality of life with minimal health problems.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Systemic inflammatory response syndrome
- Sepsis
- Multiple organ dysfunction syndrome
- Cytokines
- Immunoparalysis
Introduction
Progressive organ dysfunctions were first reported 50 years ago in the surgical literature. In 1963, adult patients with severe peritonitis were found to develop a state of high output shock and respiratory failure requiring mechanical ventilation. Biochemical and mechanical factors were presumed to explain the severe deterioration in these patients [1]. A sequential pattern of organ failures was identified during the 1970s among patients with ruptured aortic aneurysms [2]. Improvement in the medical management of shock states began to change disease progression and more reports of multiple organ failures in shock survivors emerged [3, 4]. Several studies uncovered a relationship between an increasing number of failing organs and mortality [5] or the length of stay in the intensive care unit (ICU) [5]. Multiple organ dysfunctions were found to occur with or without any identifiable infectious source [6], changing in severity over time, and being potentially reversible. Faced with this new entity, adult diagnostic criteria for the systemic inflammatory response syndrome (SIRS) [7], sepsis, and organ dysfunctions were proposed in 1992 [6] and were revisited in 2003 [8]. These definitions helped to distinguish the insult (infection, trauma, etc.), from the host response (SIRS) and the subsequent number of organ dysfunctions, while emphasizing the pathophysiological continuum culminating in these organ dysfunctions [9].
Definition of Pediatric Multiple Organ Dysfunction Syndrome (MODS)
Diagnostic criteria currently used to define the infectious insults, the host response (SIRS), and the number of organ dysfunction in children were established in 2002 [10] and are summarized here. Systemic inflammatory response syndrome [7] refers to any combination of two or more symptoms including fever or hypothermia; tachycardia or bradycardia in infants (<12 months of age); tachypnea or hypocapnia; leukocytosis or leukopenia (Tables 35.1 and 35.2) [6]. The host response is called “sepsis” when these symptoms are suspected to be triggered by an infection.
Multiple organ failure in critically ill children is defined as the simultaneous dysfunction of at least two organ systems [11, 12]. Criteria for organ failures (Table 35.3) were established according to severity of illness scoring systems used in critically ill children [10, 13]. The aim of using a common definition for MODS is to provide a reproducible assessment of organ dysfunction that allows for tracking of changes in organ function. However, the reproducibility and relative strength of these criteria has not been evaluated. MODS can be classified as primary or secondary, depending on the timing of organ dysfunctions. Primary MODS develops rapidly after pediatric ICU (PICU) admission [14–16] and is generally the consequence of a well-defined insult. In one study, the maximal number of organ failures was noted within 72 h in the majority of patients [14]. Secondary MODS corresponds to children who develop evidence of organ damages after the first week of PICU admission and/or develop a sequential pattern of organ dysfunction [17].
Pediatric MODS Scoring Systems
Two scores were developed to quantify the severity of MODS and follow its evolution over time: (1) Leteurtre et al. developed and validated the PELOD score [18, 19], which is derived from six independent physiological variables (Table 35.4) [18]; (2) Graciano et al. developed the Pediatric-MODS score, which relies exclusively on laboratory values (lactic acid, PaO2/FiO2 ratio, bilirubin, fibrinogen, blood urea nitrogen) and therefore does not take into consideration the neurological function [20]. This may be a serious limitation of the Pediatric-MODS score, since 80 % of the variability in PELOD scores is attributable to cardiovascular and neurologic dysfunctions [18]. Although both scores have good discriminative values and are useful tools to describe the severity of MODS in critically ill children, the calibration of the PELOD score has been recently criticized [21, 22]. Since mortality is low (around 5 %) and incidence of MODS higher (from 6 % to 57 %) in critically ill children (Table 35.5), the PELOD score has been used as a surrogate outcome measure in pediatric clinical trials for risk adjustment [23] or secondary outcome [24]. Daily PELOD scores of critically ill children effectively identified survivors from non survivors [25]. Fifty percent of 115 deaths were associated with an increase in the score from day 1 to day 2 and from day 2 to day 4 [25].
Epidemiology
Pediatric mortality is closely correlated with the number of organ dysfunctions [11, 12]. Conversely, the number of children who die in the PICU without reaching criteria for MODS is low [12, 15, 18]. MODS may stem from pediatric conditions, including sepsis, congenital heart diseases, trauma, and liver or bone marrow transplantations [26]. The incidence and mortality rate of MODS varies between studies in part due to disparities in case-definition and case-mix (see Table 35.5).
Risk factors for MODS in adults include delayed or inadequate resuscitation, persistent infectious or inflammatory focus, advancing age, malnutrition, or cancer [27]. In children, MODS most frequently affects children under 1 year of age [28]. The incidence and mortality of MODS is higher in neonates compared to older children [29] and a distinct pattern of organ dysfunctions was noted in the neonatal population [30, 31]. Indeed, important developmental changes occur during the first year of life that govern the maturation of renal, hepatic, gastrointestinal, and central nervous systems, which may predispose infants to MODS [32].
The presence of comorbid conditions increases the incidence of MODS and mortality. While one fourth of children with MODS were reported to have chronic condition in the mid-80s [12], now almost two thirds of pediatric ICU patients have an underlying chronic condition [33]. Not surprisingly, the incidence of MODS is twofold greater among children with a comorbid condition, which independently increases the risk of death [33].
Etiology
The initial life-threatening insult leading to MODS also influences mortality. A diagnosis of MODS compels the physician to identify the underlying cause since several diseases with multi-systemic manifestations may require a specific therapy. An overview of common and less usual causes of pediatric MODS are presented in Table 35.6.
Sepsis
Pediatric MODS in sepsis is associated with a poor prognosis compared to non-infectious SIRS [34]. Moreover, severity of organ failures and mortality rates are closely correlated with the severity of the infectious process [15, 16, 35, 36]. A detailed discussion of sepsis is found later in this textbook.
Congenital Heart Diseases
Children with congenital heart diseases sometimes develop organ dysfunction both before and after cardiac surgery requiring cardiopulmonary bypass. Pre-operative imbalances of the pulmonary and systemic circulations may lead to organ dysfunctions. This is the case of children with hypoplastic left heart syndrome and pulmonary overcirculation associated with poor systemic perfusion. Afterload reduction has been reported to improve hepatic, renal, and gastrointestinal functions pre-operatively in these patients [37]. MODS may also occur after cardiac surgery as a consequence of cardiopulmonary bypass and the surgical correction itself. Cardiovascular instability, endothelial damage, platelet and immune activations from cardiopulmonary bypass predispose to MODS [38]. Persistent renal failure, in the context of cardiac surgery has been associated with poor outcome [39, 40]. The surgical repair may sometimes exacerbate organ damage in the presence of low cardiac output syndrome, residual lesions, or a delayed adaptation to the post-operative physiology [37, 41]. Children with congenital heart diseases may be prone to “classical” adult-type MODS characterized by the development of immune paralysis, and susceptibility to a second-hit phenomenon [42]. For example, in the first week after surgery, Ben-Abraham et al. found that 80 % of mortality was due to MODS; thereafter, sepsis was believed to be the main cause of death [42].
Multiple Trauma
Multiple trauma is a cause of MODS in children, albeit less frequent than in adults. In a series of 334 children admitted to the PICU with isolated head injury, not a single patient developed MODS [43]. Only 3 % of children with multiple traumatic injuries acquired MODS 2 days after their admission to the PICU [43]. However, multiple trauma associated with abdominal compartment syndrome and MODS has a worse prognosis, with a reported mortality rate of 20 % in children [44]. Overall, the mortality from multiple trauma is threefold lower in children compared to adults [43], possibly because children have different mechanisms of injury, fewer comorbid conditions, and a different physiological response to traumatic injury.
Solid Organ or Bone Marrow Transplantations
MODS is a major determinant of early mortality after pediatric orthotopic liver transplantation due to vascular thrombosis, sepsis, or as a result of pre-transplant organ dysfunctions [45]. The extent of damage to the engrafted liver is a major contributor to organ dysfunction. In this regard, hepatic vascular thrombosis may lead to severe hemorrhagic shock and acute renal failure. This may then lead to polymicrobial sepsis due to intestinal perforation and malnutrition. Severe rejection is rare early after liver transplantation. Conversely, patients with rejections are less likely to develop MODS in the postoperative phase [45]. Long term survival depends on the underlying disease, the presence of MODS in the post-operative phase, or late sepsis [45]. The development of chronic graft failure or lymphoproliferative disease are also major determinants of outcome [45], the latter being associated with a 50 % mortality rate [46].
In bone marrow transplantation, pre-transplant conditioning leads to potentially reversible cytotoxicity including pancytopenia, capillary leak syndrome, acute graft versus host disease, and hepatic veno-occlusive disease. If important, this toxicity may create MODS. In one large prospective study, MODS was the only variable that had a negative impact on the outcome [47]. An increased mortality rate has been noted in children who developed septic shock and MODS after bone marrow transplantation, but not among those suffering from neoplasic disorders who did not have transplantation [48]. In the former group, pulmonary or neurological dysfunctions were important determinants of patient survival [49]. Respiratory insufficiency may be secondary to opportunistic infections, bronchiolitis obliterans, pulmonary edema, or toxicity. Combined neurological and renal dysfunctions may occur with cyclosporine or tacrolimus toxicity and the related bone marrow transplant thrombotic microangiopathy.
Pathogenesis
Evolutionary Ties Between Sepsis and Tissue Injury
Despite similar host responses to severe sepsis and post-traumatic SIRS suggestive of a unifying cause, the molecular mechanism has been poorly understood. Due to lower blood pressure and relative splanchnic hypoperfusion in severe trauma, the possibility of bacterial translocation from the gut was initially suggested. However, this hypothesis was later refuted. More recent evidence posits activation of the innate immune system through highly conserved molecules known as the pathogen-associated molecular patterns (PAMPs), expressed by a variety of pathogens. Similarly, host molecules released following tissue injury called damage-associated molecular patterns (DAMPs) also initiate the innate immune response through shared signalling pathways with PAMPs, even in the absence of microbial pathogens [50]. Recent evidence reveals that DAMPs, including the high mobility group protein (HMGB1) produced by nucleated cells, are released in the blood of injured patients and their levels correlate with the development of organ failures.
Mitochondria provide a plausible explanation for the common infectious and tissue injury triggers of the innate immune response (Fig. 35.1a). Mitochondrial and bacterial DNA share similar structural motifs as an evolutionary consequence of the bacterial origin of these organelles [51]. Zhang et al. therefore postulated that mitochondrial components spilled by necrotic tissue after severe trauma (DAMPs) could mimic PAMPs and activate host response [52]. Administration of mitochondrial DAMPs in rats induced acute lung injury. Severe trauma in humans caused a rapid release of mitochondrial DNA and mitochondrial DAMPs such as formyl peptides, which attracted neutrophils and initiated the immune response through pattern-recognition receptors (PRRs), such as toll-like receptor 4 (TLR-4). Conserved molecular motifs between bacteria and mitochondria may therefore provide an explanation for a shared immune response to injury and infections [52].
Sepsis, tissue injury and the inflammatory response. (Panel a) Release of molecules called pathogen-associated molecular patterns (PAMPs) from bacteria and damage-associated molecular patterns (DAMPs) from tissue necrosis and mitochondrial fragments trigger the inflammatory response. (Panel b) Activation of innate immunity and the complement cascade leads to the release of cytokines, reactive oxygen species (ROS) and highly reactive lipid mediators. Hemodynamic instability is the outcome of changes in myocardial contractility, vasodilatation and capillary leak. The endothelium begins to express tissue factor (TF) launching the coagulation cascade, while plasminogen activator inhibitor-1 (PAI-1) decreases fibrinolysis; this results in microangiopathy and DIC. Together, cellular dysoxia culminate in organ dysfunctions (Adapted from Cohen [187]. With permission from Nature Publishing Group)
Inflammation and Immune System
Sepsis and MODS were traditionally believed to result from over-activation of the immune system and the ensuing inflammatory cascade (Fig. 35.1b). Overwhelming stimulation of innate immune cells expressing PRRs rapidly initiate host defence after tissue damage or microbial infection [53]. TLRs are a subfamily of PRRs crucial to the initiation of the inflammatory response. TLR4-mediated recognition of lipopolysaccharide and DAMPs (such as mitochondrial DNA), rapidly initiates host response and facilitate crosstalk with the complement system [53]. Activated neutrophils and macrophages produce cytokines, chemokines, and complement-activation products, resulting in a markedly imbalanced cytokine response (or ‘cytokine storm’). This pro-inflammatory environment triggers the liberation of powerful secondary lipid mediators and reactive oxygen species that further amplify the inflammatory storm, leading to host tissue damage. Children who died from meningococcal sepsis presented higher concentrations of several pro-inflammatory cytokines, as well as increased serum levels of anti-inflammatory mediators (IL-10, soluble TNF receptors) [54, 55]. Hereditary markers of innate immunity influence the outcome of sepsis [56, 57]. However, if most patients die during the initial phase of sepsis and MODS, several succumb later during the second phase characterized by protracted immune suppression (Fig. 35.2).
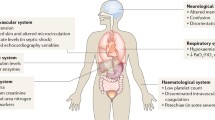
Overview of the pathophysiology of multiple organ dysfunction syndrome. The host response to injury or infection is central to the development of multiple organ dysfunction syndrome (MODS). Shock states are characterized by abnormal microcirculatory blood flow, with variable degree of peripheral vasoplegia and myocardial depression that may cause acute renal failure. The latter may aggravate capillary leak syndrome. Renal failure itself may result in worse lung injury or other organ failure. Inflammatory processes, including the cytokine and chemokine response, lead to endothelial cell activation, which is clinically recognized as disseminated intravascular coagulation, capillary leak as well as acute respiratory distress syndrome. Hypermetabolism, also called “septic autocannibalism”, may result in a state of severe malnutrition which is associated with secondary immunoparalysis. Overall, impaired mechanisms of tissue repair may lead to the development of nosocomial infections, usually 7–10 days later. The biological significance of other clinical conditions highlighted above remains to be clarified (TAMOF Thrombocytopenia associated multiple organ failure)
Adaptive Immunity and Immune Suppression
In contrast to the innate immune system, adaptive immunity develops over several days and provides a more specific line of defence against pathogens. T cells orchestrate the inflammatory response, particularly CD4+ T helper 1 (TH1) and 2 (TH2) cells, with distinct cytokine profiles. During sepsis, adaptive immunity shifts from a TH1 cell mediated inflammatory response (interferon-γL-2 and IL-12), to a TH2-cell response (IL-4, IL-5, IL-10 and IL-13), which can contribute to immunosuppresion.
Multiple cellular mechanisms underlie the immune suppression in sepsis. Increased levels of apoptosis in lymphocytes and dendritic cells contribute to immune suppression [58]. Moreover, apoptotic cells intensify the process of ‘immune paralysis’ in remaining immune cells characterized by shut-down of cytokine response and signalling capacity [59, 60], albeit not a generalized phenomenon [61]. In contrast to circulating immune cells, those derived from tissues appear to remain fully responsive, thereby indicating compartmentalization of inflammatory processes [61]. Intracellular reprogramming may be responsible for the hyporeactivity of circulating leukocytes and may represent a physiological adaptation with protective effects. This observation is reminiscent of the phenomenon of endotoxin tolerance well described in sepsis models [62–64].
Autopsies of pediatric and adult patients that died of sepsis and MODS revealed significant lymphoid depletion. An absolute lymphocyte count of less than 1,000 for more than 7 days was only observed in children with MODS [65]. Lymphopenia and lymphoid depletion predispose to anergy, a state of non-responsiveness to antigens. Together, this immune reprogramming (or ‘immunoparesis’) referred to as the compensatory anti-inflammatory response syndrome (CARS), is an adaptive mechanism to restrain the initial aggressive inflammatory burst. However, relative immune suppression also predisposes critically ill patients to viral reactivation [66], nosocomial infections and death [65].
Coagulation and Fibrinolysis
The sepsis triad refers to the activation of coagulation and inhibition of fibrinolysis triggered by inflammation [67] (see again Fig. 35.1b). The extent of pro-thrombotic and anti-fibrinolytic plasma activation is correlated with the severity of pediatric MODS and mortality [68–75]. Tissue factor (TF) is pivotal to the initiation of the coagulation cascade. In sepsis, inflammation results in the expression of TF on endothelial cells, the activation of coagulation and ensuing disseminated intravascular coagulation (DIC). Tissue factor binds and activates factor VII, X and V, thereby increasing thrombin activation, fibrin deposition, and microthrombi formation [76]. Inflammation also elevates the levels of plasminogen-activator inhibitor 1 (PAI-1) and thrombin-activatable fibrinolysis inhibitor (TAFI) which impair fibrin removal [77]. The general consumption of factors that regulate thrombin formation, such as antithrombin III, protein C and tissue-factor pathway inhibitor (TFPI) further exacerbates DIC [78].
Thrombocytopenia-associated multiple organ failure (TAMOF) is a clinical entity associated with sepsis. It comprises a spectrum of similar conditions including disseminated intravascular coagulation (DIC) and secondary thrombotic microangiopathy (TMA) [79]. Autopsies of children with TAMOF revealed a predominance of von Willebrand factor-rich (vWF) thrombi in the microvasculature of their brain, lung and kidney [80]. Recent evidence also suggests that as many as 30 % of children with severe sepsis have moderately decreased (20 % activity) ADAMTS-13 protease activity [81], which may increase the risk of thrombosis and organ dysfunction in this population.
Capillary Leak Syndrome
MODS has been associated with abnormal systemic vascular permeability resulting in the development of the capillary leak syndrome [82, 83]. In meningococcemia, the amount of circulating endotoxin and complement activation determines the severity of capillary leakage [84]. Susceptibility to the development of edema after cardiopulmonary bypass [85, 86] or bone marrow transplantation [87] is also related to activation of the complement system. More importantly, a positive fluid balance is associated with prolonged mechanical ventilation and increased mortality [88, 89]. PICU survivors had less fluid overload and were more likely to attain their target dry weight during continuous renal replacement therapy [90–92]. However, it is unclear whether endothelial dysfunction and the ensuing edema is simply an epiphenomena or contributes to the disease process. Recent work explored the role of adherens junctions that binds endothelial cells together to prevent vascular leak. Slit proteins and its receptor Robo4 are important to neuronal and vascular development. London and colleagues recently demonstrated that Slit and Robo4 proteins can stabilize VE-cadherin on endothelial adherens junction thereby decreasing vascular permeability [93]. In three different mouse model of infection, intravenous injection of Slit prevented vascular leakage and reduced mortality [93]. The role of the microvascular barrier in severe infections is now considered a therapeutic target [94]. Although confirmation in human is required, this may suggest a critical role of the endothelium and the capillary leak syndrome in sepsis.
Neuroendocrine Response
The initial phase of MODS results in a massive release of stress hormones, including adrenocorticotropic hormone (ACTH) and cortisol, catecholamines, vasopressin, glucagon, and growth hormone [95]. These hormones help supply the increased demand by maintaining circulation and the liberation of energy substrate, namely glucose, fatty acids and amino acids. Insulin resistance is a common manifestation of this overwhelming neuroendocrine response, although the mechanism remains ill-defined [95]. Intracellular metabolism, energy expenditure and tissue oxygen consumption doubles during that initial period. Concurrently, less vital systems are shut down and anabolism is halted.
In the second phase of MODS, the hormonal response recedes. Vasopressin levels are often insufficient, the adrenals become less responsive to ACTH, and sick euthyroid syndrome begins to appear [95]. Suppression of the hypothalamus-pituitary-adrenal axis is presumed to be a consequence of hypoperfusion, cytokine, and nitric oxide signalling in situ [96]. The transition between the first and second phase of the hormonal response may result from the abnormal pulsatile secretion of growth hormone, thyrotropin, and prolactin [95]. The later endocrine changes may also in part be the consequence of inhibitory feedbacks from the initial burst of hormonal activation. As such, high cortisol levels prevent the secretion of growth hormone, and together with prolactin repress the secretion of gonadotropins. Cortisol may also modulate thyroid metabolism by promoting the generation of metabolically inactive reverse T3, contributing to the development of the sick euthyroid syndrome.
In children, non-survivors from meningococcal sepsis had variable aldosterone levels [97, 98], lower serum cortisol, and severely decreased cortisol to ACTH ratio, indicating a state of adrenal insufficiency [97, 99, 100]. They also had acquired sick euthyroid syndrome (decreased total T3 and T4, increased reverse T3, normal free T4 and TSH) [96, 101, 102]. In newborns, dopamine curbs the secretion of growth hormone, thyrotropin and prolactin, which could aggravate partial hypopituitarism and sick euthyroid syndrome [103].
Hyper and Hypometabolism
At the onset of severe infections or thermal injury, a decreased metabolic rate with hypothermia and stimulation of the neuroendocrine response has been referred to as the ebb phase [104]. Hypermetabolism has then been noted during the flow phase, usually about 24 h after injury [105]. Normal metabolic requirements were noted in children with SIRS or sepsis without any organ dysfunction [106]. Briassoulis et al. noted a predominance of a hypermetabolic pattern which declined within 1 week of an acute stress [107]. In adults, hypermetabolism occurs as a result of an increased oxidation of glucose and fatty acids [108], as well as an increased rate of neoglucogenesis through the use of lactate, glycerol or amino acids (alanine, glutamine, serine, glycine).
Humoral factors released by the wound have been shown to trigger skeletal muscle proteolysis. TNF-α, also known as “cachectin”, plays a major role along with IL-1 in the development of “septic autocannibalism” [108]. Decreased lipoprotein lipase activity induced by TNF-α leads to increased serum levels of triglycerides, cholesterol and hyperglycemia, a clinical condition known as the “metabolic syndrome”. Glucose-lactate metabolism between skeletal muscle and liver is known as the Cori cycle. Under hypoxic conditions of tissue injury or infection, glucose is transformed into lactate which is further converted within liver into glucose, before returning to the injured area. This process resulted in a net loss of 4 mol of adenosine triphosphate per cycle which may explain in part the drainage of energetic reserve. In the most severely ill patients, muscle protein breakdown with consumption of branched amino acids and increased nitrogen urinary losses, may lead to muscular cachexia, atrophy of intestinal epithelium, abnormal wound healing and secondary immune dysfunction.
Cellular Dysoxia
Compromised oxygen delivery in shock is a major determinant of organ failures. Inducible nitric oxide synthase (iNOS) triggered by the inflammatory response liberates large concentrations of nitric oxide (NO), far exceeding the regional production [109]. This may lead to abnormal regional vascular blood flow and would contribute to inadequate oxygen delivery [109]. The severity of arterial hypotension in pediatric sepsis is correlated with serum concentrations of nitrites and nitrates [74]. Neuroendocrine and inflammatory factors can exacerbate hypoperfusion as discussed. Although organ failure is classically believed to result from hypoxia and cellular damage, histological inspection of dysfunctional organs is often normal [110]. This would suggest a functional rather than a structural deficit.
Cytopathic dysoxia is therefore potentially important to the pathogenesis of MODS. Mitochondrial respiration generally increases in the acute phase of critical illness, but tends to fall with prolonged inflammation [111]. The presence of glucocorticoids and thyroid receptors on mitochondria [112] suggests the integration of neurohormonal demands with corresponding energy supply at the cellular level. However, NO and cytokines have been shown to inhibit enzymes of the mitochondrial respiratory chain, which curtails energy production [113]. Markers of oxidative and nitrosative stress also correlate with decreased mitochondrial respiratory chain activity (mainly Complex I) [114]. Despite reduced ATP production from cytopathic dysoxia, ATP levels are largely maintained in surviving septic patients, thereby implying a state of diminished cellular energy consumption [115]. Based on these observations, Singer et al. have argued that multiorgan failure is a survival mechanism instating a dormant state analogous to hibernation that may increase the chances of survival when faced with a potentially overwhelming insult [116].
Organ Dysfunctions in Critically Ill Children
Cardiovascular Dysfunction and Septic Shock
Hemodynamic profiles noted in critically ill children with septic shock are more unpredictable than initially recognized [117–119]. Indeed, only 20 % of children with fluid refractory septic shock presented the classical picture of high cardiac index and low systemic vascular resistance [120]. Nearly 60 % of patients showed low cardiac index with high systemic vascular resistance, and both parameters might even be decreased [120]. During shock, sympathetic stimulation preferentially directs blood flow toward the brain and myocardium, diverting it from the splanchnic circulation (the so-called “dive reflex”). This may lead to increased serum lactate concentrations [121, 122]. In contrast to adults, most studies performed in critically ill children did not find the gastric pH to be predictive of developing MODS or death [121, 123–126]. However, decreased intestinal pH in very low birth weight infants was associated with a higher risk of developing necrotizing enterocolitis [127].
Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS)
Pulmonary congestion with protein-rich pulmonary edema is a cardinal feature of the acute respiratory distress syndrome (ARDS) [26, 128], which has been associated with a 20 % mortality rate in children [129]. This can be due to a direct pulmonary insult such as infection (so-called “direct ARDS”) or secondary to systemic inflammation (so-called “indirect ARDS”). Abnormally increased vascular pulmonary permeability has been associated with platelet activation, neutrophils and macrophage infiltration [128], as well as with fibrin exudate resulting in hyaline membrane formation [128]. During the early phase of pulmonary injury, a restrictive pattern is noted with a decrease in respiratory system compliance and forced vital capacity [130]. The natural course of ARDS has been characterized by inadequate gas exchanges requiring more aggressive mechanical ventilation. This leads to the production of inflammatory mediators that would further increase pulmonary capillary permeability and generates deleterious mechanical forces that leads to further damage of the alveolar-capillary membrane [131].
Gut Mucosal Barrier Dysfunction
Gut injury and inflammation have been proposed as the “motor of MODS” [132]. The mechanism was thought to be related to intestinal bacteria and/or endotoxin translocating to the systemic circulation via the portal vein. However, neither clinical studies nor animal studies demonstrated bacterial translocation via the portal vein [133]. Instead it appears that mesenteric lymph translocates factors which activate neutrophils and injure endothelial cells [133]. In neonates, the development of necrotizing enterocolitis resulted in increased plasma endotoxin levels [134]. Endotoxemia was more severe at the onset of illness among infants with necrotizing enterocolitis and play a critical role in the development of MODS [134]. Theorically, measures to improve gut epithelial barrier may improve or prevent MODS.
MODS is a significant risk factor to develop upper gastrointestinal bleeding [135–137]. Clinically significant upper gastrointestinal bleeding occurs in 2 % of PICU admissions [137]. It is most frequently observed among mechanically ventilated patients with a PRISM score higher than 10, and with evidence of systemic coagulopathy [137].
Neuromuscular Syndromes
Neuromuscular syndromes, including critical illness polyneuropathy, pure motor polyneuropathy, thick-filament myopathy, and necrotizing myopathy have been described [138–141]. Prolonged weakness has been identified in 2 % of critically ill children studied prospectively, of whom 63 % had MODS and 57 % had transplantation [142]. SIRS has been proposed as a common underlying pathogenic process, which may have been potentiated by the use of corticosteroids or neuromuscular blocking agents [138]. Patients showed flaccid quadriplegia with the inability to wean from ventilatory support [138]. In most severe cases, deep tendon reflexes were abolished. Electrophysiological abnormalities usually showed a pattern of axonal polyneuropathy or abnormalities of neuromuscular transmission [138]. Recovery in strength most frequently occurred over a period of weeks to months.
Outcome of Pediatric MODS
Development of MODS is associated with greater resource use and an increased length of stay in the PICU [28]. A normal quality of life with minimal health problems is reported in 60 % of children with MODS, while 32 % indicated a fair quality of life with ongoing health, emotional, social, physical or cognitive problems that required some intervention or hospitalization; 2 % had a poor quality of life [143]. The return of organ function in children who developed MODS has not been examined in a systematic manner. There are few small case series in children with ARDS or those with MODS after cardiac surgery [144, 145]. In one study, 78 % of children who left the hospital after acute renal failure in the ICU survived beyond 24 months [146].
Treatment of Pediatric MODS
The care of children with MODS is best performed by a multidisciplinary team that carefully balances multiple therapeutic modalities. These modalities include general supportive care and organ specific therapeutics. The patient clinical condition should be reassessed periodically as for the need to perform complementary exams or invasive procedures in order to distinguish between possible, probable or definitive diagnosis.
General Supportive Care
Control of the infectious focus is of major importance. Antibiotic therapy should be started early with appropriate resection of infected or necrotic tissue. However, the prolonged use of large spectrum antibiotic therapy should be avoided when cultures are negative, and the risk-benefit of invasive catheters must be re-evaluated periodically. The use of recombinant human activated protein C reduced mortality and improved organ dysfunction among adults with severe sepsis [147]. However, in the RESOLVE trial, a pediatric trial in which children with sepsis-induced cardiovascular and respiratory failure were randomly assigned to receive placebo or recombinant human activated protein, there was no difference between treatment groups in either organ failure resolution or mortality [148]. While overall bleeding events were not different between groups, there was an increased incidence of central nervous system bleeding in the treated group among children younger than 2 months. Based upon follow-up trials in adults showing no benefit, the manufacturer removed activated protein C from the market and it is no longer available for clinical use [149]. Results of the CORTICUS trial in adults suggest that although shock reversal may occur more rapidly with corticsteroids, overall survival is not improved, apparently due to an increased rate of infections [150]. In the case of a transplanted patient with active systemic infection, immunosuppressive therapy should be minimized. Lymphopenia may occur with the prolonged use of dopamine or steroids, and prolonged lymphopenia has been associated with secondary infection and MODS [65].
A large-scale multicenter clinical trial in PICU patients who were hemodynamically stable, the TRIPICU study, showed that a restrictive transfusion strategy based on an hemoglobin transfusion threshold of 70 g/L, was not inferior to a liberal approach (threshold: 95 g/L) with regard to the number of patients with “new or progressive MODS or death” [151]. The incidence rate of “new and/or progressive MODS” in the TRIPICU study was 12 %, while the death rate was, as expected, only 4 %.
Critically ill children should receive appropriate sedation and analgesia. Vet et al. have recently shown that increased disease severity resulted in lower clearance of midazolam (decreased cytochrome 3A activity), without decreasing midazolam dose requirements [152]. Several drugs used in critical care have a narrow therapeutic index. Caution should be applied when using nephrotoxic or hepatotoxic drugs, with a special emphasis on timely drug dosages, metabolic clearance and drug interaction. Iatrogenic complications may typically occur due to difficult vascular catheterization, or overactive cardio-respiratory support usually based on a blind treatment of numbers.
While inadequate oxygen delivery to tissues results in organ dysfunction initially, MODS itself may well occur as a result of mitochondrial dysfunction [153]. As children with septic shock have better outcomes than adults, it is suggestive that their mitochondrial functions are relatively preserved compared to that of adults. This is a new area of research as therapies are being developed to affect mitochondrial function in sepsis [154]. There is some evidence that blood glucose control can improve mitochondrial dysfunction in patients with sepsis [155]. What remains unclear at this point is whether therapy aimed at reversing the metabolic response is helpful in critically ill patients [156]. In medical or surgical adult ICUs, tight glycemic control with intensive insulin therapy has been reported to decrease morbidity or mortality; other studies suggested no benefit or potential harm due to hypoglycemia [157–159].
Organ Therapeutic Management
In this section, only some specificities of organ dysfunction management are reported. For more details in the management, readers should refer to the appropriate and relevant chapters later in this textbook.
Hemodynamic Management
Early goal-directed therapy has been shown to decrease mortality and the severity of MODS in adults with sepsis [160]. Guidelines developed in 2002 proposed a time-dependent flow diagram in the hemodynamic support of children with sepsis [161].
Lung Protective Ventilation
There is no clear data in children. Expert opinions recommend to keep positive inspiratory pressures below 30 cmH2O and consider small tidal volume ventilation (physiologic tidal volumes in a normal subject are in the range of 6–8 ml/kg). The other therapies such as endotracheal surfactant, high-frequency oscillatory ventilation, prone positioning, bronchodilators or corticosteroids for lung inflammation and fibrosis need further research before they can be recommended in clinical practice [162].
Renal Failure Management
Renal replacement therapy can be continuous or intermittent according to team experience and patient tolerance. High dialysis dose did not demonstrate any benefits in adults [163, 164] and no data are available in children. Although, fluid overload is a risk factor of death in adults [165, 166] and children [90, 167, 168], no data are available on the impact of negative fluid balance on critically ill children outcome [169]. Such aggressive ultrafiltration needs to be balanced with the risk of hypovolemia.
Nutritional Support
Nutritional support may allow sufficient protein-calorie intake. Early enteric feeding has been proposed to prevent intestinal disuse with secondary mucosal atrophy, decreasing the susceptibility to bacterial translocation and systemic inflammation. Indeed, the capacity to tolerate enteral feedings, as for the mobilization of third space and peripheral edema, usually represent a trend for clinical improvement.
Withdrawal of Curative Care
Despite the willingness to provide as good as possible intensive care to children with MODS, several patients simply persistently fail to improve or spontaneously further deteriorate, presenting several complications, that may ultimately be viewed as an inexorable pathway to death. Therefore, the issue of medical futility and palliative care is frequently encountered in children with MODS. The pro’s and con’s of not escalating the level of care, the withdrawal of cardiopulmonary resuscitation (CPR), or discontinuing some of the therapeutic modalities, are usually evaluated by the members of the multidisciplinary team. With the aim of reaching a consensus between the medical team and family, honest clinical information should be provided at least daily to the family, including when standard of medical care fails to lead to recovery.
References
Burke J, Pontoppidan H, Welch C. High output respiratory failure: an important cause of death ascribed to peritonitis or ileus. Ann Surg. 1963;158(4):581–95.
Tilney NL, Bailey GL, Morgan AP. Sequential system failure after rupture of abdominal aortic aneurysms: an unsolved problem in postoperative care. Ann Surg. 1973;178(2):117–22.
Pinsky M. The definition and history of multiple-system organ failure. In: Charbonneau P, Société de Réanimation de Langue Française, editors. Syndrome de défaillance multiviscérale. Paris: Expansion scientifique français; 1991. p. 3–7.
Fry D, Pearlstein L, Fulton R, Polk H. Multiple system organ failure: the role of uncontrolled infection. Arch Surg. 1980;115(2):136–40.
Knauss W, Wagner D. Multiple organ failure: epidemiology and prognosis. Crit Care Clin. 1989;5(2):221–32.
American College of Chest Physicians, Society of Critical Care Medicine. American College of Chest Physicians/society of critical care medicine consensus conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–74.
Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995;273:117–23.
Levy M, Fink M, Marshall J, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31(4):1250–6.
Montgomery VL, Strotman JM, Ross MP. Impact of multiple organ system dysfunction and nosocomial infections on survival of children treated with extracorporeal membrane oxygenation after heart surgery. Crit Care Med. 2000;28(2):526–31.
Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8.
Wilkinson JD, Pollack MM, Glass NL, Kanter RK, Katz RW, Steinhart CM. Mortality associated with multiple organ system failure and sepsis in pediatric intensive care unit. J Pediatr. 1987;111(3):324–8.
Wilkinson JD, Pollack MM, Ruttimann UE, Glass NL, Yeh TS. Outcome of pediatric patients with multiple organ system failure. Crit Care Med. 1986;14(4):271–4.
Gebara BM. Values for systolic blood pressure. Pediatr Crit Care Med. 2005;6(4):500. [Comment Letter]; author reply 500-1.
Proulx F, Grunberg F. Suicide in hospitalized patients. Sante Ment Que. 1994;19(2):131–43.
Tantalean JA, Leon RJ, Santos AA, Sanchez E. Multiple organ dysfunction syndrome in children. Pediatr Crit Care Med. 2003;4(2):181–5.
Goh A, Lum L. Sepsis, severe sepsis and septic shock in paediatric multiple organ dysfunction syndrome. J Paediatr Child Health. 1999;35(5):488–92.
Proulx F, Lacroix J, Farrell CA, Lambert M. Blood lactate and gastric intramucosal pH during severe sepsis. Crit Care Med. 1996;24(6):1092.
Leteurtre S, Martinot A, Duhamel A, Proulx F, Grandbastien B, Cotting J, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362(9379):192–7.
Leteurtre S, Martinot A, Duhamel A, Gauvin F, Grandbastien B, Nam TV, et al. Development of a pediatric multiple organ dysfunction score: use of two strategies. Med Decis Making. 1999;19(4):399–410.
Graciano AL, Balko JA, Rahn DS, Ahmad N, Giroir BP. The Pediatric Multiple Organ Dysfunction Score (P-MODS): development and validation of an objective scale to measure the severity of multiple organ dysfunction in critically ill children. Crit Care Med. 2005;33(7):1484–91.
Tibby SM. Does PELOD measure organ dysfunction…and is organ function a valid surrogate for death? Intensive Care Med. 2010;36(1):4–7.
Garcia PC, Eulmesekian P, Branco RG, Perez A, Sffogia A, Olivero L, et al. External validation of the paediatric logistic organ dysfunction score. Intensive Care Med. 2010;36(1):116–22.
Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, et al. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009;373(9663):547–56. Randomized Controlled Trial Research Support, Non-U.S. Gov’t.
Lacroix J, Hebert PC, Hutchison JS, Hume HA, Tucci M, Ducruet T, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356(16):1609–19.
Leteurtre S, Duhamel A, Grandbastien B, Proulx F, Cotting J, Gottesman R, et al. Daily estimation of the severity of multiple organ dysfunction syndrome in critically ill children. CMAJ. 2010;182(11):1181–7.
Berkowitz FE, Vallabh P, Altman DI, Diamantes F, Van Wyk HJ, Stroucken JM. Jarisch-Herxheimer reaction in meningococcal meningitis. Am J Dis Child. 1983;137(6):599.
Baue AE. Multiple, progressive, or sequential systems failure. A syndrome of the 1970s. Arch Surg. 1975;110(7):779–81.
Johnston J, Yi M, Britto M, Mrus J. Importance of organ dysfunction in determining hospital outcomes in children. J Pediatr. 2004;144(5):595–601.
Bestati N, Leteurtre S, Duhamel A, Proulx F, Grandbastien B, Lacroix J, et al. Differences in organ dysfunctions between neonates and older children: a prospective, observational, multicenter study. Crit Care. 2010;14(6):R202.
Avanoglu A, Ergun O, Bakirtas F, Erdener A. Characteristics of multisystem organ failure in neonates. Eur J Pediatr Surg. 1997;7(5):263–6.
Shah P, Riphagen S, Beyene J, Perlman M. Multiorgan dysfunction in infants with post-asphyxial hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2004;89(2):F152–5.
Kearns G, Abdel-Rahman S, Alander S, Blowey D, Leeder J, RE K. Developmental pharmacology: drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–67.
Typpo K, Petersen N, Hallman D, Markovitz B, Mariscalco M. Impact of premorbid conditions on multiple organ dysfunction syndrome in the PICU. Crit Care Med. 2007;35(12):A10.
Carvalho PR, Feldens L, Seitz EE, Rocha TS, Soledade MA, Trotta EA. Prevalence of systemic inflammatory syndromes at a tertiary pediatric intensive care unit. J Pediatr (Rio J). 2005;81(2):143–8.
Leclerc F, Leteurtre S, Duhamel A, Grandbastien B, Proulx F, Martinot A, et al. Cumulative influence of organ dysfunctions and septic state on mortality of critically ill children. Am J Respir Crit Care Med. 2005;171(4):348–53.
Aneja RK, Carcillo JA. Differences between adult and pediatric septic shock. Minerva Anestesiol. 2011;77(10):986–92.
Stieh J, Fischer G, Scheewe J, Uebing A, Dutschke P, Jung O, et al. Impact of preoperative treatment strategies on the early perioperative outcome in neonates with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2006;131(5):1122–9. e2.
Seghaye MC, Engelhardt W, Grabitz RG, Faymonville ME, Hornchen H, Messmer BJ, et al. Multiple system organ failure after open heart surgery in infants and children. Thorac Cardiovasc Surg. 1993;41(1):49–53.
Baslaim G, Bashore J, Al-Malki F, Jamjoom A. Can the outcome of pediatric extracorporeal membrane oxygenation after cardiac surgery be predicted? Ann Thorac Cardiovasc Surg. 2006;12(1):21–7.
Aharon AS, Drinkwater Jr DC, Churchwell KB, Quisling SV, Reddy VS, Taylor M, et al. Extracorporeal membrane oxygenation in children after repair of congenital cardiac lesions. Ann Thorac Surg. 2001;72(6):2095–101. discussion 101–2.
Shime N, Ashida H, Hiramatsu N, Kageyama K, Katoh Y, Hashimoto S, et al. Arterial ketone body ratio for the assessment of the severity of illness in pediatric patients following cardiac surgery. J Crit Care. 2001;16:102–7.
Ben-Abraham R, Efrati O, Mishali D, Yulia F, Vardi A, Barzilay Z, et al. Predictors for mortality after prolonged mechanical ventilation after cardiac surgery in children. J Crit Care. 2002;17(4):235–9.
Calkins CM, Bensard DD, Moore EE, McIntyre RC, Silliman CC, Biffl W, et al. The injured child is resistant to multiple organ failure: a different inflammatory response? J Trauma. 2002;53(6):1058–63.
Steinau G, Kaussen T, Bolten B, Schachtrupp A, Neumann UP, Conze J, et al. Abdominal compartment syndrome in childhood: diagnostics, therapy and survival rate. Pediatr Surg Int. 2011;27(4):399–405.
Feickert HJ, Schepers AK, Rodeck B, Geerlings H, Hoyer PF. Incidence, impact on survival, and risk factors for multi-organ system failure in children following liver transplantation. Pediatr Transplant. 2001;5(4):266–73.
Pinho-Apezzato ML, Tannuri U, Tannuri AC, Mello ES, Lima F, Gibelli NE, et al. Multiple clinical presentations of lymphoproliferative disorders in pediatric liver transplant recipients: a single-center experience. Transplant Proc. 2010;42(5):1763–8.
Diaz M, Vicent M, Prudencio M, Rodriguez F, Marin C, Serrano A, et al. Predicting factors for admission to an intensive care unit and clinical outcome in pediatric patients receiving hematopoietic stem cell transplantation. Haematologica. 2002;87(3):292–8.
Kutko M, Calarco M, Flaherty M, Helmrich R, Ushay H, Pon S, et al. Mortality rates in pediatric septic shock with and without multiple organ system failure. Pediatr Crit Care Med. 2003;4(3):333–7.
Rossi R, Shemie SD, Calderwood S. Prognosis of pediatric bone marrow transplant recipients requiring mechanical ventilation. Crit Care Med. 1999;27(6):1181–6.
Mollen KP, Anand RJ, Tsung A, Prince JM, Levy RM, Billiar TR. Emerging paradigm: toll-like receptor 4-sentinel for the detection of tissue damage. Shock. 2006;26(5):430–7.
Gray MW, Burger G, Lang BF. The origin and early evolution of mitochondria. Genome Biol. 2001;2(6):Reviews1018.
Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–7.
Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46.
Hazelzet JA, van der Voort E, Lindemans J, ter Heerdt PG, Neijens HJ. Relation between cytokines and routine laboratory data in children with septic shock and purpura. Intensive Care Med. 1994;20(5):371–4.
Kornelisse RF, Hazelzet JA, Savelkoul HF, Hop WC, Suur MH, Borsboom AN, et al. The relationship between plasminogen activator inhibitor-1 and proinflammatory and counterinflammatory mediators in children with meningococcal septic shock. J Infect Dis. 1996;173(5):1148–56.
Viktorov VV, Viktorova TV, Mironov PI, Khustnutdinova EK. Significance of hereditary factors in multiple organ dysfunction syndrome in children with infections. Anesteziol Reanimatol. 2000;1:32–4.
Mariscalco MM. Infection and the host response. In: Fuhrman BP, Zimmerman JJ, editors. Pediatric critical care. 3rd ed. Philadelphia: Mosby; 2006. p. 1299–319.
Hotchkiss RS, McConnell KW, Bullok K, Davis CG, Chang KC, Schwulst SJ, et al. TAT-BH4 and TAT-Bcl-xL peptides protect against sepsis-induced lymphocyte apoptosis in vivo. J Immunol. 2006;176(9):5471–7.
Singh-Naz N, Sprague BM, Patel KM, Pollack MM. Risk factors for nosocomial infection in critically ill children: a prospective cohort study. Crit Care Med. 1996;24(5):875–8.
Hall MW, Knatz NL, Vetterly C, Tomarello S, Wewers MD, Volk HD, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011;37(3):525–32.
Cavaillon J, Adib-Conquy M, Cloez-Tayarani I, Fitting C. Immunodepression in sepsis and SIRS assessed by ex vivo cytokine production is not a generalized phenomenon: a review. J Endotoxin Res. 2001;7(2):85–93.
Adib-Conquy M, Moine P, Asehnoune K, Edouard A, Espevik T, Miyake K, et al. Toll-like receptor-mediated tumor necrosis factor and interleukin-10 production differ during systemic inflammation. Am J Respir Crit Care Med. 2003;168(2):158–64.
Adib-Conquy M, Adrie C, Moine P, Asehnoune K, Fitting C, Pinsky MR, et al. NF-kappaB expression in mononuclear cells of patients with sepsis resembles that observed in lipopolysaccharide tolerance. Am J Respir Crit Care Med. 2000;162(5):1877–83.
Cavaillon J, Fitting C, Adib-Conquy M. Mechanisms of immunodysregulation in sepsis. Contrib Nephrol. 2004;144:76–93.
Felmet KA, Hall MW, Clark RS, Jaffe R, Carcillo JA. Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol. 2005;174(6):3765–72.
Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, Neff MJ, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300(4):413–22. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t.
Short M. Linking the sepsis triad of inflammation, coagulation and suppressed fibrinolysis to infants. Adv Neonat Care. 2004;4(5):258–73.
Doughty L, Carcillo JA, Kaplan S, Janosky J. The compensatory anti-inflammatory cytokine interleukin 10 response in pediatric sepsis-induced multiple organ failure. Chest. 1998;113(6):1625–31.
Doughty L, Carcillo JA, Kaplan S, Janosky J. Plasma nitrite and nitrate concentrations and multiple organ failure in pediatric sepsis. Crit Care Med. 1998;26(1):157–62.
Doughty LA, Kaplan SS, Carcillo JA. Inflammatory cytokine and nitric oxide responses in pediatric sepsis and organ failure. Crit Care Med. 1996;24(7):1137–43.
Hatherill M, Tibby SM, Turner C, Ratnavel N, Murdoch IA. Procalcitonin and cytokine levels: relationship to organ failure and mortality in pediatric septic shock. Crit Care Med. 2000;28(7):2591–4.
Whalen MJ, Doughty LA, Carlos TM, Wisniewski SR, Kochanek PM, Carcillo JA. Intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 are increased in the plasma of children with sepsis-induced multiple organ failure. Crit Care Med. 2000;28(7):2600–7.
Han YY, Doughty LA, Kofos D, Sasser H, Carcillo JA. Procalcitonin is persistently increased among children with poor outcome from bacterial sepsis. Pediatr Crit Care Med. 2003;4(1):21–5.
Wong HR, Carcillo JA, Burckart G, Shah N, Janosky JE. Increased serum nitrite and nitrate concentrations in children with the sepsis syndrome. Crit Care Med. 1995;23(5):835–42.
Green J, Doughty L, Kaplan SS, Sasser H, Carcillo JA. The tissue factor and plasminogen activator inhibitor type-1 response in pediatric sepsis-induced multiple organ failure. Thromb Haemost. 2002;87(2):218–23.
Anisimova IN, Shvets OL, Guliaev DV, Tsinzerling VA, Belebez’ev GI. Morphologic aspects of hemostasis disturbances in meningococcemia in children. Arkh Patol. 1993;55(5):16–22.
Zeerleder S, Schroeder V, Hack CE, Kohler HP, Wuillemin WA. TAFI and PAI-1 levels in human sepsis. Thromb Res. 2006;118(2):205–12.
Levi M, de Jonge E, van der Poll T. New treatment strategies for disseminated intravascular coagulation based on current understanding of the pathophysiology. Ann Med. 2004;36(1):41–9.
Nguyen TC, Carcillo JA. Bench-to-bedside review: thrombocytopenia-associated multiple organ failure–a newly appreciated syndrome in the critically ill. Crit Care. 2006;10(6):235.
Nguyen T, Hall M, Han Y, Fiedor M, Hasset A, Lopez-Plaza I, et al. Microvascular thrombosis in pediatric multiple organ failure: is it a therapeutic target? Pediatr Crit Care Med. 2001;2(3):187–96.
Nguyen TC, Liu A, Liu L, Ball C, Choi H, May WS, et al. Acquired ADAMTS-13 deficiency in pediatric patients with severe sepsis. Haematologica. 2007;92(1):121–4.
Foley K, Keegan M, Campbell I, Murby B, Hancox D, Pollard B. Use of single-frequency bioimpedance at 50 kHz to estimate total body water in patients with multiple organ failure and fluid overload. Crit Care Med. 1999;27(8):1472–7.
Shime N, Ashida H, Chihara E, Kageyama K, Katoh Y, Yamagishi M, et al. Bioelectrical impedance analysis for assessment of severity of illness in pediatric patients after heart surgery. Crit Care Med. 2002;30(3):518–20.
Hazelzet JA, de Groot R, van Mierlo G, Joosten KF, van der Voort E, Eerenberg A, et al. Complement activation in relation to capillary leakage in children with septic shock and purpura. Infect Immun. 1998;66(11):5350–6.
Zhang S, Wang S, Li Q, Yao S, Zeng B, Ziegelstein RC, et al. Capillary leak syndrome in children with C4A-deficiency undergoing cardiac surgery with cardiopulmonary bypass: a double-blind, randomised controlled study. Lancet. 2005;366(9485):556–62.
Zhang S, Wang S, Yao S. Evidence for development of capillary leak syndrome associated with cardiopulmonary bypass in pediatric patients with the homozygous C4A null phenotype. Anesthesiology. 2004;100(6):1387–93.
Nurnberger W, Heying R, Burdach S, Gobel U. C1 esterase inhibitor concentrate for capillary leakage syndrome following bone marrow transplantation. Ann Hematol. 1997;75(3):95–101.
Naran N, Sagy M, Bock KR. Continuous renal replacement therapy results in respiratory and hemodynamic beneficial effects in pediatric patients with severe systemic inflammatory response syndrome and multiorgan system dysfunction. Pediatr Crit Care Med. 2010;11(6):737–40.
Flori HR, Church G, Liu KD, Gildengorin G, Matthay MA. Positive fluid balance is associated with higher mortality and prolonged mechanical ventilation in pediatric patients with acute lung injury. Crit Care Res Pract. 2011;2011:854142.
Goldstein SL, Currier H, Graf C, Cosio CC, Brewer ED, Sachdeva R. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics. 2001;107(6):1309–12.
Goldstein SL, Somers MJ, Baum MA, Symons JM, Brophy PD, Blowey D, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005;67(2):653–8.
Michael M, Kuehnle I, Goldstein S. Fluid overload and acute renal failure in pediatric stem cell transplant patients. Pediatr Nephrol. 2004;19(1):91–5.
London NR, Zhu W, Bozza FA, Smith MCP, Greif DM, Sorensen LK, et al. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci Transl Med. 2010;2(23):23ra19.
Ye X, Ding J, Zhou X, Chen G, Liu SF. Divergent roles of endothelial NF-kappaB in multiple organ injury and bacterial clearance in mouse models of sepsis. J Exp Med. 2008;205(6):1303–15.
Van den Berghe G, de Zegher F, Bouillon R. Acute and prolonged critical illness as different neuroendocrine paradigms. J Clin Endocrinol Metab. 1998;83(2):1827–34.
Joosten KF, de Kleijn ED, Westerterp M, de Hoog M, Eijck FC, Hop WCJ, et al. Endocrine and metabolic responses in children with meningococcal sepsis: striking differences between survivors and nonsurvivors. J Clin Endocrinol Metab. 2000;85(10):3746–53.
den Brinker M, Joosten KF, Liem O, de Jong FH, Hop WC, Hazelzet JA, et al. Adrenal insufficiency in meningococcal sepsis: bioavailable cortisol levels and impact of interleukin-6 levels and intubation with etomidate on adrenal function and mortality. J Clin Endocrinol Metab. 2005;90(9):5110–7.
Lichtarowicz-Krynska E, Cole T, Camacho-Hubner C, Britto J, Levin M, Klein N, et al. Circulating aldosterone levels are unexpectedly low in children with acute meningococcal disease. J Endocrinol Metab. 2004;89(3):1410–4.
Riordan F, Thomson A, Ratcliffe J, Sills J, Diver M, Hart C. Admission cortisol and adrenocorticotrophic hormone levels in children with meningococcal disease; evidence for adrenal insufficiency? Crit Care Med. 1999;27(10):2257–61.
De Kleijn ED, Joosten KF, Van Rijn B, Westerterp M, De Groot R, Hokken-Koelega AC, et al. Low serum cortisol in combination with high adrenocorticotrophic hormone concentrations are associated with poor outcome in children with severe meningococcal disease. Pediatr Infect Dis J. 2002;21(4):330–6.
den Brinker M, Joosten KF, Visser TJ, Hop WC, de Rijke YB, Hazelzet JA, et al. Euthyroid sick syndrome in meningococcal sepsis: the impact of peripheral thyroid hormone metabolism and binding proteins. J Clin Endocrinol Metab. 2005;90(10):5613–20.
den Brinker M, Dumas B, Visser TJ, Hop WC, Hazelzet JA, Festen DA, et al. Thyroid function and outcome in children who survived meningococcal septic shock. Intensive Care Med. 2005;31(7):970–6.
Van den Berghe G, de Zegher F, Lauwers P. Dopamine suppresses pituitary function in infants and children. Crit Care Med. 1994;22(11):1747–53.
Herndon DN, Gore D, Cole M, Desai MH, Linares H, Abston S, et al. Determinants of mortality in pediatric patients with greater than 70% full-thickness total body surface area thermal injury treated by early total excision and grafting. J Trauma. 1987;27(2):208–12.
Frayn K. Hormonal control of metabolism in trauma and sepsis. Clin Endocrinol. 1986;24(5):577–99.
Turi RA, Petros AJ, Eaton S, Fasoli L, Powis M, Basu R, et al. Energy metabolism of infants and children with systemic inflammatory response syndrome and sepsis. Ann Surg. 2001;233(4):581–7.
Briassoulis G, Venkataraman S, Thompson A. Cytokines and metabolic patterns in pediatric patients with critical illness. Clin Dev Immunol. 2010;2010:354047.
Cerra FB. Hypermetabolism-organ failure syndrome: a metabolic response to injury. Crit Care Clin. 1989;5(2):289–302.
Schwartz D, Mendonca M, Schwartz I, Xia Y, Satriano J, Wilson CB, et al. Inhibition of constitutive nitric oxide synthase (NOS) by nitric oxide generated by inducible NOS after lipopolysaccharide administration provokes renal dysfunction in rats. J Clin Invest. 1997;100(2):439–48.
Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–51.
Singer M, Brealey D. Mitochondrial dysfunction in sepsis. Biochem Soc Symp. 1999;66:149–66.
Scheller K, Seibel P, Sekeris CE. Glucocorticoid and thyroid hormone receptors in mitochondria of animal cells. Int Rev Cytol. 2003;222:1–61.
Borutaite V, Budriunaite A, Brown GC. Reversal of nitric oxide-, peroxynitrite- and S-nitrosothiol-induced inhibition of mitochondrial respiration or complex I activity by light and thiols. Biochim Biophys Acta. 2000;1459(2–3):405–12.
Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci U S A. 1998;95(13):7631–6.
Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360(9328):219–23.
Singer M. Multiorgan failure is an adaptative, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet. 2004;364:S45–7.
Pollack MM, Fields AI, Ruttimann UE. Sequential cardiopulmonary variables of infants and children in septic shock. Crit Care Med. 1984;12(7):554–9.
Pollack MM, Fields AI, Ruttimann UE. Distributions of cardiopulmonary variables in pediatric survivors and nonsurvivors of septic shock. Crit Care Med. 1985;13(6):454–9.
Mercier JC, Beaufils F, Hartmann JF, Azema D. Hemodynamic patterns of meningococcal shock in children. Crit Care Med. 1988;16(1):27–33.
Roth BL, Suba EA, Carcillo JA, Litten RZ. Alterations in hepatic and aortic phospholipase-C coupled receptors and signal transduction in rat intraperitoneal sepsis. Prog Clin Biol Res. 1989;286:41–59.
Duke TD, Butt W, South M. Predictors of mortality and multiple organ failure in children with sepsis. Intensive Care Med. 1997;23(6):684–92.
Siegel LB, Dalton HJ, Hertzog JH, Hopkins RA, Hannan RL, Hauser GJ. Initial postoperative serum lactate levels predict survival in children after open heart surgery. Intensive Care Med. 1996;22(1):1418–23.
Casado-Flores J, Mora E, Perez-Corral F, Martinez-Azagra A, Garcia-Teresa MA, Ruiz-Lopez MJ. Prognostic value of gastric intramucosal pH in critically ill children. Crit Care Med. 1998;26(6):1123–7.
Dugas MA, Proulx F, de Jaeger A, Lacroix J, Lambert M. Markers of tissue hypoperfusion in pediatric septic shock. Intensive Care Med. 2000;26(1):75–83.
Calvo RC, Ruza TF, Delgado Dominguez MA, Lopez-Herce CJ, Dorao Martinez-Romillo P. Effectiveness of hemodynamic treatment guided by gastric intramucosal pH monitoring. An Esp Pediatr. 2000;52(4):339–45.
de Souza RL, de Carvalho WB. Preliminary study about the utility of gastric tonometry during the weaning from mechanical ventilation. Rev Assoc Med Bras. 2002;48(1):66–72.
Campbell ME, Costeloe KL. Measuring intramucosal pH in very low birth weight infants. Pediatr Res. 2001;50(3):398–404.
Matthay M, Zimmerman G. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol. 2005;33(4):319–27.
Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171(9):995–1001.
Newth C, Stretton M, Deakers T, Hammer J. Assessment of pulmonary function in the early phase of ARDS in pediatric patients. Pediatr Pulmonol. 1997;23(3):169–75.
Fishel RS, Are C, Barbul A. Vessel injury and capillary leak. Crit Care Med. 2003;31(8 Suppl):S502–11.
Fink MP, Delude RL. Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clin. 2005;21(2):177–96.
Senthil M, Brown M, Xu DZ, Lu Q, Feketeova E, Deitch EA. Gut-lymph hypothesis of systemic inflammatory response syndrome/multiple-organ dysfunction syndrome: validating studies in a porcine model. J Trauma. 2006;60(5):958–65. discussion 65–7.
Sharma R, Tepas 3rd JJ, Hudak ML, Mollitt DL, Wludyka PS, Teng RJ, et al. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. J Pediatr Surg. 2007;42(3):454–61.
Lacroix J, Nadeau D, Laberge S, Gauthier M, Lapierre G, Farrell CA. Frequency of upper gastrointestinal bleeding in a pediatric intensive care unit. Crit Care Med. 1992;20(1):35–42.
Gauvin F, Dugas MA, Chaibou M, Morneau S, Lebel D, Lacroix J. The impact of clinically significant upper gastrointestinal bleeding acquired in a pediatric intensive care unit. Pediatr Crit Care Med. 2001;2(4):294–8.
Chaibou M, Tucci M, Dugas MA, Farrell CA, Proulx F, Lacroix J. Clinically significant upper gastrointestinal bleeding acquired in a pediatric intensive care unit: a prospective study. Pediatrics. 1998;102(4 Pt 1):933–8.
Jordan I, Cambra FJ, Alcover E, Colomer J, Campistol J, Caritg J, et al. Neuromuscular pathology in a critical pediatric patient. Rev Neurol. 1999;29(5):432–5.
Sheth RD, Bolton CF. Neuromuscular complications of sepsis in children. J Child Neurol. 1995;10(5):346–52.
Petersen B, Schneider C, Strassburg HM, Schrod L. Critical illness neuropathy in pediatric intensive care patients. Pediatr Neurol. 1999;21(4):749–53.
Ohto T, Iwasaki N, Ohkoshi N, Aoki T, Ichinohe M, Tanaka R, et al. A pediatric case of critical illness polyneuropathy: clinical and pathological findings. Brain Dev. 2005;27(7):535–8.
Banwell BL, Mildner RJ, Hassall AC, Becker LE, Vajsar J, Shemie SD. Muscle weakness in critically ill children. Neurology. 2003;61(12):1779–82.
Morrison AL, Gillis J, O’Connell AJ, Schell DN, Dossetor DR, Mellis C. Quality of life of survivors of pediatric intensive care. Pediatr Crit Care Med. 2002;3(1):1–5.
Fanconi S, Kraemer R, Weber J, Tschaeppeler H, Pfenninger J. Long-term sequelae in children surviving adult respiratory distress syndrome. J Pediatr. 1985;106(2):218–22.
Ben-Abraham R, Weinbroum AA, Roizin H, Efrati O, Augarten A, Harel R, et al. Long-term assessment of pulmonary function tests in pediatric survivors of acute respiratory distress syndrome. Med Sci Monit. 2002;8(3):CR153–7.
Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL. 3–5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int. 2006;69(1):184–9.
Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344(10):699–709.
Nadel S, Goldstein B, Williams MD, Dalton H, Peters M, Macias WL, et al. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet. 2007;369(9564):836–43.
Wheeler DS. An after action report of drotrecogin alpha (activated) and lessons for the future. Pediatr Crit Care Med. 2012;13:692–4.
Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111–24.
Bailey D, Phan V, Litalien C, Ducruet T, Merouani A, Lacroix J, et al. Risk factors of acute renal failure in critically ill children: a prospective descriptive epidemiological study. Pediatr Crit Care Med. 2007;8(1):29–35.
Vet NJ, de Hoog M, Tibboel D, de Wildt SN. The effect of inflammation on drug metabolism: a focus on pediatrics. Drug Discov Today. 2011;16(9–10):435–42.
Singer M. Mitochondrial function in sepsis: acute phase versus multiple organ failure. Crit Care Med. 2007;35(9):S441–8.
Piel DA, Gruber PJ, Weinheimer CJ, Courtois MR, Robertson CM, Coopersmith CM, et al. Mitochondrial resuscitation with exogenous cytochrome c in the septic heart. Crit Care Med. 2007;35(9):2120–7.
Vanhorebeek I, Langouche L, Van den Berghe G. Glycemic and nonglycemic effects of insulin: how do they contribute to a better outcome of critical illness? Curr Opin Crit Care. 2005;11(4):304–11.
Verbruggen SC, Joosten KF, Castillo L, van Goudoever JB. Insulin therapy in the pediatric intensive care unit. Clin Nutr. 2007;26(6):677–90.
Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–61.
van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–67.
Brunkhorst FM, Reinhart K. Sepsis therapy: present guidelines and their application. Chirurg. 2008;79(4):306–14.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–77.
Carcillo JA, Fields AI. Clinical practice parameters for hemodynamic support of pediatric and neonatal patients in septic shock. Crit Care Med. 2002;30(6):1365–78.
Randolph AG. Management of acute lung injury and acute respiratory distress syndrome in children. Crit Care Med. 2009;37(8):2448–54. Review.
Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359(1):7–20. Multicenter Study Randomized Controlled Trial Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.
Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361(17):1627–38. Multicenter Study Randomized Controlled Trial Research Support, Non-U.S. Gov’t.
Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12(3):R74. Multicenter Study Research Support, Non-U.S. Gov’t.
Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76(4):422–7. Multicenter Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t.
Foland JA, Fortenberry JD, Warshaw BL, Pettignano R, Merritt RK, Heard ML, et al. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004;32(8):1771–6.
Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55(2):316–25. Multicenter Study Research Support, Non-U.S. Gov’t.
Claure-Del Granado R, Mehta RL. Assessing and delivering dialysis dose in acute kidney injury. Semin Dial. 2011;24(2):157–63. Research Support, Non-U.S. Gov’t Review.
Proulx F, Gauthier M, Nadeau D, Lacroix J, Farrell CA. Timing and predictors of death in pediatric patients with multiple organ system failure. Crit Care Med. 1994;22(6):1025–31.
Tan G, Tan T, Goh D, HK Y. Risk factors for predicting mortality in a paediatric intensive care unit. Ann Acad Med Singapore. 1998;27(6):813–8.
Khilnani P, Sarma D, Zimmerman J. Epidemiology and peculiarities of pediatric multiple organ dysfunction syndrome in New Delhi, India. Intensive Care Med. 2006;32:1856–62.
Proulx F, Fayon M, Farrell CA, Lacroix J, Gauthier M. Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest. 1996;109(4):1033–7.
Keenan HT, Bratton SL, Martin LD, Crawford SW, Weiss NS. Outcome of children who require mechanical ventilatory support after bone marrow transplantation. Crit Care Med. 2000;28(3):830–5.
Lamas A, Otheo E, Ros P, Vazquez JL, Maldonado MS, Munoz A, et al. Prognosis of child recipients of hematopoietic stem cell transplantation requiring intensive care. Intensive Care Med. 2003;29(1):91–6.
Proulx F, Joyal JS, Mariscalco MM, Leteurtre S, Leclerc F, Lacroix J. The pediatric multiple organ dysfunction syndrome. Pediatr Crit Care Med. 2009;10(1):12–22.
Mack CL, Ferrario M, Abecassis M, Whitington PF, Superina RA, Alonso EM. Living donor liver transplantation for children with liver failure and concurrent multiple organ system failure. Liver Transpl. 2001;7(10):890–5.
Kamat P, Kunde S, Vos M, Vats A, Heffron T, Romero R, et al. Invasive intracranial pressure monitoring is a useful adjunct in the management of severe hepatic encephalopathy associated with pediatric acute liver failure. Pediatr Crit Care Med. 2012;13(1):e33–8.
Ozanne B, Nelson J, Cousineau J, Lambert M, Phan V, Mitchell G, et al. Threshold for toxicity from hyperammonemia in critically ill children. J Hepatol. 2012;56(1):123–8.
Karapinar B, Yilmaz D, Balkan C, Akin M, Ay Y, Kvakli K. An unusual cause of multiple organ dysfunction syndrome in the pediatric intensive care unit: hemophagocytic lymphohistiocytosis. Pediatr Crit Care Med. 2009;10(3):285–90.
Castillo L, Carcillo J. Secondary hemophagocytic lymphohistiocytosis and severe sepsis/systemic inflammatory response syndrome/multiorgan dysfunction syndrome/macrophage activation syndrome share common intermediate phenotypes on a spectrum of inflammation. Pediatr Crit Care Med. 2009;10(3):387–92. Research Support, N.I.H., Extramural.
Chehal A, Taher A, Shamseddine A. Sicklemia with multi-organ failure syndrome and thrombotic thrombocytopenic purpura. Hemoglobin. 2002;26(4):345–51.
Brenner JL, Jadavji T, Pinto A, Trevenen C, Patton D. Severe Kawasaki disease in infants: two fatal cases. Can J Cardiol. 2000;16:1017–23.
Liet JM, Pelletier V, Robinson BH, Laryea MD, Wendel U, Morneau S, et al. The effect of short term dimethylglycine treatment on oxygen consumption in cytochrome oxidase deficiency: a double-blind randomized crossover trial. J Pediatr. 2003;142:62–6.
Brossier T, Gwinner N, Fontaine P, Girard C. Anesthetic malignant hyperthermia and multiple organ dysfunction syndrome. Ann Fr Anesth Reanim. 2001;20:647–50.
Gauvin F, Toledano B, Champagne J, Lacroix J. Reactive hemophagocytic syndrome presenting as a component of multiple organ dysfunction syndrome. Crit Care Med. 2000;28:3341–5.
Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420(6917):885–91.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Proulx, F., Leteurtre, S., Joyal, J.S., Jouvet, P. (2014). Multiple Organ Dysfunction Syndrome. In: Wheeler, D., Wong, H., Shanley, T. (eds) Pediatric Critical Care Medicine. Springer, London. https://doi.org/10.1007/978-1-4471-6362-6_35
Download citation
DOI: https://doi.org/10.1007/978-1-4471-6362-6_35
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-6361-9
Online ISBN: 978-1-4471-6362-6
eBook Packages: MedicineMedicine (R0)