Abstract
Ventricular hypertrophy is an adaptation of the heart that develops in response to congenital or acquired pathologies to reduce wall stress and to maintain cardiac output. These adaptations can be successful (compensated) or inadequate, leading to deterioration of cardiac function, resulting in heart failure. A model of compensated hypertrophy is the dog with chronic AV-block (CAVB), in which cardiac remodeling occurs after AV-block, without deterioration towards heart failure.
Electrically, the most notable change is a lengthening of the QT-interval on the ECG. This is due to a lengthening of the action potential, which in turn depends on down regulation of repolarizing potassium currents. This long-QT phenotype leads to increased propensity for Torsade de Pointes (TdP) arrhythmias.
Contractile remodeling is observed in an increased systolic calcium concentration, while diastolic levels are not elevated, leading to improved contraction and increased dP/dT in the left ventricle. The increased calcium transient is linked to an increased loading of the sarcoplasmic reticulum.
Structurally, remodeling is characterized by hypertrophy of both ventricles. However, this is not accompanied by increased fibrosis or decreased intercellular coupling or conduction.
Intracellular signaling in the CAVB dog is reminiscent of exercise-induced hypertrophy. Calcineurin is not activated, while the CaMKII signaling cascade is interrupted at the HDAC4 level. Akt however, likewise in physiological hypertrophy, is activated.
The precise mechanism of arrhythmogenesis in this model is unclear, but could be due to an L-type calcium window current or an increased sodium late current, as inhibition of these currents is anti-arrhythmic. Reentry is probably not involved, as conduction is not slowed. Also, the presence of early afterdepolarizations in isolated cardiomyocytes suggests triggered activity as a leading mechanism instead.
Concluding, the compensated hypertrophy in the CAVB dog is accompanied by electrical remodeling, leading to a severely increased sensitivity for arrhythmias.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Ventricular hypertrophy is an adaptation of the heart that develops in response to either congenital or acquired pathologies in order to reduce wall stress [1] and to maintain cardiac output. Initially, the term remodeling was reserved for structural changes (hypertrophy, dilatation). More recently, it has been recognized that adaptive mechanisms are present on different levels (structural, contractile, and electrical) [2]. After an insult, these adaptations can be successful (compensated) or inadequate leading to deterioration of cardiac function, resulting in heart failure (Fig. 23.1a). Structurally, the compensated heart has regained the balance between wall thickness and internal cavity dimension [3], whereas the failing heart becomes quite dilated in time [4] (Fig. 23.1b). On the cellular level, there is an increase in the duration of the cellular action potential [5] (electrical remodeling, Fig. 23.1c) that is accompanied by an increased intracellular calcium transient [6], or by a severely depressed one [7, 8] (contractile remodeling). In case of the latter, the intracellular diastolic calcium levels ([Ca2+]i) are often elevated too [7] (Fig. 23.1c). Also the signal transduction pathways involved in the remodeling process may differ [9, 10]. It is known that exercise leads to a physiological hypertrophy in which the pAkt pathway is dominant [11, 12], whereas heart failure or pathological hypertrophy is ruled, among others, by the calcineurin [13, 14] and possibly the Ca2+-calmodulin-kinase (CaMKII) pathways [15] (Fig. 23.2).
Differences between decompensated and compensated hypertrophy. (a) Evolvement of cardiac output over time in compensated (grey line) and decompensated (yellow and red lines) hypertrophy after an initiating event. (b) In compensated hypertrophy wall thickness and luminal volume increase (grey circle), while in decompensated hypertrophy dilatation occurs (red circle). (c) Lower part: the differences in intracellular calcium levels at baseline (black) and in compensated (grey) and decompensated (red) hearts. In the upper part the accompanying cardiac action potentials are shown for reference
Ventricular remodeling is known to increase the propensity for ventricular arrhythmias and may lead to sudden cardiac death. Despite a clear association between left ventricular mechanical dysfunction, hypertrophy and ventricular arrhythmias [16], the majority of sudden cardiac death occurs at earlier stages of the disease process, even in circumstances in which mechanical dysfunction or ventricular hypertrophy, are absent [16– 18]. The mechanisms by which contractile and electrical remodeling predispose to arrhythmias remain unclear, but the changes in [Ca2+]i handling and repolarization as seen in the compensated form (Fig. 23.1c) may contribute. Therefore, in this chapter, we will concentrate on a canine model of complete atrio-ventricular block (CAVB) in which the beneficial adaptations leading to compensated biventricular hypertrophy are accompanied by a detrimental one, being an increased susceptibility for repolarization related ventricular arrhythmias. We will discuss the specific remodeling changes that are related to this phenotype.
Ventricular Remodeling in the CAVB Dog
Creation of chronic CAVB by radiofrequency ablation results in numerous adaptations initiated to overcome, acutely and in the long run (weeks), the abrupt decrease in cardiac output (Fig. 23.3a, black line) due to the bradycardia (Fig. 23.3a, red line). The CAVB dog [19] is a model of compensated biventricular hypertrophy with a long QT phenotype [3, 6, 19, 20]. The primary compensatory parameter is illustrated in the recovery of cardiac output after the initial decline. Initially, this is reached by increasing cardiac contractility as can be seen in left ventricular (LV) pressure development over time (LV dP/dt, Fig. 23.3a, green line). However, as time progresses, a decline is seen, until it reaches a stable state at >10 weeks that accounts for 120 % of baseline values. Nonetheless, also on this longer time scale cardiac output is maintained, because biventricular hypertrophy develops (increased heart weight to body weight, Fig. 23.3a, yellow line) creating a new equilibrium between wall thickness and LV volume, which prevents deterioration into dilated cardiomyopathy (for reference: see Fig. 23.1) [3, 21].
Progress of remodeling in the CAVB dog. (a) Schematic figure summarizing the development of cardiac remodeling in the CAVB dog over time. Scale is relative. The red line shows development of heart rate. The black line is an approximation of cardiac output. The green line shows contractile remodeling as left ventricular pressure changes. The yellow line represents structural remodeling, via cardiac hypertrophy as heart weight to body weight. The blue line depicts QT interval lengthening on the ECG, as a parameter for electrical remodeling. Finally, the bars represent fraction of dogs sensitive to drug-induced TdPs (see right Y-axis) at 2, 6, or 12 weeks of chronic AV-block. (b) Summary of the mechanism of drug-induced repolarization-dependent arrhythmias. From lengthening of the QT interval/APD, to early afterdepolarizations (EADs) and extra beats (EBs), to Torsade de Pointes arrhythmias (TdP)
Aside from the structural and mechanical changes, electrical remodeling is also present. Grossly, this is identified in a lengthening of the cellular action potential duration, that in vivo is reflected in prolongation of the QT-time (Fig. 23.1, blue line) [3, 7, 17, 19–21]. This is pro-arrhythmogenic, as further drug-induced lengthening leads to early afterdepolarizations (EADs), extra beats, and ultimately life threatening Torsade de Pointes arrhythmias (TdP) (Fig. 23.3b) [17, 19, 22–24].
In the following paragraphs, we will describe in depth the electrical, contractile, and structural remodeling. Moreover, the possible mechanisms responsible for the enhanced arrhythmogeneity (arrhythmogenic outcome) and possible responsible intracellular signaling pathways will be discussed. Special focus will be on the existing intricate connections between ventricular remodeling and their effect on arrhythmogeneity.
Electrical Remodeling
As mentioned above, the most striking electrical remodeling is seen in a lengthening of the action potential duration, which can be observed via lengthening of the QT interval on the ECG or, more regionally, via an increase in the duration of the catheter-based monophasic action potentials (MAPD, Table 23.1) [17, 19, 22, 24] or the activation recovery interval (ARI) of a needle measurement [25]. The prolongation of the MAPD is larger in the LV versus the right ventricle (RV), indicating that interventricular dispersion assessed as LV MAPD – RV MAPD is increasing too (Table 23.1) [17, 19, 24, 25]. More recently, spatial dispersion was further assessed using 66 needles with 4 electrodes, demonstrating increases in transseptal, interventricular, LV transmural as LV apex to base dispersion [25]. Also temporal dispersion, quantified as short term variability of beat-to-beat repolarization was increased after CAVB [24]. So not only is the repolarization prolonged, but it is also non-uniform in space and time.
Lengthening of the QT-duration develops in the first 2 weeks after AV-block and then stabilizes (Fig. 23.3a, blue line) [23]. Cellular experiments performed at 3–9 weeks of CAVB in isolated cardiomyocytes confirm this increase in repolarization times [26, 27]. Since potassium currents are largely responsible for proper repolarization [28], the most logical assumption would be to expect a decrease in these currents in the CAVB dog. Indeed, both the slow component of the delayed rectifier current Iks, as to a lesser extend the rapid component (Ikr), are downregulated in the LV and in the RV (Table 23.2) [29]. Other repolarizing currents as the inward rectifying potassium current (Ik1) and the transient outward current (Ito) are not changed functionally in either of the two ventricles [29].
Also the inward currents are attenuated when compared to their functioning before AV-block. The peak sodium current (INa) is down in the LV (Table 23.2) [25], whereas INa late, which occurs in the plateau of the action potential, is also reduced [30]. The current through the L-type calcium current (ICa-L) does not change after AV-block [6], but a more frequent occurrence of the window current is observed [31]. Sipido et al. [6] observed an increased current via the sodium calcium exchanger (NCX), in both modes, responsible for a more active sarcolemmal exchange of calcium and natrium ions through this channel. Also this increase may be pro-arrhythmic (see further). In line with this observation, [Na+]i seems to be increased, while activity of the Na+/K+ exchanger remained similar [32]. The reduction in both peak and late sodium current are not in line with an increase in [Na+]i or [Ca2+]i nor with an increase in cellular APD. Recently we studied the relevance of the sodium-proton exchanger (Na+/H+) (van Borren et al., 2009, unpublished data). The activity of this pump seems elevated thereby possibly explaining the increased [Na+]i. Taken together, repolarization reserve in the CAVB model has been diminished.
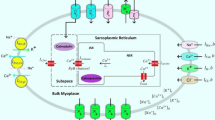
For a complete summary of the observed changes in ion currents in the CAVB dog, see Tables 23.1 and 23.2. On mRNA and protein level, appropriate changes of the (alpha)α-subunits of ion channel proteins have been observed [26, 33]. A reference for the role of ion currents in a normal action potential can be found in Fig. 23.4a.
Ion currents and calcium handling in the cardiomyocyte. (a) Schematic illustration of the depolarizing and repolarizing currents that shape the action potential in the normal mammalian ventricle. Time course of each of the currents is shown together with the course of the Ca2+ transient. (b) Depiction of intracellular calcium movement during an action potential. Upon depolarization the L-type calcium channel opens (LTCC) and a relatively small amount of calcium enters the cardiomyocyte. The ryanodine receptor (RyR) reacts to this calcium and opens as well, leading to release of calcium out of the sarcoplasmatic reticulum. The myofilaments start contracting in response to the increased calcium concentration. At the end of the action potential the intracellular calcium concentration is reduced to baseline by the sarcoplasmic reticulum calcium-ATPase (SERCA) pump, which pumps calcium back in to the sarcoplasmic reticulum, and via the sodium/calcium exchanger (NCX), which removes a smaller amount of calcium in exchange for sodium ions (three sodium ions for one calcium ion). (c) Behaviour of the intracellular calcium flux during a normal action potential, and a delayed and early afterdepolarization. The dotted line depicts the intracellular calcium concentration. Note that a delayed afterdepolarization occurs after the end of the action potential, while a early afterdepolarization occurs during the repolarization phase of an action potential
Contractile Remodeling
The ion current responsible for coupling the electrical impulse to contraction is ICaL via the L-type calcium channel (LTCC) (Fig. 23.4b) [34], which is voltage-sensitive and activates upon depolarization. The ryanodine receptor (RyR), located at the sarcoplasmic reticulum (SR), opens in accordance with the influx of calcium through LTCC, and even more calcium will be released in the cytoplasm; a process called ‘calcium-induced calcium release’ [34]. The SR functions as an intracellular calcium store. Cytosolic calcium binds to the myofilaments and myocyte shortening/contraction occurs. Thereafter [Ca2+]i returns to baseline values with the assistance of the NCX, transporting calcium out of the cell, while the (larger) remainder of cytoplasmic Ca2+ is pumped back into the SR via the sarcoplasmic reticulum calcium ATPase (SERCA). This is summarized in Fig. 23.4b and in the left part of Fig. 23.4c. Also, for a more detailed review of excitation-contraction coupling, see Bers [34].
Contractile strength can be varied under regulation of adrenergic stimuli, like epinephrine. The ß(beta)-adrenergic pathway modifies contraction by phosphorylation of a number of key proteins: (1) LTCC, (2) RyR release, and (3) SR reuptake of calcium by phosphorylation of phospholamban, the ancillary inhibitor protein of SERCA [34]. Also, CamKII has a positive, facilitating function in increasing contraction by phosphorylation of the same set of proteins, although at a different amino-acid residue [35].
In the CAVB dog, the [Ca2+]i transient is longer and in amplitude increased (Fig. 23.1c), while diastolic calcium levels are unaltered, thereby enhancing the systolic calcium fluxes as compared to non-hypertrophied, non-remodeled cardiomyocytes [6]. These increased [Ca2+]i levels result in more contractile power at the slow heart rhythm (bradycardia) in this animal model. A negative force frequency relationship occurs (in both ventricles) [6, 20], which is in contrast to normal physiological behavior [7, 36]. Also, potentiation of contraction, as achieved by extra-stimuli, is increased in the CAVB dog [20]. As mentioned before, both the Na+/Ca2+ and possibly Na+/H+ exchange, and SERCA have increased functional activity in order to handle this larger sarcolemmal and SR calcium movements. There is a close relation between electrical and contractile remodeling (see section “Arrhythmogenic Outcome”).
The stronger contractility can be measured in vivo using LV or RV dP/dt+, which is clearly increased at 2 weeks of CAVB (Fig. 23.3a, green line) [20]. Neurohumoral activation as seen in temporarily increased levels of (nor) epinephrine, angiotensin II and aldosteron in the blood plasma of these dogs is in agreement. Both contractility and neurohormonal levels are transiently increased, since after 4–6 weeks of CAVB, all measured neurohumoral plasma concentrations are back to baseline [19, 26].
Structural Remodeling
The most obvious structural change is of course the biventricular hypertrophy. This can be seen on the whole heart level as an increase in the heart to body weight (Fig. 23.5a), as well as on LV and RV weight determinations [19], as on the cellular level, where the cardiomyocytes are both lengthened and broadened (Fig. 23.5a) [3, 25, 27]. In this animal model, hypertrophy is more pronounced in the RV than in the LV as is reflected in the averaged increase in the length of the individual cardiomyocytes of Fig. 23.5a.
Structural remodeling in the CAVB dog and its consequences for conduction. (a) Cardiac hypertrophy in the CAVB dog heart and at the cellular level. (b) Comparison of ventricular fibros in sinus (SR) rhythm and chronic AV-block (CAVB) dogs as assessed by Sirius red staining. (c) Comparison of Connexin43 (Cx43) expression level and distribution in SR and CAVB dogs as assessed by immunohistochemistry. (d) Measurement of ventricular impulse propagation, both in SR and CAVB dogs via epicardial mapping. Red depicts early activation, blue late. The pacing site (indicated with the pacing symbol) was from the center of the epicardial placed electrode grid
Profound hypertrophy, especially of the pathological kind, can be accompanied by extensive fibrosis, decoupling of the cardiomyocytes and slowing of the conduction velocity [37, 38]. In the CAVB dog, neither is present: interstitial fibrosis, quantified using Sirius red staining, does not increase (Fig. 23.5b), nor is there a decrease in connexin43, a gap junction protein that constitutes channels responsible for ventricular electrical coupling (Fig. 23.5c) [37]. Finally, the capillary-fiber ratio of the myocytes remains similar [19]. As a consequence, CAVB does not affect electrical conduction over the myocardium (Fig. 23.5d) [25].
Arrhythmogenic Outcome
The enhanced susceptibility of this animal model for drug induced Torsade de Pointes arrhythmias (Fig. 23.3b) indicates that the beneficial adaptations resulting in compensated biventricular hypertrophy have deleterious effects on electrophysiology and ventricular repolarization. Especially, repolarization reserve is severely diminished in these animals.
Generally, there are roughly two ways in which arrhythmias can develop: reentry or triggered-activity [39]. Reentry based arrhythmias are dependent on conduction slowing and unidirectional block of conduction, circumstances which promote self-sustaining electrical wavefronts. This mechanism of arrhythmogeneity can probably be excluded in case of the CAVB dog, as the necessities for these kind of arrhythmias are not present, which is most clearly shown by its retained conduction velocity (see Fig. 23.5d), and inability to demonstrate contribution of reentry in the initiation and perpetuation of TdPs [25].
Triggered activity either resulting from delayed (DADs, Fig. 23.4b, middle panel) or early afterdepolarizations (EADs, Fig. 23.4b, right panel) are likely involved in the initiation of ectopic beats and ventricular arrhythmias [40]. In this model, their occurrence, alone or in combination, has been demonstrated in numerous conditions, both in vivo [19, 20], as in isolated cells [27, 41]. There is a close relation with the excitation-contraction activity, as can be seen in Fig. 23.4b: spontaneous release of Ca2+ from the (overloaded) SR through RyR can (re)trigger depolarization of the action potential, either after (delayed) or within (early) the AP. The sequence is now reversed to mechanical-electrical coupling, as calcium release induces a change in membrane potential, instead of the opposite. In case of DADs, the NCX is most likely responsible for the transient inward current (Iti) [42]. For EADs, window currents carried by the LTCC have been mentioned to underlie these events [31], probably assisted in a conditional phase induced by the NCX. Especially in conditions when intracellular Ca2+-handling is combined with a decreased repolarization reserve, these interactions may cause this arrhythmogenic mechanism. The drug-induced TdPs as observed, are both initiated and perpetuated by EADs, and triggered ectopic beats, which accumulate to self terminating polymorphic ventricular tachyarrhythmias (Fig. 23.3b, right panel), as we have recently shown in this model [25].
Controlling the [Ca2+]i is anti-arrhythmic and block of the LTCC, by verapamil or flunarizine, is clearly effective in preventing and suppressing these arrhythmias in vivo and on the cellular level [43].
This arrhythmogenic outcome is stable over time (Fig. 23.3, the bars) in the CAVB dog: 60–80 % of the dogs show reproducible TdPs, at 2, 6, or 12 weeks after AV-block, whereas no TdP can be induced in dogs without cardiac remodeling, like dogs still in sinus rhythm or directly after AV-block. When evaluating the different remodeling processes (structural, contractile, and electrical), it is clear that electrical remodeling is the only one that remains stable in the weeks after CAVB (Fig. 23.3a). Structural remodeling develops more slowly, whereas contractile adaptations after being profoundly increased transiently, are returning to less increased values in time [23].
Intracellular Signaling
As has been summarized in Fig. 23.2, hypertrophic remodeling is accompanied by activation of a number of signaling pathways in the cardiomyocyte. Of these, some, like calcineurin [13, 14] and CaMKII [15] (in the heart, CaMKII almost always refers to the CaMKIIδ variant, as this is the most expressed isoform in the heart) [44], are related to heart failure, while others, like Akt [11, 12], are more closely linked to physiological hypertrophy. In the CAVB model, mechanotransduction seems to play an prominent role [3]. Besides bradycardia, volume overload and altered ventricular activation are important in generating this different phenotype [45].
As the CAVB dog has a compensated hypertrophy, one would expect to indentify signaling reminiscent of physiological hypertrophy. This is indeed the case, as Akt was shown to be activated [3]. In contrast, calcineurin, another signaling molecule involved in pathological remodeling, appeared not to be involved in cardiac remodeling of the CAVB dog, which was assessed via calcineurin inhibition through chronic cyclosporin A treatment [46]. Cyclosporin A did not affect electrical, contractile, or structural remodeling, thereby suggesting no role of calcineurin.
CaMKII activation in the dog was more paradoxical. We have recently established that CaMKII total levels were not changed, but autophosphorylation levels were increased. This is indicative for increased CaMKII activity in the CAVB dog. However, the CaMKII-dependent pathway that leads to MEF2-dependent changes in gene expression was not activated, as HDAC4, the link between MEF2 and CaMKII [47], was not phosphorylated. On the other hand, CaMKII also phosphorylates numerous targets involved in intracellular calcium handling, like RyR, LTCC, and phospholamban (the inhibitor of SERCA) [48]. This implies a regulatory role of CamKII in [Ca2+]i handling, but no involvement in alterations of the gene expression profile associated with maladaptive remodeling. A summary of the involved signaling pathways in the CAVB dog, as we have observed, can be seen in Fig. 23.6.
Conclusion
The CAVB dog has a heart with profound adaptations on the structural, electrical, and contractile levels. This remodeling is compensatory, as the cardiac output is retained in the long-term due to stable biventricular hypertrophy and increased contractility. However, the action potential is heterogeneously lengthened, both in space and time, which is pro-arrhythmic and maladaptive, as the remodeled heart appears much more sensitive to EADs, extra beats, and TdP arrhythmias.
References
Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56(1):56–64.
Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling – concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35(3):569–82.
Donker DW, Volders PG, Arts T, Bekkers BC, Hofstra L, Spatjens RL, et al. End-diastolic myofiber stress and ejection strain increase with ventricular volume overload–Serial in-vivo analyses in dogs with complete atrioventricular block. Basic Res Cardiol. 2005;100(4):372–82.
Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7(8):589–600.
Bailly P, Benitah JP, Mouchoniere M, Vassort G, Lorente P. Regional alteration of the transient outward current in human left ventricular septum during compensated hypertrophy. Circulation. 1997;96(4):1266–74.
Sipido KR, Volders PG, de Groot SH, Verdonck F, Van de WF, Wellens HJ, et al. Enhanced Ca(2+) release and Na/Ca exchange activity in hypertrophied canine ventricular myocytes: potential link between contractile adaptation and arrhythmogenesis. Circulation. 2000;102(17):2137–44.
Davies CH, Harding SE, Poole-Wilson PA. Cellular mechanisms of contractile dysfunction in human heart failure. Eur Heart J. 1996;17(2):189–98.
Mulieri LA, Hasenfuss G, Leavitt B, Allen PD, Alpert NR. Altered myocardial force-frequency relation in human heart failure. Circulation. 1992;85(5):1743–50.
Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128(1):191–227.
Balakumar P, Jagadeesh G. Multifarious molecular signaling cascades of cardiac hypertrophy: can the muddy waters be cleared? Pharmacol Res. 2010;62(5):365–83.
DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, et al. Akt1 is required for physiological cardiac growth. Circulation. 2006;113(17):2097–104.
Sussman MA, Volkers M, Fischer K, Bailey B, Cottage CT, Din S, et al. Myocardial AKT: the omnipresent nexus. Physiol Rev. 2011;91(3):1023–70.
Bourajjaj M, Armand AS, da Costa Martins PA, Weijts B, van der Nagel R, Heeneman S, et al. NFATc2 is a necessary mediator of calcineurin-dependent cardiac hypertrophy and heart failure. J Biol Chem. 2008;283(32):22295–303.
Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93(2):215–28.
Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51(4):468–73.
Tomaselli GF, Beuckelmann DJ, Calkins HG, Berger RD, Kessler PD, Lawrence JH, et al. Sudden cardiac death in heart failure. The role of abnormal repolarization. Circulation. 1994;90(5):2534–9.
Schoenmakers M, Ramakers C, van Opstal JM, Leunissen JD, Londono C, Vos MA. Asynchronous development of electrical remodeling and cardiac hypertrophy in the complete AV block dog. Cardiovasc Res. 2003;59(2):351–9.
Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98(21):2334–51.
Vos MA, de Groot SH, Verduyn SC, van der Zande J, Leunissen HD, Cleutjens JP, et al. Enhanced susceptibility for acquired torsade de pointes arrhythmias in the dog with chronic, complete AV block is related to cardiac hypertrophy and electrical remodeling. Circulation. 1998;98(11):1125–35.
de Groot SH, Schoenmakers M, Molenschot MM, Leunissen JD, Wellens HJ, Vos MA. Contractile adaptations preserving cardiac output predispose the hypertrophied canine heart to delayed afterdepolarization-dependent ventricular arrhythmias. Circulation. 2000;102(17):2145–51.
Verduyn SC, Ramakers C, Snoep G, Leunissen JD, Wellens HJ, Vos MA. Time course of structural adaptations in chronic AV block dogs: evidence for differential ventricular remodeling. Am J Physiol Heart Circ Physiol. 2001;280(6):H2882–90.
Dunnink A, van Opstal JM, Oosterhoff P, Winckels SK, Beekman JD, van der Nagel R, et al. Ventricular remodelling is a prerequisite for the induction of dofetilide-induced torsade de pointes arrhythmias in the anaesthetized, complete atrio-ventricular-block dog. Europace. 2012;14(3):431–6.
Oros A, Beekman JD, Vos MA. The canine model with chronic, complete atrio-ventricular block. Pharmacol Ther. 2008;119(2):168–78.
Thomsen MB, Oros A, Schoenmakers M, van Opstal JM, Maas JN, Beekman JD, et al. Proarrhythmic electrical remodelling is associated with increased beat-to-beat variability of repolarisation. Cardiovasc Res. 2007;73(3):521–30.
Boulaksil M, Jungschleger JG, Antoons G, Houtman MJ, de Boer TP, Wilders R, et al. Drug-induced Torsade de Pointes arrhythmias in the chronic AV block dog are perpetuated by focal activity. Circ Arrhythm Electrophysiol. 2011;4(4):566–76.
Stengl M, Ramakers C, Donker DW, Nabar A, Rybin AV, Spatjens RL, et al. Temporal patterns of electrical remodeling in canine ventricular hypertrophy: focus on IKs downregulation and blunted beta-adrenergic activation. Cardiovasc Res. 2006;72(1):90–100.
Volders PG, Sipido KR, Vos MA, Kulcsar A, Verduyn SC, Wellens HJ. Cellular basis of biventricular hypertrophy and arrhythmogenesis in dogs with chronic complete atrioventricular block and acquired torsade de pointes. Circulation. 1998;98(11):1136–47.
Nabauer M, Kaab S. Potassium channel down-regulation in heart failure. Cardiovasc Res. 1998;37(2):324–34.
Volders PG, Sipido KR, Vos MA, Spatjens RL, Leunissen JD, Carmeliet E, et al. Downregulation of delayed rectifier K(+) currents in dogs with chronic complete atrioventricular block and acquired torsades de pointes. Circulation. 1999;100(24):2455–61.
Antoons G, Oros A, Beekman JD, Engelen MA, Houtman MJ, Belardinelli L, et al. Late na(+) current inhibition by ranolazine reduces torsades de pointes in the chronic atrioventricular block dog model. J Am Coll Cardiol. 2010;55(8):801–9.
Antoons G, Volders PG, Stankovicova T, Bito V, Stengl M, Vos MA, et al. Window Ca2+ current and its modulation by Ca2+ release in hypertrophied cardiac myocytes from dogs with chronic atrioventricular block. J Physiol. 2007;579(Pt 1):147–60.
Verdonck F, Volders PG, Vos MA, Sipido KR. Increased Na+ concentration and altered Na/K pump activity in hypertrophied canine ventricular cells. Cardiovasc Res. 2003;57(4):1035–43.
Ramakers C, Vos MA, Doevendans PA, Schoenmakers M, Wu YS, Scicchitano S, et al. Coordinated down-regulation of KCNQ1 and KCNE1 expression contributes to reduction of I(Ks) in canine hypertrophied hearts. Cardiovasc Res. 2003;57(2):486–96.
Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205.
Maier LS, Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc Res. 2007;73(4):631–40.
Hasenfuss G, Holubarsch C, Hermann HP, Astheimer K, Pieske B, Just H. Influence of the force-frequency relationship on haemodynamics and left ventricular function in patients with non-failing hearts and in patients with dilated cardiomyopathy. Eur Heart J. 1994;15(2):164–70.
Boulaksil M, Winckels SK, Engelen MA, Stein M, van Veen TA, Jansen JA, et al. Heterogeneous Connexin43 distribution in heart failure is associated with dispersed conduction and enhanced susceptibility to ventricular arrhythmias. Eur J Heart Fail. 2010;12(9):913–21.
Wolk R. Arrhythmogenic mechanisms in left ventricular hypertrophy. Europace. 2000;2(3):216–23.
Rosen MR. Mechanisms for arrhythmias. Am J Cardiol. 1988;61(2):2A–8.
Weiss JN, Garfinkel A, Karagueuzian HS, Chen PS, Qu Z. Early afterdepolarizations and cardiac arrhythmias. Heart Rhythm. 2010;7(12):1891–9.
Nalos L, Varkevisser R, Jonsson M, Houtman M, Beekman J, van der Nagel R, et al. Comparison of I(Kr) blocking drugs Moxifloxacin and Dofetilide/E-4031 in 5 screening models of pro-arrhythmia reveals insufficient specificity of isolated cardiomyocytes. Br J Pharmacol. 2012;165(2):467–78.
Volders PG, Kulcsar A, Vos MA, Sipido KR, Wellens HJ, Lazzara R, et al. Similarities between early and delayed afterdepolarizations induced by isoproterenol in canine ventricular myocytes. Cardiovasc Res. 1997;34(2):348–59.
Oros A, Houtman MJ, Neco P, Gomez AM, Rajamani S, Oosterhoff P, et al. Robust anti-arrhythmic efficacy of verapamil and flunarizine against dofetilide-induced TdP arrhythmias is based upon a shared and a different mode of action. Br J Pharmacol. 2010;161(1):162–75.
Tombes RM, Faison MO, Turbeville JM. Organization and evolution of multifunctional Ca(2+)/CaM-dependent protein kinase genes. Gene. 2003;322:17–31.
Winckels SK, Thomsen MB, Oosterhoff P, Oros A, Beekman JD, Attevelt NJ, et al. High-septal pacing reduces ventricular electrical remodeling and proarrhythmia in chronic atrioventricular block dogs. J Am Coll Cardiol. 2007;50(9):906–13.
Bourgonje VJA, Schoenmakers M, Beekman HDM, Van Der Nagel R, De Windt LJ, Van Veen AAB, et al. Discrepancy between acute and long-term effects of the calmodulin/CaMKII/calcineurin pathway on arrhythmogenesis in the CAVB dog. Eur Heart J. 2010;31:77–8.
Zhang T, Kohlhaas M, Backs J, Mishra S, Phillips W, Dybkova N, et al. CaMKIIdelta isoforms differentially affect calcium handling but similarly regulate HDAC/MEF2 transcriptional responses. J Biol Chem. 2007;282(48):35078–87.
Anderson ME. Multiple downstream proarrhythmic targets for calmodulin kinase II: moving beyond an ion channel-centric focus. Cardiovasc Res. 2007;73(4):657–66.
Acknowledgement.
This research was sponsored by Fondation Leducq: the Alliance for Calmodulin Kinase II signaling in heart disease
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag London
About this chapter
Cite this chapter
Bourgonje, V.J.A., van Veen, T.A.B., Vos, M.A. (2013). Ventricular Electrical Remodeling in Compensated Cardiac Hypertrophy. In: Gussak, I., Antzelevitch, C. (eds) Electrical Diseases of the Heart. Springer, London. https://doi.org/10.1007/978-1-4471-4881-4_23
Download citation
DOI: https://doi.org/10.1007/978-1-4471-4881-4_23
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-4880-7
Online ISBN: 978-1-4471-4881-4
eBook Packages: MedicineMedicine (R0)