Abstract
The majority of women with ovarian cancer continue to present at advanced stages and the overall 5-year survival rate remains just over 40 %. The principles of the initial management of advanced ovarian cancer consist of maximal cytoreductive surgery and platinum-based chemotherapy. Improvements in surgery and chemotherapy strategies have led to better clinical outcomes. However, disease recurrence and drug resistance present management challenges. Clinical trials evaluating the optimal schedule, mode of administration of established chemotherapy drugs and the integration of targeted agents have led to improvements in survival and practice change. This chapter provides an overview of the initial management of advanced ovarian cancer and the integration of targeted therapies in this setting.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
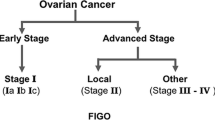
The worldwide incidence of ovarian cancer is estimated to be 225, 000 cases per year [1]. In Europe, there are approximately 65, 697 new cases and 41, 448 ovarian cancer-related deaths per year [1]. While women with disease confined to the ovaries (stage I) usually have a good outlook (5-year survival of >90 %), due to the lack of well-defined symptoms, the vast majority (75–80 %) unfortunately present with more advanced disease (FIGO III-IV) and little prospect of cure, with a 5 year survival rate of approximately 40 % [2]. Around 90 % of ovarian carcinomas are epithelial in origin whereas the remainder arise from germ cells or stromal cells. The principles of the initial management of epithelial ovarian cancer has remained largely unchanged over the years and consists of attempted maximal cytoreductive surgery and platinum-based chemotherapy. Improvements in surgical techniques and chemotherapy strategies have led to improved clinical outcomes. However, disease recurrence and drug resistance continue to pose persistent management challenges. Advances in our knowledge of the molecular biology underlying ovarian cancer coupled with development of novel agents offers promise. A number of clinical trials address the optimal schedule, mode of administration of established chemotherapy drugs and the integration of targeted agents. This chapter provides an overview of the initial management of advanced ovarian cancer and the integration of targeted therapies in this setting.
2 Systemic Therapy for the Initial Management of Advanced Ovarian Cancer
The current international standard of care for advanced ovarian cancer is either initial or interval optimal cytoreductive surgery (no residual disease) and a total of six cycles of three-weekly intravenous (IV) chemotherapy with carboplatin (area under the curve [AUC] 5–7.5) given in combination with paclitaxel (175 mg/m2) [3]. Chemotherapy given following surgery is termed ‘adjuvant’ and upfront chemotherapy followed by interval debulking surgery is referred to as ‘neoadjuvant.’ The recommendation of a platinum/paclitaxel combination is based on a series of phase III studies over the last two decades which address type of platinum, combination therapy, dosage and scheduling [4–7] (see Table 41.1). The results of the Gynecologic Oncology Group (GOG) 111 trial demonstrated the importance of incorporating taxanes into first line chemotherapy with platinum [4]. Four hundred and ten women with advanced ovarian cancer and residual masses larger than 1 cm after initial surgery were randomized to receive cisplatin (75 mg/m2) with either cyclophosphamide (750 mg/m2) or paclitaxel (135 mg/m2 over 24 h). Progression free survival (PFS), (median, 18 vs. 13 months, p < 0.001) and overall survival (OS), (median 38 vs. 24 months, p < 0.001) were significantly longer in the cisplatin/paclitaxel arm compared to cisplatin/cyclophosphamide in women with sub-optimally debulked ovarian cancer.
The results of the European and Canadian Intergroup trial (EORTC-NCIC OV-10) provided confirmatory evidence for cisplatin and paclitaxel as the standard regimen in advanced ovarian cancer [5]. Compared to GOG-111, this study had a broader selection of patients by also including patients with optimally debulked stage III or IV disease, as well as those having FIGO stage IIB or IIC disease and allowed recruitment of patients who had undergone interval debulking surgery. Additionally, the dose of paclitaxel was higher (175 mg/m2 vs. 135 mg/m2) with a shorter infusion time of 3 h, instead of 24 h, as this strategy had previously been found to be more convenient, produce less neutropenia, as well as confer a PFS advantage. After a median follow-up of 38.5 months, a longer PFS (15.5 vs. 11.5 months, p = 0.0005) and OS (35.6 vs. 25.8 months, p = 0.0016) was observed in the cisplatin and paclitaxel arm compared to the cisplatin and cyclophosphamide arm.
However, the GOG-132 trial which compared cisplatin (100 mg/m2) or paclitaxel (200 mg/m2 24 h infusion) monotherapy with the cisplatin (75 mg/m2) and paclitaxel (135 mg/m2) combination therapy in suboptimally debulked stage III or IV ovarian cancer did not find any difference in PFS or OS in the combination arm compared to either of the monotherapy arms [6]. Similarly, the International collaborative ovarian neoplasm (ICON)-3 group study which compared carboplatin (AUC5, if glomerular filtration rate (GFR) used and AUC6, if Cockcroft Gault equation used) and paclitaxel (175 mg/m2 over 3 h) against either single agent carboplatin (AUC5 or 6) or a combination of cyclophosphamide (500 mg/m2), doxorubicin (50 mg/m2) and cisplatin (50 mg/m2) (CAP) also failed to show an OS advantage for the carboplatin/paclitaxel arm [7]. A likely explanation for the lack of survival advantage seen in GOG-132 and ICON3 is that a significant proportion of patients crossed over prior to progression (>20 %) thereby diminishing any potential survival benefit in the platinum/paclitaxel arms. A possible interpretation of the results is that sequential treatment with platinum/paclitaxel is equivalent to the combination.
Once the role of paclitaxel in combination with a platinum agent was established, the AGO-OVAR-3, GOG-158 and Dutch-Danish Intergroup studies concluded that first line chemotherapy with carboplatin and paclitaxel was at least as effective and associated with a better toxicity profile than the cisplatin combination [8–10].
3 Optimizing First-Line Combination Chemotherapy
Following the adoption of carboplatin in combination with paclitaxel every 3 weeks for six cycles as the international standard of care, issues including choice of taxane, triple therapy, chemotherapy scheduling and mode of delivery to further improve outcome have been evaluated. However, many questions regarding the optimization of chemotherapy in this setting remain unclear and the results of ongoing studies are awaited.
3.1 Choice of Chemotherapy
The Scottish Randomised Trial in Ovarian Cancer (SCOTROC)-1 established that the substitution of paclitaxel (175 mg/m2) with docetaxel (75 mg/m2) was not inferior in terms of survival or clinical response and was associated with less neurotoxicity, at the expense of increased grade 3/4 neutropenia [11]. Carboplatin in combination with docetaxel may be an acceptable alternative to carboplatin/paclitaxel for some patients where neurotoxicity is a particular concern.
Several phase III trials have addressed the addition of a third cytotoxic agent to carboplatin/paclitaxel [12–14]. The GOG0182-ICON5 was a randomized, phase III trial containing five arms which incorporated gemcitabine, liposomal doxorubicin, or topotecan compared with carboplatin and paclitaxel [14]. The addition of a third cytotoxic agent has not been shown to improve long-term clinical outcomes and is associated with increased hematological toxicity.
3.2 Scheduling of Carboplatin/Paclitaxel
The standard of care is a three-weekly schedule of carboplatin and paclitaxel. However, it has suggested that a dose-fractionated schedule may enhance antitumor activity leading to improved survival. A Japanese study JGOG3016 set out to address this. The study compared six cycles of dose dense weekly paclitaxel (80 mg/m2, given IV over 1 h) in addition to 3-weekly carboplatin (AUC6) against 3-weekly carboplatin (AUC 6) and paclitaxel (180 mg/m2 IV over 3 h) in patients with advanced ovarian cancer [15]. Despite higher rates of myelosuppression, delays and dose modifications in the dose dense group, at the median follow up period of 76.8 months, the median PFS (28.2 months vs. 17.5 months, P = 0.004) and median OS (100.5 months vs. 62.2 months, P = 0.039) was longer in the dose dense group compared to the conventional group [16]. The outcome of this study could lead to change in standard of care and confirmation of the findings of the JGOG study in different study populations is required. Results of the MITO-7 study comparing 3-weekly carboplatin (AUC6) and paclitaxel (175 mg/m2) against weekly carboplatin (AUC2) and weekly paclitaxel (80 mg/m2) did not demonstrate a significant benefit in PFS with weekly administration compared to standard carboplatin/paclitaxel every 3 weeks, but was associated with better QoL and toxicity [17]. The ongoing ICON-8 trial is a randomized, phase III, three arm, study evaluating dose fractionation schedules (3 weekly carboplatin/paclitaxel vs 3 weekly carboplatin/weekly paclitaxel vs weekly carboplatin/paclitaxel) following immediate surgery or as part of delayed primary surgery.
3.3 Maintenance Chemotherapy
Despite surgery and first-line chemotherapy, at least 65 % of women who achieve a complete response will eventually relapse, at which stage the condition is deemed incurable. Maintenance chemotherapy after initial therapy has been explored as a possible strategy to prevent or delay relapse. In the phase III SWOG 9701/GOG 178 study, patients with advanced ovarian cancer who had achieved complete clinical response were randomly assigned to receive 3 or 12 additional cycles of 4-weekly paclitaxel. Based on an interim analysis which reported a significant improvement in PFS of 7 months (21 vs 28 months) in the 12 cycle arm, the study was stopped prematurely [18]. However, no OS advantage was demonstrated [19]. Potential reasons for a lack of OS benefit include the effect of subsequent therapies, crossover of patients from 3 cycles to 12 cycles and reduced sample size due to the closure of the study. The Italian Cooperative Group After-6 phase III trial evaluated six cycles of 3-weekly paclitaxel as maintenance therapy compared with observation. No significant difference in PFS (34 vs 30 months) or OS (2 year survival rate: 87 % vs 90 %) between the paclitaxel and observation arms was seen following an interim futility analysis and the study closed early [20]. The ongoing GOG-0212 study is evaluating paclitaxel or polyglutamate paclitaxel or observation in women with stage III/IV ovarian cancer who achieve a complete clinical response to primary platinum/paclitaxel chemotherapy.
4 The Role of Neoadjuvant Chemotherapy
Primary surgery aims to achieve complete tumor resection with no residual disease because it has been shown that the volume of residual disease following surgery is an independent prognostic indicator.
In some cases of advanced ovarian cancer including stage IV disease, complete cytoreductive surgery with no residual disease may not realistically be achievable. In addition, a proportion of patients may be too unwell at presentation to undergo such major, radical surgery. This has led to debate regarding whether primary surgery or neoadjuvant chemotherapy followed by interval debulking surgery after three to four cycles of chemotherapy is the preferred option [21–23]. Cytoreductive surgery is an integral component in the management of ovarian cancer, there are some concerns that delaying surgery for patients to have chemotherapy may impact on overall outcome. In addition, some subtypes of epithelial ovarian cancer, such as low-grade serous carcinomas do not respond well to chemotherapy and in such cases there is an argument for primary surgery. The European Organisation for Research and Treatment of Cancer (EORTC) 55971 trial recruited potentially operable patients with stage IIIc or IV disease and randomized them to receive either primary debulking surgery and chemotherapy or neoadjuvant chemotherapy followed by interval debulking surgery [24]. The PFS and OS were similar in the two arms but in the neoadjuvant chemotherapy arm 80.6 % had ≤1 cm residual tumor remaining compared to only 41.6 % of patients in the primary surgery arm, where post-operative morbidity was more common. The recent results of the phase III CHORUS study [25] support the findings of the EORTC 55971 trial.
As more patients are likely to be receiving neoadjuvant chemotherapy followed by interval debulking surgery, it is important that this strategy is recognized and incorporated into future trial designs of advanced ovarian cancer. At present, the decision regarding whether neoadjuvant chemotherapy followed by interval debulking surgery or primary surgery should be made on a case by case basis in a multidisciplinary setting. Upfront surgery is the preferred option in fit patients where it is believed that cytoreduction with no residual disease can be achieved. However, neoadjuvant therapy can achieve equivalent therapeutic outcomes and may be associated with less morbidity for patients with bulky disease [24].
4.1 Time to Initiate Chemotherapy Following Primary Surgery
In patients undergoing primary surgery, the optimal time to initiate chemotherapy is an important issue. While it can be argued that chemotherapy should be initiated as soon as possible to prevent metastatic re-growth, patients who have been optimally debulked may have required invasive surgery including liver and/or bowel resection, as well as diaphragmatic stripping. In an analysis of prospective phase III trials, the median time to chemotherapy was 19 days (range 1–56) and a delayed start to chemotherapy was associated with decreased OS (p = 0.038) in optimally debulked patients whereas in patients with residual disease, a longer time to initiate chemotherapy had no effect on OS (p = 0.452) [26]. This analysis provides evidence to support an earlier start to initiate chemotherapy in optimally debulked patients.
4.2 Intraperitoneal Chemotherapy
Intra-peritoneal (IP) chemotherapy is another strategy that has been investigated in an attempt to improve outcomes in ovarian cancer. The rationale behind its use stems from the concept that advanced ovarian cancer predominantly affects peritoneal surfaces. Delivering cytotoxic agents directly to the peritoneum therefore increases dose intensity while preventing systemic toxicity.
Three randomized trials provided evidence for a survival advantage with IP chemotherapy compared to IV administration in women with optimally debulked (to <0.5 cm) stage III epithelial ovarian cancer [27–29]. The GOG 104 study compared six cycles of three-weekly IV cyclophosphamide (600 mg/m2) combined with either IV or IP cisplatin (100 mg/m2) [27]. The IP arm had a significantly longer median survival, (49 vs. 41 months, P = 0.02) but at the expense of more frequent moderate to severe abdominal pain. The GOG 114 trial incorporated a taxane into the treatment arms and provided further support for IP chemotherapy [29]. Six cycles of IV paclitaxel (135 mg/m2) and cisplatin (75 mg/m2) every 3 weeks was compared with IV carboplatin (AUC 9) every 28 days for two cycles followed by six cycles of IV paclitaxel (135 mg/m2) and IP cisplatin (100 mg/m2) every 3 weeks in patients with stage III optimally debulked ovarian cancer. Median PFS was longer in the IP arm (28 months vs. 22 months, P = 0.01) and median OS was increased in this arm (63 months vs. 52 months, P = 0.05) but again patients in the IP arm experienced increased toxicity and 18 % of patients received less than two courses of IP chemotherapy as a consequence. In GOG 172, 415 patients with optimally debulked stage III ovarian cancer were randomized to receive 63 weekly cycles of IV paclitaxel (135 mg/m 2 over 24 h) followed by either IV cisplatin (75 mg/m2) on day 2 or IP cisplatin (100 mg/m2) on day 2 plus IP cisplatin (60 mg/m2) on day 8 [28]. The median survival data was impressive and again in favor of the IP arm (65.6 months vs. 49.7 months, P = 0.03). Despite the results of all three trials appearing to support the role of IP chemotherapy, it has not become routine clinical practice internationally. This is in part largely due to the increased toxicity (abdominal discomfort, infection, bowel injury, catheter-related problems, fatigue, hematological, gastrointestinal and neurological events) in the IP arms. It has been argued that the favorable outcome in GOG 114 [29] may be influenced by the increased amount of chemotherapy delivered in the IP arm (eight cycles). Furthermore, in GOG 172, the control arm did not receive the current standard of care i.e., IV carboplatin and paclitaxel and the dose and schedule of cisplatin and paclitaxel was different in the two arms of the study [28]. Therefore the higher dose of chemotherapy in the IP arm may have played a significant part in the survival benefit seen rather than the mode of delivery itself. Finally, the analysis was not a true intention-to-treat analysis and therefore it is feasible for minor imbalances in the number of excluded patients impacting on the statistical significance.
Combined data from the GOG114 and GOG172 demonstrated a significant improvement in median OS with IP administration, compared with IV administration (61.8 vs 51.4 months; P = 0.048) [30]. A subset analysis of 393 patients within the GOG172 study suggested that the survival advantage of IP chemotherapy was limited to a subset of patients with low BRCA1 expression as measured by immumohistochemistry (84 months IP vs 47 months IV; p = 0.0002) and that low BRCA1 expression was an independent prognostic factor for better survival in women randomized to IP therapy (hazard ratio (HR) = 0.67 p = 0.032) [31].
Several trials of IP chemotherapy are ongoing and include GOG-252, NCIC-CTG OV21/NCRI-PETROC and JGOG 3109. Issues addressed include the use of carboplatin/paclitaxel as the control arm, incorporating dose-dense scheduling of paclitaxel, bevacizumab and IP administration of carboplatin.
5 Novel Biological Agents
Novel biologically targeted agents aim to target tumor cells and/or the microenvironment by exploiting specific molecular abnormalities in the tumor leading to greater selectivity and a better toxicity profile than traditional chemotherapy [32]. Epithelial ovarian cancer has previously been treated as a single disease. It is recognized that ovarian cancer is a heterogenous disease rather than a single entity, made up of several histological subtypes with distinct clinical outcomes and molecular aberrations (high grade serous- p53, BRCA, homologous recombination deficiency; low grade serous- BRAF, KRAS, NRAS, HER2); clear cell-PIK3CA, PTEN; endometrioid PIK3CA, PTEN; and mucinous- KRAS, HER2). Multiple molecules involved in critical, signalling pathways which drive growth and progression of ovarian cancer can now be targeted with novel drugs [32]. Angiogenesis inhibitors and PARP inhibitors are the most developed in ovarian cancer.
6 Angiogenesis Inhibitors
Angiogenesis is the formation of new blood vessels and is a critical component of cancer growth and metastasis. Vascular endothelial growth factor (VEGF) is a key promoter in the process of angiogenesis in epithelial ovarian cancer. Strategies to target either the ligand or the receptor have been explored. Bevacizumab is a humanized monoclonal antibody that targets VEGF-A and prevents it from binding to VEGF receptors and subsequent downstream signalling. Two randomized, phase III trials, the Gynecologic Oncology Group (GOG) trial 0218 [33] and ICON-7 trial [34], set out to evaluate the addition of bevacizumab to the combination of carboplatin/paclitaxel followed by maintenance therapy as first-line treatment for advanced ovarian cancer. The GOG-0218 study was a three arm, double blind placebo-controlled trial enrolling 1,873 patients with either stage III or stage IV epithelial ovarian cancer who had undergone debulking surgery [33]. The study participants were randomized to receive either standard treatment with IV carboplatin and paclitaxel for six cycles every 3 weeks followed by placebo every 3 weeks for cycles 7–22 or standard treatment with the addition of bevacizumab (15 mg/kg) from cycle 2 until cycle 22 (a total of 15 months) or standard treatment with the addition of bevacizumab (15 mg/kg) from cycle 2–6 followed by placebo for cycles 7–22. Patients in the bevacizumab throughout arm had a significant improvement in PFS compared to the control arm (14.1 vs. 10.3 months HR 0.717; P < 0.001). The IOCN-7 study was an open label study that assigned 1,528 patients to either carboplatin and paclitaxel with concurrent bevacizumab (7.5 mg/kg) followed by maintenance bevacizumab for 12 cycles (or until disease progression) or carboplatin and paclitaxel alone [34]. This study confirmed an improvement in PFS with the addition of bevacizumab (19.0 vs. 17.3 months; HR 0.81; P = 0.004). A pre-planned analysis of the patients at highest risk of progression (stage III with >1 cm residual disease or stage IV disease), showed that bevacizumab conferred a greater magnitude of benefit in this sub-population (restricted means 18.1 vs. 14.5 months; HR 0.73; P = 0.002). Furthermore, early analyses demonstrated a significant improvement in OS in the high risk group (28.8 vs. 36.6 months HR = 0.64, 95 % CI 0.48–0.85; P = 0.002). However, the final OS data from the ICON-7 study showed no benefit from the addition of bevacizumab and an OS benefit was not evident in GOG-0218. In ICON-7, in a pre-specified sub-group analysis of poor prognosis patients, a benefit of 4.8 months in the restricted means survival time was observed [35].
In both studies, the addition of bevacizumab was relatively well-tolerated with adverse effects as expected for angiogenesis inhibitors [36]- (≥grade 2, ICON-7 18 % (bevacizumab arm) vs 2 % (chemotherapy)), thromboembolism (≥grade 3, ICON-7 7 % (bevacizumab arm) vs 3 % (chemotherapy)). Recognized complications of bevacizumab include gastrointestinal (GI) perforation and fistula formation. However, in ICON-7 and GOG-0218, the reported rates of GI perforation are low (≥grade 3 ICON-7 1 % bevacizumab arm; <3 % in GOG 218 and 1 %). The results of these studies led to the European Medicines Agency (EMA) approval of bevacizumab to be used in combination with carboplatin and paclitaxel in the front line setting of patients with advanced ovarian cancer (FIGO stage IIIB, IIIC and IV).
The role of bevacizumab has also been investigated in recurrent ovarian cancer. The OCEANS study, a double-blind, placebo-controlled trial evaluated the addition of bevacizumab (15 mg/kg) to carboplatin (AUC 4) and gemcitabine (1,000 mg/m2 on day 1 and day 8) continued until progression in women with first relapse platinum-sensitive ovarian cancer [37]. This study provided evidence for bevacizumab in the platinum sensitive setting with an improvement in PFS (12.4 vs 8.4 months, P < 0.0001). In addition, the AURELIA study provided support for the use of bevacizumab (15 mg/kg) in the platinum resistant setting [38]. Bevacizumab in combination with paclitaxel, topotecan or liposomal doxorubicin led to a significant improvement in PFS (6.7 vs. 3.4 months; HR 0.48, P < 0.001) but no statistically significant improvement in OS.
It is currently not known whether bevacizumab should be used in the first line setting or reserved for platinum-sensitive or platinum-resistant relapse. Ongoing trials of bevacizumab address the role of bevacizumab with IP chemotherapy, dose dense chemotherapy, extending the duration of maintenance therapy and the continuation of bevacizumab beyond progression. Preliminary data from the GOG-262 trial, evaluating bevacizumab in combination with dose dense chemotherapy suggests that bevacizumab does not confer any additional benefit to dose dense treatment [39].
VEGF receptor tyrosine kinase inhibitors (TKIs) inhibit downstream VEGF signalling and other pro-angiogenic molecules such as platelet derived growth factor (PDGFR) and fibroblast growth factor (FGFR). VEGFR TKIs is a potential strategy to help overcome some mechanisms of resistance to antiangiogenic therapy [40]. The AGO-OVAR 16 trial is a phase III randomized, double-blind study which involved 940 patients with FIGO stage II to IV ovarian, fallopian tube, or primary peritoneal cancer who had been initially treated with surgery and chemotherapy to receive 800 mg of pazopanib or placebo daily for up to 24 months [41]. There was a significant improvement in median PFS (17.9 months vs 12.3 months; HR 0.788, p = 0.002). However, 58 % of patients in the treatment arm required a dose reduction compared with 14 % of patients in the placebo arm and the most frequent grade 3 or 4 toxicity was hypertension (31 % vs 6 %). Nevertheless, this is the first study of a targeted agent administered as maintenance therapy only, showing a meaningful PFS benefit. The OS data remain immature. The results of the AGO-OVAR12, a phase III trial of nintedanib (BIBF1120), an inhibitor of VEGFR, FGFR and PDGFR in combination with carboplatin/paclitaxel followed by maintenance therapy in the first-line setting, showed a modest PFS benefit in the nintedanib arm (17.3 vs. 16.6 months, p = 0.024). The most significant PFS benefit with nintedanib was seen in the low risk group with low volume disease following surgery (27.1 vs. 20.8 months, p = 0.005) suggesting its role in maintenance treatment in such patients [42].
Cediranib, an oral pan-VEGFR kinase inhibitor has been evaluated in relapsed platinum sensitive disease in combination with chemotherapy followed by maintenance in the ICON-6 trial. Cediranib is the first TKI to demonstrate a statistically significant OS benefit (2.7 months) [43].
Most recently, the results of TRINOVA-1, a double blind placebo controlled phase III trial using Trebananib to target the angiopoietin axis as an alternative anti-angiogenic strategy, have been published. Trebananib is an Fc fusion protein that binds to the angiopoietins, Ang1 and Ang2 and prevents their interaction with the Tie2 receptor. Patients that had been treated with ≤ three previous regimens and had a platinum free interval of <12 months were enrolled to receive weekly paclitaxel with IV Trebananib or placebo. Median PFS was longer in the Trebananib group (7.2 vs. 5.4 months, p < 0.0001) although Trebananib was related to more adverse event-related treatment discontinuation [44].
7 PARP Inhibitors
Women with mutations in the BRCA genes (BRCA1 or BRCA2) have an increased risk of developing ovarian cancer due to defects in DNA repair pathways (called homologous recombination). Tumors in patients with a BRCA mutation are particularly susceptible to drugs called PARP inhibitors which generate specific DNA lesions that require functional BRCA1 and BRCA2 for DNA repair [45]. PARP inhibitors in clinical trials of ovarian cancer include olaparib, rucaparib and niraparib. Encouraging response rates were seen in patients with heavily pre-treated ovarian cancer that harbor a germline BRCA mutation (57.6 % RECIST and CA–125 criteria) [46, 47]. Based on the observation that up to 50 % of high-grade serous, sporadic ovarian cancers may have homologous recombination defects (including somatic BRCA mutations, BRCA methylation) which confer sensitivity to PARP inhibition, a randomized phase II trial of maintenance therapy with olaparib was performed [48]. In this study, olaparib extended PFS by almost 4 months (median 8.4 months vs. 4.8 months; HR 0.35, P < 0.001), in patients with platinum-sensitive, relapsed, high-grade serous ovarian cancer with or without BRCA1 or BRCA2 germline mutations. The improvement in PFS was greater in BRCA mutation carriers (median: 11.2 vs 4.1 months; HR, 0.17; P < 0.001) [49].
A phase III trial of maintenance olaparib or placebo in patients who have responded to first-line chemotherapy is currently recruiting.
8 Other Targeted Agents
Epidermal growth factor receptor (EGFR) inhibition has been investigated as maintenance therapy following first-line chemotherapy. Maintenance erlotinib, an EGFR inhibitor, did not improve PFS or OS in the EORTC55041/OV07 [50]. A randomized trial of oregovomab monotherapy (monoclonal antibody directed against CA-125) maintenance post first-line therapy also failed to show an improvement in clinical outcome [51].
Folate receptors are overexpressed in epithelial ovarian cancer but not in normal tissues therefore anti-folate receptor antibodies and folate chemotherapy-conjugates have been investigated as treatment strategies. Farletuzumab, a monoclonal antibody that binds to folate receptorα has been investigated in a double blind placebo-controlled phase III trial in combination with carboplatin and taxane chemotherapy in patients with first platinum sensitive relapse [52]. The results were disappointing as the study did not meet its primary end point of PFS. Vintafolide (EC145), is a folic acid-desacetylvinblastine conjugate that binds to the folate receptor. Etarfolatide is a folate receptor targeted imaging agent thought to be helpful in selecting patients likely to benefit from vintafolide. A phase II study investigated vintafolide in combination with pegylated liposomal doxorubicin (PLD) compared to PLD alone in platinum resistant patients and showed an improvement in PFS (5 vs. 2.7 months, p = 0.031) [53]. The encouraging results from this study prompted a randomized, double blind, phase III trial in platinum resistant ovarian cancer, the PROCEED study which was terminated early. The results are awaited.
Many other targeted agents are under investigation in recurrent ovarian cancer and include targeting the RAS/Raf/MEK pathway and PI3 kinase/AKT/mTOR pathway [32]. Successful strategies in recurrent ovarian cancer are likely to be developed in the first-line setting as has been the case with bevacizumab and olaparib.
9 Conclusion
Advanced ovarian cancer remains an incurable disease for the majority of patients. Improvements in first-line systemic therapies delivered in the neoadjuvant and/or adjuvant settings have the potential to prevent or at least delay disease relapse. Carboplatin in combination with paclitaxel remains the standard of care worldwide. Bevacizumab is approved in Europe as part of first line treatment and other angiogenesis inhibitors such as pazopanib may follow suit. PARP inhibitors appear promising and a trial as first-line maintenance is planned in BRCA mutation carriers. The successful integration of targeted therapy with chemotherapy will depend on the identification of the correct patient population, managing new toxicities, utilizing biomarkers to guide management and overcoming drug resistance.
Key Points
-
The majority of women present with advanced ovarian cancer and the OS is around 40 %
-
‘Adjuvant’ refers to chemotherapy given following surgery. ‘Neoadjuvant’ refers to upfront chemotherapy followed by interval debulking surgery (followed by chemotherapy)
-
The international standard of care for advanced ovarian cancer is either upfront or interval attempted optimal cytoreductive surgery and six cycles of carboplatin in combination with paclitaxel
-
Neoadjuvant chemotherapy followed by interval debulking surgery is a valid treatment option for patients with bulky stage IIIC or IV ovarian carcinoma
-
IP chemotherapy is a promising approach. It is not considered standard of care. Further clinical trials are ongoing.
-
Bevacizumab in combination with first line chemotherapy followed by maintenance therapy improves PFS
-
There is an OS benefit from bevacizumab when given in the first line setting to women at high risk of disease progression (>1 cm residual disease or stage IV)
-
Anti-angiogenic agents improve clinical outcome in the first line and recurrent (platinum sensitive and platinum resistant) setting
-
PARP inhibitors as maintenance therapy following chemotherapy for platinum-sensitive relapse significantly improve PFS. Phase III clinical trials are underway
-
BRCA mutation carriers derive the most benefit from PARP inhibitors
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917. PubMed PMID: 21351269. Epub 2011/02/26. eng.
CRUK. Cancer Research UK ovarian incidence statistics. 2010 http://info.cancerresearchuk.org/cancerstats/incidence/risk/. Accessed 2013.
du Bois A, Quinn M, Thigpen T, Vermorken J, Avall-Lundqvist E, Bookman M, et al. 2004 consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004). Ann Oncol. 2005;16 Suppl 8:viii 7–12. PubMed PMID: 16239238.
McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334(1):1–6. PubMed PMID: 7494563.
Piccart MJ, Bertelsen K, James K, Cassidy J, Mangioni C, Simonsen E, et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst. 2000;92(9):699–708. PubMed PMID: 10793106.
Muggia FM, Braly PS, Brady MF, Sutton G, Niemann TH, Lentz SL, et al. Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2000;18(1):106–15. PubMed PMID: 10623700.
Group. ICON. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360(9332):505–15. PubMed PMID: 12241653. Epub 2002/09/21. eng.
du Bois A, Luck HJ, Meier W, Adams HP, Mobus V, Costa S, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95(17):1320–9. PubMed PMID: 12953086.
Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21(17):3194–200. PubMed PMID: 12860964.
Neijt JP, Engelholm SA, Tuxen MK, Sorensen PG, Hansen M, Sessa C, et al. Exploratory phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancer. J Clin Oncol. 2000;18(17):3084–92. PubMed PMID: 10963636.
Vasey PA, Jayson GC, Gordon A, Gabra H, Coleman R, Atkinson R, et al. Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2004;96(22):1682–91. PubMed PMID: 15547181.
Kristensen GB, Vergote I, Stuart G, Del Campo JM, Kaern J, Lopez AB, et al. First-line treatment of ovarian cancer FIGO stages IIb-IV with paclitaxel/epirubicin/carboplatin versus paclitaxel/carboplatin. Int J Gynecol Cancer. 2003;13 Suppl 2:172–7. PubMed PMID: 14656276.
du Bois A, Weber B, Rochon J, Meier W, Goupil A, Olbricht S, et al. Addition of epirubicin as a third drug to carboplatin-paclitaxel in first-line treatment of advanced ovarian cancer: a prospectively randomized gynecologic cancer intergroup trial by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group and the Groupe d’Investigateurs Nationaux pour l’Etude des Cancers Ovariens. J Clin Oncol. 2006;24(7):1127–35. PubMed PMID: 16505432.
Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a phase III trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009;27(9):1419–25. PubMed PMID: 19224846. Pubmed Central PMCID: 2668552.
Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374(9698):1331–8. PubMed PMID: 19767092.
Katsumata N, Yasuda M, Isonishi S, Takahashi F, Michimae H, Kimura E, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol. 2013;14(10):1020–6. PubMed PMID: 23948349.
Pignata S, Scambia G, Katsaros D, Gallo C, Pujade-Lauraine E, De Placido S, et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2014;15(4):396–405. PubMed PMID: 24582486.
Markman M, Liu PY, Wilczynski S, Monk B, Copeland LJ, Alvarez RD, et al. Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: a Southwest Oncology Group and Gynecologic Oncology Group trial. J Clin Oncol. 2003;21(13):2460–5. PubMed PMID: 12829663.
Markman M, Liu PY, Moon J, Monk BJ, Copeland L, Wilczynski S, et al. Impact on survival of 12 versus 3 monthly cycles of paclitaxel (175 mg/m2) administered to patients with advanced ovarian cancer who attained a complete response to primary platinum-paclitaxel: follow-up of a Southwest Oncology Group and Gynecologic Oncology Group phase 3 trial. Gynecol Oncol. 2009;114(2):195–8. PubMed PMID: 19447479. Epub 2009/05/19. eng.
Pecorelli S, Favalli G, Gadducci A, Katsaros D, Panici PB, Carpi A, et al. Phase III trial of observation versus six courses of paclitaxel in patients with advanced epithelial ovarian cancer in complete response after six courses of paclitaxel/platinum-based chemotherapy: final results of the after-6 protocol 1. J Clin Oncol. 2009;27(28):4642–8. PubMed PMID: 19704064. Epub 2009/08/26. eng.
Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecol Oncol. 2006;103(3):1070–6. PubMed PMID: 16875720.
Kumar L, Hariprasas R, Kumar S. Neoadjuvant chemotherapy (NACT) followed by interval debulking surgery versus upfront surgery followed by chemotherapy (CT) in advanced epithelial ovarian carcinoma (EOC): a prospective randomized study – interim results. J Clin Oncol. 2007;25(18S):abstr 5531.
van der Burg ME, van Lent M, Buyse M, Kobierska A, Colombo N, Favalli G, et al. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. Gynecological Cancer Cooperative Group of the European Organization for research and treatment of cancer. N Engl J Med. 1995;332(10):629–34. PubMed PMID: 7845426. Epub 1995/03/09. eng.
Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–53. PubMed PMID: 20818904.
Kehoe S, Hook J, Nankivell M, Jayson G, Kitchener H, Lopes T, et al. Chemotherapy or upfront surgery for newly diagnosed advanced ovarian cancer: results from the MRC CHORUS trial. J Clin Oncol. 2013;31(Suppl):abstr 5500.
Mahner S, Eulenburg C, Staehle A, Wegscheider K, Reuss A, Pujade-Lauraine E, et al. Prognostic impact of the time interval between surgery and chemotherapy in advanced ovarian cancer: analysis of prospective randomised phase III trials. Eur J Cancer. 2013;49(1):142–9. PubMed PMID: 22921185.
Alberts DS, Liu PY, Hannigan EV, O’Toole R, Williams SD, Young JA, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335(26):1950–5. PubMed PMID: 8960474.
Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43. PubMed PMID: 16394300.
Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19(4):1001–7. PubMed PMID: 11181662.
Tewari D, Java J, Salani R, Armstrong D, Markman M, Herzog T, et al. Long-term survival advantage of intraperitoneal chemotherapy treatment in advanced ovarian cancer: an analysis of a Gynecologic Oncology Group ancillary data study. Gynecol Oncol. 2013;130:e4.
Lesnock JL, Darcy KM, Tian C, Deloia JA, Thrall MM, Zahn C, et al. BRCA1 expression and improved survival in ovarian cancer patients treated with intraperitoneal cisplatin and paclitaxel: a Gynecologic Oncology Group Study. Br J Cancer. 2013;108(6):1231–7. PubMed PMID: 23462720. Pubmed Central PMCID: 3619264. Epub 2013/03/07. eng.
Banerjee S, Kaye SB. New strategies in the treatment of ovarian cancer: current clinical perspectives and future potential. Clin Cancer Res. 2013;19(5):961–8. PubMed PMID: 23307860. Epub 2013/01/12. eng.
Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–83. PubMed PMID: 22204724. Epub 2011/12/30. eng.
Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365(26):2484–96. PubMed PMID: 22204725. Epub 2011/12/30. eng.
Oza A, Perren T, Swart A, Schroder W, Pujade-Lauraine E, Havsteen H, et al. ICON7: final overall survival results in the GCIG phase III randomized trial of bevacizumab in women with newly diagnosed ovarian cancer. Eur J Cancer. 2013;49 Suppl 3:abstr 6.
Stone RL, Sood AK, Coleman RL. Collateral damage: toxic effects of targeted antiangiogenic therapies in ovarian cancer. Lancet Oncol. 2010;11(5):465–75. PubMed PMID: 20226736. Pubmed Central PMCID: 3199129. Epub 2010/03/17. eng.
Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30(17):2039–45. PubMed PMID: 22529265.
Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302–8. PubMed PMID: 24637997.
Chan J, Brady M, Penson R, Monk B, Boente M, Walker J, et al., editors. Phase III trial of every-3-weeks paclitaxel versus dose dense weekly paclitaxel with carboplatin+/−bevacizumab in epithelial ovarian, peritoneal, fallopian tube cancer: GOG 262 (NCT0116712). Oral presentation at the 18th International Meeting of the European Society of Gynecological Oncology, Liverpool, UK; 2013.
Bottsford-Miller JN, Coleman RL, Sood AK. Resistance and escape from antiangiogenesis therapy: clinical implications and future strategies. J Clin Oncol. 2012;30(32):4026–34. PubMed PMID: 23008289. Pubmed Central PMCID: 3488272. Epub 2012/09/26. eng.
Andreas Du Bois AF, Kim JW, Rau J, Del Campo JM, Friedlander M, Pignata S, Fujiwara K, Vergote I, Colombo C, Mirza MR, Monk BJ, Wimberger P, Ray-Coquard I, Zang R, Diaz-Padilla I, Baumann KH, Kim JH, Harter P. Randomized, double-blind, phase III trial of pazopanib versus placebo in women who have not progressed after first-line chemotherapy for advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (AEOC): results of an international intergroup trial (AGO-OVAR16). J Clin Oncol. 2013;31(Suppl):abstr LBA5503.
Du Bois A, Kristensen G, Ray-Coquard I, Reuss A, Pignata S, Colombo N, et al., editors. Ago-ovar 12: a randomized placebo-controlled gcig/engot-intergroup phase iii trial of standard frontline chemotherapy plus/-nintedanib for advanced ovarian cancer. Int J Gynecol Cancer; 2013: Philadelphia: Lippincott Williams & Wilkins. 23(8 Suppl 1)7–8.
Ledermann J, Perren T, Raja F, et al. Randomised double-blind phase III trial of cediranib (AZD 2171) in relapsed platinum sensitive ovarian cancer: results of the ICON6 trial. European Society for Medical Oncology Congress 2013; September 27–October 1, 2013; Amsterdam, The Netherlands. Abstract 10
Monk BJ, Poveda A, Vergote I, Raspagliesi F, Fujiwara K, Bae DS, et al. Anti-angiopoietin therapy with trebananib for recurrent ovarian cancer (TRINOVA-1): a randomised, multicentre, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15(8):799–808. PubMed PMID: 24950985.
Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol. 2008;26(22):3785–90. PubMed PMID: 18591545. Epub 2008/07/02. eng.
Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–34. PubMed PMID: 19553641. Epub 2009/06/26. eng.
Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, Gourley C, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28(15):2512–9. PubMed PMID: 20406929. Epub 2010/04/22. eng.
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–92. PubMed PMID: 22452356. Epub 2012/03/29. eng.
Jonathan A, Ledermann PH, Gourley C, Friedlander M, Vergote I, Rustin GJS, Scott GL, Meier W, Shapira-Frommer R, Safra T, Matei T, Fielding A, Macpherson E, Dougherty B, Jürgensmeier JM, Orr M, Matulonis U. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer (SOC) and a BRCA mutation (BRCAm). J Clin Oncol. 2013;31(Suppl):abstr 5505.
Vergote IB, Joly F, Katsaros D, Corneel Coens AR, Marcia H, Steer CB, Nicoletta C, Anne L, Antonio C, Edgar P, John G, Martin B, Isabelle Laure R-C, Annamaria F, Laure F, Nicholas R, Herve C, Jimeno A, Pujade-Lauraine E. Randomized phase III study of erlotinib versus observation in patients with no evidence of disease progression after first-line platin-based chemotherapy for ovarian carcinoma: a GCIG and EORTC-GCG study. J Clin Oncol. 2012;30(Suppl):abstr LBA5000.
Berek J, Taylor P, McGuire W, Smith LM, Schultes B, Nicodemus CF. Oregovomab maintenance monoimmunotherapy does not improve outcomes in advanced ovarian cancer. J Clin Oncol. 2009;27(3):418–25. PubMed PMID: 19075271. Epub 2008/12/17. eng.
Vergote J, Armstrong D, Scambia G, Fujiwara K, Gorbunova V, Schweizer C. Phase III double-blind, placebo-controlled study of weekly farletuzumab with carboplatin/taxane in subjects with platinum-sensitive ovarian cancer in first relapse. Int J Gynecol Cancer. 2013;23(8 Suppl 1):11.
Naumann RW, Coleman RL, Burger RA, Sausville EA, Kutarska E, Ghamande SA, et al. PRECEDENT: a randomized phase II trial comparing vintafolide (EC145) and pegylated liposomal doxorubicin (PLD) in combination versus PLD alone in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2013;31(35):4400–6. PubMed PMID: 24127448.
Acknowledgments
We acknowledge support from the National Institute of Health Research (NIHR) Biomedical Research Centre.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag London
About this chapter
Cite this chapter
Khakoo, S.S., Banerjee, S. (2015). Neoadjuvant and Adjuvant Chemotherapy for Advanced Ovarian Cancer, Including Biological Agents. In: Patel, H., Mould, T., Joseph, J., Delaney, C. (eds) Pelvic Cancer Surgery. Springer, London. https://doi.org/10.1007/978-1-4471-4258-4_41
Download citation
DOI: https://doi.org/10.1007/978-1-4471-4258-4_41
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-4257-7
Online ISBN: 978-1-4471-4258-4
eBook Packages: MedicineMedicine (R0)