Abstract
Androgen deprivation therapy (ADT) remains the primary treatment modality for patients with metastatic prostate cancer (PCa) but is uniformly marked by progression to castration-resistant prostate cancer (CRPC) over a period of about 18 months, with an ensuing median survival of 1–2 years. Continued activation of androgen receptor (AR) signaling despite suppression of circulating testosterone (T) appears to remain a critical driving force in tumor progression [1]. Accumulating data emphasize that “androgen-independent” or “hormone-refractory” tumors retain a clinically relevant degree of hormone sensitivity and highlight the continued importance of AR axis activity in advanced tumors [2]. Accordingly, therapeutic strategies designed to more effectively ablate androgen signaling are required to improve clinical efficacy and prevent disease progression. Herein, we review AR-dependent mechanisms underlying PCa progression following standard androgen deprivation strategies (summarized in Table 74.1) and discuss the rationale and status of new hormone-based therapies targeting the AR axis, which are currently in clinical and preclinical development (summarized in Table 74.2).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Androgen Receptor
- Androgen Deprivation Therapy
- Androgen Receptor Expression
- Androgen Receptor Signaling
- Androgen Receptor Antagonist
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Androgen deprivation therapy (ADT) remains the primary treatment modality for patients with metastatic prostate cancer (PCa) but is uniformly marked by progression to castration-resistant prostate cancer (CRPC) over a period of about 18 months, with an ensuing median survival of 1–2 years. Continued activation of androgen receptor (AR) signaling despite suppression of circulating testosterone (T) appears to remain a critical driving force in tumor progression [1]. Accumulating data emphasize that “androgen-independent” or “hormone-refractory” tumors retain a clinically relevant degree of hormone sensitivity and highlight the continued importance of AR axis activity in advanced tumors [2]. Accordingly, therapeutic strategies designed to more effectively ablate androgen signaling are required to improve clinical efficacy and prevent disease progression. Herein, we review AR-dependent mechanisms underlying PCa progression following standard androgen deprivation strategies (summarized in Table 74.1) and discuss the rationale and status of new hormone-based therapies targeting the AR axis, which are currently in clinical and preclinical development (summarized in Table 74.2).
Significance of Intratumoral Androgens in CRPC
Ample evidence demonstrates that castration does not eliminate androgens from the prostate tumor microenvironment, that residual androgen levels are well within the range capable of activating the AR and AR-mediated gene expression [9–12], and that intratumoral androgens are clinically relevant in driving growth of castration-resistant tumors.
Persistence of Intratumoral Androgens Despite Castration
The efficacy of ADT is routinely based on achieving castrate levels of serum T, defined as <20 ng/dl. However, prostatic tissue androgen levels in the setting of benign prostatic hyperplasia (BPH), locally recurrent PCa, or metastatic CRPC have consistently demonstrated that castration does not eliminate androgens from the prostate tumor microenvironment. Geller et al. examined prostatic DHT levels by radioimmunoassay (RIA) and demonstrate that castration by orchiectomy (or megace plus DES) reduced prostatic DHT levels by 75–80 % to 1 ng/g in some but not all patients, epithelial and stromal cell protein synthesis were strongly correlated with tissue DHT levels, and prostatic DHT levels were further reduced when castration was combined with adrenal androgen blockade by ketoconazole [9, 13–17]. These and other studies led early investigators to conclude that even low amounts of residual DHT may be sufficient to stimulate tumor growth (or at least maintain cell survival) and that the goal of therapy should be to decrease prostatic DHT to as low as possible.
Incomplete suppression of tissue androgens by castration has been confirmed in numerous studies of short- and long-term castration therapy. Treatment of BPH patients for 3 months with an LHRH agonist decreased intraprostatic T levels by 75 % to about 0.1 ng/g and DHT levels by 90 % to 0.48 ng/g [18]. A similar 70–80 % decrease in prostate tissue androgens was reported after 1 month of ADT in normal healthy men [12]. In prostate tumors, 6 months of neoadjuvant ADT with castration and flutamide reduced prostatic DHT levels by 75 % to about 1.35 ng/g [11]. Moreover, tumor differentiation based on Gleason grading was correlated with change in tissue DHT, with an 85 % decrease measured in Gleason 6 cancers but only a 60 % decrement in Gleason 7–10 tumors [19]. This finding indicates that tumor type-specific changes in androgen metabolism (synthesis or utilization) may impact responses to systemic T suppression.
In advanced PCa, Mohler et al. found that prostatic T levels in castrate patients with locally recurrent tumors were equivalent to those of BPH patients and that intratumoral DHT levels were only reduced by 80 % to about 0.4 ng/g [10]. Further, T levels in metastatic tumors obtained via rapid autopsy from men with CRPC were found to be approximately threefold higher than levels within primary prostate tumors from untreated (eugonadal) patients [20]. Adrenal androgens have also been detected at significant levels in prostate tissue of castrate men. Prostatic levels of dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEA-S), and androstenedione (AED) were decreased by about 50 % in castrate patients with recurrent PCa and far exceeded values of T and DHT in recurrent tumor tissue [10]. A separate study found no decrease in prostatic levels of 5-androstenediol (a primary metabolite of DHEA and a direct precursor of T, Fig. 74.1) after castration [22], which is of particular significance as this androgen has been shown to bind wild-type AR without being inhibited by flutamide or bicalutamide [23].
The classical and backdoor pathways of androgen biosynthesis. In the classical pathway (solid gray arrow), C21 precursors (pregnenolone and progesterone) are converted to the C19 adrenal androgens DHEA and androstenedione (AED) by the sequential hydroxylase and lyase activity of CYP17A1. Circulating adrenal androgens (including the sulfated form of DHEA, DHEA-S) enter the prostate and can be converted to testosterone by a series of reactions involving the activity of HSD3B, HSD17B, and AKR1C enzymes. Testosterone is then converted to the potent androgen DHT by the activity of SRD5A. In the backdoor pathway to DHT synthesis (short gray arrows), C21 precursors are first acted upon by SRD5A and the reductive 3α−HSD activity of the AKR1C family member AKR1C2, followed by conversion to C19 androgens via the lyase activity of CYP17A1. DHT is subsequently generated by the action of HSD17B3 and an oxidative 3α−HSD enzyme, including HSD17B6 (also called RL-HSD) or HSD17B10 (as well as RODH4, RDH5, and NT 3α−HSD, not shown) (Adapted from Mostaghel and Nelson [21], with permission)
Activity of Intratumoral Androgens in CRPC
Data derived from in vitro and in vivo studies have determined that tissue DHT levels of 0.5–1.0 ng/g, the range observed in prostatic tissue of castrated patients, are sufficient to activate the AR, stimulate expression of AR-regulated genes, and promote androgen-mediated tumor growth [10, 24–27]. Activity of intratumoral androgens in CRPC tumors is generally evidenced by reconstitution of tissue and serum PSA levels. Maintenance of PSA expression in neoplastic prostate epithelial cells has also been shown at 3 or 9 months of castration therapy [28]. The importance of intratumoral androgens in mediating CRPC tumor growth is confirmed by clinical responses produced by therapeutics that target residual androgen pathway activity. These include historical responses described in response to adrenalectomy and/or hypophysectomy [29, 30], the limited but consistent ∼ 5 % overall survival benefit seen in meta-analyses of combined androgen blockade (CAB) trials [31–33], the observation that nearly 30 % of recurrent prostate tumors demonstrate at least transient clinical responses to secondary or tertiary hormonal manipulation [34], and most recently, the striking clinical response observed with the novel AR axis inhibitors abiraterone and MDV3100 (discussed below) [3, 5].
Ligand-Dependent Mechanisms Mediating AR Transactivation in CRPC
Resistance to AR pathway inhibition may include ligand and/or AR-dependent and independent mechanisms (Table 74.1). Castration-resistant tumors are characterized by elevated tumor androgens and by steroid enzyme alterations, which may potentiate de novo androgen synthesis or utilization of circulating adrenal androgens [10, 20, 35, 36]. The dependence of CRPC on intratumoral androgen metabolism has been modeled in vitro and in vivo [37–39]. These observations suggest that tissue-based alterations in steroid metabolism contribute to development of CRPC and underscore these metabolic pathways as critical targets of therapy.
In the classical pathway of androgen synthesis, C21 steroids generated from cholesterol such as pregnenolone and progesterone are first converted to C19 steroids DHEA and AED via sequential hydroxylase and lyase activity of CYP17A1 (Fig. 74.1). These adrenal steroids are then acted on by HSD3B, HSD17B3, and SRD5A to generate T and then DHT. Recent data also suggest steroidogenesis in some tumors may proceed from adrenal androgen intermediates to DHT via androstenedione rather than T [40]. In steroidogenic tissues in which both CYP17A1 and SRD5A are expressed, an alternate route to DHT is possible wherein C21 steroids are first acted upon by HSD3B and SRD5A, followed by CYP17A1 and HSD17B3 [41]. This “backdoor pathway,” wherein steroid flux to DHT bypasses conventional intermediates of AED and T, has also been postulated to be operative in prostate tumors (Fig. 74.1) [39].
Steroidogenic Enzymes in CRPC
Enhanced expression of transcripts encoding key enzymes in the cholesterol biosynthetic pathway has been demonstrated in CRPC tumors, including expression of squalene epoxidase (SQLE), the rate-limiting enzyme in cholesterol synthesis [36]. Altered expression of genes encoding many steroidogenic enzymes including upregulation of FASN, CYP17A1, HSD3B1, HSD17B3, CYP19A1, and UBT2B17 has been reported in CRPC metastases, suggesting that castration-resistant tumors have the ability to utilize progesterone as androgenic precursors [20, 35]. Differential expression of several 17beta-hydroxysteroid dehydrogenase family members (HSD17B) occurs in PCa, suggesting a shift in tumoral androgen metabolism toward formation of T and DHT, with increased expression of reductive enzymes catalyzing conversion to active androgens (HSD17B3 and HSD17B5—also known as aldo-keto reductase AKR1C3) and decreased expression of oxidative enzymes catalyzing the reverse reaction (HSD17B2) (reviewed in [21]). A selective loss of AKR1C2, which mediates catabolism of DHT to androstanediol (3α-diol), has been observed in primary prostate tumors, accompanied by a reduced capacity to catabolize DHT and an increased level of tumoral DHT. PCa cell lines and human prostate tissue have also been demonstrated to express oxidative enzymes capable of mediating back conversion of 3α-diol to DHT. Enzymes with this capacity include RODH4, RDH5, DHRS9, HSD17B6 (RODH-like 3αHSD or RL-HSD), and HSD17B10 [42, 43].
Experimental Models of De Novo Steroidogenesis
Studies of in vitro and in vivo models of CRPC support the concept of intratumoral androgen synthesis. The androgen-independent LNCaP derivative (C81) demonstrated higher expression of steroid metabolic machinery, including steroidogenic acute regulatory (StAR) protein, cytochrome P450 cholesterol side chain cleavage (P450scc), and CYP17A1 compared to its androgen-dependent counterpart (C33) and was shown to directly convert cholesterol into T [44]. Increases in expression of genes responsible for accumulation of free cholesterol and cholesterol synthesis, low-density lipoprotein receptor (LDLR), scavenger receptor (SR)B1, ATP-binding cassette ((ABC)A1), StAR, acyl-coenzyme A cholesterol acyltransferase (ACAT) 1 and 2, 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA), and side-chain cleavage enzyme (CYP11A1) were also demonstrated in a xenograft LNCaP model [45–47]. Also detected were increases in transcripts encoding CYP17A1, AKR1C1, AKR1C2, AKR1C3, HSD17B2, and SRD5A1 [45]. Conversion of acetic acid to 5α-DHT was observed in these xenografts, and tumors were shown to metabolize progesterone to six different intermediates upstream of 5α-DHT, via both classic and “backdoor” pathways [45]. Collectively, these data suggest that PCa cells may be capable of de novo steroidogenesis from cholesterol.
Stromal-Epithelial Interactions and Intratumoral Androgen Biosynthesis
Androgen metabolism in PCa cells may also be facilitated by bone marrow and PCa-associated stromal cells. Compared to monocultures of LAPC-4 PCa cells stimulated with DHEA, coculture of LAPC-4 cells with PCa-associated stromal cells resulted in marked stimulation of PSA expression. This effect was likely mediated by stromal cell generation of T from DHEA, as T was detected in a time- and dose-dependent manner in PCa stromal cell monocultures treated with DHEA [48]. Similarly, the impact of DHEA on PSA promoter activity in LNCaP cells was markedly enhanced in the presence of PCa-derived stromal cells [38]. Knockdown of AR in LNCaP cells abrogated this effect, while coculture with PCa stromal cells transfected with AR shRNA did not, suggesting paracrine factors secreted by stromal cells act on the LNCaP AR. Furthermore, following DHEA treatment, T and DHT concentrations were ∼ 5-fold higher in PCa stromal/LNCaP coculture versus LNCaP monoculture. Interestingly, PSA expression was also induced by normal prostate stroma, bone marrow stroma, lung stroma, and bone-derived stromal cells, although strongest effects were noted with PCa-derived stromal cells. Resting mesenchymal cells in a separate study of bone marrow stromal cell were also found to express HSD3B and SRD5A protein, while incubation with DHEA additionally resulted in expression of HSD17B5 [49]. These findings indicate that maintenance of intratumoral androgen levels in CRPC tumors may be facilitated by metabolism of androgen precursors in cancer-associated stromal cells.
Alterations in Cellular Uptake of Steroid Hormones
Despite the generally accepted view that steroid hormones transit from circulation to intracellular compartments via free diffusion across lipid membranes, recent studies suggest a potential role for steroid transport proteins in actively mediating uptake of androgen into PCa cells. The organic anion-transporting polypeptides (OATP; encoded by the SLCO gene family) are variably expressed throughout liver, kidney, and steroidogenic tissues, and several SLCO genes are overexpressed in CRPC metastases versus untreated PCa [50]. These transporters mediate import of substrates such as bile acids, xenobiotics, and steroidogenic precursors, and single-nucleotide polymorphisms (SNPs) in SLCO genes can markedly alter substrate-specific transport efficiency [51]. Notably, OATP1B3 actively transports T in transiently transfected COS-7 cells [52]. Furthermore, a nonsynonymous SNP of OATP1B3 displayed a twofold decrease in T uptake, which correlated with a longer median survival, improved 10-year survival, and a longer time to androgen independence in two small studies of men with CRPC [53]. In a parallel study, OATP2B1 was shown to mediate uptake of DHEA-S in transiently transfected LNCaP cells, and a nonsynonymous SNP which displayed impaired DHEA-S import was correlated with a longer time to progression in men with CRPC receiving ADT [54]. Together, these studies imply that active hormone uptake may contribute to elevated androgen levels observed in CRPC tumors and progression of advanced disease.
AR-Based Alterations Mediating AR Transactivation in CRPC
Numerous molecular features have been shown to contribute to AR signaling in context of low or absent androgen levels in CRPC (Table 74.1). Collectively, characterization of these molecular events indicates that AR activation may occur via both ligand-dependent and independent mechanisms. These include changes in expression and structure of the AR itself, as well as alterations in associated cofactors which regulate AR transactivation. As a consequence, AR ligand specificity can be broadened, and efficiency of AR activation at low or absent ligand levels can be enhanced.
Overexpression and Genomic Amplification of Wild-Type AR
AR overexpression is a well-recognized feature of CRPC and believed to be a critical driver of CRPC progression. In preclinical PCa models, Chen et al. identified AR as the most common gene upregulated following androgen deprivation. AR overexpression supported in vitro proliferation of transfected cells at fivefold lower androgen levels than untransfected cells and was both necessary and sufficient to induce tumor formation when placed in castrate SCID mice compared to untransfected controls [1]. Importantly, AR overexpression not only mediated sensitivity to low ligand concentrations but converted antiandrogens such as bicalutamide and flutamide from antagonists to agonists via changes in composition of coactivators recruited to the AR promoter. While rarely identified in primary prostate tumors, AR gene amplification leading to AR overexpression is present in approximately 30 % of clinical CRPC specimens [55]. Additional mechanisms that mediate increased AR transcription and/or AR stability are likely operative, as increased AR expression is frequently observed in the absence of AR amplification. Recent data suggest that dimerization of AR with ligand-independent AR splice variants (discussed below) may increase AR levels by preventing AR protein degradation [56].
AR Mutations
Mutations in the AR are found in approximately 20–40 % of CRPC tumors, though are rare in hormone treatment-naïve PCa [57]. Multiple mutations are frequently isolated from the same tumor, demonstrating the high degree of heterogeneity present in PCa [58]. Several hundred AR mutations have been described following ADT, but >90 % are nonsense or missense in nature and result in a nonfunctional AR. A number of clinically important AR mutations occur in the ligand-binding domain (LDB), and it is notable that none have been identified in this region in the absence of ADT. The most common mutation occurs at or around amino acid 877. The Thr877Ala mutation was originally described in the LNCaP human PCa cell line. This mutation permits binding of an expanded repertoire of steroid ligands, such as progestins and estradiol, as well as the antiandrogen flutamide, converting antiandrogen of the latter to an agonist [59]. Gain of function mutations also occur in both N- and C-termini, which can alter N/C interactions involved in cofactor recruitment. Although AR mutations are associated with castration resistance, none of them occur with a frequency that would suggest they are responsible for development of castration resistance. However, potential agonist activity of steroidal antiandrogens in the setting of AR amplification and/or AR mutation has spurred development of AR antagonists without agonist properties.
Alterations in AR Coregulators
Several hundred AR coregulators have been described which influence AR activation via multiple mechanisms, including recruitment of transcriptional machinery, modulation of chromatin-remodeling enzymes, and initiation of RNA polymerase activity [60]. A number of AR coactivators are increased in CRPC including TIF-1, MAGE-II, SRB-1, NFKB, and ARA70, while corepressors such as SMRT are downregulated. Whether alterations in the balance between AR and its coregulators can activate AR in the absence of ligand in CRPC is not clear. However, altered coregulator expression may sensitize the AR for activation under low-androgen conditions, as well as converting AR antagonists into agonists via corepressor downregulation and/or corepressor dismissal from the AR complex [61]. Inhibition of AR coregulators has been proposed as a target for suppressing AR activity in CRPC [62].
Activation of AR by Peptide Ligands
Several studies have determined that peptide growth factors can transactivate AR in absence of ligand via cross talk through well-characterized signal transduction pathways. These include insulin-like growth factors (IGF-I and IGF-II), epidermal growth factor (EGF), keratinocyte growth factor (KGF), and cytokines such as interleukin 6 (IL-6) (reviewed in [63]). The impact of these factors in vivo in terms of maintaining AR signaling is not known, although inhibition of IGF-IR by the IGF-IR inhibitory antibody A12 affects AR translocation and transactivation in preclinical models [64]. Probably, the most convincing of these potential AR peptide ligands is IL-6, which binds the LBD and transactivates AR as determined by ARE-luciferase reporter constructs or by increased expression of androgen-regulated genes. Additionally, induced nuclear translocation of AR by IL-6 has been described. However, clinical relevance of IL-6 in PCa is not clear. While IL-6 is significantly elevated in serum and bone metastases of patients with advanced PCa, a recent clinical trial of men with CRPC treated with an IL-6 inhibitory antibody showed no evidence of benefit [65].
Constitutively Active AR Splice Variants
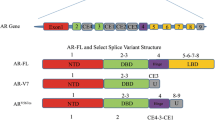
Differential splicing of pre-mRNA is a frequent mechanism for generation of protein variants with oncogenic activity [66], and the expression of posttranscriptional AR splice variants with capacity for constitutive AR transactivation has recently been recognized as a potential mechanism of CRPC progression [56, 67–72]. Approximately 25 variants have been identified in human prostate tissues and cell lines [56, 67, 69, 71–73]. Some of these variants have no predicted function, while others appear to enhance effect of the full-length wild-type receptor. Most significant are those in which the carboxy-terminal AR LBD is lost, resulting in ligand-independent constitutive AR activation. Among the variants identified to date, ARV7 (which encodes the same protein as AR3) and ARv567es appear to be the most clinically relevant, with detection of ARV7 in radical prostatectomy (RP) tissues associated with an increased risk of biochemical relapse [67, 69] and ARV7 or ARv567es in CRPC metastases associated with shorter survival [68]. Notably, markedly higher expression of ARV7 and ARv567es has been observed in CRPC versus primary PCa, with ARv567es showing nearly exclusive expression in CRPC [56, 68, 69].
Mechanisms responsible for generation of AR splice rearrangements are thought to reflect a cellular response to ligand deprivation, as variants most prevalent in human CRPC tissues are those most consistently found following androgen deprivation in vitro. The emergence of specific AR isoforms including ARV7/AR3 and ARv567es in vitro and in vivo following suppression of intratumoral androgens [56, 73] suggests growth of these tumors is dependent on AR variants in low-androgen environments. Moreover, truncated AR variants can potentiate activity of full-length AR under low-ligand conditions, essentially functioning as AR ligands themselves. Sun et al. have demonstrated that ARv567es can form a heterodimer with full-length AR, leading to efficient nuclear translocation and AR transactivation in the absence of ligand. Recently, Dehm et al. have demonstrated that high-level expression of AR variants may be associated with intragenic rearrangement of alternative AR exons, although the clinical prevalence of this mechanism remains to be established [74].
Whether truncated AR variants have a pathogenic role or simply recapitulate wild-type AR transactivation is unknown but has significant implications for understanding CRPC tumor behavior. Several studies have shown that expression of ARV7 or ARv567es portends more clinically aggressive disease [67–69]. Moreover, emerging data demonstrate that AR splice variants transactivate an overlapping but not identical repertoire of gene targets compared to wild-type AR [56, 67, 73]. Differences in transcriptional output may reflect structural changes resulting in alterations in coregulator recruitment, as in silico analyses suggest loss of the LBD may affect interactions with NCOA1, NCOA2, TIP60, and ARA54 [60, 75]. Notably, expression of ARv567es and high-level expression of ARV7 in CRPC bone metastases were associated with shorter cancer-specific survival and with gene expression changes indicative of disturbed cell cycle regulation and increased invasiveness (e.g., CDK1, CYCLINA2, CDC20, C-MYC, HSP27, and UBE2C) [68].
From a therapeutic standpoint, tumors expressing AR splice variants may present a significant clinical challenge depending on their sensitivity to AR antagonists that are designed to target the AR LBD (e.g., bicalutamide, TOK-001, or MDV3100). Emerging data suggest that truncated AR variants may function in part via binding and promoting nuclear localization of full-length AR, and thus, the presence of carboxy-terminal AR variants does not necessarily preclude a response to ligand-binding inhibitors such as MDV [73]. While expression of LBD-deficient AR variants alone results in AR transcriptional activity, expression of AR variants has generally been reported to occur in conjunction with expression of full-length AR. Watson et al. recently demonstrated that in the presence of both truncated and full-length AR variants, targeting full-length AR with the antiandrogen MDV3100 suppressed AR activity and cell growth as efficiently as when only full-length AR was present, suggesting that activity of certain AR variants is mediated through full-length AR [73]. Additional studies are required to determine if all AR variants require full-length AR to activate the AR transcriptional program and maintain cell survival and growth, as unpublished observations suggest coexpression of ARV7 or ARv567es can mediate resistance to LBD-directed AR inhibition (Stephen Plymate, personal communication 2012).
Secondary Hormonal Manipulation After Failure of First-Line ADT
The contribution of ongoing androgen pathway activity in CRPC progression is supported by response rates ranging from 20 to 60 % in studies of secondary hormonal manipulation [76]. Importantly, serum T levels <50 ng/dl should be documented prior to making a designation of CRPC. Breakthrough T levels >50 ng/dl were documented on one or more occasions in nearly 25 % of patients in a study of LHRH agonist therapy administered as a 3-monthly depot over a 6–48 month period of time [77]. Interestingly, LHRH antagonists have been reported to induce durable androgen suppression in patients in whom LHRH agonists were not effective in maintaining castrate (<50 ng/dl) serum T levels and may be of value in this setting [78].
Once CRPC has been documented, standard strategies targeting residual AR pathway activity include antiandrogen withdrawal (AAW), alternative antiandrogens such as flutamide or nilutamide (after progression on bicalutamide), high-dose bicalutamide, addition of 5-α reductase inhibitors such as finasteride or dutasteride, the nonspecific CYP17A1 inhibitor and adrenolytic agent ketoconazole, estrogenic agents such as DES or transdermal estradiol, and palliative glucocorticoids. The choice and sequence of agents is largely physician dependent and often driven by side effect profiles, as numerous studies of secondary ADT have demonstrated prolongations in PFS, but none have reported improvement in overall or cancer-specific survival, reviewed in [76]. In general, PSA responses >50 % have been observed in 20–50 % of patients undergoing secondary hormonal maneuvers, with duration of median response ranging from 2 to 8 months.
Recent observations suggest that androgen levels may be useful in stratifying patients likely to sustain durable benefit from second-line therapies. In a randomized study of AAW alone versus AAW plus ketoconazole, PSA responses were observed in 10 % of men on AAW versus 32 % treated with the combination. Importantly, men with a >50 % PSA response while on ketoconazole experienced significantly longer survival (41 vs. 13 months, p > 0.001), and patients with higher baseline levels of androstenediol were most likely to demonstrate responses to ketoconazole [79]. A small study of second-line therapy using flutamide (after progression on bicalutamide) also reported an association between PSA response and baseline androstenediol levels [80]. In a separate study of either flutamide or bicalutamide for second-line therapy, men with T levels higher than 5 ng/dl demonstrated significantly higher response rates (77 vs. 37.5 %, p = 0.04); serum T level <5 ng/dl prior to initiation of second-line therapy was reported as an independent predictor of PSA-free progression at 1 year (0 vs. 53 % in men with pretreatment T > 5 ng/dl, p = 0.002) [81].
New Agents Targeting Intratumoral Androgens
Potent therapies targeting ligand and/or AR-driven activation of the AR axis are currently in clinical development (Table 74.2). Alterations in a number of critical enzymes responsible for DHT synthesis and catabolism provide mechanistic support for the role of intracrine androgen production in maintaining the tumor androgen microenvironment in CRPC and underscore these metabolic pathways as critical therapeutic targets.
Inhibitors of CYP17A1
CYP17A1 is a single enzyme that catalyzes sequential steps in the conversion of C21 progesterone precursors to C19 adrenal androgens, DHEA and AED. Ketoconazole (a weak inhibitor of CYP11A and CYP17A1) has been utilized for suppression of residual adrenal androgens but has limited efficacy and significant treatment-related side effects. This has prompted development of a number of potent CYP17A1 inhibitors, including agents exhibiting both CYP17A1 inhibition and antiandrogen activity [82].
Abiraterone is a pregnenolone derivative that acts as a selective irreversible inhibitor of both the 17 alpha-hydroxylase and C17,20-lyase activity of CYP17A1. Abiraterone suppressed T levels by >50 % in eugonadal men, accompanied by a corresponding rise in luteinizing hormone (LH) levels, while in castrate men, abiraterone further suppressed serum T levels by >75 % [83].
Phase I/II studies in chemotherapy-naïve metastatic CRPC demonstrated durable PSA declines >50 % in approximately two-thirds of patients, with partial radiographic responses (by RECIST criteria) in 37.5 % and a median time to progression of 32 weeks [84, 85]. PSA responses >50 % were observed in 47 % of patients with prior ketoconazole treatment versus 64 % of patients without [86]. DHEA levels were suppressed by approximately 75 %, and DHEA-S, AED, and T levels became essentially undetectable [85, 86]. As observed in studies of ketoconazole, patients achieving >50 % PSA declines had higher baseline levels of DHEA-S, DHEA, and AED, and, in contrast to progression on ketoconazole, increases in T, AED, or DHEA levels were not observed on progression with abiraterone [79, 84].
In a phase II study of postdocetaxel-treated CRPC patients, PSA declines >50 % were observed in 51 % of patients, with a median time to progression of 24 weeks [87]. In a postchemotherapy study in which 41 % of patients had received prior ketoconazole, abiraterone (in combination with prednisone, 5 mg twice daily) achieved PSA declines >50 % in 45 % of ketoconazole-naïve patients and 26 % of ketoconazole-treated patients, with a median time to progression of 28 and 14 weeks, respectively [88].
Phase III studies of abiraterone in combination with prednisone versus prednisone alone are ongoing in the chemotherapy-naïve (COU-AA-302) and postdocetaxel setting (COU-AA-301). Notably, the COU-001 study was unblinded at the interim analysis as improvement in OS exceeded the preplanned criteria for study termination. Among 1195 patients randomized 2:1 to abiraterone versus placebo, OS was 14.8 in the abiraterone-treated patients versus 10.9 months in the placebo-treated group (HR = 0.646, p < 0.0001), representing a 35 % reduction in risk of death with abiraterone [3]. Interestingly, phase II data suggest that at 3 months after starting abiraterone, over 30 % of patients may demonstrate an increase in bone scan intensity followed by improvement or stability of findings at 6 months [89]. The positive results in the phase III setting strongly suggest that bone scan findings early after starting treatment should not be used as criteria for early discontinuation of therapy.
Side effects with abiraterone have been related to expected increases in C21 steroids upstream of CYP17A1 (including a 10-fold increase in deoxycorticosterone and 40-fold increase in corticosterone). These were primarily manifested as symptoms of mineralocorticoid excess (including grade 1 and 2 hypertension, hypokalemia, edema, and fatigue) and responded to treatment with eplerenone or low-dose glucocorticoids (spironolactone was avoided due to potential AR agonist activity). Decreases in serum cortisol (twofold) with concomitant elevations in ACTH (fivefold) were also observed. Interestingly, 4 of 15 patients progressing on abiraterone responded to addition of dexamethasone, which decreased ACTH and deoxycorticosterone levels to below baseline [85], consistent with reports that steroids upstream of CYP17, including progestins and corticosteroids, can stimulate AR. At present, abiraterone in combination with low-dose prednisone or dexamethasone is recommended to prevent treatment-related rise in ACTH and attendant side effects.
TAK-700 is a nonsteroidal CYP17 inhibitor designed to have selectivity against C17,20-lyase over 17-alpha hydroxylase activity of CYP17. In a phase I/II dose escalation study, 11 of 20 patients with metastatic CRPC receiving >300 mg twice daily showed PSA declines >50 %, and 4 had reductions >90 %. At 4 weeks, median T and DHEA-S levels decreased from 4.9 to 0.6 ng/dl and 53.8 ug/dl to undetectable, respectively. Adverse effects included fatigue, nausea, constipation, and anorexia. Consistent with the agent’s selective inhibition of 17,20-lyase over 17 alpha-hydroxylase activity, a significant incidence of hypertension was not observed [4]. The phase II portion is ongoing, including an arm evaluating concomitant use of prednisone.
VN/124-1, a heteroaryl steroid, is a potent dual CYP17 and AR inhibitor currently being evaluated in a phase I/II study under the trade name TOK-001. VN/124-1 exhibits three- and four-fold stronger inhibition of CYP17 activity than abiraterone and ketoconazole, respectively, and is also a potent inhibitor of the AR, both as a competitive antagonist (with a binding affinity comparable to bicalutamide) and as a dose-dependent inhibitor of AR protein expression, mediated in part via an increase in AR degradation [8] Notably, VN/124-1 has similar AR inhibitory activity against wild-type AR and the T877A AR mutant. VN/124-1 was significantly more effective than castration or bicalutamide in suppressing growth of androgen-sensitive LAPC4 xenografts. Moreover, VN/124-1 maintained potent downregulation of AR in vivo, leading to a tenfold reduction in tumor AR levels compared to castration or bicalutamide (both of which demonstrated a two- to three-fold increase in AR expression). Interestingly, this agent also inhibits growth of AR-negative PCa cells via induction of the endoplasmic reticulum stress response [90].
Inhibitors of Other Steroidogenic Enzymes
The metabolic pathway from cholesterol to DHT offers several potential candidates for targeting steroid synthesis inhibition, either singly or in combination for maximal efficacy in reducing the production of tumor androgens.
The conversion of T to the more potent androgen DHT is carried out by steroid 5-alpha reductases SRD5A1 and SRD5A2 (and possibly SRD5A3, although the function of this enzyme has not been fully established) [91]. SRD5A2 is the primary isoform in benign prostate tissue, while PCa shows a relative increase in SRD5A1 expression and activity. Finasteride (a specific inhibitor of SRD5A2) and dutasteride (a dual SRD5A inhibitor) are 4-azasteroids extensively used in the treatment of BPH and have been explored for prevention and treatment of PCa. While dutasteride alone has limited activity in men with CRPC, a phase II study of ketoconazole, hydrocortisone, and dutasteride (KHAD) demonstrated PSA responses >50 % in 56 % of men and a median time to progression of 14.5 months, nearly twice that observed in phase II studies of abiraterone, leading the authors to postulate that intratumoral DHT synthesis may contribute to abiraterone resistance [92].
The final steps in T and DHT biosynthesis (reduction of the adrenal androgens AED and androstenedione, respectively) are catalyzed by HSD17B3 and/or AKR1C3. HSD17B3 is primarily expressed in testicular Leydig cells, while AKR1C3 mediates production of T and DHT in peripheral tissues. Increased expression of these enzymes in CRPC tumors suggests they may be important targets for inhibition [20, 35, 93]. The AKR1C family members AKR1C1 and AKR1C2 mediate catabolism of DHT (to 3β and 3α − diol, respectively), and a selective loss of these enzymes has been reported in prostate tumors (accompanied by a reduced capacity to metabolize DHT and an increase in tumoral DHT levels) [94].
Agents which selectively target AKR1C3 (but not the highly related AKR1C1 and AKR1C2) and HSD17B3 are under development. AKR1C family members are inhibited by nonsteroidal anti-inflammatory drugs (NSAIDs) and the COX-2 selective inhibitor celecoxib [95]. Indoleacetic acids (e.g., indomethacin) are among the most potent agents targeting the AKR1C family. Indomethacin analogs that selectively target AKR1C3 but do not inhibit COX-1, COX-2, AKR1C1, and AKR1C2 have been reported [96]. Small molecule inhibitors of HSD17B3 have been developed and shown to reduce systemic androgen levels (reviewed in [97]). However, to date, no studies of these agents in PCa have been reported.
The conversion of delta 4 steroids such as DHEA and androstenediol to delta 5 steroids AED and T, respectively, is mediated by 3BHSD. 3BHSD is required for de novo biosynthesis of androgens from cholesterol (via either classical or backdoor pathways) as well as for pathways converting adrenal androgens to T and DHT. The type 1 isoform is expressed in adrenal, ovary, and testis and type 2 in peripheral tissues such as prostate. Transcripts encoding both isoforms have been observed in CRPC metastases. Several studies have demonstrated that DHEA or androstenediol can directly activate wild-type and mutated AR [22], while others have demonstrated a requirement for 3BHSD-mediated conversion to downstream metabolites first [98], implicating 3BHSD as a therapeutic target for CRPC. Epostane, a competitive inhibitor of 3BHDS1, has been used in human studies for medical termination of pregnancy via inhibition of progesterone synthesis and has been shown to inhibit DHEA-induced proliferation of breast cancer MCF-7 cells [99], suggesting a study in PCa may be warranted.
Hydrolysis of inactive sulfates of estrogen and DHEA to biologically active steroids is carried out by steroid sulfatase (STS). PCa cell lines express functionally active STS, as demonstrated by hydrolysis of estrone-S and DHEA-S to unconjugated forms. STS expression in prostate tumors has been confirmed by immunohistochemical analyses (reviewed in [21]). STS inhibitors have been evaluated in breast cancer and may have efficacy in preventing prostatic utilization of the adrenal androgen DHEA, which primarily circulates as the inactive sulfate DHEA-S. A phase I study of the steroid sulfatase inhibitor BN83495 in men with advanced CRPC has recently completed accrual, and results are pending (NCT00790374) [100].
Apoptone (HE3235) is a synthetic analog of 3-beta androstanediol (a naturally occurring metabolite of DHT formed in prostate tissue). This agent has been shown to suppress tumor growth, decrease AR expression and nuclear localization, and suppress levels of intratumoral androgens in CRPC xenografts [101, 102]. While its mechanism of action has not been fully elucidated, HE3235 appears to inhibit conversion of d-cholesterol to d-pregnenolone, without inhibition of CYP17A1. HE3235 is currently under study in a phase I/II clinical trial of men with CRPC [103].
Production of androgens by the testis is under control of the hypothalamic-pituitary-testicular axis via sequential release of luteinizing hormone-releasing hormone (LHRH) and luteinizing hormone (LH) from the hypothalamus and pituitary, respectively. Variants of GnRH and LHRH receptor have also been demonstrated in prostate epithelium [104]. Thus, GnRH antagonist therapy may have direct antitumor effects [105], and LHRH receptors on prostate tumors may serve as targets for LHRH analogs hybridized to cytotoxic moieties. An analog of LHRH conjugated to doxorubicin has been clinically tested in women with gynecologic tumors expressing LHRH receptors [106]. Interestingly, receptors for LH itself have also been described in PCa specimens. Exposure of both androgen-sensitive (LNCaP) and androgen-independent (22RV1 and C4-2B) PCa cell lines to LH increased protein levels of steroidogenic enzymes including STAR, CYB5B, CYP11A, and 3BHSD, and a 2.5-fold increase in progesterone synthesis was observed in LH-treated C4-2B cells compared to controls [104]. LH may have a role in the regulation of steroid biosynthesis in PCa cells, with the LH receptor serving as a potential therapeutic target.
New Agents Targeting the AR and AR Signaling Mechanisms
Androgen Receptor Antagonists
AR antagonists prevent the AR from achieving the transcriptionally active conformation required for stable DNA binding via inhibition of chaperone dissociation, alterations in subcellular AR localization, recruitment of nuclear corepressor complexes, or ineffective recruitment of coactivator proteins (reviewed in [107]). Several mechanisms by which nonsteroidal antiandrogens function as AR agonists have been described, including AR mutations and/or alterations in cofactor recruitment. This has been a critical impetus for the development of novel, potent AR inhibitors without agonist activity against wild-type or mutant ARs (Table 74.2). The recent description of constitutively active AR variants lacking the C-terminal ligand-binding domain has also raised significant interest in the development of N-terminal-targeted antiandrogens.
MDV3100 is a second-generation diarylthiohydantoin competitive AR antagonist which binds to the AR with five- to eight-fold greater affinity than bicalutamide and only two- to three-fold lower affinity than DHT. Preclinical studies in VCaP xenografts (with endogenous AR gene amplification) or LNCaP xenografts engineered to express high AR levels have demonstrated that, compared to bicalutamide, MDV3100 potently decreased the nuclear translocation of AR, markedly reduces chromatin occupancy at canonical AREs, and is significantly more effective in suppressing tumor growth [6]. Importantly, MDV3100 did not elicit agonist activity against LNCaP tumors overexpressing the AR or against T877A or W741C AR mutations, situations in which bicalutamide demonstrates agonist activity. Moreover, targeting full-length AR with MDV3100 in cells expressing both truncated and full-length AR variants led to suppression of AR activity and cell growth. These data suggest certain classes of AR variants act via an interaction with full-length AR and that MDV3100 may have clinical efficacy even in patients whose tumors express ligand-independent AR variants [73].
While preclinical studies show that MDV3100 is highly effective in tumors driven by an amplified AR, androgen was able to overcome the AR inhibitory effects of MDV3100 in vitro, raising a question as to whether MDV3100 will be equally effective in the setting of a nonamplified AR, particularly if residual tumor androgens are present. AR is amplified in about 20–25 % of CRPC cases [55] and in up to 50 % of CRPC cases when circulating tumor cells (CTC) are evaluated [108], and these may represent cases in which MDV3100 will have most efficacy.
A phase I/II study of MDV3100 in 140 men with CRPC demonstrated maximum PSA declines >50 % in 62 % of chemotherapy-naïve patients and 51 % of docetaxel-treated patients (p = 0.23). At 12 weeks, the proportion of patients with declines >50 % was greater in the chemotherapy-naïve group (57 vs. 36 %, p = 0.02), and median time to PSA progression (defined as 25 % or greater increase from nadir) was 41 versus 21 weeks, respectively [5]. PSA declines >50 % were achieved in 37 % of patients with prior ketoconazole treatment versus 71 % of those without, and 10 of 22 patients who were assessed by [18F]-FDHT PET scans showed >25 % declines in FDHT accumulation. Responses were dose dependent up to 150 mg/day. Fatigue, nausea, dyspnea, anorexia, and back pain were the most common adverse events, with 240 mg/day determined to be the maximum tolerated dose. Two seizures were observed at 360 and 600 mg doses (also observed with the experimental AR antagonist BMS-641988, and potentially due to GABA-A antagonist activity of AR antagonists) [109]. A phase III randomized placebo-controlled trial of MDV3100 in docetaxel-treated men with metastatic CRPC is ongoing.
An alternative to pharmacological approaches that target the AR ligand-binding domain is development of N-terminal domain (NTD) AR inhibitors. The NTD is essential for both ligand-dependent and independent AR activation. Agents such as MDV3100 or nonsteroidal antiandrogens do not inhibit ligand-independent transactivation of the AR NTD (such as bypass mechanisms mediated by IL-6 and other peptide growth factors) nor do they directly target constitutively active AR splice variants lacking the LBD. At present, the most promising compound that has been published is EPI-001, which is a degradation product of bisphenol A and was found by testing a library of products isolated from marine sponges [7]. EPI-001 binds to the amino terminus of the AR and inhibits AR transactivation. EPI-001 does not alter AR nuclear translocation or prevent ligand binding but disrupts the AR N/C interaction thereby inhibiting cofactor recruitment. EPI-001 blocked ligand- and nonligand-dependent AR transactivation in LNCaP cells stimulated with R1881 or IL-6, as well as blocking AR activity in 22RV1 cells which express full-length and truncated AR variants. When given to castrate mice, EPI-001 decreased the size of AR-positive LNCaP xenografts but not AR-negative PC-3 tumors. No apparent toxicity has been noted in animals, and it has 85 % bioavailability after oral administration. The combination of a LBD and ligand-targeting agents has significant potential for robustly suppressing AR activity.
Modulators of AR Expression, Stability, and Downstream Signaling
Agents which do not target the AR directly but alter cellular pathways involved in maintaining expression, stability, and downstream signaling components of the AR axis are also under investigation for PCa therapy. Heat shock protein (HSP) chaperones, histone deacetylases (HDACs), and mammalian target of rapamycin (mTOR) are among those in development for men with CRPC.
HSP90 is an ATP-dependent chaperone protein involved in maintaining stability, localization, and activity of the AR as well as other oncogenic client proteins such as Her2 and AKT. Geldanamycin is an ansamycin antibiotic which binds the ATP-binding pocket of HSP90 leading to degradation of client proteins. Tanespimycin (17-AA-geldanamycin) inhibited growth of AR-positive PCa xenografts, accompanied by an 80 % decrease in AR expression [110]. Agents with improved solubility characteristics are currently being evaluated, as phase I studies have not shown significant clinical activity with current agents in men with CRPC.
Histone deacetylase (HDAC) inhibitors have been shown to modulate AR signaling and have demonstrated antitumor effects toward several malignancies. Transcriptional activity of numerous genes involved in cell survival and differentiation is regulated by chromatin remodeling, which is determined by the balance of histone acetylation versus deacetylation. HDAC inhibitors can decrease transcription of AR, inhibit AR-mediated transcription (by blocking recruitment of RNA polymerase to the promoter of HDAC-dependent AR target genes), and promote AR degradation (via acetylation-induced inhibition of HSP90 ATP binding) [111]. The combination of the HDAC inhibitor vorinostat (SAHA) with bicalutamide has shown synergistic activity in suppressing PCa cell proliferation in vitro [112]. A phase I study of vorinostat with docetaxel and a phase II study of single-agent vorinostat in the postchemotherapy setting showed minimal clinical response and significant dose-limiting toxicity, suggesting alternative agents in this class with a more favorable toxicity profile will be required. A phase I/II study of panobinostat (LBH589) in combination with bicalutamide in men with CRPC is ongoing.
Alterations in the PI3K/Akt/mTOR pathway (including loss or mutation of the negative regulator PTEN) are present in 30–50 % of prostate tumors, and this pathway is central to a number of signaling cascades mediating cell growth and survival. The Akt/mTOR pathway can also activate AR in the absence of androgen. Many agents targeting PI3K, Akt, and mTOR have been evaluated in both in vitro and in vivo models of PCa. Multiple phase I and II trials with the mTOR inhibitors rapamycin and its analogs everolimus (RAD-001) and temsirolimus (CCI-779) are ongoing [113]. Recent studies demonstrate a reciprocal feedback between PI3K and AR signaling, such that cotargeting the AR pathway may be significantly more effective than PI3K pathway inhibition alone [114–116].
Src kinases have been implicated in androgen-induced proliferation of CRPC cells and are nonreceptor protein tyrosine kinases involved in signal transduction downstream of multiple cell surface receptors, including EGFR, PDGFR, and VEGFR. A dual Abl and Src family kinase inhibitor dasatinib has been shown to inhibit AR phosphorylation and activation in vitro [117], as well as targeting osteoclast and osteoblast activity [118]. In a phase II study of chemotherapy-naïve men with CRPC, progression occurred in 60 and 80 % of patients at 12 and 24 weeks respectively, although nearly half the patients showed a decrease in markers of bone metabolism [119]. A randomized phase III study of dasatinib in combination with docetaxel (with skeletal-related events as one end point) is ongoing.
Conclusions
Data regarding the molecular responses of PCa to therapeutics targeting the AR pathway continues to emerge, providing critical insights into cellular growth and signaling pathways that may be exploited as treatment targets. The optimal timing, sequence, and potential combinatorial strategies for novel AR pathway inhibitors entering clinical practice are critical questions in the treatment of men with CRPC. The introduction of potent steroidogenic inhibitors in combination with novel AR antagonists holds significant promise for the concept of multitargeted AR pathway blockade, as the presence of residual androgens and persistent activation of the AR signaling axis in CRPC suggest that a multitargeted treatment approach to ablate all contributions to AR signaling within the prostate tumor will be required for optimal antitumor efficacy.
While the clinical response to agents such as abiraterone and MDV3100 in men with CRPC has been impressive, the duration of response has been variable, mechanisms of resistance are not well understood, and optimal treatment strategies for men who develop resistance to abiraterone or MDV3100 have yet to be established. Whether these tumors now represent cancers that are entirely independent of AR pathway activity or still retain dependence on the AR signaling axis is a central question for selection of therapy in this setting. In this regard, recent data in preclinical models have shown that abiraterone treatment may variously result in upregulation of wild-type AR, AR splice variants, and CYP17A expression [120, 121]. Importantly, the effect of abiraterone on tumor tissue from patients is poorly understood, and the extent to which the therapeutic efficacy of agents targeting the AR axis is influenced by either baseline or treatment-induced differences in these resistance mechanisms is unknown. Delineating mechanisms and biomarkers of resistance to novel AR pathway inhibitors will be critical for rational trial design and for the stratification of men with CRPC to treatment strategies with the highest likelihood of durable efficacy.
References
Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–9.
Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23(32):8253–61.
de Bono JS. Abiraterone acetate improves survival in metastatic castration-resistant prostate cancer: phase III results. 2010 European Society for Medical Oncology, Milan, 2010.
Dreicer R, Agus DB, MacVicar GR, MacLean D, Zhang T, Stadler WM. Safety, pharmacokinetics, and efficacy of TAK-700 in castration-resistant, metastatic prostate cancer: a phase I/II, open-label study. Genitourinary cancers symposium, , San Francisco, Feb 2010.
Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 2010;375(9724):1437–46.
Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–90.
Andersen RJ, Mawji NR, Wang J, et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell. 2010;17(6):535–46.
Vasaitis T, Belosay A, Schayowitz A, et al. Androgen receptor inactivation contributes to antitumor efficacy of 17{alpha}-hydroxylase/17,20-lyase inhibitor 3beta-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene in prostate cancer. Mol Cancer Ther. 2008;7(8):2348–57.
Geller J, Liu J, Albert J, Fay W, Berry CC, Weis P. Relationship between human prostatic epithelial cell protein synthesis and tissue dihydrotestosterone level. Clin Endocrinol (Oxf). 1987;26(2):155–61.
Mohler JL, Gregory CW, Ford 3rd OH, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10(2):440–8.
Nishiyama T, Hashimoto Y, Takahashi K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin Cancer Res. 2004;10(21):7121–6.
Page ST, Lin DW, Mostaghel EA, et al. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab. 2006;91(10):3850–6.
Geller J, Albert J, Loza D, Geller S, Stoeltzing W, de la Vega D. DHT concentrations in human prostate cancer tissue. J Clin Endocrinol Metab. 1978;46(3):440–4.
Geller J, Albert J, Yen SS, Geller S, Loza D. Medical castration of males with megestrol acetate and small doses of diethylstilbestrol. J Clin Endocrinol Metab. 1981;52(3):576–80.
Liu J, Geller J, Albert J, Kirshner M. Acute effects of testicular and adrenal cortical blockade on protein synthesis and dihydrotestosterone content of human prostate tissue. J Clin Endocrinol Metab. 1985;61(1):129–33.
Liu J, Albert J, Geller J. Effects of androgen blockade with ketoconazole and megestrol acetate on human prostatic protein patterns. Prostate. 1986;9(2):199–205.
Geller J, Albert J. Effects of castration compared with total androgen blockade on tissue dihydrotestosterone (DHT) concentration in benign prostatic hyperplasia (BPH). Urol Res. 1987;15(3):151–3.
Forti G, Salerno R, Moneti G, et al. Three-month treatment with a long-acting gonadotropin-releasing hormone agonist of patients with benign prostatic hyperplasia: effects on tissue androgen concentration, 5 alpha-reductase activity and androgen receptor content. J Clin Endocrinol Metab. 1989;68(2):461–8.
Nishiyama T, Ikarashi T, Hashimoto Y, Wako K, Takahashi K. The change in the dihydrotestosterone level in the prostate before and after androgen deprivation therapy in connection with prostate cancer aggressiveness using the Gleason score. J Urol. 2007;178(4 Pt 1):1282–8; discussion 8–9.
Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447–54.
Mostaghel EA, Nelson PS. Intracrine androgen metabolism in prostate cancer progression: mechanisms of castration resistance and therapeutic implications. Best Pract Res Clin Endocrinol Metab. 2008;22(2):243–58.
Mizokami A, Koh E, Fujita H, et al. The adrenal androgen androstenediol is present in prostate cancer tissue after androgen deprivation therapy and activates mutated androgen receptor. Cancer Res. 2004;64(2):765–71.
Miyamoto H, Yeh S, Lardy H, Messing E, Chang C. Delta5-androstenediol is a natural hormone with androgenic activity in human prostate cancer cells. Proc Natl Acad Sci USA. 1998;95(19):11083–8.
Culig Z, Hoffmann J, Erdel M, et al. Switch from antagonist to agonist of the androgen receptor bicalutamide is associated with prostate tumour progression in a new model system. Br J Cancer. 1999;81(2):242–51.
Gregory CW, Johnson Jr RT, Mohler JL, French FS, Wilson EM. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 2001;61(7):2892–8.
Gregory CW, Hamil KG, Kim D, et al. Androgen receptor expression in androgen-independent prostate cancer is associated with increased expression of androgen-regulated genes. Cancer Res. 1998;58(24):5718–24.
Mohler JL, Morris TL, Ford 3rd OH, Alvey RF, Sakamoto C, Gregory CW. Identification of differentially expressed genes associated with androgen-independent growth of prostate cancer. Prostate. 2002;51(4):247–55.
Mostaghel EA, Page ST, Lin DW, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67(10):5033–41.
Greenberg E. Endocrine therapy in the management of prostatic cancer. Clin Endocrinol Metab. 1980;9(2):369–81.
Robinson MR, Shearer RJ, Fergusson JD. Adrenal suppression in the treatment of carcinoma of the prostate. Br J Urol. 1974;46(5):555–9.
Samson DJ, Seidenfeld J, Schmitt B, et al. Systematic review and meta-analysis of monotherapy compared with combined androgen blockade for patients with advanced prostate carcinoma. Cancer. 2002;95(2):361–76.
Schmitt B, Bennett C, Seidenfeld J, Samson D, Wilt T. Maximal androgen blockade for advanced prostate cancer. Cochrane Database Syst Rev. 2000;(2):CD001526.
Caubet JF, Tosteson TD, Dong EW, et al. Maximum androgen blockade in advanced prostate cancer: a meta-analysis of published randomized controlled trials using nonsteroidal antiandrogens. Urology. 1997;49(1):71–8.
Small EJ, Ryan CJ. The case for secondary hormonal therapies in the chemotherapy age. J Urol. 2006;176(6 Suppl 1):S66–71.
Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66(5):2815–25.
Holzbeierlein J, Lal P, LaTulippe E, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164(1):217–27.
Koh E, Kanaya J, Namiki M. Adrenal steroids in human prostatic cancer cell lines. Arch Androl. 2001;46(2):117–25.
Mizokami A, Koh E, Izumi K, et al. Prostate cancer stromal cells and LNCaP cells coordinately activate the androgen receptor through synthesis of testosterone and dihydrotestosterone from dehydroepiandrosterone. Endocr Relat Cancer. 2009;16(4):1139–55.
Locke JA, Guns ES, Lubik AA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68(15):6407–15.
Chang KH, Li R, Papari-Zareei M, et al. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc Natl Acad Sci USA. 2011;108(33):13728–33.
Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab. 2004;15(9):432–8.
Mohler JL, Titus MA, Bai S, et al. Activation of the androgen receptor by intratumoral bioconversion of androstanediol to dihydrotestosterone in prostate cancer. Cancer Res. 2011;71(4):1486–96.
Bauman DR, Steckelbroeck S, Williams MV, Peehl DM, Penning TM. Identification of the major oxidative 3{alpha}-hydroxysteroid dehydrogenase in human prostate that converts 5{alpha}-androstane-3{alpha},17{beta}-diol to 5{alpha}-dihydrotestosterone: a potential therapeutic target for androgen-dependent disease. Mol Endocrinol. 2006;20(2):444–58.
Dillard PR, Lin MF, Khan SA. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol. 2008;295(1–2):115–20.
Locke JA, Wasan KM, Nelson CC, Guns ES, Leon CG. Androgen-mediated cholesterol metabolism in LNCaP and PC-3 cell lines is regulated through two different isoforms of acyl-coenzyme A: Cholesterol Acyltransferase (ACAT). Prostate. 2008;68(1):20–33.
Locke JA, Nelson CC, Adomat HH, Hendy SC, Gleave ME, Guns ES. Steroidogenesis inhibitors alter but do not eliminate androgen synthesis mechanisms during progression to castration-resistance in LNCaP prostate xenografts. J Steroid Biochem Mol Biol. 2009;115(3–5):126–36. Epub 2009 Apr 5.
Leon CG, Locke JA, Adomat HH, et al. Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration-resistant prostate cancer in a mouse xenograft model. Prostate. 2009;70(4):390–400.
Arnold JT, Gray NE, Jacobowitz K, et al. Human prostate stromal cells stimulate increased PSA production in DHEA-treated prostate cancer epithelial cells. J Steroid Biochem Mol Biol. 2008;111(3–5):240–6.
Sillat T, Pöllänen R, Lopes JR, et al. Intracrine androgenic apparatus in human bone marrow stromal cells. J Cell Mol Med. 2009;13(9B):3296–302.
Wright JL, Kwon EM, Ostrander EA, et al. Expression of SLCO transport genes in castration resistant prostate cancer and impact of genetic variation in SCLO1B3 and SLCO2B1 on prostate cancer outcomes. Cancer Epidemiol Biomarkers Prev. 2011;20(4):619–27. Epub 2011 Jan 25.
Zair ZM, Eloranta JJ, Stieger B, Kullak-Ublick GA. Pharmacogenetics of OATP (SLC21/SLCO), OAT and OCT (SLC22) and PEPT (SLC15) transporters in the intestine, liver and kidney. Pharmacogenomics. 2008;9(5):597–624.
Hamada A, Sissung T, Price DK, et al. Effect of SLCO1B3 haplotype on testosterone transport and clinical outcome in caucasian patients with androgen-independent prostatic cancer. Clin Cancer Res. 2008;14(11):3312–8.
Sharifi N, Hamada A, Sissung T, et al. A polymorphism in a transporter of testosterone is a determinant of androgen independence in prostate cancer. BJU Int. 2008;102(5):617–21.
Yang M, Oh WK, Xie W, et al. Genetic variations in SLCO2B1 and SLCO1B3 and the efficacy of androgen-deprivation therapy in prostate cancer patients. J Clin Oncol. 2011;29(18):2565–73.
Visakorpi T, Hyytinen E, Koivisto P, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9(4):401–6.
Sun S, Sprenger CC, Vessella RL, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120(8):2715–30.
Taplin ME, Balk SP. Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem. 2004;91(3):483–90.
Steinkamp MP, O’Mahony OA, Brogley M, et al. Treatment-dependent androgen receptor mutations in prostate cancer exploit multiple mechanisms to evade therapy. Cancer Res. 2009;69(10):4434–42.
Veldscholte J, Berrevoets CA, Ris-Stalpers C, et al. The androgen receptor in LNCaP cells contains a mutation in the ligand binding domain which affects steroid binding characteristics and response to antiandrogens. J Steroid Biochem Mol Biol. 1992;41(3–8):665–9.
Chmelar R, Buchanan G, Need EF, Tilley W, Greenberg NM. Androgen receptor coregulators and their involvement in the development and progression of prostate cancer. Int J Cancer. 2007;120:719–33.
Zhu P, Baek SH, Bourk EM, et al. Macrophage/cancer cell interactions mediate hormone resistance by a nuclear receptor derepression pathway. Cell. 2006;124(3):615–29.
Rahman MM, Miyamoto H, Lardy H, Chang C. Inactivation of androgen receptor coregulator ARA55 inhibits androgen receptor activity and agonist effect of antiandrogens in prostate cancer cells. Proc Natl Acad Sci USA. 2003;100(9):5124–9.
Culig Z. Androgen receptor cross-talk with cell signalling pathways. Growth Factors. 2004;22(3):179–84.
Wu JD, Haugk K, Woodke L, Nelson P, Coleman I, Plymate SR. Interaction of IGF signaling and the androgen receptor in prostate cancer progression. J Cell Biochem. 2006;99(2):392–401.
Dorff TB, Goldman B, Pinski JK, et al. Clinical and correlative results of SWOG S0354: a phase II trial of CNTO328 (siltuximab), a monoclonal antibody against interleukin-6, in chemotherapy-pretreated patients with castration-resistant prostate cancer. Clin Cancer Res. 2010;16(11):3028–34.
Srebrow A, Kornblihtt AR. The connection between splicing and cancer. J Cell Sci. 2006;119(Pt 13):2635–41.
Guo Z, Yang X, Sun F, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69(6):2305–13.
Hornberg E, Ylitalo EB, Crnalic S, et al. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS One. 2011;6(4):e19059.
Hu R, Dunn TA, Wei S, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69(1):16–22.
Hu R, Isaacs WB, Luo J. A snapshot of the expression signature of androgen receptor splicing variants and their distinctive transcriptional activities. Prostate. 2011;71(15):1656–67. doi:10.1002/pros.21382. Epub 2011 Mar 28.
Marcias G, Erdmann E, Lapouge G, et al. Identification of novel truncated androgen receptor (AR) mutants including unreported pre-mRNA splicing variants in the 22Rv1 hormone-refractory prostate cancer (PCa) cell line. Hum Mutat. 2010;31(1):74–80.
Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68(13):5469–77.
Watson PA, Chen YF, Balbas MD, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci. 2010;107(39):16759–65. Epub 2010 Sep 7.
Li Y, Alsagabi M, Fan D, Bova GS, Tewfik AH, Dehm SM. Intragenic rearrangement and altered RNA splicing of the androgen receptor in a cell-based model of prostate cancer progression. Cancer Res. 2011;71(6):2108–17.
Centenera MM, Harris JM, Tilley WD, Butler LM. The contribution of different androgen receptor domains to receptor dimerization and signaling. Mol Endocrinol. 2008;22(11):2373–82.
Molina A, Belldegrun A. Novel therapeutic strategies for castration resistant prostate cancer: inhibition of persistent androgen production and androgen receptor mediated signaling. J Urol. 2011;185(3):787–94.
Morote J, Planas J, Salvador C, Raventos CX, Catalan R, Reventos J. Individual variations of serum testosterone in patients with prostate cancer receiving androgen deprivation therapy. BJU Int. 2009;103(3):332–5; discussion 5.
Raddin RS, Walko CM, Whang YE. Response to degarelix after resistance to luteinizing hormone-releasing hormone agonist therapy for metastatic prostate cancer. Anticancer Drugs. 2011;22(3):299–302.
Ryan CJ, Halabi S, Ou SS, Vogelzang NJ, Kantoff P, Small EJ. Adrenal androgen levels as predictors of outcome in prostate cancer patients treated with ketoconazole plus antiandrogen withdrawal: results from a cancer and leukemia group B study. Clin Cancer Res. 2007;13(7):2030–7.
Narimoto K, Mizokami A, Izumi K, et al. Adrenal androgen levels as predictors of outcome in castration-resistant prostate cancer patients treated with combined androgen blockade using flutamide as a second-line anti-androgen. Int J Urol. 2010;17(4):337–45.
Hashimoto K, Masumori N, Hashimoto J, Takayanagi A, Fukuta F, Tsukamoto T. Serum testosterone level to predict the efficacy of sequential Use of antiandrogens as second-line treatment following androgen deprivation monotherapy in patients with castration-resistant prostate cancer. Jpn J Clin Oncol. 2010;41(3):405–10.
Handratta VD, Vasaitis TS, Njar VCO, et al. Novel C-17-heteroaryl steroidal CYP17 inhibitors/antiandrogens: synthesis, in vitro biological activity, pharmacokinetics, and antitumor activity in the LAPC4 human prostate cancer xenograft model. J Med Chem. 2005;48(8):2972–84.
O’Donnell A, Judson I, Dowsett M, et al. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004;90(12):2317–25.
Attard G, Reid AH, A’Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27(23):3742–8.
Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26(28):4563–71.
Ryan CJ, Smith MR, Fong L, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28(9):1481–8.
Reid AH, Attard G, Danila DC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28(9):1489–95.
Danila DC, Morris MJ, de Bono JS, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28(9):1496–501.
Ryan CJ, Shah SK, Efstathiou E, et al. Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clin Cancer Res. 2011;17(14):4854–61. Epub 2011 Jun 1.
Bruno RD, Gover TD, Burger AM, Brodie AM, Njar VC. 17alpha-Hydroxylase/17,20 lyase inhibitor VN/124-1 inhibits growth of androgen-independent prostate cancer cells via induction of the endoplasmic reticulum stress response. Mol Cancer Ther. 2008;7(9):2828–36.
Godoy A, Kawinski E, Li Y, et al. 5alpha-reductase type 3 expression in human benign and malignant tissues: a comparative analysis during prostate cancer progression. Prostate. 2011;71(10):1033–46.
Taplin ME, Regan MM, Ko YJ, et al. Phase II study of androgen synthesis inhibition with ketoconazole, hydrocortisone, and dutasteride in asymptomatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15(22):7099–105.
Bauman D, Steckelbroeck S, Peehl D, Penning T. Transcript profiling of the androgen signal in normal prostate, benign prostatic hyperplasia and prostate cancer. Endocrinology. 2006;147(12):5806–16. Epub 2006 Sep 7.
Ji Q, Chang L, Stanczyk FZ, Ookhtens M, Sherrod A, Stolz A. Impaired dihydrotestosterone catabolism in human prostate cancer: critical role of AKR1C2 as a pre-receptor regulator of androgen receptor signaling. Cancer Res. 2007;67(3):1361–9.
Bauman DR, Rudnick SI, Szewczuk LM, Jin Y, Gopishetty S, Penning TM. Development of nonsteroidal anti-inflammatory drug analogs and steroid carboxylates selective for human aldo-keto reductase isoforms: potential antineoplastic agents that work independently of cyclooxygenase isozymes. Mol Pharmacol. 2005;67(1):60–8.
Byrns MC, Steckelbroeck S, Penning TM. An indomethacin analogue, N-(4-chlorobenzoyl)-melatonin, is a selective inhibitor of aldo-keto reductase 1C3 (type 2 3alpha-HSD, type 5 17beta-HSD, and prostaglandin F synthase), a potential target for the treatment of hormone dependent and hormone independent malignancies. Biochem Pharmacol. 2008;75(2):484–93. Epub 2007 Sep 14.
Day JM, Tutill HJ, Purohit A, Reed MJ. Design and validation of specific inhibitors of 17beta-hydroxysteroid dehydrogenases for therapeutic application in breast and prostate cancer, and in endometriosis. Endocr Relat Cancer. 2008;15(3):665–92.
Evaul K, Li R, Papari-Zareei M, Auchus RJ, Sharifi N. 3beta-hydroxysteroid dehydrogenase is a possible pharmacological target in the treatment of castration-resistant prostate cancer. Endocrinology. 2010;151(8):3514–20.
Thomas JL, Bucholtz KM, Kacsoh B. Selective inhibition of human 3beta-hydroxysteroid dehydrogenase type 1 as a potential treatment for breast cancer. J Steroid Biochem Mol Biol. 2011;125(1–2):57–65. Epub 2010 Aug 22.
A phase I dose escalating study evaluating the pharmacodynamic profile and safety of BN83495 in patients with prostate cancer with evidence of disease progression while on androgen ablative therapy. ClinicalTrialsgov 2011. 28 Feb 2011. Cited 1 Apr 2011, NCT00790374. Available from http://clinicaltrials.gov/ct2/show/study/NCT00790374?intr=%22BN83495%22&rank=1.
Koreckij TD, Trauger RJ, Montgomery RB, et al. HE3235 inhibits growth of castration-resistant prostate cancer. Neoplasia. 2009;11(11):1216–25.
Ahlem C, Kennedy M, Page T, et al. 17alpha-Alkynyl 3alpha, 17beta-androstanediol non-clinical and clinical pharmacology, pharmacokinetics and metabolism. Invest New Drugs. 2012;30(1):59–78. Epub 2010 Sep 3.
Montgomery RB, Morris MJ, Ryan CJ, et al. HE3235, a synthetic adrenal hormone, in patients with castration-resistant prostate cancer (CRPC): clinical phase I/II trial results. Genitourinary cancers symposium, San Francisco, Feb 2010.
Liu SV, Schally AV, Hawes D, et al. Expression of receptors for luteinizing hormone-releasing hormone (LH-RH) in prostate cancers following therapy with LH-RH agonists. Clin Cancer Res. 2010;16(18):4675–80.
Gnanapragasam V, Darby S, Khan M, Lock W, Robson C, Leung H. Evidence that prostate gonadotropin-releasing hormone receptors mediate an anti-tumourigenic response to analogue therapy in hormone refractory prostate cancer. J Pathol. 2005;206(2):205–13.
Emons G, Sindermann H, Engel J, Schally AV, Grundker C. Luteinizing hormone-releasing hormone receptor-targeted chemotherapy using AN-152. Neuroendocrinology. 2009;90(1):15–8.
Singh P, Uzgare A, Litvinov I, Denmeade SR, Isaacs JT. Combinatorial androgen receptor targeted therapy for prostate cancer. Endocr Relat Cancer. 2006;13(3):653–66.
Leversha MA, Han J, Asgari Z, et al. Fluorescence in situ hybridization analysis of circulating tumor cells in metastatic prostate cancer. Clin Cancer Res. 2009;15(6):2091–7.
Rathkopf D, Liu G, Carducci MA, et al. Phase I dose-escalation study of the novel antiandrogen BMS-641988 in patients with castration-resistant prostate cancer. Clin Cancer Res. 2011;17(4):880–7.
Solit DB, Zheng FF, Drobnjak M, et al. 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res. 2002;8(5):986–93.
Welsbie DS, Xu J, Chen Y, et al. Histone deacetylases are required for androgen receptor function in hormone-sensitive and castrate-resistant prostate cancer. Cancer Res. 2009;69(3):958–66.
Marrocco DL, Tilley WD, Bianco-Miotto T, et al. Suberoylanilide hydroxamic acid (vorinostat) represses androgen receptor expression and acts synergistically with an androgen receptor antagonist to inhibit prostate cancer cell proliferation. Mol Cancer Ther. 2007;6(1):51–60.
Morgan TM, Koreckij TD, Corey E. Targeted therapy for advanced prostate cancer: inhibition of the PI3K/Akt/mTOR pathway. Curr Cancer Drug Targets. 2009;9(2):237–49.
Wang Y, Kreisberg JI, Ghosh PM. Cross-talk between the androgen receptor and the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer. Curr Cancer Drug Targets. 2007;7(6):591–604.
Carver BS, Chapinski C, Wongvipat J, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19(5):575–86.
Mulholland DJ, Tran LM, Li Y, et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell. 2011;19(6):792–804.
Liu Y, Karaca M, Zhang Z, Gioeli D, Earp HS, Whang YE. Dasatinib inhibits site-specific tyrosine phosphorylation of androgen receptor by Ack1 and Src kinases. Oncogene. 2010;29(22):3208–16.
Koreckij T, Nguyen H, Brown LG, Yu EY, Vessella RL, Corey E. Dasatinib inhibits the growth of prostate cancer in bone and provides additional protection from osteolysis. Br J Cancer. 2009;101(2):263–8.
Yu EY, Wilding G, Posadas E, et al. Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15(23):7421–8.
Changmeng C, Balk SP. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71(20):6503–13. Epub 2011 Aug 25.
Mostaghel EA, Marck B, Plymate S, et al. Resistance to CYP17A1 inhibition with abiraterone in castration resistant prostate cancer: Induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17(18):5913–25. Epub 2011 Aug 1.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag London
About this chapter
Cite this chapter
Mostaghel, E.A., Nelson, P.S. (2013). Hormone-Based Therapies for Castration-Resistant Prostate Cancer. In: Tewari, A. (eds) Prostate Cancer: A Comprehensive Perspective. Springer, London. https://doi.org/10.1007/978-1-4471-2864-9_74
Download citation
DOI: https://doi.org/10.1007/978-1-4471-2864-9_74
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-2863-2
Online ISBN: 978-1-4471-2864-9
eBook Packages: MedicineMedicine (R0)